Abstract
In order to determine the mechanism of tumour destruction by tumour-infiltrating lymphocytes (TIL), we examined the ability of both CD4+ and CD8+ effector TIL, and TIL clones, to manifest granzyme-mediated and Fas-mediated destruction of tumour targets. In many in vitro studies TIL have been shown to manifest anti-tumour reactivity, yet many tumours escape immunological destruction. To investigate the role of Fas expression and the concomitant sensitivity to the inducibility of apoptotic death, we derived TIL from four melanomas and one glioma. The glioma, and all but one of the melanomas, expressed Fas, but Fas-mediated apoptosis could only be detected if the targets were treated with cyclohexamide. The melanomas and the glioma all expressed detectable cytoplasmic Bcl-2 protein, known to exert anti-apoptotic activity. Lysis of tumours by CD8-enriched cultures and CD8+ clones was Ca2+-dependent and could not be modified by an anti-Fas MoAb. In CD4-enriched cultures or CD4+ clones with cytotoxic potential against tumour cells, cytotoxicity was also Ca2+-dependent. As Ca2+-dependent cytotoxicity is usually the result of secretion of perforin/granzyme-B, we investigated the presence of perforin in cytotoxic CD4+ clones and demonstrated the presence of granular deposits of this enzyme in some of the CD4+ clones. Although an anti-Fas MoAb did not block the lysis of melanoma targets by CD4+ clones, the examination of Fas-dependent targets demonstrated that these clones also had the potential to kill by the Fas/Fas ligand system. These data suggest that the predominant mechanism in tumour killing by TIL appears to be perforin–granzyme-dependent, and that the solid tumour cell lines we studied are less susceptible to Fas-mediated apoptosis. As non-apoptotic pathways may enhance tumour immunogenicity, exploitation of the perforin–granzyme-dependent cytotoxic T lymphocyte (CTL) pathways may be important for achieving successful anti-tumour responses.
Keywords: tumour-infiltrating lymphocytes, cytotoxicity, perforin, Fas/CD95, FasL, Bcl-2, apoptosis
INTRODUCTION
Despite numerous investigations, the role of tumour-infiltrating lymphocytes (TIL) in controlling tumour growth remains unclear. The ability of some TIL cultures to exert strong cytotoxic activity against autologous tumour cells suggests that such cells should be able to suppress tumour growth, but the ability of some tumours to progress, even when infiltrated with tumour-specific TIL, suggests that the mechanism of killing may be impaired in vivo. The question of the mechanism of killing manifested in vivo may have particular importance. Melcher et al. [1] have recently demonstrated that apoptotic killing may result in reduced tumour immunogenicity compared with lytic mechanisms which induce heat shock protein and produce inflammation. Thus, the question of how tumours are lysed, and whether there is impairment of particular lytic pathways, may heavily influence the outcome of the immune response to tumours and our ability to modulate this response. In this study we evaluate the contribution of perforin and Fas-mediated lysis to the recognition of autologous human tumours by the T lymphocytes which infiltrate them.
Although the literature is replete with articles using murine models, including knock-out and transgenic animals, which describe the contributions of perforin and Fas-mediated lysis in the rejection of allografts [2], in the pathogenesis of viral [3,4] and autoimmune diseases [5], and in a few instances against leukaemic malignant cells [6,7], the question of which lytic pathways are taken by TIL in the destruction of solid tumours has rarely been addressed [8]. Thus, although there is much data to suggest that tumours may escape from immune recognition by a variety of pathways, there is no evaluation of the contribution of the Fas versus the perforin and granzyme pathways in the lysis of solid tumours by human TIL. In the light of tumour progression taking place even in the presence of a lymphoid infiltrate with tumour-specific cells, a better understanding of these mechanisms could help explain how tumours escape from immune recognition.
Since the Fas–FasL mechanism appears to be more sensitive to activation-induced regulation, we reasoned that down-regulation of this mechanism could be involved in the down-regulation of cytotoxicity. We therefore investigated Fas expression, and the susceptibility to Fas-mediated apoptosis in several tumour cell lines, including the tumour cells’ expression of the anti-apoptotic protein Bcl-2 [8–11]. In addition, we studied the mechanisms of killing involved in both CD8+ and CD4+ effector TIL mediating anti-tumour cytotoxicity.
Several studies have correlated the presence of TIL with a relatively better prognosis in melanomas and gliomas [12–15]. In malignant melanoma, it has been shown that TIL are able to recognize tumour-associated antigens, and in some cases to lyse the malignant cells [16–30]. These findings provided a basis for the use of in vitro cultured TIL in adoptive immunotherapy in an effort to enhance the in vivo accumulation of tumour-lytic lymphocytes [29,31]. However, there is considerable doubt concerning the efficacy of TIL in vivo, even in cases in which it is possible to demonstrate the accumulation in vivo of clones capable of exerting strong anti-tumour cytotoxicity in vitro. For example, our observation of a predominant tumour-specific cytotoxic T lymphocyte (CTL) cell clone in multiple metastatic lesions from the same patient indicated that such cells were able to propagate, circulate, home and accumulate within tumour deposits. However, these tumour-specific CTL clones were found in a patient with progressive melanoma, indicating that they were not sufficient to contain tumour growth [32]. Thus, the discrepancy between a demonstrated in vitro tumour lytic activity, and the in vivo tumour progression in the presence of this lymphoid infiltrate, remains to be clarified.
Several mechanisms of lymphocyte-mediated cytotoxicity have been described, including specific cytotoxicity mediated by CTL, as well as natural killer (NK) and lymphokine-associated killer (LAK) activities, antibody-dependent cell-mediated cytotoxicity, and cytokine-mediated tumour destruction [8,33–35]. Specific cytotoxicity requires T cell receptor (TCR) recognition of tumour-associated antigens presented in the context of MHC molecules as well as an array of accessory molecules which mediate both target binding and the delivery of regulatory ‘second signals’. Current models indicated that CTL-mediated lysis usually occurs through the release of cytolytic proteins (perforin, granzymes) accumulated in the granules abundantly present in the cytoplasm of CD8+ cells. Cell lysis then occurs in a Ca2+-dependent fashion [36].
