Abstract
Phosphatidylinositol 3-kinase inhibitors have been shown to affect the endocytosis or subsequent intracellular sorting in various receptor systems. Agonist-activated β2-adrenergic receptors undergo desensitization by mechanisms that include the phosphorylation, endocytosis and degradation of receptors. Following endocytosis, most internalized receptors are sorted to the cell surface, but some proportion is sorted to lysosomes for degradation. It is not known what governs the ratio of receptors that recycle versus receptors that undergo degradation. To determine if phosphatidylinositol 3-kinases regulate β2-adrenergic receptor trafficking, HEK293 cells stably expressing these receptors were treated with the phosphatidylinositol 3-kinase inhibitors LY294002 or wortmannin. We then studied agonist-induced receptor endocytosis and postendocytic sorting, including recycling and degradation of the internalized receptors. Both inhibitors amplified the internalization of receptors after exposure to the β-agonist isoproterenol, which was attributable to the sorting of a significant fraction of receptors to an intracellular compartment from which receptor recycling did not occur. The initial rate of β2-adrenergic receptor endocytosis and the default rate of receptor recycling were not significantly altered. During prolonged exposure to agonist, LY294002 slowed the degradation rate of β2-adrenergic receptors and caused the accumulation of receptors within rab7-positive vesicles. These results suggest that phosphatidylinositol 3-kinase inhibitors (1) cause a misrouting of β2-adrenergic receptors into vesicles that are neither able to efficiently recycle to the surface nor sort to lysosomes, and (2) delays the movement of receptors from late endosomes to lysosomes.
Keywords: PI 3-kinase, β2-adrenergic receptors, rab7, lysosomes, endosomes
Introduction
Signaling by β2-adrenergic receptors (β2ARs) upon treatment with β2-agonists is attenuated by several distinct mechanisms. Phosphorylation of receptors by G-protein-coupled receptor (GPCR) kinases and protein kinases A and C [1–3] causes partial uncoupling from G-proteins. Similar to other GPCRs, phosphorylated β2ARs then bind with high affinity to β-arrestin, which fully uncouples receptors from G proteins [4] and also interacts with clathrin and adaptor proteins, thereby facilitating rapid receptor endocytosis [5, 6]. Internalized receptors traffic through early endosomes, localizing with transferrin receptors [7] and rab5a [8] before returning to the cell surface (recycling). During prolonged exposure to agonists, β2ARs may also sort from early endosomes to lysosomes via late endosomes for degradation [9, 10]. Receptor endocytosis and subsequent intracellular sorting events are thus critical regulators of surface β2AR number, thereby influencing the intensity of tissue responses to β-agonists used as therapeutic agents.
Given the importance of proper postendocytic sorting to maintain adequate surface receptor numbers, much effort has been made to discover intracellular factors that regulate the choice between receptor degradation and receptor recycling. An important advance in the study of protein sorting was the discovery that phosphatidylinositol 3-kinase (PI 3-kinase), which phosphorylates phosphatidylinositol to phosphatidylinositol 3-phosphate (PtdIns3P), is required for the transport of membrane proteins to the yeast vacuole [11]. Subsequent work with intact mammalian cells showed that pharmacologic inhibition of PI 3-kinase by the drugs LY294002 or wortmannin inhibits the intracellular trafficking of receptors, including the recycling of transferrin receptors [12–15] and the degradation of PDGF receptors [16, 17]. A previous study also showed that wortmannin treatment reduces the recycling kinetics of the G protein-coupled angiotensin-1 receptor [18], indicating that postendocytic trafficking of GPCRs might be regulated by phosphoinositides. However, there is no information regarding the dependence of β2AR degradation or recycling on PtdIns3P production.
Since PI 3-kinase inhibitors affect the postendocytic sorting of other receptors, we sought to determine whether such compounds would affect the degradation or recycling of β2ARs. We thus treated HEK293 cells stably expressing β2ARs with PI 3-kinase inhibitors and then examined agonist-induced receptor endocytosis and postendocytic sorting, including the recycling and degradation of internalized receptors.
Materials and methods
Cells and reagents
12β6 cells, an HEK293 line expressing ∼1 pmol/mg of hemagglutinin (HA)-tagged β2ARs, were obtained from B. Kobilka. A rabbit polyclonal antibody to the human β2AR C-terminus (H-20) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A rabbit polyclonal antibody to the human Na+/K+-ATPase was obtained from Upstate Cell Signaling Solutions (Charlottesville, VA), and a monoclonal antibody mHA.11 against the HA tag from Covance (Berkeley, CA). Fluorescent secondary antibodies (goat anti-rabbit IgG-Texas Red and goat anti-mouse IgG-Alexa 488), and Alexa 594 holotransferrin, were purchased from Invitrogen-Molecular Probes (Eugene, OR). [3H]CGP12177 (33 Ci/mmol) was purchased from NEN Life Sciences (Boston, MA), (-) isoproterenol (ISO) bitartrate from ICN (Irvine, CA), and LY294002 from Biomol (Plymouth Meeting, PA). A construct expressing EGFP-rab7 was constructed by PCR amplification of a human rab7 cDNA and was subcloned into pEGFP-C1. FuGene 6 transfection reagent was obtained from Roche Applied Science. (Indianapolis, IN). All other reagents were from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted.
Measurement of β2AR endocytosis
12β6 cells growing in 24-well clusters were equilibrated in medium containing LY294002 or vehicle (0.075% ethanol) for 30 min, then with ISO (10 μM) in triplicate wells for varying times up to 20 min at 37°C. LY294002 or vehicle was present throughout the agonist incubation period. The clusters were rapidly chilled and the wells washed four times with ice-cold DMEM-H (DMEM with 20 mM HEPES, pH 7.4). The cells were then incubated at 4°C for 90 minutes with 6 nM [3H] CGP-12177, a hydrophilic radioligand that selectively binds surface β2ARs. The cells were washed twice with cold DMEM-H, and lysed with 0.1% SDS, 0.1% NP-40 for counting by scintillation spectroscopy. In dose-response experiments, cells were treated with varying concentrations of LY294002 or wortmannin, then with 10 μM ISO for 20 min prior to measuring surface receptors. In time-course experiments, LY294002 was used at 50 μM and incubations with ISO varied up to 20 min. Nonspecific binding was assessed by including 1 μM propranolol in parallel binding incubations, and was always <3% of the total binding.
Measurement of β2AR recycling
The recycling assay measured the rate at which internal receptors returned to the cell surface after removal of agonist. Cells growing in 24-well clusters were first treated with vehicle or with LY294002 for 30 min, then with 10 μM ISO in triplicate wells for 20 min to obtain a steady-state level of internalized receptors. LY294002 (50 μM) or vehicle was present throughout the agonist incubation period. The cells were washed rapidly three times with warm medium, and then incubated at 37°C in medium with vehicle or LY294002 for varying times to allow recycling. The clusters were chilled on ice, and surface receptors were measured by [3H]CGP-12177 binding as described above. The first-order recycling rate constant (kr) was determined using nonlinear regression to calculate the rate at which internalized receptors recycle to the maximal extent, as described by us previously [19]. The data were also plotted without modeling to show a time course of internalization and return of receptors to the cell surface.
