Summary
The pathways that deliver newly synthesized proteins that reside in lysosomes are well understood by comparison with our knowledge of how integral membrane proteins are sorted and delivered to the lysosome for degradation. Many membrane proteins are sorted to lysosomes following ubiquitination, which provides a sorting signal that can operate for sorting at the TGN (trans-Golgi network), at the plasma membrane or at the endosome for delivery into lumenal vesicles. Candidate multicomponent machines that can potentially move ubiquitinated integral membrane cargo proteins have been identified, but much work is still required to ascertain which of these candidates directly recognizes ubiquitinated cargo and what they do with cargo after recognition. In the case of the machinery required for sorting into the lumenal vesicles of endosomes, other functions have also been determined including a link between sorting and movement of endosomes along microtubules.
Introduction
One important signal for sending post-Golgi membrane proteins to the lysosome for degradation is ubiquitin (Ub). Ub attachment to membrane proteins correlates strongly with their degradation. Ub attachment not only helps destroy damaged proteins but, in mammalian cells, acutely regulates a variety of cell surface receptors including EGFR (epidermal growth factor receptor), PDGFR (platelet derived growth factor receptor), IL1-R (interleukin 1 receptor) and TrkA (nerve growth factor receptor) which undergo “downregulation” and lysosomal degradation to attenuate their signaling potential. Ub may act as a sorting signal for membrane proteins at the TGN, the plasma membrane and at endosomes. Although it has been recognized for many years that late endosomes are morphologically identical to MVBs (multivesicular bodies)[1], only recently have we have started to understand the mechanisms by which endocytosed, ubiquitinated membrane proteins are sorted into endosomal lumenal vesicles. This sorting occurs prior to the fusion of late endosomes with lysosomes that results in protein degradation[2,3]. Sorting into the lumenal vesicles of MVBs is mediated in part by cytosolic proteins functioning as ESCRT (endosomal sorting complexes required for transport) complexes together with ESCRT-associated proteins [4–7]. In this article we review how cells use Ub as a sorting signal for membrane proteins, the function of ESCRT complexes in linking MVB formation to endosome movement along microtubules and the relationship between MVB formation and fusion of late endosomes with lysosomes.
Ubiquitin as a sorting signal
The correlation between the degradation of membrane proteins and their ubiquitination has been established for sometime [8]. Now we understand that this correlation represents a conglomerate of ubiquitin-dependent trafficking steps that eventually send proteins to the interior of the lysosome for degradation. The first Ub-dependent sorting step that was discovered was internalization from the cell surface using the yeast G-protein coupled receptor Ste2p [9]. Removing the ubiquitinated lysines from the C-terminus of Ste2p blocked its internalization while in-frame fusion of Ub restored its internalization. Ub can also direct proteins to endosomes from the TGN, in some cases serving as a quality control device to divert damaged or functionally inappropriate proteins to lysosomes and prevent them from appearing on the cell surface. In yeast, this has been shown for nutrient transporters such as Fur4p and Gap1p, which are downregulated by an excess of their substrates as the direct result of ubiquitination [10–12]. Ub can also cause proteins to sort into the interior of endosomes by incorporating them into lumenal vesicles that accumulate to form MVBs [13,14]. These three Ub-dependent pathways, namely endocytosis, delivery from TGN to endosomes and sorting into lumenal vesicles were first described in yeast but also operate in animal cells (Figure 1). Studies in both systems are beginning to resolve the general mechanisms for how Ub is recognized as a sorting signal and how ubiquitinated proteins are incorporated into various transport intermediates.
Figure 1.
