Abstract
Aims
The aim of this study was to assess the influence of concomitant caffeine intake on the pharmacokinetics of oral melatonin, a probe drug for CYP1A2 activity.
Methods
Twelve healthy subjects, six smokers and six nonsmokers, were given melatonin (6 mg) either alone or in combination with caffeine (3 × 200 mg). Blood samples for the analysis of melatonin or caffeine and paraxanthine were taken from 1 h before until 6 h after intake of melatonin. Subjects were genotyped with respect to the CYP1A2*1F (C734A) polymorphism.
Results
When caffeine was coadministered the Cmax and AUC of melatonin were increased on average by 142% (P = 0.001, confidence interval on the difference 44, 80%) and 120% (P < 0.001, confidence interval on the difference 63, 178%), respectively. The inhibitory effect of caffeine was more pronounced in nonsmokers and in individuals with the *1F/*1F genotype.
Conclusion
The results of this study revealed a pronounced effect of caffeine on the bioavailability of orally given melatonin, most probably due to inhibition of CYP1A2 activity.
Keywords: caffeine, CYP1A2, melatonin
Introduction
Besides its role in drug metabolism, CYP1A2 has gained attention for its capability to activate procarcinogenes like aromatic- and heterocyclic amines [1, 2]. CYP1A2 activity varies widely between individuals, mostly due to the influence of environmental factors [3–6]. The only polymorphisms of functional relevance described so far have been found in intron 1 and the 5′ flanking region of the CYP1A2 gene [7, 8]. Although a recent twin study has provided evidence for a contribution of genetic factors to the catalytic activity of CYP1A2 [9], a clear association between allelic variation and enzyme function is currently lacking, and thus phenotyping has been the only way of assessing CYP1A2 activity.
The metabolism of caffeine is more than 90% dependent on CYP1A2 activity [10], and thus caffeine clearance is considered as the ‘golden standard’ for assessment of CYP1A2 activity [11]. However, the results from caffeine phenotyping can be confounded by several factors [12]. Recently it has become evident that the pituitary hormone melatonin is quite a selective substrate for CYP1A2 and thus might be used as an alternative probe for this enzyme [13, 14].
The present study in healthy volunteers was performed to investigate the effect of caffeine on the pharmacokinetics of orally administered melatonin with special reference to smoking status and the CYP1A2*1F (C734A) polymorphism.
Methods
Subjects
Twelve healthy Finnish subjects (five females), aged between 18 and 40 years participated in the study. Six were smokers (at least 20 cigarettes/day) but none of the 12 subjects used any concomitant medications, including oral contraceptives. All volunteers gave their written informed consent prior to the study. The study protocol was approved by the first ethics committee of the hospital district of Varsinais-Suomi.
Study procedure
Consumption of methylxanthine containing food and beverages was restricted for 48 h before and during each study day. Subjects were not allowed to drink alcohol or use any prescribed or nonprescribed drugs or herbal products for 2 days before and during the study. Subjects in the smoking group were required to document the number of cigarettes smoked over the last 2 days before and during the study days. The subjects were not allowed to consume charcoal grilled food or cruciferous vegetables on the study days and for 48 h preceding each study day. The investigation was conducted as an open, balanced, randomized, cross-over study with all subjects receiving the two alternative treatments (melatonin only or melatonin + caffeine) in random order. In period A of the study, subjects received a single oral dose of 6 mg melatonin (2 × 3 mg tablets, Yliopiston apteekki, Helsinki, Finland) at 08.30 h and venous blood samples (10 ml) were taken 1 and 0.5 h before, immediately before intake and 0.5, 1, 1.5, 2, 2.5, 3, 4, and 6 h after intake. In period B, 200 mg caffeine (2 × 100 mg tablets, Vitabalans, Hämeenlinna, Finland) was given 1 h before intake of 6 mg melatonin (at 08.30 h) and at 1 and 3 h afterwards. Blood was sampled at the same time points as in period A. There was a 1-week washout between the two periods. For the determination of genotype, a 10 ml whole blood sample was collected in an EDTA tube for the preparation of genomic DNA.
