Abstract
Immune function in the elderly is associated with a number of phenotypic and functional abnormalities, and this phenomenon of immune senescence is associated with increased susceptibility to infection. The immune response to pathogens frequently declines with age, but the CD8+ T-cell response to cytomegalovirus (CMV) is unusual, as it demonstrates a significant expansion over time. Here we have documented the CD4+ T-cell immune response to CMV in healthy donors of different ages. The magnitude of the CMV-specific CD4+ T-cell immune response increases from a mean of 2.2% of the CD4+ T-cell pool in donors below 50 years of age to 4.7% in donors aged over 65 years. In addition, CMV-specific CD4+ T cells in elderly donors demonstrate decreased production of interleukin-2 and less dependence on costimulation. CMV seropositivity is associated with marked changes in the phenotype of the overall CD4+ T-cell repertoire in healthy aged donors, including an increase in CD57+ expression and a decrease in CD28 and CD27 expression, a phenotypic profile characteristic of immune senescence. This memory inflation of CMV-specific CD4+ T cells contributes to evidence that CMV infection may be damaging to immune function in elderly individuals.
Healthy aging is associated with the development of a number of phenotypic and functional abnormalities of the immune system. These include the accumulation of memory T cells, impaired functional responses in vitro, and a reduction in the response rate to vaccinations (5, 8). These findings are associated with the phenomenon of immune senescence and are thought to underlie the increased rate of infectious disease that is seen in elderly individuals (7).
The magnitude of the cellular immune response to a number of pathogens has been studied in donors of different ages and has revealed that cellular immunity to viruses such as influenza virus and varicella-zoster virus decreases with advancing age (3). In marked contrast to these findings, the CD8 T-cell response to cytomegalovirus (CMV) increases markedly with age, such that it may represent over 40% of the CD8+ T-cell pool (14, 15). A similar observation has been seen with the CD8 T-cell immune response to murine cytomegalovirus (13). There has been speculation that this accumulation of memory CD8 T cells may itself contribute to features of immune senescence, and this idea has gained support from studies of elderly donors in whom CMV seropositivity is associated with the development of an immunological phenotype associated with impaired survival (16, 29).
CD4 T cells are important in the induction and regulation of the cellular immune response to pathogens, and an impaired CMV-specific CD4 T-cell immune response has been correlated with prolonged viral secretion following neonatal infection (10). Currently little is known with regard to how the magnitude or functional properties of the CD4 T-cell immune response to CMV are influenced by aging. Here we have studied the CMV-specific CD4 T-cell response to CMV viral lysate in a cohort of 30 young individuals aged less than 50 years and compared this to 40 elderly individuals aged over 60 years. We find that the CMV-specific immune response increases markedly with age and leads to the development of phenotypic and functional changes within the CD4 T-cell repertoire which have previously been associated with immune senescence.
MATERIALS AND METHODS
Donors.
Blood was taken from laboratory personnel or a cohort of healthy elderly volunteers known as “The Thousand Elders.” Ethical permission was obtained from the Local Ethical Committee for the study, and written consent was obtained in all cases.
Blood sampling.
Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll gradient centrifugation from heparinized blood, and aliquots of 106 cells were used for phenotyping studies. Sodium heparin anticoagulation and whole blood were used for cytokine flow cytometry.
Antigen stimulation.
The frequency of CMV-specific CD4 T cells was determined according to the previously described method (24). Briefly, whole blood was dispensed into 15-ml propylene tubes with or without the addition of costimulatory monoclonal antibodies anti-CD28 and anti-CD49d to a 1-μg/ml final concentration. CMV lysate (1/100 dilution) or staphylococcal enterotoxin B (SEB; 200 ng/ml) was added, and an unstimulated sample was used as a negative control. CMV lysate (strain AD169) was purchased from Microbix Biosystems, Inc., and is inactivated by sonication. A negative control of mock-infected cell lysate was used in each donor. SEB and Brefeldin A were purchased from Sigma, United Kingdom. Culture tubes were incubated at 37°C for 6 h together with Brefeldin A (10 μg/ml) for the last 4 h. EDTA was then added to a 2 mM final concentration for 15 min at room temperature. Red cells were then lysed, and leukocytes were fixed by incubation with fluorescence-activated cell sorter (FACS) lysing solution (Becton Dickinson, United Kingdom) for 10 min at room temperature. Cells were washed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin and 0.1% sodium azide and added to FACS permeabilization buffer for 10 min at room temperature. The cells then were washed in PBS with bovine serum albumin and sodium azide prior to immunofluorescent staining.
Labeling with monoclonal antibodies and flow cytometric analysis.
Cells were incubated with directly conjugated monoclonal antibodies for 30 min at room temperature in the dark. They were then washed and fixed in 1% paraformaldehyde in PBS, and flow cytometric analysis was performed by FACS. A minimum of 50,000 CD4+ cells was collected in each analysis. Cells were gated initially on side scatter versus forward scatter to collect lymphocytes and then on CD4+ cells versus side scatter. Data were analyzed using WinMDI software and are displayed as dot plots of cytokine expression versus CD69 fluorescence.
