Abstract
The Src homology 2-containing phosphotyrosine phosphatase (SHP2) is primarily a positive effector of receptor tyrosine kinase signaling. However, the molecular mechanism by which SHP2 effects its biological function is unknown. In this report, we provide evidence that defines the molecular mechanism and site of action of SHP2 in the epidermal growth factor-induced mitogenic pathway. We demonstrate that SHP2 acts upstream of Ras and functions by increasing the half-life of activated Ras (GTP-Ras) in the cell by interfering with the process of Ras inactivation catalyzed by Ras GTPase-activating protein (RasGAP). It does so by inhibition of tyrosine phosphorylation-dependent translocation of RasGAP to the plasma membrane, to its substrate (GTP-Ras) microdomain. Inhibition is achieved through the dephosphorylation of RasGAP binding sites at the level of the plasma membrane. We have identified Tyr992 of the epidermal growth factor receptor (EGFR) to be one such site, since its mutation to Phe renders the EGFR refractory to the effect of dominant-negative SHP2. To our knowledge, this is the first report to outline the site and molecular mechanism of action of SHP2 in EGFR signaling, which may also serve as a model to describe its role in other receptor tyrosine kinase signaling pathways.
The process of protein tyrosine phosphorylation and dephosphorylation is central to growth factor, cytokine, and integrin signal transduction. The enzymes that catalyze phosphorylation reactions are either the receptors themselves with intrinsic tyrosine kinase activity, known as receptor tyrosine kinases (RTKs), or cytoplasmic tyrosine kinases. The substrates for these kinases are the receptors themselves and/or downstream signaling proteins. Some of the most extensively studied RTKs include the epidermal growth factor receptor (EGFR), the platelet-derived growth factor receptor (PDGFR), and the fibroblast growth factor (FGF) receptor (13, 21, 22, 27, 29, 37, 42, 49, 55, 57). Upon the binding of a cognate ligand, RTKs dimerize and autophosphorylate tyrosine residues in their cytoplasmic domain or tyrosylphosphorylate downstream substrates (5, 6, 9, 11, 17, 20, 25, 30, 34, 41). More often than not, phosphorylated tyrosine residues serve as binding sites for Src homology 2 (SH2)-domain-containing signaling proteins (38). These interactions mediate the formation of multiprotein signaling complexes by which signals are transduced down the cascade. At least two known functions are effected by these interactions: recruitment of enzymes to substrate microdomains and/or induction of enzyme activity (3, 16, 18, 26, 30, 41, 43; M. Adachi, E. H. Fischer, J. Ihle, K. Imai, F. Jirik, B. Neel, T. Pawson, S. Shen, M. Thomas, A. Ullrich, and Z. Zhao, Letter, Cell 85:15, 1996). The binding of a cytokine to its cognate receptor also elicits similar signaling events. However, tyrosine phosphorylation of proteins, including the receptors themselves, is carried out by associated cytoplasmic tyrosine kinases, since these receptors lack intrinsic tyrosine kinase activity (7, 58, 59).
Recent advances in the area of phosphotyrosylphosphatases (PTPs) indicate that tyrosine dephosphorylation is as important as tyrosine phosphorylation in the transduction of signals elicited by growth factors and cytokines. Among the hundred or slightly more transmembrane and nontransmembrane phosphotyrosine (pY) phosphatases known to date, the ubiquitously expressed mammalian cytoplasmic phosphatase termed SH2 phosphatase 2 (SHP2) is a well-known positive effector of tyrosine kinase signaling (23, 32, 53, 54). Also, Corkskrew, the Drosophila melanogaster counterpart of SHP2, is an essential positive effector of the Torso RTK signaling (8). On the other hand, SHP1, which is expressed primarily in hematopoietic cells, is a negative regulator of tyrosine kinase signaling, although it has significant structural similarity to SHP2.
SHP2 possesses two tandemly arranged SH2 domains in its N-terminal region and a phosphatase domain in its C-terminal region (15, 16). It also possesses a stretch of proline-rich sequences and tyrosine phosphorylation sites in its extreme C-terminal region. Both the SH2 domains and the phosphatase domain have been shown to be absolutely essential for the biological activity of SHP2 (10). However, no biological role has been definitively ascribed to the tyrosine phosphorylation sites or the proline-rich region. Deletion of the N-SH2 or mutation of the conserved cysteine residue to serine in the active site of the phosphatase domain eliminates the biological activity of SHP2 (15, 16). Transgenic mice homozygous for the N-SH2 deletion mutation die in the uterus before day E10.5 from multiple defects in mesoderm development (4, 39, 40). Also, microinjection of the N-SH2 deletion or phosphatase-dead mutant mRNA into Xenopus laevis eggs causes abnormal embryonal development (48). These findings have established that SHP2 is a requisite for normal growth and development.
SHP2 interacts directly with autophosphorylated RTKs such as EGFR and PDGFR or indirectly via tyrosine-phosphorylated adaptor proteins through its SH2 domains (9, 18, 25, 26, 41, 52). These interactions have been shown to be essential for RTK signaling. However, how these interactions contribute positively to tyrosine kinase signaling is virtually unknown. Moreover, how pY dephosphorylation by SHP2 drives the signaling message forward and what substrates are acted upon by SHP2 are unknown. Recently, we developed an efficient substrate-trapping mutant of SHP2 and demonstrated that one of its physiological substrates is EGFR (2). In the present report, we describe the molecular mechanism of SHP2 in the mitogenic signaling pathway by using EGFR as a model. We find that SHP2 acts upstream of Ras in the EGFR pathway and functions by increasing the half-life of activated Ras (GTP-Ras) in the cell. It does so by interfering with the process of Ras inactivation (conversion to GDP-bound form) catalyzed by GTPase-activating protein (RasGAP) via inhibition of tyrosine phosphorylation-dependent translocation of RasGAP to the plasma membrane (PM). Furthermore, we demonstrate that Y992 of the EGFR is a negative-regulatory autophosphorylation site that acts as a binding site for RasGAP.
MATERIALS AND METHODS
Cells, cell culture, and antibodies.
The cell types used in this study were COS-1, NIH 3T3, and A431. COS-1 and NIH 3T3 were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, whereas A431 cells were grown in Dulbecco's modified Eagle's medium containing only 5% fetal calf serum. All cell types were maintained at 37°C with 7% CO2. EGF, PDGFβ, and FGF-1 were purchased from GIBCO-BRL; anti-phospho-ERK1/2 or pan-ERK2 antibodies were from New England Biolabs; anti-RasGAP antibodies were from Upstate Biotechnology; anti-PTP1D and anti-Ras monoclonal antibodies were from Transduction Laboratories, anti-pY monoclonal antibody (4G10) was a gift from D. Morrison; and anti-T7 tag monoclonal antibody was from Novagen. The polyclonal antibody to SHP2 was raised by injection of rabbits with a glutathione S-transferase (GST) fusion of the N-terminal region (36). Horseradish peroxidase-conjugated secondary antibodies were purchased from Amersham. For fluorescent quantitation of phosphoprotein band intensities, alkaline phosphatase-conjugated anti-rabbit and anti-mouse secondary antibodies were purchased from Zymed, whereas the AttoPhos fluorescent substrate system was from Promega.
Plasmid construction and site-directed mutagenesis.
Construction of plasmids that express wild-type SHP2 (WT-SHP2) or C459S-SHP2 was described previously (36). The R465E-SHP2 and the C459S/R465E-SHP2 mutants, hereinafter referred to as R/E and C/S-R/E, respectively, were produced by introducing the indicated point mutation into the WT or the C459S mutant with complementary primers that span the signature motif of the PTP domain. The sense primer for the R/E was 5′-CTGCAGTGCTGGAATTGGCGAGACAGGGACGTTCATTGTG-3′, and the antisense primer was 5′-CACAATGAACGTCCCTGTCTCGCCAATTCCAGCACTGCAG-3′. For the C/S-R/E, the sense primer was 5′-CAGCAGTGCTGGAATTGGCGAGACAGGGACGTTCATTGTG-3′, and the antisense primer was 5′-CACAATGAACGTCCCTGTCTCGCCAATTCCAGCACTGCTG-3′. The Tyr-to-Phe point mutants of the EGFR were produced using the following primers: 5′-GTGGTGGATGCCGACGAGTTCCTCATCCCACAGCAGGGCTTC-3′ and 5′-GAAGCCCTGCTGTGGGATGAGGAACTCGTCGGCATCCACCAC-3′ for Y992F, 5′-CTCCCAGTGCCTGAATTCATAAACCAGTCCGTTC-3′ and 5′-GAACGGACTGGTTTATGAATTCAGGCACTGGGAG-3′ for Y1068F, 5′-GTGCAGAATCCTGTCTTTCACAATCAGCCTCTG-3′ and 5′-CAGAGGCTGATTGTGAAAGACAGGATTCTGCAC-3′ for Y1086F, 5′-CTGGACAACCCTGACTTCCAGCAGGACTTCTTTC-3′ and 5′-GAAAGAAGTCCTGCTGGAAGTCAGGGTTGTCCAG-3′ for Y1148, and 5′-CAGCTGAAAATGCAGAATTCCTAAGGGTCGCGCCAC-3′ and 5′-GTGGCGCGACCCTTAGGAATTCTGCATTTTCAGCTG-3′ for Y1173. The Stratagene site-directed mutagenesis kit and protocol were used to produce the mutants. Introduction of the point mutation was confirmed by sequencing the relevant region with the primer 5′-GTCAAATACTGGCCTGATGAGTATGCTC-3′. A pCGT plasmid encoding full-length WT-H-Ras with T7 tag was kindly provided by Dafna Bar-Sagi (State University of New York, Stony Brook).
