One of the many conundrums of herpesvirology is why a herpesvirus is so profligate in its use of four or more envelope glycoproteins for entry into a cell when other viruses can manage very well with only one or two. Profligacy does, however, have its advantages. Epstein-Barr virus (EBV), originally recognized for its ability to infect and transform lymphocytes, is now clearly understood to infect epithelial cells as part of its normal cycle of persistence in a human host, and under some circumstances, the virus may infect T cells, natural killer cells, smooth muscle cells (47), and possibly monocytes as well (11, 50). Our understanding of how EBV enters each of these cell types is very incomplete, but some of the major players involved in B-cell and epithelial cell infections are being identified, and they provide a window into the flexibility of tropism that the use of different combinations of virus and cell membrane proteins can provide. This review summarizes what we know about these players so far.
ATTACHMENT
(i) B cells.
Eight virus glycoproteins have been implicated in some way in EBV entry into either a B cell or an epithelial cell (Table 1). One of the most abundant of these in the virus envelope, gp350/220 (22), is responsible for attachment of the virus with high affinity (36) to the complement receptor type 2 (CR2) on B cells (7, 8, 40, 41, 60, 61). An EBV recombinant lacking gp350/220 can transform B cells with much-reduced efficiency (20), so CR2 is perhaps not the only portal by which the virus can access a B cell. It is, however, clearly the predominant one (Fig. 1). Antibodies to gp350/220 that block virus binding neutralize B-cell infection, and soluble forms of either CR2 or gp350/220 can do the same (35, 61).
TABLE 1.
EBV envelope glycoproteins with roles in entry into B cells or epithelial cells
| Protein/genea | Type | Role in virus entry |
|---|---|---|
| gp350/BLLF1 | Single pass type1 | Attachment to B-cell receptor CR2/CD21 |
| gH/BXLF2 | Single pass type 1 | Fusion; attachment to epithelial cell receptor/coreceptor |
| gL/BKRF2 | Single pass type 2 | Chaperone for gH |
| gp42/BZLF2 | Single pass type 2 | Fusion; interaction with B-cell coreceptor HLA class II |
| gB/BALF4 | Single pass type 1 | Fusion |
| gN/BLRF1 | Single pass type 1 | Codependent with gM for expression; possibly involved in postfusion events |
| gM/BBRF3 | Multispanning | |
| BMRF2 | Multispanning | Binds integrins; important for infection of polarized epithelial cells |
EBV envelope proteins are conventionally named for their apparent masses (e.g., gp350); the alphabetical names of homologs in other herpesviruses, if they exist, (e.g., gH); or their gene names only (e.g., BMRF2).
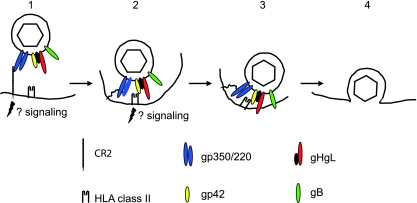
FIG. 1.
A putative model of the steps involved in entry of virus into a B lymphocyte. (1) The virus binds to CR2 via gp350, possibly initiating signaling events and triggering endocytosis. (2) CR2 may now bind to gp220 rather than gp350, and this, combined with the potential flexibility of CR2, may allow the virus to approach closer to the cell membrane, where gp42 can interact with HLA class II. (3) Interaction of gp42 with HLA class II triggers the interaction of the core fusion machinery, gHgL and gB, with the endosomal membrane and may also initiate further signaling events. (4) The virus and endosomal membranes fuse, allowing entry of the tegumented capsid into the cytoplasm. Modified from reference 18, with permission.
Glycoprotein gp350/220 is a highly glycosylated single-pass membrane protein, which as a result of alternative splicing is made in two forms, with approximate masses of 350 and 220 kDa (1, 17). Residues 500 to 757, which include three repeats of a 21-amino-acid motif with amphipathic characteristics, are lost from the full-length 907-amino-acid protein as a result of the splice. It does, however, maintain the reading frame, and both forms of the protein retain the CR2 binding domain at the amino terminus (61). Solution of the structure of an unliganded form of gp350/220 has mapped this domain to a glycan-free surface (59) that includes a sequence with some similarity to the natural ligand of CR2, the C3dg fragment of complement (26, 39, 61). The extracellular domain of CR2 is composed of tandem repeats of structural modules 60 to 75 amino acids in length known as short consensus repeats (SCR), and the binding site for gp350/220 has been genetically mapped to a region composed of residues from the membrane distal SCR1 and SCR2 (30). Attachment initially positions the virus at approximately 50 nm from the cell surface (38), but the segmental flexibility that the repeated SCRs may give to CR2 (68) and the possibility of an exchange in binding from gp350 to gp220 may contribute to moving the virus closer to the cell membrane.
Binding of gp350/220 also triggers capping of CR2 and endocytosis of the virus (38, 60). CR2 can function as a signal transducer both independently and as part of a signal transduction complex that includes CD19 and CD35 (5). Cross-linking by gp350/220 activates NF-κB (53, 58) and induces interleukin-6 via a protein kinase C pathway (4, 62). None of these signaling events may be critical to penetration of the cell membrane, but they clearly have potential consequences for downstream events in infection.