Monoclonal antibodies and antigens.
The following monoclonal antibodies were used in this study: anti-gamma interferon (anti-IFN-γ; fluorescein isothiocyanate [FITC] and phycoerythrin [PE]), anti-interleukin-2 (anti-IL-2; FITC and PE), anti-tumor necrosis factor alpha (anti-TNF-α; FITC and PE), mouse immunoglobulin G2a (IgG2a; FITC and PE), mouse IgG1 (FITC and PE), and anti-CD49d and anti-CD28 were obtained from Becton-Dickinson Immunocytometry Systems. Anti-CD4 (PE Texas Red [ECD] and PE-Cy5 [PC5]), anti-CD3 (PE), anti-CD69 (PC5), anti-CD57 (FITC and PE), anti-CD28 (FITC), anti-CD45RO (FITC and PE), anti-CD45RA (FITC), anti-CD27 (FITC), anti-CD38 (FITC), anti-HLA-DR (FITC), and mouse IgG1 (ECD and tricolor [TC]) were obtained from Coulter Immunology. Anti-CD28 (TC) and anti-CD8 (TC) were from Caltag. Anti-CCR7 (FITC) was obtained from R&D Systems.
Statistical analysis.
Intergroup comparisons were performed using the Mann-Whitney U test. All P values were two-tailed and were modified according to the Holm-Bonferroni correction. They were considered significant if the corrected P value remained <0.05.
RESULTS
The CMV-specific CD4 T-cell response increases with age.
Cytokine flow cytometry (CFC) was used to determine the CD4 T-cell response to CMV viral lysate in a cohort of 30 healthy donors aged up to 50 years (range, 22 to 50 years) and 40 healthy donors aged over 65 years (range, 65 to 84 years). CMV-specific cells were clearly identified by production of cytokine in response to antigen (Fig. 1A). A mock lysate was used as a negative control in all donors, and negligible cytokine secretion was observed in response to this agent (<0.03% of CD4+ pool). Furthermore, no significant cytokine response to CMV lysate was detected in eight CMV-seronegative donors tested (<0.05% of CD4 pool).
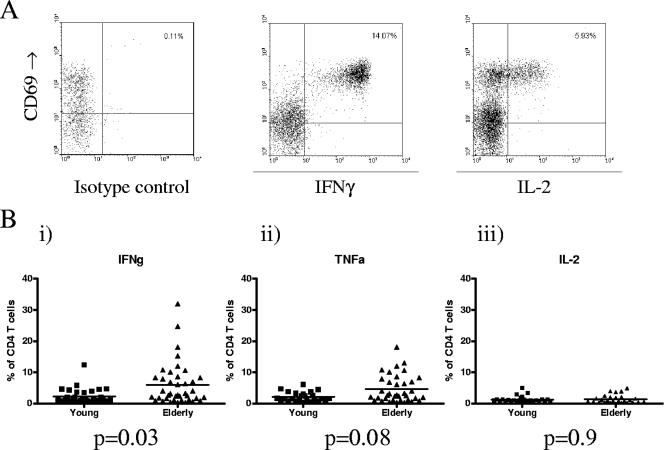
FIG. 1.
The frequency of CMV-specific CD4 T cells is increased in elderly donors. Whole blood from CMV-seropositive donors was incubated with CMV viral lysate at 37°C for 6 h or with mock lysate as a control. The blood was then lysed, fixed, permeabilized, and stained with monoclonal antibodies. The frequency of the cells responsive to CMV was determined by the fraction of CD4 T cells which upregulated CD69 and expressed detectable amounts of intracellular TNF-α, IFN-γ, and IL-2. A. Representative flow cytometric profiles from a single elderly donor for the isotype control (i) and IFN-γ (ii) and IL-2 (iii) cytokine responses to CMV lysate. B. The frequency of CMV-specific CD4 T cells in individual donors is expressed as a percentage of the total CD4 T-cell pool and shown as a single data point. The mean value within the group is indicated as a horizontal line. (i) The mean percentage of CMV-specific CD4 T cell producing IFN-γ was 2% in young donors (range, 0.4 to 5.78%) and 4.3% in elderly donors (0.3 to 32%) (P = 0.03). (ii) The mean percentage of CMV-specific CD4 T cells producing TNF-α was 2.2% in young donors (range, 0.51 to 4.7%) and 4.7% in elderly donors (0.3 to 16.7%) (P = 0.08). (iii) The mean percentage of CMV-specific CD4 T cells producing IL-2 was 1.1% in young donors (range, 0.1 to 4.8%) and 1.3% in elderly donors (0.26 to 4.9%) (P = 0.9).