Cell transfection and preparation of lysates.
Cells were transfected with various constructs by using the FuGene transfection reagent as recommended by the manufacturer (GIBCO-BRL), incubated for ∼36 h, serum starved for ∼12 h, and then stimulated with 100 ng of EGF/ml. After being washed twice with ice-cold phosphate-buffered saline (PBS), cells were lysed in 10 mM Tris-HCl (pH 7.4) buffer containing 150 mM NaCl, 50 mM NaF, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 0.5 mM sodium orthovanadate, and protease inhibitor cocktail. Lysates were cleared by centrifugation at 15,000 × g at 4°C and analyzed as desired.
Immunoprecipitation and immunoblotting.
Immunoprecipitation experiments were performed by incubation at 4°C for 2 h or overnight with an additional incubation for 2 h after addition of protein A- or G-Sepharose beads. Unless otherwise specified, immunocomplexes captured on Sepharose beads were washed three times with cell lysis buffer, eluted by being boiled with Laemmli sample buffer, and separated on a 10% denaturing polyacrylamide gel. After transfer onto nitrocellulose, blocking was done by incubation with 3% bovine serum albumin (BSA). Primary antibody staining was done by incubation overnight at 4°C or for 2 h at room temperature, and secondary antibody staining was for 1 h at room temperature. The chemiluminescence detection method was used for all Western blot experiments.
Immunofluorescent quantitation of band intensities.
To quantify band intensities of phosphorylated ERK1/2, we employed a newly developed immunofluorescence technique, which is similar to the chemiluminescence protocol. It utilizes alkaline phosphatase-conjugated anti-rabbit or anti-mouse immunoglobulin G as a secondary antibody and AttoPhos fluorescent substrate in place of chemiluminescence reagents. After addition of the AttoPhos substrate and exposure for a few minutes, membranes were put in transparent plastic bags and band intensities were quantified with a phosphoimager using ImageQuant software (Storm 860 phosphoimager; Molecular Dynamics).
Analysis of Ras activation.
Ras activation was determined by use of the interaction of GTP-Ras with Raf-1 (51). A GST fusion protein of the Ras-binding domain of Raf-1 (Raf-RBD) was kindly provided by D. Shalloway (Cornell University, Ithaca, N.Y.). Lysates of bacteria expressing the fusion protein were prepared as described previously (51) with an additional step of lysate clearance by passage through a syringe filter (0.2-μm pore size). GST-RBD was purified on glutathione Sepharose beads (20-μl packed volume, ∼1 μg of GST-RBD per sample) by incubation for 30 min at 4°C and three washings with bacterial lysis buffer and two washings with eukaryotic cell lysis buffer (see above). Lysates were added to beads and incubated further for 1 h at 4°C. Captured proteins were washed five times with cell lysis buffer, eluted by being boiled for 10 min with Laemmli sample buffer, and resolved on a sodium dodecyl sulfate-12% polyacrylamide gel. Separated proteins were transferred onto a nitrocellulose membrane, blocked with 3% BSA, and probed with anti-Ras or anti-T7 tag monoclonal antibody.
Preparation of PM-enriched fractions (PMFs).
To study EGF-induced translocation of RasGAP to the PM in the presence or absence of the WT- or the R/E-SHP2, PMFs of COS-1 or A431 cells were prepared as follows. Either cell line was transfected with two different concentrations of WT- or R/E-SHP2, incubated for ∼30 h, serum starved for ∼12 h, stimulated with 100 ng of EGF/ml for 10 min, and washed once with ice-cold PBS and once with a 10 mM Tris-HCl (pH 7.5) hypotonic buffer. Cells were then swollen with a hypotonic buffer (10 mM Tris-HCl [pH 7.5], 1 mM sodium vanadate, and protease inhibitor cocktail) for 10 min, collected by scraping, and lysed by douncing (25 strokes with a tight pestle). Lysates were cleared of cell nuclei and unbroken cells by centrifugation at 10,000 × g for 10 min in a microcentrifuge at 4°C. The supernatant was transferred to polycarbonate tubes and centrifuged at 100,000 × g for 30 min. The pellets were washed once with PBS and then solubilized in the cell lysis buffer described above. PMF lysates were analyzed by direct immunoblotting or subjected to affinity precipitation and immunoblot analysis as described for individual experiments.
Affinity precipitation and far-Western analysis.
The SH2 domains of RasGAP fused to GST (kindly provided by Bruce Myers, University of Connecticut) were used for both procedures. Preparation of bacterial lysates and capture of GST-SH2-RasGAP onto glutathione agarose were the same as in the GTP-Ras assay. For far-Western analysis, the GST-SH2-RasGAP fusion protein was purified as recommended by the manufacturer (Pharmacia). First, lysates of PMFs were separated on a 10% polyacrylamide gel, transferred onto a nitrocellulose membrane, and then blocked with 3% BSA by incubation at room temperature for 1 h. The membranes were then incubated with 10 μg of purified GST-SH2-GAP protein/ml in 3% BSA overnight at 4°C. They were then washed three times with Tris-buffered saline-Tween and incubated further with anti-RasGAP monoclonal antibody (raised against the C-SH2 domain of RasGAP; Transduction Laboratories) for 2 h at room temperature. The rest of the procedure was as described for the Western blot experiments above.
Production of NIH 3T3 cells stably expressing R/E-SHP2.
A retroviral vector termed REBNA/IRES/GFP (56) was used to produce viruses expressing SHP2 proteins. Production of NIH 3T3 cells expressing the WT or the R/E protein was as described previously (1).
RESULTS
In this work, we investigated the mechanism of action of SHP2 in the EGFR signaling pathway. Initially, we examined if C459S-SHP2, frequently referred to as dominant-negative SHP2, inhibits EGF-induced ERK1/2 activation in our system. To narrow our area of focus in the EGFR-Ras-ERK signaling cascade, we also assessed the effect of this mutant on constitutively active Ras (V12-H-Ras)-induced ERK1/2 activation. For the purpose of simplicity, we refer to C495S-SHP2 as the C/S protein and to WT-SHP2 as the WT protein hereinafter.
The C/S protein inhibits EGFR-induced ERK1/2 activation.
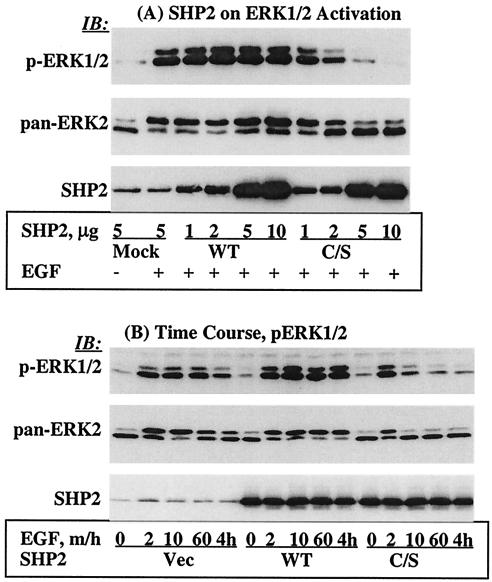
It was previously shown that the C/S protein inhibits growth factor- or cytokine-induced ERK1/2 activation (10, 28, 31, 44). Here, we wanted to reproduce this observation in our system as a starting point for our investigation of the mechanism of action of SHP2 in EGFR signaling. Thus, we expressed various amounts of the WT or C/S protein in COS-1 cells and assessed EGF-induced ERK1/2 phosphorylation with a phosphospecific antibody. The C/S protein inhibited ERK1/2 phosphorylation in a concentration-dependent manner. Maximum inhibition was observed at an expression level approximately five times the endogenous level. On the other hand, expression of the WT protein caused no inhibition but rather a slight enhancement (Fig. 1A, top). Reprobing with anti-pan-ERK2 antibody showed comparable amounts of protein in all lanes, which also confirmed the level of ERK activation in the context of phosphorylation-dependent band shift (Fig. 1A, middle). Further reprobing the membrane with anti-SHP2 antibody showed similar expression levels of both the WT and C/S proteins (Fig. 1A, bottom). Therefore, these findings are consistent with previous reports that the C/S protein inhibits EGF-induced activation of the ERK1/2 mitogen-activated protein kinase.
FIG. 1.