Colocalization of β2ARs and EGFP-rab7
12β6 cells were grown on glass coverslips in 6-well clusters to 50–60% confluence, then were transiently transfected for 24 h with EGFP-rab7 DNA using FuGene 6 transfection reagent. For colocalization, the cells were equilibrated for 30 min in fresh media containing either vehicle or 50 μM LY294002, then were treated with 0.1 mM ascorbate, 1 mM thiourea (AT: used to prevent ligand oxidation during prolonged incubations) or AT + 10 μM ISO for 1, 2, or 4 h. The cells were washed with ice-cold 1X PBSS (PBS with 1.2% sucrose), fixed with 4% paraformaldehyde at 4°C, then washed at room temperature with 1X PBSS (PBS with 0.1% sucrose). Next, the cells were incubated with 0.34% L-lysine/0.05% Na-periodate, permeabilized with 0.2% Triton X-100, then blocked with 10% normal goat serum. The cells were incubated in primary polyclonal rabbit antibody (4 μg/ml) that recognizes the carboxyl terminus of the β2AR (anti-β2AR-CT) for 1 h, then washed with 1X PBSS and incubated for 30 min with goat anti-rabbit secondary antibodies (1:2000 dilution) conjugated to Alexa 594. After additional washes, the cover slips were mounted on slides with Mowiol containing 2.5% 1,4-diazabicyclo-[2,2,2]octane. Images were captured using an inverted Olympus IX70 microscope fitted with a 100X lens (NA = 1.4) and a Quantix EEV57 back-thinned CCD camera.
Quantification of imaging analysis
Image stacks of thirty-two 0.2 μm optical sections were collected and deconvolved using software from ISee Imaging (Raleigh, NC), which uses a constrained iterative procedure and a predetermined point spread function [20]. Colocalization of EGFP-rab7 with Alexa594-labeled β2ARs was quantified using Metamorph (Universal Imaging Corp., Downingtown, PA). For each cell, three images from the top, middle and bottom of the cell (namely stacks 8, 16 and 24 from the 32 images in the stack) were used to quantify colocalization. The colocalization was measured by the overlap of the green and red fluorophores with each other, which was calculated by Metamorph® as % integrated for each image stack. Both red overlap with green and green overlap with red gave very similar patterns. For each time point of agonist exposure, 6–9 cells from two separate experiments were chosen for analysis on the basis of their displaying a vesicular EFGP-rab7 morphology and minimal EGFP-rab7 expression levels, to minimize any effects of rab7 overexpression.
Transferrin recycling
12β6 cells were incubated in DMEM + 0.1% BSA for 60 min, with LY294002 (50 μM) or vehicle present during the last 30 min at 37°C. Alexa594 holotransferrin (50 μg/ml) and ISO (10 μM) were then added for 30 min. The cells were quickly washed twice with 37°C PBS, then incubation was continued at 37°C with DMEM + 0.1% BSA, 1 μM propranolol, 50 μg/ml holotransferrin and either LY294002 or vehicle for another 40 min. The cells were then fixed and labeled with anti-β2AR-CT primary and goat anti-rabbit Alexa 488 secondary antibodies, as described above.
Measurement of β2AR degradation using surface biotinylation
12β6 cells were grown in 6-well plates, then were surface biotinylated using impermeant non-cleavable EZ-Link Sulfo-NHS-LC-Biotin (Pierce Biotechnology, Rockford, IL) at 1 mg/ml for 30 min at room temperature. Biotinylation was followed by four washes with warm PBS to remove excess reagent. The cells were then incubated in medium for varying times up to 8 h with AT only or AT plus 10 μM ISO. The cells were collected by centrifugation and then lysed in 250 μl DDM lysis buffer (20 mM HEPES, 300 mM NaCl, 0.8% n-dodecyl-β-D-maltoside [DDM], 5 mM EDTA) on ice for 15 min. The samples were centrifuged at 13,000 Xg for 15 min 4°C and the supernatants saved. Streptavidin-agarose beads (50 μl) were added to the supernatant and the mixture was rotated for 1 h at 4°C. The volume of beads used was predetermined to be in excess of that needed to recover >90 % of the intact receptors remaining in the cell lysate. The beads were recovered by centrifugation and then washed with 500 μl of cold DDM lysis buffer. The biotinylated proteins were eluted from the beads in 50 μl of SDS sample buffer. The samples were then heated at 60°C for 15 min, centrifuged to remove the resin and the supernatant, then treated with peptide:N-glycosidase F (New England Biolabs, Beverly, MA) for 1–2 h at 37°C to deglycosylate receptors. Equivalent protein amounts (based on the streptavidin bead supernatants) were electrophoresed on 10% Tris-glycine gels and electroblotted. The membranes were probed with rabbit anti-β2AR-CT antibodies (1:1000), then washed and incubated with goat anti-rabbit IgG secondary antibodies (1:80,000). The bound antibodies were detected by chemiluminescence and then visualized and quantified with a FluorChem 8800 CCD imaging system equipped with automatic bloom detection (Alpha Innotech Corporation, San Leandro, CA). Receptors were quantified in the linear range of detection, as determined by immunoblotting of serial dilutions of 12β6 cell extracts. The receptor band intensity was normalized to the band intensity of cell surface Na+/K+-ATPase used as a loading control. At each time point, the normalized levels of receptors remaining after incubation with AT + ISO were divided by the normalized levels remaining after incubation with AT only.
Results
Treatment with PI 3-kinase inhibitors augments agonist-induced β2AR internalization
To determine the effect of PI 3-kinase inhibitors on the cellular trafficking of agonist-activated β2ARs, various studies were performed on HEK293 cells stably expressing β2ARs (12β6 cells). Initially, we determined the concentration and effect of the PI3-kinase inhibitor, LY 294002 on receptor internalization. Cells were treated with varying concentrations of LY294002, followed by a saturating concentration of a full agonist, isoproterenol (ISO) for 20 min as detailed in the methods. Radioligand binding studies showed that treatment with LY294002 augmented agonist-induced receptor internalization in a dose-dependent manner, as indicated by lower levels of surface receptors after agonist treatment (Fig. 1A). These results were further confirmed in an independent line of HEK293 cells expressing β2ARs. We also found that these results are not specific to LY294002 since similar results were obtained with the structurally unrelated PI 3-kinase inhibitor wortmannin (data not shown). The decrease in surface receptors required the presence of agonist (Fig. 1A, inset, and Fig. 1 legend). A 50 μM concentration of LY294002 was used in subsequent studies since it is known to be stable during prolonged incubations [21]. We also verified that LY294002 is stable during prolonged incubation, as medium from cells treated for 24 h with the LY294002 augmented agonist-induced β2AR internalization when applied to fresh 12β6 monolayers (data not shown).
Figure 1. LY294002 augments the decrease in the number of surface β2ARs caused by exposure to agonist.