High levels of CMV-specific CD4 T cells were detected by IFN-γ or TNF-α secretion in the elderly cohort. In eight donors the CMV-specific CD4 T-cell response comprised over 10% of the CD4 T-cell pool, with a highest value of 32% in one donor. The IFN-γ cytokine response averaged 2.32% (0.2 to 12.4%) in young donors and 5.92% (0.33 to 32.0%) in the elderly group, which represents a significant increase with age (P = 0.03). Comparable values were observed for the TNF-α cytokine response, with averages of 2.2% (0.51 to 4.7%) and 4.7% (0.3 to 17%) in the young and elderly groups, respectively (P = 0.08) (Fig. 1B).
CMV-specific CD4 T cells in elderly donors show impaired secretion of IL-2.
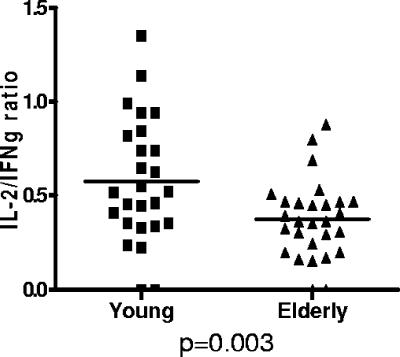
In addition to production of antiviral and proinflammatory cytokines as described above, IL-2 secretion by CD4 T cells is important for promoting T-cell proliferation and can be used as a surrogate marker for T helper function. We thus measured the frequency of IL-2-producing CD4 T cells in response to CMV lysate in our panel of young and elderly donors. In contrast to the findings with the IFN-γ and TNF-α CFC assay, the IL-2 cytokine response did not increase with age, with mean responses of only 1.1% (0.1 to 4.8%) and 1.3% (0.26 to 4.9%) in the younger and older cohorts, respectively (P = 0.9) (Fig. 1B). This observation was reflected in a significant reduction in the ratio for IL-2 versus IFN-γ cytokine production within CMV-specific CD4 T cells in association with aging. The median value was 0.63 in the younger donors but decreased to 0.37 in the elderly cohort (P = 0.003) (Fig. 2).
FIG. 2.
The ratio of IL-2/IFN-γ production by CMV-specific CD4 T cells is decreased in elderly donors. Whole blood stimulation and cytokine flow cytometry were used to determine IFN-γ and IL-2 production in response to CMV viral lysate. PBMC were stimulated with CMV lysate, and the percentage of cells which produced IL-2 or IFN-γ was determined and expressed as a ratio (IL-2/IFN-γ). Data from each donor are expressed as a single data point, with the median indicated by a horizontal line. The median value was 0.63 in the young donors and 0.37 in elderly donors (P = 0.003).
Cytokine production from CMV-specific CD4 T cells in elderly donors is less dependent on costimulation.
Costimulatory antibodies, such as anti-CD28 and anti-CD49d, are routinely used to maximize detection of the antigen-specific CD4 T-cell response in cytokine flow cytometry assays. CD28 is an important costimulatory molecule on T cells, although expression is frequently lost on highly differentiated populations, including CMV-specific T cells. CD49d is one component of the α4β1 integrin. Previous work has shown that the use of these agents increases detection of the CMV-specific CD4 T-cell response by up to threefold and that IL-2 production is more dependent on costimulation than the IFN-γ response (28).
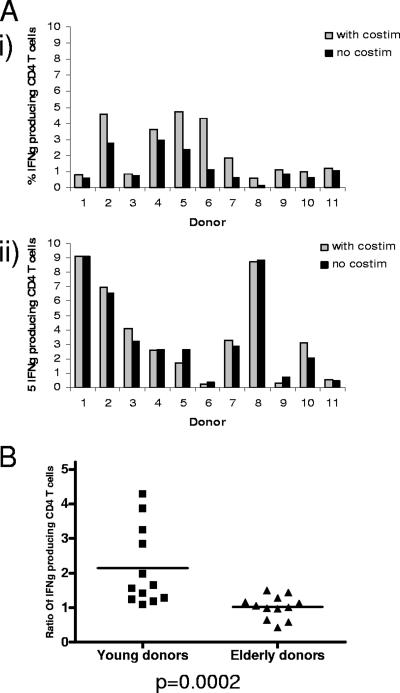
The CMV-specific CD4 T-cell response, measured by IFN-γ production in response to CMV lysate stimulation, was determined in both age cohorts in the presence or absence of costimulatory antibodies. In a panel of 11 young donors, the addition of costimulatory antibodies to the CFC assay resulted in an increase in the detection of CMV-specific CD4 T cells in all donors (Fig. 3A). In contrast, in 11 donors aged over 65 years, there was no increment in the CFC response with the addition of costimulation (Fig. 3A). The increment in the number of CMV-specific T cells detected with the addition of costimulatory antibodies averaged 2.2-fold in young donors, with no increase in elderly donors (P = 0.0002) (Fig. 3B). This independence of CMV-specific T cells in elderly donors from the requirement for costimulation indicates that the activation threshold of CMV-specific CD4 T cells in elderly donors is reduced in comparison to younger donors. To verify that the addition of costimulatory antibodies did not stimulate nonspecific cytokine production, we also tested mock lysate responses and CMV lysate responses (in CMV-seronegative donors) with costimulation. In both sets of control experiments we did not observe an increment in the response from the background levels observed without costimulation (data not shown).