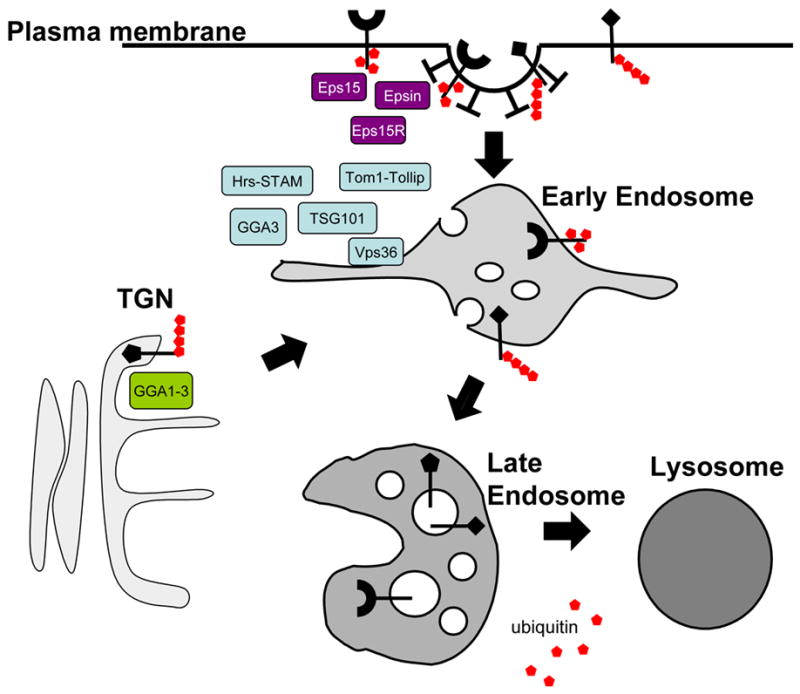
Ub is used as a sorting signal in post-Golgi membrane traffic pathways. The diagram shows three sites at which Ub is known to act as a sorting signal for membrane proteins: TGN, plasma membrane and endosome. Some known components of Ub interacting sorting machinery are indicated.
How efficient a single Ub signal is for these three sorting pathways to lysosomes has been unclear. Membrane proteins can carry multiple single Ubs attached directly to substrate protein (multiple monoubiquitination) or short polyubiquitin chains. Thus proteins such as MHC-I [15] and TrkA [16] have a single lysine that may be modified by a K63-linked polyUb chain but EGFR carries multiple monoUb [17] and/or polyUb [18]. The consensus view is that it is the presence of many Ubs rather than the presence of a particular polyubiquitin linkage that affords efficient Ub-dependent sorting to lysosomes. The physical basis of this lies in the relatively low affinity of the candidate Ub-sorting receptors for a single Ub (often in the range 10−4->10−3M). For all three of the sorting steps, monoubiquitination appears to be sufficient in yeast. Thus, fusion of Ub onto reporter proteins causes internalization of Ste2p [19], redirection of the SNARE Snc1p to endosomes, and entry of stable vacuolar membrane proteins into MVBs [20]. The problem with these studies however, is that the presence of Ub itself can promote ubiquitination of the fusion protein which could theoretically occur on any number of exposed lysines or cysteines left in the fusion protein or the glycine tail of ubiquitin at the C-terminus. Other experiments have demonstrated the inefficiency of a single in-frame fusion of Ub as either an internalization signal, a TGN-to-endosome signal, or an MVB sorting signal. One potential problem, however, with these latter experiments is that they use mutant forms of Ub in which all lysines (including lysine 27 which protrudes into the core of Ub) are altered to arginine and which contain alterations in the C-terminal tail which could negatively impact the ability of known and unknown Ub-binding protein machinery to properly recognize these Ub-fusion reporter proteins (for instance, altering the surface lysines of Ub reduces its binding to the GGA GAT domain, RCP unpublished data).
Discovering exactly what protein complexes recognize Ub as a lysosomal sorting signal has been difficult for two main reasons. The first is that many proteins that play a role in protein sorting at the plasma membrane, TGN and endosomes have been found to contain UBDs (Ub-binding domains). These include proteins such as Eps15, Epsin, CIN85, Hrs, Tsg101, Tom1, Tollip Vps36/Eap45, STAM and GGAs 1–3, which bind Ub via UIM, SH3, GAT, CUE, NZF, and GLUE domains (reviewed in [21]). This not only presents a large number of candidate Ub-sorting proteins to analyze but also implies that there may be redundant Ub-recognition complexes. The second reason is that while it is tantalizing to suppose the primary purpose of these Ub-binding motifs is to contact ubiquitinated cargo, ubiquitination can serve many other cellular roles [22]. Indeed, most of the candidate Ub-sorting proteins are themselves ubiquitinated in animal cells upon overexpression in a process termed coupled monoubiquitination [23]. Ubiquitination of the machinery is strictly by virtue of the ability of these proteins to bind ubiquitinated proteins non-covalently and likely works by positioning the machinery on or near Ub ligase activity [23,24]. Such ubiquitination could drive the assembly of UBDs into large networks. Alternatively, it could drive an intramolecular interaction between the UBD and covalently attached Ub, which causes a conformational change that prevents other intermolecular interactions [25]. For instance, ubiquitinated GGA3 and Eps15 fail to bind Ub non-covalently and ubiquitinated Epsin fails to bind clathrin [26,27]. Furthermore, overexpression of Ub ligases that ubiquitinate Eps15 or TSG101 attenuate internalization and degradation of the EGFR [24,28].