Effect of the C/S protein on ERK1/2 phosphorylation. (A) COS-1 cells were transfected with various amounts of an expression vector for the WT or C/S protein, incubated for 36 h in 10% serum-containing medium, serum starved for 12 h, and then stimulated with 100 ng of EGF/ml for 10 min. Total-cell lysates containing equivalent amounts of protein were separated on a 10% polyacrylamide gel and then analyzed by immunoblotting with the indicated antibodies. (B) Time course studies of ERK1/2 phosphorylation. A constant amount of the indicated expression plasmids was transfected into COS-1 cells. Following transfection, cells were treated as for panel A and then left unstimulated or stimulated with 100 ng of EGF/ml for the indicated time points. Total-cell lysates containing equivalent amounts of protein were analyzed by immunoblotting with the indicated antibodies. (C) Quantitation of phospho-ERK1/2 band intensities. The same set of lysates as in panel B was used to determine band intensities for ERK1/2 phosphorylation as described in Materials and Methods. IB, immunoblot.
The results presented in Fig. 1A showed inhibition of EGF-induced ERK1/2 activation by the C/S protein. However, they did not show whether the inhibition was due to blockade of signal transduction through the cascade or to premature termination of the signal before it reached a level sufficient to cause cellular responses. We reasoned that time course studies with EGF stimulation might provide an answer to these questions. We overexpressed a constant amount of the WT or the C/S protein in COS-1 cells and then stimulated the cells with EGF for various periods ranging from 2 min to 4 h; the vector alone was used as a control. Total-cell lysates prepared from these cells were analyzed for ERK1/2 phosphorylation as described for Fig. 1. In cells transfected with the C/S protein, phosphorylation of ERK1/2 occurred at 2 min, but this phosphorylation was short-lived and reached a basal level before 10 min (Fig. 1B, top). In cells transfected with the vector, the level of ERK1/2 phosphorylation was the same for about 60 min but declined after that. On the other hand, in cells transfected with the WT protein, the duration of ERK1/2 phosphorylation was increased (at least 4 h) and the intensity of the signal was slightly enhanced. Reprobing the membrane with anti-pan-ERK2 antibody showed that there were comparable amounts of protein in all lanes (Fig. 1B, middle). Further reprobing the membrane with anti-SHP2 antibody showed comparable expression levels of the WT and C/S proteins (Fig. 1B, bottom).
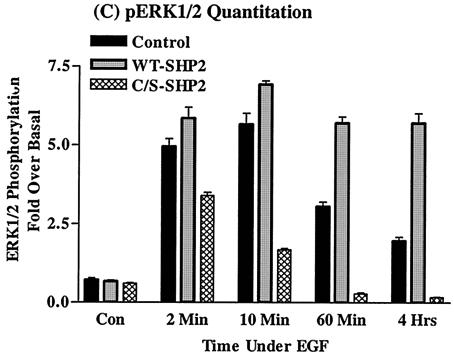
In order to better characterize ERK activation, we quantitated ERK1/2 phosphorylation by employing the immunofluorescence quantitation technique (see Materials and Methods). At 2 min, the level of ERK1/2 phosphorylation was five, six, and four times the basal level in the vector and the WT- and the C/S protein-expressing cells, respectively (Fig. 1C). And at 10 min, phosphorylation reached maximum levels in the vector and the WT-expressing cells, approximately 6.0 and 7.0 times the basal level, respectively. This continued for at least 4 h in the WT-expressing cells but gradually declined in the control. On the other hand, the level of ERK1/2 phosphorylation in cells expressing the C/S protein was only 1.8 times the basal level at 10 min, suggesting that the signal was prematurely terminated. The results with the C/S protein indicated that ERK1/2 phosphorylation does occur but becomes rapidly down regulated. Thus, SHP2 increases the duration of EGFR-induced ERK1/2 activation with slight enhancement.
The C/S protein has no effect on V12-H-Ras-induced ERK1/2 activation.
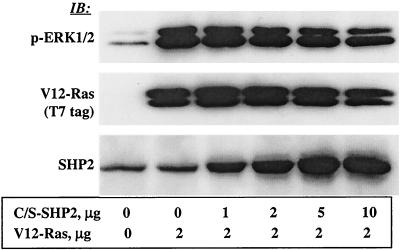
The results in Fig. 1 showed that SHP2 modulates EGFR-induced ERK1/2 activation. However, the site of action of SHP2 in the Ras-ERK signaling cascade was not clear. With the aim of pinpointing the site of action, we assessed the effect of the C/S protein on V12-H-Ras-induced ERK1/2 activation. It was reasoned that inhibition would put SHP2's site of action downstream, whereas the absence of inhibition would put it upstream of or parallel to Ras in the RTK-Ras-ERK signaling cascade. To address these questions, we transiently coexpressed various amounts of the C/S protein and a constant amount of V12-H-Ras in COS-1 cells (Fig. 2, middle and bottom) and examined ERK1/2 phosphorylation by using the phosphospecific antibody as described above for Fig. 1. In contrast to its effect on ligand-induced activation, the C/S protein showed no inhibitory effect on V12-H-Ras-induced ERK1/2 activation even at the highest concentration used (Fig. 2, top). Reprobing the membrane with anti-T7 (for Ras) antibody showed that equivalent amounts of the Ras protein were expressed in all transfectants. Further reprobing with anti-SHP2 antibody showed that the C/S protein was expressed at an increasing level. These results suggested that the site of action of SHP2 in the Ras-ERK signaling cascade is upstream of or parallel to Ras.
FIG. 2.
Effect of C/S protein on V12-H-Ras-induced ERK1/2 phosphorylation. COS-1 cells were transfected with a constant amount of an expression vector for V12-H-Ras and a varying amount for the C/S protein. Following transfection, cells were incubated for 36 h in 10% serum-containing medium and for a further 12 h in the absence of serum. Total-cell lysates containing equivalent amounts of protein were separated on a 10% polyacrylamide gel and then analyzed by immunoblotting with the indicated antibodies. IB, immunoblot.
The C/S protein inhibits EGF-induced Ras activation.
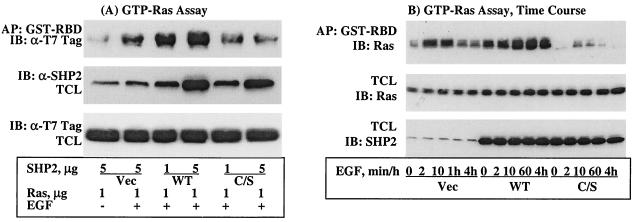
Experiments with V12-H-Ras suggested that SHP2 acts at the level of Ras in the Ras-ERK signaling cascade, but they could not differentiate between the possibilities, upstream of or parallel to Ras. Thus, we investigated the effect of the C/S protein on Ras activation. The RBD of Raf-1 fused to GST (51) was used to assay for EGF-induced Ras activation (see Materials and Methods for details). Two different concentrations of the WT or the C/S protein and a constant amount of WT-H-Ras were coexpressed in COS-1 cells, and lysates prepared from these cells were assayed for GTP-Ras levels. Overexpression of the C/S protein inhibited, whereas the WT protein enhanced, EGF-induced Ras activation (GDP-Ras to GTP-Ras) in a concentration-dependent manner (Fig. 3A, top). Probing total-cell lysates with anti-T7 tag (for Ras) and anti-SHP2 antibodies showed that all the proteins were expressed as expected (Fig. 3A, middle and bottom, respectively). Similar results were obtained when the EGF-induced endogenous GTP-Ras level was determined (data not shown). These results suggested that SHP2 functions upstream of Ras.
FIG. 3.
Effect of the C/S protein on Ras activation. (A) COS-1 cells were cotransfected with two different concentrations of an expression vector for SHP2 (WT or the C/S protein) and H-Ras. (B) Time course studies of Ras activation. COS-1 cells were cotransfected with a constant amount of WT-H-Ras and the WT or C/S protein. In either case, cells were incubated for 36 h in 10% serum-containing medium, serum starved for 12 h, and then stimulated with 100 ng of EGF/ml for 10 min in panel A or the indicated time points in panel B. Total-cell lysates (TCL) prepared from these cells were subjected to affinity precipitation (AP) with GST-RBD of Raf-1, resolved on a 10% polyacrylamide gel, and then analyzed by immunoblotting (IB) with T7 tag antibody for Ras. To examine expression of SHP2 and Ras proteins, total-cell lysates were directly analyzed with the indicated antibodies.
Data presented in Fig. 1B and C show that the C/S protein did not block initial activation of ERK1/2 but shortened its duration. These results led us to conduct time course studies of EGF-induced Ras activation to see if it correlated with ERK activation. We coexpressed the WT or C/S protein with WT-H-Ras in COS-1 cells and determined EGF-induced GTP-Ras levels at the same time points used in the ERK time course studies. In the control cells, the level of GTP-Ras reached the maximum attainable in 2 min and declined after 10 min. On the other hand, the level of GTP-Ras in WT cells was elevated throughout the time points tested. In contrast to the control and WT cells, the level of GTP-Ras in the C/S cells was approximately 30 to 40% of that of the control cells at 2 and 10 min and declined to basal level after that. Immunoblot analysis of corresponding total-cell lysates with anti-SHP2 and anti-T7 tag (Fig. 3A, top and bottom, respectively) showed that all transfected proteins were expressed as expected. These results demonstrated two aspects that were not apparent in the results presented in Fig. 3A: initial Ras activation occurred in the C/S cells but became down regulated rapidly due to lack of SHP2 activity and GTP-Ras accumulated in WT-SHP2-transfected cells possibly due to an increase in its half-life. Thus, ERK1/2 and Ras activations were highly correlated.
