Abstract
The chemokine receptor CXCR4 belongs to the large superfamily of G protein-coupled receptors, and is directly involved in a number of biological processes including organogenesis, hematopoeisis, and immune response. Recent evidence has highlighted the role of CXCR4 in a variety of diseases including HIV, cancer, and WHIM syndrome. Importantly, the involvement of CXCR4 in cancer metastasis and WHIM syndrome appears to be due to dysregulation of the receptor leading to enhanced signaling. Herein we review what is currently known regarding the regulation of CXCR4 and how dysregulation contributes to disease progression.
Keywords: CXCR4, receptor regulation, phosphorylation, cancer, WHIM syndrome, signaling
Chemokines are 8–10 kDa cytokines that are classified into four groups (CXC, CC, C, and CX3C) based on the position of the first two cysteines [1]. Chemokine receptors belong to the G protein-coupled receptor (GPCR) superfamily and couple to the pertussis toxin sensitive Gi proteins [2]. In general, chemokines/chemokine receptors exhibit promiscuity, being able to bind multiple receptors/ligands, though 6 of the 18 chemokine receptors bind a single ligand [3]. One of the best studied chemokine receptors is CXCR4, primarily due to its role as a co-receptor for HIV entry [4] as well as its ability to mediate the metastasis of a variety of cancers [5].
CXCR4 is a 352 amino acid rhodopsin-like GPCR and selectively binds the CXC chemokine Stromal Cell-Derived Factor 1 (SDF-1) also known as CXCL12 [2, 6]. Classically, two alternatively spliced isoforms of SDF have been identified. SDF-1α is an 89 amino acid protein that is the predominantly expressed form of SDF-1 while SDF-1β contains a four amino acid extension at the carboxyl terminus [7]. SDF-1α and β bind to CXCR4 with a comparable affinity (Kd of 7.5 and 13.7 nM, respectively) [8]. Recently, an additional four splice variants that contain 30 (SDF-1γ), 31 (SDF-1δ), 1 (SDF-1ε), and 51 (SDF-1φ) amino acid extensions at the carboxyl terminus compared to SDF-1α have been identified [9]. These isoforms are functional and have a differential tissue distribution, however, their functional significance is currently unknown. Mice that lack either SDF-1 or CXCR4 exhibit an almost identical phenotype of late gestational lethality and defects in B cell lymphopoiesis, bone marrow colonization, and cardiac septum formation [10, 11]. These and other studies reveal that CXCR4 is essential for development, hematopoeisis, organogenesis, and vascularization [10–15], in addition to functioning as a classical chemokine receptor in the adult [16, 17].
As a number of reviews have recently been published highlighting CXCR4 as a target in HIV [18–20] and its role in cancer metastasis [5, 21–23], this review will focus on what is known regarding those factors that shape signaling, receptor regulation, and receptor expression, and how dysregulation of these pathways may contribute to disease progression.
Regulation of CXCR4 Expression and Function
Transcriptional Control of CXCR4
In order to understand the role of CXCR4 in disease, a fundamental understanding of the factors regulating expression is critical. While CXCR4 was initially cloned from leukocytes [24, 25], it has since been shown to be expressed in a number of tissues in addition to cells of hematopoetic lineages [26]. The promoter region of CXCR4 contains a number of predicted regulatory consensus sequences [27–29], however, the basal transcription is mainly controlled by the opposing actions of two transcriptional regulators. Functional characterization of the CXCR4 promoter has revealed that Nuclear Respiratory Factor-1 (NRF-1) is the major transcription factor positively regulating the transcription of CXCR4 [28, 29], although a potential role for an additional transcription factor, SP-1, has also been suggested [29]. This work also defined a negative regulatory element upstream (near position −300 of the transcriptional start site) that may be mediated by Ying Yang 1 (YY1) [30].
In addition to the basal regulation of CXCR4 transcription, a number of signaling molecules also have been shown to affect CXCR4 transcription. For example, the expression of CXCR4 can be increased as a result of intracellular second messengers such as calcium [28] and cyclic AMP [31], by the cytokines interleukin-2 (IL-2) [28], IL-4 [32], IL-7 [32], IL-10 [33], IL-15 [32], TGF-1β [33], and simultaneous CD3 and CD28 engagement [28], and by growth factors such as basic fibroblast growth factor (bFGF) [34, 35], vascular endothelial growth factor (VEGF) [35], and epidermal growth factor (EGF) [36]. On the other hand, inflammatory cytokines such as tumor necrosis factor-α (TNF-α) [34, 37, 38], interferon-γ (INF-γ) [37], and IL-1β [37] have all been shown to attenuate CXCR4 expression.
These data clearly show that there is dynamic regulation of CXCR4 transcription as the result of physiological stimuli. Of additional interest are those factors that regulate CXCR4 expression and affect disease progression, such as modulating HIV infection. Alterations in NRF-1 or YY1 activity can lead to an increase or decrease in transcription of CXCR4, respectively, which certain viruses appear to have taken advantage of. The human T lymphotropic virus type I transactivator Tax protein interacts with and enhances NRF-1 activity, which in infected individuals may enhance susceptibility to HIV infection or disease progression [39]. In contrast, individuals infected with human herpes virus 6 have a decrease in cell surface expression of CXCR4 [40]. Investigation into the underlying mechanism has revealed that there is an increase in YY1 binding through a decreased association with c-Myc, a natural suppressor of YY1 activity [41].
Regulation of CXCR4 Protein Expression
A number of co-translational modifications contribute to the expression and function of CXCR4. Within the extracellular domain of CXCR4, there are two potential N-linked glycosylation sites, Asn11 and Asn176 [42]. Both sites undergo glycosylation when CXCR4 is expressed in Sf9 insect cells [43], however, only Asn11 appears to be glycosylated in mammalian cells [44]. SDF and the HIV-1 glycoprotein gp120 bind to a non-overlapping region of the N-terminus of CXCR4 [45–50] and glycosylation has opposing effects on each process. Mutation of Asn11 to glutamine leads to enhanced CD4-dependent binding of both CXCR4-specific and duel tropic (CCR5 and CXCR4) HIV-1 isolates [46, 51, 52]. Conversely, mutation of Asn11 to glutamine [52] or leucine [43] disrupts SDF binding and diminishes signal transduction [52]. Thus, glycosylation of CXCR4 is important for SDF binding and helps to inhibit the use of CXCR4 as an HIV-1 co-receptor.
CXCR4 has also been shown to undergo tyrosine sulfation, a modification catalyzed by tyrosyl protein sulfotransferase within the trans-golgi network. There are three extracellular tyrosines in CXCR4 that are modified by sulfation, Tyr7, Tyr12, and Tyr21, with Tyr21 accounting for the majority of sulfate incorporation [53]. Functionally, tyrosine sulfation of CXCR4 doesn’t regulate co-receptor usage by HIV-1 [53] as is observed with CCR5 [54], however, similar to CCR2b [55], CCR5 [56], and CX3CR1 [57], this is an important modification for ligand binding [53]. Indeed, the structural basis for sulfotyrosine-SDF interaction reveals that sulfotyrosine 21 binds to a specific site on SDF-1 that includes Arg47 [58]. An additional N-terminal modification that has been identified in CXCR4 is addition of a chondroitin sulfate chain at serine 18, although no functional consequence of this modification has been identified [53].
Oligomerization
An emerging theme in GPCR signaling is the formation of homo- and heterodimers [59]. CXCR4 exhibits significant heterogeneity in cells, which may be a result of ubiquitination, differential glycosylation, or the formation of oligomers [60, 61]. It’s been suggested that CXCR4 has the ability to homodimerize in the absence of ligand [62–65], an event that most likely occurs soon after protein translation [62]. However, two reports suggest that SDF can also enhance dimerization [65, 66]. There have also been reports of CXCR4 forming heterodimers with CCR2 and CD4, which may affect the functionality of CXCR4 as a co-receptor for HIV [64, 65, 67–69]. While some studies suggest that CXCR4 does not heterodimerize with CCR5 [62, 63], CD4+ cells isolated from patients with a CCR5Δ32 mutant, a loss-of function mutation that prevents cell surface expression of CCR5, have reduced expression of CXCR4 [70]. Moreover, these studies show that CCR5Δ32 and CXCR4 can interact resulting in reduced cell surface expression of CXCR4 and enhanced resistance to HIV infection [70].