(ii) Epithelial cells.
Attachment of the virus to an epithelial cell is a more-complicated affair (Fig. 2). CR2 is expressed at least at low levels on some epithelial cells in culture (6) in the absence of CD19 and CD35, and infection of an epithelial cell that has been engineered to express high levels of CR2 can be very efficient (3, 29). Notably, in this regard, EBV-mediated cross-linking of CR2 on epithelial cells, which lack the other components of the CR2 signaling complex found on B cells, stimulates relocalization and clustering of CR2 and the formin homolog overexpressed in spleen (FHOS/FHOD1). Formins are molecular scaffolds that nucleate actin, linking signal transduction to actin reorganization and gene transcription (10), and this may contribute to successful delivery of the virus to the nucleus of the cell. However, which, or even whether, epithelial cells normally express CR2 in vivo remains uncertain. Studies with monoclonal antibodies to CR2 were compromised by the finding that the antibody most commonly used cross-reacted with an unrelated epithelial cell protein (73).
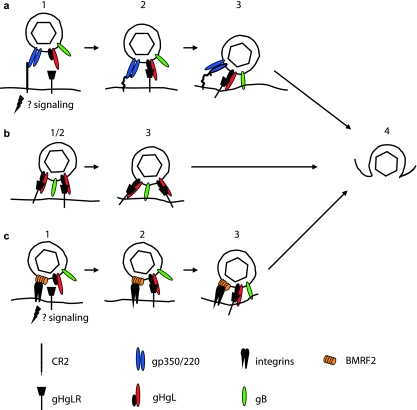
FIG. 2.
Putative models of the steps involved in entry of virus into epithelial cells after attachment via different cell proteins. (a) Entry via CR2 and gHgLR. (1) The virus binds to CR2 via gp350; signaling may occur, but endocytosis is not triggered. (2) A switch to interaction with gp220, combined with the potential flexibility of CR2, may allow the virus to approach closer to the cell membrane, where gHgL can interact with gHgLR. (3) Interaction of gHgL with gHgLR triggers the interaction of the core fusion machinery, gHgL and gB, with the cell membrane. (b) Entry via gHgLR alone (which is less efficient). (1) There is no separate attachment event. (2) gHgL interacts directly with gHgLR. (3) Interaction of gHgL with gHgLR triggers the interaction of the core fusion machinery, gHgL and gB, with the cell membrane. (c) Entry via integrins and gHgLR. (1) BMRF2 interacts with α5β1 integrins, possibly initiating signaling events. (2) gHgL interacts with gHgLR. (3) Interaction of gHgL with gHgLR triggers the interaction of the core fusion machinery, gHgL and gB, with the cell membrane. Step 4 is the same for all routes: the virus and cell membrane fuse, allowing entry of the tegumented capsid into the cytoplasm. Modified from reference 18, with permission.
At least three other possible attachment mechanisms have been proposed that involve neither gp350/220 nor CR2. The first was a demonstration that virus coated with immunoglobulin A specific to gp350/220 can bind productively to the polymeric immunoglobulin-A receptor (54). This may be particularly relevant to infection via the basolateral surface of an epithelial cell in an immune host, although, since in polarized cells virus was transported intact from the basolateral to the apical surface, it may be more relevant to trans epithelial transport than direct infection (9). The second was a demonstration that in the absence of CR2 a complex of two additional glycoproteins, gH and gL, can serve as epithelial ligands (34, 43). EBV gH, a type 1 membrane protein, is, like its homologs in other herpesviruses, misfolded and retained in the endoplasmic reticulum in the absence of the much smaller type 2 protein, gL (27, 72). No separate function in addition to facilitating folding and transport of gH has been ascribed to gL, and the two proteins, which associate noncovalently, are usually referred to as a unit, the gHgL complex. EBV derived from a B cell can bind well to a CR2-negative epithelial cell, but recombinant viruses that lack gHgL lose this ability (34, 43). A soluble form of gHgL made in baculovirus can bind specifically to epithelial cells, but not B cells, and its binding can be reduced by a monoclonal antibody specific for the gHgL complex (3). The same antibody can also reduce virus binding (34). These observations have been interpreted to mean that there is an epithelial cell receptor for gHgL that can serve in attachment. The identity of the molecule is not yet known, but operationally it has been referred to as gHgLR.
Finally, and most intriguing, on polarized epithelial cells, which presumably resemble more closely the environment that the virus encounters in vivo, an interaction between a multispan virus membrane protein encoded by the BMRF2 open reading frame and integrins has been demonstrated (63). Antibodies to integrins and to a BMRF2 fusion protein only partially block binding to polarized epithelial cells but have a significant impact on infection via the basolateral surface of the polarized monolayer. The RGD motif present in an exposed loop of BMRF2 has been shown to be a ligand for β1, α5, α3, and αv integrins (71). The protein is not required for cell-cell fusion (12, 31). However, whether the BMRF2 integrin interaction is primarily responsible for attachment or whether it is most relevant to postattachment events to which signaling may make an important contribution is not yet clear. There is apparently very little BMRF2 protein in the virion (22).