More recently, however, it has been shown that CTL can also kill targets via a Ca2+-independent mechanism. This event is mediated by the engagement of a surface molecule (Fas or CD95) capable of delivering a lethal signal. Cross-linking of Fas, physiologically mediated by cells expressing FasL or artificially mediated by the addition of anti-Fas MoAbs or soluble FasL, can be shown to induce apoptotic death [37,38]. Cell death via Fas-binding is independent of extracellular Ca2+[39] and does not require active macromolecular synthesis [40,41].
FasL is expressed by activated CD8+ and CD4+ cells, thus providing a basis for the assumption that CD8+ cells can kill by both Ca2+-dependent and -independent mechanisms. In contrast, among CD4+ cells, most Th1 cells lack perforins [42–44] and thus presumably kill primarily through the Fas-based mechanism. CD4+ cells of the Th2 subset have little killing potential [45–48]. A few studies have pointed out, however, that CD4+ cells may also lyse targets via a Ca2+-dependent mechanism [49–51]. Importantly, it has been demonstrated that some tumours may be resistant to Fas-mediated killing, by virtue of Bcl-2 expression, but remain susceptible to perforin-mediated lysis [8,10,11]
In this study, we have assessed the ability of TIL to mediate tumour destruction by both Ca2+-dependent and -independent mechanisms. Although our data demonstrate the ability of many TIL to manifest Fas-mediated target destruction, in fact the tumour cells from which they were derived were relatively insusceptible to Ca2+-independent lysis, indicating that such mechanisms are unlikely to play a major role in tumour destruction in vivo. However, both CD8+ TIL, and some CD4+ TIL, were able to manifest strong Ca2+-dependent lysis of tumour targets, and perforin-containing granules were detected in these lytic cells, indicating that this is probably the predominant tumour lytic activity among TIL.
MATERIALS AND METHODS
Cell lines
Five pairs of autologous tumour cell lines and TIL were derived: four melanoma cell lines (M-1, M-3, M-5 and Mel-EW), one glioma cell line (Gli-PI) and the corresponding TIL. M-1, M-3 and M-5 melanomas and TIL have been previously characterized [52,53]. Mel-EW was derived from a metastatic melanoma patient and Gli-PI from a glioma. Cultures supplemented with IL-2 demonstrated propagation of activated T cells in those specimens containing lymphocytic infiltrates. These cultures were kept in IL-2 and restimulated with phytohaemagglutinin (PHA) and irradiated feeder cells every 2–4 weeks to enhance proliferation, as previously described [54]. Other cell lines used included Jurkat (derived from a T cell leukaemia), U-937 (derived from a promonocytic leukaemia) and K562 (from an erythroleukaemia). These cell lines were obtained from ATCC (Rockville, MD).
Cloning of TIL cultures
T cell clones were obtained by limiting dilution as previously reported [27,55]. Limiting dilution of IL-2-propagated TIL was performed within 4 weeks from original establishment of the culture.
Antibodies
TIL (bulk cultures and clones) were studied with a panel of MoAbs which included anti-CD3, CD4, CD8 (Ortho Diagnostic System, Raritan, NJ). MoAbs were used to stain cells in an indirect immunofluorescence test followed by analysis using a Becton Dickinson FACScan (Palo Alto, CA). Fas expression was detected by anti-Fas CH-11 MoAb obtained from UBI (Lake Placid, NY). Fas expression was also studied after pre-incubation of tumour cells with interferon-gamma (IFN-γ) as previously described in detail [53]. FasL expression was detected on TIL activated for 3 h in culture with PHA, anti-CD3 (purified OKT3 from Ortho, used at appropriate dilutions) or phorbol myristate acetate (PMA) and Ca-ionophore (ionomycin, obtained from Sigma (St Louis, MO) and used at 3 μg/ml), with a polyclonal rabbit anti-FasL (N-20) serum (Santa Cruz Biotechnology, Santa Cruz, CA), in an indirect immunofluorescence test with an anti-rabbit immunoglobulin FITC serum.
Anti-Fas IgM CH-11 antibody was also used to test susceptibility to Fas-mediated lysis by tumour cell lines at the concentration of 0.5 μg/ml. Anti-Fas MoAb (IgG ZB4; Immunotech, Westbrook, ME) was used at a concentration of 1 μg/ml to inhibit Fas-mediated lysis.
Perforin accumulations were detected in the cytoplasm of cytocentrifuged smears by immunofluorescence with an anti-perforin antibody (obtained from Kamiya Biomedical Co., Thousand Oaks, CA), and used at a concentration of 150 μg/ml. The percentage of stained cells was calculated from a minimum of 200 cells counted, with cells scored as positive if they contained distinctly fluorescent granules.
Cytoplasmic staining with Bcl-2 was assessed using anti-Bcl-2 (Novacastra Labs, Newcastle upon Tyne, UK) using cells which were first fixed for 10 min in 1% paraformaldehyde, followed by membrane permeabilization with 0.1% saponin for 10 min prior to addition of the anti-Bcl-2 antibody for 45 min. Following two washes, FITC-labelled goat anti-mouse IgG was added for 30 min. All steps were performed at room temperature (22°C). Assessment of staining was made by FACScan flow cytometry (Becton Dickinson).