FIG. 3.
Influence of addition of costimulatory antibodies on detection of CMV-specific CD4 T cells in young or elderly donors. PBMC were stimulated with CMV lysate in the presence or absence of costimulatory antibodies to CD28 or CD49d. A. Magnitude of the CMV-specific CD4 T-cell response determined by CFC in the presence or absence of costimulatory antibodies in 11 young (i) and 11 elderly (ii) donors. B. Mean ratio of CMV-specific CD4 T cells detected in the presence or absence of costimulatory antibodies in young or elderly donors. The mean ratio was 2.2 (range, 1.07 to 3.90) in young donors. In elderly donors the mean ratio was 1, with a range of 0.40 to 1.48 (P = 0.0002).
CMV-specific CD4 T cells are highly differentiated effector memory cells.
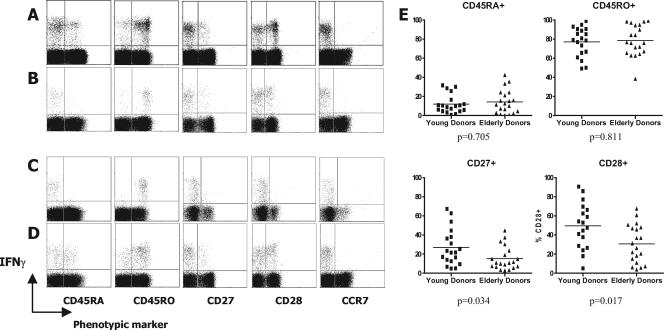
It was also necessary to characterize the cell membrane phenotype of CD4 T cells specific for CMV in young and elderly donors. As before, cells were stimulated with CMV lysate and then stained for surface expression of CD45RA, CD45RO, CD27, CD28, CCR7, and CD62 ligand (CD62L). After washing, cytoplasmic staining for IFN-γ was performed. Figure 4 describes the observed phenotypes in young and elderly donors. The expression of CD45 isoforms was generally biased towards CD45RO (approximately 80%) in both young and elderly donors (Fig. 4A to D), indicating an effector memory phenotype. The presence of a small but significant CD45RAhigh CD4 T-cell population was demonstrable in many donors, suggesting a revertant memory phenotype, although this did not appear to accumulate particularly with age (Fig. 4E). CD27 expression was lower than CD28 expression on virus-specific CD4 T cells (Fig. 4A to D), with a trend towards further reduction in elderly donors (Fig. 4E). In virtually all donors tested, the CMV-specific CD4 response was uniformly very low for CCR7 and CD62L expression (data not shown), although it is possible that CD62L may have been downregulated following stimulation.
FIG. 4.
Phenotypic analyses of CMV-specific CD4 T-cell responses in young and elderly asymptomatic virus carriers. PBMC from donors were stimulated with CMV lysate and then surface stained with monoclonal antibodies specific for each of the phenotypic markers shown, followed by cytoplasmic IFN-γ staining. Representative data are shown for two young (A and B) and two elderly (C and D) donors. (E) Summarized data showing CD45RA, CD45RO, CD27, and CD28 expression in all young and elderly donors tested are also shown. The y axis represents the percent of CMV-specific CD4 T cells expressing each marker.
CMV seropositivity is associated with a significant alteration in the phenotype of the CD4 T-cell repertoire.
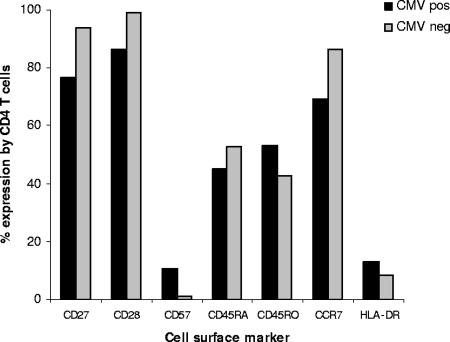
As the CD4 T-cell response to CMV accumulates with age, it was felt important to assess the contribution of CMV carriage to the phenotype of the total CD4 T-cell repertoire. The expression of eight phenotypic markers was therefore determined on CD4 T cells from 10 CMV-seropositive donors aged over 65 years and a further 10 age-matched CMV-seronegative donors (Fig. 5). The CD45RO isoform is a marker of antigen experience, and the percentage of CD45RO+ CD4 T cells was significantly increased in CMV-seropositive donors in comparison to the CMV-seronegative cohort (53 versus 43%; P = 0.012). A reduction was seen in the CD45RA+ CD4 T-cell population in CMV-seropositive donors, although this did not reach statistical significance (45 versus 53%; P = 0.07). CD27 and CD28 are costimulatory molecules but support T-cell expansion through different mechanisms. CD27 does not affect cell cycle activity but supports survival of activated T cells (11). CD27 expression was markedly reduced on CD4 T-cell populations in CMV-seropositive donors (77 versus 94%; P = 0.00016). In addition, in CMV-seropositive donors 14% of CD4 T cells had lost expression of the major costimulatory molecule CD28, whereas this population represented less than 1% of the CD4 T-cell pool in CMV-seronegative donors.