FUSION AND PENETRATION
Fusion of the EBV envelope with both a B cell and an epithelial cell requires three glycoproteins, gHgL and gB, which are conserved throughout the herpesvirus family and which have been referred to as the core fusion machinery (55). However, how and where this machinery is activated differs significantly for each cell type.
(i) B cells.
Fusion of the virus with a normal B cell (Fig. 1) requires endocytosis, which is triggered by the interaction between gp350/220 and CR2 and occurs in a low-pH compartment, although low pH itself is not required (32). The virus proteins that are both necessary and sufficient for efficient fusion are gHgL, gB, and gp42. A role for gH was first demonstrated when it was shown that virosomes made from proteins depleted of those that bound to an antibody to gH could attach to B cells but could not fuse (14). It was later confirmed by analysis of the phenotype of a recombinant gH-null virus (34). A role for EBV gB was not possible to evaluate genetically, since, unlike its homologs in other herpesviruses, it is essential for virus morphogenesis and egress (16). However, when cell-cell fusion assays were developed as models of virus-cell fusion, it became apparent that its contribution is also very important (12, 31).
The fourth and final protein involved in fusion of EBV with a B cell, gp42, has homologs only among the lymphocryptoviruses (48, 49). It is another type 2 membrane protein, and it associates noncovalently with gHgL. The original observation was that gp42 resembles a C-type lectin and that a soluble form made by replacing the signal sequence with one that is efficiently cleaved can bind to HLA class II (56). Within the three-part complex of gHgLgp42 in the virus, this interaction is now thought to provide the trigger for B-cell fusion. A monoclonal antibody that was initially mapped to gH (57) but ultimately proved to react with gp42 (28) inhibited interaction with HLA class II (27) and also blocked virus-cell fusion (33). Reciprocally, a monoclonal antibody to HLA class II that blocked interaction with gp42 also inhibited infection. The soluble form of gp42 initially used to document HLA class II binding could inhibit infection by competing with gp42 in the virus, and B cells that lacked HLA class II could be efficiently infected only if HLA class II expression was restored (27). A recombinant gp42-null virus could infect a B cell only if the cells and the bound virus were treated with an exogenous fusogen such as polyethylene glycol (65) or if the soluble form of gp42, which retained a gHgL binding domain at its amino terminus, was added in trans to reform three-part complexes (66).
Binding of gp42 to HLA class II shows some allelic specificity in that nonfunctional HLA-DQ alleles have been identified (13), but since all three alleles, HLA-DP, HLA-DQ, and HLA-DR, can be used, it is perhaps unlikely that this has a major impact in an outbred human population. A crystal structure of the ectodomain of gp42 liganded to HLA-DR1 has been solved (37). The protein is most closely related to natural killer cell receptors, such as the murine receptor LY49, which interact with major histocompatibility complex (MHC) class I molecules, but it does not use the canonical surface site which many C-type lectins use to bind to their ligands. Instead, this site forms a hydrophobic pocket that, it is suggested, may be used to accommodate another virus protein or another cell protein important for virus entry (37), perhaps after triggering by HLA class II. This is consistent with the observation that mutations in the hydrophobic pocket do not affect HLA class II binding but inhibit fusion (52). The contact region on HLA-DR1 is within the β1 domain to one side of the peptide binding groove. It was thought on theoretical grounds to be likely to interfere sterically with the binding of T-cell receptors to the peptide MHC complex and has been shown experimentally to reduce T-helper-cell recognition of B cells (45, 46, 56). Both peptide-loaded and immature MHC class II proteins that have not yet trafficked to the peptide-loading compartment can interact with gp42 (46), and, as discussed below, this has proven to have an interesting impact on virus tropism. Whether or not additional signaling events may be initiated as a result of a gp42-HLA class II interaction is not yet known, but since signaling via HLA class II molecules is well documented under other circumstances, this remains a reasonable possibility (67).
(ii) Epithelial cells.
Fusion with a normal epithelial cell, at least one that is nonpolarized, takes place at neutral pH and does not appear to require endocytosis (3, 32). Epithelial cells do not constitutively express HLA class II, and thus, not surprisingly, infection is not blocked by the monoclonal antibody that blocks gp42 and HLA class II interactions. A gp42-null virus can infect an epithelial cell as well as, or even better than, wild-type virus. However, not only is gp42 dispensable for epithelial cell infection, but its presence is actually inhibitory (66). Stoichiometric analysis of gHgL and gp42 in the virion indicated that wild-type EBV carries far more gHgL than gp42, implying that there are two kinds of gH complexes, one the three-part complex composed of gHgLgp42 and the other a two-part complex composed of only gHgL. Only three-part complexes can mediate B-cell infection, but conversion of all two-part complexes to three-part complexes by the addition of soluble gp42 in trans blocks epithelial cell infection. This was interpreted to mean that fusion with an epithelial cell is triggered by a direct interaction between gHgL and a novel epithelial cell surface molecule. Three monoclonal antibodies that interact with gH or gHgL and have no effect on B-cell infection can also inhibit infection of epithelial cells, whether virus is bound via gp350/220 to CR2 or via gHgL to gHgLR. One of them blocks gHgL binding to gHgLR, suggesting that the cell molecule that triggers fusion might be the same protein, gHgLR, which can serve as an attachment receptor in the absence of CR2. Consistent with this is the observation that soluble gp42 also blocks binding of the virus to gHgLR. Use of gHgL for attachment as well as fusion does, however, seem to compromise infection. The virus can bind efficiently to gHgLR on a CR2-negative epithelial cell, but infection levels are low, either because fusion becomes less efficient or perhaps because engagement of gHgLR does not induce a downstream event required for completion of transport of the virus genome to the nucleus (3).
