Abstract
The Dictyostelium XMAP215 family member DdCP224 is involved in centrosome duplication and cytokinesis and is concentrated at the centrosome and microtubule tips. Herein, we have created a DdCP224 promoter replacement mutant that allows both over- and underexpression. Overexpression led to supernumerary microtubule-organizing centers and, independently, an increase of the number of multinuclear cells. Electron microscopy demonstrated that supernumerary microtubule-organizing centers represented bona fide centrosomes. Live cell imaging of DdCP224-green fluorescent protein mutants also expressing green fluorescent protein-histone2B as a DNA label revealed that supernumerary centrosomes were also competent of cell cycle-dependent duplication. In contrast, underexpression of DdCP224 inhibited cell growth, reduced the number and length of astral microtubules, and caused nocodazole hypersensitivity. Moreover, microtubule regrowth after nocodazole removal was dependent on DdCP224. Underexpression also resulted in a striking disappearance of supernumerary centrosomes and multinuclear cells caused by previous overexpression. We show for the first time by live cell observation that the number of supernumerary centrosomes can be reduced either by centrosome fusion (coalescence) or by the formation of cytoplasts containing supernumerary centrosomes during cytokinesis.
INTRODUCTION
In dividing cells, control of centrosome number is essential for the fidelity of mitosis and maintenance of euploidy. Supernumerary centrosomes are a hallmark of tumor cells, although it is still a matter of discussion whether their appearance is a cause or a consequence of carcinogenesis (Lingle et al., 2002; Nigg, 2002). However, it is widely accepted that supernumerary centrosomes contribute to the formation of multipolar spindles and thus to defective chromosome segregation. Although most of the daughter cells resulting from such mitotic chaos will die, some of them will acquire the potential for neoplastic growth in spite of supernumerary centrosomes and aneuploidy, as they regain the ability to form a bipolar spindle. Brinkley (2001) suggested two mechanisms how this can be achieved: first, “deamplification” of supernumerary centrosomes by elimination or inactivation, and second, fusion or “coalescence” of supernumerary centrosomes resulting in one or two compound microtubule-organizing centers (MTOCs).
Herein, we have studied the fate of supernumerary centrosomes in a simple model system. In Dictyostelium amoebae, one molecular component involved in the formation of supernumerary centrosomes is DdCP224, a member of the XMAP215 family of microtubule-associated proteins (Gräf et al., 2000). In most species, these proteins are long, thin, monomeric molecules with a size of ∼ 230 kDa (Cassimeris et al., 2001). Their ubiquitous occurrence, even in plants, suggests indispensible functions (Ohkura et al., 2001). XMAP215, for instance, is a promoter of microtubule elongation due to a suppression of catastrophe events induced by the Kin I family kinesin XKCM1 (Kinoshita et al., 2001). XMAP215 is also a key component for the microtubule-nucleating activity of centrosomes (Popov et al., 2002). Like XMAP215, DdCP224 is both a microtubule-associated protein and a genuine centrosomal component (Gräf et al., 2000; Popov et al., 2001). It also colocalizes with DdEB1, a wellknown microtubule plus end marker, at microtubule tips, suggesting a role of DdCP224 in microtubule interactions with the cell cortex (Rehberg and Gräf, 2002; Hestermann et al., 2002). Previous studies have established that overexpression of a DdCP224-green fluorescent protein (GFP) fusion protein induces a mild cytokinesis defect, and, independently, the formation of supernumerary MTOCs (Gräf et al., 2000). This was the first evidence that XMAP215 family proteins may be involved in centrosome biogenesis.
We now provide ultrastructural evidence that supernumerary MTOCs elicited by DdCP224 overexpression are bona fide centrosomes. Furthermore, we show for the first time in living cells that supernumerary centrosomes are able to duplicate and that their number can be reduced both by coalescence and by “deamplification” through the formation of cytoplasts containing supernumerary centrosomes. Moreover, we demonstrate that the control of centrosome number in Dictyostelium is very sensitive to the level of DdCP224 protein and that DdCP224 is required for microtubule growth in vivo.
MATERIALS AND METHODS
Construction of DdCP224 Mutants
For generation of the DdCP224 promoter replacement mutant, a cDNA fragment containing the complete coding sequence of the first 813 amino acids of DdCP224 was obtained by BamHI/SacI digestion of the ΔC-GFP vector (Gräf et al., 2000). This fragment was cloned into pA6P-SSEB2, which is based on p1ABsr8 (Gräf et al., 2000). It allows expression of the protein of interest as an N-terminal fusion to superglow-GFP (QBiogene, Carlsbad, CA) under control of the actin6 promoter. The GFP sequence is followed by a SalI, SacI, EcoRI, BamHI, NsiI polylinker. The final, uncleaved promoter replacement vector was transferred into Dictyostelium AX2 cells by electroporation (Mann et al., 1998), and clones were selected in HL5c-medium with 4 μg/ml blasticidin S (ICN Biomedicals, Eschwege, Germany). Both homologous recombination at the DdCP224 gene locus and ectopic integrations elsewhere in the genome were possible. In case of homologous recombination, the resulting cells can only express N-terminally GFP-tagged full-length DdCP224 due to disruption of the endogenous gene (Figure 1). Such clones were easily discernible by their green fluorescent centrosomes. Ectopic integration of the replacement vector led to expression of the N-terminal DdCP224 fragment alone, which was known to exhibit no subcellular localization (Gräf et al., 2000). Two independent clones with targeted insertion of the replacement vector were obtained (called A3 and D1). The homologous recombination event was verified by a polymerase chain reaction (PCR) with genomic DNA (Nellen et al., 1987) as a template and primers directed against the GFP sequence and the C-terminal part of DdCP224 (Figure 1).
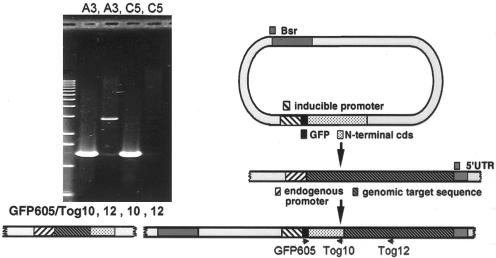
Figure 1.
