Abstract
Invasive glioma cells migrate preferentially along central nervous system (CNS) white matter fiber tracts irrespective of the fact that CNS myelin contains proteins that inhibit cell migration and neurite outgrowth. Previous work has demonstrated that to migrate on a myelin substrate and to overcome its inhibitory effect, rat C6 and human glioblastoma cells require a membrane-bound metalloproteolytic activity (C6-MP) which shares several biochemical and pharmacological characteristics with MT1-MMP. We show now that MT1-MMP is expressed on the surface of rat C6 glioblastoma cells and is coenriched with C6-MP activity. Immunodepletion of C6-MP activity is achieved with an anti–MT1-MMP antibody. These data suggest that MT1-MMP and the C6-MP are closely related or identical. When mouse 3T3 fibroblasts were transfected with MT1-MMP they acquired the ability to spread and migrate on the nonpermissive myelin substrate and to infiltrate into adult rat optic nerve explants. MT1-MMP–transfected fibroblasts and C6 glioma cells were able to digest bNI-220, one of the most potent CNS myelin inhibitory proteins. Plasma membranes of both MT1-MMP–transfected fibroblasts and C6 glioma cells inactivated inhibitory myelin extracts, and this activity was sensitive to the same protease inhibitors. Interestingly, pretreatment of CNS myelin with gelatinase A/MMP-2 could not inactivate its inhibitory property. These data imply an important role of MT1-MMP in spreading and migration of glioma cells on white matter constituents in vitro and point to a function of MT1-MMP in the invasive behavior of malignant gliomas in the CNS in vivo.
Keywords: MT1-MMP, diffuse astrocytoma, invasion, central nervous system, metalloprotease
Diffuse astrocytomas and especially glioblastoma multiforme, the most common primary intracranial tumor in humans, invade the brain preferentially along white matter fiber tracts (Pedersen et al., 1995; Giese et al., 1996; Kleihues and Cavenee, 1997). This rapid infiltrative growth renders these tumors so dangerous and prevents successful surgical resection. Interestingly, CNS myelin is known as a very inhibitory substrate for neurite outgrowth as well as for spreading and migration of several cell types including astrocytes and fibroblasts (Schwab and Caroni, 1988; Amberger et al., 1994; Spillmann et al., 1997). A high molecular weight myelin protein (NI-220/ 250/IN-1 antigen) plays a crucial role for this inhibitory property of myelin (Caroni and Schwab, 1988; Paganetti et al., 1988; Spillmann et al., 1998).
C6 rat glioma, a chemically induced highly invasive cell line (Benda et al., 1968), as well as human glioma cells can spread and migrate on a central nervous system (CNS)1 myelin substrate in culture due to the presence of a membrane-bound metalloprotease activity (C6-MP) by which C6 cells overcome the inhibitory properties of CNS myelin (Paganetti et al., 1988; Amberger et al., 1994, 1998; Hensel et al., 1998). The nature of this protease activity has remained undefined up to now.
The use of metalloproteases for tissue infiltration by tumor cells is not uncommon. A number of studies have shown that matrix metalloproteases (MMPs) play a major role in physiological as well as pathological extracellular matrix (ECM) remodeling (Rodgers et al., 1994; Stuve et al., 1996; Yamamoto et al., 1996; Uhm et al., 1996, 1997). The MMP family is subdivided into four groups based on the presence of specific protein domains: the minimal domain MMPs which contain the pre-, pro-, and catalytic domains (matrilysin/MMP-7), the hemopexin domain MMPs which contain an additional terminal domain sharing a structural similarity to hemopexin and vitronectin (collagenases: MMP-1, 8, 13, 18; stromelysins: MMP-3, 10, 11 and metalloelastase MMP-12), the fibronectin domain MMPs (gelatinase A/MMP-2, gelatinase B/MMP-9), and the transmembrane domain MMPs (MT-MMPs) (Nagase et al., 1997). MT1-MMP is an integral membrane MMP and has been cloned from human placenta and breast cancer cDNA libraries (Okada et al., 1995; Sato et al., 1994). The physiological roles of MT1-MMP are not fully understood: MT1-MMP can activate progelatinase A/MMP-2 (Atkinson et al., 1995; Sato et al., 1996b; Butler et al., 1998), but broad-spectrum proteolytic capacities have also been demonstrated (Ohuchi et al., 1997; d'Ortho et al., 1998). MT1-MMP expression (along with gelatinase A/MMP-2 and gelatinase B/MMP-9) correlates with the malignancy of gliomas and also lung, cervical, and breast cancer and melanomas (Rooprai and McCormick, 1997; Okada et al., 1995; Yamamoto et al., 1996; Yoshizaki et al., 1997; Tokuraka et al., 1995; Gilles et al., 1996; Polette et al., 1996). Interestingly, the invasion of malignant melanoma cells occurs only when MT1-MMP localizes on plasma membrane invadopodia, which are cell membrane protrusions contacting or invading the surrounding matrix (Nakahara et al., 1997).
Here we show that C6 cells express active MT1-MMP on their plasma membranes. The ability of C6 membranes to inactivate the inhibitory substrate property of CNS myelin is lost after immunoprecipitation with an anti–MT1-MMP antibody whereas immunoprecipitation with an anti–MMP-2 antibody had no effect. MT1-MMP–transfected mouse 3T3 fibroblasts behaved like C6 cells on CNS white matter and infiltrate into optic nerve explants. In addition to the spreading and migration ability on CNS white matter as well as the degradation of the inhibitory protein bNI-220 by MT1-MMP–transfected 3T3 fibroblasts and C6 glioma cells, the MT1-MMP–mediated activation of gelatinase A/MMP-2 was sensitive to tissue inhibitor of matrix metalloproteases (TIMP)2 and insensitive to TIMP1 and batimastat (BB94). Gelatinase A/MMP-2 did not mimic the effect of MT1-MMP.
We conclude that MT1-MMP, but not gelatinase A/MMP-2 was able to remodel the nonpermissive CNS myelin substrate into a permissive one thus allowing spreading, migration, and infiltration of glioma cells on a CNS myelin substrate and, possibly, the invasion of myelinated CNS fiber tracts in vivo.
Materials and Methods
Reagents and Materials
Rat C6 glioma cells were a kind gift of D. Monard (Friedrich Miescher Institute, Basle, Switzerland) and mouse NIH 3T3 fibroblasts were obtained from the American Type Culture Collection. Trypsin, iodacetamide, PMSF, leupeptin, and pepstatin A were purchased from Fluka and TIMP1, TIMP2, and the monoclonal anti–MT1-MMP antibody (113-5B7) were purchased from Fuji Chemical Industries. The tetrapeptide carbobenzoxy-Phe-Ala-Phe-Tyr-amide (cbz-FAFY-amide) was synthesized by Bachem AG and BB94 was obtained from British Biotech Pharmaceutical. Four-well cell culture dishes were bought from Greiner and teflon-printed microscope slides were bought from Cell Line Associates. The cell sedimentation manifold (Apex Manufacturing) used for the cell migration assay was a kind gift of M. Berens (St. Joseph's Hospital, Phoenix, AZ). All products for zymography were bought from Bio-Rad. Nitrocellulose membranes were from Micron Separations Inc. The enhanced chemiluminescence (ECL)-Western blot detection kit, anti–MMP-2 antibody, proMMP2, and active MMP-2 were from Chemicon. DME and Ca2+/ Mg2+-free HBSS, penicillin, and streptomycin were purchased from GIBCO BRL and FCS was purchased from PAA. All other chemicals were purchased from Sigma.
Cell Culture
Rat C6 glioma cells and mouse NIH 3T3 fibroblasts were cultured to 60– 80% confluency in DME, containing 10% heat-inactivated FCS (FCS/ DME). All culture media contained 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were harvested after a short trypsin treatment (0.1% trypsin in 1 ml HBSS for 60 s) stopped by addition of 5 vol of FCS/DME, and then followed by centrifugation (1,000 rpm, 6 min). Cells were resuspended in FCS/DME in presence of inhibitors of interest and used for the experiments.
Preparation of Plasma Membrane-enriched Cell Fractions
C6 cells were grown to confluency in roller bottles and collected as described by Amberger et al. (1994). A 1-ml aliquot of this suspension was stored immediately at −20°C (cell homogenate fraction). Subcellular fractionation was carried out as described by Hoffman et al. (1990) with some minor modifications. In brief, the homogenate was centrifuged for 10 min at 2,000 g at 4°C, the pellet was collected and resuspended in 2 vol of 2.25 M sucrose in PBS. The plasma membrane fraction was isolated by centrifugation at 150,000 g for 1 h at 4°C on a discontinuous sucrose density gradient at the 1.52–0.8 M sucrose interphase, resuspended in PBS, and then stored in 500-μl fractions at −70°C for further use. Plasma membranes were pelleted, resuspended in 1 vol PBS containing 2 M NaCl, homogenized, and then centrifuged for 1 h at 4°C at 100,000 g to remove associated proteins. This salt-washed plasma membrane fraction (PM) was resuspended in PBS and 100-μl fractions were stored at −70°C for further use. The same procedure, on a smaller scale, was used for the preparation of the fibroblast membranes.
Preparation of the bNI-220–enriched CNS Substrate
A CNS-derived inhibitory protein fraction was prepared as described by Spillmann et al. (1997, 1998) with some modifications. In brief, bovine spinal cord (obtained from Schlacthaus Der Stadt Zürich) was cleaned from the meninges, minced, and subsequently homogenized on ice in 1 vol of extraction buffer (100 mM Tris-HCl, pH 8.0, 60 mM CHAPS, 10 mM EDTA, 2.5 mM iodacetamide, 1 mM PMSF, 0.1 μg/ml aprotonin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin A) and extracted for 10 min on a rotary shaker. After pelleting the insoluble material at 100,000 g for 1 h at 4°C, the clear extract was enriched for inhibitory activity on a Q-Sepharose anion exchange column. bNI-220 is a main inhibitory protein constituent of this fraction.
