Abstract
Pulsed ultrasound, when used as an adjuvant to recombinant tissue plasminogen activator (rt-PA), has been shown to enhance thrombolysis in the laboratory as well as in clinical trials for the treatment of ischemic stroke. The exact mechanism of this enhancement has not yet been elucidated. In this work, stable and inertial cavitation (SC and IC) are investigated as possible mechanisms for this enhancement. A passive cavitation detection scheme was utilized to measure cavitation thresholds at 120 kHz (80% duty cycle, 1667 Hz pulse repetition frequency) for four host fluid and sample combinations: plasma, plasma with rt-PA, plasma with clot and plasma with clot and rt-PA. Following cavitation threshold determination, clots were exposed to pulsed ultrasound for 30 min in vitro using three separate ultrasound treatment regimes: (1) no cavitation (0.15 MPa), (2) SC alone (0.24 MPa) or (3) SC + IC combined (0.36 MPa) in the presence of rt-PA. Percent clot mass loss after each treatment was used to determine thrombolysis efficacy. The highest percent mass loss was observed in the stable cavitation regime (26%), followed by the combined stable and inertial cavitation regime (20.7%). Interestingly, the percent mass loss in clots exposed to ultrasound without cavitation (13.7%) was not statistically significantly different from rt-PA alone (13%) [p > 0.05]. Significant enhancement of thrombolysis correlates with presence of cavitation and stable cavitation appears to play a more important role in the enhancement of thrombolysis.
Keywords: Cavitation, Ultrasound-assisted thrombolysis, Cavitation detection
INTRODUCTION
The addition of ultrasound with clinically relevant intensities and frequencies has enhanced the enzymatic rate of thrombolytic therapy in vitro (Blinc et al. 1993; Francis et al. 1992; Holland et al. 2002; Lauer et al. 1992; Olsson et al. 1994; Shaw et al. 2001), in animal models (Kashyap et al. 1994; Lou et al. 1996; Suchkova et al. 2000) and in clinical trials (Alexandrov et al. 2004a, 2004b). Acceleration of thrombolysis is a particularly critical issue for acute treatment of ischemic stroke. Tissue plasminogen activator is the only scientifically-proven and FDA-approved treatment for acute ischemic stroke, but its approval is based upon administration within 3 h of stroke onset (The NINDS rt-PA Stroke study Group, 1995). A pooled analysis of all randomized stroke trials of t-PA indicates that there may be some beneficial effect of t-PA administered up to 4 h after onset, but this analysis also demonstrated that the time from onset to administration is critical to the effectiveness of the therapy, even within 3 h (Hacke et al. 2004). In addition, studies of subjects treated with t-PA indicate that IV t-PA reopens partially only 30 to 40% of occluded major intracranial trunk arteries within the first 1 to 2 h after initiation of treatment, as described by cerebral angiography (Broderick and Hacke 2002). The major risk of t-PA and other thrombolytic agents is intracerebral hemorrhage, which is symptomatic in 6% of patients (Broderick and Hacke 2002; Hacke et al. 2004). Thus, the use of ultrasound to enhance and accelerate the thrombolysis of occluded intracranial arteries is an area of active investigation. Ideally, ultrasound’s beneficial effect should be accomplished without increasing the risk of intracranial hemorrhage.
There are generally two ultrasound frequency regimes used by investigators to enhance thrombolysis, 1–5 MHz and 40–500 kHz. Recent studies demonstrate greater acceleration in fibrinolysis using frequencies in the midkilohertz range rather than in the megahertz range (Blinc et al. 1993; Suchkova et al. 1998, 2002). Apart from accelerated fibrinolysis, there are additional benefits of using kilohertz-frequency ultrasound regarding better tissue penetration, particularly past the skull (Coussios et al. 2002, 2004) and for reduced heating. Reduction in the potential for heating becomes important for minimizing thermal bioeffects in the temporal bone and brain for transcranial applications. This kilohertz ultrasound frequency regime with lower intensities has been investigated in several transcranial applications to enhance thrombolysis (Akiyama et al. 1998; Behrens et al. 1999). A 120-kHz ultrasound thrombolysis system (UTS) has also shown significant enhancement of thrombolysis in vitro when used adjuvant to rt-PA (Cheng et al. 2004, 2005; Datta et al. 2005; Holland et al. 2002; Shaw et al. 2001). A 2 MHz transcranial Doppler has also been shown to augment t-PA-induced arterial recanalization in patients (Alexandrov et al. 2004b).
Mechanisms of ultrasonic potentiation of thrombolysis need to be identified and exploited for therapeutic applications. In the kilohertz frequency range, several mechanisms have been proposed. Some studies (Datta et al. 2005; Siegel et al. 1989; Suchkova et al. 1998) have hypothesized nonthermal mechanisms of enhanced thrombolysis by increasing the transport of fibrinolytic enzymes into the clot. Suchkova et al. (2002) proffered that mechanical processes unrelated to cavitation may also be involved. Sakharov et al. (2000) attributed the increase in lysis rate to an increase in enzymatic action due to a 6°C temperature rise using 1-MHz ultrasound, but concluded that temperature rise is a minor portion of the accelerating effect of ultrasound. Sakharov et al. (2000) also concluded that the effect of ultrasound is equivalent to mild stirring, possibly caused by acoustic streaming. A recent study by Pieters et al. (2004) correlated an increase in thrombolysis to a lesser extent with temperature rise and to a greater extent with an increase in penetration of the thrombolytic agent into the clot using 40-kHz ultrasound. The results exhibited clot model dependence and the presence of multiple mechanisms resulting in increased transport of plasminogen and thrombolytic agent into the clot. The mechanism itself responsible for transport of plasminogen or thrombolytic agent was not elucidated.
Acoustic cavitation has been studied extensively by investigators to understand ultrasound bioeffects (Apfel 1981; Flynn 1982). Acoustic cavitation is the formation and collapse of gaseous and vapor bubbles in a liquid due to an acoustic pressure field. Cavitation is generally classified into two types, stable cavitation, which results in emissions at subharmonics of the main excitation frequency and inertial cavitation, which is characterized by broadband noise emissions. Stable cavitation can induce bubble-associated microstreaming (Miller 1988) and inertial cavitation can cause microjetting and pitting on solid surfaces (Bailey et al. 1999; Crum 1988). Everbach and Francis (2000) demonstrated an increase in thrombolysis due to cavitation effects, but also suggested the possibility of other unknown mechanisms. Their work did not distinguish between the effect of stable and inertial cavitation. Some investigators have clearly shown that ultrasound accelerates enzymatic fibrinolysis by increasing the transport of reactants through cavitation-related mechanisms (Francis et al. 1992; Blinc et al. 1993; Francis et al. 1995). Koch et al. (2000) provided evidence that ultrasound-assisted drug uptake is due to cavitation. In addition, Greenleaf et al. (1998) demonstrated enhanced transfection due to the addition of Albunex® as a cavitation nucleation agent in the presence of 1-MHz continuous wave ultrasound. In light of these previous studies, the objective of this study was to investigate stable and inertial cavitation as potential mechanisms for ultrasound-enhanced thrombolysis.
