Abstract
The first alkaline phosphatase (APase) structural gene mutant of Bacillus subtilis 168 was constructed by using a clone identified by hybridization to a synthetic degenerative oligonucleotide. The design of the probe was based on the first 29 amino acids of the sequenced mature APase III protein, which had been isolated from the secreted fraction of vegetative, phosphate-starved cells. DNA sequencing of the clone revealed the first 80 amino acids of the APase III protein, including a typical procaryotic signal sequence of 32 amino acids preceding the start of the mature protein. The 29 amino acids encoded by the predicted open reading frame immediately following the signal sequence are identical to the first 29 amino acids of the sequenced mature protein. This region shows 80% identity to strand A of the beta sheet that is very well conserved in Escherichia coli and mammalian APases. A phoAIII structural mutant was constructed by insertional mutagenesis with a fragment internal to the coding region. The effects of this mutation on APase production in B. subtilis 168 were analyzed under both phosphate starvation and sporulation conditions. The mutation in APase III reduced the total vegetative APase specific activity by approximately 40% and sporulation APase specific activity by approximately 45%. An APase protein was isolated from sporulating cells at stage III and was identified as APase III by protein sequencing of the amino terminus and by its absence in the phoAIII mutant. The APase III gene has been mapped to approximately 50 degrees on the B. subtilis chromosome.
Full text
PDF


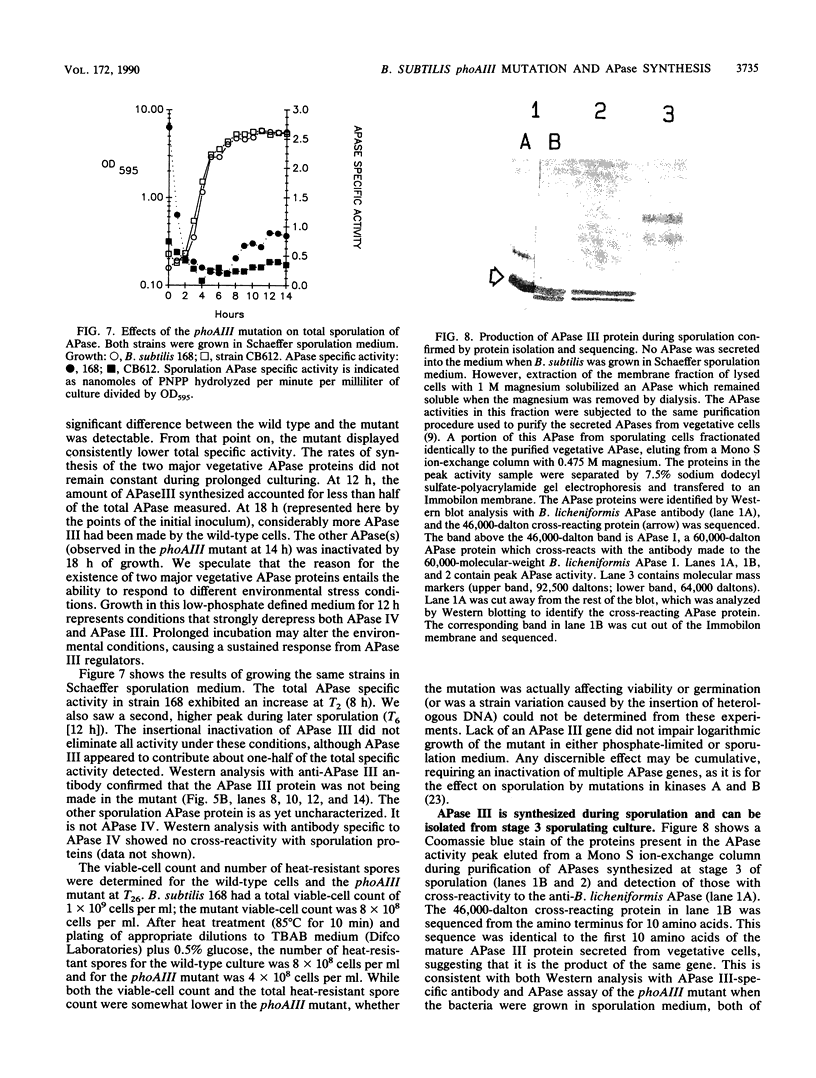


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradshaw R. A., Cancedda F., Ericsson L. H., Neumann P. A., Piccoli S. P., Schlesinger M. J., Shriefer K., Walsh K. A. Amino acid sequence of Escherichia coli alkaline phosphatase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3473–3477. doi: 10.1073/pnas.78.6.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Glenn A. R., Mandelstam J. Sporulation in Bacillus subtilis 168. Comparison of alkaline phosphatase from sporulating and vegetative cells. Biochem J. 1971 Jun;123(2):129–138. doi: 10.1042/bj1230129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D. Sporulation in Bacillus subtilis 168. Control of synthesis of alkaline phosphatase. J Gen Microbiol. 1974 Jun;82(2):363–369. doi: 10.1099/00221287-82-2-363. [DOI] [PubMed] [Google Scholar]
- Greaves H. Microbiological aspects of wood chip storage in tropical environments. Aust J Biol Sci. 1975 Jun;28(3):323–330. [PubMed] [Google Scholar]
- Harwood C. R., Williams D. M., Lovett P. S. Nucleotide sequence of a Bacillus pumilus gene specifying chloramphenicol acetyltransferase. Gene. 1983 Oct;24(2-3):163–169. doi: 10.1016/0378-1119(83)90076-8. [DOI] [PubMed] [Google Scholar]
