Abstract
The heavy chain of cytoplasmic dynein is required for nuclear migration in Aspergillus nidulans and other fungi. Here we report on a new gene required for nuclear migration, nudG, which encodes a homologue of the “8-kD” cytoplasmic dynein light chain (CDLC). We demonstrate that the temperature sensitive nudG8 mutation inhibits nuclear migration and growth at restrictive temperature. This mutation also inhibits asexual and sexual sporulation, decreases the intracellular concentration of the nudG CDLC protein and causes the cytoplasmic dynein heavy chain to be absent from the mycelial tip, where it is normally located in wild-type mycelia. Coimmunoprecipitation experiments with antibodies against the cytoplasmic dynein heavy chain (CDHC) and the nudG CDLC demonstrated that some fraction of the cytoplasmic dynein light chain is in a protein complex with the CDHC. Sucrose gradient sedimentation analysis, however, showed that not all of the NUDG protein is complexed with the heavy chain. A double mutant carrying a cytoplasmic dynein heavy chain deletion plus a temperature-sensitive nudG mutation grew no more slowly at restrictive temperature than a strain with only the CDHC deletion. This result demonstrates that the effect of the nudG mutation on nuclear migration and growth is mediated through an interaction with the CDHC rather than with some other molecule (e.g., myosin-V) with which the 8-kD CDLC might theoretically interact.
Keywords: cytoplasmic dynein, light chain, nucleus, migration, Aspergillus
Nuclear positioning is of fundamental importance to the growth and development of both higher and lower eukaryotes. In higher eukaryotes, nuclear migration plays a significant role in a wide variety of processes, which include epithelial folding (Sauer, 1935; Schoenwolf and Smith, 1990; Viebahn et al., 1995), cancer cell migration (Klominek et al., 1991), pronuclear migration during fertilization (Schatten, 1982; Reinsch and Karsenti, 1997), nuclear corticalization in insect eggs (Zalokar and Erk, 1976; Baker et al., 1993), mitotic spindle orientation in Drosophila (McGrail and Hays, 1997; Theurkauf, 1997), and development of the Drosophila eye (Fan and Ready, 1997). Among lower eukaryotes, nuclear migration is required to distribute nuclei through the hyphal mycelium in filamentous fungi (reviewed by Morris et al., 1995), to move daughter nuclei into the bud in budding yeast (reviewed by Hoyt et al., 1997; Stearns, 1997), to partition nuclei into daughter cells in fission yeast (reviewed by Hagan and Yanagida, 1997) and for karyogamy (reviewed by Rose, 1996). In the budding yeast Saccharomyces cerevisiae, cytoplasmic dynein, dynactin, and a set of kinesins play overlapping roles in nuclear positioning and migration into the bud. Deletion of dynein or any other individual motor has little effect on either mitosis or colony forming ability because of this functional redundancy. In contrast, dynein deletion in the filamentous fungi has a profound effect on both nuclear migration and colony forming ability (Plamann et al., 1994; Xiang et al., 1994). Nuclear migration is almost abolished in the absence of the cytoplasmic dynein heavy chain, yet dynein-deficient colonies are still able to grow slowly, suggesting some redundant function that can mediate low level nuclear migration. A subtle effect of kinesin deficiency on nuclear distribution in Neurospora crassa (Seiler et al., 1997) suggests that kinesin also plays a role in nuclear migration and might provide this redundancy.
In higher organisms, cytoplasmic dynein has been shown to be a multisubunit, minus-end-directed, microtubule-dependent, motor protein that is involved in the motility of a wide variety of organelles (reviewed by Sheetz, 1996; Vallee and Sheetz, 1996; Hirokawa, 1998). It consists of two or three very high molecular weight heavy chains (∼500 kD) that are responsible for microtubule (MT)1 binding and motor activity, several intermediate chains of ∼74 kD, and several light intermediate chains of 52–61 kD (Holzbauer et al., 1994; Schroer, 1994). Different heavy chains have been associated with different cellular organelles (Vaisberg et al., 1996). In addition to the heavy, intermediate, and light intermediate chains of cytoplasmic dynein, an “8-kD” light chain component was recently identified by a database search for sequences similar to flagellar outer arm dynein from Chlamydomonas reinhardii. This revealed sequences related to the outer arm light chain in organisms without cilia or flagella (King and Patel, 1995) and led to the subsequent copurification and colocalization of the 8-kD light chain with cytoplasmic dynein from mammalian brain (King et al., 1996). The 8-kD cytoplasmic dynein light chain (CDLC), which has an electrophoretic mobility of 8 kD, but a calculated molecular weight of 10.3 kD, exhibits extraordinary amino acid sequence conservation in a wide variety of organisms including Saccharomyces cerevisiae (Dick et al., 1996a ), Schistosoma mansoni (Hoffmann and Strand, 1996), Caenorhabditis elegans, (GenBank accession number U00043), Drosophila melanogaster (Dick et al., 1996b ), Chlamydomonas reinhardii (Piperno and Luck, 1979; Pfister et al., 1982; King and Patel, 1995), and Rattus norvegicus (Jaffrey and Snyder, 1996). In addition to cytoplasmic dynein, a second large multisubunit complex known as dynactin, which interacts with dynein, has been shown to be required for migration of membranous vesicles in higher eukaryotes (Allan, 1994; Sheetz, 1996). Mutations in various components of dynactin inhibit long range nuclear migration in filamentous fungi and short-range migration into the bud in yeast (Muhua et al., 1994; Plamann et al., 1994; Clark et al., 1994; Robb et al., 1995; Bruno et al., 1996; Tinsley et al., 1996; Geiser et al., 1997; Kahana et al., 1998). Thus the dynein/dynactin system is both structurally and functionally conserved between higher eukaryotes and fungi.
Early observations of nuclear migration through the hyphae of living fungi suggested that nuclei were pulled through the cytoplasm by a tractive force on their spindle pole bodies (SPBs). Because tubulin mutations in filamentous fungi affect nuclear migration, and because a yeast mutant that specifically lacks SPB microtubules has a nuclear migration defect (Oakley and Morris, 1980, 1981; Sullivan and Huffaker, 1992; Palmer et al., 1992), it is generally believed that nuclear migration is mediated by an interaction between SPB MTs and cytoplasmic dynein. Cytoplasmic dynein has been localized to astral microtubules and spindle pole bodies and has been shown to affect microtubule stability in yeast (Shaw et al., 1997; Carminati and Stearns, 1998) and in the filamentous fungus Nectria haematococca (Inoue et al., 1998a ). We have shown by staining hyphae with antibodies raised against the cytoplasmic dynein heavy chain (CDHC) that the dynein heavy chain is concentrated at the hyphal tips in A. nidulans (Xiang et al., 1995a ). This observation was recently confirmed with green fluorescent protein (GFP)–tagged CDHC, which, however, showed that, in addition to the material at the tip, some of the GFP-CDHC is associated with packets of material migrating rapidly toward the hyphal tips (Xiang et al., 1997; Xiang, X., and N.R. Morris, unpublished observations). These observations support but by no means prove a class of models in which nuclei are moved in filamentous fungi by the interaction between cortically fixed dynein/dynactin and SPB microtubules (Plamann et al., 1994; Morris et al., 1995; Carminati and Stearns, 1998; Efimov and Morris, 1998). Presumably, dynein, which is a minus end–directed motor, moves nuclei by attempting to migrate along SPB MTs toward the SPB microtubule organizing center, thereby reeling in the nucleus. In the absence of dynein, as seen in various dynein mutants, the nuclei are not reeled in. Additional support for this idea comes from laser optical trap experiments showing that nuclei are fixed in position during interphase such that the laser beam cannot move them in wild-type cells of Nectria haematococca. In contrast, nuclei are freely movable in a strain from which cytoplasmic dynein has been deleted (Inoue et al., 1998b ), presumably because they are no longer fixed to the cortex by the interaction between their SPB MTs and dynein at the cortex.