Cytotoxicity
Cytotoxic activity was assessed by the lysis of 51Cr-labelled target cells in a 4-h Cr-release assay, as previously reported [56]. Per cent specific lysis was calculated as: (experimental −spontaneous) × 100/(maximum −spontaneous), where all experimental points were calculated as the average of triplicate cultures, and the spontaneous and maximum release by the target cells was the average of six wells each. Cytotoxicity was determined at serial dilutions of effector cells at ratios of 50:1, 25:1, 12.5:1 and 6.25:1 to determine the level at which maximum lysis could be achieved for each target/effector pair. As maximum release was achieved using 25 effectors per target, the data shown are for this ratio only in order to simplify data presentation. In all cases the ‘spontaneous release’ (background) was < 15% of the maximum, including cultures in which antibodies and cyclohexamide were added. Target cells included autologous tumour cell lines, as well as the series of tumour lines described above. Effector cells were TIL maintained in IL-2 (100 U/ml) for between 8 and 20 days after the previous restimulation with PHA and irradiated feeder cells. Assays were performed at multiple dilutions resulting in effector:target ratios of 50, 25, 12 and 6:1. As maximal lysis was apparent at ratios of 25:1, results are reported as percentage of specific lysis at this ratio. Effects on cytotoxicity by a calcium chelant were measured after adding 2 mm EGTA (Sigma) to the cultures.
Detection of Fas-induced cell death
After treatment of the tumour cell lines with anti-Fas (CH-11, at 1 μg/ml), apoptosis was evaluated by flow cytometry as previously described [57] with minor modifications. Briefly, the cells were centrifuged and resuspended in 0.6 ml of propidium iodide (PI) hypotonic fluorochrome solution (PI 50 μg/ml in 0.1% sodium citrate plus 0.1% NP-40; Sigma). The tubes were incubated overnight at 4°C in the dark before the flow cytometric analysis. The PI fluorescence was measured using an Ortho Absolute flow cytometer.
Assessment of cell death was also evaluated by the reduction of tetrazolium salt, MTT, by living cells to form a blue formazan product as previously reported [58].
Fas-induced apoptosis was also evaluated after coincubation of tumour cells with CH11 and cyclohexamide (CHX; used at a concentration of 1 μg/ml) as previously reported [59].
RESULTS
Fas and Bcl-2 expression on tumour cell line
We evaluated Fas expression on eight tumour cell lines. These included Jurkat (derived from a T cell leukaemia), U-937 (derived from a promonocytic leukaemia), K562 (from erythroleukaemia), four melanoma cell lines (M-1, M-3, M-5 and Mel-EW) and one glioma cell line (Gli-PI). Our data confirmed Fas expression on the Jurkat and U937 cell lines, while K562 was negative. In addition, we observed Fas expression on three melanoma cell lines (M-3, M-5, Mel-EW) and the Gli-PI glioma cell line, while M-1 cells were negative. Pre-incubation of the tumour cells with IFN-γ had only insignificant effects on Fas expression in all the cell lines tested (not shown). All of the melanomas and the glioma cell lines showed detectable cytoplasmic Bcl-2, although levels were generally low.
Fas-mediated apoptosis in tumour cells lines
We then tested the ability of an anti-Fas MoAb (clone CH-11) to induce apoptosis of target cells. Tumour cell lines, incubated with the anti-Fas antibody CH-11, were evaluated for apoptosis using PI and flow cytometry and by MTT assays. As shown in Table 1, the MTT assay (data shown are representative of four separate assays) revealed that Fas ligation was able to induce cell death in Jurkat (80%) and, to a lesser extent, in U-937 (32%) cells. Of the other cell lines tested, only 19% of the M-3 cell line population was killed, while the other cells tested showed very little susceptibility to cytotoxicity (≤ 12%). PI staining confirmed that the cell death observed in these cultures was due to apoptosis (data not shown).
Table 1.
Fas and Bcl-2 expression (as determined by flow cytometry), and mortality rate (expressed of percentage of dead cells in the MTT test) of tumour cell lines incubated with anti-Fas MoAb (CH-11)
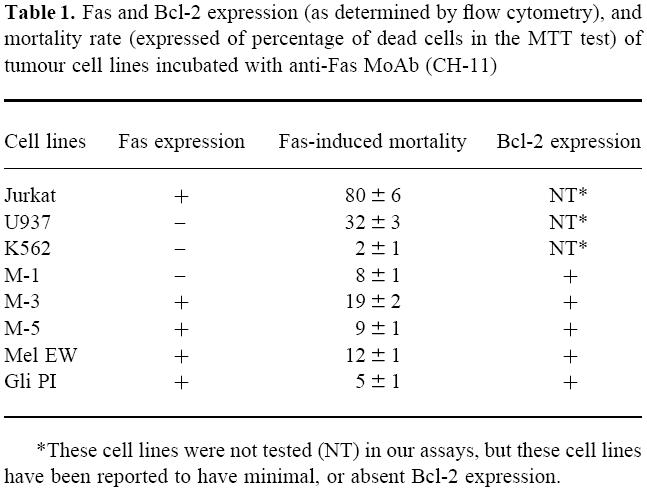
*These cell lines were not tested (NT) in our assays, but these cell lines have been reported to have minimal, or absent Bcl-2 expression.
As it has been shown that Fas-induced apoptosis in certain tumour cells may be inhibited by short lived cytoplasmic proteins [59], we also evaluated the ability of CH-11 to kill tumour targets after pre-incubation with CHX (an inhibitor of protein synthesis). Data show that, under these conditions, Fas was able to trigger increased apoptotic death in some of the cells lines (Fig. 1). (Data shown are representative of three separate assays).
Fig. 1.
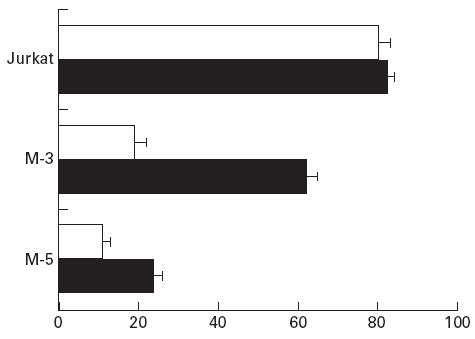
Mortality rate (expressed as percentage of dead cells in the MTT test) of tumour cell lines incubated with anti-Fas MoAb (CH-11) with or without cyclohexamide (CHX). □, CH-11; ▪, CH-11+ CHX.