Caffeine assay
The concentrations of caffeine and paraxanthine in serum were analysed by a recently published HPLC method with UV-detection [15]. The interassay coefficients of variation from the analysis of control samples at 1 and 10 µm in the case of caffeine and 10 and 60 µm in case of paraxanthine were always less than 5% for both drugs. The limit of determination was 1 µm for both caffeine and paraxanthine.
Melatonin assay
Melatonin was measured by a radioimmunoassay (RIA) adapted from the method described by English et al.[16]. The limit of determination was less than 2 pg ml−1 and the interassay coefficient of variation (CV) was 16.3% at low concentrations (less than 20 pg ml−1). To reach the linear range of the assay (2–500 pg ml−1) samples were diluted 20 to 100 times. There was no crossreaction with either caffeine and metabolites or melatonin metabolites.
All samples were analysed in duplicate and reported as means.
Genotyping
Extracted DNA was subjected to a 5′−nuclease allele-specific assay (Taqman®) test specific for the C→A polymorphism in intron 1 as described by Nordmark et al.[17].
Data analysis
All pharmacokinetic parameters (area under the concentration-time curve, AUC (µg l−1 h), oral melatonin clearance, CL (l h−1 kg−1), elimination rate constant, kel (h−1)) were calculated by noncompartmental analyses using the Top-Fit pharmacokinetic data analysis software (version 2.0, Gustav Fischer Verlag, Stuttgart, Germany). The maximum concentrations (Cmax) and the time to reach Cmax (tmax) were taken directly from the concentration vs time data.
To compare the means of the two treatment regimens, melatonin only and melatonin + caffeine, data were log transformed and a paired t-test was applied.
The relationship between the difference in the pharmacokinetic parameters for melatonin between period A and B and the caffeine and paraxanthine concentrations was tested by the parametric Pearson correlation test.
P values less than 0.05 were considered statistically significant. All statistical analyses were performed with SPSS software (version 9.0, SPSS Inc., Chicago, USA).
Results
When caffeine was coadministered the Cmax of melatonin was increased (Figure 1, Table 1) on average by 137% (P = 0.001, 95% confidence interval, CI, 44.3, 230%) and AUC by 120% (P < 0.001; 95% CI 62.7, 178%). Accordingly, oral CL was decreased, on average by 47% (P = 0.02, 95% CI −33, −61%). The apparent t1/2 of melatonin was not affected by caffeine (Table 1). The differences in Cmax and AUC between the two treatment periods were significantly correlated with caffeine concentration 2 h after intake of the first caffeine dose (r = 0.696; P = 0.012 and r = 0.659, P = 0.02, respectively) and with the last measured caffeine concentration (r = 0.717; P = 0.009 and r = 0.861, P < 0.001, respectively). No correlation was found with the paraxanthine concentrations.
Figure 1.
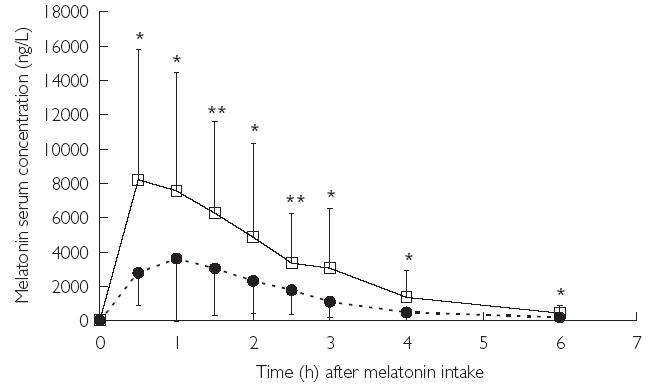
Melatonin serum concentration after oral administration of melatonin alone (closed circles, dotted line) or together with caffeine (3 × 200 mg) given 1 h before and 1 and 3 h after melatonin intake (open squares, solid line). Melatonin serum concentrations were significantly (*P < 0.05; **P < 0.01) higher at all time points between 0.5 and 6 h after melatonin intake when caffeine was coadministered
Table 1. Melatonin pharmacokinetic parameters (mean and 95% confidence interval, 95% CI), in six healthy smoking and six healthy nonsmoking subjects derived after oral administration of 6 mg melatonin alone and after coadministration with caffeine (3 × 200 mg p.o).
