Abstract
Human mast cells and basophils that express the high-affinity immunoglobulin E (IgE) receptor, Fcε receptor 1 (FcεRI), have key roles in allergic diseases. FcεRI cross-linking stimulates the release of allergic mediators1. Mast cells and basophils co-express FcγRIIb, a low affinity receptor containing an immunoreceptor tyrosine-based inhibitory motif and whose co-aggregation with FcεRI can block FcεRI-mediated reactivity2–4. Here we designed, expressed and tested the human basophil and mast-cell inhibitory function of a novel chimeric fusion protein, whose structure is γ Hinge-CHγ2-CHγ3-15aa linker-CHε2-CHε3-CHε4. This Fcγ–Fcε fusion protein was expressed as the predicted 140-kD dimer that reacted with anti-human ε- and γ-chain specific antibodies. Fcγ–Fcε bound to both human FcεRI and FcγRII. It also showed dose- and time-dependent inhibition of antigen-driven IgE-mediated histamine release from fresh human basophils sensitized with IgE directed against NIP (4-hydroxy-3-iodo-5-nitrophenylacetyl). This was associated with altered Syk signaling. The fusion protein also showed increased inhibition of human anti-NP (4-hydroxy-3-nitropheny-lacetyl) and anti-dansyl IgE-mediated passive cutaneous anaphylaxis in transgenic mice expressing human FcεRIα. Our results show that this chimeric protein is able to form complexes with both FcεRI and FcγRII, and inhibit mast-cell and basophil function. This approach, using a Fcγ–Fcε fusion protein to co-aggregate FcεRI with a receptor containing an immunoreceptor tyrosine-based inhibition motif, has therapeutic potential in IgE-and FcεRI-mediated diseases.
Allergen aggregation of IgE bound to FcεRI induces release of preformed mediators and synthesis of later acting leukotrienes and chemokines from mast cells and basophils5. This process has a major role in many of human diseases such as asthma, allergic rhinitis, chronic urticaria, angioedema and anaphylaxis. FcεRI aggregation induces release of preformed mediators and synthesis of later-acting leukotrienes, chemokines and cytokines5. The FcεRI is a heterotetramer consisting of a single IgE-binding α-subunit, a β-subunit and two disulfide-linked γ-subunits. The β and γ-subunit cytoplasmic tails each contain a conserved immunoreceptor tyrosine-based activation motif (ITAM). Cross-linking FcεRI via IgE bound to multivalent antigen activates tyrosine phosphorylation of ITAMs, thereby initiating downstream signaling6. Mast cells and basophils co-express FcγRIIb, which contains two extracellular immunoglobulin-like loops and a single conserved immunoreceptor tyrosine-based inhibition motif (ITIM) within its cytoplasmic tail7. FcγRIIb may co-aggregate with FcεRI under physiologic conditions. It is hypothesized that when FcγRIIb aggregates, it induces inhibitory signaling via SH2-domain-containing inositol 5-phosphatase (SHIP) and phosphorylation of FcγRIIb requires the co-aggregation with FcεRI (refs. 8,9). Aggregating FcγRII to FcεRI leads to the rapid tyrosine phosphorylation of the FcγRIIb ITIM tyrosine by FcεRI-associated Lyn and inhibition of FcεRI signaling10–13. To take advantage of this FcγRIIb-negative signaling in human basophils and mast cells, we constructed a single chimeric human bifunctional protein (GE2) engineered by fusing the human Fcγ1 and Fcε. Here we show that this chimeric protein is able to inhibit IgE-mediated in vitro activation of human basophils and mast cells and in vivo activation of human FcεRIα-bearing mast cells in FcεRIα transgenic mice. This approach using chimeric Fcγ proteins provides a platform for non-specific as well as future antigen-specific inhibition of IgE–FcεRI-mediated reactivity in a host of human diseases.
Human genomic DNA encoding the IgG γ1 constant region extends from the hinge through the CH3 domain. This sequence was initially cloned into a mammalian expression vector with a cytomegalovirus promoter and a murine immunoglobulin κ-chain leader sequence. Genomic DNA of the human ε heavy chain CH2 through CH4 domains was placed after the CHγ3. Between Fcγ1 and Fcε, we placed a 15 amino-acid linker (Gly4Ser)3, which has been used in single-chain Fv fragment expression14. This flexible peptide linker facilitates chain pairing and minimizes refolding and aggregation problems encountered when the two chains are expressed individually. The fusion protein contained the binding sites for FcεRI (CHε2–CHε3) and for FcγRII (CHγ2–CHγ3)15,16. The first constant region domains (CHγ1 or CHε1) were deleted as this domain associates with BiP (immunoglobulin heavy-chain binding protein) and a fusion protein containing these regions was not expressed17. SDS–PAGE demonstrated the Fcγ1–Fcε fusion protein was expressed as the predicted ~140-kD dimer. Western-blot analysis and ELISA testing demonstrated that the GE2 protein was recognized by antibodies specific for human ε and γ chains.