Western Blotting
For the evaluation of bNI-220 degradation properties, 10 μg of the samples were incubated for 1 h at 37°C with 30 μg bNI-220–enriched CNS substrate (see Cell Spreading Assay). Cell homogenates and plasma membrane fractions were prepared as described above and analyzed by 10 or 5% (MT1-MMP blot, NI-220 blot, respectively) PAGE according to Laemmli et al. (1970). The separated proteins were transferred onto a nitrocellulose membrane. The membrane was blocked with 3% gelatin in TBS containing 1% Tween 20 (TBST) for 16 h at 4°C and probed with 5 μg/ml anti–MT1-MMP antibody or a rabbit anti-NI-220 polyclonal antibody 472 (1:5,000) (Huber et al., 1997). After extensive washing with TBST, the membrane was incubated with goat anti–mouse Ig coupled to HRP or goat anti–rabbit Ig coupled to HRP for 1 h at room temperature. Finally, the blot was developed with the ECL-Western blot detection kit.
Zymography
Zymography was performed as described by Sawaya et al. (1996). In brief, 15 ng of proMMP2 were preincubated with 10 μg plasma membrane for 2 h at 37°C and electrophoresed on 10% SDS-PAGE containing gelatin. After electrophoresis, the gels were rinsed twice in 2.5% Triton X-100 and incubated at 37°C for 20 h in 50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 5 mM CaCl2, and 0.02% Brij35. For the evaluation of the different protease inhibitors, the inhibitors were added to the development buffer; for the evaluation of the effect of the inhibitors on the MT1-MMP–mediated activation of proMMP-2, the plasma membranes were preincubated with the inhibitors for 20 min at room temperature. The gels were stained with 0.5% Coomassie blue and destained in 40% methanol with 10% acetic acid in H2O. Gelatinolytic activity was detected as transparent bands on the blue background of the Coomassie-stained slab gel.
Immunocytochemistry
Cells were plated on CNS bNI-220–enriched substrate-coated wells (see Cell Spreading Assay). After 1 h the cells were fixed for 15 min with prewarmed 4% paraformaldehyde in PBS at room temperature. After blocking the nonspecific binding sites with 3% gelatin in PBS, cells were incubated over night at 4°C in 100 μl anti-human MT1-MMP antibody (5 μg/ml), washed three times with PBS, and then reacted with TRITC-conjugated goat anti–mouse antibody (Jackson ImmunoResearch) for 1 h at room temperature. Slides were embedded in Mowiol and evaluated under a Zeiss Axiophot fluorescence microscope.
Immunodepletion
C6 plasma membranes were extracted with Triton X-100 (protein detergent ratio 2:1) for 1 h at 4°C followed by centrifugation at 1 h at 100,000 g for 4°C). The supernatant was incubated for 1 h at room temperature with 0.05, 0.1, or 0.2 μg anti-human MT1-MMP monoclonal mouse antibody or with 0.2, 1, or 2 μg of an anti–MMP-2 polyclonal rabbit antibody. Anti-mouse antibody- and anti-rabbit antibody-coated magnetic beads were added and rotated for 30 min at room temperature. The beads were collected and the supernatant was evaluated for its C6 metalloproteolytic activity in the fibroblast spreading assay as described (Paganetti et al., 1988; Amberger et al., 1994), with or without o-phenanthroline to confirm metalloproteolytic activity.
Transfection of 3T3 Cells
The cDNA encoding human MT1-MMP (GenBank/EMBL/DDBJ accession number D26512) was isolated by nested polymerase chain reaction (PCR). The first amplification was performed for 30 cycles at an annealing temperature of 45°C using the oligonucleotide pair CCGTCGACAAAGGCGCCCCGAGGGA and AATCTAGAGGTAGGTGAGGCAGAGG with a human placenta cDNA library as template (Clontech). The resulting 2.5-kb cDNA fragment was reamplified with the oligonucleotides CCGGTCGACTCGGACCATGTCTC and TTTCTAGAGCGTCAGACCTTGTCC for 40 cycles with annealing at 45°C, resulting in a 1.75-kb cDNA fragment containing the whole open reading frame of MT1-MMP that was blunt end–cloned in pUC19 (Invitrogen). The restriction sites XbaI and SalI present in the oligonucleotides (underlined sequences) were used for subcloning in the pRK vector (Ruat et al., 1995).
C6 cells and 3T3 cells were cultured as described. On the day of transfection, fibroblasts were washed with HBSS. A DNA mix was prepared by adding 10 μg MT1-MMP–plasmid to 300 μl HBSS and 60 μl SuperFect, as described by the manufacturer (Qiagen). After 10 min of incubation at room temperature, 3 ml FCS/DME were added. HBSS was removed from the culture dishes and the DNA mix was added. The dishes were incubated at 37°C for 3 h, another 3 ml of FCS/DME were added, and cells were evaluated after 48 h.
Cell Spreading Assay
The Q pool protein concentration resulting in 80% inhibited 3T3 fibroblasts was determined and used for all further experiments. After 1 h of incubation, inhibited fibroblasts remained round in contrast to well-attached, flat, spreading cells on a control substrate. The Q pool fraction was coated overnight at 4°C onto four-well culture dishes; control dishes were coated with extraction buffer only. Unbound material was removed by two washes with HBSS and 8,000 fibroblasts in FCS/DME were plated per well (1 cm2). After 1 h of incubation at 37°C the cells were fixed with 4% paraformaldehyde in PBS. The portion of flat, well-spread cells was determined (200–300 cells counted per well). Protease inhibitors were added directly to the cell suspension 15 min before plating from 100× solutions (final concentrations: 1 mM EDTA, 200 μM o-phenanthroline, 300 μM phosphoramidon, 10 μM Cbz-Phe-Ala-Phe-Tyr-amide, 1 μM BB94, 100 nM TIMP1, and 100 nM TIMP2).
To investigate the influence of active gelatinase A/MMP-2 and of C6 cell homogenate (CH) and plasma membranes of C6 cells, MT1-MMP– and mock-transfected fibroblasts on the CNS myelin inhibitory properties, the coated Q pool was treated for 1 h at 37°C with 10 μM active gelatinase A/MMP-2, 50 μg CH, or 30 μg PM and washed two times with HBSS before plating fibroblasts. Every experiment was performed at least three times in triplicate.
Cell Migration Assay
The migration assay was performed as described by Berens et al. (1994) and Amberger et al. (1998), with some modifications. In brief, teflon-printed microscope slides, subdivided into 10 wells, were precoated overnight with 50 μg/ml poly-l-lysine, washed twice with PBS, air dried, and then stored at room temperature until use. Subsequently, six wells were coated overnight with the CNS inhibitory protein fraction at 4°C, four controls were coated with extraction buffer only. After two washes with HBSS, the wells were covered with 70 μl FCS/DME each (with or without inhibitors), the cell sedimentation manifold was placed on the slide, and 1 μl of cell suspension (2,000 cells) was placed in each cylinder. Protease inhibitors were added directly to the cell suspension 15 min before plating from 100× solutions. After incubation for 2 h at 37°C under 5% CO2, the cell sedimentation manifold was removed. The area covered with cells was recorded 2, 6, 12, and 24 h after seeding with a video camera (charge-coupled device [CCD]; Sony) attached to an inverted microscope (2.5× objective, IMT-2; Olympus). The cell migration area was quantified with an image analysis system (Image-1; Universal Imaging). The migration area 2 h after seeding was used as the migration reference point; all values measured were divided by the 2-h value to obtain a relative migration area. Every experiment was performed at least three times in triplicate for the CNS substrate and in duplicate for the control substrate.
Optic Nerve Explant Infiltration Assay
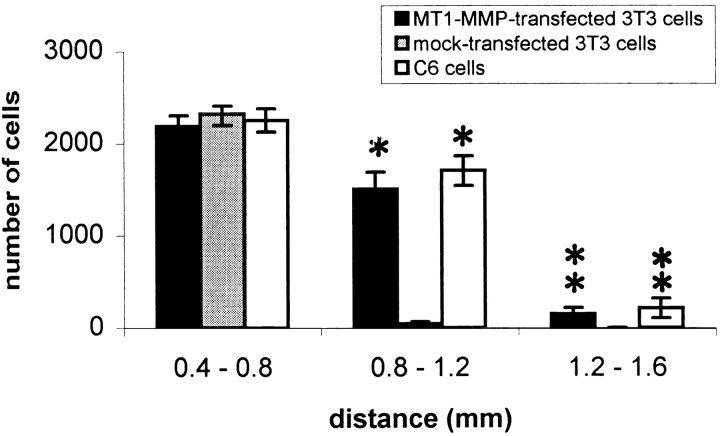
Optic nerve explants in chamber cultures were prepared as described by Schwab and Thoenen (1985). In brief, the optic nerves were rapidly dissected from 3–4-mo-old Lewis rats and then trimmed. Subsequently, the nerves were X-irradiated at 5,000 g for 7 min to reduce glial cell proliferation and placed under a teflon ring sealed to a culture dish with silicon grease. Three chambers in contact only via the explants were obtained in this way (see Fig. 9 a). C6 glioblastoma cells, MT1-MMP–transfected, or mock-transfected fibroblasts were prelabeled with Hoechst-dye for 1.5–3 h (50 μl Hoechst-dye/10 ml DME/FCS), washed three times with DME/ FCS, and then 100,000 cells were placed into the middle chamber. Cultures were incubated for 7 d. Medium was exchanged every other day. At the end of the experiment the optic nerves were fixed for 48 h in 4% PFA containing 5% sucrose, washed, removed from the cultures, flat mounted in Tissue Tek (Sakura), and then frozen at −40°C. 20-μm longitudinal sections were serially cut on a cryostate. Labeled infiltrated cells were counted under a fluorescences microscope (Zeiss Axiophot) starting from the tip of the nerve exposed to the middle, cell-containing chamber. The cells were counted at distances of 0.4–0.8, 0.8–1.2, and 1.2–1.8 mm from the middle chamber stump. Three sections were counted for each distance, and the cell numbers were extrapolated for the thickness of the nerve. 12 nerves infiltrated by MT1-MMP–transfected fibroblasts, 12 nerves with mock-transfected fibroblasts, and eight nerves with C6 cells were evaluated.
Figure 9.