Our laboratory has previously identified (Morris, 1976) and cloned three genes, nudA, nudC, and nudF that affect nuclear migration in A. nidulans. Mutations in all of these genes cause a similar phenotype. Nuclear migration is defective during both vegetative growth and differentiation. As a consequence, colony size and the production of asexual and sexual spores are severely reduced. NudA encodes the heavy chain of cytoplasmic dynein (Xiang et al., 1994). NudC encodes an evolutionarily conserved 22-kD protein of unknown biochemical function (Osmani et al., 1990; Cunniff et al., 1997; Morris et al., 1997). The nudF gene encodes a 49-kD, WD-40 protein related to the human Miller-Dieker lissencephaly (LIS1) neuronal migration protein (Reiner et al., 1993; Xiang et al., 1995 b), which binds to tubulin (Sapir et al., 1997) and purifies as part of platelet activating factor acetyl hydrolase (Hattori et al., 1994). The NUDC and NUDF proteins appear to function upstream of dynein and are believed to be part of a regulatory pathway that controls cytoplasmic dynein function (Xiang et al., 1995 b; Willins et al., 1997). In this paper, we describe the characterization of a fourth gene, nudG, required for nuclear migration in A. nidulans. NudG encodes a close homologue of the 8-kD CDLC. Here we show by analyzing the effects of the temperature-sensitive (ts) nudG8 mutation that the nudG CDLC plays a role in both nuclear migration and cytoplasmic dynein localization at the mycelial tip.
Materials and Methods
Isolation of the nudG8 Mutation and Growth Conditions
Strain ts289 (nudG8, pabaA6, biA1) carrying the nudG8 mutation was identified by fluorescence microscopic inspection of nuclear distribution in 4′,6-diamidino-2-phenylindone (DAPI)-stained germlings from a collection of 1,164 temperature sensitive mutants generated by 4-nitroquinoline oxide mutagenesis of strain FGSC (Fungal Genetics Stock Center) A28 (pabaA6, biA1). This collection of ts mutants was made by S. Harris, M. Momany, and J. Hamer of the Department of Biological Sciences of Purdue University (Lafayette, IN), who generously shared their collection with us. Crosses between ts289 and XX19 (nudA2, chaA1, pyrG89, nicA2, and/or nicB8), AO1 (nudC3, wA2, pabaA1, nicA2, and pyrG89), and XX21 (nudF7, yA2, and pyrG89) identified nudG8 as a mutation in a new nud gene. ts289 was outcrossed to GR5 (wA2, pyroA4, and pyrG89) to introduce pyrG89, a selective marker suitable for transformation selection, into the strain. The resultant strain, named SB09, (nudG8, wA2, pyroA4, pyrG89, and biA1) was crossed to A391 (chaA1 and biA1) to produce SB10 (nudG8, chaA1, pyroA4, pyrG89, and biA1). Media and growth conditions were as previously described for other nud mutations (Xiang et al., 1994; Xiang et al., 1995a ,b). Progeny from a cross between SB09 (nudG8, wA2, pyroA4, pyrG89, and biA1) and A391 (chaA1 and biA1) were used for self-crosses to test for fertility defects. A double mutant strain was generated by crossing SB10 with XX60 (ΔnudA::pyrG; pyrG89; Xiang et al., 1995a ).
DAPI Staining and Indirect Immunofluorescence
To observe nuclei of A. nidulans germlings, spores were inoculated onto coverslips overlaid with medium on the bottom of a Petri dish and grown 8–12 h at either permissive or restrictive temperatures. Cells were fixed in 100 μl of 5% glutaraldehyde in 50 mM K2HPO4, pH 6.5, 0.25 μg/ml DAPI and 0.2% Triton X-100 for 20 min at room temperature. The coverslips were then rinsed with water and air dried. Coverslips were mounted on glass slides with a drop of Citifluor (UKC Chemlab, Canterbury, UK) and observed on an epifluorescence microscope (Carl Zeiss, Inc., Thornwood, NY). CDHC indirect immunofluorescence was performed as described previously (Xiang et al., 1995a ). The nudA CDHC antiserum has been described previously (Xiang et al., 1995a ). Tubulin was stained with DM1A antibody (Sigma Chemical Co., St. Louis, MO) against alpha tubulin. Antibody against actin was a gift from Andersland et al. (1994).
Mapping and Cloning of nudG
We mapped the nudG8 mutation to chromosome VIII by conventional parasexual genetic methods (reviewed by Clutterbuck, 1992). We used a chromosome VIII–specific cosmid library (Brody et al., 1991) obtained from the Fungal Genetics Stock Center and sib selection as described in Xiang et al. (1994) to clone the wild-type nudG gene. The library was divided into seven pools, each containing 50 cosmids. The SB10 (nudG8, chaA1, pyroA4, pyrG89, and biA1) strain was transformed with each pool, and we looked for DNA-mediated complementation of the mutant phenotype. The cosmid was subcloned to the smallest fragment, 2 kb, that would complement nudG mutation in the SB10 strain.
A. nidulans Transformation Protocol
A. nidulans was transformed according to the protocol of Yelton et al. (1984) with the following modifications. We initially used ∼3 μg of cosmid DNA plus 13 μg of pyrG DNA. The final protoplast pellet was resuspended in STC buffer containing 1.2 M sucrose instead of sorbitol. An equal volume of 60% PEG solution (polyethylene glycol 4000, #81240; Fluka Chemical Corp., Milwaukee, WI) was added to the protoplast/DNA mixture, and after 20 min incubation, 1.0 ml of YGS (0.5% yeast extract, 2% glucose, and 1% sucrose) medium containing sucrose in place of sorbitol was added and the eppendorf tubes were shaken at 150 rpm for 1–2 h at room temperature. The protoplasts were plated on YAG-KCL (0.5% yeast extract, 2% agar, 2% glucose, 1.6% KCl) and incubated at 32°C for 12–20 h, after which YAG top agar was added to the plates and incubation continued at 44°C until colonies grew through the top agar. These were regridded and retested at 44°C on YAG. Rescue of the nuclear migration defect phenotype produced wild-type colony growth and abundant production of asexual spores at restrictive temperature.
cDNA Library Screening
We screened a lambda gt10 A. nidulans cDNA library (Osmani et al., 1988) using the 2-kb genomic fragment that rescued the nudG8 phenotype. The library had been transformed into Escherichia coli C600 strain using the modified procedure described in the Cloning Kit Instruction Manual (Stratagene Inc., La Jolla, CA). Prehybridization and hybridization of the filters and preparation of the probe was done according to the nonradioactive Genius kit (Boehringer Mannheim Co., Indianapolis, IN). E. coli (DH5α) were made competent and transformed according to Sambrook et al. (1989). The phage DNA was isolated as described in Sambrook et al. (1989) and subcloned into pBluescript II LS+ (Stratagene Inc.), purified through CsCl and sequenced using the 4000L Sequencer (LI-COR, Inc., Lincoln, NB).