SHP2 regulates ligand-induced RasGAP translocation to the PM.
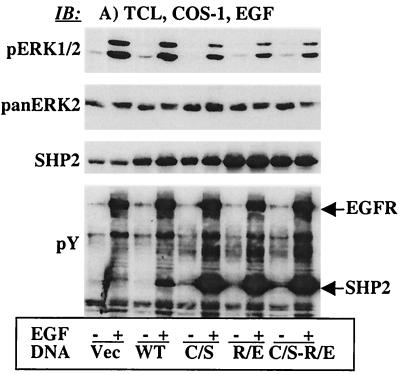
The results presented in Fig. 3 demonstrate that SHP2 was required for sustained Ras activation. Based on these findings, we postulated that SHP2 was modulating either the activation of Ras catalyzed by son of sevenless (SOS) or the down regulation catalyzed by RasGAP. To discriminate between these possibilities, we determined EGF-induced translocation of SOS or RasGAP to the PM, which is primarily cytosolic in unstimulated cells. We prepared PMFs from COS-1 cells transiently transfected with the vector alone or the WT or C/S protein (see Materials and Methods for details) and determined SOS and RasGAP levels after stimulation with EGF. EGF-induced translocation of SOS to the PMF was not affected by the WT or C/S protein compared to the controls. Also, there was no change in the amount of SOS-associated Grb2 (data not shown). On the other hand, EGF-induced translocation of RasGAP to the PMF was significantly reduced in cells overexpressing the WT protein and slightly increased in cells overexpressing C/S protein (data not shown). Although these observations were consistent with regulation of RasGAP by SHP2, they were insufficient to account for the dramatic inhibitory effect of the C/S protein on Ras activation. We reasoned that the C/S protein might not be an ideal dominant-negative mutant to assay subtle biochemical changes since it has some substrate-trapping ability. It was thus necessary to produce dominant-negative mutants of SHP2 devoid of the substrate-trapping effect. Previously, it was reported that the R residue in the signature motif (…HCGXXGRT/S…) is essential for substrate binding by PTPs (59). Hence, we replaced the R residue (positively charged) with E (negatively charged) in the WT and the C/S backgrounds to produce R465E-SHP2 and C459S/R465E-SHP2, hereinafter referred to as the R/E and C/S-R/E proteins, respectively. We evaluated these mutants in several ways: their effect on ERK1/2 activation, their ability to autodephosphorylate (46), and their capacity for PTP domain-mediated interactions. Both the R/E and the C/S-R/E proteins inhibited EGF-induced ERK1/2 activation comparably to the C/S protein (Fig. 4A, top). Anti-pan-ERK2 immunoblotting showed approximately equal amounts of protein in all lanes (Fig. 4A, second panel). Also, anti-SHP2 blotting showed that the different SHP2 proteins were expressed at approximately five times the level of the endogenous protein (Fig. 4A, third panel). Furthermore, anti-pY blotting of total-cell lysates indicated that, as in the C/S protein, the R/E and the C/S-R/E proteins were unable to autodephosphorylate (Fig. 4A, bottom), suggesting that the R-to-E mutation had disabled the PTPase activity of SHP2.
FIG. 4.
Characterization of new dominant-negative mutant of SHP2. The R/E and the C/S-R/E proteins were developed as described in Materials and Methods. An expression vector containing the WT, C/S, R/E, or C/S-R/E protein was transfected into COS-1 cells. Following transfection, cells were incubated for 36 h in 10% serum-containing medium and for a further 12 h in the absence of serum and then left unstimulated or stimulated with 100 ng of EGF/ml for 10 min. (A) Total-cell lysates were analyzed for ERK1/2 phosphorylation (top), loading control for ERK2 (second panel), level of expression of the different SHP2 proteins (third panel), or tyrosine phosphorylation pattern (bottom). (B) Time course study with PDGF stimulation (10 ng/ml). The same SHP2 constructs were transfected into NIH 3T3 cells, incubated under the same conditions as in panel A, and then stimulated with PDGF for the indicated time points. Lysates prepared from these cells were analyzed for ERK1/2 activation (top), loading control for ERK2 (middle), and level of expression of the different SHP2 proteins (bottom). (C) Time course study with FGF stimulation (100 ng/ml). All the procedures were the same as in panel B except that the ligand was FGF instead of PDGF. TCL, total-cell lysates; IB, immunoblot.
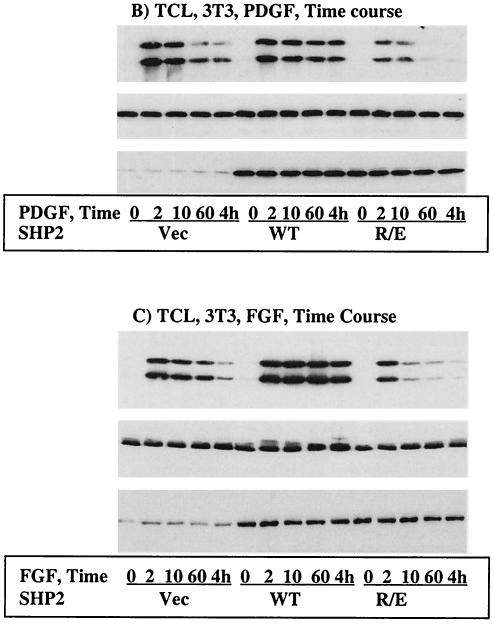
We chose the R/E protein for further characterization simply because the R/E protein was a single mutant with an inhibitory effect equal to that of the C/S-R/E protein. First, we expressed the WT, C/S, or R/E protein in COS-1 cells and then conducted immunoprecipitation with anti-SHP2 and immunoblotting with anti-EGFR to see whether the R/E protein could have PTP domain-mediated interactions; the C/S protein partially traps the EGFR (2). Although expression of each SHP2 protein was comparable, only the C/S protein showed some trapping of the EGFR. The amount of EGFR that precipitated with the R/E protein was identical to that with the WT protein, reflecting only SH2 domain-mediated interactions (data not shown). To further characterize the R/E protein, we expressed the WT or R/E protein in NIH 3T3 cells that respond to PDGF and FGF in ERK1/2 activation. Time course studies showed that the R/E protein shortened the duration of PDGF- and FGF-induced ERK1/2 activation in a manner similar to that of the C/S protein for EGF-induced ERK1/2 activation in COS-1 cells (Fig. 4B and C, top panel). Reprobing with anti-pan-ERK antibody showed comparable amounts of protein in all lanes (middle panels, Fig. 4B and C). Further reprobing with anti-SHP2 antibody indicated that the WT and the R/E proteins were expressed about four to five times more than the endogenous protein (bottom panels, Fig. 4B and C). Thus, the R/E protein is a better dominant-negative mutant since it lacks the partial trapping ability of the C/S protein while preserving an inhibitory effect on ERK1/2 activation induced by the indicated growth factors.
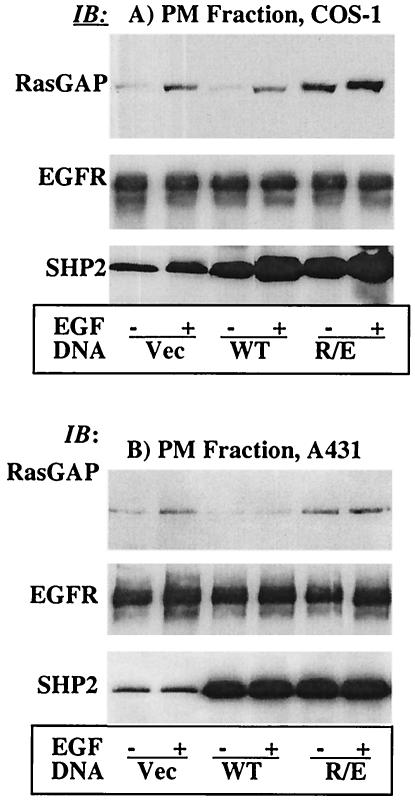
Using the newly developed dominant-negative mutant of SHP2, the R/E protein, we investigated its effect on EGF-induced translocation of RasGAP to the PM. PMFs were prepared from COS-1 cells transfected with the vector or the WT or R/E protein and assessed for the presence of RasGAP. Compared with the controls (vector), EGF-induced translocation of RasGAP to the PMF was reduced in the WT and significantly increased in the R/E protein-overexpressing cells (Fig. 5A, top). Interestingly, the basal level of RasGAP in the R/E cells was also higher. As a loading control, the membrane was reprobed with anti-EGFR antibody, which showed an equal amount of protein in each lane (Fig. 5A, middle). Further reprobing with anti-SHP2 indicated that the expression levels of the WT and R/E proteins were comparable (Fig. 5A, bottom). In order to see if the COS-1 results could be reproduced in another cell type, we conducted the same experiment in the A431 human epidermoid carcinoma cell line by infecting these cells with retroviruses expressing the indicated SHP2 proteins. Indeed, the results in A431 cells mirrored those in COS-1 cells (Fig. 5B). These results indicated that SHP2 interferes with EGF-induced translocation of RasGAP to the PM.