Ubiquitin-mediated sorting at the cell surface, TGN and endosomes
At the plasma membrane, Epsin, Eps15 and their yeast homogues mediate the endocytic internalization of a variety of ubiquitinated membrane proteins and has been well described (reviewed in [29]). Interestingly, the low affinity of Epsin for a single Ub moiety correlates with the need for multiple Ubs to constitute an efficient internalization signal, thus implicating these factors in the direct recognition of Ub-cargo [30,31]. Intriguingly, Eps15 has also been found complexed with STAM and Hrs, two endosomal proteins associated with ESCRT complexes and implicated in sorting into the MVB lumen [32–34]. Thus, Eps15 and Epsin could act as switch hitters, lending their Ub-binding/regulatory capacity to a variety of sorting events.
Ub-dependent sorting of proteins from the TGN to endosomes in yeast requires GGA proteins, which bind Ub via motifs within the GAT domain. Loss of GGA or loss of Ub binding by GGAs disrupts this sorting process [35]. Whether GGAs can similarly mediate sorting of ubiquitinated proteins from the TGN to endosomes in animal cells has yet to be convincingly demonstrated. In mammalian cell, the transport of the polytopic lysosomal membrane protein LAPTM5 requires both GGA3 and ubiquitination but this process shows some interesting twists. LAPTM5 moves from the TGN to endosomes but does not require ubiquitination [36]. Rather, LAPTM5 requires both a UIM domain that binds Ub and a PY motif, which can bind the Nedd4 ubiquitin ligase. These data imply a model whereby LAPTM5 recruits Nedd4 to ubiquitinate an associated protein to which LAPTM5 can then bind via its UIM domain. Interestingly, one of the candidate ubiquitinated partners may be GGA itself, which upon ubiquitination can associate with LAPTM5, possibly pulling the membrane protein into transport vesicles that depart the TGN.
At the endosome, a host of proteins have been found which might bind ubiquitinated cargo and sort it to the MVB interior. These include the Hrs/STAM complex, GGA3, the TOM1/Tollip complex, as well as subunits of the ESCRT-I and ESCRT-II complexes thought to participate in MVB formation (Figure 2). These studies are further complicated since these protein complexes appear to support multiple cellular functions, each of which might potentially integrate with the Ub system [6]. The idea that the Hrs/STAM complex directly binds and sorts ubiquitinated cargo is supported by the observation that mutations that inactivate Ub binding by this complex result in specific defects in sorting ubiquitinated cargo into the MVB interior [37]. If indeed the Hrs/STAM complex does serve as an endosomal Ub-cargo sorting complex, it is likely not the only one—especially since homologues cannot be found in plants and Dictyostelium [38]. Other candidate Ub-sorting receptors include GGA3, which party localizes to endosomes, binds Ub, and can influence sorting of ubiquitinated EGFR [39]. Blocking Ub binding by GGA3 with mutations in the GAT domain greatly influences the trafficking of EGFR. However, those mutations also block association with other GAT-associated factors. Also, members of the Tom1 family of proteins (Tom1, Tom1L1, Tom1L2) are likely candidates as they bind Ub, can associate with other Ub binding proteins, localize to endosomes, and are represented in both plant and Dictyostelium genomes [40–42]. Depletion of Tom1 family proteins potentiates signaling from PDGFR and retards degradation of ubiquitinated IL-1R [43,44]. Tom1 also associates with another endosomal Ub-binding protein Tollip, which associates with ubiquitinated IL-1R and which requires its Ub-binding CUE domain to effect IL-1R transport into lysosomes [44]. All of these complexes can bind to clathrin, which may allow them to localize to clathrin-rich subdomains on endosomes which are thought to be the staging ground for protein sorting into lumenal vesicles [40,45–47]. All of these complexes also interact with TSG101, a Ub binding subunit of the ESCRT-I complex. The ESCRT-I and ESCRT-II complexes are localized to endosomes and in yeast are required for MVB biogenesis. At least in yeast, ESCRT-I can associate with ESCRT-II to form a proposed supercomplex. An attractive model is that Ub-binding complexes such as Hrs/STAM or Tollip/Tom1 could transfer their Ub-cargo to the ESCRT-I/II complexes for further sorting to lumenal vesicles of MVBs. However, clear evidence that Vps23p/TSG101 (yeast and animal ESCRT-I subunits subunits that bind Ub) and Vps36p/Eap45 (yeast and animal ESCRT-II subunits that bind Ub) act as specific Ub-sorting receptors is lacking. Now that the interfaces for ESCRT-I and ESCRT-II complexed with Ub have been solved [21], better testing of whether these proteins recognize Ub-cargo during cargo sorting can be done. Similarly, the role of Ub binding by mammalian Vps36/Eap45 can be addressed now that recent structural studies provide a model for how its GLUE domain binds Ub [48,49].