The functional consequences of homo- or heterodimerization are currently not well understood. However, it has been suggested that homodimerization of CXCR4 is necessary to elicit G protein independent activation of JAK/STAT as well as enhance the response of CXCR4 to SDF (see below). Heterodimerization may be a means of achieving an additional level of regulation. For example, it has recently been proposed that non-agonist occupied CCR5 may be phosphorylated by GRK2 activated as a result of heterodimer formation and ligation of C5a [71]. Taken together, homo- and hetero-oligomerization of CXCR4 may be a way of regulating signaling while also allowing for alternative, non-classical, signaling pathways upon activation.
Regulation of CXCR4 Signaling
SDF Binding
The interaction between SDF and CXCR4 has been proposed to occur through a two-step process [72]. The initial interaction between residues 12–17 of SDF and 2–36 of CXCR4 are believed to result in a conformational change in the receptor [73]. This conformational change facilitates interaction between the first eight amino acids of SDF and an exposed binding pocket in CXCR4 that involves residues in both the second (Asp187) and third (Glu268) extracellular loops [45, 50]. As this interaction requires the integrity of both SDF and CXCR4, it is not surprising that proteases are able to inhibit this interaction. During an inflammatory response, neutrophil released cathepsin G and neutrophil elastase have the ability to inactivate SDF by cleaving the N-terminal residues necessary for interacting with CXCR4 [74, 75]. Additionally, the widely expressed cell surface protease dipeptidase 26 (CD26) is also able to cleave and inactivate SDF [76–78]. To date, only neutrophil elastase has been shown to cleave the N terminal domain of CXCR4, effectively disrupting interaction with SDF [75]. Therefore, inflammatory responses promote the release of factors that positively and negatively regulate the receptor. When taken together, these data highlight the exquisite interplay between a variety of factors that are able to shape and influence the SDF-CXCR4 signaling axis, ensuring that the proper physiological response is elicited.
SDF-1 is also able to interact with glycosaminoglycans, such as heparin sulfate, and is most likely immobilized in vivo allowing for gradient formation [79, 80]. Furthermore, this association may induce the oligomerization of SDF-1 [81], a phenomenon observed at high SDF-1 concentrations [72, 82–84], that may promote CXCR4 oligomerization and enhanced function. In fact, it has been shown that the combination of glycosaminoglycans and SDF-1 enhanced migration when compared to SDF alone [85]. Moreover, SDF-1 mediated inhibition of HIV X4 isolates was enhanced in the presence of heparin sulfate [86].
It may also be possible to sensitize CXCR4 to have a greater response to lower SDF-1 concentrations. Recent evidence suggests that products released during inflammatory responses [87] or platelet activation [88, 89] “prime” the SDF response enhancing hematopoetic stem/progenitor cell migration at lower SDF concentrations [87–89]. This phenomenon may be the result of changing the membrane localization of CXCR4 through incorporation into lipid rafts [89]. A number of studies have suggested that lipid raft localization is required for proper function of CXCR4 [90–92] and recently it has been shown that SDF stimulation promotes the incorporation of Src tyrosine kinases, focal adhesion kinase, PI3 kinase and the small G protein Rac into lipid rafts [89]. This agonist promoted clustering of receptor and effectors into lipid rafts might be a way of ensuring that the proper signaling pathways are activated.
G Protein Signaling
Upon activation of CXCR4, a number of signaling pathways are activated leading to a variety of biological responses (Figure 1) [93]. As CXCR4 couples to the Gi family of proteins, the use of pertussis toxin (PTX), which ADP-ribosylates Gαi and inhibits GPCR/Gi coupling, is a useful tool to delineate pathways that are G protein-dependent and -independent. To date, the majority of signaling pathways and biological outcomes of CXCR4 activation are PTX-sensitive and therefore dependent on activation of Gi proteins. Activated Gi is able to inhibit adenylyl cyclase as well as activate the Src family of tyrosine kinases while liberated Gβγ activates phospholipase C-β (PLC-β) and phosphoinositide-3 kinase (PI3K) ultimately leading to the regulation of processes such as gene transcription, cell migration, and cell adhesion (Figure 1).
Figure 1. Signal Transduction Pathways and Regulation of CXCR4.
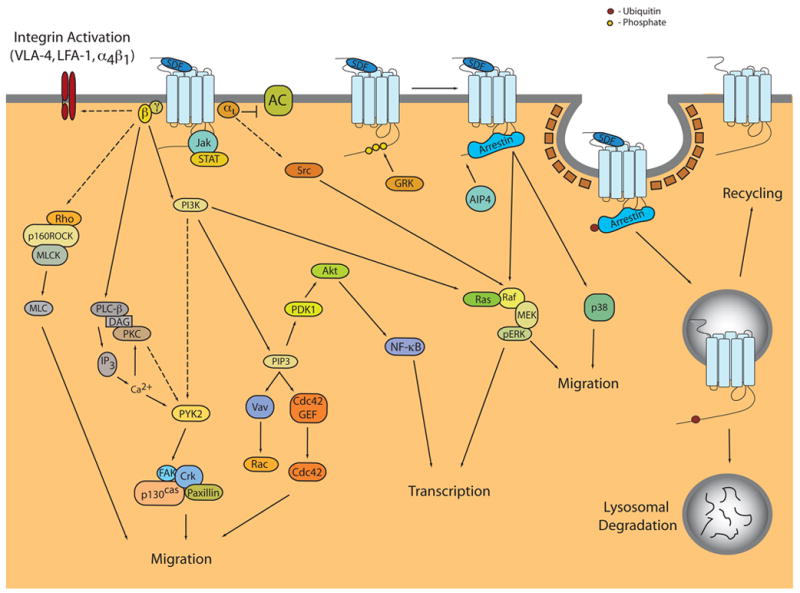
SDF binding to CXCR4 leads to the activation of multiple G protein-dependent signaling pathways, resulting in diverse biological outcomes such as migration, adhesion, and transcriptional activation. Pathways activated and outcomes elicited may differ between CXCR4+ cell types. Two potential G protein-independent pathways have been described. Tyrosine phosphorylation of CXCR4 results in the recruitment and activation of the JAK/STAT pathway, while p38 and ERK activation has been shown to be partially dependent on arrestin-3. Following activation, GRK phosphorylation results in the recruitment of arrestin 2/3 and subsequent internalization. CXCR4 is also ubiquitinated by AIP4 at the plasma membrane, which results in its sorting to and degradation in lysosomes. However, a portion of the internalized receptor may also recycle back to the plasma membrane.
G Protein Independent Signaling
Activation of the JAK/STAT pathway by CXCR4 has been proposed to be G protein independent [66]. SDF induced the transient association of JAK2 and JAK3 with CXCR4, leading to the activation and nuclear translocation of a number of STAT proteins. While JAK/STAT activation was G protein-independent, pretreatment with PTX led to a prolonged association of JAK with CXCR4 suggesting that G protein coupling is involved in JAK/STAT-receptor complex recycling [66].
The non-visual arrestins (arrestin-2 and -3, also called β-arrestin-1 and -2) have classically been considered to shut off signal transduction following receptor activation, a process termed desensitization [94]. Indeed, lymphocytes isolated from arrestin-3 knock out mice display attenuated desensitization and enhanced G protein coupling of CXCR4 [95]. However, these mice also display a decreased chemotactic response to SDF, possibly due to the ability of arrestin-3 to promote signaling [95]. In addition to signal termination, arrestins are able to act as scaffolds for a number of signaling molecules [96, 97]. These interactions may serve to propagate signaling or even create a platform to allow for activation of the proper signaling cascade [98]. Consistent with these observations, it has been reported that arrestin-2 and -3 enhance CXCR4-mediated ERK activation [99] and arrestin-3 is involved in p38 activation and migration following SDF stimulation [100]. Taken together, non-visual arrestins may play a role in regulating CXCR4/Gi interaction as well as SDF-promoted signaling and cell migration.