Beyond this, epithelial cell penetration also requires higher levels of gB than does B-cell penetration (42), presumably reflecting a need for more gB in fusion (31). However, the mechanics of fusion itself remain obscure, as they do for all herpesviruses. Mutations in an amino-terminal region of gH that is predicted to form a coiled coil significantly reduced fusion in a cell-cell fusion assay (44), and insertion or point mutations in a region close to the transmembrane domain can either differentially affect B-cell and epithelial cell fusion or abrogate fusion with either cell type (69, 70). However, some epithelial cell fusion can be mediated in the absence of gHgL by a gB construct with a mutation in the cytoplasmic tail. This has been interpreted to mean that gB may be the critical fusion protein of EBV. Certainly the crystal structure of the homolog of gB in herpes simplex virus shows structural homology with the vesicular stomatitis virus G protein, which is the virus fusogen (15). Both form trimers characterized by an alpha-helical coiled-coil core and extended β-hairpins that are characteristic of class I and class II fusion proteins, respectively. At this point, however, perhaps the most that can be definitively said is that virus fusion with a B cell requires a trigger that is transmitted via gp42 to gHgL and gB, whereas fusion with an epithelial cell requires a trigger that is transmitted directly to gHgL. Whether there is a temporal cascade of events culminating in fusion mediated by gB or whether gHgL and gB coordinately contribute to the process remains to be determined.
TRANSFER OF VIRUS BETWEEN B CELLS AND EPITHELIAL CELLS
gp42 as a switch of tropism.
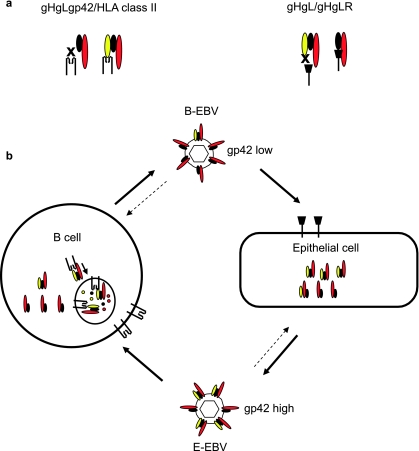
The observation that two-part gHgL and three-part gHgLgp42 complexes are mutually exclusive for epithelial and B-cell entry suggested that the relative amounts of each complex in the virion might impact virus tropism. Analyses of viruses made in B cells and epithelial cells confirmed this. In a B cell, some three-part gHgLgp42 complexes prove to interact with immature MHC class II in the endoplasmic reticulum and are targeted to the HLA class II trafficking pathway, where they are vulnerable to degradation. This does not happen in an HLA class II-negative epithelial cell. Epithelial cell viruses are, as a result, rich in complexes containing gp42 and are as much as two orders of magnitude more infectious for a B cell than viruses produced by a B cell. B-cell viruses, lower in three-part complexes but rich in two-part complexes, are better able to infect an epithelial cell, although the phenotype is not nearly as striking. This suggested a model (Fig. 3) of persistent infection in which virus reactivating from a latently infected memory B cell as it terminally differentiates into a plasmablast (23) is equipped to infect an epithelial cell, whereas virus amplified in an epithelial cell is strongly B cell tropic (2).
FIG. 3.
Glycoprotein gp42 as a switch of cell tropism. (a) B-cell infection requires an interaction between gHgLgp42 and HLA class II, whereas epithelial cell infection requires an interaction between gHgL and gHgLR. Complexes lacking gp42 cannot bind to HLA class II, and complexes containing gp42 cannot bind to gHgLR. (b) gHgLgp42 complexes can interact with HLA class II in the endoplasmic reticulum of a B cell. They may then be targeted to endosomal vesicles rich in proteases, where incoming proteins are digested into peptides for loading into the peptide binding groove of HLA class II before continuing on to the cell surface. The result is a loss of gHgLgp42 complexes to degradation and a relative decrease in gHgLgp42 complexes in viruses produced from B cells or plasmablasts (B-EBV). Complexes in epithelial cells are not lost to this degradative pathway, and viruses produced from epithelial cells (E-EBV) are richer in gp42. Epithelial cells high in gp42 are better able to infect B cells, and B cells low in gp42 are better able to infect epithelial cells. Modified from reference 18, with permission.
EBV is an orally transmitted virus, and the switch in tropism effected by levels of gp42, which could impact transmission, raised the question of the cellular origins of viruses that are shed in saliva. Viruses made in a B cell, low in gp42, have been shown to be able to bind to gHgLR on an epithelial cell almost as well as they can bind CR2 on a B cell. In contrast, virus made in an epithelial cell, high in gp42, binds gHgLR very poorly (3). A comparison of the differential abilities of viruses from saliva and viruses from transformed B cells obtained from a series of EBV-positive donors then facilitated the determination that the majority of viruses shed in saliva are derived from HLA class II-negative cells (21). For the most-efficient transmission, this either means that the virus directly accesses a B cell in the oral mucosa or that transmission to an epithelial cell for amplification before accessing a B cell is influenced by other events. Two have been proposed, and both implicate the differential use of gp350/220.
gp350/220 as an impediment to epithelial infection.