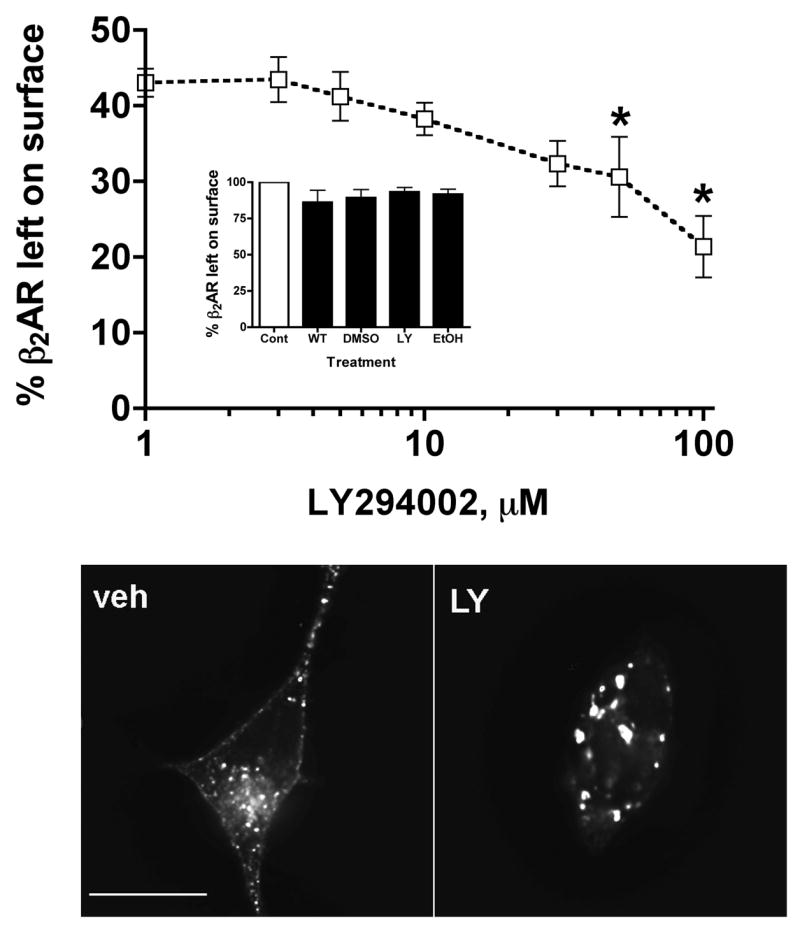
(A). 12β6 cells were treated with varying concentrations of LY294002 for 30 min, then with 10 μM ISO for 20 min followed by chilling to 4°C. Surface receptor levels were measured by binding to [3H]CGP12177 as described in the Materials and Methods. (*) Significantly different from vehicle control (P < 0.05; N=3), one-way ANOVA, Dunnett’s multiple comparisons post-test. A one-way ANOVA with a post-test for linear trends is also significant (P < 0.0001). Inset. 12β6 cells were treated for 30 min with LY294002 (LY- 50 μM), wortmannin (WT- 100nM), ethanol vehicle (EtOH – 0.075%), DMSO vehicle (DMSO – 0.1%), or not treated, then chilled and incubated on ice with [3H]CGP 12177 as described in the Materials and Methods. Compared to the untreated control, the percentage of bound radioligand remaining on the surface was LY, 93.3 ± 3.0; WT, 86.12 ± 8.32; Eth, 91.7 ± 3.49; DMSO, 89.32 ± 5.61. No pair of values was significantly different (P > 0.05, one-way ANOVA, Tukey's Multiple Comparison Test). (B). 12β6 cells were treated with 50 μM LY294002 or vehicle for 30 min, then with 10 μM ISO for 20 min followed by fixation with 4% paraformaldehyde and labeling with antibodies against the β2AR HA-tag. The cells were imaged by deconvolution immunofluorescence microscopy. Scalebar = 10 μm.
Further analysis of β2ARs by immunofluorescence microscopy of LY294002-treated cells fixed after 20 min of ISO exposure showed that internalized β2ARs localized to relatively enlarged endocytic vesicles compared to vehicle-treated cells (Fig. 1B), and similar results were obtained with wortmannin-treated cells (not shown).
The apparent increase in receptor internalization could be due to an increased rate of endocytosis or a decreased rate of β2AR recycling [19]. To determine the effect of PI 3-kinase inhibitors on the rate of receptor endocytosis, a time-course of receptor internalization following agonist addition was performed. In the presence of 50 μM LY294002, surface receptor levels reached a lower steady-state level compared with vehicle-treated cells (Fig. 2A), consistent with the dose-response data (Fig. 1A), and reflecting augmented receptor internalization. The initial endocytic rate constant was estimated by performing a semi logarithmic transformation of the data describing surface receptor loss over the first three min of endocytosis, before recycling or other sorting events are likely to have occurred to any significant extent. The slopes of the lines produced in these plots are equivalent to the first-order endocytic rate constants [19]. The initial rate of agonist-induced β2AR endocytosis is the same in the presence of vehicle or LY294002 (Fig. 2B, legend).
Figure 2. LY294002 does not affect the initial rate of β2AR endocytosis.
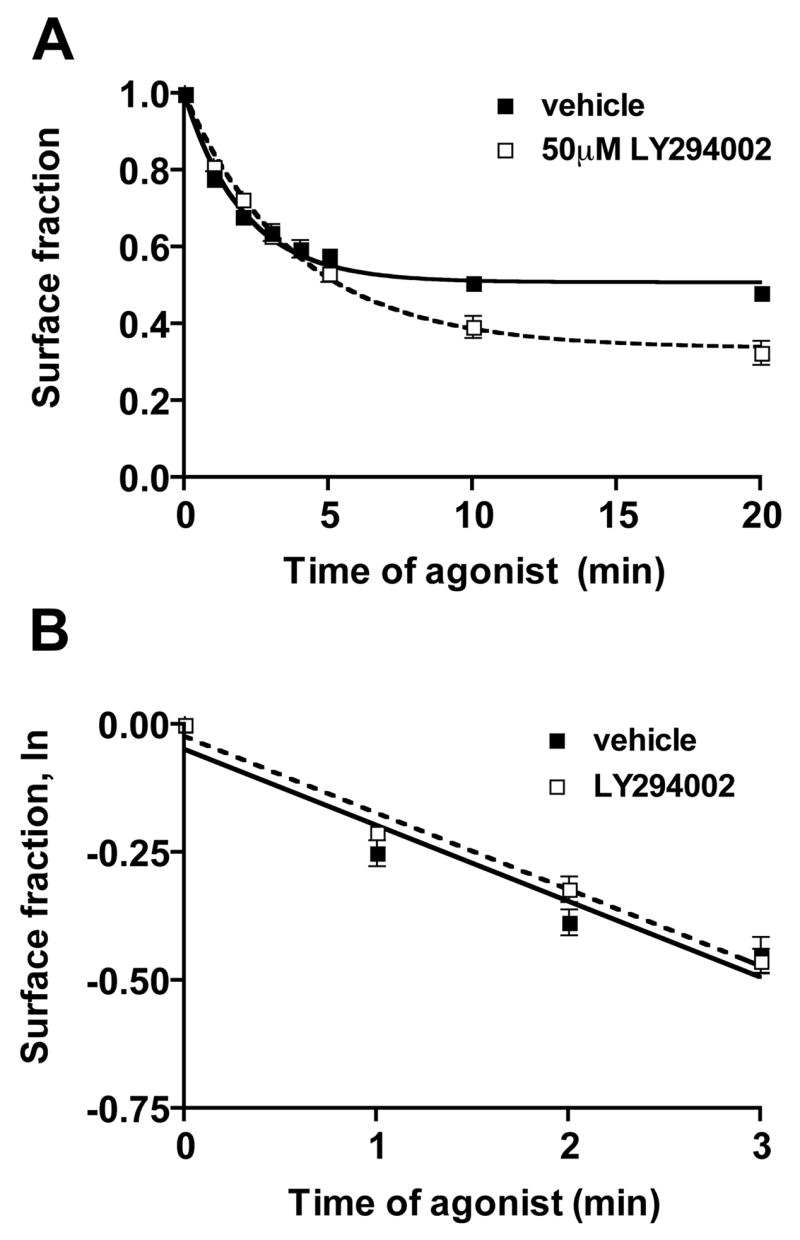
12β6 cells were treated with 50 μM LY294002 or vehicle for 30 min, then for varying times with 10 μM ISO followed by chilling to 4°C. Surface receptor levels were measured by binding to [3H]CGP12177 as described in the Materials and Methods. (A) Time-course of internalization, modeled according to Morrison et al [19]. The calculated plateau values, which represent the maximal fraction of receptors left on the cell surface, were 0.48 ± 0.01 for vehicle-treated control cells, and 0.32 ± 0.03 for LY294002-treated cells (P = 0.0097; N=3); (B) A plot of the natural logarithm of the surface receptor fraction remaining during the first three min after addition of agonist. (■) Vehicle; (□) LY294002. For vehicle treated cells, the slope of the line is −0.15 ± 0.02, and for LY295002-treated cells, − 0.15 ± 0.01, representing first order rate constants of 0.15 min−1 for both vehicle and LY294002-treated cells.