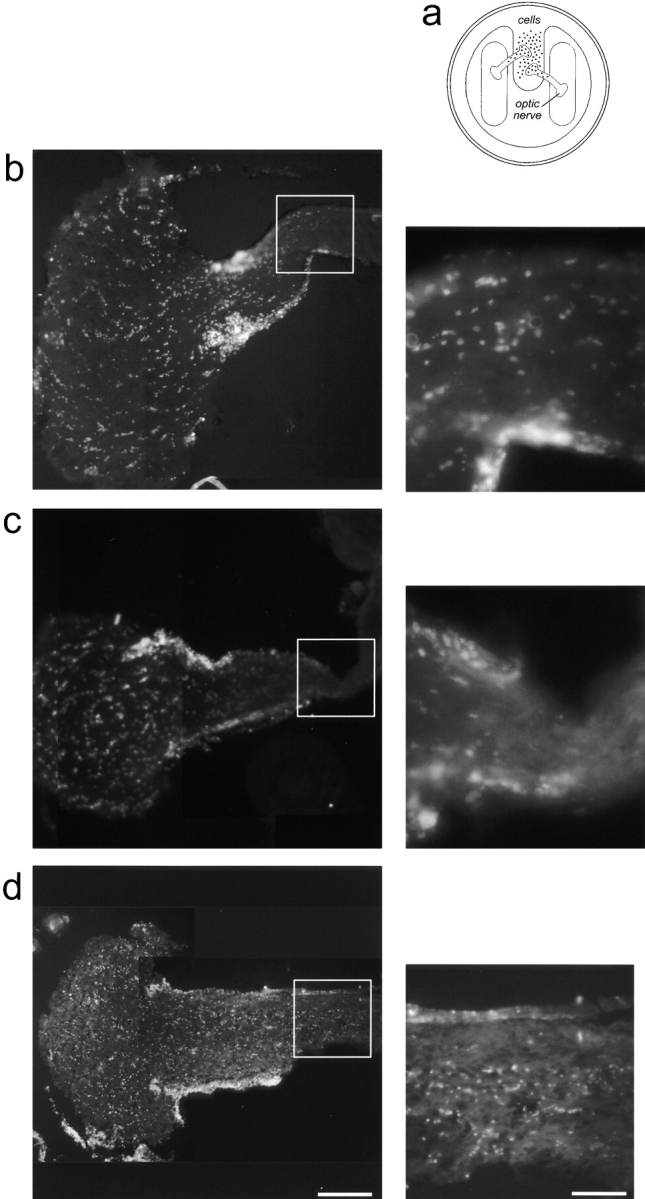
(a) Optic nerve explant infiltration assay. Adult rat optic nerves were placed underneath a teflon ring sealed to a culture dish with silicon grease. Hoechst dye-prelabeled C6 cells, MT1-MMP–transfected fibroblasts, or mock-transfected fibroblasts (100,000 cells) were placed in the middle chamber, in contact with the tips of the nerves (a). After 7 d Hoechst dye-labeled cells are observed infiltrating the optic nerve explants in longitudinal sections. MT1-MMP–transfected fibroblasts (b) but not mock-transfected fibroblasts (c) infiltrated the optic nerves. Enlargements show a high density of MT1-MMP–transfected fibroblasts at a distance of 1.6 mm. Very few mock-transfected fibroblasts were found at this depth. C6 cells infiltrated the optic nerve to the same extent as the MT1-MMP– transfected fibroblasts (d). Bar, 0.3 mm.
Results
Active MT1-MMP Is Localized on the Plasma Membrane of C6 Glioblastoma Cells and Is Coenriched with the C6 MP Activity
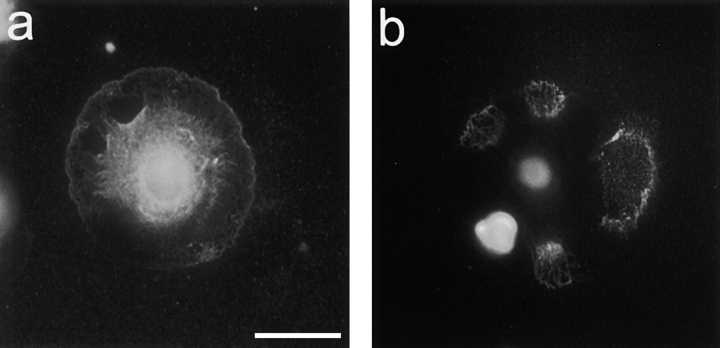
To determine if MT1-MMP is expressed on the surface of C6 glioblastoma cells, cells were plated on a CNS inhibitory protein substrate for 1 h. The cells were firmly attached and well spread assuming the typical fried egg appearance. Immunofluorescence with a monoclonal anti– MT1-MMP antibody revealed staining along the plasma membrane (Fig. 1 a) often enriched at substrate contact sites (Fig. 1 b).
Figure 1.
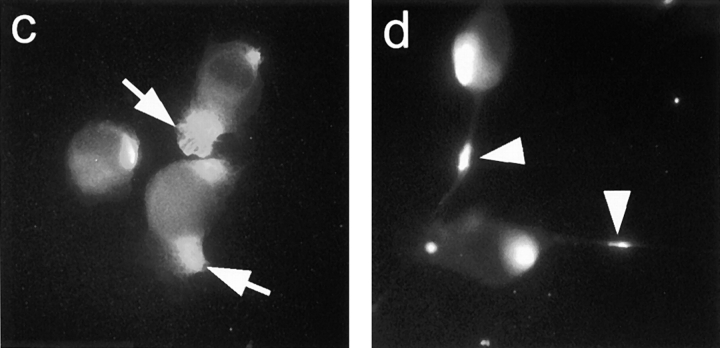
MT1-MMP immunoreactivity in C6 glioblastoma cells and MT1-MMP–transfected fibroblasts 1 h after plating the cells on CNS substrate. (a) Well-spread C6 cell shows diffuse MT1-MMP staining over the plasma membrane, especially along the cell periphery. (b) When the plane of focus is at the level of the substrate, MT1-MMP immunoreactivity is detected at the substrate contact sites. (c) MT1-MMP–transfected fibroblasts show immunoreactivity enriched on lamellipodia (arrows). (d) 24 h after plating MT1-MMP–transfected fibroblasts cells formed long processes on which foci of MT1-MMP are observed (arrowhead). Bar, 14 μm.
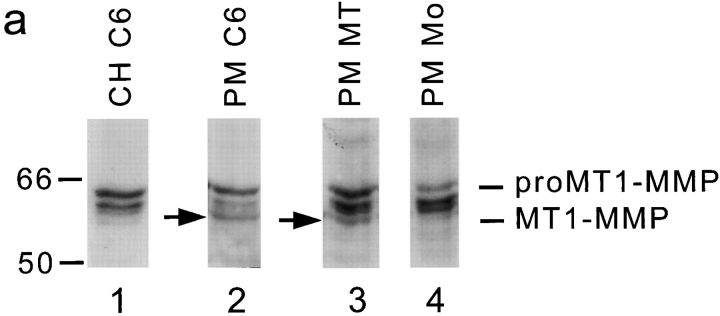
To biochemically evaluate the localization of MT1-MMP, a C6 total cell homogenate and a salt-washed C6 plasma membrane fraction were prepared. These fractions were separated on 10% SDS-PAGE and blotted. Incubation of the blots with anti–MT1-MMP antibody revealed two specific bands: one at the molecular weight of proMT1-MMP (63 kD) and another band at 58 kD, corresponding to the molecular weight of active MT1-MMP (Fig. 2 a) (Ohuchi et al., 1997). The 58-kD band was clearly more intense in the plasma membrane fraction than in the cell homogenate fraction.
Figure 2.
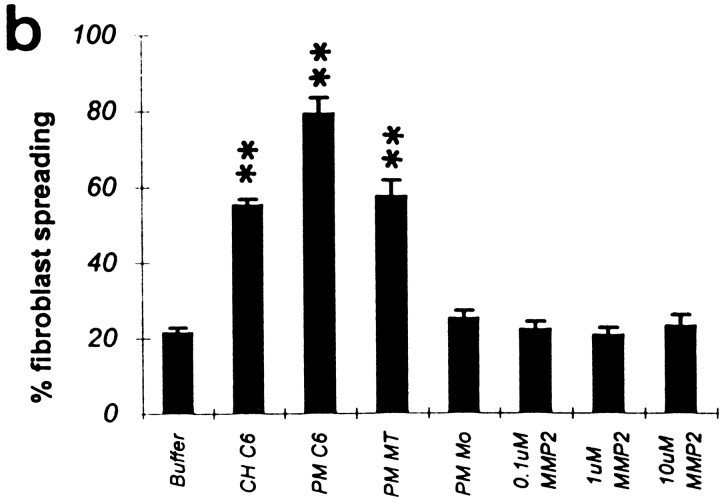
MT1-MMP Western blot and remodeling of the CNS inhibitory substrate. (a) Western blots of cell lysates and salt-washed plasma membranes using a monoclonal antibody against MT1-MMP. The protein fractions were electrophoresed on a 10% SDS polyacrylamide gel, blotted onto a nitrocellulose membrane, and probed with MT1-MMP antibody. Bound antibody was visualized by peroxidase-conjugated second antibody and chemiluminescence. Lane 1, C6 cell homogenate (CH C6); lane 2, C6 cell plasma membranes (PM C6); lane 3, plasma membranes of MT1-MMP–transfected fibroblasts (PM MT); lane 4, plasma membranes of mock-transfected fibroblasts (PM Mo). Molecular mass markers are indicated at the left side, proMT1-MMP and processed MT1-MMP (arrows) on the right. Two nonspecific bands are seen between the pro- and mature MT1-MMP forms since they are visualized also in the absence of primary antibody. (b) Culture dishes coated with inhibitory CNS membrane proteins were pretreated in different ways and their inhibitory activity assayed for 3T3 fibroblast spreading. Treatment with C6 homogenate (50 μg) or plasma membranes (30 μg) of C6 glioma cells or MT1-MMP–transfected fibroblasts reduced the inhibitory property significantly, whereas plasma membranes of mock-transfected fibroblasts did not. Pretreatment of the CNS substrate with gelatinase A/MMP-2 (0.1, 1, and 10 μM) did not influence its nonpermissive substrate property. Values are means ± SEM of three experiments performed in triplicate. Significance with the two-tailed Student's t test: P < 0.01 (**).