FIG. 5.
Expression of differentiation and activation markers on peripheral blood CD4+ T cells of healthy CMV-seropositive and -seronegative donors. PBMC from CMV-seronegative (n = 10) and CMV-seropositive (n = 10) donors aged >65 years were stained with FITC, PE, and PC5-conjugated MAb. CD4+ CD3+ lymphocytes were gated and analyzed for the expression of a range of phenotypic markers on the third color. The mean percentages of CD4 T cells expressing individual phenotypic markers in CMV-seronegative or CMV-seropositive donors were as follows: CD45RA (53 and 45%, respectively; P = 0.0.07); CD45R0 (43 and 53%; P = 0.012) CD27 (94 and 77%; P = 0.00016); CD28 (99 and 86%; P = 0.00014); CD57 (0.92 and 11%; P = 0.0006); CD38 (44 and 38%; P = 0.3); HLA-DR (8.3 and 13%; P = 0.1); CCR7 (86 and 69%; P = 0.0015).
CD57, CD38, and HLA-DR are expressed following T-cell activation, and CMV seropositivity was associated with a dramatic increase in the expression of CD57+ CD4 T cells from 0.9% in the CMV-seronegative donors to 10.6% in the CMV-seropositive cohort (P = 0.0006). The function of CD57+ CD4 T cells remains under investigation, although such populations do not support immunoglobulin production (2) but can exhibit cytotoxic activity (26). The HLA-DR+ CD4 T-cell subset was increased from 8.3% to 13% in CMV-seropositive donors, although this did not reach statistical significance.
CCR7 expression is present on naïve and central memory T cells but is absent on effector memory populations (23). The percentage of CCR7+ CD4 T cells was significantly decreased in CMV-seropositive donors (69 versus 86%; P = 0.0015) and likely reflects an accumulation of effector memory cells.
CD4 T cells from CMV-seropositive elderly donors show comparable IL-2 responses to superantigen stimulation.
The alterations in the phenotype of the CD4 T-cell repertoire associated with CMV seropositivity might be associated with impairment of the CD4 T-cell response to heterologous antigen in these donors. SEB superantigen was used as a source of antigen to stimulate CD4 T cells from CMV-seropositive or CMV-seronegative donors in vitro, and the response was measured by cytokine flow cytometry (Table 1). Our primary hypothesis was that we might expect a difference in the IFN-γ, TNF-α, and IL-2 responses to such an antigen. The mean value of the IFN-γ cytokine response to SEB was 5.6% in CMV-seropositive donors compared to 3.8% in CMV-seronegative donors, although this did not reach significance after Holm-Bonferroni correction for multiple testing (P = 0.03). Subgroup analysis of dual-cytokine-producing cells revealed that the number of cells with a combined IFN-γ+ IL-2− cytokine phenotype was increased in CMV-seropositive donors (mean, 3.6 versus 1.6%), but this result did not reach statistical significance. Polarized IFN-γ secretion is characteristic of effector/memory Th1 CD4 T cells, and this result is likely to reflect a trend towards the accumulation of this subset in CMV-seropositive donors. The number of CD4 T cells that produced IL-2 in response to SEB was reduced by 33% in CMV-seropositive compared to CMV-seronegative donors (mean, 4.1 versus 6%, respectively). Although this did not reach statistical significance, this suggests a reduction in the number of central memory and naïve T cells in CMV-seropositive donors and is supported by a nonsignificant reduction in the percentage of T cells with an IFN-γ− IL-2+ cytokine phenotype in response to SEB in these donors (mean, 2.5 versus 3.9%).
TABLE 1.
Comparative analysis of cytokine production in CD4+ T cells following SEB stimulation in elderly donors grouped according to CMV serostatus
| Cytokine profile | Mean (CI) cytokine production ina:
|
P value | |
|---|---|---|---|
| CMV seropositive (n = 15) | CMV seronegative (n = 15) | ||
| IFN-γ+ | 5.6 (1.1-11) | 3.8 (1.4-8.3) | NS |
| TNF-α+ | 6.9 (0.75-15) | 8.0 (1.1-18) | NS |
| IL-2+ | 4.1 (1.23-7.9) | 6 (1.8-10) | NS |
| IFN-γ+ IL-2+ | 1.6 (0.92-2.8) | 2.2 (0.5-7.2) | NS |
| IFN-γ+ IL-2− | 3.6 (1.1-8.1) | 1.6 (0.68-4.1) | NS |
| IFN-γ− IL-2+ | 2.5 (0.74-3.6) | 3.9 (1.1-10.3) | NS |
All data were gated on CD3+ CD4+ events. CI, 95% confidence interval.