Mechanisms of killing involved in TIL-mediated cytotoxicity of tumours. Ca2+-dependent killing by CD8+ and CD4+ cells
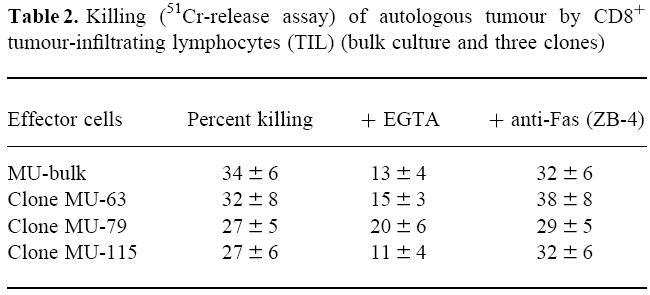
We first investigated the mechanisms involved in cell-mediated cytotoxicity by CD8+ TIL. We studied a previously described CD8+ TIL cell line, derived from a melanoma patient, with strong cytotoxic capacity against autologous tumour cells (M-1 cell line). Some clones derived from this bulk culture were also studied (MU-63, MU-79, MU-115). These CD8+ clones kill the autologous tumours through the recognition of the Melan A/MART-1 presented on HLA-A2 (not shown). As expected, killing by the bulk culture and the three derived clones was dependent on the presence of Ca2+, as shown by the inhibition of cytotoxicity in the presence of the Ca2+ chelant EGTA, whereas it could not be blocked by the addition of the anti-Fas MoAb ZB-4 (Table 2). (Data shown are representative of three experiments.)
Table 2.
Killing (51Cr-release assay) of autologous tumour by CD8+ tumour-infiltrating lymphocytes (TIL) (bulk culture and three clones)
The mechanisms involved in killing by CD4+ cells were investigated in TIL bulk cultures with a CD4+ phenotype and in CD4+ clones derived from both a melanoma and a glioma patient. TIL derived from a glioma patient, for which an autologous tumour cell line (Gli-PI) was available, were shown to be predominantly CD4+ (63%). This TIL cell line showed only marginal lytic capacity against the autologous tumour (5%, 10% and 15% in three different experiments). Three CD4+ clones (PI-A, PI-D and PI-H) derived from the glioma TIL also showed little cytotoxicity (not shown).
The expression of MHC class II antigens on glioma tumour cells was undetectable by staining with an anti-DR antibody and class II expression could not be induced by pretreatment of the glioma cells with IFN-γ (data not shown). As HLA class II molecules were not expressed on tumour cells, this precluded efficient recognition of the target by CD4 effectors. Therefore, we cross-linked effectors and target cells with PHA before performing the cytotoxic assay. Under these conditions, both the bulk culture and the three clones showed killing of the autologous tumour. In clones PI-A, -D and -H killing was 57%, 35% and 87%, respectively. Killing was Ca2+-dependent, as shown by > 90% inhibition observed with EGTA, while marginal (< 10%) effects were seen with the addition of the ZB-4 anti-Fas antibody (representative of three separate assays).
Expression of perforin on CD4+ cells
Ca2+-dependent killing manifested by CD4+ cells in the experiments with PHA-cross-linked targets suggested that Fas–FasL-mediated killing of tumour cells may not have been involved in the mechanism of lysis by these cells. Instead, the Ca2+-dependent killing observed is more consistent with perforin-mediated lysis. Therefore, we evaluated the presence of perforin in the cytoplasm of six CD4+ clones (three from the glioma and three from melanoma patient Mel-EW), by indirect immunofluorescence. In three out of six CD4+ clones examined, we observed the presence of scattered perforin staining in the cytoplasm in a granular distribution (Fig. 2). The positive clones were glioma clones PI-A and PI-H, and melanoma clone EW-2E5. This clone showed 43% killing of the autologous tumour cells, but killing was not significantly inhibited by either EGTA or ZB-4. In the three positive CD4+ clones, perforin staining was strong in only 15–50% of the cells (scored + or stronger (out of + + + +) among 200 cells counted per sample). In contrast, in CD8+ cultures the number of strongly perforin-positive cells was > 80% (most + + + or + + + +). In addition, Giemsa staining revealed the presence of the characteristic azurophilic cytoplasmic granules in the CD8+ cells, while in the CD4+ clones azurophilic granules were not detected. Glioma clones PI-D and melanoma clones EW-2E10 and 3F8 did not show detectable perforin.
Fig. 2.
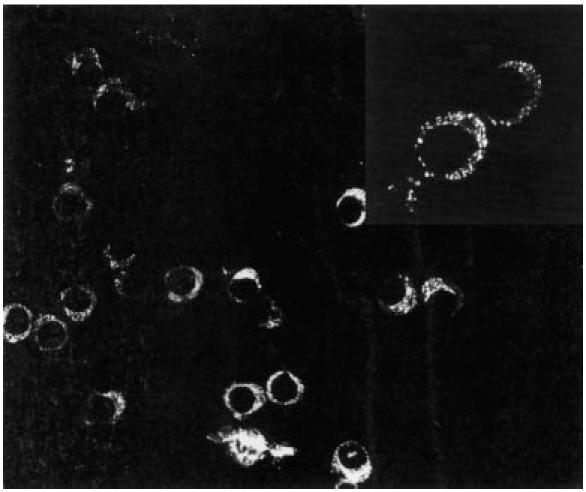
Expression of perforin by tumour-infiltrating lymphocytes (TIL) by indirect immunofluorescence on cytocentrifuged preparations. CD4+ clone PI-H (derived from a glioma lesion) showed bright cytoplasmic staining in approximately half of the cells; at higher magnification (inset) the cytoplasmic stain shows a granular pattern.
Fas-mediated killing by CD4 TIL
To determine if the CD4+ effectors derived from tumour lesions also had the potential to kill by Fas engagement, we first evaluated the ability of TIL cell lines to express FasL. Bulk cultures, maintained in IL-2 for 6 or more days after restimulation with PHA and feeder cells, showed little expression of FasL. However, when we tested the same cell lines 3 h after stimulation with either PMA and ionomycin, anti-CD3 or PHA (without feeder cells), we observed increased FasL expression in all the tested cell lines, suggesting transient expression after stimulation (Fig. 3).