- Hulett F. M., Bookstein C., Jensen K. Evidence for two structural genes for alkaline phosphatase in Bacillus subtilis. J Bacteriol. 1990 Feb;172(2):735–740. doi: 10.1128/jb.172.2.735-740.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett F. M., Jensen K. Critical roles of spo0A and spo0H in vegetative alkaline phosphatase production in Bacillus subtilis. J Bacteriol. 1988 Aug;170(8):3765–3768. doi: 10.1128/jb.170.8.3765-3768.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam W., Clauser E., Kim Y. S., Kan Y. W., Rutter W. J. Cloning, sequencing, and chromosomal localization of human term placental alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8715–8719. doi: 10.1073/pnas.82.24.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M., Binnie C., Schmidt R., Losick R. Cloned gene encoding the delta subunit of Bacillus subtilis RNA polymerase. Gene. 1988 Jul 15;67(1):13–19. doi: 10.1016/0378-1119(88)90003-0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Guss B., Uhlén M., Philipson L., Lindberg M. Gene for staphylococcal protein A. Proc Natl Acad Sci U S A. 1983 Feb;80(3):697–701. doi: 10.1073/pnas.80.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Kimura S., Nakata A., Ishihama A. Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol. 1988 Sep 5;203(1):85–95. doi: 10.1016/0022-2836(88)90093-9. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Miki T., Minami Z., Ikeda Y. The genetics of alkaline phosphatase formation in Bacillus subtilis. Genetics. 1965 Nov;52(5):1093–1100. doi: 10.1093/genetics/52.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J Biol Chem. 1970 Mar 10;245(5):1137–1145. [PubMed] [Google Scholar]
- Neugebauer K., Sprengel R., Schaller H. Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a Gram-positive bacterium. Nucleic Acids Res. 1981 Jun 11;9(11):2577–2588. doi: 10.1093/nar/9.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Cole S. P., Burbulys D., Trach K., Hoch J. A. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol. 1989 Nov;171(11):6187–6196. doi: 10.1128/jb.171.11.6187-6196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Taylor S. Y. New types of mutation affecting formation of alkaline phosphatase by Bacillus subtilis in sporulation conditions. J Gen Microbiol. 1977 Sep;102(1):69–80. doi: 10.1099/00221287-102-1-69. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Yoshikawa H., Takahashi H., Saito H. Cloning and nucleotide sequence of phoP, the regulatory gene for alkaline phosphatase and phosphodiesterase in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):2913–2916. doi: 10.1128/jb.169.7.2913-2916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. PHO-regulon of Escherichia coli K12: a minireview. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):243–249. [PubMed] [Google Scholar]
- Vandeyar M. A., Zahler S. A. Chromosomal insertions of Tn917 in Bacillus subtilis. J Bacteriol. 1986 Aug;167(2):530–534. doi: 10.1128/jb.167.2.530-534.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites W. M., Kay D., Dawes I. W., Wood D. A., Warren S. C., Mandelstam J. Sporulation in Bacillus subtilis. Correlation of biochemical events with morphological changes in asporogenous mutants. Biochem J. 1970 Jul;118(4):667–676. doi: 10.1042/bj1180667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. C. Sporulation in Bacillus subtilis. Biochemical changes. Biochem J. 1968 Oct;109(5):811–818. doi: 10.1042/bj1090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Henthorn P. S., Lafferty M. A., Slaughter C., Raducha M., Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Abrahmsén L. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 1989 Feb 27;244(2):439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]