DISCUSSION
Infectious disease remains a major cause of morbidity and mortality in all populations, and the elderly are at particular risk of clinical disease (8). This has been attributed to a number of findings, of which a functional impairment of the immune system termed immune senescence is believed to be a major cause. Immune senescence is characterized by features such as reduction in the number of naive T cells and an accumulation of memory cells (1). The combination of features such as an inverted CD4/CD8 ratio and reduced proliferative response to mitogen has been term the immune risk phenotype and has been directly associated with impaired survival in cohort studies of healthy elderly donors (20, 29). Primary CMV infection is usually silent, but the virus is not cleared from the host and enters a state of viral latency. The majority of individuals over the age of 65 years are CMV seropositive, although CMV seropositivity is generally not considered to be of clinical significance in the immunocompetent host.
However, the potential association between CMV infection and immune senescence is coming under increasing scrutiny. CMV seropositivity is associated with the development of the immune risk phenotype (21), and an impaired immune response to influenza vaccination is observed in CMV-seropositive donors (25). In contrast to the immune response to most infections, the CD8 T-cell immune response to CMV increases markedly with age, such that over 40% of the CD8 T-cell pool may comprise CMV-specific T cells in some donors (15). It is possible that the development of such large memory T-cell expansions may be associated with an impairment of the immune response to heterologous agents. Age-related CD8 T-cell clonal expansions in mice are associated with an impaired immune response to viral infection, due to constriction of the CD8 T-cell repertoire (17). However, the potential importance of the CMV-specific CD4 T-cell immune response in relation to immune function during aging has received less attention.
Here we show that in CMV-seropositive individuals there is an accumulation of CMV-specific CD4 T cells in association with aging. This supports recent data showing that the percentage of CMV-specific CD4 T cells increases with age, whereas other virus-specific CD4 T-cell responses do not (9). The cytokine flow cytometry assay revealed that the CMV-specific immune response to viral lysate constitutes nearly 6% of the CD4 T-cell repertoire in the elderly, a frequency that is considerably greater than for other pathogen-specific immune responses. Nevertheless, indirect evidence suggests that this frequency is likely to be a significant underestimate of the total CMV-specific CD4+ T-cell response. Although lysate of CMV-infected cells is widely used as an antigenic source for the identification of CMV-specific CD4 T cells, the full complement of viral proteins may not be evenly presented by antigen-presenting cells due to competition for HLA class II binding. There may also be CMV-specific CD4 T cells that go undetected due to their inability to produce cytokines during in vitro analysis, and this has been shown repeatedly in the detection of CMV-specific CD8 T cells using major histocompatibility complex class I peptide tetramers (15).
CMV-specific cytotoxic T lymphocytes in elderly donors demonstrated lower levels of IL-2 production, which is characteristic of CD4 T cells with a low activation threshold (12, 18, 28). Production of IL-2 may be necessary in states of high antigen load or inflammation in situations requiring expansion of effector T cells. CMV-specific CD4 T cells are characterized by a CD28− CD57+ effector memory phenotype, and an IFN-γ+ IL-2− cytokine profile is typical of this subset (23). Murine data have shown that a memory CD4 T-cell subset with predominant expression of IL-2 is preferentially located within lymphoid tissue, and examination of the frequency and cytokine production of CMV-specific CD4 T cells within secondary lymphoid tissue will be of interest (22).
The functional properties of the CMV-specific CD4 T-cell response show significant differences between young and elderly donors. The CMV-specific CD4+ T-cell response exhibits heterogeneity in the activation threshold based on the requirement for costimulation in the form of antibodies to CD28 or CD49d or activation of antigen-presenting cells by agents such as TNF-α (28). Such differences in threshold can be found within a single clonotype and appear to be independent of T-cell receptor stimulation and consistent with an intrinsic activation threshold that is downstream of the T-cell receptor (6). The young donors in our study exhibited this phenomenon, and the addition of costimulatory antibodies increased the magnitude of the detected CMV-specific immune response by a factor of 1.1- to 3.7-fold. In contrast, costimulation did not increase detection of the CMV-specific CD4 T-cell response in elderly donors, indicating that cells have a lower threshold level for activation.
Many CMV-specific CD4 T cells lose expression of CD28, and anti-CD28 antibody is therefore unlikely to act as a costimulatory stimulus to this population. However, CD28− CMV-specific CD4 T cells are also seen in considerable numbers in young donors (27). There was no correlation between the magnitude of the CMV-specific response and response to costimulation, suggesting that maintenance, rather than expansion, of CD4+ T-cell clones is associated with an alteration in signaling threshold.
