Abstract
The goal of this study was to develop a small-animal model to study human immunodeficiency virus type 1 (HIV-1) pathogenesis in blood and primary and secondary lymphoid organs. Rag2−/−γc−/− mice that are neonatally injected with human CD34+ cells develop a functional human immune system (HIS), with human hematopoietic cells being found in the thymuses, peripheral blood, spleens, and bone marrow of the animals (hereafter these animals are referred to as HIS-Rag2−/−γc−/− mice). HIS-Rag2−/−γc−/− mice were infected with small amounts of CCR5-tropic HIV-1. Viral replication and immunophenotypic changes in the human cells in peripheral blood and lymphoid organs were examined. The productive infection of human cells in peripheral blood, thymus and spleen tissue, and bone marrow was detected. Ratios of CD4+ T cells to CD8+ T cells in the infected animals declined. Although no specific anti-HIV-1 immune responses were detected, immunoglobulin M (IgM) and IgG antibodies to an unidentified fetal calf serum protein present in the virus preparation were found in the inoculated animals. Thus, we have shown that the HIS-Rag2−/−γc−/− mouse model can be used for infection with low doses of CCR5-tropic HIV-1, which is most commonly transmitted during primary infections. HIS-Rag2−/−γc−/− mice can serve as a small-animal model for investigating HIV-1 pathogenesis and testing potential HIV-1 therapies, and studies with this model may replace some long and costly studies with nonhuman primates.
Presently, the best animal models for human immunodeficiency virus type 1 (HIV-1) infection are arguably the nonhuman primate models for simian immunodeficiency virus and chimeric simian-human immunodeficiency virus infections (7, 8, 17). Simian immunodeficiency virus infection in rhesus macaques (Macaca mulatta) results in AIDS-like pathology and viral pathogenesis resembling that which occurs in humans. Vaccines and therapies against HIV can be investigated using this model (28). However, there are still drawbacks. Rhesus macaques are scarce, experiments are expensive, and macaques cannot be infected with HIV-1. A small-animal model to investigate HIV-1 pathogenesis would be ideal. Efforts to create transgenic mice bearing genomic or subgenomic HIV-1 and/or human cellular genes required for HIV-1 infection have generally not led to sufficiently robust HIV-1 replication (3, 6, 25). Chimeric mouse-human models such as the SCID-hu thy/liv and hu-PBL-SCID models have been successfully employed to study some aspects of HIV-1 infection (1, 5, 20), but the human lymphoid systems that develop consist of either only immature thymic progenitors (SCID-hu thy/liv) or primarily xenoreactive T cells (hu-PBL-SCID) (18, 21). Recently, we and others have described new chimeric mouse models wherein a human hematopoietic system is reconstituted following the injection of human CD34+ hematopoietic progenitor cells into neonatal Rag2−/−γc−/− (9, 10, 27) or NOD/SCID/γc−/− (14, 29) mice. Interestingly, these animals generate a more complete human immune system (HIS) consisting of mature and immature T cells (including regulatory T cells), B cells, natural killer (NK) cells, dendritic cells, macrophages, granulocytes, and erythrocytes in lymphoid organs and in the peripheral circulation than animals in previous chimeric mouse models (16). Importantly, immune responses to foreign antigens have been reported previously, suggesting at least a partially functional adaptive HIS (10, 14, 27).
In this study, we demonstrate that mice with reconstituted human hematopoietic systems as described above (hereafter referred to as HIS-Rag2−/−γc−/− mice) can be infected with low doses of CCR5-tropic (R5) HIV-1. HIV-1 can be detected in multiple lymphoid tissues, including the thymus.
MATERIALS AND METHODS
Generation of HIS-Rag2−/−γc−/− mice.
H-2d Rag2−/− mice were originally obtained from Anton Rolink and Shunichi Takeda (Basel Institute for Immunology, Basel, Switzerland) and were crossed with mice with a knockout of the interleukin-2 receptor gamma chain (γc−/− mice) to obtain H-2d Rag2−/−γc−/− mice (15). These double-knockout mice have no functional T and B cells and no NK cells, and in contrast to NOD/SCID mice they do not develop thymus sarcomas. Rag2−/−γc−/− mice were originally bred at The Netherlands Cancer Institute and transferred to the University of California-Los Angeles for the HIV-1 studies. Mice were maintained in isolators and fed autoclaved food and water, and all manipulations were done under laminar flow.