FIG. 5.
SHP2 regulates EGF-induced translocation of RasGAP to the PM. Immunoblot analysis of PMFs prepared from COS-1 cells transfected with the vector, the WT, or the R/E protein (A) or from A431 cells infected with a retrovirus expressing the same set of proteins (B). In both panels A and B, samples of PMFs containing equivalent amounts of protein were separated on a 10% polyacrylamide gel and then analyzed with anti-RasGAP antibody (top), anti-EGFR antibody as a loading control (middle), or anti-SHP2 antibody to show expression (bottom). IB, immunoblot.
SHP2 dephosphorylates RasGAP binding sites on the EGFR.
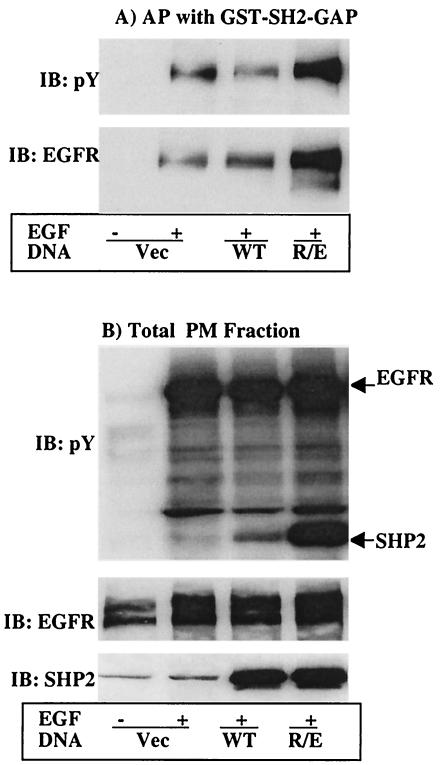
The experiments presented above demonstrated that SHP2 may positively regulate Ras activation through its effect on RasGAP translocation to the PM. It was previously shown that RasGAP interacts with the EGFR, and recently, we reported that the EGFR is one of the target substrates of SHP2 (2). We thus reasoned that SHP2 might be dephosphorylating pY residues on the EGFR that serve as binding sites for RasGAP SH2 domains. To address this possibility, we conducted affinity precipitation analysis with the GST fusion of the SH2 domains of RasGAP on PMFs prepared from COS-1 cells transfected with the vector or the WT or R/E protein. Captured proteins were separated on a 10% polyacrylamide gel and then analyzed by immunoblotting first with anti-pY and second with anti-EGFR antibodies (see Materials and Methods for details). A prominent phosphoprotein of the size of the EGFR was detected in cells overexpressing the R/E protein (Fig. 6A, top). On the other hand, a significantly smaller amount of this protein was detected in the control or the WT cells. No signal was detected in the unstimulated control cells, suggesting that the interaction required tyrosine phosphorylation. Reprobing the same membrane with anti-EGFR antibody confirmed that it was the EGFR (Fig. 6A, bottom). To evaluate if the observed differences were due to differences in total protein levels, total PMFs were analyzed by immunoblotting. Anti-pY blotting showed that the EGFR was highly tyrosine phosphorylated in all stimulated cells with a moderate increase in cells expressing the R/E protein (Fig. 6B, top). Reprobing the membrane with anti-EGFR antibody revealed that the total EGFR protein level was comparable in all lanes (Fig. 6B, middle). Further reprobing with anti-SHP2 antibody showed that expression levels of the WT and R/E proteins were comparable (Fig. 6B, bottom). These results demonstrate that RasGAP interacts with the EGFR via its SH2 domain depending on the presence or absence of specific pY residues and that these pYs could be target substrates of SHP2.
FIG. 6.
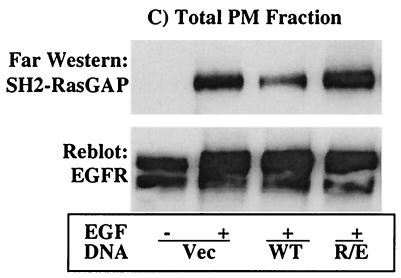
Affinity precipitation and far-Western analysis for the interaction of RasGAP with the EGFR. COS-1 cells were transfected with expression vector for the indicated SHP2 proteins. Following transfection, cells were incubated for 36 h in 10% serum-containing medium and for a further 12 h in the absence of serum and then left unstimulated or stimulated with 100 ng of EGF/ml for 10 min. PMFs were prepared as described in Materials and Methods, subjected to affinity precipitation with GST-SH2-RasGAP (A), or directly separated on a 10% polyacrylamide gel (B and C). In panels A and B, the membranes were probed first with anti-pY antibody and second with anti-EGFR after stripping. Membrane B was further probed with anti-SHP2 antibody to show expression of the different SHP2 proteins. In panel C, total PMFs were analyzed by far-Western blotting with 10 μg of GST-SH2-GAP/ml as described in Materials and Methods. Membrane C was further reprobed with anti-EGFR antibody to show the presence of approximately equal amounts of the EGFR protein in all lanes. IB, immunoblot; AP, affinity precipitation.
The results presented above cannot rule out the possibility of an indirect interaction via an intermediate that could bind to both RasGAP and the EGFR. To discriminate between these possibilities, we conducted a far-Western analysis of the remaining PMFs using the GST-SH2-GAP. The SH2 domain of RasGAP bound strongly to the EGFR from cells overexpressing R/E, but less so from cells overexpressing the WT protein despite the presence of tyrosine-phosphorylated EGFR (Fig. 6C, top). Significant binding was also observed to the EGFR from vector-transfected cells, perhaps due to the high-level expression of the EGFR in these cells, which may be too much for the endogenous SHP2 to act upon in 10 min of stimulation time. Reprobing the membrane with anti-EGFR antibody showed that the amount of EGFR protein in each lane was comparable (Fig. 6C, bottom). Therefore, RasGAP directly interacts with the EGFR depending on the presence of a specific pY residue or residues.
Phospho-Y992 (pY992) of EGFR is the target substrate of SHP2.
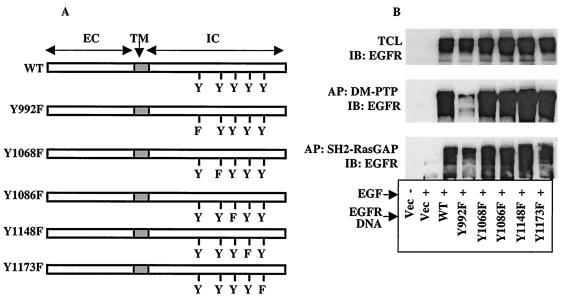
Our recent report (2) and the results presented above suggested that SHP2 targets specific pY residues on the EGFR, which serve as binding sites for RasGAP. However, they did not show which pY residue or residues out of the five or more autophosphorylation sites in the C-terminal region were target substrates of SHP2. To address these questions, we produced single Tyr-to-Phe mutants of EGFR at the five known autophosphorylation sites (Fig. 7A). These constructs were transfected into NIH 3T3 cells, which express undetectable amounts of endogenous EGFR. Immunoblot analysis of total-cell lysates from these cells showed comparable expression of all constructs (Fig. 7B, top). To identify the target pY residue on the EGFR, we conducted affinity precipitation studies on these lysates using a GST fusion of the PTP domain of the substrate-trapping mutant of SHP2, hereinafter referred to as the DM-PTP (2). The precipitates were resolved on a 10% polyacrylamide gel and then analyzed by immunoblotting with anti-EGFR antibody. As shown in Fig. 7B (middle panel), the DM-PTP precipitated all EGFR constructs except Y992F, suggesting that pY992 could be the target substrate of SHP2. Previous in vitro phosphatase studies have also shown that the recombinant PTP domain of SHP2 preferentially dephosphorylated a phosphopeptide derived from Y992 of the EGFR (33, 47).
FIG. 7.
Identification of SHP2 substrate pY residue(s) on the EGFR. (A) Schematic representation of WT or the single Tyr-to-Phe mutants of EGFR used in this experiment. (B) NIH 3T3 cells were transfected with the vector or the constructs depicted in panel A and incubated for 48 h in 10% serum-containing medium and for a further 6 h in serum-free medium. Cells were then stimulated with 100 ng of EGF/ml for 10 min as indicated. Total-cell lysates (TCL) prepared from these cells were directly analyzed by immunoblotting (IB) with anti-EGFR antibody (top) or subjected to affinity precipitation (AP) with GST fusion of DM-PTP (middle) or SH2-RasGAP (bottom) and then immunoblotting with anti-EGFR antibody.