Binding of GE2 to both the human FcεRI and FcγRII was demonstrated using CHO3D10, a human FcεRIα-transfected cell line and HMC-1, which expresses human FcγRII but not FcεR (ref. 18). GE2 protein bound to both FcεRI and FcγRII in a fashion equivalent to human IgE and IgG, respectively, as assessed by flow cytometry (data not shown). These results demonstrate that expressed GE2 protein is properly folded so as to preserve bifunctional heterotypic FcR binding.
Fresh human basophils expressing both FcεRI and FcγRII can be passively sensitized with chimeric human IgE specific for NIP (4-hydroxy-3-iodo-5-nitrophenylacetyl) and then histamine release induced by cross-linking the anti-NIP IgE–FcεRI complex with NIP-BSA (ref. 19). We sensitized basophils with 1 μg/ml human anti-NIP IgE, plus doses of GE2 ranging from 0.01 to 10 μg/ml for 1 hour before activation with 50 ng/ml NIP-BSA (Fig. 1a). Myeloma IgE (PS myeloma) was used as a control. We found that 1 μg GE2 at 1 μg per ml inhibited almost half of histamine release, whereas at 10 μg/ml GE2 gave an average of 84% inhibition. 10 μg of non-specific PS IgE only decreased histamine release by 19%. Adding the GE2 at the same time as the IgE anti-NIP gave optimal inhibition. The longer the delay in GE2 addition following sensitization with IgE anti-NIP, the less the inhibition (Fig. 1b). These results show that the inhibition of antigen-driven histamine release induced by GE2 is dependent on time and dosage. We obtained similar results with cultured human mast cells; a dose of 1 μg caused 50% inhibition of IgE-mediated degranulation (data not shown). Cross-linking of GE2 by adding an anti-IgG antibody in vitro along with GE2 caused enhanced inhibition of IgE-mediated release (data not shown). In performing this experiment, we sought to mimic what may occur in vivo should subjects given GE2 make an antibody response against it. The actual fate of antibody bound GE2, should it occur in vivo, is not possible to predict.
Fig. 1.
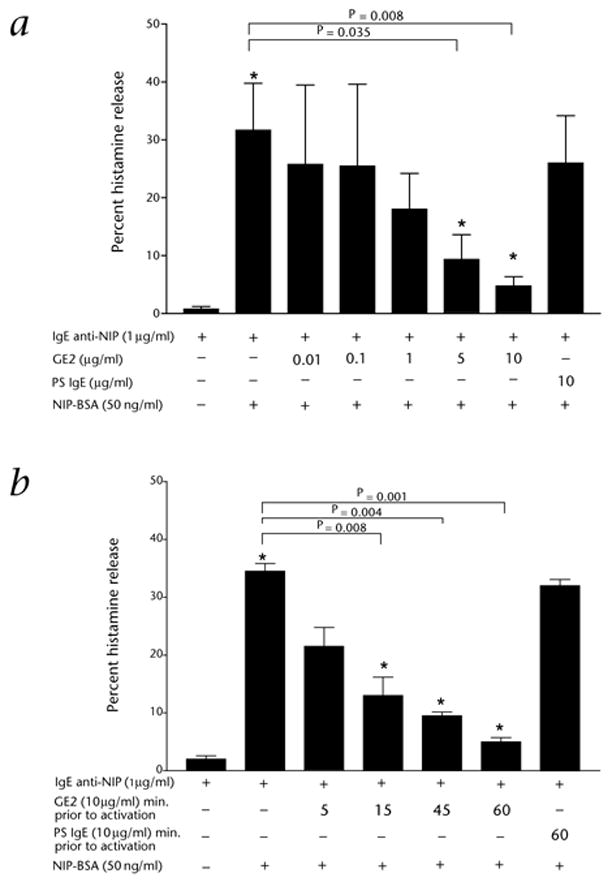
Dose- and time- dependent inhibition of basophil histamine release using GE2. a, Dose-dependence. Results are representative of 3 separate donors, each done in duplicate. b, Time-dependence. Results are representative of 2 separate donors, each done in duplicate. For both panels: *, significant differences in histamine release (P < 0.05), comparing the two indicated conditions. Total histamine in the donor basophils was 1.2 μg per 1 × 106 basophils.