As described previously, HIS-Rag2−/−γc−/− mice were generated by injecting CD34+ cells isolated from human fetal liver tissue into the mice in the first week of life, when the conditions for the engraftment, expansion, and reconstitution of a human immune system are optimal (9). We and others have shown that the CD34+ cells develop into T lymphocytes on the mouse thymus stromal remnant that becomes a thymic organ and gives rise to human T cells (αβ and γδ, as well as regulatory T cells) (9, 16, 27). In addition, B cells, NK cells, monocytes, plasmacytoid dendritic cells, granulocytes, and low but consistent percentages of erythrocytes can be found in the peripheral blood and organs of the Rag2−/−γc−/− mice (9, 27). At 1 to 3 days after birth, mice were irradiated (3.5 Gy) once and received human CD34+ cells (5 × 105) through intraperitoneal injection. Peripheral blood was taken biweekly from the HIS-Rag2−/−γc−/− mice starting 4 weeks after injection to determine the presence of human cells.
HIV-1 infection of HIS-Rag2−/−γc−/− mice.
CCR5-tropic molecular clone HIV-1NFN-SX(SL9) stocks were prepared as previously described (24). The CCR5 tropism of the virus stocks was confirmed with CCR5+CD4+ GHOST cells (National Institutes of Health AIDS Research and Reference Program) by using green fluorescent protein expression with flow cytometry and cytopathicity, as described by Morner et al. (19). No signs of infection with HIV-1NFN-SX(SL9) in parental or CXCR4+CD4+ GHOST cells were observed. All infections were standardized by determining 50% tissue culture infective doses (TCID50) (24). HIS-Rag2−/−γc−/− mice were infected by intraperitoneal injection with small amounts of HIV-1 (500 or 5,000 TCID50 of HIV-1) at 14 to 36 weeks of age (ages were as follows: experiment 1, 14 weeks; experiment 2, 14 weeks; experiment 3, 17 weeks; and experiment 4, 36 weeks). The 500-TCID50 viral inoculum contained 375 pg of p24, and the 5,000-TCID50 viral inoculum contained 3,750 pg of p24. The volume of each viral inoculum was 250 μl and contained 2% fetal bovine serum. Controls consisted of mock-infected mice or mice infected with heat-inactivated HIV-1. Peripheral blood, thymuses, spleens, and bone marrow were taken from the HIV-1-infected and control HIS-Rag2−/−γc−/− mice for immunophenotyping of the human cells and coculturing with phytohemagglutinin- and interleukin-2-activated CD8-depleted human peripheral blood mononuclear cells (PBMC; 5 ×105 cells) for 10 days to detect HIV-1. Virus replication was assessed by measuring the level of the p24 antigen in the supernatants of the cocultures by using a specific p24 antigen enzyme-linked immunosorbent assay (ELISA; Coulter, Hialeah, FL).
Surface and intracellular immunophenotyping.
Surface immunophenotyping of human thymocytes and peripheral lymphoid cells with directly conjugated antibodies was performed as previously described (26). Fluorescent conjugated monoclonal antibodies to CD3, CD4, CD8, CD19, and CD45 and isotype control antibodies of mouse immunoglobulin G1 (IgG1) and mouse IgG2 conjugated with fluorescein isothiocyanate, phycoerythrin, and/or allophycocyanin were obtained from Becton Dickinson Immunocytometry Systems (BDIS; San Jose, CA). Monoclonal antibodies to CCR5 (clone 2D7) and CXCR4 (clone 12G5) conjugated to fluorescein isothiocyanate, phycoerythrin, or allophycocyanin were obtained from BD-Pharmingen (La Jolla, CA). Cells were acquired on a dual-laser FACSCalibur flow cytometer (BDIS, San Jose, CA). Multiparameter data acquisition and analysis were performed with Cell Quest software (BDIS).
ELISA and Western blotting.