Construction of a DdCP224 promoter replacement mutant. After insertion of the replacement vector into the endogenous DdCP224 gene by homologous recombination, the plasmid encoded DdCP224 fragment becomes completed by the missing C-terminal part and cells can only express N-terminally GFP-tagged full-length DdCP224 under control of the actin6 promoter. The endogenous promoter drives expression of an untagged N-terminal DdCP224 fragment that shows no specific cellular localization (Gräf et al., 2000). In case of ectopic plasmid insertions, only this N-terminal fragment instead of full-length DdCP224 is expressed as a GFP-fusion protein. The agarose gel on the left shows confirmation of the desired recombination event by PCR on genomic DNA. The primer combination GFP605/TOG12 leads to a 2.9-kb PCR product only in case of homologous recombination (clone A3) but not in case of ectopic integrations (clone C5), whereas the primer combination GFP605/TOG10 gives a 1.3-kb PCR product in both cases.
DdCP224-GFP/GFP-histone2B cells were generated by transformation of DdCP224-GFP mutants (Gräf et al., 2000) with a GFP-histone2B vector. This plasmid, which was kindly provided by Günther Gerisch (MPI für Biochemie, Martinsried, Germany), allows expression of Dictyostelium histone2B with an N-terminal GFP-tag under control of the actin15 promoter. Clones were selected with 10 μg/ml G418 (Sigma Chemie, Deisenhofen, Germany) in HL5c-medium.
Cell Culture
Dictyostelium cells were cultivated as described previously (Gräf et al., 1998, 2000). For DdCP224 underexpression, cells were cultured in Klebsiella aerogenes suspensions in phosphate buffer (14.6 mM KH2PO4, 2 mM Na2HPO4), essentially as described by Liu et al. (2002). For 30 ml of buffer, one 90-mm Petri-dish of Klebsiella overnight culture was used. Klebsiella suspensions were inoculated with Dictyostelium cells at a final density of ∼70,000 cells/ml in 100-ml Erlenmeyer flasks and incubated on a rotary shaker (150 rpm) at 21°C. For underexpression experiments, cells were used ∼36 h after inoculation (cell density <106/ml).
Nocodazole Treatment
Cells were allowed to settle on coverslips and treated with 10 μg/ml nocodazole in phosphate buffer for 3 h. Microtubule regrowth was induced by exchange of the nocodazole solution against phosphate buffer. Cells were fixed with glutaraldehyde (see “Electron Microscopy” below) after washout for 0, 10, and 20 min, respectively.
Light Microscopy
Light microscopy was essentially performed as described previously (Gräf et al., 1998). Confocal images were acquired on an Axiovert 200M/510META laser scanning system (live and fixed cells) (Carl Zeiss, Jena, Germany) equipped with a 63×/1.4 lens, or on an Ultraview spinning disk confocal system (PerkinElmer Wallac, Cambridge, United Kingdom) with a 100×/1.3 objective and a 12-bit charge-coupled device camera (live cells only). Live cells were viewed under agar overlay (Fukui et al., 1987) at very low laser intensity. At the Ultraview microscope, z-stacks were recorded at a frame rate of 1.4 frames/s by using 2 × 2 binning. At the 510META microscope, images were acquired at a frame rate of 2/s and a resolution of 512 × 256 pixels. Z-stacks were projected onto one plane by using a brightest point algorithm and ImageJ software (National Institutes of Health, Bethesda, MD).
Electron Microscopy
GFP-DdCP224–overexpressing Dictyostelium cells were placed on CeLLocate coverslips (Eppendorf, Hamburg, Germany). After a brief rinse in phosphate buffer, they were fixed for 15 min with 0.5% glutaraldehyde in modified PHEM buffer (12 mM PIPES, 5 mM HEPES, 1,6 mM EGTA, 1 mM MgCl2, pH 7; Schliwa and van Blerkom, 1981) supplemented with 0.5% Triton X-100. Suitable cells containing one or more free centrosomes, as judged by GFP and 4,6-diamidino-2-phenylindole (DAPI) fluorescence, were selected and photo-graphed. They were processed for electron microscopy according to standard procedures. The coverslips were removed from the epoxy blocks by using liquid nitrogen and the preselected cells were identified using the microgrid present on the epoxy surface. The cells were sectioned, stained, and viewed in a 1200 EX II electron microscope (JOEL, Tokyo, Japan).
Other Methods
SDS gel electrophoresis and Western blotting were carried out according to Gräf et al., 1998. Cell extracts of AX2 cells and A3-mutants were prepared as described previously (Daunderer and Gräf, 2002). Over- and underexpression levels of DdCP224 estimated from immunoblot band intensities and centrosomal GFP fluorescence in microscopic images were evaluated with ImageJ image analysis software.
RESULTS
To study the dynamics of supernumerary MTOCs elicited by DdCP224 overexpression, we have created two new Dictyostelium mutants. The first mutant was generated by transformation of the DdCP224-GFP overexpression mutant (Gräf et al., 2000) with a GFP-histone2B plasmid. In this mutant, both centrosomes and nuclei are labeled with GFP. The second mutant is a DdCP224 promoter replacement mutant generated by homologous recombination.