| Melatonin only | Melatonin + caffeine | |||||
|---|---|---|---|---|---|---|
| Cmaxa(ng ml−1) (95% CI) | CLb(l h−1kg−1) (95% CI) | t1/2c(h) (95% CI) | Cmax(% change) (95% CI) | CL (% change) (95% CI) | t1/2 (% change) (95% CI) | |
| All | 4.48 | 2.46 | 1.77 | + 137%** | −46.9%** | + 6.50 |
| (n = 12) | (2.20, 6.77) | (0.89, 4.04) | (1.12, 2.42) | (44, 230) | (−32.7, −61.0) | (−20.4, 33.4) |
| Smoking status | ||||||
| Nonsmoker | 5.61 | 1.48 | 1.11 | + 176.6% | −56.2%* | + 2.52% |
| (n = 6) | (0.97, 10.2) | (0.76, 2.19) | (0.86, 1.35) | (−23.2, 376.4) | (32.4, 79.9) | (−22.1, 27.2) |
| Smoker | 3.36 | 3.45 | 2.43 | + 97.2%* | −37.5%* | + 10.5% |
| (n = 6) | (0.82, 5.89) | (0.02, 6.89) | (1.28, 3.58) | (11.5, 183) | (17.3, 57.8) | (−50.2, 71.2) |
maximum concentration,
total clearance,
apparent half-life,
P < 0.05,
P < 0.01.
Smokers had a lower Cmax (3.36 ng ml−1, 95% CI 0.82, 5.89) and AUC (5.5 µg l−1 h, 95% CI 2.13, 8.62 µg l–1 h) compared with nonsmokers (Cmax = 5.61 ng ml−1 95% CI 0.97, 10.2; AUC = 9.65 µg l−1 h, 95% CI 4.52, 18.55 µg l−1 h) when melatonin was given alone. As shown in Table 1, the inhibition of melatonin metabolism was in general more pronounced in nonsmokers (mean increase in Cmax+ 197% and decrease in CL −56%, respectively) than in smokers (mean increase in Cmax+ 97% and decrease in CL −38%, respectively).
Our sample comprised seven subjects homozygous for the *1F allele (*1F/*1F), four heterozygous (*1F/*1 A) and one homozygous for the *1 A allele (*1 A/*1 A). The nonsmoking subject carrying two *1 A alleles had by far the highest Cmax of 14.3 ng ml−1 and AUC of 23.9 µg l−1 h and lowest oral CL of 0.63 l h−1 kg−1. The mean increase in Cmax was higher in subjects with the *1F/*1F genotype (202%) compared with subjects carrying *1F/*1 A alleles (36%).
Discussion
The most prominent effect of caffeine on melatonin disposition was on the Cmax, which was on average more than doubled. However, apparent half-life was not affected, suggesting an effect on the first-pass metabolism of melatonin leading to a higher oral bioavailability, the magnitude of which is similar to the previously reported effect of fluvoxamine, a known CYP1A2 inhibitor [18]. The caffeine dose of 200 mg corresponds to one large cup or two small cups of coffee and is comparable with that ingested at breakfast in many western countries [19].
The results of this study support concerns on concomitant intake of CYP1A2 substrates like theophylline, clozapine or olanzapine with caffeine. Moreover, if melatonin is used as a CYP1A2 probe concomitant caffeine intake is a confounding factor and needs to be avoided.
In summary, caffeine was found to increase the oral bioavailability of melatonin probably due to an inhibition of the CYP1A2 catalysed first-pass metabolism of melatonin. The effect was more pronounced in nonsmokers and subjects with the *1F/*1F genotype.
This study was supported by grants from the Swedish Medical Research Council (3902) and the National Institutes of Health, USA (IR0I GM60548–01A2).
Acknowledgments
Annika Ahlström is gratefully acknowledged for HPLC analysis of caffeine and paraxanthine and Andreas Faldum for statistical assistance.