Sera obtained from mice injected with human fetal liver CD34+ cells were tested for the presence of total human IgM and IgG starting at 14 to 16 weeks after injection by using a standard ELISA (Alerchek). Sera from uninfected and HIV-1-infected mice were also examined for HIV-specific IgM and IgG at 6, 9, and 11 weeks postinfection by Western blotting. Supernatant from 293T cells [mock-transfected or transfected with HIVNFN-SX(SL9)] was harvested at 48 h posttransfection and filtered through a 0.22-μm-pore-size filter. Equivalent volumes of samples (5 μl) were mixed with 2× Laemmli buffer containing 100 mM dithiothreitol and separated on an 8 to 16% Tris-glycine gel (Cambrex). Sera from mock-infected or HIV-1-infected mice (1:200 dilution) were used for Western blotting, followed by secondary horseradish peroxidase-conjugated goat anti-human IgG or IgM antibody (Sigma), and blots were developed with the enhanced chemiluminescence assay (Amersham, Arlington Heights, IL).
ELISPOT assays.
Enzyme-linked immunospot (ELISPOT) assays were performed as described by Helms et al. (13). In brief, immunospot plates (96 wells; Cellular Technology, Cleveland, OH) were coated overnight at 4°C with capture antibodies dissolved in phosphate-buffered saline (PBS) that were specific for gamma interferon. The wells were blocked with PBS-bovine serum albumin for 1 h and washed three times with PBS. Lymphocytes were plated in medium at 2 × 105 to 3 × 105 cells per well, and stimulant CD2/CD2R plus CD28 (BDIS) or HIV peptides (National Institutes of Health AIDS Research and Reference Reagent Program) were added to duplicate wells. After 24 h, the plates were washed and biotinylated detection antibodies were added for 12 h at 4°C, followed by streptavidin-horseradish peroxidase in PBS-bovine serum albumin-Tween for 2 h at room temperature. The spots were developed using AEC (3-amino-9-ethyl-carbazole) solution. Quantitative analysis of the ELISPOT assay results by using computerized image analysis to allow background subtraction and artifact exclusion was performed with the immunospot image analyzer (Cellular Technology). A positive response was defined as an increase of twofold or more above the background.
Statistics.
The nonparametric Mann-Whitney test was used to statistically analyze differences in the ratios of CD4+ T cells to CD8+ T cells in HIS- Rag2−/−γc−/− mice that were productively infected and the ratios of these cells in uninfected and nonproductively infected mice.
RESULTS
Reconstitution of human hematopoietic system in neonatal Rag2−/−γc−/− mice.
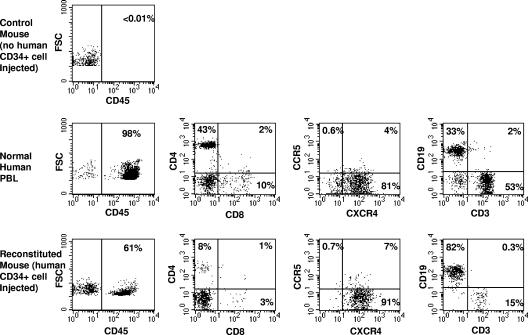
As we and others previously reported (9, 27), the injection of human CD34+ cells into neonatal Rag2−/−γc−/− mice leads to the reconstitution of a human hematopoietic system. The percentages of CD45+ cells (including CD19+ B cells and CD4+ and CD8+ T cells) in our present study performed at the University of California-Los Angeles ranged from 9.5 to 74.2% (average ± standard deviation, 48.5% ± 23%) at 8 to 32 weeks after injection with human CD34+ cells. Figure 1 shows results from a representative experiment comparing the immunophenotype of CD45+ cells in the peripheral blood of HIS-Rag2−/−γc−/− mice with that of CD45+ cells among normal human PBMC. The percentages of human cells expressing HIV-1 coreceptors CCR5 and CXCR4 were similar to those in normal human peripheral blood (Fig. 1). Ratios of CD4+ T cells to CD8+ T cells in the peripheral blood of the HIS-Rag2−/−γc−/− mice increased or were relatively stable over a 2- to 10-week time period after reconstitution was observed. Human lymphoid cells were found in the thymuses, spleens, and bone marrow of the mice, similar to the results in our previous report (9). These data indicate that these Rag2−/−γc−/− mice bred at different institutions show similar patterns of reconstitution after injection with human CD34+ fetal liver cells.
FIG. 1.
