Abstract
Background and purpose:
Protein synthesis-dependent late-long term potentiation (L-LTP) is an enduring form of synaptic plasticity that has been shown to rely on, at least partly, protein synthesis at synaptic and/or dendritic sites. Evidence suggests that somatic transcription of new mRNAs may provide a significant contribution to the availability of mRNAs at synaptic sites where they are made available for dendritic translation. Transport of mRNAs from somatic to dendritic sites might be expected to involve movement along a microtubule network. In this study we examined whether it was possible to maintain L-LTP in hippocampal slices with destabilized microtubule networks.
Experimental approach:
Extracellular field excitatory postsynaptic potentials (fEPSPs) were recorded from rat hippocampal slices and following a period of baseline recording, stimuli were given that induced LTP. LTP was monitored for 5 h in both control slices and slices treated with vincristine to depolymerize tubulin.
Key results:
L-LTP was induced and maintained in vincristine-treated slices. Four hours after tetanic stimulation fEPSPs were 196±19% of baseline values. The magnitude of potentiation was similar to that seen in untreated slices (175±15%). L-LTP in vincristine-treated slices was, however, not maintained in the presence of the protein synthesis inhibitor, rapamycin. Immunohistochemistry and confocal microscopy of vincristine-treated slices verified that the microtubule network had been destabilized.
Conclusions and Implications:
Communication between somatic and synaptic sites through protein and/or mRNA trafficking via an intact microtubule network is not required for protein synthesis dependent L-LTP.
Keywords: dendrite, field potential, hippocampus, LTP, microtubule, mRNA, NMDA receptor, protein synthesis, synaptic plasticity, vincristine
Introduction
Alterations in synaptic efficacy are thought to underpin the formation and storage of new memories. Long-term potentiation (LTP) is the most widely studied cellular model for changes in synaptic strength that might underlie the process of memory acquisition and maintenance. If LTP or related phenomena underpin memory formation, it is to be expected that processes involved in bringing about changes in synaptic strength are such that they can persist for as long as is necessary for permanent change in synaptic connections to be established. Studies have shown that LTP can persist for many hours in vitro (Schwartzkroin and Wester, 1975; Frey et al., 1988) and for several weeks and even months in vivo (Bliss and Gardner-Medwin, 1973; Abraham, 2003). It is now widely accepted that the phenomenon of NMDA receptor-dependent LTP possesses both protein synthesis-independent and protein synthesis-dependent components. Thus early (E-) LTP, lasting up to 2 h, is not dependent on de novo protein synthesis, whereas late (L-) LTP is defined by its sensitivity to inhibitors of protein synthesis (for reviews see Kelleher et al., 2004b; Lynch, 2004). Recent studies have shown that the protein synthesis required for the maintenance of L-LTP in vitro can be derived from the translation of pre-existing dendritic mRNAs (Bradshaw et al., 2003; Tsokas et al., 2005; Vickers et al., 2005). Indeed, mRNAs have been identified in dendrites and synaptic locations (Steward and Levy, 1982; Steward and Fass, 1983; Bagni et al., 2000), although the identification of the specific mRNAs and proteins involved in L-LTP remains to be determined.
Proteins and dendritic mRNAs can move throughout cells attached to kinesin motors, which travel along a network of microtubules, which are in turn composed of polymerized tubulin (Bassell et al., 1994; Setou et al., 2002; Hirokawa, 2006). Specifically, cis-acting sequences on mRNAs are recognized by RNA-binding proteins (RBP), which can then bind the mRNAs to the cytoskeletal network for transport (Zhang et al., 2001). An example of such an RBP is zipcode-binding protein 1 (ZBP1) which has been demonstrated to bind to β-actin mRNA and regulate its activity-dependent location to dendritic sites in neurons (Eom et al., 2003). The majority of transport throughout dendrites is along microtubule networks composed of tubulin, whereas at dendritic spines, the cytoskeleton becomes more actin dense and is comprised of a microfilamentous network.