The factors that determine the threshold of an antigen-specific response are uncertain, but repeated episodes of antigenic stimulation may be one factor. CMV latency is associated with episodes of subclinical viremia (19), and it is likely that CMV carriage for many decades exposes the donor to intermittent viral reactivation with consequent repeated activation of T-cell immunity. The physiological significance of this change in activation threshold is uncertain, but T cells with lower thresholds for activation are more likely to be able to control viral reactivation in the setting of minimal inflammation and low antigen load. They may thus be well adapted to control low levels of CMV reactivation with minimal bystander damage and exhibit a form of pathogen control based on a high cytotoxic T-lymphocyte-low-antigen-load model. It has also been shown by others that CMV-specific CD4 T cells are driven to replicative exhaustion in elderly CMV-seropositive hosts (9), although incidents of virus-associated disease in such patients do not occur. Whether this becomes problematic at a later stage for such individuals remains unknown.
The phenotype of CMV-specific CD4+ T cells indicated that cells had an effector memory profile with almost complete loss of CCR7 and CD62L. Most cells expressed the memory marker CD45RO, but a significant minority had a revertant phenotype with high-level expression of CD45RA although, in contrast to CD8+, this population did not increase with age. The proportion of cells which had lost expression of CD27 and CD28 showed an increase with age, in agreement with a previous report (9). The accumulation of effector memory CMV-specific CD4+ T cells was reflected in a marked alteration in the global CD4+ T-cell repertoire in elderly donors. CD27− and CD28− CD4 T-cell populations were increased from 6% and 1% of the CD4 T-cell pool, respectively, in CMV-seronegative donors to over 23% and 13% in the CMV-seropositive cohort (4). It is likely that most of the CD4+ CD28− CD4 T-cell population has specificity for CMV, as documented in a recent report (27). CD57 expression is associated with a highly differentiated cytotoxic phenotype (18) and increases markedly from less than 1% of the CD4 T-cell repertoire to over 10% in CMV-seropositive donors. These data suggest that CMV-specific CD4 T cells may represent up to 10% of the T-cell pool in healthy elderly donors, although it is also possible that CD27 and CD28 loss is induced on T cells which are not specific for CMV peptides due to cytokine-mediated effects (9). An accumulation of CD4 T cells expressing the activation marker HLA-DR or CD45RO, the CD45 isoform expressed on antigen-experienced T cells, is also observed in association with CMV seropositivity. Such an expansion of memory T cells may impair the number of naïve T cells within the CD4 T-cell repertoire, and this may be reflected in the 17% decrease in the percentage of CD4+ CCR7+ T cells in CMV-seropositive donors.
CMV-specific CD8 T cells are markedly expanded in elderly donors, and it may be possible that the CMV-specific CD4 T cells are providing help for CD8 T-cell expansion.
No correlation was observed between the magnitude of CMV-specific CD8 and CD4 immune responses in individual donors in our study (data not shown). However, an association cannot be formally ruled out, because the CD8 data were derived using a panel of tetramers that covers common but, importantly, not all HLA class I types (15).
CMV seropositivity is associated with the accumulation of large numbers of virus-specific CD4 T cells and a profound alteration of the global T-cell repertoire. These features may impair the ability of the immune response to respond to heterologous antigen, and this was assessed by analysis of the response of CD4 T cells to stimulation in vitro with superantigen. Among CMV-seropositive donors, a nonsignificant reduction was observed in the percentage of T cells that produced IL-2 in response to superantigen, and while further studies are required this may reflect a reduction in the naïve and central memory CD4 T cells in CMV-seropositive donors.
CMV infection in immunocompetent donors has generally been regarded as being of negligible consequence for the health of the host, but accumulating data indicate that this may be a naïve assumption. Efforts to control the spread of CMV infection or to limit levels of CMV viremia within an infected host may serve to protect the immune system from the accumulation of potentially crippling levels of cellular immune responses.
Acknowledgments
This work was supported by the Medical Research Council, United Kingdom. B.P. was supported by a scholarship from the Government of Iran.
