Summary
As acute infections resolve, effector CD8 T cells differentiate into IL-7Rlo short-lived effector cells (SLECs) and IL-7Rhi memory precursor effector cells (MPECs) capable of generating long-lived memory CD8 T cells. Using another SLEC marker, KLRG1, we found that KLRG1hi effector cells began appearing early during infection and were committed to downregulating IL-7R. Unlike IL-7Rhi MPECs, KLRG1hi IL-7Rlo SLECs relied on IL-15, but IL-15 could not sustain their long-term maintenance or homeostatic turnover. The SLEC/MPEC fate decision was regulated by the amount of inflammatory cytokines (i.e. IL-12) present during T cell priming. According to the amount of inflammation, a gradient of T-bet was created in which high T-bet levels induced SLECs and low levels promoted MPECs. These results elucidate a novel mechanism by which the innate immune system sets the relative levels of a lineage-determining transcription factor in activated CD8 T cells and correspondingly, regulates their memory cell potential.
Introduction
In response to acute viral or bacterial infections, antigen-specific CD8 T cells rapidly expand and differentiate into effector cells to help clear infection. Subsequently most effector cells die, leaving behind a few memory CD8 T cells that protect from reinfection. Conceptually, effector CD8 T cells can be divided into at least two subsets, memory precursor effector cells (MPECs) that can become long-lived memory CD8 T cells and short-lived effector cells (SLECs) that do not. Currently, it is not well known when during their differentiation the effector cells make the critical decision to become MPECs or SLECs or what factors control this process.
Historically, identification of MPECs and SLECs within the effector CD8 T cell population has proven difficult because most of the T cell attributes first studied (e.g. CD44hi and CD11ahi) were acquired uniformly by effector CD8 T cells (Dutton et al., 1998). However, recent analyses have found subsets of CD8 T cells that differentially express the IL-7 receptor alpha-chain (IL-7R), L-selectin (CD62L), CCR7, Killer cell lectin-like receptor G1 (KLRG1), CD27/28 and others, that depending on the attribute examined, differ according to their localization, effector functions or potential to become protective memory CD8 T cells (de Bree et al., 2005; Huster et al., 2004; Kaech et al., 2003; Sallusto et al., 1999; Schluns et al., 2000; Voehringer et al., 2001; Wherry et al., 2003). Nevertheless, a greater understanding of how these different subsets form during infection is needed.
Recent work identified IL-7R as a marker of MPECs following acute infections because at the peak of effector CD8 T cell expansion, ∼5−20% of antigen-specific CD8 T cells expressed IL-7R (referred to as IL-7Rhi) and had a significantly greater potential to form memory CD8 T cells when compared to IL-7Rlo effector CD8 T cells (Huster et al., 2004; Kaech et al., 2003; Schluns et al., 2000). This finding raises two pertinent questions: when during infection do the activated CD8 T cells commit to becoming IL-7Rhi MPECs or IL-7Rlo SLECs and what signals regulate this decision?
An effector CD8 T cell will see many signals during infection that could affect its gene expression and longevity. Some signals that can influence this process are the strength/duration of antigenic stimulation, costimulation, CD4 T cell help and inflammatory cytokines (e.g., IL-12, IFNα/β, IFNγ) (Bachmann et al., 2004; Badovinac and Harty, 2006; Khanolkar et al., 2004; Kolumam et al., 2005; Lang et al., 2005; Mescher et al., 2006; Wherry et al., 2003). Due to spatial and temporal differences in antigen load and local cytokine milieus, it is likely that two individual effector CD8 T cells will be exposed to unique sets of signals and this will lead them to differentiate to varying degrees or along separate cell lineages. Similarly, in the specification of developing CD4 T cells, differential exposure to cytokines and antigenic stimuli can instruct cell fate decisions towards TH1, TH2, TH17 or Treg lineages (Weaver et al., 2006). Because of their profound effects, these lineage-determining cytokines are now referred to as signal 3 during T cell priming (Mescher et al., 2006).
This study investigated how and when IL-7Rhi MPECs and IL-7Rlo SLECs formed during a primary immune response to an acute viral infection. We found that the NK cell inhibitory receptor KLRG1 can serve as an early indicator of effector CD8 T cells committed to adopting an SLEC fate. Moreover, a critical determinant of the SLEC/MPEC fate decision was the level of inflammation the CD8 T cells were exposed to at the time of priming, and in particular IL-12 was an instructive signal in this process. IL-12 could modulate the expression of the transcription factor T-bet (tbx21) in a dose-dependent manner and, in accordance, we identified that the relative levels of T-bet regulated the SLEC/MPEC fate decision. High levels of T-bet induced KLRG1hi IL-7Rlo SLECs, but lower levels promoted the development of KLRG1lo IL-7Rhi MPECs. Our data presented here detail a novel model of how inflammatory cytokines, through a gradient of T-bet expression, regulate the formation of memory CD8 T cells.
Results
KLRG1 is a marker of short-lived effector CD8 T cells
To understand how MPECs and SLECs formed and acquired different cell fates during acute viral infection, we transferred ∼1×104 Thy1.1+ naïve P14 (LCMV GP33−41 specific) TCR transgenic (tg) CD8 T cells into naïve wt recipients to make “P14 chimeric mice” that were subsequently infected with LCMV. Next, we compared day 7 IL-7Rhi and IL-7Rlo P14 effector CD8 T cells using Affymetrix GeneChips. Of the ∼400 genes that differed significantly between IL-7Rhi and IL-7Rlo effector CD8 T cells, the expression of several NK cell receptors (KLRG1, Ly49c, KLRE1, 2B4 and Ly49h) was increased in IL-7Rlo SLECs (Figure 1A). However, this phenotype did not extend to other NK cell receptors such as NKG2A/C, NKG2D and CD94 (data not shown). We focused on KLRG1 because of the large differential in its mRNA and protein expression (Figure 1A, B). Moreover, previous reports showed that KLRG1 was a marker of “terminally differentiated” mature NK cells, CD8 and CD4 T effector memory (TEM) and mast cells (Chtanova et al., 2005; Kaech et al., 2003; Ortega et al., 1991; Robbins et al., 2005). It is possible that KLRG1 expression may indicate a common program of terminal-differentiation in these cell types.
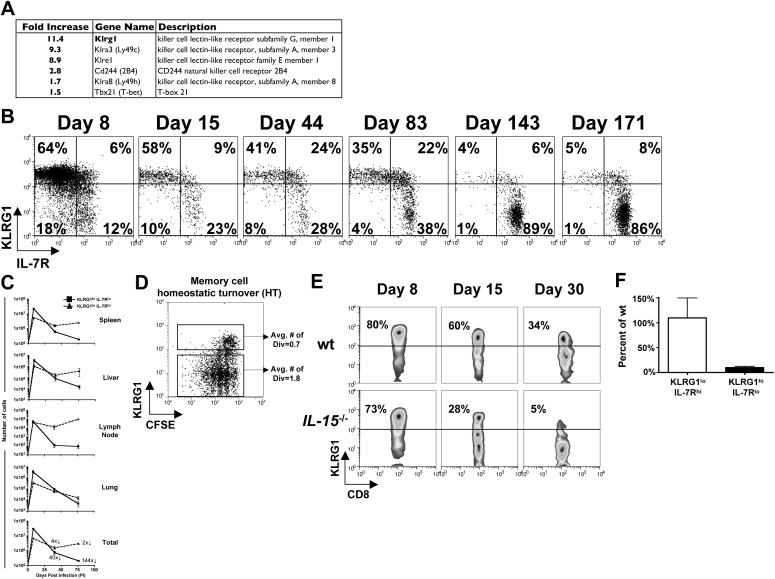
Figure 1.