Regulation of Signaling
Three processes primarily regulate GPCRs: desensitization (homologous and heterologous), internalization, and degradation. The process of homologous desensitization, or becoming refractory to continued stimulation, is initiated by G protein-coupled receptor kinase (GRK) phosphorylation of serine/threonine residues of the third intracellular loop (TIL) or cytoplasmic tail (C-tail) following receptor activation [94]. This phosphorylation allows for the subsequent binding of arrestin-2 and/or arrestin-3, effectively uncoupling the receptor from further G protein activation and often targeting the receptor for internalization [94].
Upon SDF activation, CXCR4 is rapidly phosphorylated and internalized [101–104]. Removing the 45 amino acid C-tail of CXCR4, which contains 15 serine and 3 threonine residues, eliminates agonist-promoted phosphorylation [101], enhances receptor activity, and attenuates receptor internalization [103]. Truncation and alanine scanning mutagenesis has suggested multiple regions in the CXCR4 C-tail as potential phospho-acceptor sites [102, 104]. Mutation of Ser338 and Ser339 resulted in reduced SDF-promoted phosphorylation of CXCR4 as did truncation of the C-terminal 7 amino acids, which removes serines 346, 347, 348, 351, and 352 (Figure 2A) [102]. Recently, a phospho-specific antibody directed against phospho-Ser339 also revealed increased phosphorylation of Ser339 following SDF stimulation [105]. Interestingly, increased phosphorylation of Ser339 was also observed following EGF or phorbol ester treatment [105], suggesting that this may be a potential PKC phosphorylation site. To date, the GRKs responsible for phosphorylation of CXCR4 have not been identified, although GRK2 [99, 102] and GRK6 [95, 106] have been implicated. Overexpression of GRK2 was able to enhance SDF-mediated internalization of CXCR4, which was further increased by the co-expression of arrestin-3 [99, 102]. Interestingly, GRK2 has also been suggested to negatively regulate CXCR4 signal transduction at a level downstream of the receptor, possibly via interaction with MEK [107]. Lymphocytes and neutrophils isolated from mice with a targeted disruption of GRK6 showed enhanced CXCR4 function and a lack of desensitization [95, 106], which was not seen in cells isolated from mice lacking GRK5 [95]. These data suggest that there may be multiple kinases regulating CXCR4 in response to SDF stimulation. As has recently been suggested for the angiotensin [108, 109], vasopressin [110], and β2-adrenergic receptors [111, 112], the coordinated action of these kinases may be necessary for proper receptor regulation by dictating specific interactions through alternative phosphorylation patterns.
Figure 2. Amino Acid Sequence of the C-terminal Tail of CXCR4.
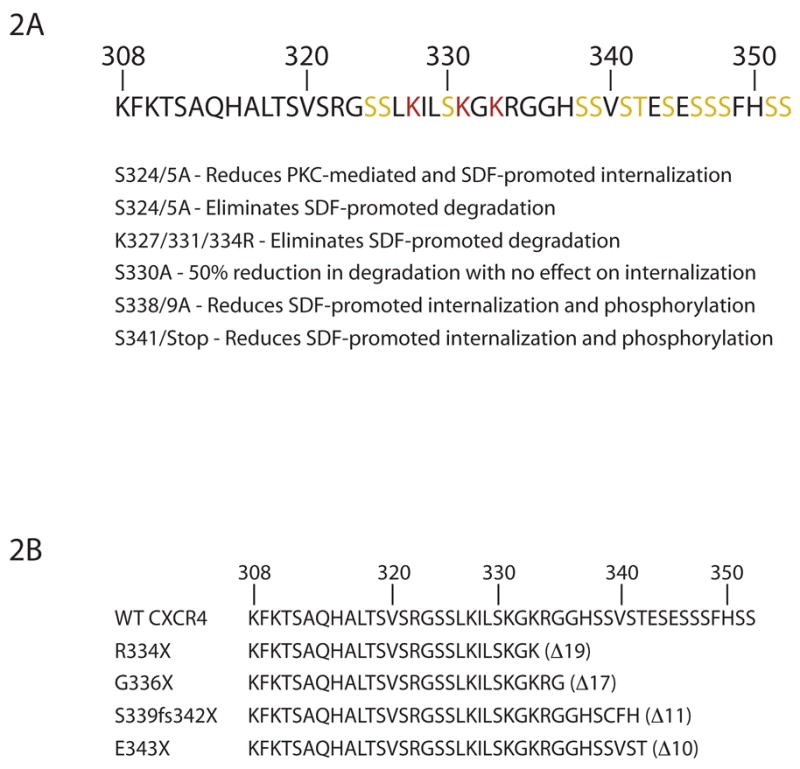
A) The C-terminal tail of CXCR4 contains 15 serine and 3 threonine residues. Truncation and alanine scanning mutagenesis has identified multiple residues as potential phospho-acceptor sites (highlighted in yellow) as well as those residues important for degradation (highlighted in red). Evidence to date suggests that multiple GRKs are responsible for homologous desensitization of CXCR4. Additionally, multiple residues are potential PKC phosphorylation sites. B) Amino acid sequence of CXCR4 as a result of the various germline mutations identified to date resulting in WHIM syndrome.
Many GPCRs also undergo a process termed heterologous desensitization, which is mediated by the activation of second messenger dependent protein kinases such as PKA and PKC. Sequence analysis of CXCR4 shows that multiple serines in the C-tail are potential PKC phosphorylation sites. Consistent with this, direct activation of PKC using phorbol esters results in phosphorylation [101] and internalization [102–104] of CXCR4. Although the sites of phorbol ester induced phosphorylation of CXCR4 have not been completely determined, a significant decrease in phorbol ester induced internalization was observed when either Ser324 and Ser325 or Ser338 and Ser339 were mutated [104] while phorbol ester treatment induced phosphorylation of Ser339 [105]. More physiologically relevant stimuli that lead to PKC activation such as T or B cell receptor engagement [113, 114], formyl peptide receptor activation [115, 116], CXCR1 activation [117], CXCR2 activation [118], or CCR5 activation [119] are also able to induce CXCR4 internalization.
Phosphorylation of tyrosine residues in CXCR4 has also been observed following both SDF [66] and cytokine activation [33], although the residues that are phosphorylated are currently unknown. SDF-promoted tyrosine phosphorylation may promote the activation of the JAK/STAT pathway [66, 120], while cytokine-induced tyrosine phosphorylation may be a way of promoting ligand-independent internalization of CXCR4 [33].
Internalization and Degradation
Upon internalization, GPCRs can be recycled back to the plasma membrane or sorted to the lysosome for degradation [121]. CXCR4 can recycle back to the plasma membrane following PKC-mediated internalization [103], however, the receptor recycles poorly following SDF stimulation [122]. In fact, CXCR4 has been shown to be ubiquitinated, sorted to the lysosome, and degraded [123], a process mediated by the E3 ubiquitin ligase AIP4 [124]. Based on electrophoretic mobility shift, the receptor is most likely mono-ubiquitinated on one of three lysines residues (Lys327, Lys331, or Lys333) in the C-tail. Mutation of these three residues to arginine eliminates ubiquitination and degradation of the receptor [123]. Interestingly, mutation of Ser330 to alanine partially inhibited degradation of CXCR4 without affecting receptor internalization while mutation of Ser324 and Ser325 partially inhibited SDF-promoted internalization but completely disrupted degradation [123]. Taken together, these data suggest that phosphorylation of specific residues may dictate the fate of the receptor following internalization.
CXCR4 Dysregulation in Disease
WHIM Syndrome
Heterozygous mutations in the gene encoding CXCR4 leads to a rare combined immunodeficiency characterized by warts, hypogammaglobulinemia, recurrent bacterial infection, and myelokathexis, known as WHIM syndrome [125, 126]. WHIM syndrome is currently the only immunological disease associated with mutations to a chemokine receptor [125]. The mutations identified to date (one frameshift and three nonsense mutations) all truncate the C-terminal tail of CXCR4 (Figure 2B) eliminating 10 to 19 of the distal tail amino acids, including a number of potential phosphorylation sites [127, 128]. This leads to the expression of a receptor with altered regulation. Following activation, there is a lack of desensitization [128, 129], enhanced chemotaxis [128, 130], an increase in F-actin polymerization [129], enhanced calcium mobilization [130], and a decrease in SDF promoted internalization [129, 130], although one report found no difference in calcium mobilization or internalization [128].