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Almanzar G., S. Schwaiger, B. Jenewein, M. Keller, D. Herndler-Brandstetter, R. Wurzner, D. Schonitzer, and B. Grubeck-Loebenstein. 2005. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79:3675-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, E., M. Ohlin, C. A. Borrebaeck, and R. Carlsson. 1995. CD4+ CD57+ T cells derived from peripheral blood do not support immunoglobulin production by B cells. Cell. Immunol. 163:245-253. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma, H., M. Sharp, H. T. Maecker, V. C. Maino, and A. M. Arvin. 2000. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis. 181:859-866. [DOI] [PubMed] [Google Scholar]
- 4.Baars, P. A., M. M. Maurice, M. Rep, B. Hooibrink, and R. A. van Lier. 1995. Heterogeneity of the circulating human CD4+ T cell population. Further evidence that the CD4+ CD45RA− CD27− T cell subset contains specialized primed T cells. J. Immunol. 154:17-25. [PubMed] [Google Scholar]
- 5.Bernstein, E., D. Kaye, E. Abrutyn, P. Gross, M. Dorfman, and D. M. Murasko. 1999. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 17:82-94. [DOI] [PubMed] [Google Scholar]
- 6.Bitmansour, A. D., D. C. Douek, V. C. Maino, and L. J. Picker. 2002. Direct ex vivo analysis of human CD4+ memory T cell activation requirements at the single clonotype level. J. Immunol. 169:1207-1218. [DOI] [PubMed] [Google Scholar]
- 7.Castle, S. C. 2000. Impact of age-related immune dysfunction on risk of infections. Z. Gerontol. Geriatr. 33:341-349. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarti, B., and G. N. Abraham. 1999. Aging and T-cell-mediated immunity. Mech. Ageing Dev. 108:183-206. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher, J., M. Vukmanovic-Stejic, P. Dunne, K. Birch, J. Cook, S. Jackson, M. Salmon, M. Tustin, and A. Akbar. 2005. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 175:8218-8225. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, N., H. Kimura, T. Morishima, N. Tanaka, T. Tsurumi, and K. Kuzushima. 2003. Flow cytometric analysis of cytomegalovirus-specific cell-mediated immunity in the congenital infection. J. Med. Virol. 71:251-258. [DOI] [PubMed] [Google Scholar]
- 11.Hendriks, J., Y. Xiao, and J. Borst. 2003. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 198:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh, Y., and R. N. Germain. 1997. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+ T cells. J. Exp. Med. 186:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U. H. Koszinowski, R. E. Phillips, and P. Klenerman. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022-2029. [DOI] [PubMed] [Google Scholar]
- 14.Khan, N., N. Shariff, M. Cobbold, R. Bruton, J. A. Ainsworth, A. J. Sinclair, L. Nayak, and P. A. Moss. 2002. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 169:1984-1992. [DOI] [PubMed] [Google Scholar]
- 15.Khan, N., A. Hislop, N. Gudgeon, M. Cobbold, R. Khanna, L. Nayak, A. B. Rickinson, and P. Moss. 2004. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 173:7481-7489. [DOI] [PubMed] [Google Scholar]
- 16.Looney, R. J., A. Falsey, D. Campbell, A. Torres, J. Kolassa, C. Brower, R. McCann, M. Menegus, K. McCormick, M. Frampton, W. Hall, and G. Abraham. 1999. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin. Immunol. 90:213-219. [DOI] [PubMed] [Google Scholar]
- 17.Messaoudi, I., J. Lemaoult, J. A. Guevara-Patino, B. M. Metzner, and J. Nikolich-Zugich. 2004. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J. Exp. Med. 200:1347-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murtaza, A., V. K. Kuchroo, and G. J. Freeman. 1999. Changes in the strength of co-stimulation through the B7/CD28 pathway alter functional T cell responses to altered peptide ligands. Int. Immunol. 11:407-416. [DOI] [PubMed] [Google Scholar]
- 19.Musiani, M., M. Zerbini, D. Zauli, G. Cometti, and M. La Placa. 1988. Impairment of cytomegalovirus and host balance in elderly subjects. J. Clin. Pathol. 41:722-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsson, J., A. Wikby, B. Johansson, S. Lofgren, B.-O. Nilsson, and F. Ferguson. 2000. Age related change in peripheral blood T lymphocyte subpopulations and cytomegalovirus in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121:187-201. [DOI] [PubMed] [Google Scholar]
- 21.Pawelec, G., A. Akbar, C. Caruso, R. Effros, B. Grubeck-Loebenstein, and A. Wikby. 2004. Is immunosenescence infectious? Trends Immunol. 25:406-410. [DOI] [PubMed] [Google Scholar]
- 22.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101-105. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 24.Suni, M. A., L. J. Picker, and V. C. Maino. 1998. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J. Immunol. Methods 212:89-98. [DOI] [PubMed] [Google Scholar]
- 25.Trzonkowski, P., J. Mysliwska, E. Szmit, J. Wieckiewicz, K. Lukaszuk, L. B. Brydak, M. Machala, and A. Mysliwski. 2003. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine 21:3826-3836. [DOI] [PubMed] [Google Scholar]
- 26.Van den Hove, L. E., S. W. Van Gool, P. Vandenberghe, M. A. Boogaerts, and J. L. Ceuppens. 1998. CD57+/CD28− T cells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendency to apoptosis. Leukemia 12:1573-1582. [DOI] [PubMed] [Google Scholar]
- 27.van Leeuwen, E. M., E. B. Remmerswaal, M. T. Vossen, A. T. Rowshani, P. M. Wertheim-van Dillen, R. A. van Lier, and I. J. ten Berge. 2004. Emergence of a CD4+ CD28− granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J. Immunol. 173:1834-1841. [DOI] [PubMed] [Google Scholar]
- 28.Waldrop, S. L., K. A. Davis, V. C. Maino, and L. J. Picker. 1998. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 161:5284-5295. [PubMed] [Google Scholar]
- 29.Wikby, A., B. Johansson, J. Olsson, S. Lofgren, B. O. Nilsson, and F. Ferguson. 2002. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol. 37:445-453. [DOI] [PubMed] [Google Scholar]