Abstract
By using a Brome mosaic virus (BMV)-Saccharomyces cerevisiae system, we previously showed that the cellular Lsm1p-7p/Pat1p/Dhh1p decapping-activator complex functions in BMV RNA translation and replication. As a first approach in investigating whether the corresponding human homologues play a similar role, we expressed human Lsm1p (hLsm1p) and RCK/p54 in yeast. Expression of RCK/p54 but not hLsm1p restored the defect in BMV RNA translation and replication observed in the dhh1Δ and lsm1Δ strains, respectively. This functional conservation, together with the common replication strategies of positive-stranded RNA viruses, suggests that RCK/p54 may also play a role in the replication of positive-stranded RNA viruses that infect humans.
The group of positive-stranded RNA viruses includes major human pathogens such as hepatitis C virus and severe acute respiratory syndrome coronavirus. Since positive-stranded RNA viruses do not encapsidate the viral polymerase, upon entering the cell the viral RNA is first translated to produce viral replicases. Then, the viral RNA is recruited to membrane-associated replication complexes and used as a template for replication. This transition from translation to replication is a key step mediated by viral proteins as well as host factors (9, 15).
The replication of Brome mosaic virus (BMV) in the yeast Saccharomyces cerevisiae is a well-established model system for studying common steps of positive-stranded RNA virus biology in a relatively simple genetic background (12). BMV is a plant virus with a tripartite positive-stranded RNA genome (1). RNA1 and RNA2 encode the replicases 1a, a helicase, and 2a, the polymerase. In the absence of 2a, 1a recruits the BMV positive-stranded RNA templates out of translation and into the endoplasmic reticulum, the site of replication. This recruitment dramatically increases the stability and accumulation of BMV positive-stranded RNAs (2, 11, 22). Finally, RNA3 encodes the movement and the capsid proteins. In this system, translation effects can easily be measured by following the translation efficiency of BMV RNA2 via Western blotting with 2a-specific antibodies (17), while recruitment of the viral RNA out of translation for replication can be studied via 1a-dependent RNA3 accumulation in Northern blots (12, 15, 19, 20).
With this BMV-yeast model system, we have recently shown that the yeast Lsm1p-7p/Pat1p/Dhh1p decapping-activator complex, which functions in cellular mRNA degradation (6), is required for both translation and recruitment of BMV RNA to the replication complex (8, 15, 17). The Lsm1p-7p heptameric ring and the helicase Dhh1p belong to the highly conserved families of Sm/Sm-like proteins and DEAD-box helicase, respectively (13, 23). All the components of the Lsm1p-7p/Pat1p/Dhh1p complex localize in cytoplasmic foci called P bodies. These are dynamic structures and sites of mRNA degradation, mRNA storage, and translation control (3, 6, 7). Interestingly, the Lsm1p-7p/Pat1p/Dhh1p complex mediates the movement of cellular mRNAs out of translation into P bodies (6, 7). With the exception of Pat1p, the human homologues of Lsm1p-7p and Dhh1p, named hLsm1p-7p and RCK/p54, respectively, have been identified presenting functions similar to those of their yeast counterparts, including their localization in P bodies (3).
Since all positive-stranded RNA viruses must regulate the transfer of the genomic RNA from translation to replication, an ensuing question is whether the human homologues of the yeast Lsm1p-7p/Pat1p/Dhh1p complex play a similar role in the replication of human positive-stranded RNA viruses. As a first step in answering this question, we cloned and expressed hLsm1p and RCK/p54 in yeast cells in which the corresponding yeast gene had been deleted. From the hLsm1p-7p heptameric ring, we tested Lsm1p because it is the protein that determines the cellular function of the ring. In addition, we expressed Xp54, the Xenopus counterpart of Dhh1p, to test functional conservation through evolution.