Interestingly, WHIM syndrome has recently been reported in two patients expressing a wild type CXCR4 [129]. Functional assays using cells isolated from these patients revealed that, consistent with classical WHIM cases, there was a lack of desensitization and internalization of CXCR4 following SDF stimulation. The lack of germline mutations in these receptors suggests that there is a change in some downstream regulator such as a GRK or arrestin. Interestingly, mice lacking either GRK6 [95, 106] or arrestin-3 [95] have enhanced receptor function in response to SDF stimulation, similar to those seen in WHIM syndrome, suggesting that these two proteins may play a primary role in regulating CXCR4.
Cancer
The expression of CXCR4 has been detected in 23 different cancers of various origins [131] and is the most common chemokine receptor expressed on cancer cells [23]. The expression of CXCR4 on hematopoetic malignancies is not surprising given the critical role of the receptor in development of these cells [11, 15, 132–134]. However, in a variety of other cancers, CXCR4 expression is enhanced compared to the adjacent normal tissue, which may have little or no CXCR4 [135–137]. A potential underlying mechanism for this may result from changes that occur within the vasculature or O2 carrying capacity of cells leading to hypoxic conditions during tumor progression [138]. Hypoxia induces the activation of hypoxia inducible factor 1 (HIF-1) which in turn promotes expression of a number of target genes [138] including CXCR4 [139–141]. Further evidence regarding the role of HIF-1 came from studies of the tumor suppressor von Hipple Lindau (VHL). Inactivating mutations of VHL, which normally targets HIF-1 for degradation, account for the increased CXCR4 expression in renal cell carcinomas [139–141].
A number of other factors also have the ability to enhance CXCR4 expression specifically during cancer progression. For example, vascular endothelial growth factor (VEGF) [142] or activation of nuclear factor kappa B (NF-κB) [143] enhances CXCR4 expression in breast cancer promoting invasion and metastasis, respectively. Additionally, it has been shown that CXCR4 expression can be induced by the oncoproteins PAX3-FKHR [144, 145] and RET/PTC [146]. CXCR4 expression as a result of the PAX3-FKHR fusion leads to enhanced migration and adhesion of rhabdomyosarcoma cells [145], while RET/PTC induced expression enhanced the transforming ability of breast cancer cells [146]. Increased cell surface expression of CXCR4 may also be the result of altered regulation, independent of effects on transcription/translation. Ubiquitination of CXCR4 is a modification regulating the expression of CXCR4 post-translationally [123, 124]. It has been found that breast cancer cells that are HER2/neu positive have increased expression of CXCR4 as a result of inhibition of receptor ubiquitination [147]. Expression of AIP4, the E3 ubiquitin ligase responsible for ubiquitination of CXCR4 [124], was able to reverse this effect [147]. Moreover, the recent finding that cytokine-independent survival kinase (CISK) associates with and inhibits AIP4 function [148] provides a potential link between HER2 positive breast cancers and the attenuated degradation of CXCR4 [147]. It will be interesting to examine if altered CXCR4 ubiquitination is a global phenomenon in CXCR4-overexpressing cancers or if this effect is specific to HER2/neu expressing cancers.
It is expected that the functional consequence of CXCR4 expression on cancer cells would be varied based on the numerous roles of the CXCR4-SDF signaling axis. For example, the combination of CXCR4 expression and interaction with stromal or nurse-like cells in chronic lymphocytic leukemia [149] and multiple myeloma [150] may account for resistance to spontaneous/drug induced apoptosis and cell adhesion-mediated drug resistance, essentially providing a protective niche. Tumor progression is also affected by CXCR4-SDF-1 signaling through the induction of tumor-associated integrin activation and signaling [151].
Since SDF is a chemokine, an attractive hypothesis is that CXCR4 expression correlates with metastasis. Consistent with this, activation of CXCR4 stimulates the production of matrix metalloproteases [152–155] potentially facilitating the ability of cancers to egress from the primary tumor site. Furthermore, SDF signaling is also able to enhance integrin activity [156–158] enhancing cell adhesion under flow conditions. Upon entering the blood or lymphatic systems, if CXCR4 truly mediates metastasis, tumors would preferentially migrate and adhere to areas that highly express SDF-1. Breast cancer follows this distinct pattern of metastasis, namely to lymph nodes, lung, liver, and bone marrow all of which highly express SDF-1 [135, 159]. Accordingly, neutralizing antibodies to CXCR4 [135] or siRNA knock down [160, 161] inhibit metastasis and growth of breast cancer cells. Other cancers, such as small cell lung cancer, thyroid, neuroblastoma, hematological and hepatic malignancies also metastasize to areas with high SDF-1 expression [162–166]. In spite of this evidence, studies attempting to correlate expression with metastatic potential have yielded mixed results. Whereas CXCR4 expression increased with aggressiveness of prostate tumors [137] there was not a significant correlation of CXCR4 expression and distant breast cancer cell metastasis [167], although the extent of nodal metastasis was greater in cells expressing high levels of CXCR4 compared to those with lower levels [167]. Recently, CXCR4 expression on hepatocellular carcinoma was suggested to correlate with local tumor progression, lymphatic and distant metastasis, as well as negatively impact the 3-year survival rate of these patients [166].
On the other hand, cancers such as lymphomas, glioma, ovarian, and pancreatic have a high expression of SDF-1 at the primary site [168–171]. Additionally, colonic epithelia normally express CXCR4 [172]. Thus, the CXCR4-SDF-1 interaction could be retaining tumor cells that originate at these sites, analogous to the retention of B-cells and neutrophils in the bone marrow during development. Epigenetic mechanisms that negatively regulate the expression of SDF or CXCR4 may be necessary in order for metastasis to occur. One example is DNA methylation, a modification typically associated with inactivation of tumor suppressors [173]. It has recently been shown that methylation of the SDF promoter in colonic epithelium promotes metastasis of these tumors [174]. The CXCR4 promoter is also methylated in a number of pancreatic cancers, decreasing mRNA and protein levels [175]. Though not addressed in the study, this may be a mechanism that allows pancreatic cancers to metastasize from these sites.
As detailed above, the C-tail is absolutely critical for proper regulation of CXCR4. Interestingly, expression of a C-tail truncated mutant of CXCR4 in MCF-7 mammary carcinoma cells led to an epithelial-to-mesenchymal transition [176]. Oligomicroarray analysis showed that there was a down regulation of E-cadherin and Zonula occludens, thereby disrupting cell-to-cell contacts, with a concomitant increase in ERK activation. There was also an increased expression of a number of growth factor receptors. While there have been no cancers described as a result of truncation of CXCR4, this may give insight into the signaling pathways critical for cancer progression and metastasis.
Recent evidence also suggests that, in some breast cancers, receptor expression and functional activity are not linked [177]. Examining a variety of breast cancer cell lines, ranging from untransformed but immortalized to highly invasive, it was concluded that receptor expression alone does not lead to the acquisition of an invasive phenotype. Specifically, it was speculated that there were alterations in G protein coupling to the receptor. Untransformed or transformed non-invasive cells were not able to properly couple to Gi, and therefore, were not able to elicit Ca2+ mobilization, ERK activation or migration; signaling pathways conserved in the invasive lines. Interestingly, as B cells develop into mature cells, they progressively lose the ability to respond to SDF-1 even though surface expression of CXCR4 remains relatively high [178, 179]. However, as they further differentiate into plasma cells, they regain responsiveness to SDF [180]. The underlying mechanisms regulating this phenomenon in B cells are currently not known, though similar mechanisms may be occurring as a result of the transition to a more malignant phenotype in these breast cancer cells.