Preparation of A. nidulans Protein Extract
Spores were inoculated into YAG medium containing uridine and uracil and grown overnight at restrictive temperature (44°C). The mycelia were harvested by filtration through Miracloth (Calbiochem-Novabiochem, La Jolla, CA), pressed dry, and frozen in liquid nitrogen. The frozen mycelia were ground to a powder with a mortar and pestle in liquid nitrogen. Proteins were extracted by vortexing the powder in a 30-ml capped tube containing 2 ml of acid-washed glass beads (425–600-μm diameter; Sigma Chemical Co.) and Tris/KCl buffer (20 mM Tris HCl, pH 7.6, 50 mM KCl, 5 mM MgCl2, and 0.5 mM EDTA) (Collins and Vallee, 1989). After a 4°C centrifugation at 3,000 g in an HB-4 Sorvall rotor for 5 min to remove cell wall debris, the supernatant was recentrifuged at 17,000 g in a Sorvall centrifuge (Newtown, CT) for 15 min at 4°C. The protein concentration ranged from 10 to 30 mg/ml as measured by the Bradford assay (Bradford, 1976).
Antibody Preparation, Western Blotting, and Immunoprecipitation
NUDG antiserum was generated against the first 14 amino acids of the CDLC sequence, H2N-MASEKKDKLEPQIK-COOH, conjugated to keyhole limpet hemagglutinin and injected into rabbits by Alpha Diagnostic Int. (San Antonio, TX). Each of two rabbits produced antiserum that recognized the 8-kD CDLC band by Western blotting of protein extracts at 1:15,000 dilution. Because only a single band was detected, we did not find it necessary to affinity purify the NUDG antiserum. The preparation of affinity purified anti–NUDA antibodies was described previously (Xiang et al., 1995a ). For immunoprecipitation experiments, we incubated 4 mg of protein extract in 1 ml of Tris/KCl buffer for 2 h with 40 μl of A. nidulans NUDG (CDLC) undiluted antiserum at 4°C on a rotating wheel. 100 μl of 50% Protein A Sepharose (Pharmacia LKB Biotechnology Inc., Piscataway, NJ) was added, and incubation continued for an additional 2 h at 4°C. The mixture was then washed six times with 1 ml Tris/KCl buffer by centrifugation for 4 s in a microfuge. The immunoprecipitate was resuspended in Laemmli sample buffer, subjected to electrophoresis on a 4–20% gradient SDS-PAGE gel (Bio-Rad Laboratories, Richmond, CA) and transferred to an Immobilon-P membrane (Millipore Corp., Bedford, MA). The transfer efficiency was monitored by Ponceau S (Sigma Chemical Co.) staining. After primary antibody and goat anti–rabbit alkaline phosphatase-conjugated secondary antibody incubations, the blots were developed colorimetrically with nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphate from Kirkegaard & Perry Laboratories, Inc. (Gaithersburg, MD).
Sedimentation of NUDA and NUDF Proteins
Wild-type and nudG3 mutant cells were grown in YG medium 15 h at 42°C and protein extracts prepared as described above. 1 ml of extract was sedimented through a 12 ml linear 17–51% sucrose density gradient made with the same buffer in which the extract was prepared. The gradient was centrifuged in a SW41 rotor for 4 h at 270,000 g at 4°C. 600-μl fractions were collected from the top of the gradient and 20 μl of each fraction was subjected to gradient (4–20%) SDS-PAGE and transferred to membrane for Western blotting as described above.
Results
Characterization of nudG8
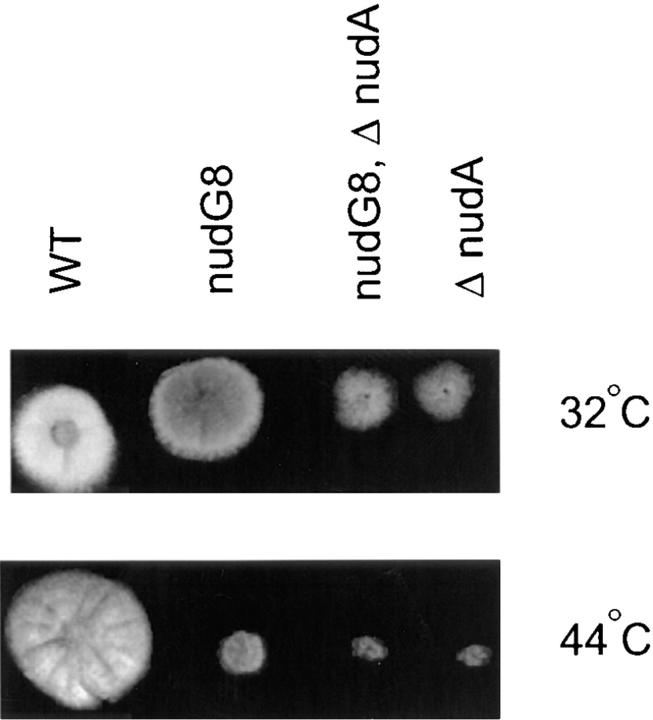
A new collection of temperature-sensitive mutants of A. nidulans (see Materials and Methods) was screened by DAPI staining of nuclei and fluorescence microscopy at restrictive temperature (42°C) to identify mutants with a defect in nuclear migration (nud mutants). Several mutants with this phenotype were identified and crossed with previously characterized nud mutants (nudA, nudC, and nudF) to determine whether they represented new genes. One such mutant was identified as a new nud gene because it produced 25% wild-type progeny when crossed with strains carrying either a nudA, nudC, or nudF mutation. This new mutation was designated as nudG8. Colonies of the nudG8 mutant strain grew slowly at restrictive temperature with a growth rate 10–15% that of wild type and a tight colony morphology indistinguishable from that of other previously described Aspergillus nud mutations (Fig. 1) (see also Osmani et al., 1990). DAPI staining of hyphae germinated at restrictive temperature revealed the typical nuclear migration defect characteristic of nud mutants. The nuclei divided normally, but failed to migrate from the spore end of the germling into the germ tube, leaving a cluster of nuclei at the spore end. When crossed with a wild-type strain, the temperature sensitivity of nudG8 segregated 1:1, confirming it was a single gene mutation. Diploids heterozygous for nudG8 grew as well as their wild-type parental strains at restrictive temperature (44°C), indicating that the nudG8 mutation was recessive and therefore presumably a loss of function mutation. NudG8, like other previously characterized nud mutants of A. nidulans, was also defective in the production of asexual spores (conidia). Because the asexual spores are responsible for colony color (Clutterbuck, 1990), this was easily seen by inspection of colonies growing on YAG agar, which were gray-brown rather than brightly colored. Because other previously characterized nud mutants of A. nidulans exhibited low fertility in matings, we compared the ascospores (sexual spore) production of self-crosses of wild-type strains and nudG8 strains. The nudG8 mutation was linked to a reduction in fertility, as demonstrated by the low numbers of ascospores (sexual spores) produced in self-crosses in contrast to wild-type ascospore production. Among eleven ascospores examined, the nudG mutant produced ∼3–10% the number of conidia as the wild type.
Figure 1.
Comparison of the growth at permissive and restrictive temperatures of a wild- type strain, nudG8, ΔnudA, and a nudG8/ΔnudA double mutant strain.