Fig. 3.
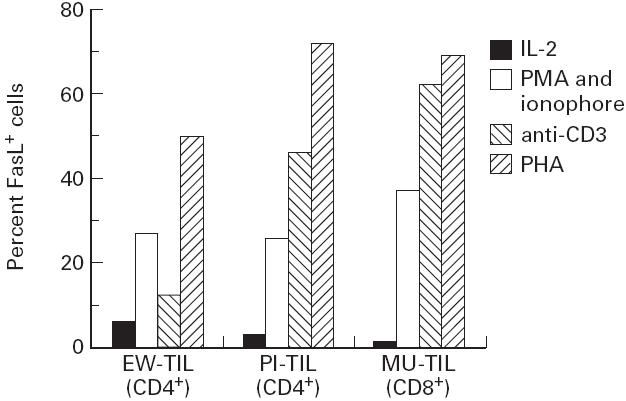
FasL expression by tumour-infiltrating lymphocytes (TIL). Sample 1 is a CD4+ bulk population derived from a melanoma lesion (EW). Sample 2 is a CD4+ bulk population derived from a glioma lesion (PI). Sample 3 is a CD8+ bulk population derived from a melanoma lesion (MU). Cells in IL-2 were stained 6 days after restimulation with phytohaemagglutinin (PHA) and feeder. For other conditions, cells were stained 3 h after restimulation with phorbol myristate acetate (PMA)/ionophore, anti-CD3, or PHA.
To assess the ability of CD4+ TIL cells to kill via Fas–FasL interactions, we developed a model using a Fas-sensitive target. As shown in Table 1, among the tumour cell lines derived in our laboratory from fresh tumours, only the M-3 targets were susceptible to some Fas-mediated apoptosis (19%). We therefore tested the ability of the melanoma-derived TIL CD4+ cell line M-3 and some of the Mel-EW CD4+ clones to induce killing of the Fas-sensitive target cell lines Jurkat and M-3. The K562 cell line was used as a Fas-insensitive control. FasL expression on effector cells was enhanced by pre-incubation with anti-CD3 antibodies for 3 h before performing the cytotoxic assay (representative of duplicate experiments). As shown in Fig. 4, the M3 (Fig. 4a) and Jurkat targets (Fig. 4b) were lysed in the presence of anti-CD3 antibody with or without anti-Fas or EDTA (P < 0.05 versus IL-2 alone). In contrast, K562 cells (Fig. 4c) were not lysed significantly by the EW clones, even in the presence of anti-CD3 antibody and added anti-Fas and EGTA. The M-3 TIL bulk had anti-K562 activity even in IL-2, which was not enhanced significantly by anti-CD3 or anti-Fas antibody, indicating the Fas-independent lysis of this target.
Fig. 4.
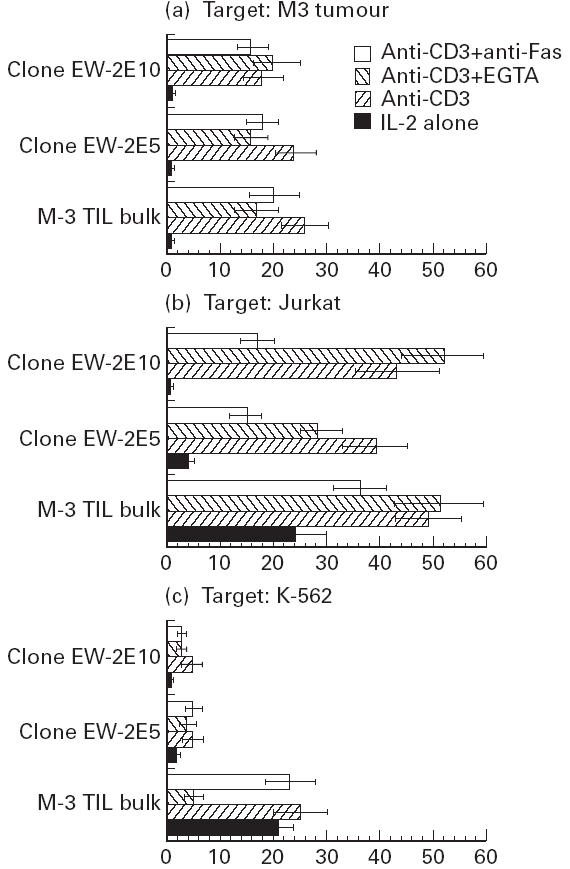
Killing of the Fas-sensitive targets (Jurkat and M-3) and Fas-resistant target (K-562) by CD4+ tumour-infiltrating lymphocyte (TIL) effectors kept in IL-2 at least 6 days after restimulation (IL-2 alone), or preactivated with anti-CD3 for 3 h. Anti-Fas (ZB-4) or EGTA were added to these CD3-stimulated cultures to determine the effect of these agents on tumour lysis.
Cells cultured in IL-2 alone (which expressed low levels of FasL, see Fig. 3) showed no killing of the M-3 target (Fig. 4a). With Jurkat (Fig. 4b) and K562 (Fig. 4c) targets, the M-3 TIL bulk cultured in IL-2 alone showed some cytotoxic activity. After activation with anti-CD3, M-3 cells and two EW clones (EW-2E5 and EW-2E10) could lyse the Jurkat (Fig. 4b) and (to a lesser degree), the M-3 targets (Fig. 4a). Killing was consistent with the differential Fas sensitivity of Jurkat and M3 cell lines (Table 1).
Killing of Jurkat by M-3 bulk and EW clones was inhibited by anti-Fas antibody, but EGTA induced little or no inhibition (Fig. 4b). On the other hand, lysis of the M-3 target by M-3 bulk and clone EW-2E5 appeared to be mediated by both Ca2+- and Fas-dependent mechanisms, since some inhibition could be observed with both EGTA and anti-Fas (Fig. 4a). Lysis of this target by clone 2E10 could not be blocked by either EGTA or anti-Fas, thus precluding a delineation of the mechanism involved in this interaction. Killing of the Fas-resistant K562 target by M-3 bulk could be inhibited only by EGTA, and not by anti-Fas, confirming that killing of this target occurred in a Ca2+-dependent, Fas-independent fashion.