Tyrosine phosphorylation of Syk is a critical step in human mast cell and basophil mediator release19. Cross-linking FcεRI on human basophils with IgE directed to NIP and NIP-BSA induces substantial tyrosine phosphorylation of Syk which was markedly reduced in cells pre-incubated with GE2 (Fig. 2). Thus, GE2 co-aggregation of FcεRI and FcγRII inhibits IgE-mediated Syk phosphorylation, which may contribute to the inhibition of histamine release.
Fig. 2.
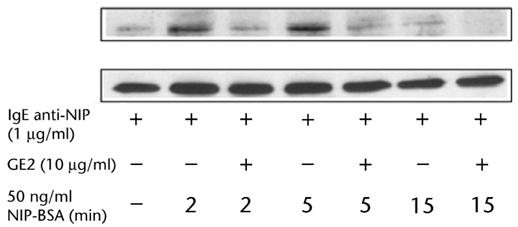
Co-aggregation of FcγRII and FcεR1 by GE2 inhibits FcεRI-mediated Syk phosphorylation. Immunoprecipitates were analyzed by western blotting with antibody against phosphotyrosine (top row), followed by anti-Syk antibody (bottom row). The top row represents phosphorylated Syk and the bottom row represents total Syk. Each lane shows Syk immunoprecipitated from 5 × 106 basophils. Results represent 2 separate experiments.
Transgenic mice altered to express the human FcεRIα and with the murine FcεRIα chain knocked out demonstrate allergic reactivity mediated by passively administered human IgE antibody along with antigen20,21. The human FcεRIα chain is coupled with the mouse β and γ chains to form a functional chimeric human/mouse FcεRI. This model takes advantage of the fact that murine mast cells also express FcγRIIb that can interact well with human IgG. This is in contrast to the lack of interaction between the murine FcεRI and human IgE. We used passive cutaneous anaphylaxis (PCA) to test the GE2 protein’s ability to block IgE-driven FcεRI-mediated mast-cell release. We intradermally primed transgenic mice with 250 ng of chimeric human anti-NP (4-hydroxy-3-nitrophenylacetyl) IgE and simultaneously injected individual sites with saline, GE2 or IgE myeloma protein. Four hours later, mice were given a systemic challenge with 0.5 mg NP-BSA plus 1% Evans Blue intravenously. The intensity of the PCA at each site was assessed by the size of the skin bluing after 30 min. The size and color intensity of the reaction at the sites of GE2 injection were decreased compared with sites injected with an equivalent amount of human IgE myeloma (Fig. 3). Similar results were obtained using genetically engineered human IgE anti-dansyl antibody and dansyl-BSA as an antigen. A total of 30 mice were tested and the GE2 was 2–4 times more potent than purified control human IgE in its ability to block PCA.
Fig. 3.
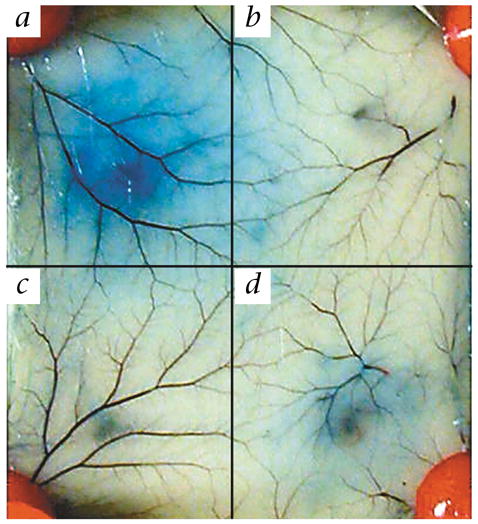
In vivo immunoglobulin Fcγ–Fcε fusion protein inhibits IgE-mediated degranulation in transgenic mice. a–d, Mice were injected intradermally with the following: a, anti-NP IgE; b, saline; c, anti-NP IgE and GE2; d, anti-NP IgE and IgE myeloma.