To determine whether the nuclear migration defect caused by nudG was mediated by an effect on cytoplasmic dynein or by an effect on some other motility system involved in nuclear migration, we constructed a doubly mutant strain carrying both nudG8 and a ΔnudA CDHC deletion mutation (Xiang et al., 1995a ). If the function of the nudG gene product was similar to that of the nudA CDHC, the growth defect of the double mutant should be no more severe than that caused by the more deleterious of the single mutations. Alternatively, if the mutations affected different processes, the effects of the mutations would be expected to be additive and the phenotype of the double mutant more extreme than either single mutant. The result of this experiment was that the nudG8, ΔnudA double mutant grew no more slowly than the worse of the singly mutant parental strains, suggesting that nudG was involved in the same motility function as the nudA-encoded CDHC (Fig. 1).
nudG Encodes a Homologue of the 8-kD Dynein Light Chain
The nudG8 mutation was mapped to chromosome VIII. A cosmid that rescued the mutant phenotype was identified by DNA-mediated complementation of nudG8 temperature sensitivity using sib selection from pools of chromosome VIII cosmids. The rescuing cosmid, SW27C04, is located on the right arm of chromosome VIII near bimG, abaA, and can67 (Brody et al., 1991; Wang et al., 1994). The 40-kb cosmid clone was subcloned to the smallest fragment, 2 kb, that was able to rescue the nudG8 mutant phenotype. Southern blot analysis using the 2-kb fragment as a probe showed a single band when genomic DNA was cut with BamHI and EcoRI, and two bands when cut with HindIII (data not shown). We later determined that the nudG sequence contains an internal HindIII site, which explains the presence of two bands. Thus, nudG appears to be represented by a single gene in the A. nidulans genome. The 2-kb genomic fragment was used to isolate two cDNA clones of 572 and 619 bp from an A. nidulans lambda gt10 phage cDNA library. The two clones encoded sequences that differed by a 54-bp sequence, possibly the result of a splicing abnormality. Both clones were able to completely rescue the growth and nuclear migration defects of nudG8 by DNA-mediated complementation (data not shown). The sequence data for the 572-bp cDNA clone is available from GenBank/EMBL/DDBJ under accession number U81827.
Characterization of the Putative NUDG Protein
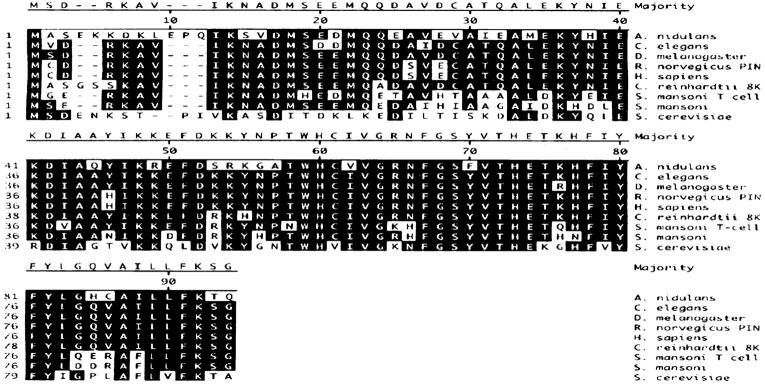
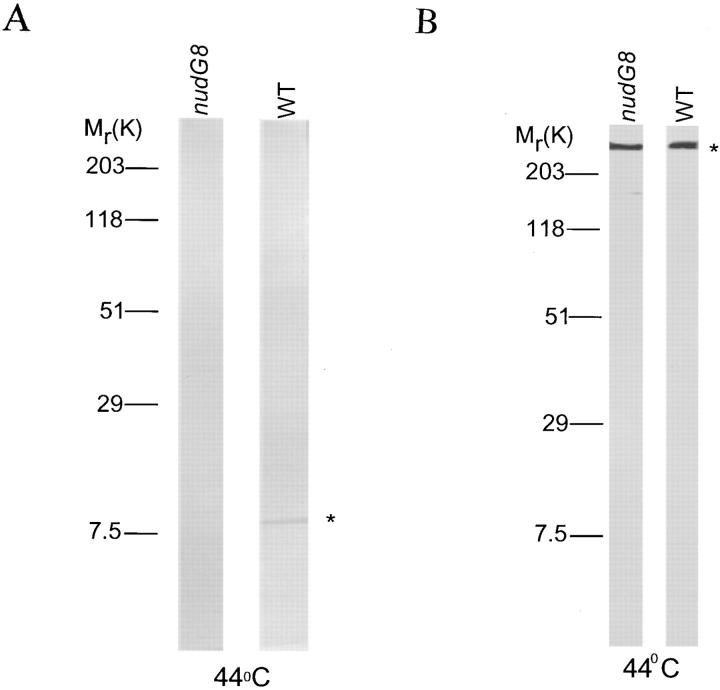
The nudG 572-bp cDNA encodes a putative protein of 94 amino acids with a predicted molecular weight of 11,031 D and an isoelectric point of 6.49. It shares 67% identity with the 8-kD outer arm CDLC from Chlamydomonas reinhardii flagella (King and Patel, 1995), 66.3% with the Caenorhabditis elegans cosmid T26A5 sequence (GenBank accession number U00043), the CDLC1 of Drosophila melanogaster (Dick et al., 1996b ), the Rattus norvegicus protein inhibitor of neuronal nitric oxide synthase (Jaffrey and Snyder, 1996) and the Homo sapiens CDLC1 (Dick et al., 1996b ), 53.9% with the Schistosoma mansoni CDLC (Hoffmann and Strand, 1996), and 43.5% identity with the SLC1 of Saccharomyces cerevisiae (Dick et al., 1996a ) (Fig. 2). An antibody was generated against the first 14 amino acids of the wild-type nudG CDLC sequence. Western blotting of extracts from wild-type strains identified a NUDG protein band migrating at 8 kD on SDS-PAGE gels even though the molecular weight predicted by the sequence is 11 kD. This is similar to the anomalous migration of CDLC protein from other species. The 8-kD CDLC band was absent from Western blots of nudG8 protein extracts grown at restrictive temperature (Fig. 3 A). Thus, the effect of the nudG8 mutation is either to increase the rate of degradation of the NUDG protein, to decrease its rate of synthesis, or both. We attempted to use our antibody to localize the nudG light chain within the A. nidulans germling; however, this antibody was not useful for immunocytochemistry.
Figure 2.
Sequence comparison of the A. nidulans 8-kD CDLC (NUDG) with other GenBank sequences. The deduced amino acid sequence of the 572-bp cDNA compared with Caenorhabditis elegans cosmid T26A5 sequence (U00043), Drosophila melanogaster CDLC1 (U32855), Homo sapiens CDLC1 (U32944), the Rattus norvegicus protein inhibitor of neuronal nitric oxide synthase (U66461), the 8-kD outer arm dynein light chain from Chlamydomonas reinhardii flagella (U19490), the T cell–stimulating antigen from the blood fluke Schistosoma mansoni (X98619), the Schistosoma mansoni DLC (U55992), and the DLC1 of Saccharomyces cerevisiae (U36468). The sequences were aligned with DNASTAR using the Clustal method with the PAM250 residue weight table. Residues that match the consensus sequence are shaded in black.
Figure 3.
(A) Western blot of A. nidulans total protein stained with anti–NUDG antibody. The NUDG/CDLC antiserum recognized a single protein with an apparent molecular weight of 8-kD in cell-free extracts from a wild-type strain (GR5) grown at restrictive temperature (44°C), as indicated by the star. Under the same conditions, no 8-kD CDLC band was seen in the nudG8 mutant strain. (B) Western blot of A. nidulans total protein stained with anti–NUDA CDHC antibody.