Formation of Supernumerary MTOCs Upon DdCP224 Overexpression Is Reversible
In the promoter replacement mutant, the endogenous DdCP224 gene was disrupted and the mutant cells can only express N-terminally GFP-tagged DdCP224 under control of the regulatable actin6 promoter (Figure 1). Promoter activity is high when cells are grown in axenic medium and low if they are grown at low density in bacterial suspension (Knecht and Loomis, 1987; Liu et al., 2002). Two independent clones with the desired promoter replacement were selected; one of them (named A3) was used for all experiments. Figure 2A shows that A3-mutants can only express GFP-tagged DdCP224 instead of the shorter, endogenous protein. When grown in shaking culture with axenic medium, A3-mutants showed overexpression of GFP-DdCP224 by a factor of ∼2.2 compared with endogenous DdCP224 in untransformed cells, as determined by quantitative evaluation of Western blots (Figure 2A). In contrast, when A3-mutants were cultivated in shaking culture with bacteria, GFP-DdCP224 was underexpressed at a level of ∼45% compared with the endogenous DdCP224 level in control cells kept under the same growth conditions (Figure 2A). The growth rate of the A3-cell culture was extremely low when DdCP224 was underexpressed (Figure 2B), suggesting that DdCP224 is essential. However, upon GFP-DdCP224 overexpression, A3-mutants grew as fast as control cells (our unpublished data). Under these conditions, A3-mutants were indistinguishable from the DdCP224-GFP mutant with an ectopically integrated plasmid and constitutive overexpression of the fusion protein (Gräf et al., 2000). Among all cells (n = 144), ∼42% contained more than one MTOC and ∼40% were multinuclear (Figure 2C). About 60% of all A3-mutants with supernumerary MTOCs contained more than one nucleus. Thus, there was no significant difference in the frequency of supernumerary MTOCs in monoversus multinuclear A3-mutants. Under the same growth conditions (shaking culture), about one-third of untransformed wild-type cells (n = 248) had more than one nucleus as well. This behavior seems to be a stable feature of the AX2 strain we are using, and it was independent of the growth media used. However, the incidence of wild-type cells containing more than two nuclei was very low and supernumerary centrosomes were not observed (n = 248). In most cases, the occurrence of supernumerary centrosomes in GFP-DdCP224 overexpressing A3-mutants was not accompanied by altered ploidy. More than 75% of all cells containing supernumerary MTOCs showed a normal DNA content as judged after quantitative evaluation of fluorescence intensities of DAPI-stained nuclei (our unpublished data).
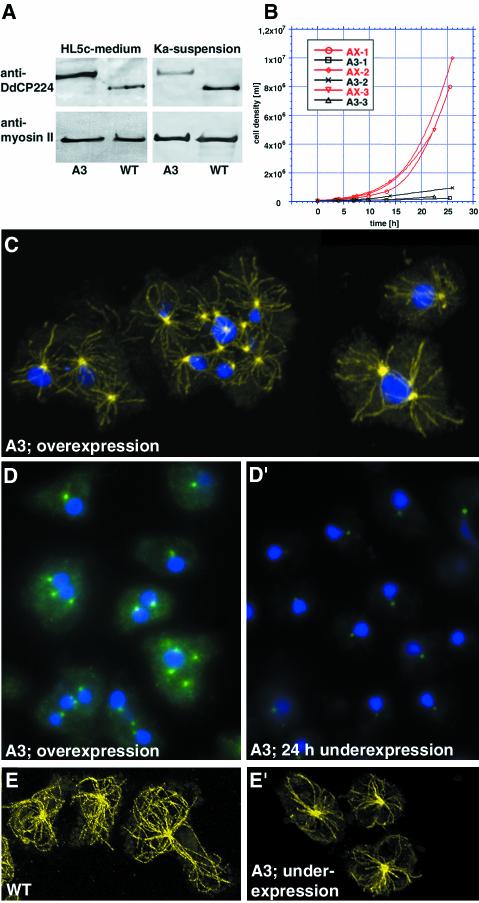
Figure 2.
Phenotypes of altered GFP-DdCP224 expression. (A) Immunoblot of cell extracts prepared from wild-type cells (WT; strain AX2) and GFP-DdCP224 cells (clone A3) under overexpression (HL5c-medium) and underexpression (Ka-suspension) conditions. A protein equivalent of 107 cells was loaded onto each lane, and the blot was stained with the monoclonal DdCP224 antibody 2/165 (Gräf et al., 1999). To show equal loading on each lane, blots were also stained with the monoclonal Dictyostelium myosin II antibody mAb96 (Claviez et al., 1982). Endogenous DdCP224 migrates faster than GFP-DdCP224 and it is absent in A3-mutants. (B) Reduced growth of A3-mutants in Klebsiella aerogenes-suspension culture. Growth curves of GFP-DdCP224–underexpressing cells (black; A3–1, 2, and 3) and AX2 wild-type cells (red; AX-1, 2, and 3) from three independent experiments are shown. (C) Supernumerary MTOCs and nucleus-associated centrosomes nucleate microtubules. Confocal immunofluorescence microscopy of fixed A3-mutants overexpressing GFP-DdCP224. Microtubules were labeled with the α-tubulin antibody YL1/2 (yellow; Chemicon, Hofheim, Germany), and nuclei were stained with TOPRO3 (Rehberg and Gräf, 2002). (D and D′) Reversibility of the GFP-DdCP224 overexpression phenotype (D) after shift to underexpression conditions for 24 h (D′). Both specimens were processed in parallel and images were acquired with the same camera settings to allow evaluation of centrosomal GFP-fluorescence. (E and E′) GFP-DdCP224 underexpression in A3-mutants (E′) leads to less and shorter microtubules compared with wild-type cells (WT; strain AX2) (E).
When A3-mutants were shifted from over- to underexpression conditions, supernumerary MTOCs virtually disappeared (∼3% of all cells; n = 224), and almost all cells were mononuclear (∼94%) within 24 h (Figure 2, D and D′). As expected, and in correspondence to the Western blot (Figure 2A), the fluorescence intensity of the centrosomal GFP-staining had decreased by ∼58% (Figure 2, D and D′). Thus, the occurrence of both supernumerary MTOCs and multiple nuclei is fully reversible and only dependent on the DdCP224 expression level.
Underexpression Reveals a DdCP224 Function in Microtubule Dynamics
Although cells underexpressing GFP-DdCP224 were almost unable to grow, their morphology was relatively normal. However, a closer look at the microtubule cytoskeleton revealed that the number and length of microtubules were reduced (Figures 2, E and E′, and 3, A and B). The function of DdCP224 in microtubule dynamics was even more evident upon treatment with the microtubule-depolymerizing drug nocodazole (Figure 3). In GFP-DdCP224–overexpressing A3-mutants and wild-type Dictyostelium cells, this treatment rarely lead to a complete depolymerization of the interphase microtubules (Figure 3D). The majority of cells retained an “incomplete” microtubule cytoskeleton with short microtubules. However, A3-mutants kept under DdCP224-underexpression conditions were hypersensitive to nocodazole treatment and showed complete depolymerization of their microtubules in approximately two-thirds of all cases (Figure 3C). In wild-type cells, the microtubule cytoskeleton was recovered completely within 10 min after drug removal. In contrast, none of the A3-mutants showed a complete recovery after this time, and approximately one-third of all cells still had no microtubules at all. After 20 min of recovery, almost 30% of the cells still showed no complete microtubule network, and the majority of these cells still had no microtubules at all. Thus, DdCP224 seems to be required for microtubule regrowth.