C6-MP activity, as determined in a cell spreading assay, was also highest in the C6 plasma membrane fraction and lower in the C6 cell homogenate (Fig. 2 b) (Paganetti et al., 1988, Amberger et al., 1994). The presence of active MT1-MMP was confirmed by zymography where plasma membranes of C6 cells as well as of MT1-MMP–transfected fibroblasts showed conversion of progelatinase A/proMMP-2 to mature MMP-2 (Fig. 3, lanes 1–7). These results show the presence of active MT1-MMP on the plasma membranes of C6 glioblastoma cells.
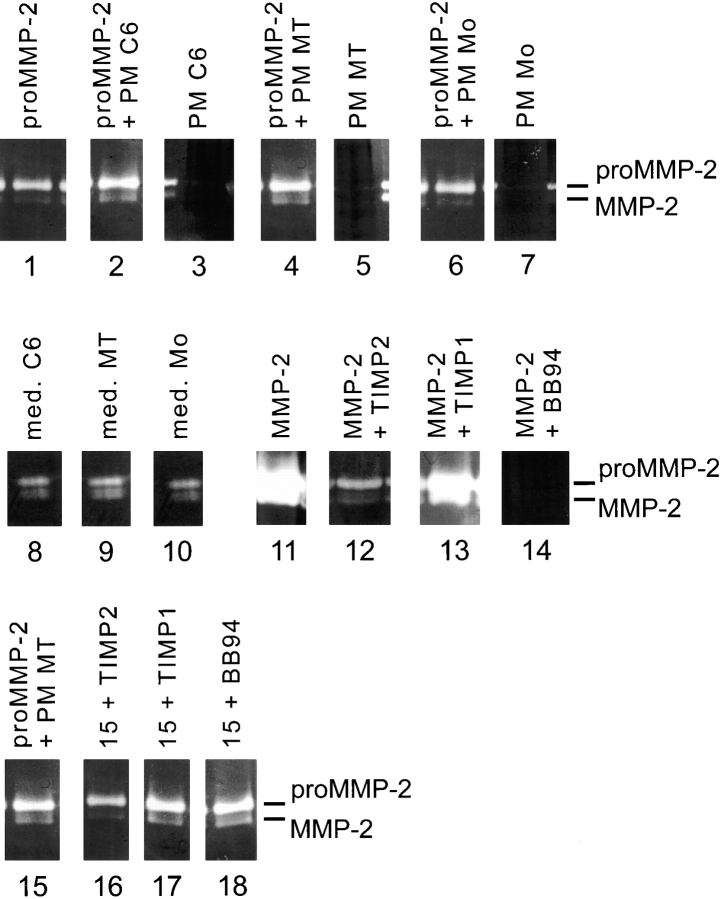
Figure 3.
Gelatin zymography. ProMMP-2 (lane 1) is partially converted to mature MMP-2 by C6 plasma membranes (PM C6) (lane 2), or MT1-MMP–transfected fibroblast plasma membranes (PM MT) (lane 4), but not by mock-transfected fibroblast plasma membranes (PM Mo) (lane 6). Plasma membranes alone (PM C6, lane 3; PM MT, lane 5; PM Mo, lane 7) neither contain mature nor proMMP-2. Conditioned media of C6 cells (lane 8), MT1-MMP–transfected fibroblasts (lane 9) and mock-transfected fibroblasts (lane 10) contain active and proMMP-2. Effects of protease inhibitors: MMP-2 is strongly inhibited by 100 nM TIMP2 (lane 12 versus lane 11) and 1 μM BB94 (lane 14), but not by 100 nM TIMP1 (lane 13). Conversion of proMMP-2 to mature MMP-2 by MT1-MMP–transfected fibroblast plasma membranes is decreased by TIMP2 (100 nM, lane 16 versus lane 15), but not by TIMP1 (100 nM, lane 17) or BB94 (1 μM).
Depletion of C6 Glioblastoma Plasma Membrane Extract with MT1-MMP Antibody Leads to Loss of Remodeling Ability of the Inhibitory CNS Myelin Substrate
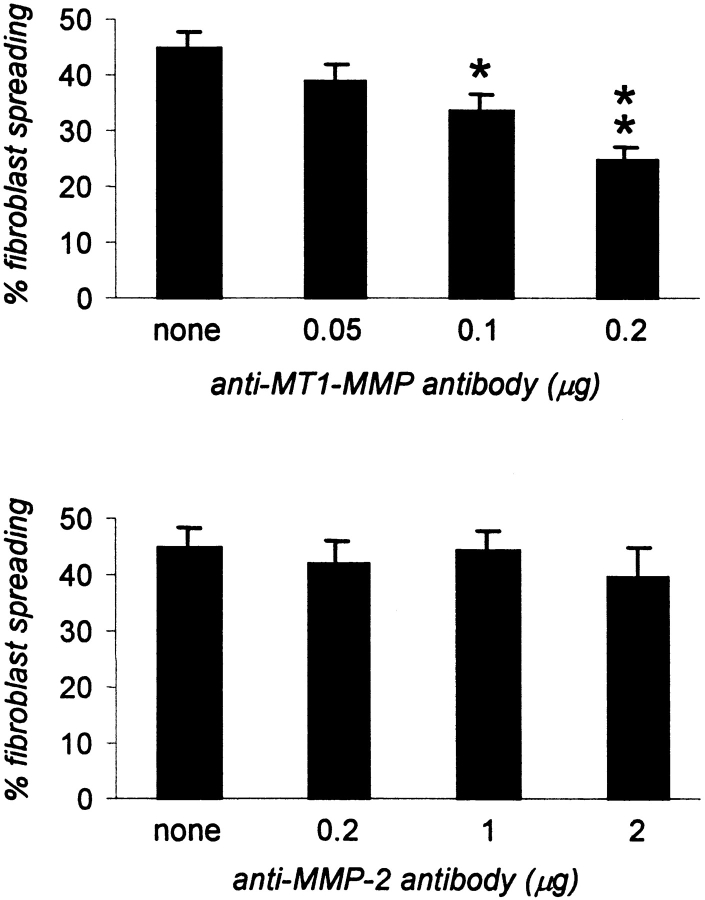
C6 plasma membrane detergent extracts, containing C6 metalloproteolytic activity (Amberger et al., 1994), were treated with a MT1-MMP antibody or a MMP-2 antibody. After immunoprecipitation with MT1-MMP antibody, the extracts showed a large decrease of their ability to inactivate the inhibitory effect of CNS membrane proteins for fibroblast spreading (Fig. 4). This effect was dose dependent. Immunoprecipitation with a MMP-2 antibody had no effect (Fig. 4).
Figure 4.
C6 plasma membrane extracts depleted of MT1-MMP by immunoprecipitation can no more inactivate the inhibitory activity of CNS myelin for fibroblast spreading, whereas immunodepletion of gelatinase A/MMP-2 did not affect the inactivation potential of the extract. 40 μg of C6 plasma membrane detergent extracts were immunodepleted with the indicated amounts anti–MT1-MMP antibody or anti–MMP-2 antibody. Inhibitory CNS substrate-coated wells were subsequently incubated for 30 min at 37°C with the immunodepleted extracts (supernatants), washed, and then used to evaluate the spreading ability of normal fibroblasts. Values are means ± SEM of two experiments performed in triplicate. Significance with the two-tailed Student's t test: P < 0.1 (*); P < 0.01 (**).
Active MT1-MMP Is Present on the Plasma Membrane of MT1-MMP–transfected Fibroblasts
1 h after plating on a bNI-220–enriched CNS substrate, MT1-MMP immunoreactivity was mainly localized on the lamellipodia of the transfected fibroblasts (Fig. 1 c). This lamellipodial staining was absent in mock-transfected cells (data not shown). About 15% of the cells showed this typical surface staining. 24 h after plating of the cells on CNS myelin, the MT1-MMP–transfected fibroblasts showed long processes on which foci of high MT1-MMP expression were found (Fig. 1 d).
Western blots demonstrated that the plasma membranes of MT1-MMP–transfected fibroblasts as well as those of mock-transfected fibroblasts contained proMT1-MMP, but only the MT1-MMP–transfected fibroblasts expressed the mature form (Fig. 2 a). Pretreatment of the inhibitory CNS protein substrate with plasma membranes of MT1-MMP–transfected fibroblasts revealed a large decrease in the inhibitory substrate property as observed by an increase in the spreading of fibroblasts on the pretreated substrate (Fig. 2 b). Pretreatment of the substrate with membranes of mock-transfected fibroblasts had no effect (Fig. 2 b).
Zymography showed that only the plasma membranes of MT1-MMP–transfected fibroblasts were able to mediate progelatinase A/proMMP-2 conversion to mature gelatinase A/MMP-2 and a band shift from the 72-kD progelatinase A/proMMP-2 to 59-kD gelatinase A/MMP-2. Again plasma membranes of mock-transfected fibroblasts were inactive in this assay (Fig. 3).