Coimmunoprecipitation of the A. nidulans 8-kD CDLC with the CDHC
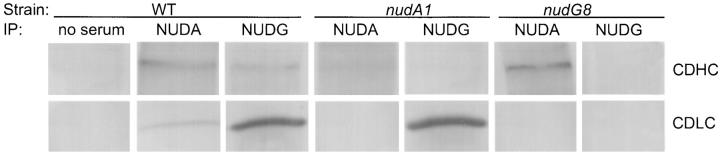
To determine whether the nudG CDLC interacts with the CDHC in A. nidulans, we used antibodies against the nudG CDLC and the nudA CDHC to immunoprecipitate proteins from cell-free extracts and analyzed the immunoprecipitates by Western blotting (Fig. 4). The NUDG antiserum precipitated both the nudG 8-kD CDLC and the nudA CDHC from wild-type extracts. Similarly, antibody against the nudA CDHC also precipitated both the CDHC and the 8-kD CDLC from wild-type extracts. Control experiments without antiserum or with protein extracts prepared from a nudA1 mutant grown at restrictive temperature, which lacks CDHC protein, or the nudG8 mutant grown at restrictive temperature, which lacks the 8-kD CDLC protein, demonstrated the specificity of the interaction. In the absence of NUDA protein (the nudA1 strain), antibody against NUDA failed to precipitate NUDG protein and, in the absence of NUDG protein (the nudG8 strain), antibody against NUDG failed to precipitate NUDA protein. These coimmunoprecipitation data show that nudG CDLC and nudA CDHC are associated in a protein complex in A. nidulans. They do not, however, necessarily indicate that the CDHC is bound directly to the light chain. The association between the nudG 8-kD CDLC and the nudA CDHC could be mediated by another chain of cytoplasmic dynein or by some as yet uncharacterized protein. Nor does this result necessarily mean that all of the CDLC is bound to the CDHC or vice versa. In fact, sedimentation analysis (see below) indicates that most of the 8-kD light chains are not associated with the CDHC.
Figure 4.
Immunoprecipitation of proteins from a cell-free extract of A. nidulans grown at 44°C. The A. nidulans strains and the antibodies used for immunoprecipitation appear above the rows of blots. The antibodies used to detect NUDA and NUDC proteins in the blots are indicated to the right. The blots in the top row were stained with affinity purified A. nidulans NUDA/CDHC antibody and the bottom row were stained with A. nidulans NUDG/CDLC antiserum. From left to right, the seven columns of blots represent (from left) a control IP without antiserum, immunoprecipitation of a wild type extract with antibody against NUDA, immunoprecipitation of a wild-type extract with antibody against NUDG, immunoprecipitation of a nudA1 extract grown at restrictive temperature with antibody against NUDA, immunoprecipitation of the same extract with antibody against NUDG, immunoprecipitation of a nudG8 extract grown at restrictive temperature with antibody against NUDA, and immunoprecipitation of the same extract with antibody against NUDG.
Sedimentation Analysis of the Cytoplasmic Dynein Heavy and 8-kD Light Chains
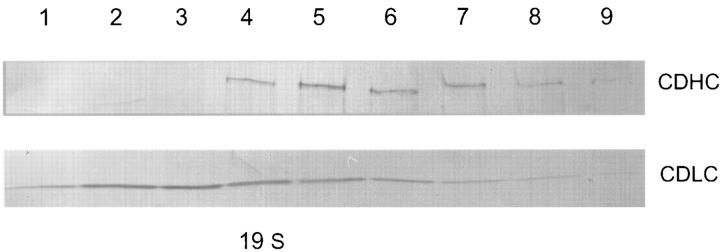
Sedimentation of cell free extracts of wild-type A. nidulans showed that the NUDA cytoplasmic heavy chain protein sediments as a large molecular weight complex at ∼20 S. The NUDG CDLC for the most part sedimented faster than expected from its calculated molecular weight and exhibited a very broad distribution, suggesting that it was involved in multiple protein complexes. Although there was an overlap between the sedimentation profiles of the NUDA and NUDG proteins, most of the NUDG protein did not cosediment with the 20-S cytoplasmic dynein complex (Fig. 5), indicating that the major fraction of NUDG is not associated with the cytoplasmic dynein complex under the conditions of this experiment. Sedimentation of extracts from nudG8 mutant cells grown at 42°C produced NUDA and NUDG sedimentation profiles that were indistinguishable from that of wild-type extract grown under the same conditions (data not shown). Thus, the nudG8 mutation has no observable effect on the structure of the cytoplasmic dynein complex as detectable by this assay.
Figure 5.
Sucrose gradient sedimentation profiles of the NUDA and NUDG proteins. A sample from each of the top nine fractions from the gradient was subjected to SDS-PAGE and Western blotted with antibody against the nudA CDHC or the nudG 8-kD CDLC.
The nudG8 Mutation Causes a Defect in Dynein Localization
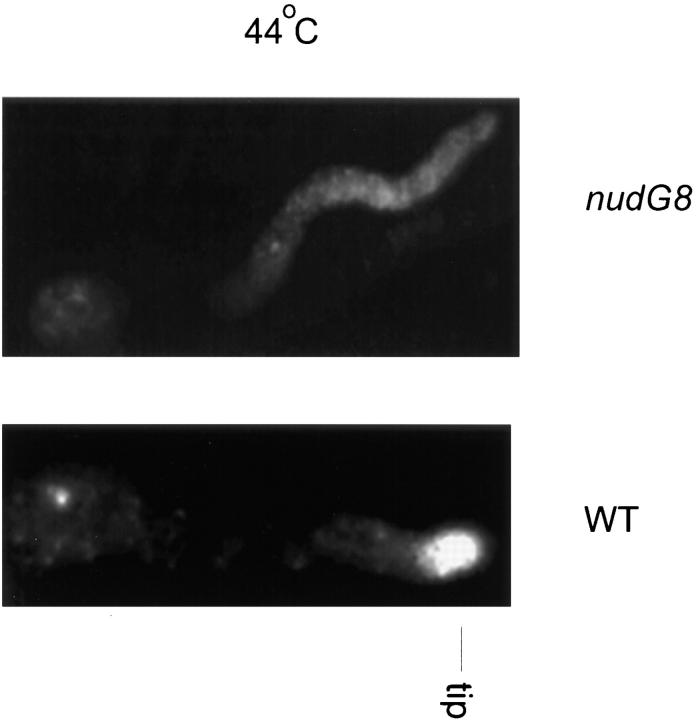
We have shown that cytoplasmic dynein is concentrated at the growing tips of wild-type A. nidulans germlings by immunocytochemistry, using an affinity purified rabbit polyclonal antibody directed against the CDHC (Xiang et al., 1995a ), and more recently by observation of a green fluorescent protein tagged–CDHC fusion protein in living cells (Xiang et al., 1997). To determine whether the nudG8 mutation affected the localization of cytoplasmic dynein, we stained nudG8 germlings with affinity purified anti– NUDA (anti–CDHC) antibody. In wild-type germlings, antibody against the CDHC stains a dot at the hyphal tips at both permissive and restrictive temperatures. A similar dot of stain is also seen at the hyphal tips of nudG8 germlings at permissive temperature. At restrictive temperature, however, the dot of CDHC at the tip disappears (Fig. 6), and the anti–NUDA antibody produces only a faint, diffuse staining throughout the cytoplasm. Because the loss of CDHC staining from the tip might represent a decrease in the total amount of heavy chain in the cells, we compared the amount of CDHC in wild-type and nudG8 cells by Western blotting. Equal amounts of protein were loaded on the gels. Western blot analysis of protein extracts from wild-type and mutant cells revealed that the CDHC protein level was similar in the wild-type and nudG8 mutant strains (Fig. 3 B). Another potential source of artifact was the possibility that the nudG8 mutation might affect the accessibility of the tip to antibody. To control for this possibility, we stained hyphae with antibodies against actin and tubulin. Both antibodies stained structures at the hyphal tip. We found no discernible difference in actin or tubulin staining at the tips of the nudG8 mutant as compared with wild-type cells (data not shown). Thus, the nudG8 mutation did not cause a difference in CDHC tip staining either by altering the amount of CDHC in the cell or by altering tip permeability to antibody, but caused a loss of CDHC protein from the hyphal tip. Because the 8-kD CDLC band was absent from protein extracts of nudG8 grown at restrictive temperature (Fig. 3 A), these data suggest that the nudG 8-kD CDLC is required for targeting of the nudA CDHC to the hyphal tip in A. nidulans. We were unable to determine whether the 8-kD CDLC was located at the hyphal tip in wild-type cells because our anti–light chain antibody did not stain the mycelium.