MATERIALS AND METHODS
Ultrasound thrombolysis system (UTS)
A prototype 120-kHz UTS transducer (Sonic Concepts Inc., Woodinville, WA, USA) was developed using a 6.14 cm-diameter single circular element made of 1 to 3 piezocomposite material. The transducer is optimized for maximum electroacoustic power conversion efficiency. The transducer output was calibrated in water with a 0.5 mm hydrophone (Reson, TC 4038, Goleta, CA, USA) mounted on a computer-controlled three-axis positioner (Velmex, NF-90 series, Bloomfield, NY, USA) and the beam pattern was carefully mapped. The transducer has a 2.5-cm -3-dB beam width and a Rayleigh distance of 8.45 cm. The 120-kHz single-element ultrasound transducer was driven by a function generator (Agilent, 33120A, Palo Alto, CA, USA), power amplifier (T & C Power Conversions Inc., Ultra 2021, Rochester, NY, USA) and custom-built impedance matching network (Sonic Concepts Inc.). For all experiments described in this study, the UTS transducer was driven in either continuous wave (CW) mode or pulsed mode with an 80% duty cycle and 1667 Hz pulse repetition frequency. These two exposure conditions were found to lead to maximum clot mass loss in preliminary ultrasound-enhanced thrombolysis studies (Holland et al., 2002).
General experimental arrangement
The experiments were conducted in a Lucite tank (40 cm × 53 cm × 27 cm) filled with degassed water. A water filtering and degassing system was developed in the laboratory to purify and degas water in the tank, to ensure that the cavitation threshold inside the tank was significantly higher than within the sample holder. Water is passed first through a 0.2 μm filter (Cole Parmer, Fin-L-Filters A-01509-90, Vernon Hills, IL, USA) and then through a cartridge containing many hydrophobic porous microfibers (Liqui-Cel Membrane Contractor, X-40, Charlotte, NC, USA). The water flows inside the lumen of the microfibers and a vacuum is applied outside the fibers with a vacuum pump (Welch 2254B, Skokie, IL, USA). Thus, dissolved gases are separated from the water within the pores of the hydrophobic microfibers. It is essential to purify the water in order to avoid nucleation of cavitation in the water bath. The dissolved oxygen level (PO2) of water in the tank was maintained at 0.8 mg/L. The water in the tank was maintained at 37°C at all times using an immersion heater with feedback control (Fisher-Scientific Isotemp 2100, Pittsburgh, PA, USA).
The overall experimental arrangement is shown in Fig. 1. The tank wall opposite the 120-kHz transducer was lined with acoustically matched (rho-C) absorber (Aptflex F36, Precision Acoustics, Dorchester, UK) to prevent the formation of standing waves and to stop reflections from reaching the sample region. The sample holder consisted of an acoustically-transparent latex condom wrapped over a plastic frame, which was filled with 35 ml of thawed, porcine fresh frozen plasma and maintained at 37°C in the temperature-controlled bath for at least 20 min before use. The sample holder was mounted on a three-axis translation stage (Newport 423, Irvine, CA, USA). This was used to position the lower portion of the sample holder, which forms a hemispherical region that is about 1.4 cm in diameter, acoustically at the Rayleigh distance (8.45 cm) of the 120-kHz UTS transducer. In experiments where a blood clot was exposed to ultrasound, the blood clot, which is about 1 cm in diameter, would sit in the condom tip, with a marginal layer of plasma between the clot and the latex interface.
Fig. 1.
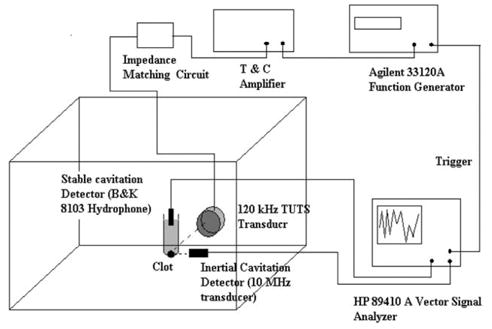
Experimental apparatus showing the confocally aligned 120-kHz UTS transducer, stable cavitation detector and inertial cavitation detector, along with the electronic equipment used for cavitation detection. The water in the tank was kept at 37°C during all experiments.
Passive cavitation detection system
Previous investigators have used both active and passive cavitation detection techniques (Everbach 1997; Holland and Apfel 1990; Holland et al. 1992; Leighton 1997; Roy et al. 1990; Thomas et al. 2005). Active cavitation detection is preferable when very high spatial resolution is required and it is desirable to observe individual cavitation events. However, in the context of this study, observation of individual cavitation events was not required. In addition, the use of a sample holder immersed in highly degassed and purified water ensured that cavitation events, if present, were bound to occur in the sample region. The same water in the sample holder also had a very high threshold of cavitation. Spatial resolution was therefore not critical and a passive cavitation detection scheme was chosen for this study, as it enables differentiation of stable from inertial cavitation within the confocal sample volume through analysis of received pulses in the frequency domain. This was achieved by using two separate detectors, one tuned to receive broadband noise emissions resulting from inertial cavitation and one listening for subharmonic emissions representative of stable cavitation.
Inertial cavitation (IC) was detected using a broadband transducer (Valpey Fisher, 46654, Hopkinton, MA, USA) with a 10-MHz center frequency, a 19-mm focal length, a 1-mm -3 dB transverse beamwidth at the focus and a 1.5 mm focal depth. The IC detection transducer was mounted onto micrometer-controlled translation stages and immersed in the tank water orthogonally to the main axis of the UTS transducer. Initially, a 1.1-cm diameter brass ball was used as an acoustic target to ensure that the focus of the IC detection transducer coincided with the center of the volume enclosed by the condom tip, which was itself positioned at the Rayleigh distance of the UTS transducer along its axis. In experiments performed using a blood clot, the IC detector was moved incrementally toward the UTS transducer so that its focus overlapped with the edge of the clot nearest to the UTS transducer. The clot itself was used as an acoustic target when driving the IC detection transducer in pulse-echo mode, to ensure that its focal region encompassed the marginal plasma layer separating the edge of the clot from the latex container and about 0.75 mm into the clot itself. Thus, the exterior layer of the clot and surrounding aqueous layer were interrogated by the cavitation detector during thrombolysis.