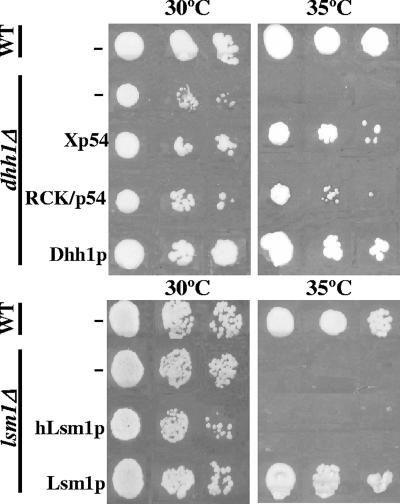
Although the LSM1 and DHH1 genes are not essential for yeast cell viability, the lsm1Δ and dhh1Δ strains have a temperature-sensitive (TS−) growth phenotype. Accordingly, we tested whether the expression of hLsm1p, Xp54, and RCK/p54 could complement the TS− phenotype of the respective deletion mutants. For this, we cloned the genes of interest into low (centromeric)- and high-copy-number (2μm) yeast plasmids and, under the different strengths promoters GAL1, TEF, and GPD (data not shown). As positive controls, we expressed the yeast LSM1 and DHH1 genes from centromeric plasmids under their native promoters in pLSM1 (8) and p414PDHH1, respectively. The wild-type (WT) strain YPH500 (12) and its derivatives, the dhh1Δ and lsm1Δ deletion mutants, were transformed with the corresponding expression plasmids, grown to mid-log phase, serially diluted, and spotted onto two plates, which were then incubated at 30°C or 35°C (Fig. 1). Confirming prior results for other yeast genetic backgrounds (4, 21), RCK/p54 and Xp54 also restored the capacity to grow at 35°C in the dhh1Δ strain. Since expression of Xp54 and RCK/p54 achieved with 2μm and the GPD promoter gave the highest level of TS− complementation, we chose these vectors for the experiments throughout. In contrast, expression of hLsm1p from any of the generated plasmids in the lsm1Δ strain had no effect on thermosensitivity (Fig. 1). A possible explanation for this lack of complementation is the low percentage of identity between yeast and human Lsm1p, which have only 42% identity and 68% similarity, whereas Dhh1p and Xp54 or RCK/p54 have 64% identity and 83% similarity.
FIG. 1.
Overexpression of Xp54 and RCK/p54 complements the temperature-sensitive phenotype of the yeast dhh1Δ strain. By contrast, expression of human Lsm1p in the lsm1Δ strain has no effect. Yeast cells were serially diluted and spotted onto two plates, which were then separately placed at 30°C and 35°C. −, empty plasmid; Xp54 and RCK/p54, 2μm GPD promoter-driven Xenopus Xp54 and human RCK/p54 expression plasmids; Dhh1p, centromeric plasmid expressing yeast Dhh1p from its native promoter; hLsm1p, 2μm GPD promoter-driven human Lsm1p expression plasmid; Lsm1p, centromeric plasmid expressing yeast Lsm1p from its native promoter.
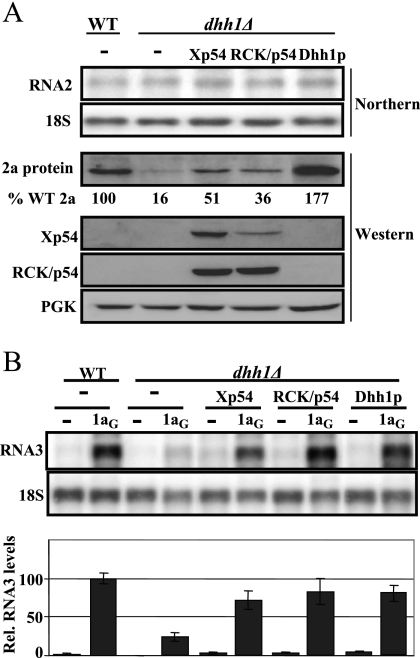
To investigate whether the complementation of Xp54 and RCK/p54 extends to BMV RNA translation, we tested whether these proteins could complement the defect in BMV RNA2 translation observed in the dhh1Δ strain. For this purpose, WT and dhh1Δ cells were transformed with plasmid pB2NR3, harboring BMV RNA2 (5) plus a vector expressing either RCK/p54, Xp54, Dhh1p, or the corresponding empty vector. Yeast growth and Northern- and Western-blot analysis to detect BMV RNA2 and 2a protein were performed as before (10, 15, 18). Expression of Xp54 and RCK/p54 was corroborated with Xp54- and RCK/p54-specific antibodies (16) (Medical and Biological Laboratories, Japan). As expected, in the dhh1Δ strain, we observed a strong inhibition of RNA2 translation, and the expression of the yeast DHH1 gene fully complemented the defect (Fig. 2A). Interestingly, when the RCK/p54 and Xp54 helicases were expressed, the levels of 2a protein increased two- and threefold, respectively. Since the levels of RNA2 were similar, this suggests a functional complementation at the level of translation. We made parallel experiments with the human Lsm1p protein. Similarly to the TS− test, there was no complementation (data not shown).