Many hypotheses on the role and mechanisms of protein synthesis in synaptic plasticity and in particular, L-LTP, suggest that trafficking of proteins and mRNAs from the soma and distal dendritic sites to the activated synapses provides the pool of new proteins necessary for the maintenance of L-LTP (Kelleher et al., 2004b). There is also evidence that there is a readily available pool of dendritic mRNAs and translation machinery local to activated synapses that could provide the locus for de novo protein synthesis (Sutton and Schuman, 2006). In this study, using the microtubule-destabilizing drug vincristine, we investigated whether an intact microtubule network that could provide a pathway for the transport of mRNAs to synaptic sites for local translation was necessary for the maintenance of the protein synthesis-dependent phase of L-LTP. Our data show that application of vincristine to hippocampal slices does not alter baseline synaptic transmission and that L-LTP can be maintained in slices with destabilized microtubule networks. These results imply that transport of proteins and/or mRNAs along such networks is not required for the maintenance of L-LTP in the in vitro preparation.
Materials and methods
Preparation of slices
Male Wistar rats (6–12 weeks old) were decapitated under halothane anaesthesia (in accordance with current UK Home Office procedures), their brains removed rapidly and placed in ice-cold (2–4°C) recording solution (mM: NaCl 120, KCl 2.5, MgSO4 1.3, CaCl2 2.5, NaH2PO4 1, NaHCO3 26 and glucose 11), gassed with 95% O2 and 5% CO2. The hippocampus was isolated and transverse slices (400 μm thick) were prepared using a tissue slicer (Stoelting, Wood Dale, IL, USA). Slices were incubated in recording solution for 1 h before being transferred to an interface humidified chamber (FST, Heidelberg, Germany) where they were perfused, at a flow rate of 3 ml min−1, with continuously gassed recording solution. All experiments were carried out at 33°C.
Electrophysiological recording of field potentials
Field excitatory postsynaptic potentials (fEPSPs) were recorded via an electrode placed in the stratum radiatum. Recording electrodes were made from thick-walled borosilicate glass, filled with the same extracellular recording solution as detailed above and had resistances of ∼2 MΩ. Two tungsten bipolar electrodes were placed either side of the recording electrode to evoke fEPSPs in two independent pathways. The stimulus intensities used to evoke fEPSPs ranged from 30 to 150 μA. The S1 pathway was used to apply the tetanus stimulation to evoke LTP, while the S2 pathway acted as a control in order that we could assess the stability of responses during prolonged periods of recording. Constant current stimuli were used to evoke fEPSPs whose slopes were approximately one-third of the magnitude of those evoked by a maximal stimulus. S1 and S2 pathways were stimulated, alternately, at a frequency of 0.033 Hz. Two pulses, 50 ms apart, were applied to measure paired-pulse facilitation (PPF) ratios before and after the induction of LTP. fEPSPs were amplified via an EXT-102F amplifier (NPI, Tamm, Germany). The LTP Program (Anderson and Collingridge, 2001) was used to display fEPSPs and allow the monitoring of online measurements of the slopes of fEPSPs. Subsequent off-line analysis was carried out to provide an accurate measure of these slopes. Before evoking LTP, stable baselines were recorded for at least 1 h. LTP was induced by the application of three high-frequency trains of stimuli 5 min apart, each comprising a single 1 s 100 Hz tetanus. The assignment of S1 and S2 pathways, relative to the position of the recording electrode, was changed regularly to ensure that there was no bias in attempting to induce LTP in only one particular recording configuration. Several criteria had to be satisfied in order for an experiment to be included. Baseline recordings of fEPSPs from both S1 and S2 pathways had to be stable for at least 50 min before the application of the tetanus, and any slice that showed a >>10% change in the slope of the fEPSP in either pathway was discarded from further analysis. In addition, following the application of the tetanic pulses to the S1 pathway, the slope of fEPSPs in the control (S2) pathway was not allowed to drift by more than 15% in any 60 min recording period. Finally, LTP was deemed to have been established if the slopes of fEPSPs in the S1 pathway were at least 150% of baseline values 60 min post tetanus. In our recordings of L-LTP in non-drug-treated slices, we monitored fEPSPs for at least 5 h following the application of the tetanus. Vincristine and rapamycin (Sigma, Poole, UK) were dissolved in dimethylsulphoxide (DMSO), the final volume of which did not exceed 1% of the recording solution. This concentration of DMSO did not affect the duration or magnitude of L-LTP. Both drugs were applied 1 h before the delivery of the L-LTP-inducing tetanus and were present for the duration of the experiment.