Summary
In this review, we have highlighted what is currently known regarding the regulation of CXCR4 and the consequences of dysregulation. Given the multifaceted role CXCR4 plays in diverse processes from development to cancer metastasis, CXCR4 is a very intriguing therapeutic target. An ample body of work has been generated in delineating potential pathways that mediate specific effects (e.g., leading to metastasis), however, a detailed basic understanding of receptor regulation is lacking. Understanding the precise mechanisms regulating CXCR4 function at the receptor level should provide insight into attractive therapeutic targets in this pathway. Furthermore, this will allow for translational research opportunities to dissect the specifics of how receptor regulation is altered in disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52(1):145–76. [PubMed] [Google Scholar]
- 3.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 5.Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib Microbiol. 2006;13:191–9. doi: 10.1159/000092973. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63(6):1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 7.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28(3):495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 8.Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass LF, Orsini MJ, Taub D, Horuk R. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998;160(2):877–83. [PubMed] [Google Scholar]
- 9.Yu L, Cecil J, Peng SB, Schrementi J, Kovacevic S, Paul D, Su EW, Wang J. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–9. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 11.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95(16):9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213(2):442–56. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 14.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10(3):179–85. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 15.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 16.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 17.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2(2):123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 18.Lusso P. HIV and the chemokine system: 10 years later. Embo J. 2006;25(3):447–56. doi: 10.1038/sj.emboj.7600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal L, Lu X, Jin Q, Alkhatib G. Anti-HIV Therapy: Current and Future Directions. Curr Pharm Des. 2006;12(16):2031–55. doi: 10.2174/138161206777442100. [DOI] [PubMed] [Google Scholar]
- 20.Reeves JD, Piefer AJ. Emerging drug targets for antiretroviral therapy. Drugs. 2005;65(13):1747–66. doi: 10.2165/00003495-200565130-00002. [DOI] [PubMed] [Google Scholar]
- 21.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 22.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 23.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119(9):2026–9. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 24.Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269(1):232–7. [PubMed] [Google Scholar]
- 25.Nomura H, Nielsen BW, Matsushima K. Molecular cloning of cDNAs encoding a LD78 receptor and putative leukocyte chemotactic peptide receptors. Int Immunol. 1993;5(10):1239–49. doi: 10.1093/intimm/5.10.1239. [DOI] [PubMed] [Google Scholar]
- 26.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 27.Caruz A, Samsom M, Alonso JM, Alcami J, Baleux F, Virelizier JL, Parmentier M, Arenzana-Seisdedos F. Genomic organization and promoter characterization of human CXCR4 gene. FEBS Lett. 1998;426(2):271–8. doi: 10.1016/s0014-5793(98)00359-7. [DOI] [PubMed] [Google Scholar]
- 28.Moriuchi M, Moriuchi H, Turner W, Fauci AS. Cloning and analysis of the promoter region of CXCR4, a coreceptor for HIV-1 entry. J Immunol. 1997;159(9):4322–9. [PubMed] [Google Scholar]
- 29.Wegner SA, Ehrenberg PK, Chang G, Dayhoff DE, Sleeker AL, Michael NL. Genomic organization and functional characterization of the chemokine receptor CXCR4, a major entry co-receptor for human immunodeficiency virus type 1. J Biol Chem. 1998;273(8):4754–60. doi: 10.1074/jbc.273.8.4754. [DOI] [PubMed] [Google Scholar]
- 30.Moriuchi M, Moriuchi H, Margolis DM, Fauci AS. USF/c-Myc enhances, while Yin-Yang 1 suppresses, the promoter activity of CXCR4, a coreceptor for HIV-1 entry. J Immunol. 1999;162(10):5986–92. [PubMed] [Google Scholar]
- 31.Cristillo AD, Highbarger HC, Dewar RL, Dimitrov DS, Golding H, Bierer BE. Up-regulation of HIV coreceptor CXCR4 expression in human T lymphocytes is mediated in part by a cAMP-responsive element. Faseb J. 2002;16(3):354–64. doi: 10.1096/fj.01-0744com. [DOI] [PubMed] [Google Scholar]
- 32.Jourdan P, Vendrell JP, Huguet MF, Segondy M, Bousquet J, Pene J, Yssel H. Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol. 2000;165(2):716–24. doi: 10.4049/jimmunol.165.2.716. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Guan E, Roderiquez G, Calvert V, Alvarez R, Norcross MA. Role of tyrosine phosphorylation in ligand-independent sequestration of CXCR4 in human primary monocytes-macrophages. J Biol Chem. 2001;276(52):49236–43. doi: 10.1074/jbc.M108523200. [DOI] [PubMed] [Google Scholar]
- 34.Feil C, Augustin HG. Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun. 1998;247(1):38–45. doi: 10.1006/bbrc.1998.8499. [DOI] [PubMed] [Google Scholar]
- 35.Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol. 1999;154(4):1125–35. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem. 2005;280(23):22473–81. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 37.Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273(7):4282–7. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 38.Han Y, Wang J, He T, Ransohoff RM. TNF-alpha down-regulates CXCR4 expression in primary murine astrocytes. Brain Res. 2001;888(1):1–10. doi: 10.1016/s0006-8993(00)02924-3. [DOI] [PubMed] [Google Scholar]
- 39.Moriuchi M, Moriuchi H, Fauci AS. HTLV type I Tax activation of the CXCR4 promoter by association with nuclear respiratory factor 1. AIDS Res Hum Retroviruses. 1999;15(9):821–7. doi: 10.1089/088922299310728. [DOI] [PubMed] [Google Scholar]
- 40.Yasukawa M, Hasegawa A, Sakai I, Ohminami H, Arai J, Kaneko S, Yakushijin Y, Maeyama K, Nakashima H, Arakaki R, Fujita S. Down-regulation of CXCR4 by human herpesvirus 6 (HHV-6) and HHV-7. J Immunol. 1999;162(9):5417–22. [PubMed] [Google Scholar]
- 41.Hasegawa A, Yasukawa M, Sakai I, Fujita S. Transcriptional down-regulation of CXC chemokine receptor 4 induced by impaired association of transcription regulator YY1 with c-Myc in human herpesvirus 6-infected cells. J Immunol. 2001;166(2):1125–31. doi: 10.4049/jimmunol.166.2.1125. [DOI] [PubMed] [Google Scholar]
- 42.Berson JF, Long D, Doranz BJ, Rucker J, Jirik FR, Doms RW. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70(9):6288–95. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou H, Tai HH. Characterization of recombinant human CXCR4 in insect cells: role of extracellular domains and N-glycosylation in ligand binding. Arch Biochem Biophys. 1999;369(2):267–76. doi: 10.1006/abbi.1999.1368. [DOI] [PubMed] [Google Scholar]
- 44.Chabot DJ, Chen H, Dimitrov DS, Broder CC. N-linked glycosylation of CXCR4 masks coreceptor function for CCR5-dependent human immunodeficiency virus type 1 isolates. J Virol. 2000;74(9):4404–13. doi: 10.1128/jvi.74.9.4404-4413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brelot A, Heveker N, Montes M, Alizon M. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J Biol Chem. 2000;275(31):23736–44. doi: 10.1074/jbc.M000776200. [DOI] [PubMed] [Google Scholar]
- 46.Brelot A, Heveker N, Pleskoff O, Sol N, Alizon M. Role of the first and third extracellular domains of CXCR-4 in human immunodeficiency virus coreceptor activity. J Virol. 1997;71(6):4744–51. doi: 10.1128/jvi.71.6.4744-4751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chabot DJ, Zhang PF, Quinnan GV, Broder CC. Mutagenesis of CXCR4 identifies important domains for human immunodeficiency virus type 1 X4 isolate envelope-mediated membrane fusion and virus entry and reveals cryptic coreceptor activity for R5 isolates. J Virol. 1999;73(8):6598–609. doi: 10.1128/jvi.73.8.6598-6609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doranz BJ, Orsini MJ, Turner JD, Hoffman TL, Berson JF, Hoxie JA, Peiper SC, Brass LF, Doms RW. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73(4):2752–61. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kajumo F, Thompson DA, Guo Y, Dragic T. Entry of R5X4 and X4 human immunodeficiency virus type 1 strains is mediated by negatively charged and tyrosine residues in the amino-terminal domain and the second extracellular loop of CXCR4. Virology. 2000;271(2):240–7. doi: 10.1006/viro.2000.0308. [DOI] [PubMed] [Google Scholar]