Figure 3.
DdCP224 is required for microtubule growth. Comparison of microtubule cytoskeletons in GFP-DdCP224–underexpressing A3-mutants and wild-type cells (WT; strain AX2) grown in Klebsiella aerogenes-suspension culture. Cells were either untreated (no noco.) or treated with nocodazole. Cells were fixed without washout of the drug or 10 and 20 min after drug removal, respectively. (A–H) Indirect immunofluorescence microscopy showing microtubules stained with the α-tubulin antibody YL1/2 and nuclei stained with DAPI. (A′–H′) Pie diagrams illustrating percentages of cells with no microtubules (light gray), an incomplete microtubule cytoskeleton with very short microtubules (dark gray), and a complete microtubule cytoskeleton with normal microtubules (i.e., as in untreated cells; A and B). The total number of cells counted for each condition is given within each diagram. One representative experiment is shown (n = 3).
DdCP224-elicited Supernumerary MTOCs Are Bona Fide Centrosomes
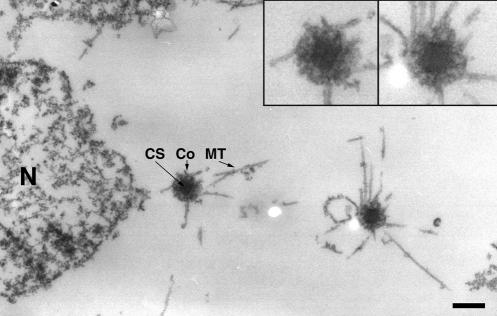
Supernumerary MTOCs induced by DdCP224 overexpression were fully competent of microtubule nucleation because they were always located at the center of microtubule asters (Figure 2C). By electron microscopy, the morphology of supernumerary, cytosolic MTOCs was indistinguishable from that of nucleus-associated centrosomes. Both were composed of a central, three-layered core structure surrounded by a corona containing microtubule nucleation sites (Euteneuer et al., 1998) (Figure 4). Thus, these supernumerary MTOCs are bona fide centrosomes.
Figure 4.
Supernumerary MTOCs are bona fide centrosomes. Representative electron microscopic image showing one nucleus-associated centrosome on the left and one supernumerary centrosome on the right. The two insets in the upper right corner illustrate that both centrosomes contain all ultrastructural features of a bona fide Dictyostelium centrosome. N, nucleus; CS, layered core structure; Co, corona; MT, microtubules. Bar, 0.5 μm.
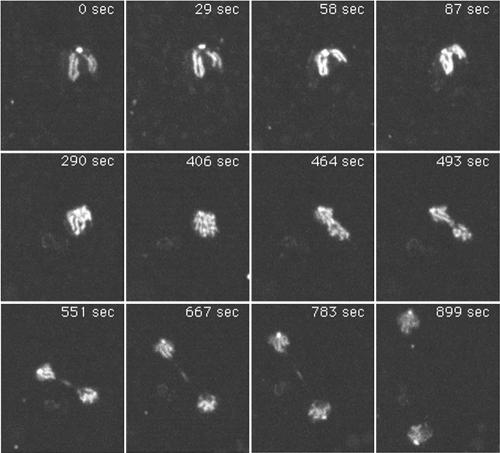
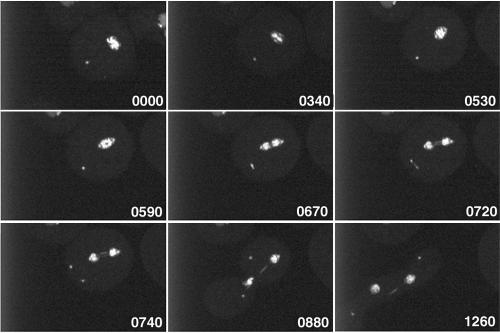
Furthermore, live observation of mitotic DdCP224-GFP/GFP-histone2B cells revealed that supernumerary centrosomes were also able to perform the complex process of centrosome duplication in ∼50% of all cases. In Dictyostelium, duplication of the nucleus-associated centrosome starts in early prophase and not at the G1/S transition as in most other organisms (Figure 5, Movie 1; Ueda et al., 1999). The freshly duplicated centrosomes insert into a fenestra in the nuclear envelope and organize a central spindle and kinetochore microtubules (Moens, 1976). In contrast to nucleus-associated centrosomes, cytosolic, supernumerary centrosomes duplicated in anaphase (Figure 6, Movie 2; 18 observations). Duplication of supernumerary centrosomes in earlier or later mitotic stages was never observed. Separation of the freshly duplicated supernumerary centrosomes seems to involve formation of a tiny spindle, because some GFP-fluorescent material was detectable between the two separating supernumerary daughter centrosomes (Figure 6, time point 0720 s). This staining pattern may correspond to the distribution of DdCP224-GFP during regular mitosis where it associates with spindle microtubules, especially in the midbody region (Figure 5).
Figure 5.
Live observation (Movie 1) of normal mitosis in a mitotic DdCP224-GFP/GFP-histone2B cell. Image acquisition started in prophase. GFP-histone2B labels chromosomes and DdCP224-GFP labels centrosomes and the midbody region. The time is indicated in seconds. Each image represents a brightest point z-projection of five confocal slices with a distance of 1 μm each. Images were acquired at the Ultraview spinning disk confocal microscope.
Figure 6.
Duplication of supernumerary centrosomes. Live observation (Movie 2) of a mitotic DdCP224-GFP/GFP-histone2B cell with initially one supernumerary centrosome. Image acquisition started in metaphase after duplication of the nucleus associated centrosome. The time is indicated in seconds. Each image represents a brightest point z-projection of five confocal slices with a distance of 0.5 μm each. Images were acquired at the Ultraview spinning disk confocal microscope.