Immunohistochemistry
Hippocampal slices were prepared as described above and then incubated in extracellular recording solution in the absence or presence of vincristine (5 μM) for 2 h before being submerged overnight in fixative (4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB), pH 7.4). Transverse sections (30 μm) were then cut on a freezing microtome (Leica, Wetzlar, Germany). Sections were then washed in phosphate-buffered saline (PBS), before being permeabilized and quenched in 50 mM NH4Cl and 0.2% (w/v) saponin in PBS. Sections were washed in PBS before being incubated for 1 h in a 1:1000 dilution of an antibody against α-tubulin (DMA clone; Upstate, Hampshire, UK) in solution A (0.2% (w/v) gelatin, 0.02% (w/v) saponin, 0.02% (w/v) NaN3 in PBS). Sections were washed with solution A and incubated with a secondary antibody (Cy2 goat anti-mouse IgG; Jackson ImmunoResearch, West Grove, PA, USA) diluted 1:500 in solution A for 1 h. Sections were then washed again in PBS before being incubated in a 1 mM solution of TOPRO-3 (Molecular Probes, Eugene, OR, USA) for 10 min. Sections were then mounted onto glass slides in Mowoil (Calbiochem, Nottingham, UK, 40% in glycerol) and coverslipped. Slides were stored at 4°C until analysis. Labelling was visualized using laser scanning confocal microscope (Biorad Radiance 2000) with × 40 oil-immersion objective. Confocal z-series were merged using Biorad LaserSharp and Adobe Photoshop software.
Statistical analysis
Results are presented as mean±s.e.m. and statistical comparison between data sets was assessed using paired t-tests. Origin 6.0 software was used for graphical presentation.
Results
Application of the microtubule-destabilizing drug vincristine disrupts α-tubulin organization in rat hippocampal CA1 pyramidal cell dendrites.
To establish that we were able to depolymerize the microtubule network in CA1 pyramidal neurons, we performed confocal image analysis of vincristine-treated and untreated control hippocampal slices. Figure 1a shows a confocal image of a control slice where labelling of α-tubulin is shown in green and the labelling of CA1 pyramidal cell nuclei by TOPRO-3 is indicated in blue. Figure 1b shows a similar image but taken from a hippocampal slice that has been treated with the microtubule-destabilizing drug vincristine (5 μM). Comparison of these two images shows that α-tubulin staining is more diffuse in the vincristine-treated slice when compared to the staining seen in the control slice. Indeed in the control slices, the α-tubulin shows a more organized distribution, whereas in the vincristine-treated slice the α-tubulin labelling is more dispersed and is consistent with the ‘particle effect' seen when neurons are treated with this toxin (Weber et al., 1975; Allison et al., 2000). Moreover, if one compares the cell bodies of CA1 pyramidal neurons in control and vincristine-treated slices, there is evidence of ‘pooling' of α-tubulin label in the drug-treated slices that is absent in control slices. The results illustrated in Figure 1 are typical of those seen in a series of similar experiments (n=6 in each group) and demonstrate that application of vincristine (5 μM) under these conditions is sufficient to induce depolymerization of α-tubulin molecules and results in the dispersion of the microtubule network in CA1 pyramidal neuron dendrites.
Figure 1.
Incubation of acute hippocampal slices in vincristine destabilizes the microtubule network in CA1 pyramidal cells. (a) Immunofluorescent labelling of the CA1 region of the hippocampus with α-tubulin (green) co-stained with TOPRO-3 (blue) in a ‘control' (untreated slice). (b) Immunofluorescent labelling of the CA1 region of the hippocampus with α-tubulin co-stained with TOPRO-3 in a slice exposed to vincristine (5 μM). Note that the α-tubulin staining is more diffuse throughout the section in (b) compared to that seen in (a). The boxed areas in (a) and (b) highlight the fact that in (b) there is diffuse pooling of α-tubulin around the cell soma, whereas in (a) there is a lack of α-tubulin staining. Scale bar in (a) and (b) is 50 μm. (c) Comparison of input–output curves for vincristine-treated and control slices. No significant differences were observed over the stimulus intensities examined.