FIG. 2.
Xp54 and RCK/p54 functionally replace yeast Dhh1p in BMV RNA2 translation and RNA3 recruitment to the site of replication. (A) Detection of BMV RNA2 and 2a protein in WT and dhh1Δ yeast strains expressing Xp54, RCK/p54, or Dhh1p. Total RNA was extracted from yeast cells, and the accumulation of RNA2 was detected by Northern blotting, using a 32P-labeled RNA probe specific to positive-stranded BMV RNA2. A probe specific to 18S rRNA was used to control equal loading of total RNA. Total proteins were extracted from an equal number of yeast cells and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with 2a-specific monoclonal antibodies, Xp54- and RCK/p54-specific antiserum, and 3-phosphoglycerate kinase (PGK)-specific antiserum to control equal loading of total protein. The percentages of WT (% WT) 2a protein are the averages of three or more experiments. Note that antibodies raised against Xp54 cross-react with human RCK/p54 and antibodies raised against RCK/p54 cross-react with Xenopus Xp54. (B) Detection of 1a-dependent RNA3 recruitment in WT and dhh1Δ yeast strains expressing Xp54, RCK/p54, or Dhh1p. Total RNA was extracted from yeast cells, and accumulation of RNA3 was detected by Northern blotting, using a 32P-labeled RNA probe specific to positive-stranded BMV RNA3. Histograms show averages and standard errors of the means of the relative (Rel.) accumulations of RNA3 from three or more experiments. The average accumulation of RNA3 in WT yeast in the presence of 1a protein was set to 100. −, no expression of 1a protein; 1aG, GAL1 promoter-driven 1a expression plasmid; Xp54 and RCK/p54, 2μm GPD promoter-driven Xenopus Xp54 and human RCK/p54 expression plasmids; Dhh1p, centromeric plasmid expressing yeast Dhh1p from its native promoter.
Finally, to test whether Xp54 and RCK/p54 can complement the defect in 1a-dependent RNA3 recruitment to the replication complex observed in the dhh1Δ yeast strain, we expressed both genes together with the viral RNA3 in the presence and absence of the 1a protein. Positive-stranded RNA3 accumulation and 1a protein expression were analyzed by Northern and Western blotting as previously described (11, 15). Similar 1a protein expression levels in all strains were achieved as before by using the GAL1-driven RNA1 construct (15) (data not shown). According to prior results, in WT cells expression of 1a dramatically increases RNA3 accumulation, while this effect is inhibited in the dhh1Δ strain (Fig. 2B). Importantly, expression of both RCK/p54 and Xp54 largely replaced the function of yeast Dhh1p in recruitment. In fact, the magnitude of complementation was similar to that achieved by expression from a plasmid of the yeast DHH1 gene with 72%, 82%, and 80% RNA3 accumulation levels for dhh1Δ yeast expressing Xp54, RCK/p54, and yeast Dhh1p, respectively.
The high level of homology between the yeast and human genes often allows functional complementation between the yeast gene and its human homologue. This so-called humanized yeast system has already been proven to be valuable for studying basic cellular processes (14). Here we extend this system to study human proteins during viral replication. Our results indicate that there is a link between the ability of the human homologue to restore cellular function and the ability to complement BMV RNA translation and replication. As positive-stranded RNA viruses have a common replication strategy, it is tempting to suggest an important function of RCK/p54 in positive-stranded human viruses.
Acknowledgments
We thank Y. Akao, T. H. Chang, R. Lührmann, R. Lill, P. Burgers, and F. Posas for plasmids pIRES-RCK, pDHH1017, YFP-hLsm1, pRS424GPD, pRS424GAL, and pRS416TEF. We also thank N. Standart for anti-Xp54s and A. Meyerhans and M. Giménez-Barcons for critical readings of the manuscript.
This work was supported by a grant from the Spanish Ministerio de Educación y Ciencia (BFU2004-00654). I.A.-R. was supported by Fundação para a Ciência e Tecnología (SARH/BD/9630/2002), Portugal.