The Number of Supernumerary Centrosomes Is Reduced by Coalescence and by Cytoplast Formation
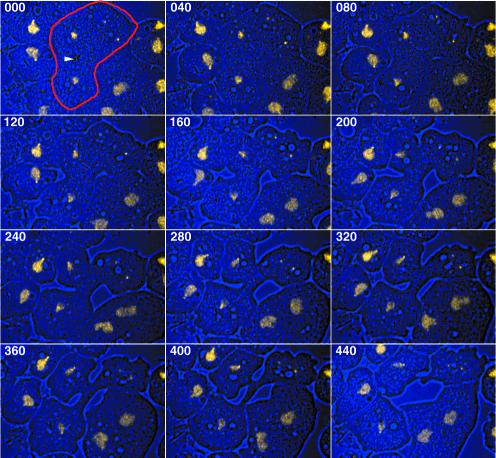
Although supernumerary centrosomes duplicate frequently, we have rarely encountered more than two extra centrosomes per nucleus. Moreover, the fraction of DdCP224-overexpressing cells with supernumerary centrosomes remains relatively constant at ∼50%. Thus, there must be mechanisms allowing mutant cells to reduce their number of supernumerary centrosomes. Theoretically, there are four possible scenarios how this can be achieved. 1) Cells with supernumerary centrosomes have reduced viability. This is rather unlikely, because both DdCP224-GFP cells and overexpressing A3-mutants have approximately the same growth rate as untransformed cells. 2) Supernumerary centrosomes are degraded. Yet, this was never observed in live cells. 3) Two or more supernumerary centrosomes fuse into one centrosome (=centrosome coalescence). 4) Supernumerary centrosomes are expelled in cytoplasts during cytokinesis or cytofission. Analysis of DdCP224-GFP/GFP-histone2B cells by confocal four-dimensional microscopy demonstrated that the latter two scenarios are indeed realized in living cells. The example shown in Figure 7 (Movie 3) shows a telophase cell with two daughter nuclei and two supernumerary centrosomes that are packaged and expelled in a cytoplast. In all cases observed (n = 6), cytoplast formation was coordinated with cytokinesis, but it cannot be excluded that it can also occur by cytofission during interphase. The low frequency of cytoplasts in a given cell population (below 1%; our unpublished data) could be due to the short life span of these nucleus-free cell fragments.
Figure 7.
Supernumerary centrosome elimination by cytoplast formation. An overlay of bright field (blue) and fluorescence images (yellow) is shown. Live observation (Movie 3) of a mitotic DdCP224-GFP/GFP-histone2B cell (labeled with a red margin in the first image) with two supernumerary centrosomes. Image acquisition started in telophase where the central spindle is still present (arrowhead at midbody region). Cytokinesis yields two daughter cells and one cytoplast containing the supernumerary centrosomes. The time is indicated in seconds. Each image represents a brightest point z-projection of five confocal slices with a distance of 1 μm each. Images were acquired on a 510META confocal laser scanning microscope (Carl Zeiss). In Movie3.mov fluorescence images (left) and bright field images (right) were separated.
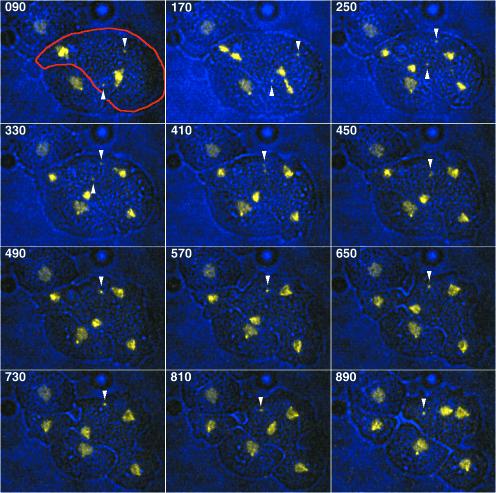
Figure 8 (movie 4) illustrates an example of centrosome coalescence in a binucleated cell. Its two supernumerary centrosomes clearly fuse into one centrosomal entity in telophase. Cytokinesis yields two normal, mononucleated daughter cells and one binucleated cell with the supernumerary, fused centrosome.
Figure 8.
Reduction of supernumerary centrosome number by centrosome coalescence. An overlay of bright field (blue) and fluorescence images (yellow) is shown. Live observation (Movie 4) of a binuclear mitotic DdCP224-GFP/GFP-histone2B cell (labeled with a red margin in the first image) with two supernumerary centrosomes (arrowheads). Image acquisition started in metaphase. The two supernumerary centrosomes fuse into one centrosomal entity in telophase. Cytokinesis yields three daughter cells, one binuclear cell with the single supernumerary centrosome and two normal, mononucleated cells. The time is indicated in seconds. Each image represents a brightest point z-projection of five confocal slices with a distance of 1 μm each. Images were acquired on a 510META confocal laser scanning microscope (Carl Zeiss). In movie4.mov fluorescence images (left) and bright field images (right) were separated.
DISCUSSION
We have shown that overexpression of DdCP224 causes the formation of bona fide supernumerary centrosomes. We could demonstrate for the first time in living cells that supernumerary centrosomes are able to duplicate and that their number can be regulated both by centrosome coalescence and by elimination through the formation of cytoplasts containing supernumerary centrosomes. Down-regulation of DdCP224 reverses the phenotype of supernumerary centrosomes, indicating that it depends only on the expression level of DdCP224. Moreover, our underexpression experiments demonstrated that DdCP224 is an essential protein required for microtubule dynamics in vivo.