MT1-MMP–transfected Fibroblasts Spread on an Inhibitory CNS Protein Substrate and Are Sensitive to the Same Protease Inhibitors as C6 Glioblastoma Cells
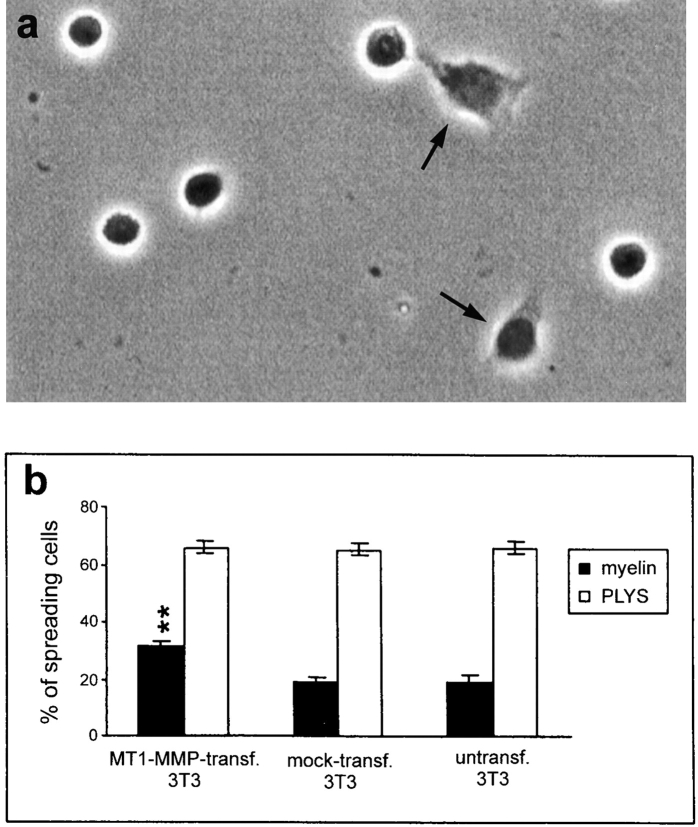
To evaluate the ability of MT1-MMP–transfected, mock-transfected, and nontransfected fibroblasts to spread on an inhibitory CNS membrane protein extract (Spillmann et al., 1998), the cells were plated on this substrate, fixed after 1 h of incubation, and then the percentage of flat, well-attached, spread cells was determined (Fig. 5). Of the nontransfected and mock-transfected fibroblasts 19 ± 2 and 20 ± 2%, respectively, of the cells were able to spread on the CNS protein substrate. Upon transfection with MT1-MMP the percentage of spread cells increased to 35 ± 3% (Fig. 5 b). This increase of 15% correlates well with the transfection rate of about 15% as seen in immunofluorescence for MT1-MMP. On the control substrate (PLYS), MT1-MMP–, mock-, and nontransfected fibroblasts spread equally well.
Figure 5.
(a) Spreading of MT1-MMP–transfected fibroblasts on culture dishes coated with inhibitory CNS membrane protein extracts. 1 h after plating ∼35% of the MT1-MMP–transfected cells were well attached and showed a flat appearance (arrow). The nonspreading cells appear as round, phase-bright cells. (b) Quantification of the spreading of MT1-MMP–, mock-, and nontransfected fibroblast on CNS inhibitory substrate and control substrate (PLYS). Significantly more MT1-MMP–transfected fibroblasts spread on CNS myelin as compared with mock- or nontransfected fibroblasts. In all three conditions, cells spread equally well on the control substrate PLYS. Values are means ± SEM of three experiments, performed in triplicate. Significance with the two-tailed Student's t test: P < 0.01 (**).
The effects of several metalloprotease inhibitors on the spreadings of MT1-MMP–transfected fibroblasts (Fig. 6 a) and of C6 glioblastoma cells (Fig. 6 b) plated on an inhibitory CNS protein substrate were studied. In contrast to normal 3T3 fibroblasts (Fig. 2 b, Fig. 4, and Fig. 5 b), MT1-MMP–transfected fibroblasts as well as C6 cells show a large proportion of spreading cells. Addition of the chelators EDTA and o-phenanthroline greatly reduced the spreading ability of both cell types (P < 0.01). The more specific C6-MP inhibitors phosphoramidon and cbz-FAFY-amide (Amberger et al., 1994) also significantly reduced the spreading ability of MT1-MMP–transfected fibroblasts similar to what was observed with the C6 glioblastoma cells.
Figure 6.
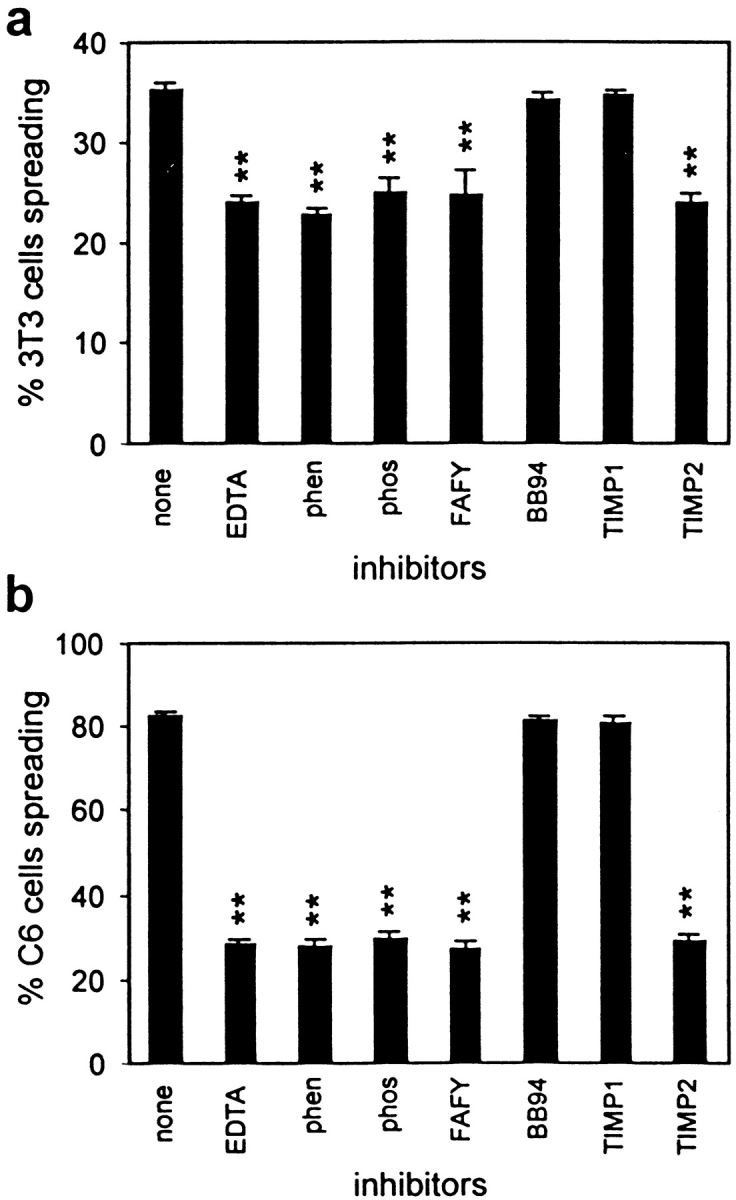
Effect of various metalloprotease inhibitors on the spreading ability of MT1-MMP–transfected fibroblasts and C6 glioma cells on CNS myelin. (a) Transiently MT1-MMP–transfected fibroblasts of which only ∼15% express high MT1-MMP levels. The proportion of spreading cells on CNS myelin substrate was reduced by 15% by 1 mM EDTA, 200 μM o-phenanthroline (phen), 300 μM phosphoramidon (phos), 10 μM Cbz-Phe-Ala-Phe-Tyr-amide (FAFY), and 100 nM TIMP2. No inhibition was observed in presence of 1 μM BB94 or 100 nM TIMP1. (b) About 80% of the C6 cells spread on the inhibitory CNS myelin substrate. This was reduced to ∼25% by the same inhibitors found to affect the MT1-MMP–transfected fibroblasts. Values are means ± SEM of three experiments, performed in triplicate. Significance with the two-tailed Student's t test: P < 0.01 (**).
In vivo, the activity of MMPs is regulated by tissue metalloproteinase inhibitors (TIMP1 to TIMP4). TIMP2 exerts activity against several MMPs, but preferentially against gelatinase A/MMP-2 by binding both its latent and active forms (Imai et al., 1996). The TIMP2 effect on MT1-MMP has been extensively studied and optimal inhibitory concentrations for TIMP2 have been assessed (Atkinson et al., 1995; Sato et al., 1996b; Butler et al., 1998). TIMP1 is active against several MMPs, but preferentially against gelatinase B/MMP-9 (Birkedal-Hansen et al., 1993). BB94, which is a synthetic general inhibitor of MMPs, has been used in clinical trials for treatment of several tumors based on its inhibitory effect on gelatinase A/MMP-2 (Wojtowicz-Praga et al., 1997). 1 μM BB94 and 100 nM TIMP1 had no effect on spreading of C6 cells as well as MT1-MMP–transfected fibroblasts (Fig. 6). Interestingly, 100 nM TIMP2 strongly reduced the spreading capacity of the MT1-MMP–transfected 3T3 fibroblasts as well as that of the C6 glioblastoma (Fig. 6). Spreading on control substrate was not influenced by these inhibitors (data not shown). The activity profile of the inhibitors at the concentrations used in the bioassays was confirmed by zymography: gelatin degradation by MMP-2 was strongly reduced by 100 nM TIMP2 and 1 μM BB94. In contrast, 100 nM TIMP1 had only a small effect (Fig. 3). These data strongly suggest an involvement of MT1-MMP in the mechanism that allows C6 cells to overcome the inhibitory property of CNS membranes, and that gelatinase A/MMP-2 and gelatinase B/MMP-9 do not play a major role in spreading of these cells on CNS myelin.
Gelatinase A/MMP-2 Does Not Account for the Inactivation of the bNI-220–enriched Inhibitory CNS Protein Substrate
Pretreatment of an inhibitory bovine spinal cord protein fraction (Spillmann et al., 1998) with active gelatinase A/MMP-2 (activity status confirmed by zymography: Fig. 3, lanes 11–14) did not transform this nonpermissive CNS protein substrate into a permissive one, since no increase in the spreading ability of fibroblasts on this substrate was observed (Fig. 2 b). On the other hand, the MMP inhibitor BB94 had neither effect on the spreading of C6 glioma cells, nor on that of MT1-MMP–transfected fibroblasts (Fig. 6). Mock-transfected fibroblasts were not able to spread on this inhibitory CNS protein substrate although they do express active gelatinase A/MMP-2 as shown by zymography (Fig. 3, lane 10). In addition, by zymography we did not detect active gelatinase A/MMP-2 in the plasma membrane fractions of C6 or MT1-MMP–transfected cells (Fig. 3, lanes 3 and 5). Thus, the ability of cells to spread on a CNS myelin protein substrate correlates well with the presence of active MT1-MMP, but not with that of active gelatinase A/MMP-2.