Figure 6.
Immunofluorescence and Western blot analysis of the CDHC in wild-type and the nudG8 mutant strains. GR5 wild-type (WT) and nudG8 germlings grown at restrictive temperature (44°C) were stained with antibody against the NUDA CDHC protein. The cytoplasmic dynein heavy chain stains intensely at the tip of wild-type germ tubes. In contrast, there is little or no anti–CDHC antibody staining at the tip of the germ tube in the nudG8 mutant strain.
Discussion
Here we report on the cloning and characterization of a new gene, nudG, required for nuclear migration in A. nidulans. The wild-type gene was cloned by DNA-mediated complementation of the temperature-sensitive nudG8 mutation and encodes a homologue of the 8-kD cytoplasmic dynein light chain protein. Experiments to determine how the nudG8 mutation affects cytoplasmic dynein showed that the nudG8 mutation caused a large decrease in the intracellular concentration of the 8-kD CDLC when the mutant fungus was grown at restrictive temperature. This decrease had two observable effects on the cell. It inhibited nuclear migration through the mycelium, and it caused CDHC staining to disappear from the hyphal tip. Western blotting showed that the loss of tip staining was not caused by a decrease in the intracellular concentration of CDHC, as the total amount of CDHC protein was not reduced in the mutant at restrictive temperature. Nor was the failure of anti–CDHC antibody to stain the tip caused by decreased antibody permeability, since anti–actin and anti– tubulin antibodies stained the tip of the nudG8 mutant as well as they stained the wild-type tip. Thus, altered CDHC tip staining caused by the nudG8 mutation apparently reflects a real change in the distribution of CDHC within the cell.
The effect of the nudG8 mutation at restrictive temperature is to cause a decrease in the amount of 8-kD CDLC in the cell. Whether this is the result of decreased synthesis or increased degradation is unknown. Precisely how the deficiency of 8-kD CDLC affects tip localization and nuclear migration is also unknown. Among the possibilities, the 8-kD CDLC could be required for assembly of the cytoplasmic dynein complex, it could be required for the interaction of dynein with dynactin, or it could be required for dynein motor function per se. Any of the aforementioned defects might prevent dynein from reaching and becoming localized at the hyphal tip. The fact that the nudG8 mutation does not affect the sedimentation of the CDHC, however, indicates that the lack of NUDG protein does not cause a gross disruption of the cytoplasmic dynein complex. Alternatively, the 8-kD CDLC might be involved in anchoring the dynein/dynactin complex to the hyphal tip. Whatever the mechanism, our results are consistent with a model in which cortically anchored dynein moves nuclei by pulling on astral MTs attached to nuclei (Palmer et al., 1992; Plamann et al., 1994; Morris et al., 1995; Carminati and Stearns, 1998; Efimov and Morris, 1998). However, it is important to recognize that they by no means prove the model.
In higher eukaryotes, cytoplasmic dynein is bound to vesicles (Sheetz, 1996; Vallee and Sheetz, 1996; Hirokawa, 1998). The tips of fungal hyphae are packed with vesicles, and the CDHC tip staining in the wild type could simply represent vesicle-associated dynein. Were this the case, decreased tip staining in the nudG8 mutation could result from any CDLC requirement for dynein translocation of vesicles to the tip. Interpretation of the role of the nudG CDLC is further complicated by the fact that the 8-kD CDLC has recently been shown to be associated with myosin-V (Espindola et al., 1996), with I kappaB alpha (Crepieux et al., 1997), and with the tegument of the blood fluke, Shistosoma (Hoffman and Strand, 1996). It has also been shown to act as an inhibitor of the neuronal form of nitric oxide synthase (Jaffrey and Snyder, 1996). The difficulty of definitively understanding the molecular function of the CDLC protein in higher eukaryotes is exemplified by phenotypic analysis of mutations in genes for the 8-kD CDLC in Drosophila melanogaster and Chlamydomonas reinhardii. Mutations in the Drosophila gene (known as DLC1 or cut up) cause axon projections to follow abnormal pathways in the central nervous system (Phillis et al., 1996). Other partial loss of function mutations in DLC1 cause disorganization of the ovaries and female sterility. Total loss of function mutations in D. melanogaster are lethal. Because the 8-kD protein has been shown to interact with myosin-V, I kappaB alpha, and nitric oxide synthase, these interesting phenotypes do not necessarily implicate the 8-kD light chain in cytoplasmic dynein function in D. melanogaster. In C. reinhardii, the 8-kD light chain has been shown to be essential for the retrograde intraflagellar transport of migrating “rafts” by a mechanism unrelated to its flagellar dynein function and therefore is likely to be specific for cytoplasmic dynein (Pazour et al., 1998), but an interaction with one of the other molecules cannot rigorously be excluded. In principle, the effect of nudG8 on tip staining and nuclear migration in A. nidulans could similarly be mediated through myosin-V or the nuclear factor– κB transcription factor rather than via cytoplasmic dynein. An effect mediated via nitric oxide synthase is unlikely, as no NO synthase activity has been detected in A. nidulans (Beckwith, S.M., N.R. Morris, and D. Wolff, unpublished observations). Because the nudG8 CDLC mutant accurately phenocopies the nuclear migration, colony growth, and sporulation defects of nudA CDHC mutants, it seems probable that nudG CDLC function is mediated via cytoplasmic dynein. The fact that the phenotype of the nudA, nudG double mutant is the same as that of the single mutations provides additional evidence that nudG functions via dynein.
In summary, the characterization of the nudG 8-kD CDLC of A. nidulans has extended the evolutionary range of this dynein light chain to the filamentous fungi, has demonstrated that it is an essential component of the main machinery responsible for nuclear migration, and has provided strong evidence that its effect is mediated through a physical interaction with the cytoplasmic dynein complex.
Acknowledgments
We thank S. Harris and J. Hamer for sharing their collection of temperature sensitive A. nidulans mutants with us. The nudG mutation was isolated from this collection. We also thank Peter D'Arpa and Xin Xiang for constructive comments on this manuscript.
This work was supported by grants to N.R. Morris, S.M. Beckwith, and B. Liu from the National Institutes of Health and to N.R. Morris from the American Cancer Society.
Abbreviations used in this paper
- CDHC
cytoplasmic dynein heavy chain
- CDLC
cytoplasmic dynein light chain
- DAPI
4′,6-diamidino-2-phenylindone
- MT
microtubule
- SPB
spindle pole bodies
- ts
temperature sensitive
Footnotes
Dr. Beckwith's present address is U.S.U.H.S., 15104 Dufief Dr., N. Potomac, MD 20878. Dr. Roghi's present address is University of Manchester, School of Biological Sciences, 205 Stopford Building, Manchester, M13 9PT, UK, and Dr. Liu's present address is Section of Plant Biology, University of California at Davis, Davis, CA 95616.