Our results demonstrate that a single chimeric molecular human protein consisting of Fcγ1 plus Fcε directly inhibits in vitro histamine release from human basophils and also inhibits humanized FcεRI-mediated mast-cell degranulation in transgenic mice. GE2 was more potent than control human IgE in all assays. Even if the GE2 protein did not have a 15 amino-acid linker between the two segments of ‘normal’ human Fcγ1 and Fcε, it would likely be recognized as foreign and induce an antibody response. With the 15 amino-acid linker, it is highly likely that antibody against GE2 would be expressed and will be primarily directed at this linker region. In fact, such an antibody response to GE2 may well prove advantageous as antibodies against GE2 will lead to increased cross-linking of Fcγ and Fcε receptors and should enhance inhibition of basophil and mast-cell function as we have observed in vitro using an anti-IgG antibody. This truncated Fcγ–Fcε fusion protein, GE2, provides a novel approach for the immunotherapy of diseases mediated via the FcεR such as allergic asthma, allergic rhinitis, chronic urticaria, angioedema and anaphylaxis. Replacement of the Fcε sequence in our construct with specific allergen genes will result in chimeric bifunctional proteins. We predict that these proteins will have the ability to inhibit mast-cell and basophil reactivity in an antigen-specific manner rather than in the global fashion of GE2. Such novel antigen-specific reagents may be useful in allergen immunotherapy.
Methods
Construction and expression
To construct the human Fcγ–Fcε chimeric gene, the human IgE Fc region (CH2-CH3-CH4) was amplified from pAG vector (provided by S.L. Morrison), containing the whole ε genomic DNA. The 5′ end primer was 5′-GCTCGAGGGTGGAGGCGGTTCAGGCGGAG-GTGGCTCTGGCGGTGGCGGATCGTTCACCCCGCCCACCGTGAAG-3′, containing a flexible linker sequence and a XhoI site. The 3′ end primer was 5′-GGCGGCCGCTCATTTACCGGGATTTACAGACAC-3′, containing a NotI site. After amplification, PCR products were cloned into pCR2.1 vector (Invitrogen, Carlsbad, California) and sequenced. Then the XhoI-NotI fragment was inserted into SalI-NotI site of pAN expression vector (from S.L. Morrison), containing the human genomic IgG γ1 constant region from hinge through the end of CH3. The IgE Fc region was placed downstream of the IgG γ1 constant region in frame with the CH3 and joined by a (Gly4Ser)3 flexible linker. The expression vector containing the immunoglobulin Fcγ–Fcε chimeric gene was transfected into SP2/0 cells. The Fcγ–Fcε fusion protein GE2 was expressed in cell-culture supernatants and purified by using an anti-human IgE affinity column.
Western-blot analysis
The purified GE2 protein was run on 7.5% SDS–PAGE and then transferred into Immobilon transfer membranes (Millipore, Bedford, Massachusetts). For protein detection, blots were probed with either goat anti-human IgE (ε chain-specific) or goat anti-human IgG (γ chain-specific) conjugated to alkaline phosphatase (KPL, Gaithersburg, Maryland). Color development was performed with an alkaline phosphatase conjugated substrate kit (BIO-RAD, Hercules, California).
Binding analysis
Human FcR binding of the purified GE2 protein was tested by binding to FcεRI-transfected CHO3D10 cells and HMC-1 cells expressing FcγRII and then analyzed by flow cytometry (FACSCalibur) using the FITC-conjugated anti-human IgE and anti-human IgG1 reagents (Biosource, Camarillo, California).
Histamine release
Acid-stripped Percoll-enriched human blood basophils were sensitized with chimeric human anti-NIP IgE (10 μg/ml) at 37 °C in a 5% CO2 incubator and 1 h later, challenged with 50 ng of NIP-BSA (ref. 19). Histamine release was measured in the supernatants 30 min later. GE2 or control human myeloma IgE was added at various doses and times to test the effects on histamine release.
Measurement of Syk phosphorylation
NIP-sensitized basophils were incubated with or without GE2 (10 μg/ml), washed and challenged for 2, 5 or 15 min with 50 ng/ml NIP-BSA. Treated or control NIP-IgE sensitized basophils were lysed in 400 μl of 50 mM Tris (pH 7.5), 150 mM NaCl, 1% Brij, 1 mM sodium orthovanadate and 1 μg/ml each of antipain, leupeptin, aprotinin and phenylmethanesulfonyl fluoride (PMSF) and incubated for 10 min on ice. Immune complexes were generated by incubating clarified supernatants with anti-Syk pre-adsorbed to protein A-Sepharose beads (Pharmacia, Uppsala, Sweden). Proteins were separated by SDS–PAGE, transferred to nitrocellulose and phosphoproteins identified by anti-phosphotyrosine immunoblotting using enhanced chemiluminescence detection reagents (ECL, Amersham, Piscataway, New York). After probing with anti-phosphotyrosine antibody, blots were stripped for 30 min with 100 mM 2-mercaptoethanol, 2% SDS (w:v), and 0.5 M Tris-HCl buffer (pH 6.8) at 50 °C, and then reprobed with anti-Syk antibody (1 μg/ml).