To confirm that pY992, which was targeted by SHP2, was a binding site for RasGAP, we conducted affinity precipitation studies using the GST-SH2-GAP on the same set of lysates used in Fig. 6B and C. The SH2 domain of RasGAP precipitated all EGFR proteins except Y992F-EGFR (Fig. 7B, bottom). These results further confirm that pY992, a target substrate of SHP2, is a binding site for RasGAP. Together, these results provide evidence for the first time that the mechanism by which SHP2 positively effects EGFR signaling is by interfering with the action of RasGAP on Ras.
Y992F-EGFR activates Ras and ERK1/2 independently of SHP2.
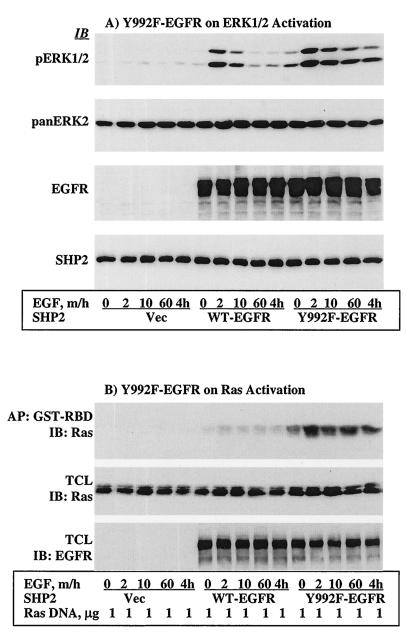
As shown in Fig. 7, pY992 of the EGFR is a binding site for RasGAP and a target substrate of SHP2. These results suggest that the role of SHP2 in EGFR signaling is to dephosphorylate negative-regulatory autophosphorylation sites. If this is the case, then Y992F-EGFR should activate the Ras-ERK signaling cascade independently of functional SHP2. To address this point, we first produced NIH 3T3 cells stably expressing the R/E protein by infecting them with a retrovirus (see Materials and Methods). Immunoblot analysis of total-cell lysates showed that the R/E protein expression level was approximately three times that of the endogenous protein (data not shown). NIH 3T3 cells were chosen for this purpose because they express undetectable levels of endogenous EGFR. These cells were then transfected with WT- or Y992F-EGFR. After incubation for ∼36 h and serum starvation for 12 h, they were stimulated with 100 ng of EGF/ml for the indicated time points, and lysates prepared from these cells were analyzed for ERK1/2 activation by using the phosphospecific antibody. Similar to the effect of C/S protein on endogenous EGFR in COS-1 cells (Fig. 1B), activation of ERK1/2 occurred at 2 min in WT-EGFR-transfected cells on the background of R/E protein expression. However, the duration of the signal was short, reaching basal levels shortly afterward. In contrast, ERK1/2 activation induced by Y992F-EGFR was higher and more prolonged under the same background (Fig. 8A, top panel). Reprobing the membrane with anti-pan-ERK2 and anti-SHP2 antibodies showed that the amount of each protein in each lane was comparable (Fig. 8A, second and third panels, respectively). Further reprobing the membrane with anti-EGFR antibody indicated that the expression levels of WT-EGFR and Y992F-EGFR were similar (Fig. 8A, third panel).
FIG. 8.
Y992F-EGFR activates ERK1/2 and Ras independently of functional SHP2. NIH 3T3 cells stably expressing the R/E protein were produced as described in Materials and Methods. (A) These cells were transfected with vector alone, WT-EGFR, or Y992F-EGFR; incubated for ∼36 h; serum starved for 12 h; and then stimulated with 100 ng of EGF/ml for the indicated time points. Lysates prepared from these cells were analyzed for ERK1/2 activation (top), for level of ERK2 as loading control (second panel), for expression of EGFR proteins (third panel), or for R/E protein expression (bottom). (B) The same cells were cotransfected with T7-tagged WT-H-Ras and WT-EGFR or Y992F-EGFR and incubated and treated under the same conditions as in panel A. Lysates prepared from these cells were assayed for GTP-Ras levels as described for Fig. 3. Shown are anti-T7 tag immunoblots for GTP-Ras level (top) and total Ras expression (middle) and anti-EGFR immunoblot for expression of EGFR proteins (bottom). IB, immunoblot; AP, affinity precipitation; TCL, total-cell lysate.
In order to determine the effect of Y992F-EGFR on Ras activation, WT-H-Ras and WT-EGFR or Y992F-EGFR were coexpressed in the same NIH 3T3 cells stably expressing the R/E protein. Transfected cells were incubated and stimulated under identical conditions as in Fig. 8A, and GTP-Ras levels were assayed as in Fig. 3. Similar to the results of ERK1/2 activation, the R/E protein inhibited Ras activation induced by WT-EGFR. This was consistent with the effect of the C/S protein on endogenous EGFR in COS-1 cells (Fig. 3). However, the R/E protein showed no effect on Ras activation induced by Y992F-EGFR at any of the time points tested. Therefore, Y992F-EGFR prolongs the duration of the activated state of the Ras-ERK signaling cascade independently of functional SHP2.
DISCUSSION
Experiments with knockout and transgenic mice, as well as Xenopus cells, have shown that SHP2 is absolutely required for normal development. Furthermore, it was shown previously that SHP2 is an essential mediator of cell transformation induced by v-Src (19) and constitutively active FGF receptor 3 (1). Recent reports also show that gain-of-function SHP2 mutations were detected in myeloid leukemias, which may represent an early initiating lesion (50). Therefore, SHP2 is a very important tyrosine phosphatase involved in both biological and disease conditions. However, the molecular mechanism by which SHP2 effects its function has remained an enigma. With the aim of defining the molecular mechanism of SHP2 in RTK signaling, we investigated its role in EGFR-induced activation of the Ras-ERK cascade. We demonstrate that SHP2 acts upstream of Ras. We specifically show that SHP2 dephosphorylates pY992, which is a RasGAP recruitment site on the EGFR, suggesting that Y992 is a negative-regulatory site. Based on the results reported in this study, we propose that the mechanism by which SHP2 functions is by temporally regulating the level of GTP-Ras via modulating ligand-induced translocation to the PM of RasGAP, the down regulator of GTP-Ras.
Consistent with previous reports, the C/S protein inhibited ERK1/2 activation induced by EGF, whereas the WT protein did not. In fact, the WT protein increased the duration of ERK1/2 activation with slight enhancement of the signal (Fig. 1). As revealed in time course studies, ERK1/2 activation did occur in cells overexpressing the C/S protein but was short-lived and submaximal. These results indicated that EGF-induced signals were initially transduced from the receptor to ERK1/2 in the absence of SHP2 function. Thus, it is apparent that SHP2 modulates the duration of the Ras-ERK cascade in EGFR signaling. On the other hand, overexpression of the C/S protein did not inhibit V12-Ras-induced ERK1/2 activation (Fig. 2), indicating that SHP2 acts upstream of or parallel to Ras in the EGFR signaling cascade. However, further investigation of the effect of the C/S protein on EGF-induced Ras activation revealed that SHP2 acts upstream of Ras. Similar observations were made by Neel's group using “constitutively active” SHP2 mutants (35).
Based on the above observations, we reasoned that SHP2 might be acting at the level of SOS or RasGAP. It was thus a logical step to study the effect of SHP2 on EGF-induced translocation of SOS or RasGAP to the PM, to their substrate microdomain. To accomplish this task, it was necessary to develop a new dominant-negative mutant of SHP2 since the C/S protein poses technical problems due to its trapping on target substrates, which may block SH2-mediated interactions (12, 32, 53). We thus developed the R/E protein, which showed an inhibitory effect equal to that of the C/S protein but lacked the partial substrate-trapping characteristic. The loss of substrate binding by the R/E protein could be explained by (i) loss of positive charge that coordinates the phosphate moiety on the substrate and (ii) electrostatic repulsion on the phosphate moiety by the acidic group of the substitute, the glutamate. Two observations that support this explanation are that the R/E protein was unable to autodephosphorylate and that it could not have a PTP domain-mediated interaction with the EGFR. Given that the R/E protein also inhibits PDGF- and FGF-induced ERK1/2 activation, it would be a better dominant-negative mutant than the C/S in future SHP2 studies.
Using the R/E protein, we showed that SHP2 modulates EGF-induced translocation of RasGAP to the PM with no apparent effect on SOS (Fig. 5). Because the two SH2 domains were intact in all SHP2 proteins used in this study, the observed changes in RasGAP levels at the PM must reflect the role played by the PTP domain. In unstimulated cells, RasGAP is primarily localized to the cytosol, but upon stimulation, it translocates to the PM. This is mediated through its two SH2 domains, which interact with phosphotyrosyl residues on proteins at the level of the PM. Some RasGAP interacting proteins are RhoGAP, EGFR, and PDGFR (14, 24, 45). Thus, SHP2 PTP activity regulates RasGAP recruitment to the PM most probably by dephosphorylating binding sites on interacting proteins.