The extent but not the rate of β2AR recycling is decreased by LY294002 treatment
Since our results show that LY294002 does not affect the initial rate of β2AR endocytosis, we next measured the rate and extent of receptor recycling. 12β6 cells were treated with agonist for 20 min to achieve a steady-state fraction of internal receptors in the presence of vehicle or LY294002. The cells were then washed extensively and incubated at 37°C for varying times in the presence of vehicle or LY294002, prior to measuring surface receptor levels using [3H]CGP12177. The recycling data in Fig. 3A were transformed so that the curves describe the rate of loss of internal receptors via their recycling to the plasma membrane. The most striking difference between the recycling curves (Fig. 3A) is that the number of nonrecyclable receptors is increased in LY294002-treated cells. The plateau values of the recycling curves were determined by nonlinear regression, and were significantly different (40.0 ± 2.5% for LY294002-treated cells and 23.8 ± 2.7% for vehicle-treated control cells; P = 0.047). In contrast, the first-order recycling rate constants, as determined by nonlinear regression, did not differ significantly (Fig. 3, legend). When plotted as time course to show the return of receptors to the surface after the removal of agonist, the recycling curves are very similar, and differ mainly due to the differing extents of receptors internalization (Fig. 3B).
Figure 3. LY294002 treatment inhibits the recycling of β2ARs.
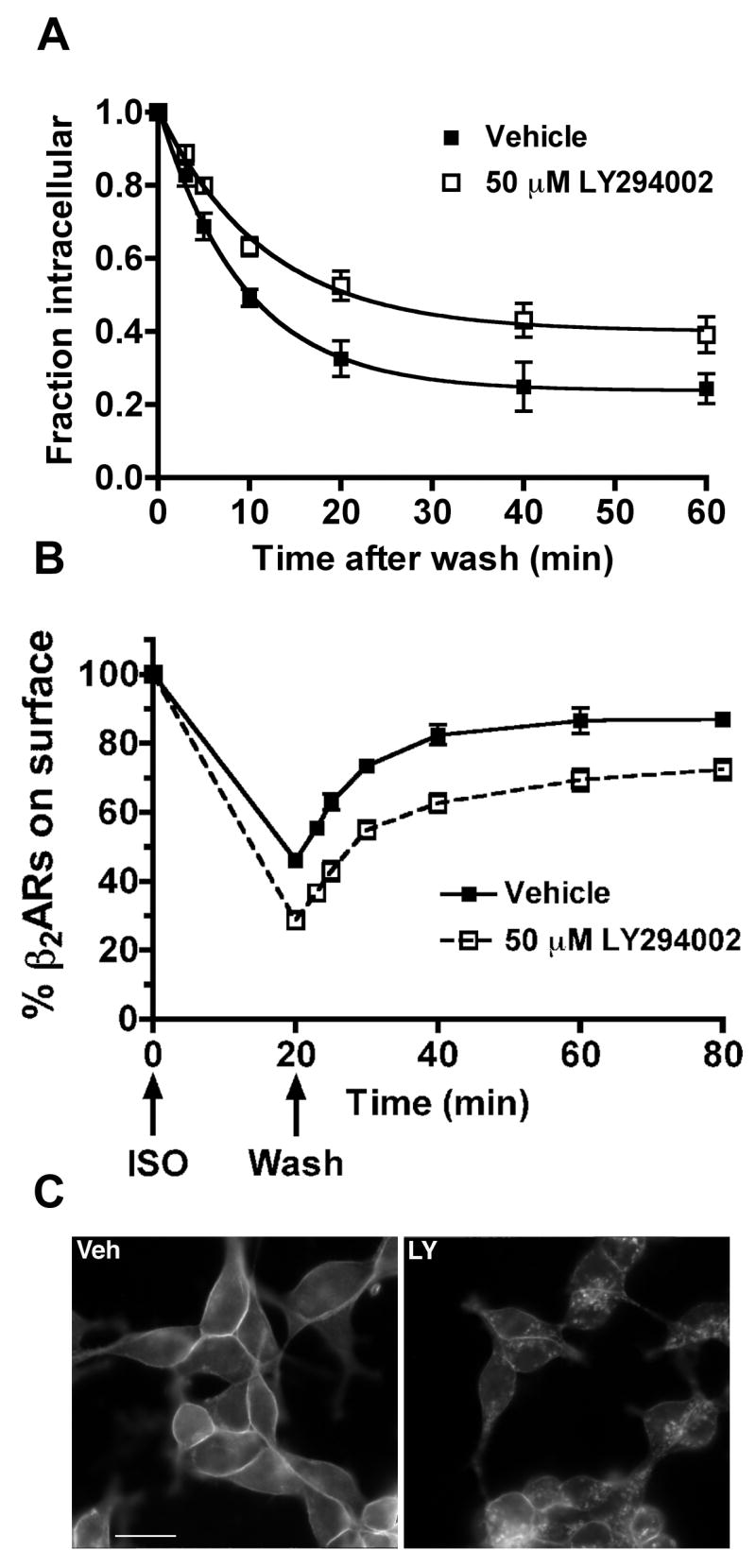
(A). 12β6 cells were treated with 50 μM LY294002 or vehicle for 30 min, then with 10 μM ISO for 20 min. The cells were washed extensively and returned to medium for varying times in the presence of 50 μM LY294002 or vehicle. After chilling on ice, the cells were incubated with [3H]CGP12177 to selectively label surface receptors. The fractional increases in surface receptors as a function of time after agonist removal were transformed to allow the plotting of the loss of internal receptors. The plots were then modeled using a first-order exponential function as described in Morrison et al [19] to obtain a plateau value for the number of receptors that fail to recycle, and the first order recycling rate constant. (■) Vehicle; (□) LY294002. The plateau values of the recycling curves were 40.0 ± 2.5% for LY294002-treated cells and 23.8 ± 2.7% for vehicle-treated control cells (P = 0.047; N=34). The recycling rate constants were 0.107 ± 0.014 min−1 for vehicle-treated cells and 0.085 ± 0.012 min−1 for LY294002-treated cells (the rates are not significantly different). (B). Recycling was plotted as a time course showing the return of receptors to the cell surface. (C). Immunofluorescence imaging of recycled β2ARs. Veh: 12β6 cells were treated with vehicle alone for 30 min, followed by 10 μM ISO for 20 min. The cells were then washed and incubated at 37°C in the presence of vehicle. Recycling was stopped after 40 min by fixation with 4% formaldehyde. LY: 12β6 cells were treated with 50 μM LY294002 for 30 min, followed by 10 μM ISO for 20 min. The cells were then washed and incubated at 37°C in the continuing presence of 50 μM LY294002. Recycling was stopped after 40 min by fixation with 4% formaldehyde. The fixed cells were labeled with antibodies against the β2AR C-terminus and imaged by conventional epifluorescence microscopy. Scalebar, 10 μm.