KLRG1hi IL-7Rlo effector CD8 T cells are short-lived and require IL-15 for survival.
(A) IL-7Rhi and IL-7Rlo Thy1.1+ effector CD8 T cells from P14 chimeric mice were compared on day 7 of LCMV infection using Affymetrix GeneChips. Table shows the fold increase in expression for selected NK receptors (IL-7Rlo > IL-7Rhi cells).
(B and C) Analysis of MPEC and SLEC subsets following LCMV infection of P14 chimeric mice. (B) Plots are gated on Thy1.1+ P14 T cells and show expression of KLRG1 and IL-7R in blood over time. (C) Line graphs show KLRG1hi IL-7Rlo (■) and KLRG1lo IL-7Rhi (▲) P14 CD8 T cell numbers in the spleen, liver, lung, inguinal lymph node (LN) and total from all tissues. The magnitude of contraction between days 8−40 and 8−75 is indicated.
(D) CFSE-labeled P14 memory CD8 T cells from day ∼40 pi were transferred into naïve mice and then analyzed for CFSE and KLRG1 expression 4−6 weeks later.
(E) wt and IL-15−/− mice were infected with LCMV and Db GP33−41 MHC class I tetramer+ CD8 T cells were analyzed for KLRG1 expression 8, 15 and 30 days pi. Similar data were observed for NP396−404-specific CD8 T cells (data not shown).
(F) KLRG1hi IL-7Rlo or KLRG1lo IL-7Rhi P14 CD8 T cells were sorted day 8 pi and transferred in equal numbers into wt or IL-15−/− recipients for 10−15 days. Bar graph shows the number of donor cells recovered from IL-15−/− recipients normalized to the number recovered from wt recipients.
We examined day 8 LCMV-specific endogenous and P14 CD8 T cells in the PBL, spleen, liver, lung, and inguinal lymph node (LN) and found mostly KLRG1hi IL-7Rlo effector CD8 T cells (Figures 1B, S1, and S3 and data not shown). During “the contraction phase” (days 8−30 post infection, pi), KLRG1hi IL-7Rlo cells sharply declined in number (∼40 fold) and continued to gradually decay thereafter (t1/2 ∼65−80 days, Figures 1C and S1). In contrast, the magnitude of KLRG1lo IL-7Rhi effector cell contraction was considerably less between days 8−30 pi (∼4 fold), and after that, KLRG1lo IL-7Rhi cell numbers remained stable in all tissues except for the lung. Consequentially, KLRG1lo IL-7Rhi CD8 T cells predominate the memory cell population at later times (Figures 1B and C). Notably, KLRG1hi memory cells were reduced in their ability to homeostatically turnover (HT), which likely contributed to their decline over time (Figure 1D).
To confirm that KLRG1hi IL-7Rlo CD8 T cell decline was due to death rather than conversion, equal numbers of FACS sorted day 8 P14 KLRG1hi IL-7Rlo and KLRG1lo IL-7Rhi effector cells were transferred into day 8 infected recipients and followed for 2−3 months post transfer (pt). There was little to no evidence of conversion between donor KLRG1hi IL-7Rlo and KLRG1lo IL-7Rhi cells during this time and ∼8 fold more KLRG1lo IL-7Rhi cells survived (Figure S2 and data not shown). Therefore, similar to previous results (Huster et al., 2004; Kaech et al., 2003), these data showed that the short-lived fate of KLRG1hi IL-7Rlo effector CD8 T cells was fixed following acute infection.
KLRG1hi IL-7Rlo CD8 T cells require IL-15 for survival
Although KLRG1hi IL-7Rlo cells were relatively short-lived, we examined if IL-15 sustains their survival because they cannot receive IL-7 signals and together, IL-7 and IL-15 promote memory CD8 T cell longevity and self-renewal (Hand et al., 2007; Schluns and Lefrancois, 2003). We tested the generation and survival of SLECs and MPECs after LCMV infection in wt and IL-15−/− mice. KLRG1hi IL-7Rlo and KLRG1lo IL-7Rhi cells had formed equally in both groups of mice (day 8 pi), but subsequently the KLRG1hi IL-7Rlo cells in IL-15−/− mice rapidly disappeared (Figure 1E and data not shown). Similar to previous work, this suggested IL-15 was required for KLRG1hi IL-7Rlo cell survival after infection (Yajima et al., 2006). To test this directly, equal numbers day 8 KLRG1hi IL-7Rlo and KLRG1lo IL-7Rhi P14 effector CD8 T cells were sorted and transferred into wt and IL-15−/− mice. While the KLRG1lo IL-7Rhi cells survived equally well in the IL-15−/− and wt animals, in the absence of IL-15, >90% of the transferred KLRG1hi IL-7Rlo cells rapidly died (Figure 1F). Thus, SLECs could not re-express IL-7R to survive in IL-15−/− mice, and consequentially they were acutely dependent on IL-15. In light of the data in Figures 1C and D, this shows that SLECs can see IL-15, but it was insufficient to maintain their long-term survival or HT.
KLRG1 marks cells early during infection that are committed to become IL-7Rlo SLECs
To determine when SLECs and MPECs emerged during infection, we followed KLRG1 expression on LCMV-specific endogenous and P14 effector CD8 T cells during LCMV infection. KLRG1hi effector CD8 T cells first appeared 4−5 days pi and after at least 7−10 cell divisions, and these cells progressively increased in number and frequency until the peak of expansion (7−8 days pi) in all tissues examined (Figures 2A and S3 and data not shown; note: “KLRG1lo” includes both intermediate and low expression). Next, we examined IL-7R expression on KLRG1hi and KLRG1lo effector CD8 T cells during infection. By days 4−5 pi, IL-7R levels were decreased compared to naïve CD8 T cells, but were indistinguishable between KLRG1hi and KLRG1lo cells (Figure 2B; Note histograms in bottom row). Between days 6−8 pi, IL-7R expression gradually increased on the KLRG1lo cells and decreased on the KLRG1hi cells, albeit ∼10% were IL-7Rhi (Figure 2B). Thus, between days 5−8 pi, KLRG1hi and KLRG1lo effector CD8 T cells appeared to differentiate and mature along two distinct cell lineages: KLRG1hi IL-7Rlo SLECs and KLRG1lo IL-7Rhi MPECs.
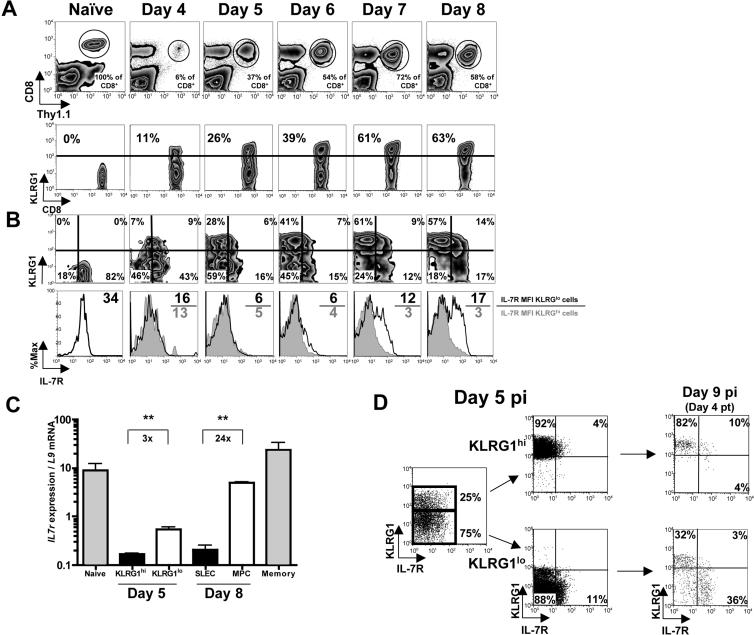
Figure 2.