The use of an IC detection transducer with a center frequency much higher than the insonifying pulse (120 kHz) allowed the differentiation of broadband noise emissions due to inertial cavitation from the fundamental (120 kHz) and superharmonic (240 kHz, 360 kHz etc.) components due to nonlinear propagation and scattering of the 120-kHz field. The signal captured by the 10-MHz transducer was analyzed by using a vector signal analyzer (HP89410A) and broadband noise and superharmonic emissions were monitored over the frequency range 2 to 7 MHz, as shown in Fig. 2.
Fig. 2.
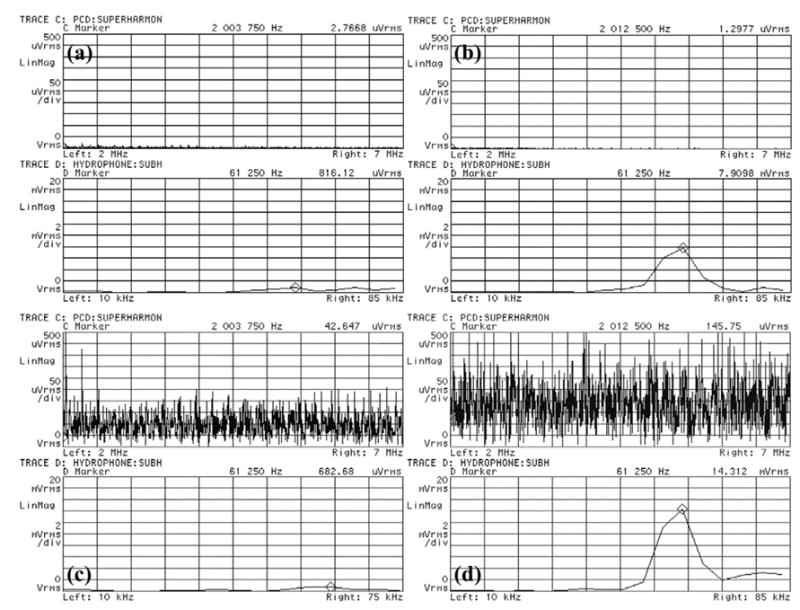
Representative frequency spectrum traces from the HP89410A vector signal analyzer used for cavitation detection caused by exposure to 120-kHz ultrasound. The top trace in each panel is the frequency spectrum from 2 MHz to 7 MHz and the bottom trace is the frequency spectrum from 10 kHz to 75 kHz. (a) Shows the typical background noise level for plasma alone at 0.24 MPa P- when no cavitation is present. (b) Shows stable cavitation in plasma exposed to 0.36 MPa P- pressure amplitude. Note the 60-kHz peak on bottom trace corresponding to subharmonic emissions, but no broadband superharmonic emissions on the top trace typical of inertial cavitation. (c) Shows inertial cavitation in plasma with a clot exposed to 0.26 MPa P- pressure amplitude. Note the broadband superharmonic emissions corresponding to inertial cavitation. (d) Shows both inertial and stable cavitation present in plasma exposed to 0.40 MPa P-.
Stable cavitation (SC) was detected using a (nondirectional) hydrophone (B & K 8103, Norcross, GA, USA), which was immersed vertically into the liquid contained in the sample holder and placed orthogonal to the 120-kHz transducer beam (Fig. 1). The signal from the B & K hydrophone was also analyzed by the vector signal analyzer over a frequency range of 10 kHz to 75 kHz. Stable cavitation was indicated by the presence of a subharmonic peak in the frequency spectrum at 60 kHz.
The results shown in Fig. 2(b) clearly indicate the presence of stable cavitation within the sample. When a bubble oscillates nonlinearly about its equilibrium radius, Faraday waves are excited along the bubble wall, causing these subharmonic emissions (Phelps and Leighton 1997). Broadband noise emissions appear above the background noise level and indicate the presence of inertial cavitation, as shown in Fig. 2(c). Such emissions are generated when bubbles undergo large radial oscillations and collapse violently (ANSI 2002; Holland et al. 1990, 1996).
By slowly ramping up the pressure amplitude generated by the 120-kHz transducer, the detected and analyzed signals were used to measure the thresholds of stable and inertial cavitation. In the present work, the cavitation threshold is defined as the lowest peak negative pressure amplitude at which subharmonic emissions (for SC threshold) or broadband noise emissions (for IC threshold) are detected. While the cavitation threshold is primarily dependent upon the properties of liquid, it is also significantly affected by the presence of impurities in the liquid (Holland and Apfel 1990) and the dissolved gas concentration. We measured the cavitation threshold for plasma alone, plasma with rt-PA, a blood clot immersed in plasma and, finally, a clot immersed in plasma and rt-PA without the addition of nucleation agents, such as hydrophobic particles, or specific manipulation of the gas concentration of these biologic fluids and tissue.
Ex vivo clot model, rt-PA dose and immunohistochemistry
To compare the efficacy of different treatment protocols, 30 porcine whole blood clots were prepared. Whole blood clots were made by aliquoting 1.5 mL fresh porcine blood into 1.3-cm inner diameter Vacutainer tubes, incubating the tubes in a 37°C water bath for 3 h, and storing the clots at 5°C for at least 3 d before use in experiments (Holland et al. 2002; Shaw et al. 2001). This ensured complete clot maturation and retraction. Porcine clots prepared with this standard method have an average diameter of 1 cm. The whole blood clots were blotted and weighed before and after each treatment to determine the mass loss.
Clots were placed in the sample holder with thawed porcine fresh frozen plasma and held at 37°C. Reconstituted rt-PA (0.107 mg/mL) was added to the plasma for sham (rt-PA exposure only) and combined ultrasound and rt-PA treatments. The treatment times were fixed at 30 min. Following exposure, each clot was again weighed, and the extent of thrombolysis was determined as a percent mass loss. Randomly-selected clots within each treatment regime were fixed in 10% formalin for at least 36 h. These clots were dehydrated in 70% ethanol, embedded in paraffin and cut in 4-μm-thick sections. Sections were immunochemically labeled with mouse antifibrinogen antibody (DakoCytomation, Carpinteria, CA) and visualized with horseradish peroxidase-linked goat antimouse IgG. This gives the fibrin mesh present in the clots a reddish brown color in the slides (shown in Fig. 3). The intensity of the reddish brown color is proportional to the local fibrin or fibrinogen density and is an indication of the amount of clot lysis. The slides were imaged using a digital imaging system (Nikon D2X single lens reflex digital camera), using strictly consistent lighting and exposure conditions.
Fig. 3.