Figure 2.
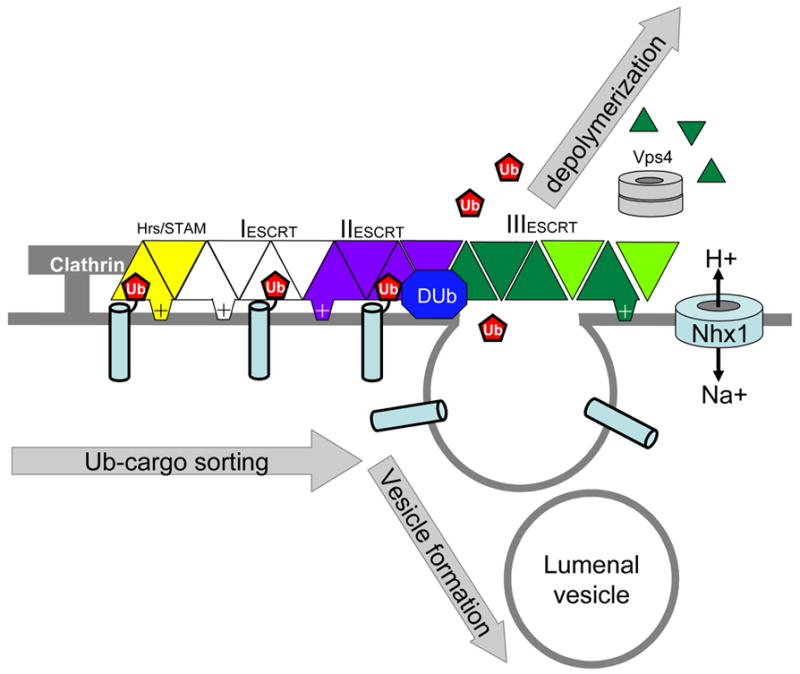
The ESCRT pathway for sorting ubiquitinated proteins at the endosome. In yeast genetic screens have identified 18 genes encoding Vps proteins required to sort ubiquitinated membrane proteins into the lumenal vesicles of MVBs. Thirteen of these form 3 ESCRT complexes, including an extended ESCRT-III complex of 6 similar alpha-helical proteins. Also shown is the Na+/H+ exchanger, which contributes in an unkown way to a pH-dependent process involved in MVB formation. The diagram shows a model in which the Hrs-STAM complex binds to ubiquitinated cargo within clathrin enriched endosomal subdomains. Ub-cargo is then recognized by Ub-binding domains of ESCRT-I and/or ESCRT-II prior to delivery into forming intralumenal vesicles. Following recruitment of ESCRT-III and ESCRT-III associated proteins, ATP hydrolysis by Vps4p results in depolymerisation. Dub indicates a de-ubiquitinating enzyme.
Studies in yeast defined a set of ESCRT protein complexes comprised of class E vps (vacuolar protein sorting) proteins that are in some way required for the biogenesis of MVBs (Figure 2). Loss of TSG101, an ESCRT-I component, from animal cells causes many of the same phenotypes that arise in yeast—accumulation of multicisternal endosomes, a block in degradation of ubiquitinated cargo proteins such as EGFR and a block in gag-dependent virus budding, a process functionally related to the budding of intralumenal vesicles into the endosomal lumen [50,51]. However, loss-of-function studies of other ESCRT components in animal cells have yielded a more complex picture. A dramatic example is depletion of ESCRT-II which does not result in gross morphological changes to the endocytic system, does not affect viral budding, the transport of ligands such as EGF to lysosomes, or the degradation of ubiquitinated membrane proteins such as MHC-I [52,53]. While there is a modest effect of ESCRT-II loss on EGFR degradation, it appears that the process of ushering ubiquitinated proteins into properly forming MVBs is largely intact. As a potential Ub-sorting receptor, ESCRT-II does not appear to be an obligate part of the sorting process, indicating that it may be only used for a subset of ubiquitinated cargo
Endosome sorting, movement and delivery to lysosomes
Microtubules play an important role in both cargo sorting and delivery in the endocytic pathway. Receptor sorting in early endosomes is retarded in the absence of dynein[54]. Movement along microtubules plays a key role in delivering cargo to lysosomes and lysosome biogenesis. The maturation of Rab5-positive early endosomes to Rab7-positive endosomes does not require microtubules [55]. However, endosomes move bidirectionally along microtubules using both dynein and kinesin motors, and efficient transport of endocytosed material from early endosomes to lysosomes is dependent on an intact microtubule cytoskeleton. One of the key proteins required for microtubule movement of endosomes is RILP (Rab7 interacting lysosomal protein) [56,57]. RILP appears to coordinate several events with regard to endosome movement since it binds the dynein-dynactin complex [56]. Intriguingly, RILP has recently been found to also bind to ESCRT-II, expanding the repertoire of possible functions with which it is involved [58,59]. The RILP Rab7 complex also binds the oxysterol-binding protein homologue ORP1L and overexpression of either RILP or ORP1L clusters lysosomes around the MTOC (microtubule organizing centre) [60]. Recently, a detailed biochemical mechanism has been proposed to explain the recruitment dynein-dynactin to late endosomes and its activation to drive endosome movement toward the minus-end of microtubules. In this model Rab7 binds RILP to recruit dynein-dynactin via p150 glued and localizes this complex in endosomal patches via an association between Rab7, ORP1L, and beta-III