KLRG1 marks effector CD8 T cells committed to an SLEC fate.
(A and B) P14 chimeric mice were infected with LCMV and on days 4−8 pi the Thy1.1+ P14 effector CD8 T cells were analyzed for expression of (A) KLRG1 and (B) KLRG1 and IL-7R. (B) Bottom row, histograms show IL-7R expression on KLRG1hi (filled) or KLRG1lo (open) P14 CD8 T cells. IL-7R MFI is shown (KLRG1lo/KLRG1hi).
(C) Bar graph shows IL-7R mRNA levels (normalized to the ribosomal gene L9) in the indicated cell populations measured by real-time PCR. **=p<0.001.
(D) Day 5 pi KLRG1hi and KLRG1lo P14 CD8 T cells were sorted and transferred in equal number back into day 5 LCMV infected recipients and analyzed 4 days post transfer (pt) for KLRG1 and IL-7R expression.
Despite similar IL-7R protein levels, day 5 KLRG1hi effector CD8 T cells expressed ∼3 fold less IL-7R mRNA than KLRG1lo cells (Figure 2C). This suggested that by day 5 KLRG1hi cells were already repressing IL-7R transcription to a greater degree and raised the possibility that they were already fully committed to becoming IL-7Rlo SLECs. To test this question, we transferred equal numbers of purified day 5 KLRG1hi and KLRG1lo effector CD8 T cells into day 5 infected animals and followed their development 4 and >60 days later. Both donor cell populations had similar engraftment and continued clonal expansion. On day 9 pi, almost all donor KLRG1hi cells were IL-7Rlo (Figure 2D). In contrast, ∼20−30% of the donor KLRG1lo cells became KLRG1hi IL-7Rlo cells between days 5−9 pi, but many remained KLRG1lo became IL-7Rhi (Figure 2D). Approximately 60 days later, these cells gave rise to ∼5 fold more memory CD8 T cells than donor KLRG1hi cells (Figure S4). Thus, as early as day 5 pi, KLRG1 upregulation marks a critical developmental decision in which effector CD8 T cells have committed to a shortened lifespan.
KLRG1hi and KLRG1lo effector CD8 T cells have similar functional properties
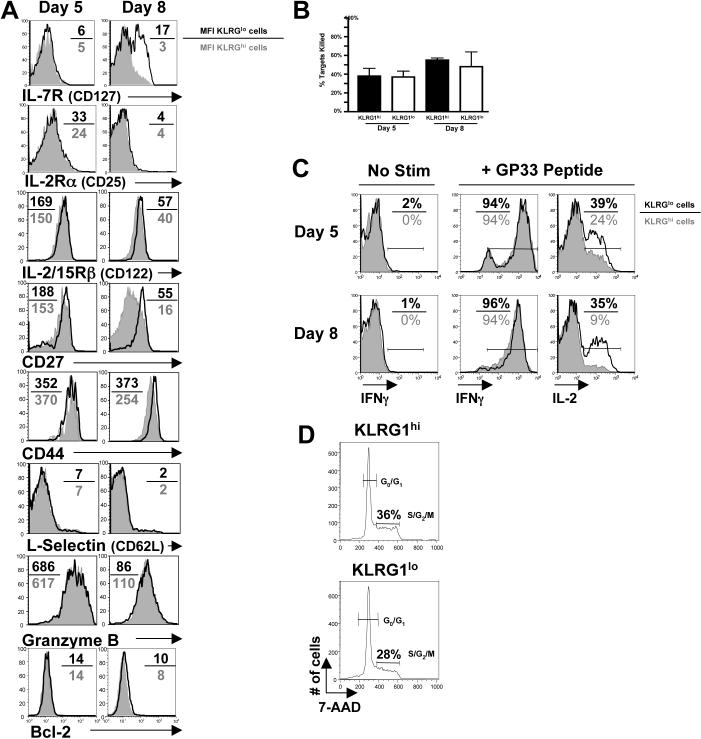
To determine other phenotypic and functional differences between KLRG1hi and KLRG1lo effector CD8 T cells, we compared their expression of several effector/memory markers, functional capabilities, and rates of division during LCMV infection. The expression of most proteins on day 5 and 8 KLRG1hi and KLRG1lo cells was similar, although KLRG1lo cells expressed more CD27, IL-7R, and IL-2/15Rβ-chain (CD122) at day 8 (Figure 3A). Functionally, day 5 and 8 KLRG1hi and KLRG1lo effector CD8 T cells had comparable cytotoxic activity and ability to produce IFNγ when restimulated (Figure 3B and C). However, ∼2−4 fold more KLRG1lo cells produced IL-2 at all time points examined (Figure 3C). Lastly, KLRG1hi and KLRG1lo effector cells had comparable rates of proliferation between days 4−8 pi, although we consistently observed a slightly greater fraction of KLRG1hi cells in cycle at most times (Figure 3D). Therefore, KLRG1hi and KLRG1lo effector cells appeared functionally and phenotypically similar during LCMV infection and were distinguished only by small differences in expression of CD27 and IL-7R and their ability to produce IL-2 that evolved between days 5−8 pi. Given their similarities, we tested if KLRG1 functioned in SLEC/MPEC lineage commitment using an shRNAi retrovirus (RV) to “knock-down” KLRG1 in effector CD8 T cells during infection. shKLRG1 efficiently decreased KLRG1 expression, but had no other noticeable effects on SLEC formation or cytokine production, suggesting that KLRG1 does not control MPEC/SLEC fate decisions (Figure S5).
Figure 3.
Phenotypic and functional comparisons between KLRG1hi and KLRG1lo effector CD8 T cells.
(A) Histograms show expression of the indicated proteins on KLRG1hi (filled) and KLRG1lo (open) P14 CD8 T cells on days 5 and 8 pi. The MFI of KLRG1lo/KLRG1hi cells is shown.
(B) In vivo CTL assay comparing day 5 and 8 KLRG1hi (black) and KLRG1lo (white) P14 effector CD8 T cells. The percent killing over 4 hrs was normalized to the on Effector:Target (E:T) ratio.
(C) Splenocytes from day 5 and 8 LCMV infected P14 chimeric were stimulated with GP33−41 peptide and analyzed for IFNγ and IL-2 production by intracellular cytokine staining. Histograms show IFNγ (left and center) and IL-2 (right) production by KLRG1hi (filled) and KLRG1lo (open) Thy1.1+ CD8 P14 CD8 T cells. IL-2 plots are gated on IFNγ-producing cells. Similar results were found in endogenous LCMV-specific CD8 T cells stimulated with NP396−404, GP33−41 and GP276−284. Note, KLRG1 expression does not change during 5 hr stimulation (data not shown).
(D) Histograms show the percent of KLRG1hi (top) and KLRG1lo cells (bottom) P14 CD8 T cells in S/G2/M phases of the cell cycle on day 5 pi using 7-AAD.