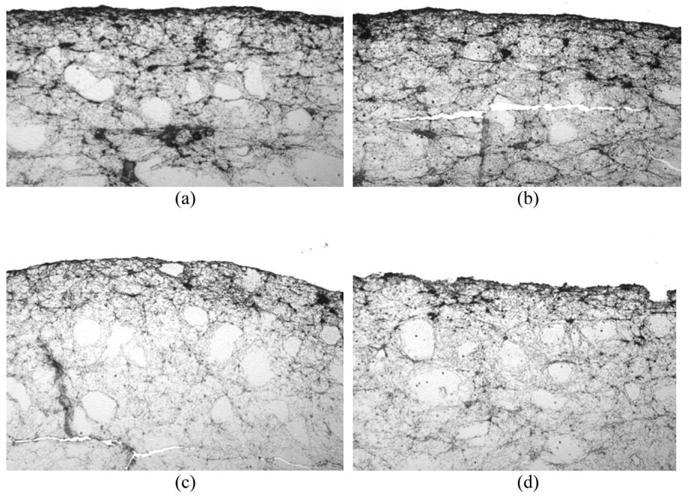
Immunohistochemical imaging analysis of treated clots. Following treatment, clots were fixed in formalin, dehydrated, embedded in paraffin, and cut in 4 micron sections. Clot slices were labeled with mouse antifibrinogen antibody and stained with horseradish peroxidase-linked goat antimouse IgG. Clot surfaces were photographed at 20 × magnification. All treated clots demonstrate a relatively densely staining band along the outermost surface. Relative to (a) Control, (b) The surface of the clot treated with ultrasound alone is essentially no different. (c) After treatment with rt-PA alone, the densely stained band at the surface is much thinner, but remains smooth in contour. (d) After treatment with rt-PA and ultrasound at 0.24 MPa P- (stable cavitation regime), the surface appears markedly irregular and porous.
To quantify the fibrin degradation from the images, mean gray-scale analysis was conducted in three regions-of-interest within the clot: (1) on the surface, (2) in the interior of the clot away from the surface and (3) encompassing both the surface and the interior of the clot. The areas of the regions-of-interest were kept fixed for each treatment, as shown in Fig. 4. The mean gray-scale values were computed using MATLAB (Mathworks Inc.) by first converting the color images into gray-scale and then computing the mean gray-scale value for the representative area. Care was taken not to include areas where there was little apparent staining by the antibody.
Fig. 4.
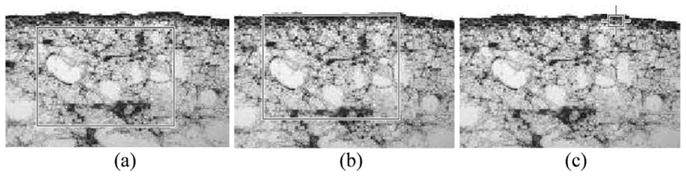
Representative areas taken for mean gray-scale analysis to evaluate fibrin degradation. (a) Interior of the clot, (b) Surface and interior of the clot and (c) Surface of the clot.
Correlation of cavitation and thrombolysis
The increase in thrombolysis noted in clots exposed to a combination of rt-PA and ultrasound was hypothesized to be correlated with cavitation activity. Thus, the stable and inertial cavitation thresholds were identified for the following host fluid and sample combinations: (1) water bath alone with no sample, (2) plasma alone, (3) plasma and rt-PA, (4) plasma and clot and (5) clot, plasma and rt-PA. The mean and standard deviation were calculated from measurements of the stable and inertial cavitation thresholds in each of the five host fluid and sample combinations. A single factor fixed effect ANOVA analysis was performed on the stable and inertial cavitation threshold measurements using MINITAB statistical analysis software (MINITAB Inc., PA, USA). The null hypothesis tested was that the means of the cavitation thresholds for the treatment protocols were equal (or that the data came from the same parent distribution). The cavitation thresholds identified in this set of experiments were subsequently used to explore the relationship between cavitation and enhanced clot mass loss.
To determine whether enhanced thrombolysis was correlated with the presence of stable cavitation, inertial cavitation or both, clot mass loss was measured for six exposure conditions: (1) control (plasma alone without rt-PA or ultrasound), (2) sham (rt-PA without ultrasound), (3) pulsed ultrasound alone (without rt-PA) at a peak negative (P-) amplitude of 0.36 MPa (above the threshold of stable and inertial cavitation), (4) combined rt-PA and pulsed ultrasound below either cavitation threshold (0.15 MPa P-), (5) combined rt-PA and pulsed ultrasound above the stable cavitation threshold but below the inertial cavitation threshold (0.24 MPa P-) and (6) rt-PA and pulsed ultrasound above the stable and inertial cavitation thresholds (0.36 MPa P-). The pulsed ultrasound exposures utilized 80% duty cycle and a 1667-Hz pulse repetition frequency. Five clots were treated for each exposure condition and the mean and standard deviation of the clot mass loss was calculated.
RESULTS
Cavitation thresholds
Cavitation threshold data were collected, based on the presence of subharmonics (stable cavitation) or broadband noise emissions (inertial cavitation) in the frequency spectrum detected by the vector signal analyzer (shown in Fig. 1). Table 1 shows the stable and inertial cavitation threshold data, averaged over the number of measurements denoted by N, for continuous wave 120-kHz ultrasound exposure in the five host fluid and sample combinations: (1) purified degassed water, (2) plasma alone, (3) plasma with rt-PA, (4) plasma and clot without rt-PA and (5) plasma and clot with rt-PA. ANOVA analysis showed that the mean of each threshold was statistically different for all five fluids (or fluid and clot combinations) (p < 0.001). Neither stable nor inertial cavitation could be detected in the water bath alone or in the presence of purified degassed water in the sample holder, up to the maximum power output of the UTS transducer (0.65 MPa P-). This, therefore, ensures that any cavitation observed for all other cases occurs within the sample holder, not in the water bath. The stable cavitation threshold is lower than the inertial cavitation threshold for each fluid and clot combination. For continuous wave ultrasound the cavitation thresholds are highest for plasma alone (0.39 MPa P- for stable cavitation and 0.50 MPa P- for inertial cavitation). The cavitation thresholds are lower in the presence of clot or rt-PA. Interestingly, there is a significant drop in cavitation thresholds when both clot and rt-PA are present.
Table 1.