References
- 1.Landi MT, Sinha R, Lang NP, Kadlubar FF. Human cytochrome P4501A2. In: Ryder W, editor. Metabolic Polymorphisms and Susceptibility to Cancer. Lyon: IARC; 1999. pp. 173–195. Scientific Publications no. 148. [PubMed] [Google Scholar]
- 2.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci USA. 1989;86:7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock KW, Schrenk D, Forster A, et al. The influence of environmental and genetic factors on CYP2D6, CYP1A2 and UDP-glucuronosyltransferases in man using sparteine, caffeine, and paracetamol as probes. Pharmacogenetics. 1994;4:209–218. doi: 10.1097/00008571-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lampe JW, King IB, Li S, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21:1157–1162. [PubMed] [Google Scholar]
- 5.Rost KL, Roots I. Accelerated caffeine metabolism after omeprazole treatment is indicated by urinary metabolite ratios. Coincidence with plasma clearance and breath test. Clin Pharmacol Ther. 1994;55:402–411. doi: 10.1038/clpt.1994.49. [DOI] [PubMed] [Google Scholar]
- 6.Fuhr U, Anders EM, Mahr G, Sorgel F, Staib AH. Inhibitory potency of quinolone antibacterial agents against cytochrome P4501A2 activity in vivo and in vitro. Antimicrob Agents Chemother. 1992;36:942–948. doi: 10.1128/aac.36.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima M, Yokoi T, Mizutani M, Knoshita M, Funayama M, Kamataki T. Genetic polymorphism in the 5'-flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem. 1999;125:803–808. doi: 10.1093/oxfordjournals.jbchem.a022352. [DOI] [PubMed] [Google Scholar]
- 8.Sachse C, Brockmöller J, Bauer S, Roots I. Functional significance of a C→A polymorphism in intron I of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–449. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen BB, Brix TH, Kyvik KO, Brøsen K. The interindividual differencees in the 3-demethylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics. 2002;12:473–478. doi: 10.1097/00008571-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Kalow W, Tang BK. Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase activities. Clin Pharmacol Ther. 1991;50:508–519. doi: 10.1038/clpt.1991.176. [DOI] [PubMed] [Google Scholar]
- 11.Streetman DS, Bertino JS, jr Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Tantcheva-Poór I, Zaigler M, Rietbrock S, Fuhr U. Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999;9:131–144. [PubMed] [Google Scholar]
- 13.Facciolá G, Hidestrand M, von Bahr C, Tybring G. Cytochrome P450 isoforms involved in melatonin metabolism in human liver microsomes. Eur J Clin Pharmacol. 2001;56:881–888. doi: 10.1007/s002280000245. [DOI] [PubMed] [Google Scholar]
- 14.Härtter S, Ursing C, Morita S, et al. Orally given melatonin may serve as a probe drug for CYP1A2 activity in vivo. Clin Pharmacol Ther. 2001;70:10–16. doi: 10.1067/mcp.2001.116512. [DOI] [PubMed] [Google Scholar]
- 15.Christensen M, Andersson K, Dalen P, et al. The Karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther. 2003;73:517–528. doi: 10.1016/S0009-9236(03)00050-X. [DOI] [PubMed] [Google Scholar]
- 16.English J, Middleton BA, Arendt J, Wirz-Justice A. Rapid direct measurement of melatonin in saliva using an iodinated tracer and solid phase second antibody. Ann Clin Biochem. 1993;30:415–416. doi: 10.1177/000456329303000414. [DOI] [PubMed] [Google Scholar]
- 17.Nordmark A, Lundgren S, Ask B, Granath F, Rane A. The effect of CYP1A2*1F on CYP 1A2 inducibility in pregnant women. Br J Clin Pharmacol. 2002;54:504–510. doi: 10.1046/j.1365-2125.2002.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Härtter S, Grözinger M, Weigmann H, Röschke J, Hiemke C. Increased bioavailability of oral melatonin after fluvoxamine coadministration. Clin Pharmacol Ther. 2000;67:1–6. doi: 10.1067/mcp.2000.104071. [DOI] [PubMed] [Google Scholar]
- 19.Hameleers PAHM, van Boxtel MPJ, Hogervorst E, et al. Habitual caffeine consumption and its relation to memory, attention, planning capacity and psychomotor performance across multiple age groups. Hum Psychopharmacol Clin Exp. 2000;15:573–581. doi: 10.1002/hup.218. [DOI] [PubMed] [Google Scholar]