spectrin [61]. Beta-III spectrin can then associate with Arp1 of dynactin to lock and load the dynein-dyactin complex onto endosomes. Given the ability of mammalian ESCRT-II subunits Eap30/Vps22p and Eap45/Vps36p to directly interact with different regions of RILP, it will be interesting to determine how the molecular events that RILP orchestrates are regulated at the molecular level of ESCRT-II association. Further evidence for an interaction of ESCRT II with the microtubule cytoskeleton has come from experiments showing that it is required for proper localization of Drosophila bicoid mRNA, which encodes a homeo-domain transcription factor [62]. Bicoid mRNA is transported along microtubules by dynein and accumulates at the anterior end of the oocyte, coincident with the minus ends of the oriented microtubules. Bicoid binds directly to the GLUE domain of Eap45/Vps36p of ESCRT-II, and fails to localize properly upon loss of any of the ESCRT-II subunits [62]. ESCRT-II has also been implicated in other microtubule-dependent processes such as proper formation of the MTOC during meiosis, which is altered in fission yeast lacking the ESCRT-II Vps22p protein [63].
Movement of endosomes along microtubules may simply place organelles near subsequent fusion partners, thus fostering the next step. For instance, overexpressing the Rab5-dependent early endosomal kinesin KIF16B shunts early endosomes towards the cell periphery and accelerates recycling while depleting KIF16B clusters endosomes around the MTOC, retards recycling and accelerates delivery to lysosomes [64]. Alternatively, microtubules may help define morphological features of endosomes that promote sorting and fusion events. For instance, phagosomes form RILP-positive tubular structures along microtubules that then fuse with lysosomes [65]. Similar microtubule-dependent tubular structures also mediate some kissing and fusion events between late endosomes and lysosomes [3]. Perhaps these tubules are enriched in fusogenic proteins such as SNAREs and various tethering factors. The connection between the microtubule system and the MVB biogenesis pathway afforded by ESCRT-II may provide a potential mechanism to ensure proper timing of lysosomal fusion such that it occurs when MVB biogenesis is complete. Further evidence suggesting that MVB formation must be complete before fusion with lysosomes comes from the observation that depletion of the mammalian ESCRT-III protein Vps24p results in accumulation of EGFR in MVBs [66].
Conclusions
The protein machinery responsible for sorting ubiquitinated membrane proteins is rapidly being identified and its molecular structure solved. However, it is not at all clear exactly which complexes move ubiquitinated membrane proteins and how many observations concerning ubiquitination of Ub sorting proteins themselves reflect bonafide physiological regulatory events. Thus, there is some way to go before it is possible to decipher which Ub-binding proteins actually recognize ubiquitinated cargo for sorting and which recognize Ub to do something else. The links between sorting machinery for ubiquitinated proteins and other machinery such as that for moving organelles along microtubules and the fusion of endosomes with lysosomes are similarly at an early stage of understanding.
Acknowledgments
Experimental work in our laboratories is supported by the Medical Research Council (G9310915) and the Wellcome Trust (079895) for JPL and by NIH R01 GM58202 for RCP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert C. Piper, Department of Physiology and Biophysics, University of Iowa, Iowa City, IA 52242, USA, Email: robert-piper@uiowa.edu
J. Paul Luzio, Cambridge Institute for Medical Research and Department of Clinical Biochemistry, University of Cambridge,Wellcome Trust/MRC Building, Addenbrooke's Hospital, Hills Road, Cambridge CB2 0XY, UK, Email: JPL10@CAM.AC.UK.