DISCUSSION
In this study we investigated the cytotoxic mechanisms involved in tumour killing by TIL derived from melanoma or glioma patients. Some TIL cultures or clones (expressing either the CD8+ or the CD4+ phenotype) were able to exert cytotoxic activity against autologous targets. The CD8+ TIL, and some of the CD4+ TIL showed cytoplasmic perforin-containing granules and lysed their targets in a Ca2+-dependent fashion. In contrast, our tumour lines were generally insensitive to Fas-mediated lysis, although several of our fresh tumour-derived cell lines expressed Fas. As these tumours also expressed cytoplasmic Bcl-2, it is possible that their insensitivity to Fas-mediated destruction was due to the anti-apoptotic activity of this protein.
It is not surprising that the cytotoxicity manifested by the CD8+ TIL was shown to be Ca2+-dependent. However, most of the available literature argues that CD4+ cells, which are commonly devoid of perforin-containing cytoplasmic granules, kill targets through a Ca2+-independent, Fas-mediated mechanism [42–48]. Our data demonstrate that killing by CD4+ bulk cultures and clones (derived from both a melanoma and a glioma) could occur in a Ca2+-dependent fashion, and was not affected by anti-Fas antibody. In fact, the demonstration of perforin-containing granules in a proportion of these CD4+ clones adds a novel model for TIL/tumour interaction, in which CD4+ cells kill in a Fas-independent fashion.
Although our data suggest that CD4+ clones do have the potential to kill by the Fas/FasL mechanisms, it appears that the autologous tumour targets we tested are not susceptible to this lytic activity. CD4+ TIL can easily express FasL upon activation and can kill Fas-sensitive targets (such as the Jurkat cell line) by a mechanism which can be inhibited by anti-Fas antibodies. However, this potential activity did not take place in the interactions of TIL with the autologous tumours we investigated. This conclusion was further supported by the fact that the melanoma and glioma tumour cell lines we derived were also resistant to Fas-mediated killing by the apoptosis-inducing antibody, CH-11. This antibody had activity only when the target cells were treated with CHX. The staining of these tumours with Bcl-2 antibody offers a likely explanation for this lack of apoptosis induction [11], further emphasizing the need for Ca2+-dependent mechanisms of tumour lysis to effect tumour destruction.
In conclusion, our data suggest that the Ca2+-dependent, perforin-associated mechanism appears to be the principle cytotoxic pathway used by TIL-tumour pairs, although we cannot exclude other lytic mechanisms, including cytokine-induced tumour destruction [34], which could also play a role in TIL–tumour interactions. However, it is noteworthy that when Kuwano et al. blocked cytokine-induced killing, the ability to lyse by a perforin–granzyme mechanism was retained [35]. The lack of Fas-dependent lysis in our assays suggests that this is unlikely to play a major role in tumour destruction, but does not preclude another, perhaps counterproductive role for Fas. For example, it has been observed that melanoma cell lines can express FasL, and it has been proposed that they can destroy TIL by this mechanism, contributing to the immune privilege of tumours [60,61]. However, when we tested three available melanoma cell lines (M-1, M-5, Mel-EW), all were FasL−. Thus, the killing of TIL by FasL-expressing tumour cells did not play a role in the tumour–TIL interactions we studied, as TIL were not destroyed by contact with tumour cells, and were able to deliver an efficient cytotoxic signal when the TCR was engaged and perforin-mediated lysis could occur. With the recent demonstration by Melcher [1] that apoptotic killing of tumour cells may render them less immunogenic, it is ironic that the tumour killing we have observed was defi-cient in Fas-mediated lysis, indicating that any killing which does occur is more likely to be via conventional cell lysis. The fact that we were able to isolate tumour-specific CTL from these tumours perhaps reflects the fact that these tumours are indeed, at least partially, immunogenic. However, the ultimate failure of these CTL to control tumour growth remains a hurdle to be surmounted.
Acknowledgments
This work was supported in part by NIH (grants HL-43793 and CA51345), NATO (grant CRG940029), AIRC (Milan) and MURST-COFIN (Italy).