Truncating infection impairs KLRG1hi IL-7Rlo SLEC formation
Next, we studied how SLEC/MPEC fate decisions are regulated. One hypothesis was that excessive exposure of effector CD8 T cells to antigenic or inflammatory stimulation during infection diminished their memory cell developmental potential and drove their development into KLRG1hi IL-7Rlo SLECs. We tested this by shortening the duration of Listeria infection with antibiotic treatment. P14 chimeric mice infected with Listeria expressing GP33−41 (LM-GP33) were either left untreated (No Rx) or treated with ampicillin (Amp Rx) on day 1 pi to stop infection. Amp Rx specifically decreased the number of SLECs formed during LM-GP33 infection because similar numbers of MPECs, and subsequently memory CD8 T cells, formed both groups of animals (Figure 4A and data not shown). Therefore, shortening the duration of infection had a greater impact on the formation of KLRG1hi IL-7Rlo SLECs.
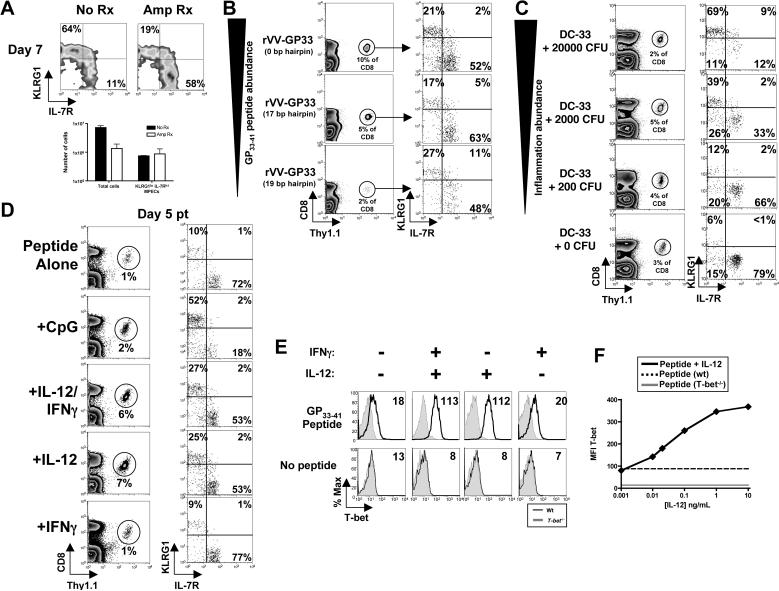
Figure 4.
Levels of inflammation regulate KLRG1hi IL-7Rlo SLEC formation.
(A) P14 chimeric mice were infected with LM-GP33 and 1 day pi were either left untreated (No Rx, black bars) or treated with Ampicillin (Amp Rx, white bars). FACS plots show KLRG1 and IL-7R expression and bar graphs show the number of total or KLRG1lo IL-7Rhi MPECs on day 7 pi.
(B) P14 chimeric mice were infected with rVVhp −0, −17, or −19 (see text) and 8 days pi Thy1.1+ P14 CD8 T cells were analyzed for KLRG1 and IL-7R expression.
(C) P14 chimeric mice were concurrently immunized with DC-33 and varying doses of Listeria (not expressing GP33−41). FACS plots show KLRG1 and IL-7R expression on day 7 P14 effector CD8 T cells.
(D) Purified naive Thy1.1+ P14 CD8 T cells were stimulated with GP33−41 peptide-loaded cells ± CpG ODN or the indicated cytokines for 24−48h and then transferred into naïve recipients. Thy1.1+ P14 CD8 T cells were analyzed for KLRG1 and IL-7R expression 5−6 days pt.
(E and F) Naïve wt or T-bet−/− P14 CD8 T cells were stimulated as in (D) with IL-12 or IFNγ or both (E) or decreasing concentrations of IL-12 (F). (E) Histograms show T-bet expression in wt (line) or T-bet−/− (shaded) Thy1.1+ CD44hi CD8 T cells and the T-bet MFI is indicated. (F) Line graph shows the MFI of T-bet with either peptide alone (dashed), the indicated concentration of IL-12 (solid), or T-bet−/− P14 CD8 T cells + IL-12 (gray). Data in graph is representative of 3 independent experiments.
Inflammatory signals regulate development or KLRG1hi IL-7Rlo effector cells
The above experiment did not discriminate how the duration of infection regulated SLEC generation because Amp Rx reduced both antigenic and inflammatory signals. To examine the role of antigenic stimulation specifically, we used three recombinant vaccinia virus strains (referred to as rVVhp), each expressing different amounts of the LCMV epitope GP33−41 via hairpin-mediated attenuation of protein expression (Wherry et al., 1999). In such, GP33−41 abundance is varied while the kinetics of infection and viral titers are not. In accordance with previous results (Wherry et al., 1999), the frequency and number of P14 effector CD8 T cells at day 7 pi was proportional to antigen abundance (Figure 4B and data not shown). However, the frequencies of KLRG1hi IL-7Rlo and KLRG1lo IL-7Rhi cells formed were not greatly affected by antigen abundance (Figure 4B).
Next, we investigated how exposure to inflammation affected SLEC development by varying the amount of “bystander” inflammation that P14 CD8 T cells were exposed during priming. P14 chimeric mice were immunized with LPS-matured dendritic cells (DCs) loaded with GP33−41 peptide (referred to as DC-33) with or without increasing different doses of a Listeria strain that does not express GP33−41 (referred to as LM). Concurrent DC-33 immunization with “high dose” LM infection resulted in ∼60−75% KLRG1hi IL-7Rlo effector CD8 T cells. However, as the LM dose was lowered, there was a corresponding decrease in the frequency of KLRG1hi IL-7Rlo cells; in the absence of any overt inflammation, mostly KLRG1lo IL-7Rhi effector CD8 T cells formed (Figure 4C). Therefore, the percentage of effector CD8 T cells that became KLRG1hi IL-7Rlo SLECs was proportional to the level or duration of inflammation.
LM infection produces many inflammatory cytokines, so the effect of a simpler adjuvant, CpG oligodeoxynucleotides (ODNs), was tested. Naïve P14 CD8 T cells were stimulated with GP33−41 peptide-loaded splenocytes with and without CpG ODN for 24−48 hrs, transferred into naïve recipients, and analyzed 5 days later. Without CpG, few KLRG1hi IL-7Rlo effector CD8 T cells formed, but with CpG, ∼25−50% of the effector cells became KLRG1hi and IL-7Rlo (Figure 4D). CpG ODN is a potent generator of both type I/II interferons and IL-12 (Krieg, 2002) and data not shown), therefore, we examined the effects of CD8 T cell priming with IL-12 and IFNγ directly. Priming P14 CD8 T cells with IL-12+IFNγ or IL-12, but not IFNγ alone, generated KLRG1hi IL-7Rlo effector cells (Figure 4D). Thus, IL-12 was a critical signal that could induce the formation of KLRG1hi IL-7Rlo effector CD8 T cells.
IL-12, but not IFNγ, induces T-bet expression in a dose-dependent manner
The transcription factor T-bet is critical for TH1 CD4 T cell differentiation and terminal maturation of KLRG1hi NK cells (Robbins et al., 2005; Szabo et al., 2000). T-bet expression is also regulated by IFNγ and IL-12 (Weaver et al., 2006), so we tested if T-bet was involved in the SLEC/MPEC cell fate decision. First, we examined whether IL-12 or IFNγ could induce T-bet expression in early-activated CD8 T cells using flow cytometry. IL-12, but surprisingly not IFNγ, could induce T-bet to significantly higher levels than peptide alone, and the amount of T-bet induced directly corresponded to the concentration of IL-12 in the media (Figures 4E and F). These data extend from previous work (Takemoto et al., 2006) by showing that T-bet expression in activated CD8 T cells could be controlled by IL-12 in a dose-dependent manner.