- 50.Zhou N, Luo Z, Luo J, Liu D, Hall JW, Pomerantz RJ, Huang Z. Structural and functional characterization of human CXCR4 as a chemokine receptor and HIV-1 co-receptor by mutagenesis and molecular modeling studies. J Biol Chem. 2001;276(46):42826–33. doi: 10.1074/jbc.M106582200. [DOI] [PubMed] [Google Scholar]
- 51.Thordsen I, Polzer S, Schreiber M. Infection of cells expressing CXCR4 mutants lacking N-glycosylation at the N-terminal extracellular domain is enhanced for R5X4-dualtropic human immunodeficiency virus type-1. BMC Infect Dis. 2002;2:31. doi: 10.1186/1471-2334-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Babcock GJ, Choe H, Farzan M, Sodroski J, Gabuzda D. N-linked glycosylation in the CXCR4 N-terminus inhibits binding to HIV-1 envelope glycoproteins. Virology. 2004;324(1):140–50. doi: 10.1016/j.virol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Farzan M, Babcock GJ, Vasilieva N, Wright PL, Kiprilov E, Mirzabekov T, Choe H. The role of post-translational modifications of the CXCR4 amino terminus in stromal-derived factor 1 alpha association and HIV-1 entry. J Biol Chem. 2002;277(33):29484–9. doi: 10.1074/jbc.M203361200. [DOI] [PubMed] [Google Scholar]
- 54.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96(5):667–76. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 55.Preobrazhensky AA, Dragan S, Kawano T, Gavrilin MA, Gulina IV, Chakravarty L, Kolattukudy PE. Monocyte chemotactic protein-1 receptor CCR2B is a glycoprotein that has tyrosine sulfation in a conserved extracellular N-terminal region. J Immunol. 2000;165(9):5295–303. doi: 10.4049/jimmunol.165.9.5295. [DOI] [PubMed] [Google Scholar]
- 56.Farzan M, Chung S, Li W, Vasilieva N, Wright PL, Schnitzler CE, Marchione RJ, Gerard C, Gerard NP, Sodroski J, Choe H. Tyrosine-sulfated peptides functionally reconstitute a CCR5 variant lacking a critical amino-terminal region. J Biol Chem. 2002;277(43):40397–402. doi: 10.1074/jbc.M206784200. [DOI] [PubMed] [Google Scholar]
- 57.Fong AM, Alam SM, Imai T, Haribabu B, Patel DD. CX3CR1 tyrosine sulfation enhances fractalkine-induced cell adhesion. J Biol Chem. 2002;277(22):19418–23. doi: 10.1074/jbc.M201396200. [DOI] [PubMed] [Google Scholar]
- 58.Veldkamp CT, Seibert C, Peterson FC, Sakmar TP, Volkman BF. Recognition of a CXCR4 Sulfotyrosine by the Chemokine Stromal Cell-derived Factor-1alpha (SDF-1alpha/CXCL12) J Mol Biol. 2006;359(5):1400–9. doi: 10.1016/j.jmb.2006.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol. 2002;42:409–35. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 60.Lapham CK, Romantseva T, Petricoin E, King LR, Manischewitz J, Zaitseva MB, Golding H. CXCR4 heterogeneity in primary cells: possible role of ubiquitination. J Leukoc Biol. 2002;72(6):1206–14. [PubMed] [Google Scholar]
- 61.Sloane AJ, Raso V, Dimitrov DS, Xiao X, Deo S, Muljadi N, Restuccia D, Turville S, Kearney C, Broder CC, Zoellner H, Cunningham AL, Bendall L, Lynch GW. Marked structural and functional heterogeneity in CXCR4: separation of HIV-1 and SDF-1alpha responses. Immunol Cell Biol. 2005;83(2):129–43. doi: 10.1111/j.1440-1711.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- 62.Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem. 2003;278(5):3378–85. doi: 10.1074/jbc.M210140200. [DOI] [PubMed] [Google Scholar]
- 63.Issafras H, Angers S, Bulenger S, Blanpain C, Parmentier M, Labbe-Jullie C, Bouvier M, Marullo S. Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J Biol Chem. 2002;277(38):34666–73. doi: 10.1074/jbc.M202386200. [DOI] [PubMed] [Google Scholar]
- 64.Percherancier Y, Berchiche YA, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, Bouvier M, Heveker N. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280(11):9895–903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 65.Toth PT, Ren D, Miller RJ. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J Pharmacol Exp Ther. 2004;310(1):8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- 66.Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. Faseb J. 1999;13(13):1699–710. [PubMed] [Google Scholar]
- 67.Basmaciogullari S, Pacheco B, Bour S, Sodroski J. Specific interaction of CXCR4 with CD4 and CD8alpha: Functional analysis of the CD4/CXCR4 interaction in the context of HIV-1 envelope glycoprotein-mediated membrane fusion. Virology. 2006 doi: 10.1016/j.virol.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 68.Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, de Ana AM, Martinez AC. Chemokine control of HIV-1 infection. Nature. 1999;400(6746):723–4. doi: 10.1038/23382. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Frade JM, del Real G, Serrano A, Hernanz-Falcon P, Soriano SF, Vila-Coro AJ, de Ana AM, Lucas P, Prieto I, Martinez AC, Mellado M. Blocking HIV-1 infection via CCR5 and CXCR4 receptors by acting in trans on the CCR2 chemokine receptor. Embo J. 2004;23(1):66–76. doi: 10.1038/sj.emboj.7600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agrawal L, Lu X, Qingwen J, VanHorn-Ali Z, Nicolescu IV, McDermott DH, Murphy PM, Alkhatib G. Role for CCR5Delta32 protein in resistance to R5, R5X4, and X4 human immunodeficiency virus type 1 in primary CD4+ cells. J Virol. 2004;78(5):2277–87. doi: 10.1128/JVI.78.5.2277-2287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huttenrauch F, Pollok-Kopp B, Oppermann M. G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem. 2005;280(45):37503–15. doi: 10.1074/jbc.M500535200. [DOI] [PubMed] [Google Scholar]
- 72.Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. Embo J. 1997;16(23):6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang X, Shen J, Cui M, Shen L, Luo X, Ling K, Pei G, Jiang H, Chen K. Molecular dynamics simulations on SDF-1alpha: binding with CXCR4 receptor. Biophys J. 2003;84(1):171–84. doi: 10.1016/S0006-3495(03)74840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delgado MB, Clark-Lewis I, Loetscher P, Langen H, Thelen M, Baggiolini M, Wolf M. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. Eur J Immunol. 2001;31(3):699–707. doi: 10.1002/1521-4141(200103)31:3<699::aid-immu699>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 75.Valenzuela-Fernandez A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, Delaunay T, Lazarini F, Virelizier JL, Chignard M, Pidard D, Arenzana-Seisdedos F. Leukocyte elastase negatively regulates Stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277(18):15677–89. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- 76.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169(12):7000–8. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 77.Huhn J, Ehrlich S, Fleischer B, von Bonin A. Molecular analysis of CD26-mediated signal transduction in T cells. Immunol Lett. 2000;72(2):127–32. doi: 10.1016/s0165-2478(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 78.Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, Scharpe S, Van Damme J, De Meester I. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276(32):29839–45. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- 79.Hoogewerf AJ, Kuschert GS, Proudfoot AE, Borlat F, Clark-Lewis I, Power CA, Wells TN. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36(44):13570–8. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka Y, Adams DH, Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993;14(3):111–5. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 81.Sadir R, Baleux F, Grosdidier A, Imberty A, Lortat-Jacob H. Characterization of the stromal cell-derived factor-1alpha-heparin complex. J Biol Chem. 2001;276(11):8288–96. doi: 10.1074/jbc.M008110200. [DOI] [PubMed] [Google Scholar]
- 82.Dealwis C, Fernandez EJ, Thompson DA, Simon RJ, Siani MA, Lolis E. Crystal structure of chemically synthesized [N33A] stromal cell-derived factor 1alpha, a potent ligand for the HIV-1 "fusin" coreceptor. Proc Natl Acad Sci U S A. 1998;95(12):6941–6. doi: 10.1073/pnas.95.12.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–99. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 84.Holmes WD, Consler TG, Dallas WS, Rocque WJ, Willard DH. Solution studies of recombinant human stromal-cell-derived factor-1. Protein Expr Purif. 2001;21(3):367–77. doi: 10.1006/prep.2001.1402. [DOI] [PubMed] [Google Scholar]
- 85.Netelenbos T, Zuijderduijn S, Van Den Born J, Kessler FL, Zweegman S, Huijgens PC, Drager AM. Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J Leukoc Biol. 2002;72(2):353–62. [PubMed] [Google Scholar]
- 86.Valenzuela-Fernandez A, Palanche T, Amara A, Magerus A, Altmeyer R, Delaunay T, Virelizier JL, Baleux F, Galzi JL, Arenzana-Seisdedos F. Optimal inhibition of X4 HIV isolates by the CXC chemokine stromal cell-derived factor 1 alpha requires interaction with cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276(28):26550–8. doi: 10.1074/jbc.M100411200. [DOI] [PubMed] [Google Scholar]