REFERENCES
- 1.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile R. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nature Med. 1998;4:581–7. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 2.Muller K, Mariani S, Matiba B, Kyewski B, Krammer P. Clonal deletion of major histocompatibility complex class I-restricted CD4+CD8+ thymocytes in vitro is independent of the CD95 (APO-1/Fas) ligand. Eur J Immunol. 1995;25:2996–9. doi: 10.1002/eji.1830251043. [DOI] [PubMed] [Google Scholar]
- 3.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–2. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann M, Ohteki T, Faienza K, Zakarian A, Kagi D, Speiser D, Ohashi P. Altered peptide ligands trigger perforin- rather than Fas-dependent cell lysis. J Immunol. 1997;159:4165–70. [PubMed] [Google Scholar]
- 5.Fukuyama H, Adachi M, Suematsu S, Miwa K, Suda T, Yoshida N, Nagata S. Transgenic expression of Fas in T cells blocks lymphoproliferation but not autoimmune disease in MRL-lpr mice. J Immunol. 1998;160:3805–11. [PubMed] [Google Scholar]
- 6.Dilloo D, Brown M, Roskrow M, Zhong W, Holladay M, Holden W, Brenner M. CD40 ligand induces an antileukemia immune response in vivo. Blood. 1997;90:1927–33. [PubMed] [Google Scholar]
- 7.Tsujimura K, Takahashi T, Iwase S, Matsudaira Y, Kaneko Y, Yagita H, Obata Y. Two types of anti-TL (thymus leukemia) CTL clones with distinct target specificities: differences in cytotoxic mechanisms and accessory molecule requirements. J Immunol. 1998;160:5253–61. [PubMed] [Google Scholar]
- 8.Jaattela Benedict M, Tewari M, Shayman J, Dixit V. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–305. [PubMed] [Google Scholar]
- 9.Panayiotidis P, Ganeshaguru K, Foroni L, Hoffbrand A. Expression and function of the Fas antigen in B chronic lymphocytic leukemia and hairy cell leukemia. Leukemia. 1995;9:1227–32. [PubMed] [Google Scholar]
- 10.Lee R, Spielman J, Podack ER. Bcl-2 protects against Fas-based but not perforin-based T cell-mediated cytolysis. Int Immunol. 1996;8:991–1000. doi: 10.1093/intimm/8.7.991. [DOI] [PubMed] [Google Scholar]
- 11.Schroter M, Lowin B, Borner C, Tschopp J. Regulation of Fas (Apo-1/CD95)- and perforin-mediated lytic pathways of primary cytotoxic T lymphocytes by the protooncogene bcl-2. Eur J Immunol. 1995;25:3509–13. doi: 10.1002/eji.1830251245. [DOI] [PubMed] [Google Scholar]
- 12.Clark WH, Elder DE, Guerry D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 13.Day C, Lew R, Mihm M, et al. A multivariate analysis of prognostic factors for melanoma patients with lesions >3.65 mm in thickness. Ann Surg. 1982;195:44. doi: 10.1097/00000658-198201001-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihm M, Clemente C, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 15.Ridley A, Cavanagh JB. Lymphocytic infiltration in gliomas: evidence of possible host resistance. Brain. 1971;94:117–24. doi: 10.1093/brain/94.1.117. [DOI] [PubMed] [Google Scholar]
- 16.Mukherji B, Guha A, Chakraborty G, Sivanandham M, Nashed A, Sporn J, Ergin M. Clonal analysis of cytotoxic and regulatory T cell responses against human melanoma. J Exp Med. 1989;169:1961–76. doi: 10.1084/jem.169.6.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh K, Platsoucas CD, Balch CM. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988;168:1419–41. doi: 10.1084/jem.168.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sensi M, Salvi S, Castelli C, et al. T cell receptor (TCR) structure of autologous melanoma-reactive cytotoxic T lymphocyte (CTL) clones: tumor-infiltrating lymphocytes overexpress in vivo the TCR beta chain sequence used by an HLA-A2-restricted and melanocyte-lineage-specific CTL clone. J Exp Med. 1993;178:1231–46. doi: 10.1084/jem.178.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sensi M, Traversari C, Radrizzani M, et al. Cytotoxic T-lymphocyte clones from different patients display limited T-cell-receptor variable-region gene usage in HLA-A2-restricted recognition of the melanoma antigen Melan-A/MART-1. Proc Natl Acad Sci USA. 1995;92:5674–8. doi: 10.1073/pnas.92.12.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurnick J, Kradin R, Blumberg R, Schneeberger E, Boyle L. Functional characterization of T lymphocytes propagated from human lung carcinomas. Clin Immunol Immunopathol. 1986;38:367–80. doi: 10.1016/0090-1229(86)90247-3. [DOI] [PubMed] [Google Scholar]
- 21.Platsoucas C. Human autologous tumor-specific T cells in malignant melanoma. Cancer Metastasis Rev. 1991;10:151–76. doi: 10.1007/BF00049412. [DOI] [PubMed] [Google Scholar]
- 22.Caignard A, Dietrich P, Morand V, et al. Evidence for T-cell clonal expansion in a patient with squamous cell carcinoma of the head and neck. Cancer Res. 1994;54:1292–7. [PubMed] [Google Scholar]
- 23.Gervois N, Heuze F, Diez E, Jotereau F. Selective expansion of a specific anti-tumor CD8+ cytotoxic T lymphocyte clone in the bulk culture of tumor infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol. 1990;20:825–31. doi: 10.1002/eji.1830200417. [DOI] [PubMed] [Google Scholar]
- 24.Ioannides C, Freedman R. Selective usage of TCR V beta in tumor-specific CTL lines isolated from ovarian tumor-associated lymphocytes. Anticancer Res. 1991;11:1919–25. [PubMed] [Google Scholar]
- 25.Mackensen A, Ferradini L, Carcelain G, Triebel F, Faure F, Viel S, Hercend T. Evidence for in situ amplification of cytotoxic T-lymphocytes with antitumor activity in a human regressive melanoma. Cancer Res. 1993;53:3569–73. [PubMed] [Google Scholar]
- 26.Miescher S, Whiteside T, Moretta L, VonFliedner V. Clonal and frequency analyses of tumor-infiltrating T lymphocytes from human solid tumors. J Immunol. 1987;138:4004. [PubMed] [Google Scholar]
- 27.Pandolfi F, Boyle L, Trentin L, Oliva A, Kurnick J. T cell receptor gene rearrangement and cytotoxic activities of clones isolated from tumor infiltrating lymphocytes from melanoma patients. Clin Exp Immunol. 1994;95:141–7. doi: 10.1111/j.1365-2249.1994.tb06028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peoples GE, Schoof DD, Andrews JV, Goedegebuure PS, Eberlein TJ. T-cell recognition of ovarian cancer. Surg. 1993;114:227–34. [PubMed] [Google Scholar]