T-bet controls formation of KLRG1hi IL-7Rlo effector CD8 T cells
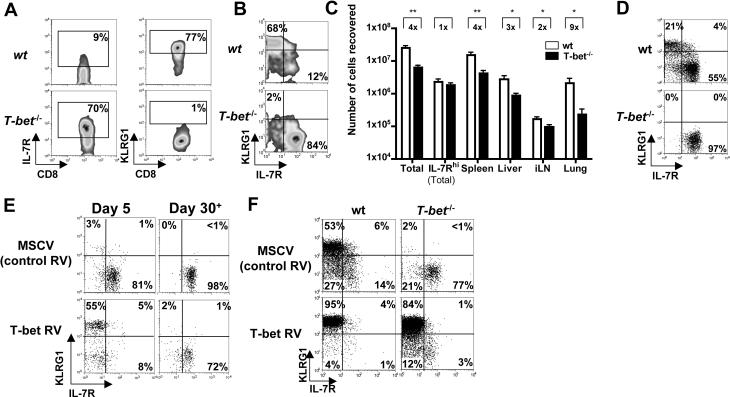
Because IL-12 induced T-bet expression and SLEC formation, we tested if T-bet was necessary for SLEC development. Therefore, we infected wt and T-bet−/− mice directly with LCMV (Figure 5A) or, to examine the cell-autonomous role of T-bet, we transferred small numbers of wt and T-bet−/− P14 CD8 T cells into wt mice that were subsequently infected with LCMV (Figure 5B). Compared to wt effector CD8 T cells, <10% of T-bet−/− effector cells were KLRG1hi and IL-7Rlo (Figures 5A and B). This likely accounted for the ∼4−9 fold reduction in the total number of T-bet−/− effector cells. Interestingly, similar numbers of KLRG1lo IL-7Rhi MPECs formed in wt and T-bet−/− mice (Figure 5C). Similar to a previous study, T-bet−/− effector CD8 T cells expressed relatively normal levels of Granzyme B and IFNγ, but IL-2 production was greatly elevated (Juedes et al., 2004 and data not shown). Most T-bet−/− effector CD8 T cells were CD27hi and expressed lower levels of Ly49c, KLRE1, CD244 (2B4) mRNA, suggesting multiple SLEC-associated attributes were dependent on T-bet (data not shown). These data suggested that T-bet plays a necessary cell-intrinsic role in SLEC formation. In support of this, IL-12 induction of KLRG1hi IL-7Rlo effector CD8 T cells required T-bet (Figure 5D).
Figure 5.
T-bet expression is necessary and sufficient for development of KLRG1hi SLECs.
(A) wt or T-bet−/− mice or (B) wt mice containing ∼1x104 wt or T-bet−/− P14 CD8 T cells were infected with LCMV and analyzed 7−8 days later for IL-7R and KLRG1 expression on GP33−41-specific CD8 T cells (A) or Thy1.1+ P14 CD8 splenocytes (B). Similar data to (A) were observed with NP396−404-specific CD8 T cells (data not shown). (C) Bar graph compares the total combined number of endogenous GP33−41 and NP396−404-specific CD8 T cells between wt (black) or T-bet−/− (white) animals on day 8 of LCMV infection. Note, similar numbers of IL-7Rhi effector cells in wt and T-bet−/− animals. **=p<0.001, *=p<0.01.
(D) As in Figure 4D, wt or T-bet−/− Thy1.1+ P14 CD8 T cells were stimulated with IL-12, transferred into naïve recipients and analyzed for KLRG1 and IL-7R expression 5−6 days later. (E) wt P14 CD8 T cells were transduced with control (MSCV) or T-bet-expressing RVs, transferred into naïve recipients, and analyzed 5 or 30+ days later for KLRG1 and IL-7R expression on Thy1.1+ GFP+ CD8 T cells.
(F) wt or T-bet−/− P14 CD8 T cells were transduced with MSCV or T-bet RVs and transferred into recipients that were subsequently infected with LCMV. Seven days later, Thy1.1+ GFP+ splenocytes were analyzed for IL-7R and KLRG1 expression.
Next, we determined if T-bet expression was sufficient for KLRG1hi IL-7Rlo effector CD8 T cell generation by transducing activated wt P14 CD8 T cells with MSCV RV expressing T-bet and GFP or GFP alone, and transferring them into naïve recipients. Transduction of cells with control MSCV RV did not induce KLRG1hi IL-7Rlo effector CD8 T cells, however if T-bet was over expressed, >50% of the cells became KLRG1hi IL-7Rlo SLECs by day 5 pt (Figure 5E). Strikingly, >80−90% of the wt or T-bet−/− P14 CD8 T cells transduced with T-bet RV became KLRG1hi and IL-7Rlo when transferred into LCMV infected recipients (Figure 5F). Furthermore, most KLRG1hi IL-7Rlo effector CD8 T cells formed by T-bet over expression were short-lived and contracted by day 50 pt (Figure 5E and data not shown). Together with that above, these data show that T-bet is necessary and (with over expression) sufficient to specify a population of naturally arising short-lived KLRG1hi IL-7Rlo effector CD8 T cells.
A gradient of T-bet expression specifies an SLEC or MPEC fate
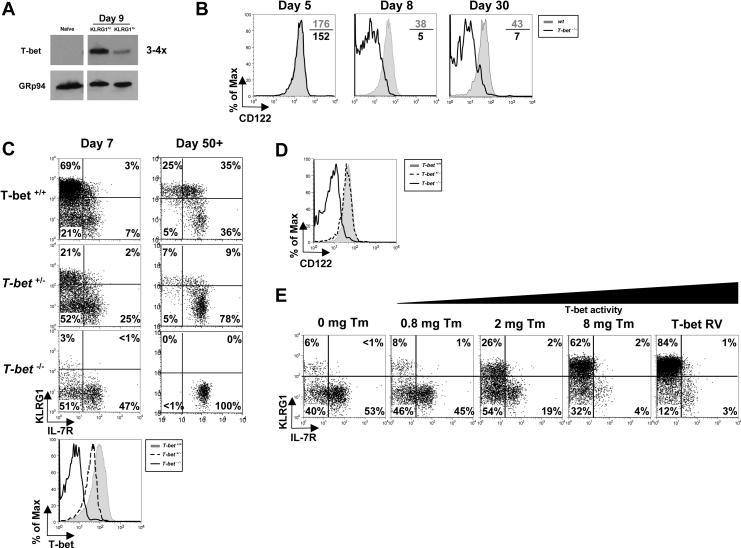
In light of the T-bet RV over expression data, we next examined if KLRG1hi IL-7Rlo effector CD8 T cells naturally express more T-bet than KLRG1lo IL-7Rhi cells. DNA microarrays and real-time PCR showed that T-bet mRNA was increased ∼1.5−2 fold in IL-7Rlo SLECs compared to IL-7Rhi MPECs at day 7 pi and Western blotting confirmed that KLRG1hi IL-7Rlo effector CD8 T cells contained ∼3−4 fold more T-bet protein than KLRG1lo IL-7Rhi cells (Figures 1A, 6A, and data not shown).
Figure 6.
T-bet functions in both MPECs and SLECs according to an expression gradient.
(A) Naïve and day 8 KLRG1hi IL-7Rlo SLECs or KLRG1lo IL-7Rhi MPECs were sorted and examined by Western blotting T-bet and GRp94 levels.
(B) wt and T-bet−/− P14 CD8 T cells were analyzed 5, 8 and 30 days pi for CD122 expression.
(C) T-bet+/+, T-bet+/− or T-bet−/− P14 CD8 T cells were analyzed 7 and 30 days pi for KLRG1 and IL-7R expression and T-bet expression (bottom histogram plot).