The internal receptors that remained after 40 min of recycling were readily visualized by immunofluorescence microscopy in cells treated with LY294002 compared with vehicle-treated cells (Fig. 3C). Approximately 90% of the cells showed such intracellular receptors, and similar results were obtained using wortmannin (data not shown).
The effect of LY294002 on transferrin recycling
Inhibition of PI 3-kinase reduces the rate of transferrin recycling in some studies [22], and there is overlap in the endosome compartment between internalized β2ARs and transferrin [7]. We thus determined whether the extent of transferrin and β2AR recycling were similarly inhibited by LY294002 in 12β6 cells under our experimental conditions. A pulse-chase procedure was performed, where we fed cells with fluorescent transferrin during a 30 min exposure to ISO, then washed and incubated for 40 min in the presence of 1 μM propranolol and excess unlabeled holotransferrin to permit the recycling of both β2ARs and transferrin receptors. Although β2ARs were still trapped inside cells after 40 min of recycling in the presence of LY294002, transferrin recycling under these experimental conditions was relatively unaffected (Fig. 4).
Figure 4. The effect of LY294002 treatment on the recycling of transferrin.
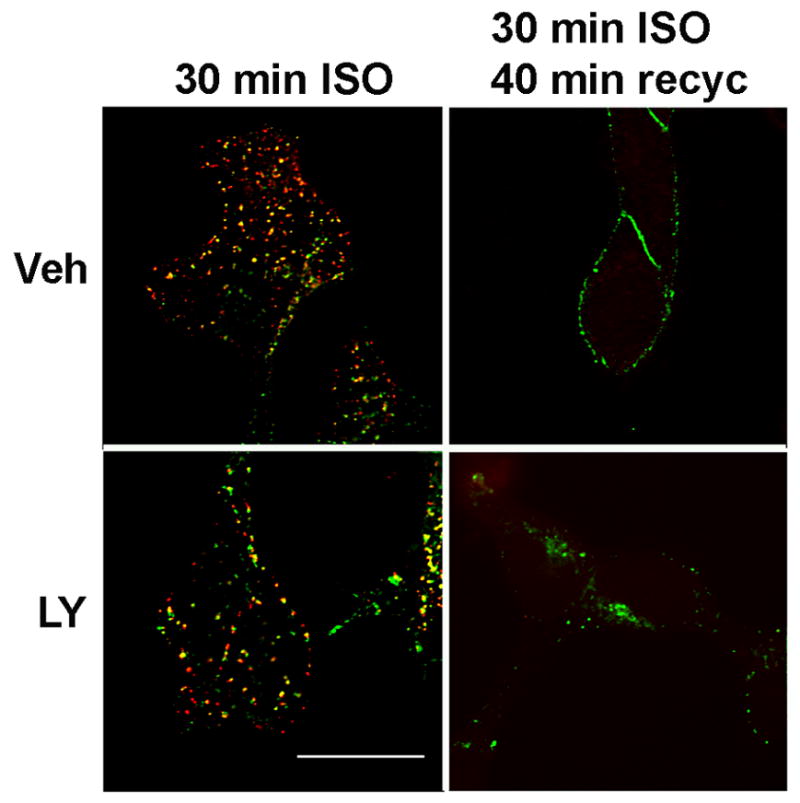
12β6 cells were treated with 50 μM LY294002 or vehicle for 60 min in DMEM + 0.1% BSA at 37°C. ISO (10μM) and 50 μg/ml Alexa 594-holotransferrin were then added for 30 min at 37°C. The medium was removed and the cells were washed twice with PBS, and then were incubated for 40 min at 37°C with 1 μM propranolol and 50 μg/ml holotransferrin with either vehicle or 50 μM LY294002. The cells were fixed and then imaged using deconvolution fluorescence microscopy. The experiment was performed twice with similar results.
β2AR degradation is slowed by LY294002
PI 3-kinase inhibitors reduce the rate of degradation of some internalized growth factor receptors [16, 17]. During prolonged exposure to agonist, β2ARs undergo downregulation, a loss of receptors that correlates with their movement through late endosomes and into lysosomes [9, 10]. Given that our results above show that PI 3-kinase inhibitors cause the intracellular retention of β2ARs, we expected that there might be changes in the rate of β2AR degradation or a change in the final extent of degradation. We treated 12β6 cells for up to 8 h with vehicle or LY294002 in the presence or absence of agonist. There was a significant reduction in the initial rate of β2AR degradation, though the final extent of β2ARs degraded after 8 h was similar in both LY294002 and vehicle-treated cells (Fig. 5).
Figure 5. LY294002 delays agonist-induced degradation of β2ARs.
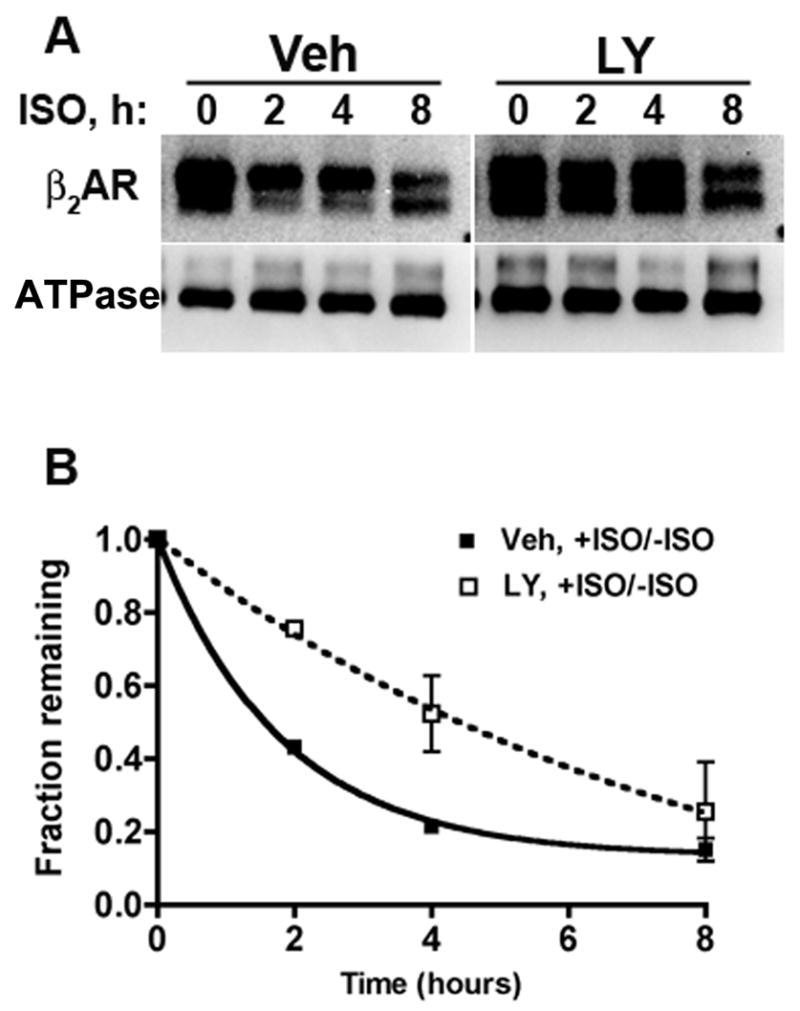
12β6 cells were incubated for 30 min with 50 μM LY294002 or vehicle, then surface biotinylated as described in Materials and Methods. The cells were incubated up to 8 h in medium with or without 10 μM ISO and with 50 μM LY294002 or vehicle. The cells were lysed and biotinylated proteins were recovered with streptavidin agarose beads. After elution from the beads, the proteins were deglycosylated and immunoblotted with antibodies to the β2AR C-terminus and to the cell-surface Na+,K+ATPase (ATPase). Blots were imaged and quantified using a CCD camera system as described in Materials and Methods. (A). Representative blots, showing deglycosylated β2ARs migrating at ∼35 kDa, and Na+,K+ ATPase migrating at ∼100 kDa. (B). Plot of the density of the receptor bands corrected for incubation after the same time period with no agonist present, and normalized to the Na+,K+ ATPase signal. The error bars show the range of two determinations.