Antibodies to gp350/220, which inhibit infection of a B cell dependent on CR2 for entry, can enhance infection of an epithelial cell, which is not (64). The effect is not mediated by Fc receptor binding but is further increased by antibody cross-linking, which may patch gp350/220 in the virus envelope. Saliva from EBV-seropositive individuals has similar effects that can be reversed by depletion of antibodies. The interpretation of these results was that, if gp350/220 is not absolutely required, then its presence may impede access of other essential proteins to the cell surface. Antibodies to gp350/220, an abundant and immunogenic protein, can overcome this in the saliva of an immune host to facilitate reinfection of an epithelial cell or to facilitate transmission through the epithelium in a new host.
There are long-standing observations that epithelial infection is more efficient if epithelial cells are cocultivated with B cells that are making the virus (19). A novel approach to investigation of the phenomenon demonstrated that binding EBV to a B cell before adding both to epithelial cells significantly enhanced infection of the epithelial cells, even in the absence of internalization and infection of the B cell (51). The mechanism of this B-cell transfer is uncertain but intriguing. The authors also suggested that gp350/220 is inhibitory to direct epithelial cell infection and proposed that the gp350/220 interaction with CR2 on the B cells unmasks envelope components necessary for epithelial cell infection. This parallels the hypothesis for enhanced infection in the presence of antibodies to gp350/220 in saliva. Neither study addressed what the envelope components necessary for efficient epithelial cell infection might be, but one likely candidate would be gB, the protein that is needed in higher amounts for epithelial than for B cell fusion.
POSTFUSION EVENTS
There is regrettably very little concrete information about what happens between fusion and the arrival of the virus genome in the nucleus, only speculation based on what is known for other herpesviruses. Allusion has been made throughout this brief review to the likelihood that signaling events triggered by interactions between the virus envelope and cell proteins have important consequences for postfusion events, but how they may impact intracellular transport and uncoating of the virion is a subject that has not yet been substantively addressed. Two glycoproteins in the virus envelope have, however, also been tentatively implicated in what are presumably immediate postfusion events that may perhaps have no connection to cell signaling. These are glycoprotein gN, a small type I membrane protein, and glycoprotein gM. Glycoprotein gM is a multispan membrane protein with a long, highly charged cytoplasmic tail rich in prolines and replete with potential phosphorylation sites that might provide a mechanism for the regulation of protein-protein interactions. Glycoproteins gN and gM are codependent for expression (24, 25) and play important roles in virus assembly and acquisition of the final virus envelope. Only very little enveloped virus is made by a recombinant EBV that lacks gN and gM. However, that enveloped virus which is made and is able to bind specifically to CR2 on a B cell is impaired in infection in a way that cannot be rescued by addition of exogenous fusogens such as polyethylene glycol. One hypothesis is that a complex that is necessary for assembly of an enveloped particle when a tegumented capsid associates with a membrane is also essential to its disassembly when a tegumented capsid must disassociate from a membrane with which its envelope has fused.
SUMMARY
The major players involved in moving EBV through a B-cell membrane have been identified, at least on the part of the virus. The model emerging is that gp350/220 binds to CR2, gp42 binds to HLA class II, and fusion is mediated by gHgL and gB. The possibility clearly exists, however, that there are additional cell partners for gHgL and gB. Some of the players involved in moving EBV through an epithelial cell membrane have been identified. Fusion involves gHgL and gB, but attachment may involve gp350/220, gHgL, or BMRF2, or possibly, if the virus is transferred from the surface of a B cell, no attachment receptor specific for any viral protein at all. With the possible exception of CR2, no cell partners have yet been identified on an epithelial cell, though there is clear evidence that they exist. There is also no conceptual understanding of the mechanism of fusion and very little insight into events inside the cell that may be triggered by interactions occurring at the cell surface. Much is still to be learned.
Despite the incompleteness of our understanding, however, what we know already provides a fascinating glimpse into differential use of virus proteins and cell partners to manipulate virus tropism and influence virus trafficking between one cell compartment and another. EBV is associated with human malignancies, and primary infection in a minority of individuals causes acute, but self-limiting, mononucleosis. The majority of humans, however, live without incident with a virus that continually infects their lymphocytes and epithelial cells. The strategies that have evolved to make this possible also continue to be remarkable.