References
- 1.Piper RC, Luzio JP. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic. 2001;2:612–621. doi: 10.1034/j.1600-0854.2001.20904.x. [DOI] [PubMed] [Google Scholar]
- 2.Luzio JP, Poupon V, Lindsay MR, Mullock BM, Piper RC, Pryor PR. Membrane dynamics and the biogenesis of lysosomes. Mol Membr Biol. 2003;20:141–154. doi: 10.1080/0968768031000089546. [DOI] [PubMed] [Google Scholar]
- 3.Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr Biol. 2005;15:360–365. doi: 10.1016/j.cub.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 5.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 6.Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolling R, Hollenberg CP. The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. Embo J. 1994;13 :3261–3271. doi: 10.1002/j.1460-2075.1994.tb06627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 10.Helliwell SB, Losko S, Kaiser CA. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J Cell Biol. 2001;153:649–662. doi: 10.1083/jcb.153.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soetens O, De Craene JO, Andre B. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J Biol Chem. 2001;276:43949–43957. doi: 10.1074/jbc.M102945200. [DOI] [PubMed] [Google Scholar]
- 12.Blondel MO, Morvan J, Dupre S, Urban-Grimal D, Haguenauer-Tsapis R, Volland C. Direct sorting of the yeast uracil permease to the endosomal system is controlled by uracil binding and Rsp5p-dependent ubiquitylation. Mol Biol Cell. 2004;15:883–895. doi: 10.1091/mbc.E03-04-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reggiori F, Pelham HR. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. Embo J. 2001;20:5176–5186. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 15.Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. Embo J. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geetha T, Jiang J, Wooten MW. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell. 2005;20 :301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 18.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. ** This paper rigorously examines the correlation between ubiquitination of the EGFR with regard to its internalization and subsequent degradation in lysosomes. Substitution of most of the ubiquitination sites of the EGFR tail severely blocks lysosomal degradation without greatly impairing internalization, suggesting that only low levels of ubiquitination are required for internalization. Alternatively, EGFR may use other signals apart from Ub for internalization. Interestingly, mass spectrometry reveals that the EGFR is modified by short polyUb chains upon activation. [DOI] [PubMed] [Google Scholar]
- 19.Shih SC, Sloper-Mould KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. Embo J. 2000;19 :187–198. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reggiori F, Pelham HR. A transmembrane ubiquitin ligase required to sort membrane proteins into multivesicular bodies. Nat Cell Biol. 2002;4:117–123. doi: 10.1038/ncb743. [DOI] [PubMed] [Google Scholar]
- 21.Hurley JH, Lee S, Prag G. Ubiquitin-binding domains. Biochem J. 2006;399:361–372. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 23.Woelk T, Oldrini B, Maspero E, Confalonieri S, Cavallaro E, Di Fiore PP, Polo S. Molecular mechanisms of coupled monoubiquitination. Nat Cell Biol. 2006;8:1246–1254. doi: 10.1038/ncb1484. [DOI] [PubMed] [Google Scholar]
- 24.Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. ** This study and the study by Woelk et al. [21] detail a mechanism for the phenomenon of coupled ubiquitination, whereby association with a Ub ligase via a protein’s UIM domain renders it susceptible to ubiquitination. This study goes on to show that such ubiquitination impacts the activity of Eps15 and might represent a physiological mode of regulation. [DOI] [PubMed] [Google Scholar]
- 25.Hoeller D, Crosetto N, Blagoev B, Raiborg C, Tikkanen R, Wagner S, Kowanetz K, Breitling R, Mann M, Stenmark H, et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol. 2006;8:163–169. doi: 10.1038/ncb1354. * This study shows that ubiquitination of Ub-binding proteins can negatively impact their function presumably by inducing an intramolecular association. [DOI] [PubMed] [Google Scholar]
- 26.Yogosawa S, Kawasaki M, Wakatsuki S, Kominami E, Shiba Y, Nakayama K, Kohsaka S, Akazawa C. Monoubiquitylation of GGA3 by hVPS18 regulates its ubiquitin-binding ability. Biochem Biophys Res Commun. 2006;350:82–90. doi: 10.1016/j.bbrc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Polo S, Di Fiore PP, De Camilli PV. Rapid Ca2+-dependent decrease of protein ubiquitination at synapses. Proc Natl Acad Sci U S A. 2003;100:14908–14913. doi: 10.1073/pnas.2136625100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amit I, Yakir L, Katz M, Zwang Y, Marmor MD, Citri A, Shtiegman K, Alroy I, Tuvia S, Reiss Y, et al. Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev. 2004;18:1737–1752. doi: 10.1101/gad.294904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci. 2007;120:543–553. doi: 10.1242/jcs.03385. [DOI] [PubMed] [Google Scholar]