We recently showed that the EGFR is one of the target substrates of SHP2 (2). Thus, it was reasonable to hypothesize that SHP2 dephosphorylates pY residues on the EGFR that serve as binding sites for RasGAP. The finding that the SH2 domains of RasGAP efficiently precipitated the EGFR obtained from cells overexpressing the R/E protein supports this hypothesis. The mechanistic explanation is that the R/E protein dominant-negatively interfered with the dephosphorylation of target pY(s) on the EGFR that serves as a binding site for RasGAP. Thus, it is the presence of specific pYs on the EGFR, not the total pY content, that determines RasGAP interaction.
Affinity precipitation studies could not rule out the possibility of an indirect interaction via an intermediate that binds to both RasGAP and the EGFR. However, far-Western analysis showed that RasGAP directly interacts with the EGFR (Fig. 6C). The demonstrations that EGFR is a target substrate of SHP2 and that RasGAP directly interacts with the EGFR only when it comes from cells expressing the R/E protein clearly show that SHP2 is dephosphorylating RasGAP binding sites on the EGFR. These results concur with the findings for Drosophila, where Corkscrew, the Drosophila homologue of SHP2, counteracts RasGAP's interaction with the Torso RTK by specifically dephosphorylating pY918, which is a binding site for RasGAP (8). Therefore, it is possible that SHP2 might be functioning in a similar manner in EGFR signaling as Corkscrew does in Torso signaling.
The results presented in Fig. 6 could not show which tyrosine residue(s) out of the five major autophosphorylation sites on the cytoplasmic region of the EGFR was a target of SHP2 PTP activity. This question was addressed by employing the recently developed substrate-trapping mutant of SHP2 termed DM-PTP (2). DM-PTP showed remarkable specificity toward pY992 (Fig. 7B, middle), suggesting that it is the target substrate of SHP2. Interestingly, the SH2 domains of RasGAP also showed significant specificity toward pY992 (Fig. 7B, bottom). These results were consistent with Y992 of EGFR being a negative-regulatory site. Thus, we hypothesized that if Y992 of the EGFR is a negative-regulatory autophosphorylation site in EGF-induced activation of the Ras-ERK signaling cascade, then Y992F-EGFR must activate this signaling cascade independently of SHP2. Remarkably, that was exactly what we found, a reciprocal of the results presented in Fig. 1 and 3. Y992F-EGFR was refractory to the effects of the R/E protein as evidenced by prolongation of the activated state of the Ras-ERK cascade in the absence of functional SHP2 (Fig. 8). To our knowledge, this is the first report showing that Y992 of the EGFR is a negative-regulatory site by serving as a binding site for RasGAP, the down regulator of GTP-Ras.
Based on the results reported in this study and other previous reports on the subject, we have proposed a possible molecular mechanism for the biological role of SHP2 in the EGFR-Ras-ERK signaling pathway, which may also serve as a model for SHP2's action in other RTK pathways. As depicted in Fig. 9, the binding of EGF to EGFR induces receptor dimerization and autophosphorylation on the five sites including Y992. These phosphorylated tyrosines serve as binding sites for SH2 domain-containing proteins including RasGAP. Because RasGAP preferentially binds to pY992 and this site is a target substrate of SHP2, the binding of RasGAP to pY992 is counteracted by the PTP activity of SHP2. The net effect would be an equilibrium shift toward an increased GTP-Ras level that results in prolongation of the signal to cause the desired cellular responses.
FIG. 9.
Schematic representation of the molecular mechanism of SHP2 in the EGFR signaling pathway. The five Y's represent autophosphorylation sites on the C-terminal region of the EGFR. SHP2 PTP activity dephosphorylates the most proximal pY, which corresponds to pY992, a binding site of RasGAP shown as GAP SH2.
In summary, the mechanism by which SHP2 increases the duration and intensity of EGFR signals is by increasing the half-life of activated Ras (GTP-Ras) in the cell by interfering with the process of Ras inactivation by RasGAP via inhibition of tyrosine phosphorylation-dependent translocation of RasGAP to the PM. Thus, SHP2 is not a relayer but a modulator of EGFR signals at the level of Ras so that these signals can attain levels sufficient to induce corresponding cellular responses. Based on the findings in this report and similar reports for Drosophila (8), we propose that the role of SHP2 in RTK-induced activation of the Ras-ERK cascade is to act on negative-regulatory pYs on the receptors themselves and/or on scaffolding proteins to prolong the duration of the signal.
Acknowledgments
We thank D. Shalloway, D. Bar-Sagi, B. Myers, O. Petrenko, and J. Schlessinger for providing valuable reagents.
This work was supported by NIH Public Service grants CA28146 and CA42573 to M.J.H.
REFERENCES
- 1.Agazie, Y. M., and M. J. Hayman. Oncogene, in press. [DOI] [PubMed]
- 2.Agazie, Y. M., and M. J. Hayman. 2003. Development of an efficient “substrate-trapping” mutant of Src homology phosphotyrosine phosphatase 2 and identification of the epidermal growth factor receptor, Gab1, and three other proteins as target substrates. J. Biol. Chem. 278:13952-13958. [DOI] [PubMed] [Google Scholar]
- 3.Bardelli, A., P. Longati, D. Gramaglia, M. C. Stella, and P. M. Comoglio. 1997. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene 15:3103-3111. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, A. M., S. F. Hausdorff, A. M. O'Reilly, R. M. Freeman, and B. G. Neel. 1996. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol. Cell. Biol. 16:1189-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlberg, K., and L. R. Rohrschneider. 1997. Characterization of a novel tyrosine phosphorylated 100-kDa protein that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid cells. J. Biol. Chem. 272:15943-15950. [DOI] [PubMed] [Google Scholar]
- 6.Chardin, P., J. H. Camonis, N. W. Gale, L. van Aelst, J. Schlessinger, M. H. Wigler, and D. Bar-Sagi. 1993. Hum. Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science 260:1338-1343. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee-Kishore, M., F. van den Akker, and G. R. Stark. 2000. Association of STATs with relatives and friends. Trends Cell Biol. 10:106-111. [DOI] [PubMed] [Google Scholar]
- 8.Cleghon, V., P. Feldmann, C. Ghiglione, T. D. Copeland, N. Perrimon, D. A. Hughes, and D. K. Morrison. 1998. Opposing actions of CSW and RasGAP modulate the strength of Torso RTK signaling in the Drosophila terminal pathway. Mol. Cell 2:719-727. [DOI] [PubMed] [Google Scholar]
- 9.Cunnick, J. M., J. F. Dorsey, T. Munoz-Antonia, L. Mei, and J. Wu. 2000. Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem. 275:13842-13848. [DOI] [PubMed] [Google Scholar]
- 10.Deb, T. B., L. Wong, D. S. Salomon, G. Zhou, J. E. Dixon, J. S. Gutkind, S. A. Thompson, and G. R. Johnson. 1998. A common requirement for the catalytic activity and both SH2 domains of SHP-2 in mitogen-activated protein (MAP) kinase activation by the ErbB family of receptors. A specific role for SHP-2 in map, but not c-Jun amino-terminal kinase activation. J. Biol. Chem. 273:16643-16646. [DOI] [PubMed] [Google Scholar]
- 11.DeMali, K. A., E. Balciunaite, and A. Kazlauskas. 1999. Integrins enhance platelet-derived growth factor (PDGF)-dependent responses by altering the signal relay enzymes that are recruited to the PDGF beta receptor. J. Biol. Chem. 274:19551-19558. [DOI] [PubMed] [Google Scholar]
- 12.Di Fiore, P. P., J. H. Pierce, T. P. Fleming, R. Hazan, A. Ullrich, C. R. King, J. Schlessinger, and S. A. Aaronson. 1987. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell 51:1063-1070. [DOI] [PubMed] [Google Scholar]
- 13.Dionne, C. A., M. Jaye, and J. Schlessinger. 1991. Structural diversity and binding of FGF receptors. Ann. N. Y. Acad. Sci. 638:161-166. [DOI] [PubMed] [Google Scholar]
- 14.Emlet, D. R., D. K. Moscatello, L. B. Ludlow, and A. J. Wong. 1997. Subsets of epidermal growth factor receptors during activation and endocytosis. J. Biol. Chem. 272:4079-4086. [DOI] [PubMed] [Google Scholar]
- 15.Feng, G. S., C. C. Hui, and T. Pawson. 1993. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science 259:1607-1611. [DOI] [PubMed] [Google Scholar]
- 16.Feng, G. S., and T. Pawson. 1994. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 10:54-58. [DOI] [PubMed] [Google Scholar]