DdCP224 Is Required for Microtubule Growth
In most species, including Caenorhabditis elegans, Arabidopsis, Drosophila, and yeasts, a reduced level of the respective DdCP224 homolog leads to mitotic spindle defects and, during interphase, to shorter and/or reduced numbers of microtubules (Kemphues et al., 1986; Whittington et al., 2001). In HeLa cells, depletion of the DdCP224 homolog colonic and hepatic tumor overexpressed gene (ch-TOG) by RNA interference caused a fivefold increase in mitotic index, concomitant with failure of proper metaphase plate organization (Gergely et al., 2003). Like Drosophila msps mutants, ch-TOG–depleted cells were characterized by defective spindles with extraspindle poles (Cullen et al., 1999; Gergely et al., 2003). In this respect, they are reminiscent of Dictyostelium cells with supernumerary centrosomes overexpressing DdCP224 (this work; Gräf et al., 2000). Surprisingly, underexpression of DdCP224 at approximately the same level as in human cells (∼60% reduction (Gergely et al., 2003) did not cause mitotic defects or a mitotic arrest, although DdCP224-underexpressing cells exhibited a drastically reduced growth rate (Figure 2B). All mitotic cells present in cell cultures underexpressing DdCP224 showed normal spindles when stained with antibodies against tubulin or various centrosomal proteins (our unpublished data). The only abnormal phenotype observed was a slight reduction of length and number of cytoplasmic microtubules, a result comparable with other XMAP215 family mutants. Unfortunately, an investigation of microtubule dynamics in vivo is very difficult in Dictyostelium, because interphase microtubules show mainly a high lateral motility and no microtubule dynamics with regard to growth and shrinkage (Kimble et al., 2000; Koonce and Khodjakov, 2002). In vitro studies are hampered by the lack of a protocol for the purification of polymerization-competent Dictyostelium tubulin. Therefore, we have circumvented these problems by treatment of mutant and control cells with nocodazole to investigate the microtubule de- and repolymerization process. In wild-type cells, microtubules are rather insensitive to nocodazole and other microtubule-depolymerizing agents (Kitanishi et al., 1984). However, A3-mutants underexpressing DdCP224 were clearly hypersensitive to nocodazole and showed complete microtubule depolymerization in the majority of cells. Furthermore, microtubule regrowth after drug removal was considerably slowed down. In theory, DdCP224 could promote microtubule regrowth by an increase in either microtubule nucleation or polymerization. If DdCP224 were required only for microtubule polymerization, we would expect rapid reappearance of many short, but slow-growing microtubules at virtually all centrosomes. However, the relatively large fraction of cells without any microtubules, even 20 min after drug removal, strongly suggests that DdCP224 is involved in microtubule nucleation. This is in agreement with recent results by Popov et al., 2002) who have shown convincingly that XMAP215 is essential for microtubule nucleation in Xenopus egg extracts and that it can promote microtubule aster formation at XMAP215-coated beads even in the absence of γ-tubulin. In addition to this function in microtubule nucleation, XMAP215 is a strong stimulator of microtubule elongation in vitro. In vivo, however, it mainly seems to suppress microtubule catastrophe events as an antagonist of XKCM1 (Kinoshita et al., 2001). Based on the substantial similarity between XMAP215 and DdCP224 in primary structure and localization pattern, it is likely that DdCP224 also functions as a promoter of microtubule elongation in addition to its role in microtubule nucleation.
Origin and Replication of Supernumerary Centrosomes
In addition to its role in microtubule growth, our studies also suggest an additional function for DdCP224 in the centrosome duplication cycle, because overexpression of DdCP224-GFP leads to the formation of supernumerary centrosomes. The appearance of supernumerary centrosomes was solely a result of an increased DdCP224 protein level. It was unrelated to failures of cytokinesis that were encountered in cells grown in shaking culture. First, compared with mononuclear cells, supernumerary centrosomes were not significantly more frequent in multinuclear cells. Second, the incidence of supernumerary centrosomes was unchanged when the cells were grown in adherent culture where the number of multinuclear cells was strongly reduced (our unpublished data). We can imagine three pathways for how DdCP224 overexpression leads to the formation of supernumerary centrosomes.
First, the appearance of supernumerary centrosomes could be a secondary effect resulting from a failure of chromosome segregation. DdCP224 is also present at kinetochores (Gräf et al., 2000), and one could suppose that DdCP224 overexpression interferes with proper attachment of spindle microtubules at kinetochores. Because Dictyostelium cells have no spindle checkpoint (Ma et al., 1999), cells could reenter interphase without karyokinesis but with a duplicated set of centrosomes. In this case, cells with supernumerary centrosomes should be at least diploid compared with the haploid wild-type cells. However, only a few cells with supernumerary centrosomes contained unusually large nuclei or mininuclei, whereas the majority showed a normal DNA content. Thus, the major pathway for the generation of supernumerary centrosomes must be another one.
Second, overexpressed DdCP224 could serve as a seed for the acquisition of preformed centrosomal material such as γ-tubulin complexes from the cytosol, leading to the assembly of supernumerary centrosomes. Such a role of DdCP224 is plausible because, in Xenopus egg extracts, XMAP215 is known to be essential for self-organization of MTOCs induced by the small GTPase Ran (Ohba et al., 1999; Wilde and Zheng, 1999).
Finally, DdCP224 overexpression could destabilize spindle poles and lead to spindle pole splitting. If only one of the two freshly split spindle poles is involved in spindle formation, then the second one could be released into the cytoplasm as a supernumerary centrosome. A comparable phenomenon of spindle pole splitting leading to multipolar spindles has been observed in Drosophila msps mutants and HeLa cells after ch-TOG depletion (Cullen et al., 1999; Gergely et al., 2003). Multipolar spindles have been observed in DdCP224 overexpressors, but at a relatively low frequency. This could be attributed to the fact that the nuclear envelope is not broken down during mitosis in Dictyostelium. Thus, supernumerary spindle poles can only interfere with chromosome segregation if they stay associated with the nuclear envelope. It is possible that all three scenarios contribute to the formation of supernumerary centrosomes in DdCP224-overexpressing mutants. So far, it was not possible to observe the de novo generation of supernumerary centrosomes in live cells. However, we frequently observed the duplication of supernumerary, cytosolic centrosomes. This tells us that supernumerary centrosomes contain the complete protein machinery of a bona fide centrosome. It also raises the question of how supernumerary centrosome duplication is regulated, because it takes place in anaphase, long after the nucleus-associated centrosome has duplicated. In animal cells, centrosome duplication is initiated by CDK2 and regulated by a set of kinases, including aurora-, polo-, and NIMA-like kinases (Fry et al., 2000). One interpretation of the delayed duplication of supernumerary centrosomes is that it engages different regulatory factors. Alternatively, one of the regulators of centrosome duplication could be retained at the spindle or spindle poles until it is allowed to diffuse to the cytosolic, supernumerary centrosomes and induce their duplication.