Address all correspondence to N.R. Morris, Department of Pharmacology, 675 Hoes Lane, Piscataway, NJ 08854. Tel.: (732) 235-4166. Fax: (732) 235-4073. E-mail: morrisnr@umdnj.edu
References
- Allan V. Organelle movement. Dynactin: portrait of dynein regulator. Curr Biol. 1994;4:1000–1002. doi: 10.1016/s0960-9822(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Andersland JM, Fisher DD, Wymer CL, Cyr RJ, Parthasarathy MV. Characterization of a monoclonal antibody prepared against plant actin. Cell Motil Cytoskel. 1994;29:339–344. doi: 10.1002/cm.970290406. [DOI] [PubMed] [Google Scholar]
- Baker J, Theurkauf WE, Schubiger G. Dynamic changes in microtubule configuration correlate with nuclear migration in the preblastoderm Drosophilaembryo. J Cell Biol. 1993;122:113–121. doi: 10.1083/jcb.122.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brody H, Griffith J, Cuticchia AJ, Arnold J, Timberlake WE. Chromosome-specific recombinant DNA libraries from the fungus Aspergillus nidulans. . Nucleic Acids Res. 1991;19:3105–3109. doi: 10.1093/nar/19.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno KS, Tinsley JH, Minke PF, Plamann M. Genetic interactions among cytoplasmic dynein, dynactin, and nuclear distribution mutants of Neurospora crassa. . Proc Natl Acad Sci USA. 1996;93:4775–4780. doi: 10.1073/pnas.93.10.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati JL, Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J Cell Biol. 1998;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SW, Staub O, Clark B, Hozbauer EL, Paschal BM, Vallee RB, Meyer DI. Beta-centractin: characterization and distribution of a new member of actin-related proteins. Mol Biol Cell. 1994;5:1301–1310. doi: 10.1091/mbc.5.12.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck AJ. The genetics of conidiophore pigmentation in Aspergillus nidulans. . J Gen Microbiol. 1990;136:1731–1738. doi: 10.1099/00221287-136-9-1731. [DOI] [PubMed] [Google Scholar]
- Clutterbuck AJ. Sexual and parasexual genetics of Aspergillusspecies. Biotechnology. 1992;23:3–18. [PubMed] [Google Scholar]
- Collins CA, Vallee RB. Preparation of microtubules from rat liver and testis: cytoplasmic dynein is a major microtubule associated protein. Cell Motil Cytoskelet. 1989;14:491–500. doi: 10.1002/cm.970140407. [DOI] [PubMed] [Google Scholar]
- Crepieux P, Kwon H, Leclerc N, Spencer W, Richard S, Lin R, Hiscott J. I kappa B alpha physically interacts with a cytoskeleton-associated protein through its signal response domain. Mol Cell Biol. 1997;17:7375–7385. doi: 10.1128/mcb.17.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunniff J, Chiu Y, Morris NR, Warrior R. Characterization of DnudC, the Drosophila homolog of an Aspergillusgene that functions in nuclear motility. Mech Dev. 1997;66:55–68. doi: 10.1016/s0925-4773(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Dick T, Urana U, Chia W. Molecular and genetic characterization of SLC1, a putative Saccharomyces cerevisiaehomolog of the metazoan cytoplasmic dynein light chain 1. Mol Gen Genet. 1996a;251:38–43. doi: 10.1007/BF02174342. [DOI] [PubMed] [Google Scholar]
- Dick T, Ray K, Salz HK, Chia W. Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. . Mol Cell Biol. 1996b;16:1966–1977. doi: 10.1128/mcb.16.5.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov VP, Morris NR. A screen for dynein synthetic lethals in Aspergillus nidulansidentifies spindle assembly checkpoint genes and other genes involved in mitosis. Genetics. 1998;149:101–116. doi: 10.1093/genetics/149.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espindola FS, Cheney RE, King SM, Suter DM, Mooseker MS. Myosin-V and dynein share a similar light chain. Mol Biol Cell. 1996;7:372a. [Google Scholar]
- Fan SS, Ready DF. Glued participates in distinct microtubule-based activities in Drosophilaeye development. Development (Camb) 1997;124:1497–1507. doi: 10.1242/dev.124.8.1497. [DOI] [PubMed] [Google Scholar]
- Geiser JR, Schott EJ, Kingsbury TJ, Cole NB, Totis LJ, Bhattacharyya G, He L, Hoyt MA. Saccharomyces cerevisiaegenes required in the absence of the CIN8 encoded spindle motor act in functionally diverse mitotic pathways. Mol Biol Cell. 1997;8:1035–1050. doi: 10.1091/mbc.8.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. Evidence for cell cycle-specific, spindle pole body–mediated, nuclear positioning in the fission yeast Schizosaccharomyces pombe. . J Cell Sci. 1997;110:1851–1866. doi: 10.1242/jcs.110.16.1851. [DOI] [PubMed] [Google Scholar]
- Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor. Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hoffmann KF, Strand M. Molecular identification of a Schistosoma mansonitegumental protein with similarity to cytoplasmic dynein light chains. J Biol Chem. 1996;271:26117–26123. doi: 10.1074/jbc.271.42.26117. [DOI] [PubMed] [Google Scholar]
- Holzbauer, E.L.F., A. Mikami, B.M. Paschal, and R.B. Vallee. 1994. Molecular characterization of cytoplasmic dynein. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. Wiley-Liss, Inc., New York. 251–267.
- Hoyt MA, Hyman AA, Bahler M. Motor proteins of the eukaryotic cytoskeleton. Proc Natl Acad Sci USA. 1997;94:12747–12748. doi: 10.1073/pnas.94.24.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Yoder OC, Turgeon BG, Aist JR. A cytoplasmic dynein required for mitotic aster formation in vivo. J Cell Sci. 1998a;111:2607–2614. doi: 10.1242/jcs.111.17.2607. [DOI] [PubMed] [Google Scholar]
- Inoue S, Yoder OC, Turgeon BG, Aist JR. Role of fungal dynein in hyphal growth, microtubule organization, spindle pole body motility and nuclear migration. J Cell Sci. 1998b;111:1555–1566. doi: 10.1242/jcs.111.11.1555. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- Kahana JA, Schlenstedt G, Evanchuk DM, Geiser JR, Hoyt MA, Silver PA. The yeast dynactin complex is involved in partitioning the mitotic spindle between mother and daughter cells during anaphase B. Mol Biol Cell. 1998;9:1741–1756. doi: 10.1091/mbc.9.7.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Barbarese E, Dillman JF, II, Patel-King RS, Carson JH, Pfister KK. Brain cytoplasmic and flagellar outer arm dynein share a highly conserved M(r) 8,000 light chain. J Biol Chem. 1996;271:19358–19366. doi: 10.1074/jbc.271.32.19358. [DOI] [PubMed] [Google Scholar]
- King SM, Patel KR. The M(r) = 8,000 and 11,000 outer arm dynein light chains from Chlamydomonasflagella have cytoplasmic homologues. J Biol Chem. 1995;270:11445–11452. doi: 10.1074/jbc.270.19.11445. [DOI] [PubMed] [Google Scholar]
- Klominek J, Sundqvist K-G, Robert K-H. Nucleokinesis: distinct pattern of cell translocation in response to an autocrine motility factor–like substance or fibronectin. Proc Natl Acad Sci USA. 1991;88:3902–3906. doi: 10.1073/pnas.88.9.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation. Development (Camb) 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- Morris NR. Mitotic mutants of Aspergillus nidulans. . Genet Res. 1976;26:237–254. doi: 10.1017/s0016672300016049. [DOI] [PubMed] [Google Scholar]
- Morris NR, Xiang X, Beckwith SM. Nuclear migration advances in fungi. Trends Cell Biol. 1995;5:278–282. doi: 10.1016/s0962-8924(00)89039-x. [DOI] [PubMed] [Google Scholar]