(D) T-bet+/+, T-bet+/− or T-bet−/− P14 memory CD8 T cells were analyzed 30 days pi for CD122 expression
(E) T-bet−/− P14 CD8 T cells were transduced with T-bet RV or one expressing T-bet fused to the estrogen receptor (T-bet:ER) and transferred into mice subsequently infected with LCMV. Mice were treated with 0 – 8 mg of Tamoxifen (Tm) during infection and on day 7 pi, GFP+ donor splenocytes were analyzed for expression of KLRG1 and IL-7R.
Although T-bet expression was lower in MPECs, we tested if it functions in these cells. Comparing wt and T-bet−/− “MPECs” using Affymetrix GeneChips, we found that T-bet regulated ∼20% of the MPEC-specific genes (data not shown). Moreover, while T-bet−/− effector cells initially expressed relatively normal levels of CD122 at day 5 pi, T-bet was required for sustained CD122 expression following this time point (Figure 6B). Thus, low levels of T-bet were necessary for normal MPEC development. This finding suggested a model in which T-bet functions distinctly in SLECs and in MPECs: high T-bet levels induced terminal SLEC differentiation, whereas low levels promoted MPEC development.
To directly test if T-bet expression levels dictate SLEC/MPEC formation, we varied the amount of T-bet in LCMV-specific effector CD8 T cells during infection using two separate methods. First, we examined the effects of T-bet gene dosage by comparing T-bet+/+, T-bet+/−, and T-bet−/− P14 CD8 T cells for their ability to form SLECs and MPECs 7 days pi and memory cells ∼50 days pi. These experiments showed that as the T-bet copy number decreased so did the frequency and number of KLRG1hi IL-7Rlo SLECs (Figure 6C and S6). Conversely, the frequency of KLRG1lo IL-7Rhi “MPECs” increased (T-bet−/− >T-bet+/− >T-bet+/+), however, similar numbers formed in all 3 groups of effector CD8 T cells (Figure 6C and S6). These data show that 2-fold differences in T-bet expression can affect SLEC/MPEC lineage commitment.
Following clearance of LCMV infection, the magnitude of T-bet+/+, T-bet+/− and T-bet−/− effector CD8 T cell contraction was proportionally decreased according to T-bet copy number, but similar sized memory CD8 T cell populations were yielded (as may have been predicted by MPEC numbers; Figure S6). Thus, at the population level, T-bet+/− and T-bet−/− effector cells appeared more efficient at generating memory CD8 T cells, but it is likely that this effect was due to their impaired SLEC formation rather than increased MPEC formation. It may be that during LCMV infection the number of MPECs formed is capped by other mechanisms. Importantly, the lowered expression of T-bet in T-bet+/− P14 cells was sufficient to maintain CD122 expression as well as to partially suppress IL-2 production in the memory CD8 T cells (Figure 6D and data not shown). It is not known yet, if the T-bet−/− memory CD8 T cells, with reduced CD122 expression, will be maintained long-term or gradually decay as in IL-15−/− mice (Schluns and Lefrancois, 2003). T-bet−/− memory CD8 T cells express higher levels of IL-7R compared to wt cells (Figure 6C), and therefore, it is possible that IL-7 may compensate for the loss of CD122 as described previously (Kieper et al., 2002) and data not shown).
Second, we regulated T-bet activity (via its nuclear localization) by fusing it to the estrogen receptor α (T-bet:ER, (Matsuda et al., 2007). T-bet−/− P14 CD8 T cells were reconstituted with T-bet:ER RV and transferred into LCMV infected recipients that were subsequently treated with different doses of tamoxifen (Tm) during infection. After 7 days, we found that the frequency of KLRG1hi IL-7Rlo SLECs directly correlated with the amount of tamoxifen administered, ranging from ∼10% to >60% at the lowest and highest Tm doses, respectively (Figure 6D). As expected, all doses of Tm rescued T-bet−/− MPEC CD122 expression, showing again that low levels of T-bet were required for normal MPEC gene expression (data not shown).
Discussion
During acute viral or bacterial infections, effector CD8 T cells undergo a complex and coordinated differentiation program, part of which involves the generation of at least two effector cell subsets that have different fates and memory cell developmental potential (Badovinac et al., 2004; Huster et al., 2004; Kaech et al., 2003). This study was designed to investigate how and when during infection these cell fate decisions occur. We found that inflammatory signals (like IL-12) present during CD8 T cell priming dictate a T-bet expression-gradient and regulate effector CD8 T cell fate determination (Figure 7). Higher amounts of T-bet induced a terminally differentiated SLEC state, associated with increased KLRG1 expression and stable IL-7R repression, and acute dependence on IL-15 for survival, but not long-term persistence or HT. In contrast, effector cells expressing lower amounts of T-bet remained KLRG1lo and some were capable of becoming IL-7Rhi MPECs that responded to both IL-7 and IL-15 and developed into long-lived, self-renewing memory CD8 T cells.
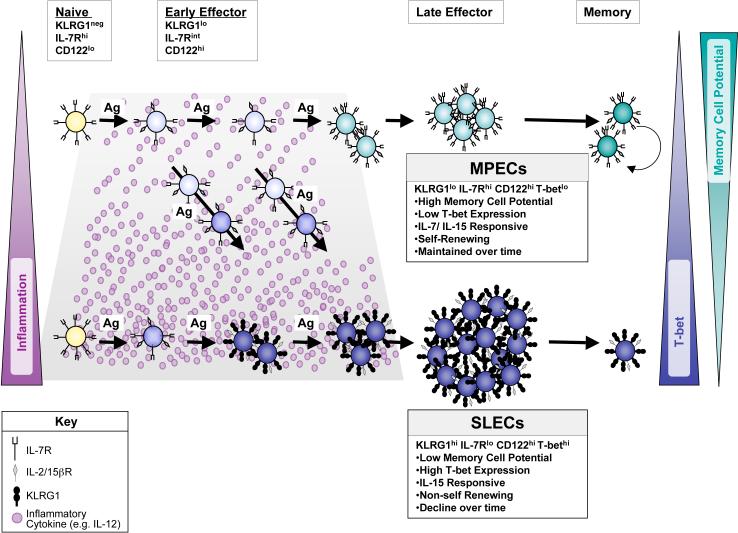
Figure 7.
Model of SLEC and MPEC development during acute viral infection.
Naive CD8 T cells are IL-7Rhi, CD122lo (IL-2/15βR), KLRG1neg and T-betneg and are IL-7 dependent. Early during infection, most effector CD8 T cells become CD122hi and downregulate IL-7R to an intermediate-to-low level, but expression of T-bet and KLRG1 is set depending on their exposure to inflammatory cytokines (e.g. IL-12). Effector CD8 T cells that are exposed to lower levels of inflammation express less T-bet (light blue cells) and begin to upregulate IL-7R to become KLRG1lo IL-7Rhi MPECs (turquoise cells). Effector CD8 T cells that encounter higher levels of inflammatory cytokines express relatively more T-bet and KLRG1 (dark blue cells), stably repress IL-7R and consequentially become KLRG1hi IL-7Rlo SLECs. SLECs become IL-15 dependent, however, IL-15 alone cannot support their long-term persistence or homeostatic turnover and they decline over time. In contrast, MPECs remain dually responsive to IL-7 and IL-15 and preferentially develop into long-lived memory CD8 T cells that can self-renew.