MT1-MMP–transfected Fibroblasts Migrate on an Inhibitory CNS Myelin Substrate and Are Sensitive to the Same Inhibitors as the C6 Glioblastoma Cells
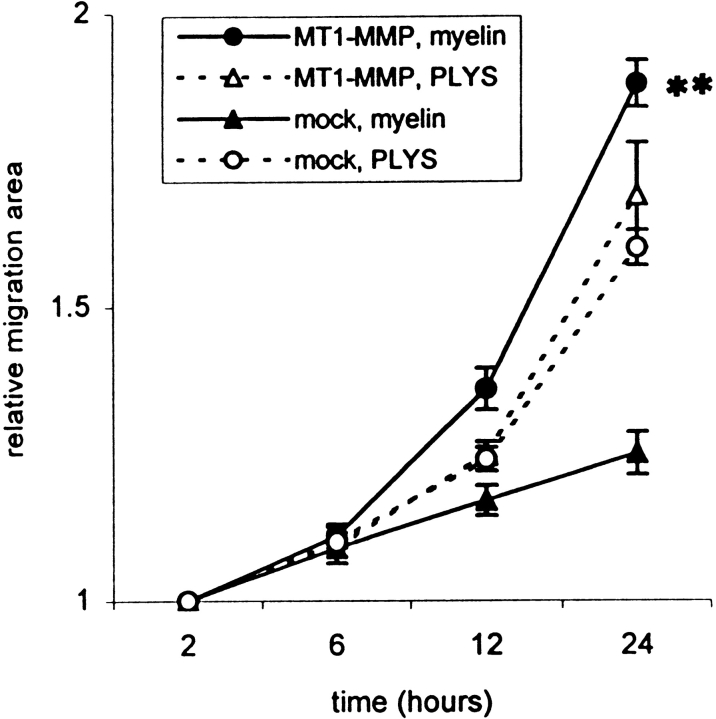
To determine the involvement of MT1-MMP in cell migration on CNS myelin, cells were seeded on the inhibitory CNS protein substrate or on PLYS control substrate with the help of the cell manifold holder in a round, well-defined area. 24 h after seeding, MT1-MMP–transfected 3T3 cells showed a large increase in the migration area compared with 2 h after seeding (Fig. 7 a and Fig. 8). In contrast, mock-transfected fibroblasts had hardly migrated out of the starting area (Fig. 8). At the rim of the migration zone single MT1-MMP–transfected cells were moving on the CNS myelin substrate (Fig. 7, b and c). This strongly indicates that these cells were not pushed out of the center due to proliferation effects. Since the transfection rate was ∼15%, only a minor population of MT1-MMP–transfected fibroblasts would be expected to migrate out of the starting area. However, more than 15% migrating cells were observed. Typically, the cells at the front of the migration zone often were characterized by long processes (Fig. 7 c). Immunofluorescence for MT1-MMP revealed that these cells were strongly MT1-MMP positive (Fig. 7 d). Cells in the center of the migration area often had shorter processes on which less MT1-MMP was expressed. The “pioneers” may have proteolytically modified the inhibitory CNS substrate, allowing also non- or low MT1-MMP– expressing cells to migrate. The time-course of fibroblast migration is shown in Fig. 8. MT1-MMP–transfected fibroblasts almost doubled the area covered with cells within 24 h, whereas mock-transfected cells could hardly migrate on the inhibitory substrate. Both cell types were migrating equally well on the control substrate.
Figure 7.
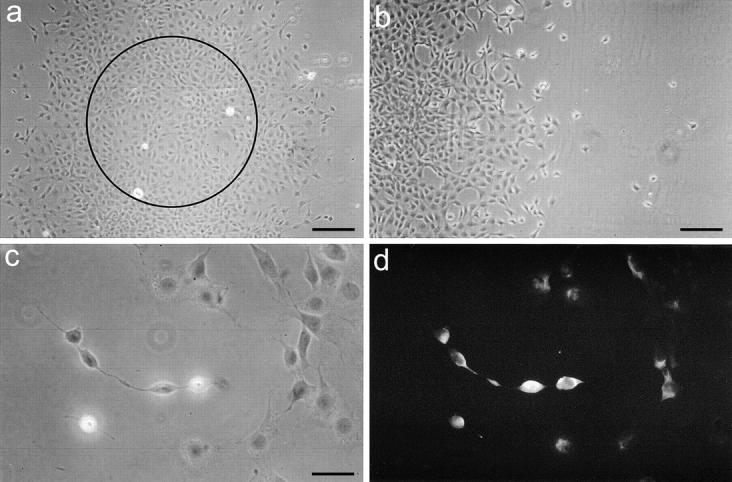
MT1-MMP–transfected fibroblasts migrate on CNS myelin. (a) MT1-MMP– transfected fibroblasts were plated in a defined area (marked by circle). After 24 h the area covered with cells doubled in size. (b and c) Enlargements of the rim of the migration zone show the migration of single MT1-MMP– transfected cells. (d) These “pioneer” cells are expressing high levels of MT1-MMP with foci of MT1-MMP on their processes as shown by MT1-MMP immunostaining (same field as c). Bars: (a) 300 μm; (b) 150 μm; (c and d) 50 μm.
Figure 8.
Time-course and quantification of 3T3 fibroblast migration. MT1-MMP–transfected fibroblasts migrated extensively on inhibitory CNS protein substrate within 24 h, in contrast to mock-transfected fibroblasts, whereas both migrated equally well on the control substrate (PLYS). Values are means ± SEM of four experiments, performed in quadruplicate. Significance with the two-tailed Student's t test: P < 0.01 (**).
The influence of the metalloprotease inhibitors BB94 (1 μM), TIMP1 (100 nM), and TIMP2 (100 nM) on the migration of MT1-MMP–transfected fibroblasts (Table I, top) and C6 glioblastoma cells (Table I, bottom) was evaluated. BB94 and TIMP1 did not show a significant effect. In contrast, TIMP2 decreased the migration capacity of both C6 glioblastoma cells and MT1-MMP–transfected fibroblasts massively (P < 0.01). For either cell type, migration on the control substrate was not influenced by these inhibitors (data not shown).
Table I.
Effect of Metalloprotease Inhibitors on Cell Migration
| Inhibitor | Relative migration area | Percent reduction in migration ability | ||||
|---|---|---|---|---|---|---|
| MT1-MMP–transfected fibroblasts on myelin | None | 1.88 ± 0.04 | 0 ± 4.5 | |||
| BB94 | 1.78 ± 0.03 | 11.4 ± 3.4 | ||||
| TIMP1 | 1.82 ± 0.06 | 6.8 ± 6.8 | ||||
| TIMP2 | 1.28 ± 0.07 | 68.2 ± 8.0 | ||||
| C6 glioblastoma cells on myelin | None | 2.02 ± 0.06 | 0 ± 5.9 | |||
| BB94 | 1.94 ± 0.05 | 7.8 ± 4.9 | ||||
| TIMP1 | 2.03 ± 0.06 | 0 ± 5.9 | ||||
| TIMP2 | 1.21 ± 0.07 | 78.4 ± 6.9 |
Effect of specific metalloprotease inhibitors on the migration capacity of MT1-MMP–transfected fibroblasts and C6 glioblastoma cells on an inhibitory CNS protein substrate (24 h). Top, the migration of MT1-MMP–transfected fibroblasts was not affected by 1 μM BB94 or 100 nM TIMP1, but was greatly decreased by 100 nM TIMP2. Bottom, C6 cells showed an inhibitor profile very similar to that of the MT1-MMP–transfected fibroblasts. Values are means ± SEM of three experiments performed in quadruplicate. Significance with the two-tailed Student's t test: P < 0.01 (
).
MT1-MMP–transfected Fibroblasts Infiltrate into Optic Nerve Explants
Pairs of adult rat optic nerves were cultured in a three-compartment chamber culture and Hoechst dye-labeled cells were placed at one end of the nerve explants (Fig. 9 a). After 7 d in vitro the distance of cell infiltration was evaluated on longitudinal histological sections. Mock-transfected fibroblasts did not infiltrate the CNS explants for more than 0.8 mm. In contrast, large numbers of the MT1-MMP–transfected fibroblasts infiltrated over 1–1.6 mm into the optic nerve, distances very similar to those seen with C6 glioblastoma cells (Fig 9, b–d). Quantification and distance of fibroblast infiltration is shown in Fig. 10. MT1-MMP expression thus enables fibroblasts to infiltrate this adult CNS tissue rich in myelin.
Figure 10.
MT1-MMP–transfected fibroblasts infiltrated the optic nerves over longer distances than mock-transfected fibroblasts. Values are means ± SEM of 12 nerves infiltrated with MT1-MMP–transfected fibroblasts, 12 nerves with mock-transfected fibroblasts, and eight nerves with C6 cells. Significance with the two-tailed Student's t test: P < 0.01 (**).
Plasma Membranes of MT1-MMP–transfected Fibroblasts Can Degrade the Potent CNS Myelin Inhibitory Protein bNI-220
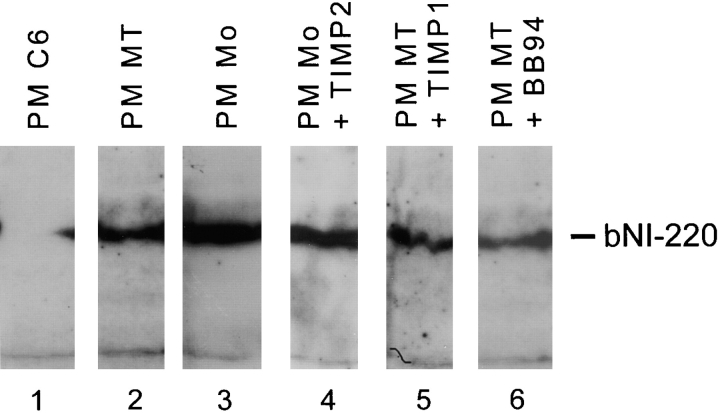
A bovine spinal cord detergent extract enriched for bNI-220 over an ion-exchange column was preincubated with plasma membranes of mock- and MT1-MMP–transfected fibroblasts. Western blots with an affinity-purified rabbit polyclonal antibody against a bNI-220 peptide sequence (Ab 472; Huber et al., 1998) revealed disappearance or diminution of the bNI-220 band after treatment with plasma membranes of MT1-MMP–transfected fibroblasts or of C6 glioma cells, but not after treatment with membranes of mock-transfected fibroblasts (Fig. 11). This degradation could be inhibited by the addition of 100 nM TIMP2, but not by the addition of 1 μM BB94 or 100 nM TIMP1 (Fig. 11). We conclude that NI-220 can be degraded specifically by MT1-MMP–enriched plasma membranes.