- Morris SM, Anaya P, Xiang X, Morris NR, May GS, Yu-Lee L-Y. A prolactin-inducible T cell gene product is structurally similar to the Aspergillus nidulansnuclear movement protein NUDC. Mol Endocrinol. 1997;11:229–236. doi: 10.1210/mend.11.2.9892. [DOI] [PubMed] [Google Scholar]
- Muhua L, Karpova TS, Cooper JA. A yeast actin-related protein homologous to that in vertebrate dynactin complex is important for spindle orientation and nuclear migration. Cell. 1994;78:669–679. doi: 10.1016/0092-8674(94)90531-2. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Morris NR. Nuclear movement is β-tubulin-dependent in Aspergillus nidulans. . Cell. 1980;19:255–262. doi: 10.1016/0092-8674(80)90407-9. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Morris NR. A β-tubulin mutation in Aspergillus nidulansthat blocks microtubule function without blocking assembly. Cell. 1981;24:837–845. doi: 10.1016/0092-8674(81)90109-4. [DOI] [PubMed] [Google Scholar]
- Osmani AH, Osmani SA, Morris NR. The molecular cloning and identification of a gene product specifically required for nuclear movement in Aspergillus nidulans. . J Cell Biol. 1990;111:543–551. doi: 10.1083/jcb.111.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani SA, Pu RT, Morris NR. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell. 1988;53:237–244. doi: 10.1016/0092-8674(88)90385-6. [DOI] [PubMed] [Google Scholar]
- Palmer RE, Sullivan DS, Huffaker T, Koshland D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae. . J Cell Biol. 1992;119:583–593. doi: 10.1083/jcb.119.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG George Witman. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister KK, Fay RB, Witman GB. Purification and polypeptide composition of dynein ATPases from Chlamydomonasflagella. Cell Motil. 1982;2:525–547. doi: 10.1002/cm.970020604. [DOI] [PubMed] [Google Scholar]
- Phillis R, Statton D, Caruccio P, Murphey RK. Mutations in the “8 kDa” dynein light chain gene disrupt sensory axon projections in the Drosophilaimaginal CNS. Development (Camb) 1996;122:2955–2963. doi: 10.1242/dev.122.10.2955. [DOI] [PubMed] [Google Scholar]
- Piperno J, Luck DJL. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii. Purification of two dyneins. J Biol Chem. 1979;254:3084–3090. [PubMed] [Google Scholar]
- Plamann M, Minke PF, Tinsley JH, Bruno KS. Cytoplasmic dynein and actin-related protein Arp1are required for normal nuclear distribution in filamentous fungi. J Cell Biol. 1994;127:139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Reinsch S, Karsenti E. Movement of nuclei along microtubules in Xenopusegg extracts. Curr Biol. 1997;7:211–214. doi: 10.1016/s0960-9822(97)70092-7. [DOI] [PubMed] [Google Scholar]
- Robb MJ, Wilson MA, Vierula PJ. A fungal actin-related protein involved in nuclear migration. Mol Gen Genet. 1995;247:583–590. doi: 10.1007/BF00290350. [DOI] [PubMed] [Google Scholar]
- Rose MD. Nuclear fusion in the yeast Saccharomyces cerevisiae. . Annu Rev Cell Dev Biol. 1996;12:663–695. doi: 10.1146/annurev.cellbio.12.1.663. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Section 1.76. Cold Spring Harbor Press, Plainview, NY.
- Sapir T, Elbaum M, Reiner O. Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit. EMBO (Eur Mol Biol Organ) J. 1997;16:6977–6984. doi: 10.1093/emboj/16.23.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer FC. Mitosis in the neural tube. J Comp Neurol. 1935;62:377–405. [Google Scholar]
- Schatten G. Motility during fertilization. Int Rev Cytol. 1982;79:59–163. doi: 10.1016/s0074-7696(08)61673-3. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanisms of neurulation: traditional viewpoint and recent advances. Development (Camb) 1990;109:243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Schroer TA. Structure, function and regulation of cytoplasmic dynein. Curr Opin Cell Biol. 1994;6:69–73. doi: 10.1016/0955-0674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Seiler S, Nargang FE, Steinberg G, Schliwa M. Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa. . EMBO (Eur Mol Biol Organ) J. 1997;16:3025–3034. doi: 10.1093/emboj/16.11.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL, Yeh E, Maddox P, Salmon ED, Bloom K. Astral microtubule dynamics in yeast: a microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud. J Cell Biol. 1997;139:985–994. doi: 10.1083/jcb.139.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MP. Microtubule motor complexes moving membranous organelle. Cell Struct Funct. 1996;21:369–373. doi: 10.1247/csf.21.369. [DOI] [PubMed] [Google Scholar]
- Stearns T. Motoring to the finish: kinesin and dynein work together to orient the yeast mitotic spindle. J Cell Biol. 1997;138:957–960. doi: 10.1083/jcb.138.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DS, Huffaker TC. Astral microtubules are not required for anaphase B in Saccharomyces cerevisiae. . J Cell Biol. 1992;119:379–388. doi: 10.1083/jcb.119.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE. Oocyte differentiation: a motor makes a difference. Curr Biol. 1997;7:R548–R551. doi: 10.1016/s0960-9822(06)00278-8. [DOI] [PubMed] [Google Scholar]
- Tinsley JH, Minke PF, Bruno KS, Plamann M. p150 Glued, the largest subunit of the dynactin complex is nonessential in Neurospora, but required for nuclear distribution. Mol Biol Cell. 1996;7:731–742. doi: 10.1091/mbc.7.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Biol Chem. 1996;133:831–842. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Sheetz MP. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Lane EB, Ramaekers FC. Cytoskeleton gradients in three dimensions during neurulation in the rabbit. J Comp Neurol. 1995;363:235–248. doi: 10.1002/cne.903630206. [DOI] [PubMed] [Google Scholar]
- Wang Y, Prade RA, Griffith J, Timberlake WE, Arnold J. A fast random cost algorithm for physical mapping. Proc Natl Acad Sci USA. 1994;91:11094–11098. doi: 10.1073/pnas.91.23.11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DA, Liu B, Xiang X, Morris NR. Mutations in the heavy chain of cytoplasmic dynein suppress the nudF nuclear migration mutation of Aspergillus nidulans. Mol Gen Genet. 1997;255:194–200. doi: 10.1007/s004380050489. [DOI] [PubMed] [Google Scholar]
- Xiang X, Beckwith SM, Morris NR. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. . Proc Natl Acad Sci USA. 1994;91:2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Roghi C, Morris NR. Characterization and localization of the cytoplasmic dynein heavy chain in Aspergillus nidulans. . Proc Natl Acad Sci USA. 1995a;92:9890–9894. doi: 10.1073/pnas.92.21.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Osmani AH, Osmani SA, Xin M, Morris NR. b. NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol Biol Cell. 1995;6:297–310. doi: 10.1091/mbc.6.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Zhuo W, Morris NR. Dynein localization in living cells of Aspergillus nidulans. . Mol Biol Cell. 1997;8:161a.. doi: 10.1091/mbc.8.9.1735. (Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton MM, Hamer JE, Timberlake WE. Transformation of Aspergillus nidulans by using a trpCplasmid. Proc Natl Acad Sci USA. 1984;81:1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalokar M, Erk I. Division and migration of nuclei during early embryogenesis of Drosophila melanogaster. . J Microsc Biol Cell. 1976;25:97–106. [Google Scholar]