Passive cutaneous anaphylaxis
Transgenic mice expressing the human FcεRIα chain and with the murine FcεRIα chain knocked out20 were primed intradermally with 250 ng of NP-specific recombinant human IgE (ref. 21) in 50 μl saline. Individual sites were injected with saline, GE2 or IgE myeloma protein simultaneously. 4 h later mice were then given an intravenous challenge with 1.5 mg/ml of NP-BSA plus 1% Evans blue in 300 μl saline solution. Cutaneous anaphylaxis was assessed visually by the blue dye leakage from blood vessels into the skin.
Acknowledgments
We thank S.L. Morrison and R. Trinh for technical assistance and providing some experimental materials. We also thank Jean-Pierre Kinet for providing the transgenic mice expressing the human FcεRIα. We are grateful to M. Lipscomb and J. Oliver from the UNM Asthma SCOR programs. This study was supported by NIH grant (AI-15251) to A.S. C.L.K. was supported by an American Lung Association-funded UNM Asthma Research Center grant, an Interest Section grant from the AAAAI and a Fellowship from the Parker B. Francis Foundation.
Footnotes
Competing interests statement
The authors declare competing financial interests: see the website (http://medicine.nature.com) for details.
References
- 1.Ott VL, Cambier JC. Activating and inhibitory signaling in mast cells: New opportunities for therapeutic intervention? J Allergy Clin Immunol. 2000;106:429–440. doi: 10.1067/mai.2000.109428. [DOI] [PubMed] [Google Scholar]
- 2.Ravetch JV. Fc receptors. Curr Opin Immunol. 1997;9:121–125. doi: 10.1016/s0952-7915(97)80168-9. [DOI] [PubMed] [Google Scholar]
- 3.Daeron M, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of FcγRIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 4.Daeron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995;95:577–585. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver JM, Kepley CL, Ortega E, Wilson BS. Immunogically mediated signaling in basophils and mast cells: Finding therapeutic targets for allergic diseases in the human FcεRI signaling pathway. Immunopharmacology. 2000;48:269–281. doi: 10.1016/s0162-3109(00)00224-1. [DOI] [PubMed] [Google Scholar]
- 6.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 7.Daeron M. Building up the family of ITIM-bearing negative co-receptors. Immunol Lett. 1996;54:73–76. doi: 10.1016/s0165-2478(96)02652-1. [DOI] [PubMed] [Google Scholar]
- 8.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 9.Fong DC, et al. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated FcγR IIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol Lett. 1996;54:83–91. doi: 10.1016/s0165-2478(96)02654-5. [DOI] [PubMed] [Google Scholar]
- 10.Daeron M. Negative regulation of mast cell activation by receptors for IgG. Int Arch Allergy Immunol. 1997;113:138–141. doi: 10.1159/000237528. [DOI] [PubMed] [Google Scholar]
- 11.Malbec O, et al. Fcε Receptor I-associated lyn-dependent phosphorylation of Fcγ Receptor IIB during negative regulation of mast cell activation. J Immunol. 1998;160:1647–1658. [PubMed] [Google Scholar]
- 12.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 13.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in FcγR II-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 14.Huston JS, et al. Protein engineering of antibody binding site: Recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helm B, et al. The mast cell binding site on human immunoglobulin E. Nature. 1988;331:180–183. doi: 10.1038/331180a0. [DOI] [PubMed] [Google Scholar]
- 16.Hulett MD, Witort E, Brinkworth RI, Mckenzie IF, Hogarth PM. Identification of the IgG binding site of the human low affinity receptor for IgG Fc gamma RII. J Biol Chem. 1994;269:15287–15293. [PubMed] [Google Scholar]
- 17.Hendershot LM, Kearney JF. A role for human heavy binding protein in the developmental regulation of immunoglobulin transport. Mol Immunol. 1988;25:585–593. doi: 10.1016/0161-5890(88)90081-8. [DOI] [PubMed] [Google Scholar]
- 18.Wedi B, Lewrick H, Butterfield JH, Kapp A. Human HMC-1 mast cells exclusively express the FcγR II subtype of IgG receptor. Arch Dermatol Res. 1996;289:21–27. doi: 10.1007/s004030050147. [DOI] [PubMed] [Google Scholar]
- 19.Kepley CL, et al. Negative regulation of FcεRI signaling by FcγR II costimulation in human blood basophils. J Allergy Clin Immunol. 2000;106:337–348. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 20.Dombrowicz D, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- 21.Fung-Leung WP, et al. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor α chain respond to human IgE in mast cell degranulation and in allergic reactions. J Exp Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]