Figure 11.
Western blot of bNI-220–enriched CNS protein extract using a polyclonal rabbit antibody against bNI-220 (Huber et al., 1998). CNS protein extract enriched for bNI-220 was preincubated for 1 h at 37°C in the presence of plasma membranes of C6 cells and transfected fibroblasts. The protein fractions then were electrophoresed on a 5% SDS polyacrylamide gel, blotted onto a nitrocellulose membrane, and then probed with purified anti–bNI-220 polyclonal rabbit antibody. Bound antibody was visualized with an peroxidase-conjugated secondary antibody followed by chemiluminescent detection. Plasma membranes of C6 glioma cells (PM C6, lane 1) and MT1-MMP–transfected fibroblasts (PM MT, lane 2) could degrade the bNI-220 completely (C6 glioma cells) or partially (MT1-MMP–transfected fibroblasts), whereas mock-transfected fibroblast plasma membranes could not (PM Mo, lane 3). Digestion of bNI-220 in the presence of PM MT was blocked by 100 nM TIMP2 (lane 4), but not by 100 nM TIMP1 (lane 5) or 1 μM BB94 (lane 6). Arrow indicates the bNI-220 level, arrowheads point to a nonspecific band, indicating that in all lanes equal amounts of protein were loaded.
Discussion
Neoplastic cells of peripheral origin which produce metastases in the brain rarely infiltrate the parenchyma of the CNS. Instead, they grow as solid, well-circumscribed tumors (Laerum et al., 1984; Bindal et al., 1994). Diffuse astrocytomas, including glioblastoma multiforme, on the other hand massively invade the brain by migrating preferentially along the white matter fiber tracts (Pedersen et al., 1995; Kleihues and Cavenee, 1997). In vitro, the motility of most cells, e.g., astrocytes or fibroblasts, is strongly restricted on a CNS white matter substrate due to the presence of inhibitory constituents, in particular the potent inhibitory protein NI-220/250 (Spillmann et al., 1997, 1998; Caroni and Schwab, 1988). Rat C6 glioma cells were shown to spread and migrate on CNS myelin and infiltrate CNS tissue in vitro and in vivo (Paganetti et al., 1988; Goldberg et al., 1991). C6 cells as well as human glioma cell lines use a membrane-bound MP activity (C6-MP) to overcome the inhibitory properties of CNS myelin (Paganetti et al., 1988; Amberger et al., 1994, 1998; Hensel et al., 1998). This C6-MP activity correlates with the malignancy of gliomas (Amberger et al., 1998), a characteristic shared by an other membrane bound metalloprotease: the MT1-MMP (Okada et al., 1995; Yamamoto et al., 1996; Yoshizaki et al., 1997). We therefore suspected a similarity (or even identity) of the C6-MP activity with MT1-MMP. This hypothesis is supported by the present results: (a) immunohistochemistry, Western blots, and zymography revealed the expression of active MT1-MMP by C6 cells; (b) C6-MP activity was immunodepleted from C6 plasma membrane extracts with an antibody against MT1-MMP but not by an antibody against gelatinase A/MMP-2; and (c) 3T3 fibroblasts, which can not spread or migrate on a CNS inhibitory protein substrate, were able to do so upon transfection with MT1-MMP. Furthermore, (d) spreading and migration of MT1-MMP–transfected fibroblasts and of C6 glioblastoma cells on a CNS myelin substrate were affected in the very same way by several inhibitors of metalloprotease: both cell types were sensitive to the chelators EDTA and o-phenanthroline, to phosphoramidon and the tetrapeptide cbz-FAFY-amide, inhibitors which were shown earlier to affect the C6-MP activity (Amberger et al., 1994). Both cell types were also affected by 100 nM TIMP2. At this concentration TIMP2 acts as an inhibitor for MT1-MMP and gelatinase A/MMP-2 (Atkinson et al., 1995; Sato et al., 1996b; Butler et al., 1998). Neither C6 cells nor MT1-MMP–transfected cells were affected by 100 nM TIMP1 or by 1 μM BB94. Both strongly inhibit gelatinase A/MMP-2 and gelatinase B/MMP-9, respectively, at this concentration.
Plasma membranes of MT1-MMP–transfected fibroblasts were able to mediate progelatinase A/MMP-2 activation, an ability which was blocked by the addition of 100 nM TIMP2, whereas 100 nM TIMP1 had no effect. Interestingly, 1 μM BB94 had no effect on MT1-MMP–mediated proMMP-2 activation. The effects of TIMP1 and TIMP2 confirm an earlier report: d'Ortho et al. (1998) showed that expression of MT1-MMP on the cell surface caused proteolysis of a gelatin film, which is inhibited by TIMP2 but not by TIMP1.
Although MT1-MMP can act as an activator of proMMP-2, it has been hypothesized that the ability of MT1-MMP–transfected cells for an elevated invasive potential may not necessarily be linked exclusively to progelatinase A/MMP-2 activation; MT1-MMP possesses direct degrading activity for number of ECM components and receptors (Ohuchi et al., 1997; d'Ortho et al., 1997; Pei and Weiss, 1996). The present results show the inability of soluble gelatinase A/MMP-2 to remodel the nonpermissive CNS protein substrate into a permissive one. Gelatinase A/MMP-2 immunodepleted C6 plasma membrane detergent extract, containing C6 metalloproteolytic activity, was still able to inactivate the inhibitory effect of the CNS white matter substrate, whereas MT1-MMP depleted extract could not. Potent gelatinase A/MMP-2 and gelatinase B/MMP-9 inhibitors (BB94 and TIMP1) did not affect C6 glioma cells or MT1-MMP–transfected fibroblasts in their spreading and migration ability on CNS myelin, thus supporting the view that MT1-MMP may directly remodel the CNS substrate and allow C6 cell motility without the involvement of soluble MMPs.
Plasma membranes of C6 cells and of MT1-MMP–transfected fibroblasts were able to convert the nonpermissive substrate into a permissive one for cell spreading, whereas mock-transfected fibroblast plasma membranes failed to do so. Furthermore, degradation of the inhibitory protein bNI-220 occurred by C6 plasma membranes and by the MT1-MMP–transfected fibroblasts, but not by plasma membranes of mock-transfected fibroblasts. These results show that MT1-MMP is crucial for the inactivation of inhibitory constituents of the CNS, a process which allows the migration of glioma cells and of MT1-MMP–transfected fibroblasts. Gelatinase A/MMP-2 did not seem to play a role in this particular process since mock-transfected fibroblasts were found to express active gelatinase A/MMP-2 but did not inactivate the inhibitory CNS substrate. Whether MT1-MMP directly modifies the inhibitory CNS proteins or whether it acts as an activator in a more complex, plasma membrane-localized protease cascade remains to be elucidated (proteases sensitive to low concentrations of BB94 and TIMP1 are excluded, however). Gelatinase A/ MMP-2 is known to be upregulated in glioblastoma multiforme, especially in endothelial cells of blood vessels, suggesting a role for gelatinase A/MMP-2 rather on the level of angiogenesis (massive neovascularization is a hallmark of glioblastomas; Sawaya et al., 1996). Itoh et al. (1998) reported that gelatinase A–deficient mice showed reduced angiogenesis and progression of tumors originating from implanted melanoma or lung carcinoma cells.
The fact that MT1-MMP–transfected fibroblasts were able to infiltrate adult optic nerve explants to an extent very similar to C6 glioma cells suggests that MT1-MMP is crucial for cell migration not only on a two-dimensional CNS protein substrate, but also within the CNS tissue and, therefore, probably also in vivo. Stably MT1–MMP transfected cell lines may be used to investigate the role of this protease after implantation into adult rat brains.
The low expression level of MT1-MMP in healthy intact brain (Yamada et al., 1995) and the high levels of MT1-MMP found in human gliomas (Okada et al., 1995; Yamamoto et al., 1996; Uhm et al., 1997), together with the results of the present study, make MT1-MMP an interesting potential target for future therapeutic interventions for diffuse astrocytomas, especially glioblastoma multiforme. Furthermore, MT1-MMP may serve as a marker and prognostic indicator for the malignancy of astrocytomas.
Acknowledgments
We thank R. Schöb and E. Hochreutner for their photographical and graphical help, M. van der Haar, I. Klusman, A. Huber, B. Niederöst, and R. Schneider (Brain Research Institute, Zurich, Switzerland) for practical assistance and fruitful discussions, and especially C. Bandtlow (Zurich, Switzerland) for critically reading the manuscript.
This work was supported by the Swiss National Science Foundation (31-45549.95) and by the Cancer League of the Canton Zurich.