Reconstitution of human lymphocytes in the Rag2−/−γc−/− mouse. At 22 weeks postinjection with human CD34+ cells, peripheral blood was collected and cells were stained for human CD45, CD4, CD8, CCR5, CXCR4, CD19, and CD3 lymphoid cell surface markers, as indicated, and analyzed by flow cytometry. The control mouse (top panel) was an animal that did not receive human CD34+ cells, and as a result, no human CD45+ cells, including human T and B cells, could be detected in this mouse. Normal human blood was analyzed in parallel for comparison (middle panels). The injection of human CD34+ cells into a neonatal Rag2−/−γc−/− mouse reconstitutes a lymphocyte subset in the peripheral blood with a profile similar to that of human peripheral blood lymphocytes (PBL) when gated on human CD45+ cells (bottom panels). FSC, forward scatter.
As we detected cells that expressed the B-cell marker CD19, we examined human immunoglobulin production in animals with reconstituted systems by measuring the total human IgG and IgM levels in the mouse sera by using a sandwich ELISA. The total human IgG levels ranged from 10 to 980 μg/ml in the mice with reconstituted systems, and the levels of total human IgM ranged from 5 to 116 μg/ml (data not shown). Thus, our mice with reconstituted HIS were capable of producing human IgG and IgM.
HIS-Rag2−/−γc−/− mice can be productively infected with small amounts of R5 HIV-1.
The infection of the HIS-Rag2−/−γc−/− mice with 5,000 TCID50 of R5 HIV-1NFN-SX(SL9) (24) resulted in productive infection in three of three animals as determined by coculturing (Table 1). HIV-1 replication was detected in peripheral blood, thymuses, and spleens. Bone marrow, tested from two animals, was also positive for HIV-1. HIV-1 infection was independent of the level of reconstitution of human CD45+ cells in the peripheral blood of the HIS mice immediately before HIV-1 infection. HIS-Rag2−/−γc−/− mice with proportions of CD45+ cells in the peripheral blood of less than or more than 50% were productively infected (Table 1). Likewise, the percentages of human CD4+ T cells in the peripheral blood before HIV-1 infection did not predict a productive HIV-1 infection (data not shown).
TABLE 1.
Productive infection in R5 HIV-1-infected HIS micea
| Expt and mouse | % of CD45 cells | HIV dose (TCID50) | Amt of p24 (ng/ml) in supernatant from coculture of cells from:
|
|||
|---|---|---|---|---|---|---|
| Blood | Spleen | Thymus | Bone marrow | |||
| Expt 1 | ||||||
| Mouse 1 | ND | None | <0.05 | <0.05 | <0.05 | ND |
| Mouse 2 | ND | 5,000 | <0.05 | 12 | 24 | ND |
| Expt 2 | ||||||
| Mouse 3 | 10 | None | <0.05 | <0.05 | <0.05 | ND |
| Mouse 4 | 53 | HI HIV | <0.05 | <0.05 | <0.05 | ND |
| Mouse 5 | 50 | 500 | <0.05 | <0.05 | <0.05 | ND |
| Mouse 6 | 55 | 500 | 393 | 529 | 464 | ND |
| Expt 3 | ||||||
| Mouse 7 | 34 | None | <0.05 | <0.05 | <0.05 | <0.05 |
| Mouse 8 | 19 | 500 | <0.05 | <0.05 | <0.05 | <0.05 |
| Mouse 9 | 74 | 500 | <0.05 | <0.05 | <0.05 | <0.05 |
| Mouse 10 | 57 | 500 | 0.78 | 291 | 24 | 263 |
| Expt 4 | ||||||
| Mouse 11 | 63 | 5,000 | 0.118 | 166 | 150 | 315 |
| Mouse 12 | 37 | 5,000 | 236 | 358 | 140 | 361 |
Cells obtained from peripheral blood, bone marrow, thymuses, and spleens of mock-infected and HIV-1-infected mice at 4 to 11 weeks postinfection (expt 1, 4 weeks; expt 2, 8 weeks; expt 3, 11 weeks; and expt 4, 5 weeks) were cocultured with activated CD8-depleted PBMC for 10 days in vitro to detect HIV-1. Virus replication as assessed by measuring the levels of the p24 antigen in the supernatants of the cocultures is indicated. The percentages of CD45+ cells 1 week before infection are shown. ND, not determined; HI HIV, heat-inactivated HIV-1.