As a test of the electrophysiological integrity of hippocampal slices following vincristine treatment, we compared input–output curves for control and drug-treated slices (n=5 in each group). Figure 1c illustrates pooled data from these experiments and shows that treatment of slices with vincristine did not alter significantly input–output relationships compared to untreated slices.
Induction and maintenance of L-LTP is not affected by destabilization of the microtubule network
We next examined the effect of destabilizing the microtubule network on the ability to induce and maintain L-LTP. Figure 2a shows examples of fEPSP waveforms recorded from an experiment where a hippocampal slice had been treated with vincristine. Vincristine was applied 1 h before tetanus and remained in the bath for the duration of the experiment. The slope (and amplitude) of the fEPSP is increased, compared to baseline levels, 4 h after the delivery of the tetanus to the S1 pathway. Example, fEPSP waveforms recorded from the control (S2) pathway do not show any significant change before and after tetanic stimulation. Figure 2b shows pooled data from a number of such L-LTP experiments and demonstrates clearly that L-LTP can be established in vincristine-treated slices (n=10) and that the magnitude of the potentiation seen is not different from that observed in control slices (n=9). Thus at 1 h following the application of the tetanus, vincristine-treated slices exhibited potentiation of 215±20% compared to baseline (pre-tetanus) levels, whereas control slices showed 216±10% potentiation. Four hours after the application of the tetanus, vincristine-treated slices exhibited potentiation of 196±19%, which was not significantly different from the 175±15% potentiation observed in control slices. Potentiation of fEPSPs was observed in all slices for at least 5 h. The levels of potentiation seen in the present series of experiments are comparable with that reported in a previous study (Vickers et al., 2005). As described above, for all L-LTP experiments, we monitored a second ‘non-tetanized' pathway (S2) in our recordings to ensure that during prolonged recording periods there was no generalized drift in the amplitude of fEPSPs. For both control and vincristine-treated slices, these S2 pathways remained stable for both conditions and gave mean fEPSP slopes of 97±8% (1 h) and 95±11% (4 h) for control slices and values of 109±8% (1 h) and 95±11% (4 h) for vincristine-treated slices.
Figure 2.
L-LTP can be induced and maintained in hippocampal slices with destabilized microtubule networks. (a) Upper panel, typical fEPSP waveforms (average of five consecutive samples) recorded in the S1 pathway before and 4 h after the application of the LTP-inducing tetanus. Lower panel, typical fEPSP waveforms (average of five consecutive samples) recorded in the S2 (non-tetanized) pathway at similar time points shown in the upper traces. (b) Pooled data showing the time course of L-LTP induced in vincristine-treated (n=10) and control (n=9) hippocampal slices. The extent and magnitude of L-LTP is similar for both groups. Non-tetanized (S2) pathways in both vincristine-treated and control slices show no significant increase in fEPSP slopes during these recordings. (c) Comparison of PPF ratios recorded before and 1 and 4 h after the application of the LTP-inducing tetanus in vincristine-treated and control slices. There is no significant difference in the PPF ratios at the different time points shown or between the two groups. fEPSP, field excitatory postsynaptic potential; L-LTP, late-long term potentiation; PPF, paired-pulse facilitation.
Following the induction of NMDA receptor-dependent LTP in the CA1 region, PPF ratios did not change (see for example Manabe et al., 1993). We measured PPF ratios before and 1 and 4 h following the induction of LTP in both control and vincristine-treated slices. No significant changes in PPF ratios were observed (Figure 2c). For control slices, PPF ratios for baseline and 1 and 4 h post tetanus were 1.4±0.1, 1.5±0.1 and 1.4±0.1, respectively. The corresponding values for vincristine-treated slices were 1.3±0.1, 1.4±0.1 and 1.6±0.2.