- 17.Frearson, J. A., and D. R. Alexander. 1998. The phosphotyrosine phosphatase SHP-2 participates in a multimeric signaling complex and regulates T cell receptor (TCR) coupling to the Ras/mitogen-activated protein kinase (MAPK) pathway in Jurkat T cells. J. Exp. Med. 187:1417-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadari, Y. R., H. Kouhara, I. Lax, and J. Schlessinger. 1998. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 18:3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hakak, Y., Y. S. Hsu, and G. S. Martin. 2000. Shp-2 mediates v-Src-induced morphological changes and activation of the anti-apoptotic protein kinase Akt. Oncogene 19:3164-3171. [DOI] [PubMed] [Google Scholar]
- 20.Honegger, A. M., A. Schmidt, A. Ullrich, and J. Schlessinger. 1990. Evidence for epidermal growth factor (EGF)-induced intermolecular autophosphorylation of the EGF receptors in living cells. Mol. Cell. Biol. 10:4035-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, D. E., and L. T. Williams. 1993. Structural and functional diversity in the FGF receptor multigene family. Adv. Cancer Res. 60:1-41. [DOI] [PubMed] [Google Scholar]
- 22.Kazlauskas, A., D. L. Durden, and J. A. Cooper. 1991. Functions of the major tyrosine phosphorylation site of the PDGF receptor beta subunit. Cell Regul. 2:413-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keyse, S. M. 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 12:186-192. [DOI] [PubMed] [Google Scholar]
- 24.Klinghoffer, R. A., and A. Kazlauskas. 1995. Identification of a putative Syp substrate, the PDGF beta receptor. J. Biol. Chem. 270:22208-22217. [DOI] [PubMed] [Google Scholar]
- 25.Kuhne, M. R., T. Pawson, G. E. Lienhard, and G. S. Feng. 1993. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J. Biol. Chem. 268:11479-11481. [PubMed] [Google Scholar]
- 26.Lechleider, R. J., S. Sugimoto, A. M. Bennett, A. S. Kashishian, J. A. Cooper, S. E. Shoelson, C. T. Walsh, and B. G. Neel. 1993. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor. J. Biol. Chem. 268:21478-21481. [PubMed] [Google Scholar]
- 27.Margolis, B., N. Li, A. Koch, M. Mohammadi, D. R. Hurwitz, A. Zilberstein, A. Ullrich, T. Pawson, and J. Schlessinger. 1990. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. EMBO J. 9:4375-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maroun, C. R., M. A. Naujokas, M. Holgado-Madruga, A. J. Wong, and M. Park. 2000. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol. Cell. Biol. 20:8513-8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massoglia, S., A. Gray, T. J. Dull, S. Munemitsu, H. J. Kun, J. Schlessinger, and A. Ullrich. 1990. Epidermal growth factor receptor cytoplasmic domain mutations trigger ligand-independent transformation. Mol. Cell. Biol. 10:3048-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melillo, R. M., M. Santoro, S. H. Ong, M. Billaud, A. Fusco, Y. R. Hadari, J. Schlessinger, and I. Lax. 2001. Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol. Cell. Biol. 21:4177-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milarski, K. L., and A. R. Saltiel. 1994. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J. Biol. Chem. 269:21239-21243. [PubMed] [Google Scholar]
- 32.Neel, B. G., and N. K. Tonks. 1997. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 9:193-204. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi, T., T. Matozaki, K. Horita, Y. Fujioka, and M. Kasuga. 1994. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell. Biol. 14:6674-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong, S. H., G. R. Guy, Y. R. Hadari, S. Laks, N. Gotoh, J. Schlessinger, and I. Lax. 2000. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 20:979-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Reilly, A. M., S. Pluskey, S. E. Shoelson, and B. G. Neel. 2000. Activated mutants of SHP-2 preferentially induce elongation of Xenopus animal caps. Mol. Cell. Biol. 20:299-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, C. Y., and M. J. Hayman. 1999. The tyrosines in the bidentate motif of the env-sea oncoprotein are essential for cell transformation and are binding sites for Grb2 and the tyrosine phosphatase SHP-2. J. Biol. Chem. 274:7583-7590. [DOI] [PubMed] [Google Scholar]
- 37.Park, M., M. Dean, K. Kaul, M. J. Braun, M. A. Gonda, and G. Vande Woude. 1987. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl. Acad. Sci. USA 84:6379-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawson, T., and G. D. Gish. 1992. SH2 and SH3 domains: from structure to function. Cell 71:359-362. [DOI] [PubMed] [Google Scholar]
- 39.Saxton, T. M., B. G. Ciruna, D. Holmyard, S. Kulkarni, K. Harpal, J. Rossant, and T. Pawson. 2000. The SH2 tyrosine phosphatase shp2 is required for mammalian limb development. Nat. Genet. 24:420-423. [DOI] [PubMed] [Google Scholar]
- 40.Saxton, T. M., M. Henkemeyer, S. Gasca, R. Shen, D. J. Rossi, F. Shalaby, G. S. Feng, and T. Pawson. 1997. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 16:2352-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaeper, U., N. H. Gehring, K. P. Fuchs, M. Sachs, B. Kempkes, and W. Birchmeier. 2000. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149:1419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 43.Schlessinger, J., M. Mohammadi, B. Margolis, and A. Ullrich. 1992. Role of SH2-containing proteins in cellular signaling by receptor tyrosine kinases. Cold Spring Harbor Symp. Quant. Biol. 57:67-74. [DOI] [PubMed] [Google Scholar]
- 44.Shi, Z. Q., D. H. Yu, M. Park, M. Marshall, and G. S. Feng. 2000. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell. Biol. 20:1526-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorkin, A., K. Helin, C. M. Waters, G. Carpenter, and L. Beguinot. 1992. Multiple autophosphorylation sites of the epidermal growth factor receptor are essential for receptor kinase activity and internalization. Contrasting significance of tyrosine 992 in the native and truncated receptors. J. Biol. Chem. 267:8672-8678. [PubMed] [Google Scholar]
- 46.Stein-Gerlach, M., A. Kharitonenkov, W. Vogel, S. Ali, and A. Ullrich. 1995. Protein-tyrosine phosphatase 1D modulates its own state of tyrosine phosphorylation. J. Biol. Chem. 270:24635-24637. [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto, S., R. J. Lechleider, S. E. Shoelson, B. G. Neel, and C. T. Walsh. 1993. Expression, purification, and characterization of SH2-containing protein tyrosine phosphatase, SH-PTP2. J. Biol. Chem. 268:22771-22776. [PubMed] [Google Scholar]
- 48.Tang, T. L., R. M. Freeman, Jr., A. M. O'Reilly, B. G. Neel, and S. Y. Sokol. 1995. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell 80:473-483. [DOI] [PubMed] [Google Scholar]
- 49.Tapley, P., A. Kazlauskas, J. A. Cooper, and L. R. Rohrschneider. 1990. Macrophage colony-stimulating factor-induced tyrosine phosphorylation of c-fms proteins expressed in FDC-P1 and BALB/c 3T3 cells. Mol. Cell. Biol. 10:2528-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tartaglia, M., C. M. Niemeyer, A. Fragale, X. Song, J. Buechner, A. Jung, K. Hahlen, H. Hasle, J. D. Licht, and B. D. Gelb. 2003. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 34:148-150. [DOI] [PubMed] [Google Scholar]
- 51.Taylor, S. J., and D. Shalloway. 1996. Cell cycle-dependent activation of Ras. Curr. Biol. 6:1621-1627. [DOI] [PubMed] [Google Scholar]
- 52.Tomic, S., U. Greiser, R. Lammers, A. Kharitonenkov, E. Imyanitov, A. Ullrich, and F. D. Bohmer. 1995. Association of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells. Phosphatidic acid activates receptor dephosphorylation by PTP1C. J. Biol. Chem. 270:21277-21284. [DOI] [PubMed] [Google Scholar]
- 53.Tonks, N. K., and B. G. Neel. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13:182-195. [DOI] [PubMed] [Google Scholar]
- 54.Tonks, N. K., and B. G. Neel. 1996. From form to function: signaling by protein tyrosine phosphatases. Cell 87:365-368. [DOI] [PubMed] [Google Scholar]
- 55.Tulasne, D., R. Paumelle, K. M. Weidner, B. Vandenbunder, and V. Fafeur. 1999. The multisubstrate docking site of the MET receptor is dispensable for MET-mediated RAS signaling and cell scattering. Mol. Biol. Cell 10:551-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueki, N., and M. J. Hayman. 2003. Signal-dependent N-CoR requirement for repression by the Ski oncoprotein. J. Biol. Chem. 278:24858-24864. [DOI] [PubMed] [Google Scholar]
- 57.Ullrich, A., L. Coussens, J. S. Hayflick, T. J. Dull, A. Gray, A. W. Tam, J. Lee, Y. Yarden, T. A. Libermann, J. Schlessinger, et al. 1984. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309:418-425. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe, S., and K. Arai. 1996. Roles of the JAK-STAT system in signal transduction via cytokine receptors. Curr. Opin. Genet. Dev. 6:587-596. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, Z. Y., Y. Wang, L. Wu, E. B. Fauman, J. A. Stuckey, H. L. Schubert, M. A. Saper, and J. E. Dixon. 1994. The Cys(X)5Arg catalytic motif in phosphoester hydrolysis. Biochemistry 33:15266-15270. [DOI] [PubMed] [Google Scholar]