Acknowledgments
Research by L.M.H.-F. is supported by Public Health Service grants AI061017 from the National Institute of Allergy and Infectious Diseases and DE016669 from the National Institute of Dental and Craniofacial Research.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Beisel, C., J. Tanner, T. Matsuo, D. Thorley-Lawson, F. Kezdy, and E. Kieff. 1985. Two major outer envelope glycoproteins of Epstein-Barr virus are encoded by the same gene. J. Virol. 54:665-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 3.Borza, C. M., A. J. Morgan, S. M. Turk, and L. M. Hutt-Fletcher. 2004. Use of gHgL for attachment of Epstein-Barr virus to epithelial cells compromises infection. J. Virol. 78:5007-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Addario, M., T. A. Libermann, J. Xu, A. Ahmad, and J. Menezes. 2001. Epstein-Barr virus and its glycoprotein-350 upregulate IL-6 in human B cells via CD21, involving activation of NF-kB and different signaling pathways. J. Mol. Biol. 308:501-514. [DOI] [PubMed] [Google Scholar]
- 5.Fearon, D. T., and R. H. Carter. 1995. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu. Rev. Immunol. 13:127-149. [DOI] [PubMed] [Google Scholar]
- 6.Fingeroth, J. D., M. E. Diamond, D. R. Sage, J. Hayman, and J. L. Yates. 1999. CD-21 dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 73:2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fingeroth, J. D., J. J. Weis, T. F. Tedder, J. L. Strominger, P. A. Biro, and D. T. Fearon. 1984. Epstein-Barr virus receptor of human B lymphocytes is the C3d complement CR2. Proc. Natl. Acad. Sci. USA 81:4510-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frade, R., M. Barel, B. Ehlin-Henricksson, and G. Klein. 1985. Gp140, the C3d receptor of human B lymphocytes, is also the Epstein-Barr virus receptor. Proc. Natl. Acad. Sci. USA 82:1490-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan, Y., J. Chodosh, A. Morgan, and J. W. Sixbey. 1997. Epithelial cell polarization is a determinant in the infectious outcome of immunoglobulin A-mediated entry by Epstein-Barr virus. J. Virol. 71:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill, M. B., J. Roecklein-Canfield, D. R. Sage, M. Zambela-Soediono, N. Longtine, M. Uknis, and J. D. Fingeroth. 2004. EBV attachment stimulates FHOF/FHOD1 redistribution and co-aggregation with CD21:formin interactins with the cytoplasmic domain of human CD21. J. Cell Sci. 117:2709-2720. [DOI] [PubMed] [Google Scholar]
- 11.Guerreiro-Cacais, A. O., L. Li, D. D. Donat, M. T. Bejarano, A. J. Morgan, M. Masucci, L. M. Hutt-Fletcher, and V. Levitsky. 2004. The capacity of Epstein-Barr virus to infect monocytes and inhibit their development into dendritic cells is affected by the cell type supporting virus replication. J. Gen. Virol. 85:2767-2778. [DOI] [PubMed] [Google Scholar]
- 12.Haan, K. M., S. K. Lee, and R. Longnecker. 2001. Different functional domains in the cytoplasmic tail of glycoprotein gB are involved in Epstein-Barr virus-induced membrane fusion. Virology 290:106-114. [DOI] [PubMed] [Google Scholar]
- 13.Haan, K. M., and R. Longnecker. 2000. Coreceptor restriction within the HLA-DQ locus for Epstein-Barr virus infection. Proc. Natl. Acad. Sci. USA 97:9252-9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddad, R. S., and L. M. Hutt-Fletcher. 1989. Depletion of glycoprotein gp85 from virosomes made with Epstein-Barr virus proteins abolishes their ability to fuse with virus receptor-bearing cells. J. Virol. 63:4998-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 16.Herrold, R. E., A. Marchini, S. Frueling, and R. Longnecker. 1995. Glycoprotein 110, the Epstein-Barr virus homolog of herpes simplex virus glycoprotein B, is essential for Epstein-Barr virus replication in vivo. J. Virol. 70:2049-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hummel, M., D. Thorley-Lawson, and E. Kieff. 1984. An Epstein-Barr virus DNA fragment encodes messages for the two major envelope glycoproteins (gp350/300 and gp220/200). J. Virol. 49:413-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutt-Fletcher, L. M. 2005. EBV entry and epithelial infection, p. 359-378. In E. S. Robertson (ed.), Epstein-Barr virus. Caister Academic Press, Norfolk, England.