L-LTP in the presence of vincristine is dependent on protein synthesis
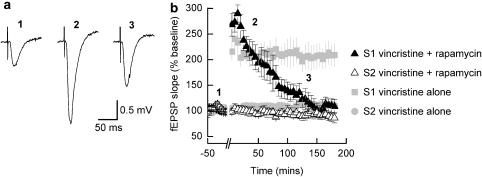
In a previous publication (Vickers et al., 2005), we have demonstrated that under our recording conditions, protein synthesis-dependent LTP is evident within 2 h following the application of a tetanus. Thus given that L-LTP lasting for at least 5 h was observed in vincristine-treated slices, one might assume that protein synthesis-dependent L-LTP is not inhibited in slices where the microtubule network has been destabilized. Nevertheless, application of vincristine might cause changes in the dependency of L-LTP for de novo protein synthesis that could result in long-lasting potentiation being obtained which was insensitive to protein synthesis inhibition. However, as is illustrated in Figure 3, L-LTP in vincristine-treated slices is inhibited when recordings are made in the presence of the mRNA translation inhibitor rapamycin (1 μM; n=5). In this set of experiments, rapamycin was applied 1 h before the delivery of the tetanus and remained present throughout the duration of the experiment. Figure 3a shows examples of average fEPSP waveforms recorded during the baseline period, around the peak of the potentiation and 2 h after tetanic stimulation. Thus, although the initial potentiation (1 h following the application of the tetanus) seen in slices treated with vincristine and rapamycin is 190±28%, which is comparable to that seen in slices treated with vincristine only (shown as grey symbols and reproduced from Figure 2a), the extent of the potentiation wanes. Indeed this potentiation is not maintained and at 3 h following the application of the tetanus, the size of fEPSPs has decayed to near baseline levels (109±14%; Figure 3b). This profile for rapamycin-induced inhibition of L-LTP in vincristine-treated slices is similar to our previous study (Vickers et al., 2005).
Figure 3.
L-LTP in vincristine-treated slices is dependent on protein synthesis. (a) Typical fEPSP waveforms (average of five consecutive traces) recorded in the S1 pathway before the delivery of the tetanus (trace 1), around the peak of the potentiation (trace 2) and 2 h following the tetanus (trace 3). (b) Pooled data (n=5) showing the time course of potentiation of fEPSPs in vincristine-treated slices in the presence of rapamycin (1 μM). After an initial large potentiation, slope values of fEPSPs decay to near baseline levels within 3 h. The non-tetanized (S2) pathway remains stable throughout the recording period indicating that the decrease in the fEPSPs seen in the S1 pathway is not due to a deterioration in the slice preparation when exposed to the protein synthesis inhibitor. For comparison, the magnitudes and time courses of L-LTP obtained in slices treated with vincristine only are reproduced from Figure 2b. fEPSP, field excitatory postsynaptic potential; L-LTP, late-long term potentiation.
Discussion
In this study, we have demonstrated that the induction and maintenance of protein synthesis-dependent L-LTP in acute CA1 hippocampal slices does not require an intact microtubule network. Furthermore, we show that L-LTP in the presence of vincristine is protein synthesis dependent. These data suggest that trafficking of mRNAs from either somatic or dendritic sites distal to activated synapses, via an intact microtubule network, is not necessary for the de novo protein synthesis required for long-lasting forms of synaptic plasticity in in vitro hippocampal slice preparations.
Understanding the mechanisms underlying protein synthesis in LTP is crucial if we are to identify the molecular and structural changes involved in these persistent changes in synaptic efficacy, which may also underpin processes involved in the formation and consolidation of memory. Indeed, disruption in the translation of mRNAs has been shown to be involved in the manifestation of disorders such as fragile X syndrome, whereby mutations on the FMRP gene result in altered protein synthesis and trafficking. Recent research has suggested that this translation deficiency has a significant dendritic component (for review see Bagni and Greenough, 2005). Thus, the molecular mechanisms of enduring synaptic modification could provide important advances in the understanding of such disorders.
Studies have shown that the translation of pre-existing dendritically located mRNAs plays an important role in the protein synthesis involved in LTP that persists for several hours (Tsokas et al., 2005; Vickers et al., 2005). Moreover, many in vivo studies have reported that the synthesis of new proteins is required for the maintenance of long-lasting changes in synaptic strength at the cellular level and for the consolidation of memory at the systems level (Frey et al., 1996; Kelleher et al., 2004a; Morris et al., 2006).