- 30.Hawryluk MJ, Keyel PA, Mishra SK, Watkins SC, Heuser JE, Traub LM. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 31.Barriere H, Nemes C, Lechardeur D, Khan-Mohammad M, Fruh K, Lukacs GL. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in Mammalian cells. Traffic. 2006;7:282–297. doi: 10.1111/j.1600-0854.2006.00384.x. * This study and the one by Hawryluk [30] use sets of reporter fusion proteins to demonstrate that multiple Ub must be present to serve as an efficient signal for clathrin-mediated internalization. [DOI] [PubMed] [Google Scholar]
- 32.Bache KG, Raiborg C, Mehlum A, Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem. 2003;278:12513–12521. doi: 10.1074/jbc.M210843200. [DOI] [PubMed] [Google Scholar]
- 33.Lohi O, Poussu A, Merilainen J, Kellokumpu S, Wasenius VM, Lehto VP. EAST, an epidermal growth factor receptor- and Eps15-associated protein with Src homology 3 and tyrosine-based activation motif domains. J Biol Chem. 1998;273:21408–21415. doi: 10.1074/jbc.273.33.21408. [DOI] [PubMed] [Google Scholar]
- 34.Sorkina T, Bild A, Tebar F, Sorkin A. Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors. J Cell Sci. 1999;112 ( Pt 3):317–327. doi: 10.1242/jcs.112.3.317. [DOI] [PubMed] [Google Scholar]
- 35.Pelham HR. Membrane traffic: GGAs sort ubiquitin. Curr Biol. 2004;14:R357–359. doi: 10.1016/j.cub.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Pak Y, Glowacka WK, Bruce MC, Pham N, Rotin D. Transport of LAPTM5 to lysosomes requires association with the ubiquitin ligase Nedd4, but not LAPTM5 ubiquitination. J Cell Biol. 2006;175:631–645. doi: 10.1083/jcb.200603001. * This study impliess that a Ub-dependent transport route from the TGN to endosomes may operate in mammalian cells and may be controlled by the Ub binding activity of the GGA3 adaptor proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bilodeau PS, Urbanowski JL, Winistorfer SC, Piper RC. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat Cell Biol. 2002;4:534–539. doi: 10.1038/ncb815. [DOI] [PubMed] [Google Scholar]
- 38.Winter V, Hauser MT. Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci. 2006;11:115–123. doi: 10.1016/j.tplants.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puertollano R, Bonifacino JS. Interactions of GGA3 with the ubiquitin sorting machinery. Nat Cell Biol. 2004;6:244–251. doi: 10.1038/ncb1106. [DOI] [PubMed] [Google Scholar]
- 40.Katoh Y, Imakagura H, Futatsumori M, Nakayama K. Recruitment of clathrin onto endosomes by the Tom1-Tollip complex. Biochem Biophys Res Commun. 2006;341:143–149. doi: 10.1016/j.bbrc.2005.12.156. [DOI] [PubMed] [Google Scholar]
- 41.Katoh Y, Shiba Y, Mitsuhashi H, Yanagida Y, Takatsu H, Nakayama K. Tollip and Tom1 form a complex and recruit ubiquitin-conjugated proteins onto early endosomes. J Biol Chem. 2004;279:24435–24443. doi: 10.1074/jbc.M400059200. [DOI] [PubMed] [Google Scholar]
- 42.Yamakami M, Yoshimori T, Yokosawa H. Tom1, a VHS domain-containing protein, interacts with tollip, ubiquitin, and clathrin. J Biol Chem. 2003;278:52865–52872. doi: 10.1074/jbc.M306740200. [DOI] [PubMed] [Google Scholar]
- 43.Franco M, Furstoss O, Simon V, Benistant C, Hong WJ, Roche S. The adaptor protein Tom1L1 is a negative regulator of Src mitogenic signaling induced by growth factors. Mol Cell Biol. 2006;26:1932–1947. doi: 10.1128/MCB.26.5.1932-1947.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brissoni B, Agostini L, Kropf M, Martinon F, Swoboda V, Lippens S, Everett H, Aebi N, Janssens S, Meylan E, et al. Intracellular trafficking of interleukin-1 receptor I requires Tollip. Curr Biol. 2006;16:2265–2270. doi: 10.1016/j.cub.2006.09.062. * This study implicates the Tom1-Tollip complex as a possible Ub-cargo receptor that works on a subset of proteins that include the IL-1 receptor. [DOI] [PubMed] [Google Scholar]
- 45.Sachse M, Urbe S, Oorschot V, Strous GJ, Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol Biol Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 47.Boman AL. GGA proteins: new players in the sorting game. J Cell Sci. 2001;114:3413–3418. doi: 10.1242/jcs.114.19.3413. [DOI] [PubMed] [Google Scholar]