Mean stable and inertial cavitation thresholds for 120-kHz continuous wave ultrasound (N is the number of samples and the number in parentheses is standard deviation.)
| Mean stable cavitation threshold
|
Mean inertial cavitation threshold
|
|||||
|---|---|---|---|---|---|---|
| Sample fluid | N | (MPa P-) | (MPa P-P) | N | (MPa P-) | (MPa P-P) |
| Water bath | 10 | >0.65 | >1.3 | 10 | >0.65 | >1.3 |
| Plasma Alone | 32 | 0.39 (0.04) | 0.76 (0.07) | 32 | 0.50 (0.05) | 0.98 (0.09) |
| Plasma with t-PA | 15 | 0.35 (0.03) | 0.70 (0.06) | 15 | 0.47 (0.04) | 0.92 (0.07) |
| Plasma with clot | 33 | 0.26 (0.03) | 0.52 (0.06) | 32 | 0.45 (0.03) | 0.89 (0.05) |
| Plasma with clot and t-PA | 19 | 0.20 (0.02) | 0.39 (0.04) | 16 | 0.27 (0.06) | 0.57 (0.11) |
Similarly, Table 2 shows cavitation threshold data for pulsed ultrasound exposures (80% duty cycle, 1667 Hz PRF). The threshold data follows the same trends as continuous wave ultrasound exposure data, with the highest thresholds exhibited for plasma alone (0.39 MPa P- for stable cavitation and 0.47 MPa P- for inertial cavitation). Again, the lowest cavitation thresholds were observed for plasma with clot and t-PA (0.20 MPa P- for stable cavitation and 0.30 MPa P- for inertial cavitation). Comparison across Table 1 and Table 2 using paired Student’s t-tests using a p = 0.05 significance level indicate that the cavitation thresholds are not significantly different for each treatment when comparing 80% duty cycle with CW ultrasound exposure.
Table 2.
Mean stable and inertial cavitation thresholds for 120-kHz pulsed ultrasound with 80 % duty cycle and 1667 Hz pulse repetition frequency. (N is the number of samples and the number in parentheses is standard deviation.)
| Mean stable cavitation threshold
|
Mean inertial cavitation threshold
|
|||||
|---|---|---|---|---|---|---|
| Sample fluid | N | (MPa P-) | (MPa P-P) | N | (MPa P-) | (MPa P-P) |
| Water bath | 10 | >0.65 | >1.3 | 10 | >0.65 | >1.3 |
| Plasma Alone | 21 | 0.39 (0.05) | 0.76 (0.09) | 21 | 0.47 (0.07) | 0.93 (0.12) |
| Plasma with t-PA | 9 | 0.34 (0.03) | 0.67 (0.05) | 9 | 0.46 (0.03) | 0.90 (0.06) |
| Plasma with clot | 20 | 0.36 (0.02) | 0.62 (0.04) | 20 | 0.47 (0.02) | 0.92 (0.04) |
| Plasma with clot and t-PA | 12 | 0.20 (0.02) | 0.40 (0.04) | 12 | 0.30 (0.03) | 0.60 (0.07) |
Mass loss
The pulsed ultrasound exposure parameters selected for the clot mass loss study were culled from the cavitation threshold data shown in Table 2. The pressure amplitude chosen for the pulsed ultrasound exposure condition above the stable cavitation threshold, namely 0.24 MPa P-, exceeded the threshold for plasma alone by twice the standard deviation of the threshold for plasma alone. The pressure amplitude selected for the pulsed ultrasound exposure above both the stable and inertial cavitation thresholds, namely 0.36 MPa P-, was selected to be two standard deviations above the inertial cavitation threshold for plasma with clot and rt-PA. The clot mass loss results for the six separate 30 min treatment protocols are presented in Fig. 5. The data suggest that ultrasound alone does not significantly enhance clot mass loss when compared with handling in the absence of rt-PA. The clot mass loss is significantly higher (p < 0.001) for rt-PA alone (sham) when compared with handling (control) in a paired Student’s t-test. However, clot mass loss is not significantly different from sham when ultrasound exposure is below either cavitation thresholds (0.15 MPa P-) adjuvant to rt-PA. For ultrasound exposure above the stable cavitation threshold but below the inertial cavitation threshold (0.24 MPa P-) adjuvant to t-PA, the clot mass loss is significantly higher (p = 0.002) compared with rt-PA alone in a paired Student’s t-test. For ultrasound exposure above the inertial and stable cavitation thresholds (0.36 MPa P-) adjuvant to rt-PA, the clot mass was again significantly higher (p < 0.001) than rt-PA alone (sham) in a paired t-test. Most interesting to note is that clot mass loss is statistically significantly higher (p = 0.046) with pulsed ultrasound exposure above the stable cavitation but below inertial cavitation (0.24 MPa P-), compared with exposure above the inertial cavitation threshold (0.36 MPa P-). The presence of cavitation is strongly correlated with enhanced clot mass loss. However, this enhancement is more pronounced in the presence of stable cavitation alone than in the presence of both stable and inertial cavitation.
Fig. 5.
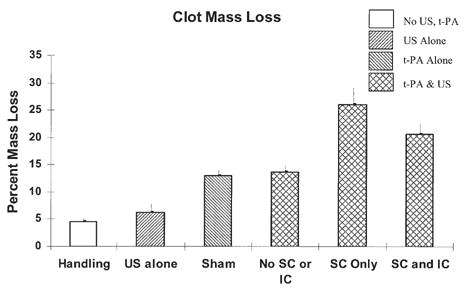
Percent clot mass loss after 30 min exposure. n = 5 for each treatment protocol. Error bars indicate one standard deviation. “Handling” refers to clots in plasma alone (without rt-PA or pulsed ultrasound). “US alone” refers to clots without rt-PA, but with pulsed ultrasound (0.36 MPa P-), “Sham” refers to clots exposed to rt-PA alone, but without pulsed ultrasound. “No SC or IC” refers to clots exposed to rt-PA and pulsed ultrasound below both the stable cavitation (SC) and inertial cavitation (IC) thresholds (0.15 MPa P-). “SC Only” refers to clots with rt-PA and pulsed ultrasound above the stable but below the inertial cavitation threshold (0.24 MPa P-), “SC and IC” refers to clots exposed to rt-PA and pulsed ultrasound above the stable and inertial cavitation thresholds (0.36 MPa P-).
Immunohistochemical analysis
The images generated from the immunohistochemical analysis of the clots provided a way to visualize the fibrin network in the clots exposed to different treatment combinations. It was observed that all the clots had a band of fibrin mesh around the surface of the clot, as shown in Fig. 3. Areas inside the clot exhibited comparatively less staining by the antibody, which was observed in almost all the treatments. For the handling, sham or ultrasound treatment below any cavitation threshold, the surface roughness and general appearance of fibrin band on the surface appears to be similar to one another. The general fibrin mesh orientation is circumferential or irregular. Exposure to stable or inertial cavitation conditions in the presence of rt-PA resulted in a relatively rough surface and the band of fibrin appears to be thinner, irregular, lighter in color and even porous at some locations (shown in Fig. 6). These qualitative observations are consistent with the mass loss data obtained for each treatment. The measurements of mean gray-scale values for the representative areas are shown in Table 3. The results indicate greatest change in mean gray-scale value on the surface for the combined ultrasound treatments with rt-PA.