Over time, somatic transcription of new mRNAs would be expected to contribute to the ongoing protein synthesis, but the delineation between the two mechanisms – local translation of pre-existing dendritic mRNAs and somatic transcription and/or transport – is important if we are to advance our understanding of the mechanisms of synaptic modification. Direct evidence that mRNAs and translational machinery local to specific synaptic sites can provide the new proteins to facilitate lasting synaptic potentiation has been difficult to obtain. Elegant studies in culture have shown that the RBP, ZBP1, can bind to β-actin mRNA and regulate its transport to distal dendritic sites in an activity-dependent manner, and studies in synaptosome preparations have shown activity-dependent protein synthesis (Bagni et al., 2000; Tiruchinapalli et al., 2003; Huttelmaier et al., 2005). However, direct evidence for translation at synaptic sites in the in vivo or in vitro preparation has not been possible to obtain. A recent study has shown an increase in the size and number of polyribosomes in dendritic spines after the induction of LTP (Bourne et al., 2007). Moreover, this study showed that the increase in polyribosomes correlated with the increase in the size of specific postsynaptic densities, 2 h after LTP induction. The data we report in this study are consistent with a ‘spine-delimited' process where the translation of pre-existing (and locally available) mRNAs is sufficient to allow increases in synaptic strength that last several hours.
Studies of protein and mRNA trafficking along microtubule networks have provided evidence that proteins bind to tubulin via chaperone proteins and kinesins, and that these complexes can travel along dendritic and axonal processes to specific destinations. Kinesins such as KIF1 and KIF17 have been shown to bind, albeit indirectly, to AMPA receptors and NMDA receptors respectively and facilitate their trafficking through the cytoplasm (Setou et al., 2000, 2002; Kim and Lisman, 2001). Direct evidence for the dendritic transport of mRNAs via molecular motors has come from the isolation of an RNAase-sensitive granule as a binding partner for KIF5. This granule contained mRNAs for the proteins CAMKII and Arc, both of which had previously been identified as mRNAs important in local protein synthesis and synaptic plasticity (Kanai et al., 2004). Thus, there is evidence that dendritic trafficking of proteins and mRNAs is a potential mechanism for alteration of the protein complement at synaptic sites that is necessary for enduring forms of synaptic plasticity and perhaps for the consolidation of memory. Our data suggest that the trafficking of mRNAs along such networks is not necessary for the early stages of L-LTP and that these events may become important when L-LTP persists such that it is sensitive to transcriptional inhibition (in our studies, at least 5–6 h post tetanus; see Vickers et al., 2005).
Previous studies have shown that vincristine, at the concentrations used in the present study, efficiently destabilizes microtubule networks. Intriguingly, one study examining the components of the postsynaptic density, including NMDA receptor subunits and scaffolding proteins, found that destabilization of the microtubule network had no effect on the composition of dendritic spines and the postsynaptic density in cultured neurons (Allison et al., 2000). These data suggest several important possibilities. First, it provides evidence that the application of the drug vincristine had no effect on the composition of the synapses in our preparation. Second, it suggests that local newly synthesized proteins in the presence of vincristine will not be prevented from accessing the postsynaptic density or surrounding sites.
A recent study has examined the role of another important cytoskeletal protein, actin, in the induction and maintenance of L-LTP (Kelly et al., 2007). The authors found that in the presence of the actin-destabilizing agent lantrunculin B, LTP was not induced with field potentials returning to near baseline within 60 min. However, application of the inhibitor after the induction of LTP had no effect on the maintenance of LTP up to 1 h after application. These data suggest that the actin-rich cytoskeleton, most likely that seen in dendritic spines, is vital for the synaptic changes involved in the induction and immediate expression of LTP, but that once established, is not required for maintenance in its protein synthesis-dependent phase. We hypothesize that the cellular mechanisms involved in the maintenance of L-LTP in the acute slice preparation can be orchestrated from mRNAs and translation machinery located proximal to the synaptic sites involved, and that it is only as the potentiation persists that recruitment of new RNAs from somatic sources and possibly distal dendritic sites is required.