Enhanced colocalization of β2ARs and EGFP-rab7 after treatment with LY294002
Our β2AR degradation studies showed that the degradation of β2ARs was delayed in the presence of LY294002, which implies that receptors were eventually sorted to lysosomes. We next sought to determine whether the reduction in the initial β2AR degradation rate caused by LY294002 was due to a delay in the sorting of receptors to late endosomes, an intermediate compartment between early endosomes and lysosomes. We transfected 12β6 cells with EGFP-rab7 to mark the late endosomal compartment, then treated the cells with LY294002 or vehicle for 30 min prior to the addition of ISO for 0–4 h. The cells were fixed and labeled with anti-β2AR antibodies, then imaged by deconvolution fluorescence microscopy. As shown in Fig. 6A, β2ARs rapidly accumulated within EGFP-rab7-containing vesicles in LY294002-treated cells compared with vehicle-treated cells. The imaging data are quantified in Fig. 6B, as a percentage integrated of overlap between the two fluorophores (red over green). The results are similar whether red over green or green over red are measured (Table 1).
Figure 6. LY294002 increases β2AR and EGFP-rab7 colocalization after prolonged agonist treatment.
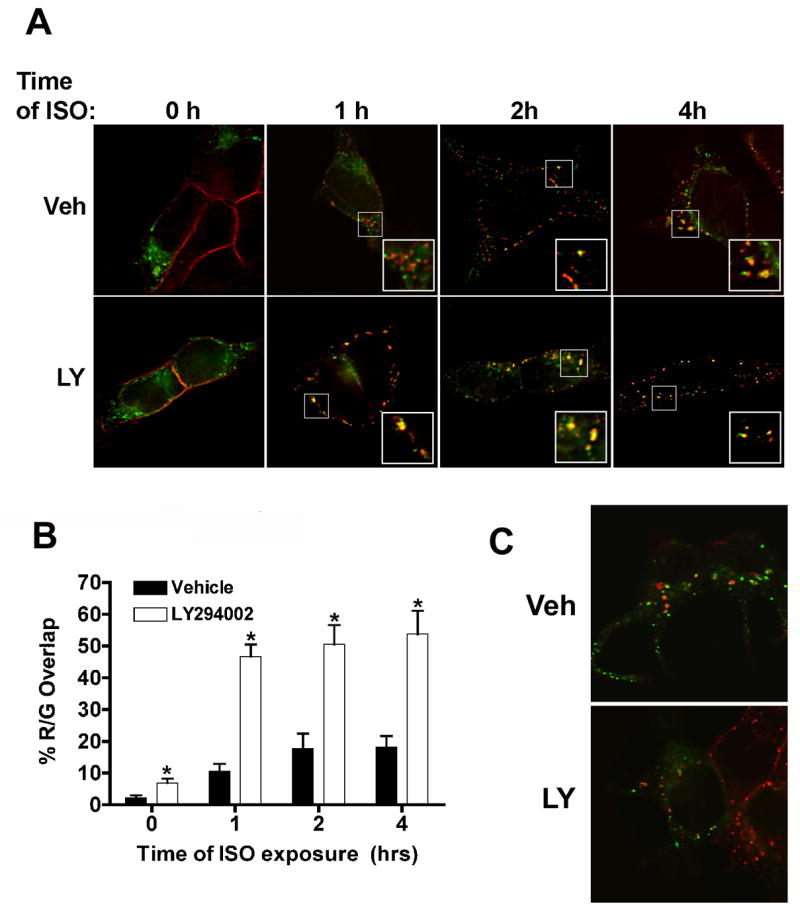
12β6 cells were transfected with a plasmid expressing EGFP-rab7 for 24 h, then treated with 50 μM LY294002 or vehicle for 30 min prior to the addition of 10μM ISO + AT. (A) After 1–4 h of agonist exposure, the cells were fixed and then labeled with antibodies to the β2AR C-terminus (Alexa 594 secondary). Red, β2AR; Green, EGFP-rab7. The labeled cells were imaged by deconvolution microscopy as described in Materials and Methods. Insets: Two-fold magnifications of boxed areas in the panels. Scale bar = 10μm. (B) Quantification of β2AR and EGFP-rab7 overlap during prolonged agonist exposure. The overlap between β2AR and EGFP-rab7 (red over green) at various times following the addition of AT alone or AT + ISO was quantified using Metamorph as described in the Materials and Methods. The Y-axis is the percentage of β2AR labeling that overlaps with EGFP-rab7. (*) Significantly different from vehicle control: 1 h, P<0.0001; 2 h, P< 0.0007; 4 h, P< 0.005. (C) The overlap between EGFP-rab5a and β2ARs after 2 h of agonist exposure. LY, LY294002; Veh, vehicle. 12β6 cells were transfected, treated and imaged as described above in (A), except that anti-HA antibody mHA.11 was used to detect receptors.
Table 1.
Colocalization of EGFP-rab7 (green) and β2AR (red)
| Red over green | ||||
|---|---|---|---|---|
| ISO, h | Vehicle, % | N | LY294002, % | N |
| 0 | 2.11 ± 0.82 | 9 | 6.83 ± 1.38 | 6 |
| 1 | 10.59 ± 2.29 | 8 | 46.65 ± 3.83 | 8 |
| 2 | 17.60 ± 4.84 | 9 | 50.47 ± 6.11 | 9 |
| 4 | 18.05 ± 3.62 | 9 | 53.74 ± 7.34 | 9 |
| Green over red | ||||
| ISO, h | Vehicle, % | N | LY294002, % | N |
| 0 | 2.57 ± 0.78 | 9 | 9.21 ± 1.23 | 6 |
| 1 | 9.94 ± 2.64 | 8 | 43.09 ± 4.42 | 8 |
| 2 | 15.75 ± 2.35 | 9 | 43.99 ± 4.62 | 9 |
| 4 | 18.08 ± 3.67 | 9 | 49.30 ± 6.38 | 9 |
To provide evidence against nonspecific colocalization of intracellular markers caused by LY294002, we transfected cells with EGFP-rab5a and treated with agonist for 2 h. Inhibition of PI 3-kinase did not cause significant colocalization of rab5a and β2ARs (Fig. 6C).