To determine whether even a 10-fold-smaller amount of HIV-1 could be used for the infection of HIS-Rag2−/−γc−/− mice, the mice were infected with 500 TCID50 of R5 HIV-1NFN-SX(SL9). Two out of five mice were found to be productively infected with HIV-1. As observed in the mice infected with the 10-fold-larger amount of HIV-1, peripheral blood and all lymphoid organs in productively infected mice were positive for HIV-1 by coculture (Table 1). The virus was undetectable in the mouse sera.
HIV-1-infected HIS-Rag2−/−γc−/− mice show a decrease in the ratios of CD4+ T cells to CD8+ T cells.
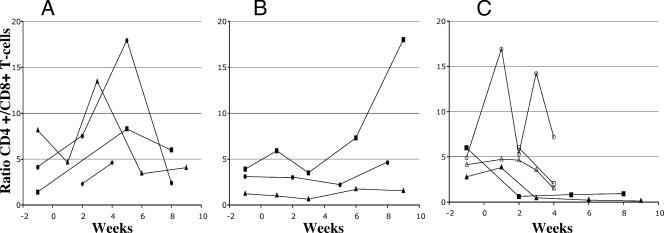
Immunophenotyping of peripheral blood and lymphoid organs of the HIV-1-infected and uninfected mice was done to determine the extent of CD4+-T-cell depletion among the human CD45+ cells. Four out of five productively infected mice showed a decline in ratios of CD4+ T cells to CD8+ T cells in the peripheral blood 2 to 11 weeks postinfection (Fig. 2; Table 2). In contrast, the CD4+-T-cell/CD8+-T-cell ratios in uninfected mice or mice injected with heat-inactivated virus did not decrease. There was also no decline in CD4+-T-cell/CD8+-T-cell ratios in HIV-1-inoculated mice that did not show productive infection (Fig. 2; Table 2). However, at weeks 4 to 6 postinfection, there was a statistically significant difference in the CD4+-T-cell/CD8+-T-cell ratios in mice that were productively infected with HIV-1 and the ratios in uninfected and nonproductively infected mice (P = 0.042). Although the patterns of change in CD4+-T-cell levels were not so consistent among the mice, the decline in CD4+-T-cell/CD8+-T-cell ratios was likely due to CD4+-T-cell depletion.
FIG. 2.
Kinetics of ratios of CD4+ T cells to CD8+ T cells in uninfected and HIV-1-infected mice. Mice were mock injected or injected with heat-inactivated (HI) or 500 or 5,000 TCID50 of HIV-1NFN-SX(SL9). At various time points pre- or postinjection, as indicated, the percentages of CD4+ and CD8+ T cells among human CD45+ lymphocytes were obtained by flow cytometric analysis and the ratio of CD4+ T cells to CD8+ T cells was calculated. Results from four independent experiments are shown. (A) Animals inoculated with heat-inactivated HIV-1 (black circles) and uninfected animals (other symbols). (B) HIV-1-inoculated, nonproductively infected (500 TCID50) animals. (C) HIV-1-inoculated, productively infected animals. Closed symbols, 500 TCID50; open symbols, 5,000 TCID50.
TABLE 2.