- 87.Majka M, Janowska-Wieczorek A, Ratajczak J, Kowalska MA, Vilaire G, Pan ZK, Honczarenko M, Marquez LA, Poncz M, Ratajczak MZ. Stromal-derived factor 1 and thrombopoietin regulate distinct aspects of human megakaryopoiesis. Blood. 2000;96(13):4142–51. [PubMed] [Google Scholar]
- 88.Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, Ratajczak J, Emerson SG, Kowalska MA, Ratajczak MZ. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98(10):3143–9. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 89.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak MZ. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105(1):40–8. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 90.Le Y, Honczarenko M, Glodek AM, Ho DK, Silberstein LE. CXC chemokine ligand 12-induced focal adhesion kinase activation and segregation into membrane domains is modulated by regulator of G protein signaling 1 in proB cells. J Immunol. 2005;174(5):2582–90. doi: 10.4049/jimmunol.174.5.2582. [DOI] [PubMed] [Google Scholar]
- 91.Nguyen DH, Giri B, Collins G, Taub DD. Dynamic reorganization of chemokine receptors, cholesterol, lipid rafts, and adhesion molecules to sites of CD4 engagement. Exp Cell Res. 2005;304(2):559–69. doi: 10.1016/j.yexcr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen DH, Taub D. CXCR4 function requires membrane cholesterol: implications for HIV infection. J Immunol. 2002;168(8):4121–6. doi: 10.4049/jimmunol.168.8.4121. [DOI] [PubMed] [Google Scholar]
- 93.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35(3):233–45. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 94.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 95.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99(11):7478–83. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148(6):1267–81. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–61. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 98.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 99.Cheng ZJ, Zhao J, Sun Y, Hu W, Wu YL, Cen B, Wu GX, Pei G. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275(4):2479–85. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 100.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277(51):49212–9. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 101.Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, Ali H, Snyderman R. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem. 1997;272(45):28726–31. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 102.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274(43):31076–86. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 103.Signoret N, Oldridge J, Pelchen-Matthews A, Klasse PJ, Tran T, Brass LF, Rosenkilde MM, Schwartz TW, Holmes W, Dallas W, Luther MA, Wells TN, Hoxie JA, Marsh M. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139(3):651–64. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Signoret N, Rosenkilde MM, Klasse PJ, Schwartz TW, Malim MH, Hoxie JA, Marsh M. Differential regulation of CXCR4 and CCR5 endocytosis. J Cell Sci. 1998;111( Pt 18):2819–30. doi: 10.1242/jcs.111.18.2819. [DOI] [PubMed] [Google Scholar]
- 105.Woerner BM, Warrington NM, Kung AL, Perry A, Rubin JB. Widespread CXCR4 activation in astrocytomas revealed by phospho-CXCR4-specific antibodies. Cancer Res. 2005;65(24):11392–9. doi: 10.1158/0008-5472.CAN-05-0847. [DOI] [PubMed] [Google Scholar]
- 106.Vroon A, Heijnen CJ, Raatgever R, Touw IP, Ploemacher RE, Premont RT, Kavelaars A. GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo. J Leukoc Biol. 2004;75(4):698–704. doi: 10.1189/jlb.0703320. [DOI] [PubMed] [Google Scholar]
- 107.Jimenez-Sainz MC, Murga C, Kavelaars A, Jurado-Pueyo M, Krakstad BF, Heijnen CJ, Mayor F, Jr, Aragay AM. G protein-coupled receptor kinase 2 negatively regulates chemokine signaling at a level downstream from G protein subunits. Mol Biol Cell. 2006;17(1):25–31. doi: 10.1091/mbc.E05-05-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J Biol Chem. 2004;279(9):7807–11. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 109.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A. 2005;102(5):1442–7. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A. 2005;102(5):1448–53. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281(2):1261–73. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 112.Violin JD, Ren XR, Lefkowitz RJ. G-protein-coupled receptor kinase specificity for beta-arrestin recruitment to the beta2-adrenergic receptor revealed by fluorescence resonance energy transfer. J Biol Chem. 2006;281(29):20577–88. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- 113.Peacock JW, Jirik FR. TCR activation inhibits chemotaxis toward stromal cell-derived factor-1: evidence for reciprocal regulation between CXCR4 and the TCR. J Immunol. 1999;162(1):215–23. [PubMed] [Google Scholar]
- 114.Guinamard R, Signoret N, Ishiai M, Marsh M, Kurosaki T, Ravetch JV. B cell antigen receptor engagement inhibits stromal cell-derived factor (SDF)-1alpha chemotaxis and promotes protein kinase C (PKC)-induced internalization of CXCR4. J Exp Med. 1999;189(9):1461–6. doi: 10.1084/jem.189.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li BQ, Wetzel MA, Mikovits JA, Henderson EE, Rogers TJ, Gong W, Le Y, Ruscetti FW, Wang JM. The synthetic peptide WKYMVm attenuates the function of the chemokine receptors CCR5 and CXCR4 through activation of formyl peptide receptor-like 1. Blood. 2001;97(10):2941–7. doi: 10.1182/blood.v97.10.2941. [DOI] [PubMed] [Google Scholar]
- 116.Selleri C, Montuori N, Ricci P, Visconte V, Carriero MV, Sidenius N, Serio B, Blasi F, Rotoli B, Rossi G, Ragno P. Involvement of the urokinase-type plasminogen activator receptor in hematopoietic stem cell mobilization. Blood. 2005;105(5):2198–205. doi: 10.1182/blood-2004-06-2424. [DOI] [PubMed] [Google Scholar]
- 117.Richardson RM, Tokunaga K, Marjoram R, Sata T, Snyderman R. Interleukin-8-mediated heterologous receptor internalization provides resistance to HIV-1 infectivity. Role of signal strength and receptor desensitization. J Biol Chem. 2003;278(18):15867–73. doi: 10.1074/jbc.M211745200. [DOI] [PubMed] [Google Scholar]
- 118.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104(2):565–71. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 119.Hecht I, Cahalon L, Hershkoviz R, Lahat A, Franitza S, Lider O. Heterologous desensitization of T cell functions by CCR5 and CXCR4 ligands: inhibition of cellular signaling, adhesion and chemotaxis. Int Immunol. 2003;15(1):29–38. doi: 10.1093/intimm/dxg002. [DOI] [PubMed] [Google Scholar]
- 120.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97(11):3342–8. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 121.Marchese A, Chen C, Kim YM, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci. 2003;28(7):369–76. doi: 10.1016/S0968-0004(03)00134-8. [DOI] [PubMed] [Google Scholar]
- 122.Tarasova NI, Stauber RH, Michejda CJ. Spontaneous and ligand-induced trafficking of CXC-chemokine receptor 4. J Biol Chem. 1998;273(26):15883–6. doi: 10.1074/jbc.273.26.15883. [DOI] [PubMed] [Google Scholar]
- 123.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276(49):45509–12. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 124.Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5(5):709–22. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 125.Diaz GA, Gulino AV. WHIM syndrome: a defect in CXCR4 signaling. Curr Allergy Asthma Rep. 2005;5(5):350–5. doi: 10.1007/s11882-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 126.Gulino AV. WHIM syndrome: a genetic disorder of leukocyte trafficking. Curr Opin Allergy Clin Immunol. 2003;3(6):443–50. doi: 10.1097/00130832-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 127.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34(1):70–4. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 128.Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, Imberti L, Pirovano S, Notarangelo LD, Soresina R, Mazzolari E, Nelson DL, Notarangelo LD, Badolato R. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004;104(2):444–52. doi: 10.1182/blood-2003-10-3532. [DOI] [PubMed] [Google Scholar]