- 19.Imai, S., J. Nishikawa, and K. Takada. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janz, A., M. Oezel, C. Kurzeder, J. Mautner, D. Pich, M. Kost, W. Hammerschmidt, and H. J. Delecluse. 2000. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 74:10142-10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, R., R. S. Scott, and L. M. Hutt-Fletcher. 2006. Epstein-Barr virus shed in saliva is high in B-cell-tropic gp42. J. Virol. 80:7281-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 101:16286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laichalk, L. L., and D. A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lake, C. M., and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus that lacks glycoprotein gN is impaired in assembly and infection. J. Virol. 74:11162-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lake, C. M., S. J. Molesworth, and L. M. Hutt-Fletcher. 1998. The Epstein-Barr virus (EBV) gN homolog BLRF1 encodes a 15-kilodalton glycoprotein that cannot be authentically processed unless it is coexpressed with the EBV gM homolog BBRF3. J. Virol. 72:5559-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambris, J. D., V. S. Ganu, S. Hirani, and H. J. Muller-Eberhard. 1985. Mapping of the C3d receptor (CR2) binding site and a neoantigenic site in the C3d domain of the third component of complement. Proc. Natl. Acad. Sci. USA 82:4235-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Q. X., M. K. Spriggs, S. Kovats, S. M. Turk, M. R. Comeau, B. Nepom, and L. M. Hutt-Fletcher. 1997. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 71:4657-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, Q. X., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Q. X., L. S. Young, G. Niedobitek, C. W. Dawson, M. Birkenbach, F. Wang, and A. B. Rickinson. 1992. Epstein-Barr virus infection and replication in a human epithelial system. Nature 356:347-350. [DOI] [PubMed] [Google Scholar]
- 30.Martin, D. R., A. Yuryev, K. R. Kalli, D. T. Fearon, and J. M. Ahearn. 1991. Determination of the structural basis for selective binding of Epstein-Barr virus to human complement receptor type 2. J. Exp. Med. 174:1299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McShane, M. P., and R. Longnecker. 2004. Cell-surface expression of a mutated Epstein-Barr virus glycoprotein B allows fusion independent of other viral proteins. Proc. Natl. Acad. Sci. USA 101:17474-17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, N., and L. M. Hutt-Fletcher. 1992. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 66:3409-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, N., and L. M. Hutt-Fletcher. 1988. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J. Virol. 62:2366-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molesworth, S. J., C. M. Lake, C. M. Borza, S. M. Turk, and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J. Virol. 74:6324-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore, M. D., M. J. Cannon, A. Sewall, M. Finlayson, M. Okimoto, and G. R. Nemerow. 1991. Inhibition of Epstein-Barr virus infection in vitro and in vivo by soluble CR2 (CD21) containing two short consensus repeats. J. Virol. 65:3559-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore, M. D., R. G. DiScipio, N. R. Cooper, and G. R. Nemerow. 1989. Hydrodynamic, electron microscopic, and ligand-binding analysis of the Epstein-Barr virus/C3dg receptor (CR2). J. Biol. Chem. 34:20576-20582. [PubMed] [Google Scholar]
- 37.Mullen, M. M., K. M. Haan, R. Longnecker, and T. S. Jardetzky. 2002. Structure of the Epstein-Barr virus gp42 protein bound to the MHC class II receptor HLA-DR1. Mol. Cell 9:375-385. [DOI] [PubMed] [Google Scholar]
- 38.Nemerow, G. R., and N. R. Cooper. 1984. Early events in the infection of human B lymphocytes by Epstein-Barr virus. Virology 132:186-198. [DOI] [PubMed] [Google Scholar]
- 39.Nemerow, G. R., R. A. Houghton, M. D. Moore, and N. R. Cooper. 1989. Identification of the epitope in the major envelope proteins of Epstein-Barr virus that mediates viral binding to the B lymphocyte EBV receptor (CR2). Cell 56:369-377. [DOI] [PubMed] [Google Scholar]
- 40.Nemerow, G. R., C. Mold, V. K. Schwend, V. Tollefson, and N. R. Cooper. 1987. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J. Virol. 61:1416-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemerow, G. R., R. Wolfert, M. McNaughton, and N. R. Cooper. 1985. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2). J. Virol. 55:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuhierl, B., R. Feederle, W. Hammerschmidt, and H. J. Delecluse. 2002. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. USA 99:15036-15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oda, T., S. Imai, S. Chiba, and K. Takada. 2000. Epstein-Barr virus lacking glycoprotein gp85 cannot infect B cells and epithelial cells. Virology 276:52-58. [DOI] [PubMed] [Google Scholar]
- 44.Omerovic, J., L. Lev, and R. Longnecker. 2005. The amino terminus of Epstein-Barr virus glycoprotein gH is important for fusion with B cells and epithelial cells. J. Virol. 79:12408-12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ressing, M. E., D. van Leeuwen, F. A. W. Verreck, R. Gomez, B. Heemskerk, M. Toebes, M. M. Mullen, T. S. Jardetzky, R. Longnecker, M. W. Schilham, T. H. M. Ottenhoff, J. Neefjes, T. N. Schumacher, L. M. Hutt-Fletcher, and E. J. H. J. Wiertz. 2003. Interference with T cell receptor-HLA-DR interactions by Epstein-Barr virus gp42 results in reduced T helper cell recognition. Proc. Natl. Acad. Sci. USA 100:11583-11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ressing, M. E., D. van Leeuwen, F. A. W. Verreck, S. Keating, R. Gomez, K. L. M. C. Franken, T. H. M. Ottenhoff, M. Spriggs, T. N. Schumacher, L. M. Hutt-Fletcher, M. Rowe, and E. J. H. J. Wiertz. 