Abbreviations used in this paper
- BB94
batimastat
- C6-MP
rat C6 glioblastoma metalloprotease
- cbz-FAFY-amide
carbobenzoxy-Phe-Ala-Phe-Tyr-amide
- CH
cell homogenate
- CNS
central nervous system
- MMP
matrix metalloprotease
- MT1-MMP
membrane-type 1 matrix metalloprotease
- TIMP
tissue inhibitor of matrix metalloproteases
References
- Amberger VR, Paganetti PA, Seulberger H, Eldering JA, Schwab ME. Characterization of a membrane-bound metalloendoprotease of rat C6 glioblastoma cells. Cancer Res. 1994;54:4017–4025. [PubMed] [Google Scholar]
- Amberger VR, Hensel T, Ogata N, Schwab ME. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998;58:149–158. [PubMed] [Google Scholar]
- Atkinson SJ, Crabbe T, Cowell S, Ward RV, Butler MJ, Sato H, Seiki M, Reynolds JJ, Murphy G. Intermolecular autolytic cleavage can contribute to the activation of progelatinase A by cell membranes. J Biol Chem. 1995;270:30479–30485. doi: 10.1074/jbc.270.51.30479. [DOI] [PubMed] [Google Scholar]
- Benda ME, Lightbody J, Sato G, Levine L, Sweet W. Differential rat glia cell strain in tissue culture. Science. 1968;161:370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Berens ME, Rief MD, Loo MA, Giese A. The role of extracellular matrix in human astrocytoma migration and proliferation studied in a microliter scale assay. Clin Exp Met. 1994;12:405–415. doi: 10.1007/BF01755884. [DOI] [PubMed] [Google Scholar]
- Bindal AK, Hammoud M, Ming SW, Wu SZ, Sawaya R, Roa JS. Prognostic significance of proteolytic enzymes in human brain tumors. J Neuro Oncol. 1994;22:101–110. doi: 10.1007/BF01052886. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Burger PC, Heinz ER, Shibata T, Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurgery. 1988;68:698–704. doi: 10.3171/jns.1988.68.5.0698. [DOI] [PubMed] [Google Scholar]
- Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, van Westrum SS, Crabbe T, Clements J, d'Ortho MP, Murphy G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central nervous system myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Bourdon MA, Luo G, Reisfeld RA, Strongin A. Matrix-metalloproteinase-2 activation modulates glioma cell migration. J Cell Sci. 1997;110:2473–2482. doi: 10.1242/jcs.110.19.2473. [DOI] [PubMed] [Google Scholar]
- Giese A, Kluwe L, Laube B, Meissner H, Berens ME, Westphal M. Migration of human glioma cells on myelin. Neurosurgery. 1996;38:755–764. [PubMed] [Google Scholar]
- Gilles C, Polette M, Piette J, Munaut C, Thompson EW, Birembaut P, Foidart JM. High levels of MT1-MMP expression is associated with invasiveness of cervical cancer cells. Int J Cancer. 1996;65:209–213. doi: 10.1002/(SICI)1097-0215(19960117)65:2<209::AID-IJC14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Goldberg WJ, Laws ER, Jr, Bernstein JJ. Individual C6 glioma cells migrate in adult rat brain after neural homografting. Int J Dev Neurosci. 1991;9:427–437. doi: 10.1016/0736-5748(91)90064-s. [DOI] [PubMed] [Google Scholar]
- Hensel T, Amberger VR, Schwab ME. A metalloprotease activity from C6 cells inactivates the myelin-associated neurite growth inhibitors and can be neutralized by antibodies. Br Cancer J. 1998;78:1564–1572. doi: 10.1038/bjc.1998.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S, Sorkin BC, White PC, Brackenbury R, Mailhammer R, Rutishauser U, Cunningham BA, Edelman GM. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982;257:7720–7729. [PubMed] [Google Scholar]
- Huber AB, Chen MS, Van Der Haar ME, Schwab ME. Developmental expression pattern and functional analysis of Nogo (formarly NI-35/250), a major inhibitor of CNS regeneration. Soc Neurosci Abstr. 1998;24:1559. [Google Scholar]
- Imai K, Ohuchi E, Aoki T, Nomura H, Fujii Y, Sato H, Seiki M, Okada Y. Membrane-type matrix metalloproteinase 1 is a gelatinolytic enzyme and is secreted in a complex with tissue inhibitor of metalloproteinase 2. Cancer Res. 1996;56:2707–2710. [PubMed] [Google Scholar]
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Kleihues P, Cavenee WK. Pathology and genetics of tumours of the nervous system. IARC. 1997;56:1–9. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophages. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laerum OD, Bjerkvig R, Steinsvag SK, De Ridder L. Invasiveness of primary brain tumors. Cancer Met Rev. 1984;3:223–236. doi: 10.1007/BF00048386. [DOI] [PubMed] [Google Scholar]
- Nagase H. Activation mechanism of matrix metalloproteases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh YY, Chen WT. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci USA. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani H, Motohashi H, Sato H, Seiki M, Nagura H. Dual over-expression of membrane-type metalloproteinase-1 in cancer and stromal cells in human gastrointestinal carcinoma revealed by in situ hybridization and immunoelectron microscopy. Int J Cancer. 1996;68:565–570. doi: 10.1002/(SICI)1097-0215(19961127)68:5<565::AID-IJC2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ohuchi E, Imai K, Fuji Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci USA. 1995;92:2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- d'Ortho MP, Stanton H, Butler M, Atkinson SJ, Murphy G, Hembry RM. MT1-MMP on the cell surface causes focal degradation of gelatin films. FEBS (Fed Eur Biochem Soc) Lett. 1998;421:159–164. doi: 10.1016/s0014-5793(97)01555-x. [DOI] [PubMed] [Google Scholar]
- Paganetti PA, Caroni P, Schwab ME. Glioblastoma infiltration into central nervous system tissue in vitro: involvement of a metalloprotease. J Cell Biol. 1988;107:2281–2291. doi: 10.1083/jcb.107.6.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PH, Edvardsen K, Garcia-Cabrera I, Mahesparan R, Thorson J, Mathisen B, Rosenblum ML, Bjerkvig R. Migratory patterns of lac-ztransfected human glioma cells in the rat brain. Int J Cancer. 1995;62:767–771. doi: 10.1002/ijc.2910620620. [DOI] [PubMed] [Google Scholar]
- Pei D, Weiss SJ. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- Pilkington GJ. Tumour cell migration in the central nervous system. Brain Pathol. 1994;4:157–166. doi: 10.1111/j.1750-3639.1994.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Polette M, Nawrocki B, Gilles C, Sato H, Seiki M, Tournier JM, Birembaut P. MT1-MMP expression and localisation in human lung and breast cancers. Virchows Arch. 1996;428:29–35. doi: 10.1007/BF00192924. [DOI] [PubMed] [Google Scholar]
- Rodgers WH, Matrisian LM, Guidice LC, Dsupin B, Cannon P, Svitek C, Gorstein F, Osteen KG. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994;94:946–953. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooprai HK, McCormick D. Proteases and their inhibitors in human brain tumours: a review. Anticancer Res. 1997;17:4151–4162. [PubMed] [Google Scholar]
- Ruat M, Molliver ME, Snowman AM, Snyder SH. Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proc Natl Acad Sci USA. 1995;92:3161–3165. doi: 10.1073/pnas.92.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix matalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Sato H, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem. 1996a;119:209–215. doi: 10.1093/oxfordjournals.jbchem.a021223. [DOI] [PubMed] [Google Scholar]
- Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS (Fed Eur Biochem Soc) Lett. 1996b;393:101–104. doi: 10.1016/0014-5793(96)00861-7. [DOI] [PubMed] [Google Scholar]
- Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG, Nicolson GL, Rao JS. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Met. 1996;14:35–42. doi: 10.1007/BF00157684. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillmann AA, Amberger VR, Schwab ME. High molecular weight protein of human central nervous system inhibits neurite outgrowth: an effect which can be neutralized by the monoclonal antibody IN-1. Eur J Neurosci. 1997;9:549–555. doi: 10.1111/j.1460-9568.1997.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Spillmann AA, Bandtlow CE, Lottspeich F, Keller F, Schwab ME. Identification and characterization of a bovine neurite growth inhibitor (bNI-220) J Biol Chem. 1998;273:19283–19293. doi: 10.1074/jbc.273.30.19283. [DOI] [PubMed] [Google Scholar]
- Strongin AY, Marmer BL, Grant GA, Goldberg GI. Plasma membrane-dependent activation of the 72-kDa Type IV collagenase is prevented by complex formation with TIMP-2. J Biol Chem. 1993;268:14033–14039. [PubMed] [Google Scholar]
- Stuve O, Dooley NP, Uhm JH, Antel JP, Francis GS, Williams G, Yong VW. Interferon beta-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann Neurol. 1996;40:853–863. doi: 10.1002/ana.410400607. [DOI] [PubMed] [Google Scholar]
- Tokuraka M, Sato H, Murakami S, Okada Y, Watanabe Y, Seiki M. Activation of the precursor of gelatinase A/72 kDa typeIV collagenase/MMP-2 in lung carcinomas correlated with the expression of membrane-type matrix metalloproteinase (MT-MMP) and with lymph node metastasis. Int J Cancer. 1995;64:355–359. doi: 10.1002/ijc.2910640513. [DOI] [PubMed] [Google Scholar]
- Uhm JH, Dooley NP, Villemure J-G, Yong VW. Glioma invasion in vitro: regulation by matrix metalloprotease-2 and protein kinase C. Clin Exp Met. 1996;14:421–433. doi: 10.1007/BF00128958. [DOI] [PubMed] [Google Scholar]
- Uhm JH, Dooley NP, Villemure J-G, Yong VW. Mechanisms of glioma invasion: role of matrix-metalloproteinases. Cancer J Neurol Sci. 1997;24:3–15. doi: 10.1017/s0317167100021028. [DOI] [PubMed] [Google Scholar]
- Wojtowicz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs. 1997;15:61–75. doi: 10.1023/a:1005722729132. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Yoshiyama Y, Sato H, Seiki M, Shinagawa A, Takahashi M. White matter microglia produce membrane-type matrix metalloprotease, an activator of gelatinase A, in human brain tissue. Acta Neuropathol. 1995;90:421–424. doi: 10.1007/BF00294800. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Mohanam S, Sawaya R, Fuller GN, Seiki M, Sato H, Gokaslan ZL, Liotta LA, Nickolson GL, Rao JS. Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase A activation in human malignant brain tumors in vivo and in vitro. . Cancer Res. 1996;56:384–392. [PubMed] [Google Scholar]
- Yoshizaki T, Sato H, Maruyama Y, Murono S, Furukawa M, Park CS, Seiki M. Increased expression of membrane type 1-matrix metalloproteinase in head and neck carcinoma. Cancer. 1997;79:139–144. doi: 10.1002/(sici)1097-0142(19970101)79:1<139::aid-cncr20>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]