To date, KLRG1 is the best-described SLEC marker during acute infection, although it is unlikely that it plays a significant role in their formation (Figure S5). In humans and mice, most acute infections result in mainly KLRG1lo IL-7Rhi memory CD8 T cells, but chronic or latent viral infections can produce greater frequencies of KLRG1hi IL-7Rlo virus-specific CD8 T cells that are likely to be continually generated due to repeated exposure to viral antigens and inflammatory cytokines (Ibegbu et al., 2005; Lang et al., 2005; Sierro et al., 2005; van Leeuwen et al., 2006; Wherry et al., 2004). Furthermore, consecutive waves of infection generate mostly KLRG1hi TEM cells , some of which express IL-7R and are long-lived (Jabbari and Harty, 2006; Masopust et al., 2006; Voehringer et al., 2001). Therefore, it is important to emphasize that the strong correlation between KLRG1 expression and reduced CD8 T cell longevity may not hold true for CD8 T cells that have encountered antigen with inflammation repeatedly or persistently, especially if they co-express IL-7R as seen in a small number of KLRG1hi IL-7Rhi primary effector CD8 T cells appear long-lived (Figure 1B). Moreover, it is important to emphasize that KLRG1hi memory CD8 T cells have a reduced proliferative capacity and this calls into question their contribution to long-term immunologic protection (Voehringer et al., 2001).
The “decreasing potential model” for the development of memory CD8 T cells suggests that “memory T cells can only arise under conditions in which the antigenic load is limited and the stimulation of precursors ceases before a point of no return” (Ahmed and Gray, 1996). In support of this model, we and others found that altering the overall amount of stimulation per CD8 T cell (either by modulating tg CD8 T cell precursor frequency or shortening/lengthening the duration of infection that T cells are exposed to) can profoundly affect the types of effector T cells produced or their longevity (Figures 4A and S7; (Bachmann et al., 2006; Badovinac et al., 2007; Badovinac et al., 2005; Badovinac et al., 2004; D'Souza and Hedrick, 2006; Jelley-Gibbs et al., 2005; Lang et al., 2005). We further explored this model by separating antigenic and inflammatory signals. Although these findings do not rule out the possibility that excessive or persistent antigenic stimulation might affect SLEC/MPEC fate, they show that inflammatory signals (e.g. IL-12), through regulation of T-bet expression, play a dominant role in regulating memory cell potential. The findings presented here provide the decreasing potential model with both an early molecular signature (KLRG1) as well as a mechanism (IL-12 → T-bet → reduced longevity) for identifying and generating SLECs during primary immune responses to infection. In support of this, effector CD8 T cells from IL-12−/− mice have reduced T-bet levels and generate more memory CD8 T cells (Pearce and Shen, 2007; Takemoto et al., 2006), W.C. and S.M.K unpublished data). Moreover, it is worth noting that during infection IL-12 is primarily produced by activated mature CD8α+ dendritic cells (DCs), but over time their production of IL-12 declines (Langenkamp et al., 2000). Because a larger number of “newly” activated DCs are present early during infection, perhaps, the timing of when a naïve CD8 T cell encounters a DC is critical in determining IL-12 exposure and SLEC/MPEC fate.
How does T-bet regulate the SLEC/MPEC fate decision? Similar to TH1 CD4 T cells (Szabo et al., 2000), T-bet may serve as the “master regulator” of CD8 T cell SLEC lineage commitment. However, unlike its asymmetric role in TH1/TH2 specification, we believe that a gradient of T-bet is critical with low levels promoting MPECs and high levels inducing SLECs (Figure 7). A similar model has been described for PU.1 regulation of macrophage vs. neutrophil development (Dahl et al., 2003). Accordingly, T-bet influenced expression of ∼50% of the SLEC-specific genes compared to ∼20% of the MPEC-specific genes (data not shown). These data showed a heightened dependence on T-bet for lineage-specific gene expression in SLECs. Thus, we propose the increased abundance of T-bet in SLECs is necessary for proper expression of these SLEC-specific genes and this explains why SLEC development is more overtly affected by T-bet deficiency. Additionally, T-bet regulates IL-12Rβ2 expression and this positive-feedback loop can make T cells more receptive to IL-12 signals, however, it is also possible that IL-12 and T-bet act in parallel to provide separate signals to control SLEC/MPEC development.
Signals from IL-12 not only enhance T-bet expression, but might also repress Eomesodermin (Eomes), another T-box family member whose function can overlap with T-bet in CD8 T cells, leading to the speculation that T-bet and Eomes might direct SLEC and MPEC development, respectively (Takemoto et al., 2006). However, it is certainly more complicated than that because KLRG1hi and KLRG1lo effector cells expressed similar amounts of eomes mRNA and T-bet was required for normal MPEC gene expression (data not shown). Previous work showed that in naïve mice T-bet-deficiency did not affect CD122 expression in polyclonal memory CD8 T cells (Intlekofer et al., 2005). However, as shown here for antigen-specific memory CD8 T cells and as described previously for NKT cells, T-bet acts in a non-redundant manner to sustain CD122 expression (Matsuda et al., 2007; Townsend et al., 2004).
Murine cytomegalovirus (MCMV) and Listeria infection produce copious amounts of IL-12p70, whereas LCMV produces very little (Dalod et al., 2002), yet similar frequencies of IL-7Rlo SLECs form during these infections (Huster et al., 2004; Kaech et al., 2003; Sierro et al., 2005). Therefore, we predict that other inflammatory cytokines besides IL-12, which induce T-bet (Agnello et al., 2003), will also influence memory cell developmental potential. IFNγ alone was insufficient to induce T-bet or KLRG1hi IL-7Rlo cells and this was surprising because IFNγ induces T-bet in CD4 T cells and recent work has suggested IFNγ programs effector T cell contraction and represses IL-7R expression (Badovinac et al., 2004; Badovinac et al., 2000). Still, it is possible IFNγis necessary, but not sufficient, because fewer KLRG1hi IL-7Rlo SLECs formed in IFNγ−/− mice during LCMV infection (data not shown). In support of this, in vivo CpG mediated IL-12p70 production and SLEC formation requires IFNγ (data not shown). Accordingly, IL-12 treatment of IFNγ−/− mice is sufficient to rescue CpG-mediated KLRG1hi IL-7Rlo effector CD8 T cell induction, indicating that IFNγ is necessary for optimal IL-12 production during immunization and infection (W.C. and S.M.K unpublished data). Moreover, our data suggest an alternative explanation for the proposed programming of effector CD8 T cell contraction by inflammation: instead of inflammation directly controlling effector CD8 T cell contraction per se, we suggest that inflammation induces the formation of, what would naturally be, a short-lived subset of effector cells.
Although exposure to inflammatory cytokines may reduce effector CD8 T cell longevity, signals from IL-12 and type I/II IFNs also play a critical role in enhancing effector CD8 T cell expansion and function (Mescher et al., 2006). Thus, we are not proposing that better memory CD8 T cells will develop in the absence of inflammation, but rather that low exposure to inflammatory signals may promote development of functional effector cells that have greater memory potential. In contrast, excessive or prolonged exposure to inflammatory signals during infection or immunization may generate potent effector cells, but as a trade-off, their memory cell potential is decreased. This model offers a reliable mechanism to maintain T cell homeostasis because it allows the T cell response to be made in proportion to the amount and/or duration of infection, while simultaneously, ensures restoration of normal T cell numbers once infection and inflammation ceases. Perhaps, this is why KLRG1hi effector cells become dependent on IL-15, a cytokine modulated by Type 1 inflammatory cytokines (Doherty et al., 1996). In the future, it may become possible to tailor larger and more durable memory T cell populations by modulating the types and levels of inflammatory signals they receive at the time of T cell priming, but this remains to be investigated.
Experimental Procedures
Mice, infections and treatments.