In conclusion, our study has shown that in the in vitro hippocampal slice preparation, the protein synthesis required for the maintenance of synaptic plasticity is not dependent on mRNA trafficking from somatic or distal dendritic sites, and that local protein synthesis, possibly directly beneath the activated synapse, is sufficient to establish long-lasting changes in synaptic efficacy.
Acknowledgments
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council (15/C19143). We thank Dr Paul Skehel for helpful scientific discussion and Dr Tom Wishart for assistance in confocal microscopy techniques.
Abbreviations
- fEPSP
field excitatory postsynaptic potential
- L-LTP
late-long term potentiation
- RBP
RNA-binding protein
- ZBP1
zipcode-binding protein 1
Conflict of interest
The authors state no conflict of interest.
References
- Abraham WC. How long will long-term potentiation last. Philos Trans R Soc London B. 2003;358:735–744. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DW, Chervin AS, Gelfand VI, Craig AM. Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules. J Neurosci. 2000;20:4545–4554. doi: 10.1523/JNEUROSCI.20-12-04545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Bagni C, Mannucci L, Dotti CG, Amaldi F. Chemical stimulation of synaptosomes modulates alpha -Ca2+/calmodulin-dependent protein kinase II mRNA association to polysomes. J Neurosci. 2000;20:RC76. doi: 10.1523/JNEUROSCI.20-10-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Singer RH, Kosik KS. Association of poly(A) mRNA with microtubules in cultured neurons. Neuron. 1994;12:571–582. doi: 10.1016/0896-6273(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Sorra KE, Hurlburt J, Harris KM. Polyribosomes are increased in spines of CA1 dendrites 2 h after the induction of LTP in mature rat hippocampal slices. Hippocampus. 2007;17:1–4. doi: 10.1002/hipo.20238. [DOI] [PubMed] [Google Scholar]
- Bradshaw KD, Emptage NJ, Bliss TV. A role for dendritic protein synthesis in hippocampal late LTP. Eur J Neurosci. 2003;18:3150–3152. doi: 10.1111/j.1460-9568.2003.03054.x. [DOI] [PubMed] [Google Scholar]
- Eom T, Antar LN, Singer RH, Bassell GJ. Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J Neurosci. 2003;23:10433–10444. doi: 10.1523/JNEUROSCI.23-32-10433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Frey S, Schollmeier F, Krug M. Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol. 1996;490:703–711. doi: 10.1113/jphysiol.1996.sp021179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. mRNA transport in dendrites: RNA granules, motors, and tracks. J Neurosci. 2006;26:7139–7142. doi: 10.1523/JNEUROSCI.1821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, et al. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438:512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Kelleher III RJ, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004a;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kelleher III RJ, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004b;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kelly MT, Yao Y, Sondhi R, Sacktor TC. Actin polymerization regulates the synthesis of PKMzeta in LTP. Neuropharmacology. 2007;52:41–45. doi: 10.1016/j.neuropharm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lisman JE. A labile component of AMPA receptor-mediated synaptic transmission is dependent on microtubule motors, actin, and N-ethylmaleimide-sensitive factor. J Neurosci. 2001;21:4188–4194. doi: 10.1523/JNEUROSCI.21-12-04188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, et al. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Wester K. Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Res. 1975;89:107–119. doi: 10.1016/0006-8993(75)90138-9. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, et al. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Steward O, Fass B. Polyribosomes associated with dendritic spines in the denervated dentate gyrus: evidence for local regulation of protein synthesis during reinnervation. Prog Brain Res. 1983;58:131–136. doi: 10.1016/S0079-6123(08)60013-8. [DOI] [PubMed] [Google Scholar]
- Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Tiruchinapalli DM, Oleynikov Y, Kelic S, Shenoy SM, Hartley A, Stanton PK, et al. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci. 2003;23:3251–3261. doi: 10.1523/JNEUROSCI.23-08-03251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, et al. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CA, Dickson KS, Wyllie DJ. Induction and maintenance of late-phase long-term potentiation in isolated dendrites of rat hippocampal CA1 pyramidal neurones. J Physiol. 2005;568:803–813. doi: 10.1113/jphysiol.2005.092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K, Bibring T, Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975;95:111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Eom T, Oleynikov Y, Shenoy SM, Liebelt DA, Dictenberg JB, et al. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31:261–275. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]