- 29.Rosenberg S, Packard B, Aebersold P. Use of tumor-infiltrating lymphocytes and interleukin 2 in the immunotherapy of patients with metastatic melanoma. N Eng J Med. 1988;25:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 30.Wong JT, Pinto CE, Gifford JD, Kurnick JT, Kradin RL. Characterization of the CD4+ and CD8+ tumor infiltrating lymphocytes propagated with bispecific monoclonal antibodies. J Immunol. 1989;143:3404–11. [PubMed] [Google Scholar]
- 31.Kradin R, Kurnick J, Lazarus D, et al. Tumour-infiltrating lymphocytes and interleukin-2 in treatment of advanced cancer. Lancet. 1989;i:577–80. doi: 10.1016/s0140-6736(89)91609-7. [DOI] [PubMed] [Google Scholar]
- 32.Hishii M, Andrews D, Boyle L, Wong J, Pandolfi F, van den Elsen P, Kurnick J. In vivo accumulation of the same anti-melanoma T cell clone in two different metastatic sites. Proc Natl Acad Sci USA. 1997;94:1378–83. doi: 10.1073/pnas.94.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes. Annu Rev Immunol. 1994;12:735–73. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 34.Hehner S, Hofmann T, Ratter F, Dumont A, Droge W, Schmitz M. Tumor necrosis factor-alpha-induced cell killing and activation of transcription factor NF-kappaB are uncoupled in L929 cells. J Biol Chem. 1998;273:18117–21. doi: 10.1074/jbc.273.29.18117. [DOI] [PubMed] [Google Scholar]
- 35.Kuwano K, Akashi A, Arai S. An anergic cytotoxic T lymphocyte clone exhibits granule exocytosis-mediated cytotoxicity. Cell Immunol. 1998;185:114–22. doi: 10.1006/cimm.1998.1289. [DOI] [PubMed] [Google Scholar]
- 36.Henkart P. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–6. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 37.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–9. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen JJ, Jenkinson EJ. Apoptosis and T-cell repertoire selection in the thymus. Ann NY Acad Sci. 1992;663:305–10. doi: 10.1111/j.1749-6632.1992.tb38673.x. [DOI] [PubMed] [Google Scholar]
- 39.Rouvier E, Luciani MF, Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 41.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994;152:1127–33. [PubMed] [Google Scholar]
- 42.Strack P, Martin C, Saito S, Dekruyff R, Ju S. Metabolic inhibitors distinguish cytolytic activity of CD4 and CD8 clones. Eur J Immunol. 1990;20:179–83. doi: 10.1002/eji.1830200126. [DOI] [PubMed] [Google Scholar]
- 43.Lancki D, Hsieh C, Fitch F. Mechanisms of lysis by cytotoxic T lymphocyte clones. J Immunol. 1991;146:3242–9. [PubMed] [Google Scholar]
- 44.Ozdemirli M, El-Khatib M, Bastiani L, Akdeniz H, Kuchroo V, Ju S. The cytotoxic process of CD4 Th1 clones. J Immunol. 1992;149:1889–95. [PubMed] [Google Scholar]
- 45.Ju ST, Cui H, Panka DJ, Ettinger R, Marshak RA. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA. 1994;91:4185–9. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Intern Immunol. 1994;6:1545–53. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 47.Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995;181:781–6. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anel A, Buferne M, Boyer C, Schmitt VA, Golstein P. T cell receptor-induced Fas ligand expression in cytotoxic T lymphocyte clones is blocked by protein tyrosine kinase inhibitors and cyclosporin A. Eur J Immunol. 1994;24:2469–76. doi: 10.1002/eji.1830241032. [DOI] [PubMed] [Google Scholar]
- 49.Grogg D, Hahn S, Erb P. CD4+ T cell mediated killing of major histocompatibility complex class II-positive antigen-presenting cells. III. CD4+ cytotoxic T cells induce apoptosis of APC. J Immunol. 1992;22:267–79. doi: 10.1002/eji.1830220139. [DOI] [PubMed] [Google Scholar]
- 50.Miskovsky E, Liu A, Pavlat W, et al. Studies of the mechanism of cytolysis by HIV-1 specific CD4+ human CTL clones induced by candidate AIDS vaccines. J Immunol. 1994;153:2787–99. [PubMed] [Google Scholar]
- 51.Susskind B, Shornick M, Iannotti M, Duffy B, nee Tanden P, Siegel J, Mohanakumar T. Cytolytic effector mechanisms of human CD4+ cytotoxic T lymphocytes. Human Immunol. 1996;45:64–75. doi: 10.1016/0198-8859(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 52.Pandolfi F, Boyle LA, Trentin L, Kurnick JT, Isselbacher KJ, Gattoni Celli S. Expression of HLA-A2 antigen in human melanoma cell lines and its role in T-cell recognition. Cancer Res. 1991;51:3164–70. [PubMed] [Google Scholar]
- 53.Pandolfi F, Trentin L, Boyle LA, Stamenkovic I, Byers HR, Colvin RB, Kurnick JT. Expression of cell adhesion molecules in human melanoma cell lines and their role in lymphocyte mediated cytotoxicity. Cancer. 1992;69:1165–73. doi: 10.1002/cncr.2820690517. [DOI] [PubMed] [Google Scholar]
- 54.Kurnick J, Kradin R, Blumberg R, Schneeberger E, Boyle L. Functional characterization of T lymphocytes propagated from human lung carcinomas. Clin Immunol Immunopathol. 1986;38:367–80. doi: 10.1016/0090-1229(86)90247-3. [DOI] [PubMed] [Google Scholar]
- 55.Kurnick J, Hayward A, Altevogt P. Helper and suppressor/inducer activity of human T cells and their cloned progeny maintained in long-term culture. J Immunol. 1981;126:1307. [PubMed] [Google Scholar]
- 56.Pandolfi F, Strong DM, Slease RB, Smith ML, Ortaldo JR, Herberman RB. Characterization of a suppressor T-cell chronic lymphocytic leukemia with ADCC but not NK activity. Blood. 1980;56:653–60. [PubMed] [Google Scholar]
- 57.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 58.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–7. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 59.Weller M, Frei K, Groscurth P, Krammer P, Yonekawa Y, Fontana A. Anti-Fas/Apo-1 antibody-mediated apoptosis of cultured human glioma cells. J Clin Invest. 1994;94:954–64. doi: 10.1172/JCI117462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hahne M, Rimoldi D, Schroter M, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implication for tumor escape. Science. 1996;274:1363–6. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 61.Yamauchi A, Taga K, Mostowski HS, Bloom ET. Target cell-induced apoptosis of interleukin-2-activated human natural killer cells: roles of cell surface molecules and intracellular events. Blood. 1996;87:5127–35. [PubMed] [Google Scholar]