Thy1.1+ P14 TCR tg mice have been described previously (Kaech et al., 2003). To make “P14 chimeric mice”, ∼1×104 Thy1.1+ wt, T-bet+/−, or T-bet−/− CD8 T cells were transferred into naïve Thy1.2+ C57BL/6 (B6) mice (National Cancer Institute, Fredrick, MD and Jackson Laboratories, Bar Harbor, ME). IL-15−/− mice were obtained from M. Caligiuri (Ohio State University, Columbus, OH). T-bet−/− mice were obtained from L. Glimcher (Harvard School of Medicine, Cambridge, MA) and crossed to P14 tg mice. All animal experiments were done with approved Institutional Animal Care and Use Committee protocols. Details of infections and treatments are found in supplemental experimental procedures.
CFSE, 7-AAD labeling, surface/intracellular staining, and antibodies.
Lymphocyte isolation, CFSE labeling (Invitrogen, Eugene, OR), LCMV peptide stimulations, production of MHC class I tetramers and surface/intracellular staining was performed as described (Kaech et al., 2003). 7-AAD staining was performed according to manufacturers directions (BD Biosciences, San Diego, CA). All antibodies were purchased from E-biosciences (San Diego, CA) except anti-Granzyme B-PE (Caltag, Burlingame, CA), Bcl-2-FITC (BD) and T-bet 647 (Santa Cruz Biotechnology, Santa Cruz, CA). Anti-KLRG1 (2F1) hybridoma was a generous gift from D. Raulet (University of California, Berkley, CA) and was conjugated to alexa-647 (Invitrogen, Eugene, OR). For T-bet staining, cells were permeabilized with 0.01% Triton X-100 in PBS + 0.5% FBS followed by intracellular staining. All flow cytometry was analyzed on a FACSCalibur (BD) with FloJo software (Treestar, San Carlos, CA).
Cell isolations, separations, and adoptive transfer.
Details of for these procedures are found in supplemental experimental procedures.
In vitro T cell stimulations for in vivo transfer and DC immunization.
DCs and naïve wt B6 splenocytes were loaded with GP33−41 peptide and either ∼1×106 peptide loaded splenocytes were cultured with ∼5×104 MACS purified naïve P14 CD8 T cells for 48 hours ± 12.5 μg/mL CpG oligodeoxynucleotide 1826 (Badovinac et al., 2005), 10 ng/mL IL-12 (R&D Systems Inc., Minneapolis, MN) and/or 100 ng/mL IFNγ (R&D Systems). For in vivo transfers, ∼4×105 activated P14 CD8 T cells from cultures stimulated for 24−48 hours with 100 ng/mL peptide-loaded splenocytes ± cytokines were iv transferred into naïve recipients and analyzed 5−6 days later. Details of DC preparation and P14 T cell stimulations are found in supplemental experimental procedures.
Retroviral constructs and transduction.
T-bet- and GFP-MSCV vectors were obtained from L. Glimcher (Szabo et al., 2000). shKLRG1-pSM2c RNAi vector (Open Biosystems, Huntsville, AL) was subcloned into LMP vector using XhoI and EcoRI. Generation of the T-bet:ER MSCV vector has been described previously (Matsuda et al., 2007). Details of the retroviral transduction are found in supplemental experimental procedures.
In vivo CTL and homeostatic turnover assay.
Details of for these procedures are found in supplemental experimental procedures.
Gene expression, real-time PCR and Western blotting analyses.
Gene expression analysis on IL-7Rhi and IL-7Rlo P14 effector CD8 T cells was performed with Affymetrix Mouse 430 2.0 Array chips (Affymetrix, Santa Clara, CA). Details for the cell isolation, RNA preparation, gene expression analysis and Western blotting are found in supplemental experimental procedures.
Statistical Analyses.
Where indicated, p-values were determined using a two-tailed unpaired student's t-test. p-values <0.05 were considered significant. All graphs show averages of the mean ± s.e.m.
Acknowledgements
The authors would like to thank Dr. M. Shlomchik, Dr. R. Medzhitov, G. Lyons, G. Tokmoulina, E. Hinson, and the Kaech lab for comments and technical expertise; L. Glimcher for T-bet RV constructs, antibody and T-bet−/− mice; E.J. Wherry and L.C. Eisenlohr for rVVhp strains; This work supported by the Burroughs-Wellcome Fund 1004313 (S.M.K.), NIH RO1 AI 066232-01 (S.M.K.), Edward Mallinckrodt, Jr. Foundation (S.M.K.), NIH T32 A1055403 (N.S.J.), Richard K. Gershon Predoctoral Fellowship (N.S.J), the Korean Ministry of Science and Technology (H.K.L), R01 AI057485 (L.G.) and P01 AI22295, R01 AI054661, and R01 AI056322 (to J.H.). The authors apologize for citation omissions due to length constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
References
- Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O'Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006;36:842–854. doi: 10.1002/eji.200535730. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Schwarz K, Wolint P, Meijerink E, Martin S, Manolova V, Oxenius A. Cutting edge: distinct roles for T help and CD40/CD40 ligand in regulating differentiation of proliferation-competent memory CD8+ T cells. J Immunol. 2004;173:2217–2221. doi: 10.4049/jimmunol.173.4.2217. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Haring JS, Harty JT. Initial T Cell Receptor Transgenic Cell Precursor Frequency Dictates Critical Aspects of the CD8+ T Cell Response to Infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8 T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- Chtanova T, Newton R, Liu SM, Weininger L, Young TR, Silva DG, Bertoni F, Rinaldi A, Chappaz S, Sallusto F, et al. Identification of T cell-restricted genes, and signatures for different T cell responses, using a comprehensive collection of microarray datasets. J Immunol. 2005;175:7837–7847. doi: 10.4049/jimmunol.175.12.7837. [DOI] [PubMed] [Google Scholar]
- D'Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R, Walsh JC, Lancki D, Laslo P, Iyer SR, Singh H, Simon MC. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat Immunol. 2003;4:1029–1036. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RA, Out TA. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005;191:1710–1718. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. Expression of interleukin-7 receptor a is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0705007104. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juedes AE, Rodrigo E, Togher L, Glimcher LH, von Herrath MG. T-bet controls autoaggressive CD8 lymphocyte responses in type 1 diabetes. J Exp Med. 2004;199:1153–1162. doi: 10.1084/jem.20031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- Lang KS, Recher M, Navarini AA, Harris NL, Lohning M, Junt T, Probst HC, Hengartner H, Zinkernagel RM. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- Masopust D, J. HS, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, George TC, Hagman J, Gapin L. Temporal dissection of T-bet functions. J Immunol. 2007;178:3457–3465. doi: 10.4049/jimmunol.178.6.3457. [DOI] [PubMed] [Google Scholar]
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8 T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Ortega E, Schneider H, Pecht I. Possible interactions between the Fc epsilon receptor and a novel mast cell function-associated antigen. Int Immunol. 1991;3:333–342. doi: 10.1093/intimm/3.4.333. [DOI] [PubMed] [Google Scholar]
- Pearce E, Shen H. Generation of CD8 T cell memory is regulated by interleukin-12. J Immunol. 2007 doi: 10.4049/jimmunol.179.4.2074. In Press. [DOI] [PubMed] [Google Scholar]
- Robbins SH, Tessmer MS, Van Kaer L, Brossay L. Direct effects of T-bet and MHC class I expression, but not STAT1, on peripheral NK cell maturation. Eur J Immunol. 2005;35:757–765. doi: 10.1002/eji.200425797. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- van Leeuwen EM, de Bree GJ, ten Berge IJ, van Lier RA. Human virus-specific CD8+ T cells: diversity specialists. Immunol Rev. 2006;211:225–235. doi: 10.1111/j.0105-2896.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.