Fig. 6.
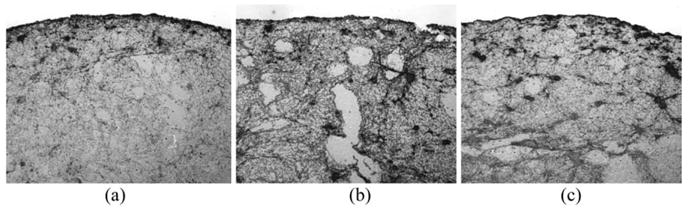
Immunohistochemical analysis of the effects of cavitation on treated clots. Clots were exposed to (a) rt-PA and ultrasound without cavitation, (b) With stable cavitation or (c) With stable and inertial cavitation for 30 min. Following treatment, the clots were prepared for immunohistochemical analysis. After treatment without any cavitation (a), the clot surface has a slightly irregular contour, but remains essentially smooth and intact. After treatment with stable cavitation but without inertial cavitation (b), the clot surface is notably irregular and discontinuous. After treatment with both stable and inertial cavitation (c), the clot surface appears thin but relatively smooth and continuous, similar in appearance to the clot treated with rt-PA alone (see Fig. 3c).
Table 3.
Mean gray-scale values within different representative regions-of-interest on the surface as well as interior of clot. A lower number indicates darker region representing a higher density of fibrin network.
| Handling | US alone | Sham | US and rt-PA | |
|---|---|---|---|---|
| Interior of clot | 157 | 159 | 174 | 169 |
| Interior and surface both | 154 | 155 | 170 | 166 |
| Surface alone | 111 | 116 | 178 | 158 |
DISCUSSION
The cavitation thresholds were measured for porcine plasma, plasma with clot, plasma with rt-PA and plasma with clot and rt-PA. To the best of our knowledge, there are no published values of cavitation thresholds in the above fluid conditions or in clotted blood. However, a previous in vitro study provided measurements of the inertial cavitation threshold in human blood (Deng et al. 1996) at 2.5 MHz and 4.3 MHz. The inertial cavitation threshold was measured using an active detection scheme. These authors reported a higher inertial cavitation threshold at 4.3 MHz of more than 6.2 MPa P-pressure compared with 2.95 MPa P- at 2.5 MHz. These authors also noted that the cavitation threshold was lowered significantly by the addition of nucleation agents.
Holland et al. (1990) reported an inertial cavitation threshold in blood of 1.41 MPa P- pressure at 0.757 MHz. The observation of this lower threshold is consistent with the expectation of a lower threshold at a lower frequency. While studying the effect of 1 MHz ultrasound on hemolysis in whole blood (Chen et al. 2003; Brayman et al. 1996), a peak negative pressure threshold of 0.5 MPa was reported in the presence of contrast agents. This threshold was significantly lower than the 4.5 MPa reported by Deng et al. (1996) in the absence of contrast agents, or stabilized microbubbles which act as cavitation nuclei. Fowlkes et al. (1988) reported cavitation thresholds for an aqueous solution measured at 1 MHz for different duty cycles and pulse repetition frequencies (PRF). They reported a cavitation threshold as low as 0.46 MPa and concluded that the threshold decreases as the duty cycle or PRF is increased. In our studies, the cavitation thresholds are similar for 80% duty cycle or CW exposures.
The measurements of acoustic cavitation thresholds have been reported in tissue at 0.2 MHz exposed to lithotriptor fields (Coleman et al. 1995). These authors measured a threshold of 1.5 to 3.5 MPa peak negative pressure for soft tissues for a single cycle acoustic shock wave. The values obtained for cavitation thresholds are specific to the particular lithotriptor, detector and fluid conditions. However, the threshold values obtained in our experiment agree with the general trend reported in the literature.
Cavitation thresholds also depend on the center frequency of the pulse and the presence of cavitation nuclei or stabilized gas bodies in the fluid which lower the threshold (Brayman et al. 1996; Chen et al. 2003; Deng et al. 1996). We observed that the presence of a clot in combination with rt-PA decreased the cavitation threshold significantly. The interaction of rt-PA with the clot results in the release of fibrin degradation products (Kimura et al. 1994; Suchkova et al. 2002) which may provide nucleation sites to facilitate cavitation. The same effect was not observed when a clot was present in plasma in the absence of rt-PA. This observation is in agreement with earlier studies (Pieters et al. 2004; Sakharov et al. 2000; Blinc et al. 1993) indicating that the presence of plasminogen and rt-PA is essential for release of fibrin degradation products and that ultrasound alone does not cause clot lysis.
Our cavitation detection apparatus has the capability of monitoring both stable and inertial cavitation simultaneously in opaque samples. The mass loss data suggest that the presence of stable cavitation seems to be more beneficial for ultrasound-assisted thrombolysis. This is an important distinction in light of the observation of lower mass loss in the inertial cavitation regime, which indicates that some of this enhancement is lost when inertial cavitation is present. Everbach et al. (2000) applied a static overpressure to eliminate inertial cavitation, which resulted in a 50% reduction in enhancement of fibrinolysis. In addition, inertial cavitation from pulsed diagnostic ultrasound has been associated with harmful bioeffects, including increased vascular permeability in the heart and lungs (Li et al. 2003; Tarantal and Canfield 1994). Stable cavitation, with smaller radial excursions of the bubble wall, potentially may cause less of this type of damage to the vessel walls.
Our observation of both the cavitational mechanisms simultaneously provides the ability to distinguish the relative impact of these types of cavitation. Temporal observation of cavitation activity by the detectors during the entire 30 min exposure could provide information about the duration of cavitation activity, or dose. In most of our experiments conducted above the stable cavitation but below the inertial cavitation threshold, cavitation was present almost throughout the entire 30 min exposure duration. In the ultrasound exposure condition above the inertial cavitation threshold, the inertial cavitation activity was sporadic and was often preceded or followed by stable cavitation activity. It was not possible to control the type of cavitation independently in our experimental set-up.