Discussion
Our major finding is that inhibitors of PI 3-kinase disrupt the postendocytic sorting of β2ARs. This is manifested in three ways: (1) internalized β2ARs are prevented from recycling normally to the cell surface (Fig, 3C); (2) the rate of agonist-induced receptor degradation is slowed (Fig. 5); and (3) β2ARs accumulate in EGFP-rab7-positive vesicles (Fig. 6). The effect on recycling is different for β2ARs compared with transferrin receptors (Fig. 4), as the latter appear to enter a recycling pathway that is insensitive to the inhibitor during a simultaneous 30 min period of internalization for both receptors. All of these effects are caused by a concentration of PI 3-kinase inhibitor (LY294002) that has no detectable effect on the initial rate of agonist-induced β2AR endocytosis (Fig. 2).
Although PI 3-kinase inhibitors reduce the number of recyclable β2ARs, the intrinsic or default rate of receptor recycling is not detectably altered (Fig. 3A and B). This suggests that while PI 3-kinase inhibitors inhibit receptor sorting into the recycling pathway, these drugs do not appear to significantly affect the machinery of the β2AR recycling pathway itself. Also, after recycling for 40 min in the absence of agonist and in the presence of LY294002 or wortmannin, a significant number of β2ARs become trapped within an intracellular compartment (Fig. 3C). This compartment seems to consist of aberrant endosomes, because these internal receptors did not localize with several markers of intracellular compartments, namely EGFP-galactosyltransferase (trans-Golgi network), EGFP-rab5 (early endosome), EGFP-rab11 (perinuclear recycling endosome), EGFP-rab7 (late endosome) or LAMP-1 (lysosome) (data not shown). Only after prolonged and continuous agonist exposure driving receptor endocytosis in the presence of LY294002 was β2AR colocalization with EGFP-rab7 significant (Fig. 6).
In previously published studies, treatment of cells with wortmannin or LY294002 reduced transferrin recycling rates [14, 15, 22], or had minimal effects [13, 23], while the recycling kinetics of AT1 angiotensin receptors were reduced [18]. In contrast, LY294002 had no measurable effect on the recycling kinetics (kr) of β2ARs (Fig. 3A, B), although the extent of β2AR recycling was reduced due to the intracellular retention of receptors (Fig. 3C). In comparing the recycling of transferrin and β2AR, it should be noted that our transferrin labeling protocol was sufficiently prolonged such that most of the label had entered recycling endosomes, which are known to be refractory to the effects of PI 3-kinase inhibitors. However, our data do suggest that transferrin and β2ARs do not follow identical intracellular itineraries during recycling.
In terms of receptor trafficking, GPCRs are classified as class A, which rapidly and completely recycle, and class B, which do not [24]. A further distinction is that β-arrestin internalizes into punctate vesicles with class B receptors, but do not with class A. It is unlikely that PI 3-kinase inhibition causes the class A β2AR to behave like a class B receptor, because we do not observe punctate intracellular β-arrestin in the presence of LY294006 after treatment with the agonist, ISO (data not shown).
In addition to reducing the extent of β2AR recycling, LY294002 significantly reduces the rate of agonist-induced degradation as assessed by a cell-surface biotinylation assay (Fig. 5). This finding is consistent with previous studies on the effects of PI 3-kinase inhibitors on the degradation of other signaling receptors, such as the PDGF receptor [16] and the EGF receptor [25, 26] There are at least three PI 3-kinases in mammalian cells, and the most commonly used PI 3-kinase inhibitors act on all three [27]. Evidence from other studies implicates the type III PI 3-kinase hVps34 in controlling traffic to lysosomes [28, 29]. Whether hVps34 is the PI 3-kinase responsible for controlling β2AR sorting to lysosomes will require additional studies.
PtdIns3P localizes to specific microdomains of endosomes [30, 31], recruiting proteins that contain PtdIns3P-binding domains and control receptor sorting. The two most important PtdIns3P-binding domains are the FYVE (Fab1, YOTB, Vac1 and EEA1) zinc finger domain [32] and the PH (phox-homology) domain [33]. Proteins with these domains play a role in sorting various ubiquitinated receptors from late endosomes to lysosomes. Agonist induced β2AR degradation could occur by this mechanism, because these receptors are ubiquitinated after agonist activation, and removal of all potential sites of β2AR ubiquitination reduces receptor downregulation [34]. Our finding that LY294002 inhibits β2AR degradation supports this possibility. Further studies are needed to identify those PtdIns3P -binding proteins that are needed for the proper postendocytic sorting of β2ARs.
In the presence of PI 3-kinase inhibitors and prolonged agonist activation, the reduction in β2AR degradation was accompanied by an accumulation of receptors in EGFP-rab7-containing vesicles. Rab7 localizes to late endosomes [35] and controls the traffic of membrane proteins from the early endosome to lysosomes [36-41]. The enhanced colocalization of β2ARs and EGFP-rab7 in late endosomes in the presence of LY294002 suggests that PI 3-kinase is required for the transport of membrane proteins from late endosomes to lysosomes. The delay we observe in agonist-induced β2AR degradation (Fig. 5) is consistent with this interpretation. The molecular mechanisms underlying rab7 action are not clear at present, however a recent study shows that rab7 binds to hVps34 (a PI 3kinase) and localizes it to late endosomes [29].
PI 3-kinase inhibitors did not affect the initial rate of β2AR endocytosis in our system (Fig. 2B). This is consistent with other studies where the endocytosis of transferrin receptors [14, 15], β2ARs [42] and AT1 angiotensin receptors [18] were measured in the presence of concentrations of wortmannin low enough to prevent nonspecific effects. Other studies have suggested that wortmannin inhibits agonist-induced β2AR endocytosis, and that agonist-dependent recruitment of a PI 3-kinase to the plasma membrane is important for this process [43]. The discrepancy is most likely due to the different β2AR expression methods used. Our study was performed in stably transfected HEK293 cells, which have a much higher initial rate of β2AR endocytosis and higher coupling efficiencies than the transiently transfected HEK293 cell systems utilized in the other studies [43]. Furthermore, we observed the same results on endocytosis using an independently derived stably transfected line of HEK293 cells (data not shown). Therefore, it does not appear that cell line variation explains the difference between our study and those previously published. An inhibitory effect of PI 3-kinase inhibition on β2AR endocytosis may be much more apparent when the endocytic rate constant is low to begin with, and when endocytic machinery is near saturation due to very high levels of receptor expression due to transient transfection [44]. We cannot rule out that PI 3-kinase inhibitors produce a slight reduction of endocytosis in our stably transfected cells that is not detectable by our methods. However, we can clearly conclude from our study that PI3-kinase inhibitors have a much more prominent effect on the postendocytic sorting of β2ARs than on β2AR endocytosis per se. Our data suggest an important role for PI 3-kinases in the sorting of β2ARs between the recycling and degradative pathways.