- 48.Alam SL, Langelier C, Whitby FG, Koirala S, Robinson H, Hill CP, Sundquist WI. Structural basis for ubiquitin recognition by the human ESCRT-II EAP45 GLUE domain. Nat Struct Mol Biol. 2006;13:1029–1030. doi: 10.1038/nsmb1160. [DOI] [PubMed] [Google Scholar]
- 49.Hirano S, Suzuki N, Slagsvold T, Kawasaki M, Trambaiolo D, Kato R, Stenmark H, Wakatsuki S. Structural basis of ubiquitin recognition by mammalian Eap45 GLUE domain. Nat Struct Mol Biol. 2006;13:1031–1032. doi: 10.1038/nsmb1163. [DOI] [PubMed] [Google Scholar]
- 50.Doyotte A, Russell MR, Hopkins CR, Woodman PG. Depletion of TSG101 forms a mammalian "Class E" compartment: a multicisternal early endosome with multiple sorting defects. J Cell Sci. 2005;118:3003–3017. doi: 10.1242/jcs.02421. [DOI] [PubMed] [Google Scholar]
- 51.Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006;17:3469–3483. doi: 10.1091/mbc.E05-11-1054. * This study and the study from Doyette et al. [50] clearly document similar morphological changes in the endocytic system of animal cells upon loss of TSG101 to those that occur in yeast lacking the TSG101 homologue Vps23p (ESCRT-I). Interestingly, while similar morphological features are observed in yeast mutants lacking most of the other class E Vps proteins, this study showed significant differences between loss of TSG101 and Hrs, suggesting diversity in their respective functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, Luzio JP. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem. 2006;281:5094–5105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- 53.Langelier C, von Schwedler UK, Fisher RD, De Domenico I, White PL, Hill CP, Kaplan J, Ward D, Sundquist WI. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J Virol. 2006;80:9465–9480. doi: 10.1128/JVI.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Driskell OJ, Mironov A, Allan VJ, Woodman PG. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat Cell Biol. 2007;9:113–120. doi: 10.1038/ncb1525. [DOI] [PubMed] [Google Scholar]
- 55.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 56.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 57.Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. Embo J. 2001;20:683–693. doi: 10.1093/emboj/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Progida C, Spinosa MR, De Luca A, Bucci C. RILP interacts with the VPS22 component of the ESCRT-II complex. Biochem Biophys Res Commun. 2006;347:1074–1079. doi: 10.1016/j.bbrc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Wang T, Hong W. RILP interacts with VPS22 and VPS36 of ESCRT-II and regulates their membrane recruitment. Biochem Biophys Res Commun. 2006;350:413–423. doi: 10.1016/j.bbrc.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 60.Johansson M, Lehto M, Tanhuanpaa K, Cover TL, Olkkonen VM. The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol Biol Cell. 2005;16:5480–5492. doi: 10.1091/mbc.E05-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor {beta}lll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. ** This study nicely describes how Rab7 might recruit microtubule motors to endosomes by coordinating the activities of RILP, ORP1L and betaIII-spectrin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irion U, St Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445:554–55. doi: 10.1038/nature05503. * This study shows that the entire ESCRT-II complex is required for the polarized localization of bicoid mRNA, which is established by movement along microtubules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin Y, Mancuso JJ, Uzawa S, Cronembold D, Cande WZ. The fission yeast homolog of the human transcription factor EAP30 blocks meiotic spindle pole body amplification. Dev Cell. 2005;9:63–73. doi: 10.1016/j.devcel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 64.Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. ** This study underscores the dual function of endosomes to carry out recycling of some proteins and degradation of others. This study shows that blocking the ability of endosomes to associate with a kinesin simultaneously delayed recycling while stimulating the degradation pathway. The study reveals a dynamic interplay between endosome motility and the regulation of recycling and degradative pathways. [DOI] [PubMed] [Google Scholar]
- 65.Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol Cell Biol. 2003;23:6494–6506. doi: 10.1128/MCB.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bache KG, Stuffers S, Malerod L, Slagsvold T, Raiborg C, Lechardeur D, Walchli S, Lukacs GL, Brech A, Stenmark H. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol Biol Cell. 2006;17:2513–2523. doi: 10.1091/mbc.E05-10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]