- 129.Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, Nicolas JF, Latger-Cannard V, Bensoussan D, Bordigoni P, Baleux F, Le Deist F, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105(6):2449–57. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 130.Kawai T, Choi U, Whiting-Theobald NL, Linton GF, Brenner S, Sechler JM, Murphy PM, Malech HL. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp Hematol. 2005;33(4):460–8. doi: 10.1016/j.exphem.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 131.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14(3):171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 132.Ara T, Itoi M, Kawabata K, Egawa T, Tokoyoda K, Sugiyama T, Fujii N, Amagai T, Nagasawa T. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170(9):4649–55. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 133.Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, Kishimoto T, Katsura Y, Nagasawa T. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15(2):323–34. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 134.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16(10):1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 135.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 136.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61(13):4961–5. [PubMed] [Google Scholar]
- 137.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 138.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59(1):15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198(9):1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425(6955):307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 141.Zagzag D, Krishnamachary B, Yee H, Okuyama H, Chiriboga L, Ali MA, Melamed J, Semenza GL. Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65(14):6178–88. doi: 10.1158/0008-5472.CAN-04-4406. [DOI] [PubMed] [Google Scholar]
- 142.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62(24):7203–6. [PubMed] [Google Scholar]
- 143.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278(24):21631–8. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 144.Tomescu O, Xia SJ, Strezlecki D, Bennicelli JL, Ginsberg J, Pawel B, Barr FG. Inducible short-term and stable long-term cell culture systems reveal that the PAX3-FKHR fusion oncoprotein regulates CXCR4, PAX3, and PAX7 expression. Lab Invest. 2004;84(8):1060–70. doi: 10.1038/labinvest.3700125. [DOI] [PubMed] [Google Scholar]
- 145.Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG, Janowska-Wieczorek A, Ratajczak MZ. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100(7):2597–606. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 146.Castellone MD, Guarino V, De Falco V, Carlomagno F, Basolo F, Faviana P, Kruhoffer M, Orntoft T, Russell JP, Rothstein JL, Fusco A, Santoro M, Melillo RM. Functional expression of the CXCR4 chemokine receptor is induced by RET/PTC oncogenes and is a common event in human papillary thyroid carcinomas. Oncogene. 2004;23(35):5958–67. doi: 10.1038/sj.onc.1207790. [DOI] [PubMed] [Google Scholar]
- 147.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6(5):459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 148.Slagsvold T, Marchese A, Brech A, Stenmark H. CISK attenuates degradation of the chemokine receptor CXCR4 via the ubiquitin ligase AIP4. Embo J. 2006;25(16):3738–49. doi: 10.1038/sj.emboj.7601267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–63. [PubMed] [Google Scholar]
- 150.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93(5):1658–67. [PMC free article] [PubMed] [Google Scholar]
- 151.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24(27):4462–71. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 152.Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23(1):157–67. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- 153.Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Exp Hematol. 2000;28(11):1274–85. doi: 10.1016/s0301-472x(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 154.Samara GJ, Lawrence DM, Chiarelli CJ, Valentino MD, Lyubsky S, Zucker S, Vaday GG. CXCR4-mediated adhesion and MMP-9 secretion in head and neck squamous cell carcinoma. Cancer Lett. 2004;214(2):231–41. doi: 10.1016/j.canlet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 155.Spiegel A, Kollet O, Peled A, Abel L, Nagler A, Bielorai B, Rechavi G, Vormoor J, Lapidot T. Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103(8):2900–7. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 156.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279(5349):381–4. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 157.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003;197(4):461–73. doi: 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wright N, Hidalgo A, Rodriguez-Frade JM, Soriano SF, Mellado M, Parmo-Cabanas M, Briskin MJ, Teixido J. The chemokine stromal cell-derived factor-1 alpha modulates alpha 4 beta 7 integrin-mediated lymphocyte adhesion to mucosal addressin cell adhesion molecule-1 and fibronectin. J Immunol. 2002;168(10):5268–77. doi: 10.4049/jimmunol.168.10.5268. [DOI] [PubMed] [Google Scholar]
- 159.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 160.Lapteva N, Yang AG, Sanders DE, Strube RW, Chen SY. CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther. 2005;12(1):84–9. doi: 10.1038/sj.cgt.7700770. [DOI] [PubMed] [Google Scholar]
- 161.Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65(3):967–71. [PMC free article] [PubMed] [Google Scholar]
- 162.Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, Kipps TJ, Burger JA. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22(50):8093–101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 163.Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP, Ben-Baruch A. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167(8):4747–57. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 164.Hwang JH, Hwang JH, Chung HK, Kim DW, Hwang ES, Suh JM, Kim H, You KH, Kwon OY, Ro HK, Jo DY, Shong M. CXC chemokine receptor 4 expression and function in human anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 2003;88(1):408–16. doi: 10.1210/jc.2002-021381. [DOI] [PubMed] [Google Scholar]
- 165.Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62(21):6304–11. [PubMed] [Google Scholar]
- 166.Schimanski CC, Bahre R, Gockel I, Muller A, Frerichs K, Horner V, Teufel A, Simiantonaki N, Biesterfeld S, Wehler T, Schuler M, Achenbach T, Junginger T, Galle PR, Moehler M. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95(2):210–7. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5(5):R144–50. doi: 10.1186/bcr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Corcione A, Ottonello L, Tortolina G, Facchetti P, Airoldi I, Guglielmino R, Dadati P, Truini M, Sozzani S, Dallegri F, Pistoia V. Stromal cell-derived factor-1 as a chemoattractant for follicular center lymphoma B cells. J Natl Cancer Inst. 2000;92(8):628–35. doi: 10.1093/jnci/92.8.628. [DOI] [PubMed] [Google Scholar]
- 169.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, Fujii N, Imamura M. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6(9):3530–5. [PubMed] [Google Scholar]
- 170.Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62(20):5930–8. [PubMed] [Google Scholar]
- 171.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277(51):49481–7. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 172.Jordan NJ, Kolios G, Abbot SE, Sinai MA, Thompson DA, Petraki K, Westwick J. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104(8):1061–9. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 174.Wendt MK, Johanesen PA, Kang-Decker N, Binion DG, Shah V, Dwinell MB. Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis. Oncogene. 2006;25(36):4986–97. doi: 10.1038/sj.onc.1209505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sato N, Matsubayashi H, Fukushima N, Goggins M. The chemokine receptor CXCR4 is regulated by DNA methylation in pancreatic cancer. Cancer Biol Ther. 2005;4(1):70–6. doi: 10.4161/cbt.4.1.1378. [DOI] [PubMed] [Google Scholar]
- 176.Ueda Y, Neel NF, Schutyser E, Raman D, Richmond A. Deletion of the COOH-terminal domain of CXC chemokine receptor 4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells. Cancer Res. 2006;66(11):5665–75. doi: 10.1158/0008-5472.CAN-05-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Holland JD, Kochetkova M, Akekawatchai C, Dottore M, Lopez A, McColl SR. Differential functional activation of chemokine receptor CXCR4 is mediated by G proteins in breast cancer cells. Cancer Res. 2006;66(8):4117–24. doi: 10.1158/0008-5472.CAN-05-1631. [DOI] [PubMed] [Google Scholar]
- 178.Fedyk ER, Ryyan DH, Ritterman I, Springer TA. Maturation decreases responsiveness of human bone marrow B lineage cells to stromal-derived factor 1 (SDF-1) J Leukoc Biol. 1999;66(4):667–73. doi: 10.1002/jlb.66.4.667. [DOI] [PubMed] [Google Scholar]
- 179.Honczarenko M, Douglas RS, Mathias C, Lee B, Ratajczak MZ, Silberstein LE. SDF-1 responsiveness does not correlate with CXCR4 expression levels of developing human bone marrow B cells. Blood. 1999;94(9):2990–8. [PubMed] [Google Scholar]
- 180.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194(1):45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