Acknowledgments
We thank D. Eikenburg and A. Bean for critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (R01HL064934, R.H.M. and R01 HL50047, B.J.K.) and the American Heart Association, Texas Affiliate (0455072Y, B.J.K.). Important technical assistance was provided by L. Iles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tran TM, Friedman J, Qunaibi E, Baameur F, Moore RH, Clark RB. Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the ®2-adrenergic receptor using phosphoserine-specific antibodies. Mol Pharmacol. 2004;65:196–206. doi: 10.1124/mol.65.1.196. [DOI] [PubMed] [Google Scholar]
- 2.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. ®-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan N, Friedman J, Whaley BS, Clark RB. cAMP-dependent protein kinase and protein kinase C consensus site mutations of the ®-adrenergic receptor. Effect on desensitization and stimulation of adenylyl cyclase. J Biol Chem. 1994;269:23032–23038. [PubMed] [Google Scholar]
- 4.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. ®-arrestin: a protein that regulates ®-adrenergic function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 5.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. ®-arrestin acts as a clathrin adaptor in endocytosis of the ®2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 6.Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The ®-adrenergic receptor/®arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Zastrow M, Kobilka BK. Ligand-regulated internalization and recycling of human ®2- adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 8.Moore RH, Sadovnikoff N, Hoffenberg S, Liu S, Woodford P, Angelides K, Trial J, Carsrud NDV, Dickey BF, Knoll BJ. Ligand-stimulated ®2-adrenergic receptor internalization via the constitutive endocytic pathway into rab5-containing endosomes. J Cell Sci. 1995;108:2983–2991. doi: 10.1242/jcs.108.9.2983. [DOI] [PubMed] [Google Scholar]
- 9.Gagnon AW, Kallal L, Benovic JL. Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the ®2-adrenergic receptor. J Biol Chem. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- 10.Moore RH, Tuffaha A, Millman EE, Hall HS, Dai W, Dickey BF, Knoll BJ. Agonist-induced sorting of human ®2-adrenergic receptors to lysosomes during downregulation. J Cell Sci. 1999;112:329–338. doi: 10.1242/jcs.112.3.329. [DOI] [PubMed] [Google Scholar]
- 11.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 12.Jess TJ, Belham CM, Thomson FJ, Scott PH, Plevin RJ, Gould GW. Phosphatidylinositol 3'-kinase, but not p70 ribosomal S6 kinase, is involved in membrane protein recycling: wortmannin inhibits glucose transport and downregulates cell-surface transferrin receptor numbers independently of any effect on fluid-phase endocytosis in fibroblasts. Cell Signal. 1996;8:297–304. doi: 10.1016/0898-6568(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 13.Shpetner H, Joly M, Hartley D, Corvera S. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J Cell Biol. 1996;132:595–605. doi: 10.1083/jcb.132.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martys JL, Wjasow C, Gangi DM, Kielian MC, McGraw TE, Backer JM. Wortmannin-sensitive trafficking pathways in Chinese hamster ovary cells. Differential effects on endocytosis and lysosomal sorting. J Biol Chem. 1996;271:10953–10962. doi: 10.1074/jbc.271.18.10953. [DOI] [PubMed] [Google Scholar]
- 16.Joly M, Kazlauskas A, Corvera S. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet–derived growth factor receptor trafficking. J Biol Chem. 1995;270:13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- 17.Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3- kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- 18.Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, Lodge R, Catt KJ, Balla T. Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol. 2002;157:1211–1222. doi: 10.1083/jcb.200111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison KJ, Moore RH, Carsrud NDV, Millman EE, Trial J, Clark RB, Barber R, Tuvim M, Dickey BF, Knoll BJ. Repetitive endocytosis and recycling of the ®2-adrenergic receptor during agonist-induced steady-state redistribution. Mol Pharmacol. 1996;50:692–699. [PubMed] [Google Scholar]
- 20.Hiraoka Y, Sedat JW, Agard DA. The use of a charge-coupled device for quantitative optical microscopy of biological structures. Science. 1987;238:36–41. doi: 10.1126/science.3116667. [DOI] [PubMed] [Google Scholar]
- 21.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 22.van Dam EM, Ten Broeke T, Jansen K, Spijkers P, Stoorvogel W. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2002;277:48876–48883. doi: 10.1074/jbc.M206271200. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd PR, Soos MA, Siddle K. Inhibitors of phosphoinositide 3-kinase block exocytosis but not endocytosis of transferrin receptors in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1995;211:535–539. doi: 10.1006/bbrc.1995.1846. [DOI] [PubMed] [Google Scholar]
- 24.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of ®-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248– 32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 25.Futter CE, Collinson LM, Backer JM, Hopkins CR. Human VPS34 is required for internal vesicle formation within multivesicular endosomes. J Cell Biol. 2001;155:1251–1264. doi: 10.1083/jcb.200108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petiot A, Faure J, Stenmark H, Gruenberg J. PI3P signaling regulates receptor sorting but not transport in the endosomal pathway. J Cell Biol. 2003;162:971–979. doi: 10.1083/jcb.200303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 28.Siddhanta U, McIlroy J, Shah A, Zhang Y, Backer JM. Distinct roles for the p110®and hVPS34 phosphatidylinositol 3'-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J Cell Biol. 1998;143:1647–1659. doi: 10.1083/jcb.143.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein MP, Feng Y, Cooper KL, Welford AM, Wandinger-Ness A. Human VPS34 and p150 are rab7 interacting partners. Traffic. 2003;4:754–771. doi: 10.1034/j.1600-0854.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 30.Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillooly DJ, Raiborg C, Stenmark H. Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem Cell Biol. 2003;120:445–453. doi: 10.1007/s00418-003-0591-7. [DOI] [PubMed] [Google Scholar]
- 32.Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R. FYVE fingers bind PtdIns(3)P. Nature. 1998;394:432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- 33.Ellson CD, Andrews S, Stephens LR, Hawkins PT. The PX domain: a new phosphoinositide-binding module. J Cell Sci. 2002;115:1099–1105. doi: 10.1242/jcs.115.6.1099. [DOI] [PubMed] [Google Scholar]
- 34.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2- adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 35.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Press B, Wandinger-Ness A. Rab7: An important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukhopadhyay A, Funato K, Stahl PD. Rab7 regulates transport from early to late endocytic compartments in Xenopus oocytes. J Biol Chem. 1997;272:13055–13059. doi: 10.1074/jbc.272.20.13055. [DOI] [PubMed] [Google Scholar]
- 38.Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni CB, Bucci C. Role of the small GTPase RAB7 in the late endocytic pathway. J Biol Chem. 1997;272:4391–4397. doi: 10.1074/jbc.272.7.4391. [DOI] [PubMed] [Google Scholar]
- 39.Press B, Feng Y, Hoflack B, Wandinger-Ness A. Mutant rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dale LB, Seachrist JL, Babwah AV, Ferguson SS. Regulation of angiotensin II type 1A receptor intracellular retention, degradation, and recycling by Rab5, Rab7, and Rab11 GTPases. J Biol Chem. 2004;279:13110–13118. doi: 10.1074/jbc.M313333200. [DOI] [PubMed] [Google Scholar]
- 41.Ceresa BP, Bahr SJ. rab7 activity affects epidermal growth factor:epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J Biol Chem. 2006;281:1099–1106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen SD, Linseman DA, McEwen EL, Heacock AM, Fisher SK. Inhibition of ®2-adrenergic and muscarinic cholinergic receptor endocytosis after depletion of phosphatidylinositol bisphosphate. J Pharmacol Exp Ther. 1999;290:603–610. [PubMed] [Google Scholar]
- 43.Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by ®-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem. 2001;276:18953–18959. doi: 10.1074/jbc.M102376200. [DOI] [PubMed] [Google Scholar]
- 44.Marullo S, Faundez V, Kelly RB. β2-adrenergic receptor endocytic pathway is controlled by a saturable mechanism distinct from that of transferrin receptor. Receptors Channels. 1999;6:255–269. [PubMed] [Google Scholar]