Control of Supernumerary Centrosome Number
Even after many passages, the fraction of DdCP224-overexpressing cells with supernumerary centrosomes remains ∼50%, although supernumerary centrosomes replicate, and it is reasonable to assume that they constantly arise de novo as well. Furthermore, the appearance of supernumerary centrosomes was completely reversible when overexpressing cells were shifted to underexpression conditions. This implies the existence of effective mechanisms for the control of centrosome number as they have been postulated for tumor cells with supernumerary centrosomes. These cells have to down-regulate the number of functional centrosomes to be able to form a bipolar spindle. So far, there are only data from fixed cells, indicating that coalescence, i.e., centrosome fusion, could be one mechanism (for review, see Brinkley, 2001). Herein, we have shown for the first time that there exist at least two different mechanisms for the reduction of supernumerary centrosome number in living cells: formation of nucleus-free, centrosome-containing cell fragments (cytoplasts) and centrosome coalescence. The regulation and exact mechanism of centrosome coalescence remains to be investigated. It is likely that the process involves more than just a tight association of two formerly individual centrosomal entities. If this were the case, centrosome coalescence should lead to a dumbbell-shaped centrosome, which should be recognizable at the light-microscopic level. Our data show instead that the two supernumerary centrosomes fuse into one single, centrosomal entity.
Cytoplast formation was always encountered as an event linked to cytokinesis. This is in agreement with the observation that astral microtubules organized from mitotic centrosomes are required for spindle positioning and determination of the cleavage furrow (Rieder et al., 2001). This is accomplished through interactions of microtubule tips with cortical dynein/dynactin (Busson et al., 1998). Because DdCP224-elicited supernumerary centrosomes behaved like fully competent MTOCs, they could also participate in cleavage furrow formation. Cortical interactions of astral microtubules emanating from supernumerary centrosomes could induce formation of extracleavage furrows and, hence, cytoplasts. Because the mechanism of cleavage furrow determination is conserved in Dictyostelium and higher cells (Neujahr et al., 1998), this mode of supernumerary centrosome elimination may be found in mammalian cells as well.
Is There a Role of XMAP215 Family Proteins in Carcinogenesis?
The reversible appearance of supernumerary centrosomes depending on the expression level of DdCP224 is a good example for how tightly protein levels must be controlled in eukaryotic cells. The human homolog of DdCP224, ch-TOG, was originally identified in a screen for proteins overexpressed in certain tumor cells, hence its name, which stands for “colonic and hepatic tumor overexpressed gene” (Charrasse et al., 1995). It is tempting to speculate that overexpression of ch-TOG could be directly involved in carcinogenesis. To date, it is unknown whether ch-TOG overexpression is directly linked to centrosome amplification in tumor cells. Analogous to increased DdCP224 levels in Dictyostelium cells, ch-TOG overexpression could also induce centrosome amplification. Together with mutations in the adenomatous polyposis coli protein (Fodde et al., 2001) and a possible interference of ch-TOG overexpression with kinetochore function (Gergely et al., 2003), ch-TOG–elicited supernumerary centrosomes could contribute to chromosomal instability which is a hallmark of most colorectal cancers.
Supplementary Material
Acknowledgments
We thank Manfred Schliwa for critical comments and continuous support. We thank Günther Gerisch for the GFP-histone2B plasmid. We acknowledge Rainer Pepperkok and everyone at the Advanced Light Microscopy Facility of the EMBL in Heidelberg for the opportunity to use the Ultraview spinning disk confocal microscope. We also thank Manfred Schliwa and Alexandra Lepier for critically reading the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (SFB413 and GR1642/1-1).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–04–0242. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-04-0242.
Online version of this article contains video material for some figures. Online version is available at www.molbiolcell.org.
References
- Brinkley, B.R. (2001). Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 11, 18–21. [DOI] [PubMed] [Google Scholar]
- Busson, S., Dujardin, D., Moreau, A., Dompierre, J., and DeMey, J.R. (1998). Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 8, 541–544. [DOI] [PubMed] [Google Scholar]
- Cassimeris, L., Gard, D., Tran, P.T., and Erickson, H.P. (2001). XMAP215 is a long thin molecule that does not increase microtubule stiffness. J. Cell Sci. 114, 3025–3033. [DOI] [PubMed] [Google Scholar]
- Charrasse, S., Mazel, M., Taviaux, S., Berta, P., Chow, T., and Larroque, C. (1995). Characterization of the cDNA and pattern of expression of a new gene over-expressed in human hepatomas and colonic tumors. Eur. J. Biochem. 234, 406–413. [DOI] [PubMed] [Google Scholar]
- Claviez, M., Pagh, K., Maruta, H., Baltes, W., Fisher, P., and Gerisch, G. (1982). Electron microscopic mapping of monoclonal antibodies on the tail region of Dictyostelium myosin. EMBO J. 1, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, C.F., Deak, P., Glover, D.M., and Ohkura, H. (1999). Mini spindles: a gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J. Cell Biol. 146, 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunderer, C., and Gräf, R. (2002). Molecular analysis of the cytosolic Dictyostelium gamma-tubulin complex. Eur. J. Cell Biol. 81, 175–184. [DOI] [PubMed] [Google Scholar]
- Euteneuer, U., Gräf, R., Kube-Granderath, E., and Schliwa, M. (1998). Dictyostelium gamma-tubulin: molecular characterization and ultrastructural localization. J. Cell Sci. 111, 405–412. [DOI] [PubMed] [Google Scholar]
- Fodde, R., et al. (2001). Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 3, 433–438. [DOI] [PubMed] [Google Scholar]