It has been demonstrated earlier that stable cavitation can be sustained for a long duration with the appropriate acoustic parameters (Mestas et al. 2003). The enhanced thrombolytic effect in the stable cavitation regime, as opposed to the inertial cavitation regime, may simply be attributed to the fact that this stable cavitation activity was sustained for a longer period of time. Another speculative explanation is that the large acoustic cross-section of inertially cavitating bubbles may effectively shield the clot surface and reduce the cavitation activity where needed. The enhancement due to stable cavitation inside the clot may be due to the mechanical effects of oscillating microbubbles near the fibrin mesh to expose new binding sites for plasmin that were previously not exposed. The stable cavitation bubbles near the surface may also act as micropumps to facilitate the removal of fibrin degradation products away from the clot, resulting in a decreased clot mass. The effect may be similar to a stirring mechanism, as suggested by Sakharov et al. (2000). Our observed correlation with stable cavitation suggests that bubble activity is important for enhanced thrombolysis.
It was noted that inertial cavitation activity often resulted in a quiescent period of low bubble activity thereafter. These data suggest that enhanced thrombolysis can be optimized by encouraging stable cavitation and avoiding inertial cavitation activity. The correlation of stable cavitation with enhanced thrombolysis, however, is not necessarily a causal one. Thus, definitive studies of the mechanisms of enhanced thrombolysis are still warranted.
CONCLUSION
Stable and inertial cavitation thresholds for combinations of porcine plasma, clot and rt-PA are reported here for 120-kHz ultrasound. The percent clot mass loss was also measured for exposure of whole blood clots to 120 kHz, 80% duty cycle ultrasound without any cavitation present, with stable cavitation alone, or with both stable and inertial cavitation. Ultrasound exposure without any cavitation does not enhance thrombolysis any more than exposure to rt-PA alone. In all cases, any marked enhancement in thrombolysis was strongly correlated with the presence of cavitation. However, the presence of both inertial and stable cavitation caused less of an enhancement than when stable cavitation alone was present. Stable cavitation, therefore, appears to play an important role in ultrasound-assisted thrombolysis.
Acknowledgments
This research was supported by the National Institutes of Health, grant number NIH/NINDS R01-NS047603. We gratefully acknowledge the assistance of Drs. Joseph Broderick and Daniel Kanter for editing this manuscript.
References
- Akiyama M, Ishibashi T, Yamada T, Furuhata H. Low frequency ultrasound penetrates the cranium and enhances thrombolysis in vitro. Neurosurgery. 1998;43:828–833. doi: 10.1097/00006123-199810000-00062. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351(21):2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Demchul AM, Burgin WS, et al. Ultrasound-enhanced thrombolysis for acute ischemic stroke: Phase I. Findings of the CLOTBUST trial. J Neuroimaging. 2004;14(2):113–117. [PubMed] [Google Scholar]
- ANSI Technical Report—Bubble detection and Cavitation Monitoring. ANSI. S1.24:TR-2002. [Google Scholar]
- Apfel RE. Acoustic cavitation prediction. J Acoust Soc Am. 1981;69:1624–1633. [Google Scholar]
- Bailey MR, Blackstock DT, Cleveland RO, Crum LA. Comparison of electrohydraulic lithotripters with rigid and pressure-release ellipsoidal reflectors. J Acoust Soc Am. 1999;106:1149–1160. doi: 10.1121/1.427123. [DOI] [PubMed] [Google Scholar]
- Behrens S, Daffertshofer M, Spiegel D, Hennerici M. Low-frequency, low-intensity ultrasound accelerates thrombolysis through the skull. Ultrasound Med Biol. 1999;25:269–273. doi: 10.1016/s0301-5629(98)00158-6. [DOI] [PubMed] [Google Scholar]
- Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood. 1993;81:2636–2643. [PubMed] [Google Scholar]
- Brayman AA, Azadniv M, Cox C, Miller MW. Hemolysis of Albunex-supplemented, 40% hematocrit human erythrocytes in vitro by 1-MHz pulsed ultrasound: Acoustic pressure and pulse length dependence. Ultrasound Med Biol. 1996;22:927–938. doi: 10.1016/0301-5629(96)00108-1. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Hacke W. Treatment of acute ischemic stroke. Part I: Recanalization strategies. Circulation. 2002;106(12):1563–1569. doi: 10.1161/01.cir.0000030406.47365.26. [DOI] [PubMed] [Google Scholar]
- Chen WS, Brayman TJ, Matula TJ, Crum LA. Inertial cavitation dose and hemolysis produced in vitro with or without Optison©. Ultrasound Med Biol. 2003;9:725–737. doi: 10.1016/s0301-5629(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Cheng JY, Holland CK, Shaw GJ. Microscopic imaging study of recombinant tissue plasminogen activator thrombolysis with 120-KHz ultrasound in an in vitro human clot model. J Ultrasound Med. 2004;23:S18. [Google Scholar]
- Cheng JY, Shaw GJ, Holland CK. In vitro microscopic imaging of enhanced thrombolysis with 120-kHz ultrasound in a human clot model. Acoustics Res Lett Online. 2005;6:25–29. doi: 10.1121/1.1815039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman AJ, Kodama T, Choi MJ, Adams T, Saundes JE. The cavitation threshold of human tissue exposed to 0.2 MHz pulsed ultrasound: preliminary measurements based on a study of clinical lithotripsy. Ultrasound Med Biol. 1995;21:405–417. doi: 10.1016/0301-5629(94)00116-u. [DOI] [PubMed] [Google Scholar]
- Coussios C-C, Holland CK, Shaw GJ. Transmission of a large unfocussed 120-kHz and 1-MHz ultrasound beam through the human skull. J Acoust Soc Am. 2002;112:2433. [Google Scholar]
- Coussios C-C, Holland CK, Jakubowska L, et al. In vitro characterization of liposomes and Optison by acoustic scattering at 3.5 MHz. Ultrasound Med Biol. 2004;30:181–190. doi: 10.1016/j.ultrasmedbio.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum LA. Cavitation microjets as a contributory mechanism for renal calculi disintegration in ESWL. J Urol. 1988;140:1587–1590. doi: 10.1016/s0022-5347(17)42132-x. [DOI] [PubMed] [Google Scholar]
- Datta S, McAdory LE, Tan J, Holland CK. Cavitation detection during ultrasound-assisted thrombolysis in porcine blood clots. J Acoust Soc Am. 2005;117:2558. [Google Scholar]