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Ahlquist, P. 1992. Bromovirus RNA replication and transcription. Curr. Opin. Genet. Dev. 2:71-76. [DOI] [PubMed] [Google Scholar]
- 2.Ahola, T., J. A. den Boon, and P. Ahlquist. 2000. Helicase and capping enzyme active site mutations in brome mosaic virus protein 1a cause defects in template recruitment, negative-strand RNA synthesis, and viral RNA capping. J. Virol. 74:8803-8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, P., and N. Kedersha. 2006. RNA granules. J. Cell Biol. 172:803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergkessel, M., and J. C. Reese. 2004. An essential role for the Saccharomyces cerevisiae DEAD-box helicase DHH1 in G1/S DNA-damage checkpoint recovery. Genetics 167:21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., A. Noueiry, and P. Ahlquist. 2001. Brome mosaic virus protein 1a recruits viral RNA2 to RNA replication through a 5′ proximal RNA2 signal. J. Virol. 75:3207-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coller, J., and R. Parker. 2004. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 73:861-890. [DOI] [PubMed] [Google Scholar]
- 7.Coller, J., and R. Parker. 2005. General translational repression by activators of mRNA decapping. Cell 122:875-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diez, J., M. Ishikawa, M. Kaido, and P. Ahlquist. 2000. Identification and characterization of a host protein required for efficient template selection in viral RNA replication. Proc. Natl. Acad. Sci. USA 97:3913-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamarnik, A. V., and R. Andino. 1998. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 12:2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa, M., M. Janda, M. A. Krol, and P. Ahlquist. 1997. In vivo DNA expression of functional brome mosaic virus RNA replicons in Saccharomyces cerevisiae. J. Virol. 71:7781-7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janda, M., and P. Ahlquist. 1998. Brome mosaic virus RNA replication protein 1a dramatically increases in vivo stability but not translation of viral genomic RNA3. Proc. Natl. Acad. Sci. USA 95:2227-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janda, M., and P. Ahlquist. 1993. RNA-dependent replication, transcription, and persistence of brome mosaic virus RNA replicons in S. cerevisiae. Cell 72:961-970. [DOI] [PubMed] [Google Scholar]
- 13.Khusial, P., R. Plaag, and G. W. Zieve. 2005. LSm proteins form heptameric rings that bind to RNA via repeating motifs. Trends Biochem. Sci. 30:522-528. [DOI] [PubMed] [Google Scholar]
- 14.Mager, W. H., and J. Winderickx. 2005. Yeast as a model for medical and medicinal research. Trends Pharmacol. Sci. 26:265-273. [DOI] [PubMed] [Google Scholar]
- 15.Mas, A., I. Alves-Rodrigues, A. Noueiry, P. Ahlquist, and J. Diez. 2006. Host deadenylation-dependent mRNA decapping factors are required for a key step in brome mosaic virus RNA replication. J. Virol. 80:246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minshall, N., and N. Standart. 2004. The active form of Xp54 RNA helicase in translational repression is an RNA-mediated oligomer. Nucleic Acids Res. 32:1325-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noueiry, A. O., J. Diez, S. P. Falk, J. Chen, and P. Ahlquist. 2003. Yeast Lsm1p-7p/Pat1p deadenylation-dependent mRNA-decapping factors are required for brome mosaic virus genomic RNA translation. Mol. Cell. Biol. 23:4094-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Restrepo-Hartwig, M. A., and P. Ahlquist. 1996. Brome mosaic virus helicase- and polymerase-like proteins colocalize on the endoplasmic reticulum at sites of viral RNA synthesis. J. Virol. 70:8908-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz, M., J. Chen, M. Janda, M. Sullivan, J. den Boon, and P. Ahlquist. 2002. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell 9:505-514. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan, M. L., and P. Ahlquist. 1999. A brome mosaic virus intergenic RNA3 replication signal functions with viral replication protein 1a to dramatically stabilize RNA in vivo. J. Virol. 73:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng-Rogenski, S. S., J. L. Chong, C. B. Thomas, S. Enomoto, J. Berman, and T. H. Chang. 2003. Functional conservation of Dhh1p, a cytoplasmic DExD/H-box protein present in large complexes. Nucleic Acids Res. 31:4995-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, X., W. M. Lee, T. Watanabe, M. Schwartz, M. Janda, and P. Ahlquist. 2005. Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates. J. Virol. 79:13747-13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weston, A., and J. Sommerville. 2006. Xp54 and related (DDX6-like) RNA helicases: roles in messenger RNP assembly, translation regulation and RNA degradation. Nucleic Acids Res. 34:3082-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]