2005. Epstein-Barr virus gp42 is posttranslationally modified to produce soluble gp42 that mediates class II immune evasion. J. Virol. 79:841-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rickinson, A. B., and E. Kieff. 2007. Epstein-Barr virus, p. 2655-2700. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 48.Rivailler, P., Y.-G. Cho, and F. Wang. 2002. Complete genomic sequence of an Epstein-Barr virus-related herpesvirus naturally infecting a New World primate: a defining point in the evolution of oncogenic lymphocryptoviruses. J. Virol. 76:12055-12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivailler, P., H. Jiang, Y.-G. Cho, C. Quink, and F. Wang. 2002. Complete nucleotide sequence of the rhesus lymphocryptovirus: genetic validation for an Epstein-Barr virus animal model. J. Virol. 76:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savard, M., C. Belanger, M. Tardif, P. Gourde, L. Flamand, and J. Gosselin. 2000. Infection of primary human monocytes by Epstein-Barr virus. J. Virol. 74:2612-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shannon-Lowe, C. D., B. Neuhierl, G. Baldwin, A. B. Rickinson, and H.-J. Delecluse. 2006. Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc. Natl. Acad. Sci. USA 103:7065-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva, A. L., J. Omerovic, T. S. Jardetzky, and R. Longnecker. 2004. Mutational analysis of Epstein-Barr virus glycoprotein gp42 reveals functional domains not involved in receptor binding but required for membrane fusion. J. Virol. 78:5946-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinclair, A. J., and P. J. Farrell. 1995. Host cell requirements for efficient infection of quiescent primary B lymphocytes by Epstein-Barr virus. J. Virol. 69:5461-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sixbey, J. W., and Q.-Y. Yao. 1992. Immunoglobulin A-induced shift of Epstein-Barr virus tissue tropism. Science 255:1578-1580. [DOI] [PubMed] [Google Scholar]
- 55.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spriggs, M. K., R. J. Armitage, M. R. Comeau, L. Strockbine, T. Farrah, B. MacDuff, D. Ulrich, M. R. Alderson, J. Müllberg, and J. I. Cohen. 1996. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR β chain and inhibits antigen presentation. J. Virol. 70:5557-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strnad, B. C., T. Schuster, R. Klein, R. F. Hopkins III, T. Witmer, R. H. Neubauer, and H. Rabin. 1982. Production and characterization of monoclonal antibodies against the Epstein-Barr virus membrane antigen. J. Virol. 41:258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugano, N., W. Chen, M. L. Roberts, and N. R. Cooper. 1997. Epstein-Barr virus binding to CD21 activates the initial viral promoter via NF-κB induction. J. Exp. Med. 186:731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szakonyi, G., M. G. Klein, J. P. Hannan, K. A. Young, R. Z. Ma, R. Asokan, V. M. Holers, and X. S. Chen. 2006. Structure of the Epstein-Barr virus major envelope glycoprotein. Nat. Struct. Mol. Biol. 13:996-1001. [DOI] [PubMed] [Google Scholar]
- 60.Tanner, J., J. Weis, D. Fearon, Y. Whang, and E. Kieff. 1987. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell 50:203-213. [DOI] [PubMed] [Google Scholar]
- 61.Tanner, J., Y. Whang, J. Sample, A. Sears, and E. Keiff. 1988. Soluble gp350/220 and deletion mutant glycoproteins block Epstein-Barr virus adsorption to lymphocytes. J. Virol. 62:4452-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanner, J. E., C. Alfieri, T. A. Chatila, and F. Diaz-Mitoma. 1996. Induction of interleukin-6 after stimulation of human B-cell CD21 by Epstein-Barr virus glycoproteins gp350 and gp220. J. Virol. 70:570-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tugizov, S. M., J. W. Berline, and J. M. Palefsky. 2003. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 9:307-314. [DOI] [PubMed] [Google Scholar]
- 64.Turk, S. M., R. Jiang, L. S. Chesnokova, and L. M. Hutt-Fletcher. 2006. Antibodies to gp350/220 enhance the ability of Epstein-Barr virus to infect epithelial cells. J. Virol. 80:9628-9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, X., and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 72:158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, X., W. J. Kenyon, Q. X. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watt, T. H. 1997. Signaling via MHC class II molecules, p. 141-161. In M. Harnete and K. P. Rigley (ed.), Lymphocyte signaling: mechanisms, subversion and manipulation. John Wiley and Sons Ltd., New York, NY.
- 68.Weisman, H. F., T. Bartow, M. K. Leppo, H. C. Marsh, Jr., G. R. Carson, M. F. Concino, M. P. Boyle, K. H. Roux, M. L. Weisdeldt, and D. T. Fearon. 1990. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249:146-151. [DOI] [PubMed] [Google Scholar]
- 69.Wu, L., C. M. Borza, and L. M. Hutt-Fletcher. 2005. Mutations of Epstein-Barr virus gH that are differentially able to support fusion with B cells or epithelial cells. J. Virol. 79:10923-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu, L., and L. M. Hutt-Fletcher. 20 February 2007. Point mutations in EBV gH that abrogate or differentially affect B cell and epithelial cell fusion. Virology 363:148-155. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao, J., J. M. Palefsky, R. Herrera, and S. M. Tugizov. 2007. Characterization of the Epstein-Barr virus glycoprotein BMRF2. Virology 359:382-396. [DOI] [PubMed] [Google Scholar]
- 72.Yaswen, L. R., E. B. Stephens, L. C. Davenport, and L. M. Hutt-Fletcher. 1993. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology 195:387-396. [DOI] [PubMed] [Google Scholar]
- 73.Young, L. S., C. W. Dawson, K. W. Brown, and A. B. Rickinson. 1989. Identification of a human epithelial cell surface protein sharing an epitope with the C3d/Epstein-Barr virus receptor molecule of B lymphocytes. Int. J. Cancer 43:786-794. [DOI] [PubMed] [Google Scholar]