- Deng CX, Xu Q, Apfel RE, Holland CK. In vitro measurements of inertial cavitation thresholds in human blood. Ultrasound Med Biol. 1996;22(7):939–948. doi: 10.1016/0301-5629(96)00104-4. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Makin IRS, et al. Correlation of ultrasound-induced hemolysis with cavitation detector output in vitro. Ultrasound Med Biol. 1997;23:619–624. doi: 10.1016/s0301-5629(97)00039-2. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol. 2000;26:1153–1160. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- Flynn HG. Generation of transient cavities in liquids by microsecond pulses of ultrasound. J Acoust Soc Am. 1982;72:1926–1932. [Google Scholar]
- Fowlkes JB, Crum LA. Cavitation threshold measurements for microsecond length pulses of ultrasound. J Acoust Soc Am. 1988;83:2190–2201. doi: 10.1121/1.396347. [DOI] [PubMed] [Google Scholar]
- Francis CW, Onundarson PT, Carstensen EL, et al. Enhancement of fibrinolysis in vitro by ultrasound. J Clin Invest. 1992;90:2063–2068. doi: 10.1172/JCI116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- Greenleaf WJ, Bolander ME, Sarkar G, et al. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med Biol. 1998;24:587–595. doi: 10.1016/s0301-5629(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- Holland CK, Roy RA, Apfel RE, Crum LA. In vitro detection of cavitation induced by a diagnostic ultrasound system. IEEE UFFC. 1992;39:95–101. doi: 10.1109/58.166815. [DOI] [PubMed] [Google Scholar]
- Holland CK, Vaidya SS, Coussios C-C, Shaw GJ. Thrombolytic effects of 120-kHz and 1-MHz ultrasound and tissue plasminogen activator on porcine whole blood clots. J Acoust Soc Am. 2002;112:2370. [Google Scholar]
- Kashyap A, Blinc A, Marder VJ, et al. Acceleration of fibrinolysis by ultrasound in a rabbit ear model of small vessel injury. Thromb Res. 1994;76:475–485. doi: 10.1016/0049-3848(95)90179-j. [DOI] [PubMed] [Google Scholar]
- Kimura M, Iijima S, Kobayashi K, Furuhata H. Evaluation of the thrombolytic effect of tissue-type plasminogen activator with ultrasonic irradiation: In vitro experiment involving assays of the fibrin degradation products from the clot. Biol Pharm Bull. 1994;17:126–130. doi: 10.1248/bpb.17.126. [DOI] [PubMed] [Google Scholar]
- Koch S, Pohl P, Cobet U, Rainov NG. Ultrasound enhancement of liposome-mediated cell transfection is caused by cavitation effects. Ultrasound Med Biol. 2000;26:897–903. doi: 10.1016/s0301-5629(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Lauer CG, Burge R, Tang DB, et al. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257–1264. doi: 10.1161/01.cir.86.4.1257. [DOI] [PubMed] [Google Scholar]
- Leighton TJ. A strategy for the development and standardization of measurement methods for high power/cavitating ultrasonic fields: Review of cavitation monitoring techniques. ISVR Technical Report. 1997;No. 263 [Google Scholar]
- Li P, Cao L-Q, Dou C-Y, et al. Impact of myocardial contrast echo-cardiography on vascular permeability: an in vivo dose response study of delivery mode, pressure amplitude and contrast dose. Ultrasound Med Biol. 2003;29:1341–1349. doi: 10.1016/s0301-5629(03)00988-8. [DOI] [PubMed] [Google Scholar]
- Lou H, Nishioka T, Fishbein MC, et al. Transcutaneous ultrasound augments lysis of arterial thrombi in vivo. Circulation. 1996;94:775–778. doi: 10.1161/01.cir.94.4.775. [DOI] [PubMed] [Google Scholar]
- Mestas JL, Lenz P, Cathignol D. Long-lasting stable cavitation. J Acoust Soc Am. 2003;113(3):1426–1430. doi: 10.1121/1.1538198. [DOI] [PubMed] [Google Scholar]
- Miller DL. Particle gathering and microstreaming near ultrasonically activated gas-filled micropores. J Acoust Soc Am. 1988;84:1378–1387. doi: 10.1121/1.396636. [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke. rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Olsson SB, Johnsson B, Hilsson AM, et al. Enhancement of thrombolysis by ultrasound. Ultrasound Med Biol. 1994;20:375–382. doi: 10.1016/0301-5629(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Phelps AD, Leighton TG. The subharmonic oscillations and combination-frequency subharmonic emissions from a resonant bubble: Their properties and generation mechanisms. Acta Acoustica. 1997;83:59–66. [Google Scholar]
- Pieters M, Hekkenberg RT, Barrett-Bergshoeff M, et al. The effect of 40 kHz ultrasound on tissue plasminogen activator-induced clot lysis in three in vitro models. Ultrasound Med Biol. 2004;30:1545–1552. doi: 10.1016/j.ultrasmedbio.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Roy RA, Madanshetty S, Apfel RE. An acoustic backscattering technique for the detection of transient cavitation produced by microsecond pulses of ultrasound. J Acoust Soc Am. 1990;87:2451–2455. doi: 10.1121/1.399091. [DOI] [PubMed] [Google Scholar]
- Sakharov DV, Hekkenberg RT, Rijken DC, et al. Acceleration of fibrinolysis by high frequency ultrasound: The contribution of acoustic streaming and temperature rise. Thromb Res. 2000;100:333–40. doi: 10.1016/s0049-3848(00)00319-4. [DOI] [PubMed] [Google Scholar]
- Shaw GJ, Hahn NL, Wagner KR, et al. Ultrasound assisted thrombolysis in an in vitro clot model. Acad Emerg Med. 2001;8 [Google Scholar]
- Siegel RJ, Ariani M, Forester JS, et al. Cardiovascular applications of therapeutic ultrasound. J Invas Cardiol. 1989;4:219–229. [Google Scholar]
- Suchkova VN, Baggs RB, Francis CW. Effect of 40 kHz ultrasound on acute thrombotic ischemia in a rabbit femoral artery thrombosis model: Enhancement of thrombolysis and improvement in capillary muscle perfusion. Circulation. 2000;101:2296–2301. doi: 10.1161/01.cir.101.19.2296. [DOI] [PubMed] [Google Scholar]
- Suchkova V, Siddiqi FN, Carstensen EL, et al. Enhancement of fibrinolysis with 40 kHz ultrasound. Circulation. 1998;98:1030–1035. doi: 10.1161/01.cir.98.10.1030. [DOI] [PubMed] [Google Scholar]
- Suchkova VN, Carstensen EL, Francis CW. Ultrasound enhancement of fibrinolysis at frequencies of 27 to 100 kHz. Ultrasound Med Biol. 2002;28:377–382. doi: 10.1016/s0301-5629(01)00522-1. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Canfield DR. Ultrasound-induced lung hemorrhage in the monkey. Ultrasound Med Biol. 1994;20:65–72. doi: 10.1016/0301-5629(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Thomas CR, Farny CH, Coussios C-C, et al. Dynamics and control of cavitation during high-intensity focused ultrasound application. ARLO. 2005;6:182. [Google Scholar]