- Fry, A.M., Mayor, T., and Nigg, E.A. (2000). Regulating centrosomes by protein phosphorylation. Curr. Top. Dev. Biol. 49, 291–312. [DOI] [PubMed] [Google Scholar]
- Fukui, Y., Yumura, S., and Yumura, T.K. (1987). Agar-overlay immunofluorescence: high resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 28, 347–356. [DOI] [PubMed] [Google Scholar]
- Gergely, F., Draviam, V.M., and Raff, J.W. (2003). The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 17, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf, R., Daunderer, C., and Schliwa, M. (1999). Cell cycle-dependent localization of monoclonal antibodies raised against isolated Dictyostelium centrosomes. Biol. Cell 91, 471–477. [DOI] [PubMed] [Google Scholar]
- Gräf, R., Daunderer, C., and Schliwa, M. (2000). Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J. Cell Sci. 113, 1747–1758. [DOI] [PubMed] [Google Scholar]
- Gräf, R., Euteneuer, U., Ueda, M., and Schliwa, M. (1998). Isolation of nucleation-competent centrosomes from Dictyostelium discoideum. Eur. J. Cell Biol. 76, 167–175. [DOI] [PubMed] [Google Scholar]
- Hestermann, A., Rehberg, M., and Gräf, R. (2002). Centrosomal microtubule plus end tracking proteins and their role in Dictyostelium cell dynamics. J. Muscle Res. Cell Motil. 23, 621–630. [DOI] [PubMed] [Google Scholar]
- Kemphues, K.J., Wolf, N., Wood, W.B., and Hirsh, D. (1986). Two loci required for cytoplasmic organization in early embryos of Caenorhabditis elegans. Dev. Biol. 113, 449–460. [DOI] [PubMed] [Google Scholar]
- Kimble, M., Kuzmiak, C., McGovern, K.N., and de Hostos, E.L. (2000). Microtubule organization and the effects of GFP-tubulin expression in Dictyostelium discoideum. Cell Motil. Cytoskeleton 47, 48–62. [DOI] [PubMed] [Google Scholar]
- Kinoshita, K., Arnal, I., Desai, A., Drechsel, D.N., and Hyman, A.A. (2001). Reconstitution of physiological microtubule dynamics using purified components. Science 294, 1340–1343. [DOI] [PubMed] [Google Scholar]
- Kitanishi, T., Shibaoka, H., and Fukui, Y. (1984). Disruption of microtubules and retardation of development of Dictyostelium with ethyl N-phenylcarbamate and thiabendazole. Protoplasma 120, 185–196. [Google Scholar]
- Knecht, D.A., and Loomis, W.F. (1987). Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science 236, 1081–1085. [DOI] [PubMed] [Google Scholar]
- Koonce, M.P., and Khodjakov, A. (2002). Dynamic microtubules in Dictyostelium. J. Muscle Res. Cell Motil. 23, 613–619. [DOI] [PubMed] [Google Scholar]
- Lingle, W.L., Barrett, S.L., Negron, V.C., D'Assoro, A.B., Boeneman, K., Liu, W., Whitehead, C.M., Reynolds, C., and Salisbury, J.L. (2002). Centrosome amplification drives chromosomal instability in breast tumor development. Proc. Natl. Acad. Sci. USA 99, 1978–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T., Mirschberger, C., Chooback, L., Arana, Q., Dal Sacco, Z., MacWilliams, H., and Clarke, M. (2002). Altered expression of the 100 kDa subunit of the Dictyostelium vacuolar proton pump impairs enzyme assembly, endocytic function and cytosolic pH regulation. J. Cell Sci. 115, 1907–1918. [DOI] [PubMed] [Google Scholar]
- Ma, S., Trivinos Lagos, L., Gräf, R., and Chisholm, R.L. (1999). Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J. Cell Biol. 147, 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, S.K.O., et al. (1998). Cell biological, molecular genetic, and biochemical methods used to examine Dictyostelium. In: Cell Biology: A Laboratory Handbook, vol. 1, ed. J.E. Celis, San Diego: Academic Press, 431–465. [Google Scholar]
- Moens, P.B. (1976). Spindle and kinetochore morphology of Dictyostelium discoideum. J. Cell Biol. 68, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen, W., Datta, S., Reymond, C., Sivertsen, A., Mann, S., Crowley, T., and Firtel, R.A. (1987). Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 28, 67–100. [DOI] [PubMed] [Google Scholar]
- Neujahr, R., Albrecht, R., Kohler, J., Matzner, M., Schwartz, J.M., Westphal, M., and Gerisch, G. (1998). Microtubule-mediated centrosome motility and the positioning of cleavage furrows in multinucleate myosin II-null cells. J. Cell Sci. 111, 1227–1240. [DOI] [PubMed] [Google Scholar]
- Nigg, E.A. (2002). Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer 2, 815–825. [DOI] [PubMed] [Google Scholar]
- Ohba, T., Nakamura, M., Nishitani, H., and Nishimoto, T. (1999). Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science 284, 1356–1358. [DOI] [PubMed] [Google Scholar]
- Ohkura, H., Garcia, M.A., and Toda, T. (2001). Dis1/TOG universal microtubule adaptors - one MAP for all? J. Cell Sci. 114, 3805–3812. [DOI] [PubMed] [Google Scholar]
- Popov, A., Severin, F., and Karsenti, E. (2002). XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr. Biol. 12, 1326. [DOI] [PubMed] [Google Scholar]
- Popov, A.V., Pozniakovsky, A., Arnal, I., Antony, C., Ashford, A.J., Kinoshita, K., Tournebize, R., Hyman, A.A., and Karsenti, E. (2001). XMAP215 regulates microtubule dynamics through two distinct domains. EMBO J. 20, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg, M., and Gräf, R. (2002). Dictyostelium EB1 is a genuine centrosomal component required for proper spindle formation. Mol. Biol. Cell 13, 2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., Faruki, S., and Khodjakov, A. (2001). The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 11, 413–419. [DOI] [PubMed] [Google Scholar]
- Schliwa, M., and van Blerkom, J. (1981). Structural interaction of cytoskeletal components. J. Cell Biol. 90, 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, M., Schliwa, M., and Euteneuer, U. (1999). Unusual centrosome cycle in Dictyostelium: correlation of dynamic behavior and structural changes. Mol. Biol. Cell 10, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington, A.T., Vugrek, O., Wei, K.J., Hasenbein, N.G., Sugimoto, K., Rashbrooke, M.C., and Wasteneys, G.O. (2001). MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610–613. [DOI] [PubMed] [Google Scholar]
- Wilde, A., and Zheng, Y. (1999). Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 284, 1359–1362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.