Kinetics of CD4+-T-cell/CD8+-T-cell ratios in uninfected and R5 HIV-1-infected micea
| Expt and mouse | HIV dose (TCID50) | HIV status | Ratio of CD4+ T cells/CD8+ T cells at wk:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 1 | 2 | 3 | 4 | 5 | 6 | 8 | 9 | 11 | |||
| Expt 1 | ||||||||||||
| Mouse 1 | None | − | ND | 2.3 | 4.6 | |||||||
| Mouse 2 | 5,000 | + | ND | 6.0 | 2.0 | |||||||
| Expt 2 | ||||||||||||
| Mouse 3 | None | − | 1.4 | ND | 8.3 | 6.0 | ||||||
| Mouse 4 | HI HIV | − | 4.1 | 7.5 | 17.9 | 2.6 | ||||||
| Mouse 5 | 500 | − | 3.1 | 3.0 | 2.2 | 4.6 | ||||||
| Mouse 6 | 500 | + | 6.0 | 0.6 | 0.8 | 0.9 | ||||||
| Expt 3 | ||||||||||||
| Mouse 7 | None | − | 8.2 | 4.7 | 13.5 | 3.5 | 4.1 | 3.0 | ||||
| Mouse 8 | 500 | − | 3.9 | 5.9 | 3.5 | 7.3 | 18.0 | 6.0 | ||||
| Mouse 9 | 500 | − | 1.3 | 1.1 | 0.7 | 1.8 | 1.6 | 1.5 | ||||
| Mouse 10 | 500 | + | 2.8 | 3.9 | 0.5 | 0.3 | 0.2 | 0.6 | ||||
| Expt 4 | ||||||||||||
| Mouse 11 | 5,000 | + | 4.2 | 4.8 | 4.7 | 3.6 | 1.6 | |||||
| Mouse 12 | 5,000 | + | 4.9 | 16.9 | 5.3 | 14.2 | 7.2 | |||||
Mice were uninfected or inoculated with heat-inactivated HIV-1 (HI HIV) or 500 or 5,000 TCID50 of HIV-1NFN-SX(SL9) as indicated. At various time points pre- (wk −1) or postinjection, as indicated, the percentages of CD4+ and CD8+ T cells among human CD45+ lymphocytes were obtained by flow cytometric analysis and the ratio of CD4+ T cells to CD8+ T cells was calculated. Results from fourindependent experiments are shown. −, coculture negative for HIV; +, coculture positive for HIV; ND, not determined.
Immune responses in HIS-Rag2−/−γc−/− mice.
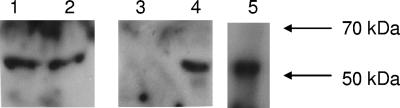
Immune responses to foreign antigens in this mouse model have been reported previously (27). We examined mice from experiments 2 and 3 for antigen-specific immune responses (mice from experiments 1 and 4 were not tested for antibody responses). We did not observe antibody responses specific to HIV-1, as assayed by Western blotting, either to viral proteins prepared from virions or to recombinant pr55 Gag. However, in four out of four HIV-1-inoculated animals that were not productively infected (mice 4, 5, 8, and 9), we observed an antibody response (IgM and IgG) directed to an unidentified 51-kDa antigen of fetal bovine serum present in virus preparations (representative data from mouse 5 are shown in Fig. 3). The antibody response was detectable starting at week 6 postinfection and continued until week 11 postinfection, when the mice were sacrificed. Antibody responses to the fetal bovine serum antigen were not detected in two out of two mice that were productively infected (mice 6 and 10) or in uninfected mice (mice 1, 3, and 7). It is noteworthy that antibody responses to the fetal bovine serum antigen were detected only in those inoculated animals in which T-cell depletion and productive viral infection were not observed, suggesting that active viral replication leading to the depletion of the lymphoid system inhibits the formation of functional immune responses.
FIG. 3.
Western blot analysis using serum from an HIV-1-inoculated HIS-Rag2−/−γc−/− mouse (mouse 5 in Tables 1 and 2) for the assessment of reactive proteins present in media from mock-transfected 293T cells (lane 1) and HIV-1NFN-SX-transfected 293T cells (lane 2), Iscove's medium alone (lane 3), and 10% fetal bovine serum (lanes 4 and 5). Molecular size standards are indicated on the right. Goat anti-human IgM was used as a secondary antibody for lanes 1 to 4, and goat anti-human IgG was used as a secondary antibody for lane 5. Western blotting of sera from mice productively infected with HIV-1 or uninfected mice did not show an antibody response to fetal bovine serum proteins.
Cellular T-cell responses were measured using gamma interferon ELISPOT assays. No cellular T-cell responses to HIV-1 Gag and Nef peptides were detected by gamma interferon ELISPOT assays (data not shown).
DISCUSSION
The reconstitution of immune systems in several immunodeficient-mouse models (16), including the Rag2−/−γc−/− (9, 27) and NOD/SCID/IL-2rγ-null (14, 29) mouse models with human CD34+ cells from fetal liver tissue or cord blood has been reported previously by us and other investigators. After the injection of human CD34+ fetal liver cells into neonatal Rag2−/−γc−/− mice, we detected human lymphocytes, including T and B cells, in murine thymuses, spleens, bone marrow, and peripheral blood, as we and others have described previously (9, 27). In addition, in our present experiments, we detected human IgM as well as IgG in the peripheral blood of the mice with reconstituted systems (IgM, 5 to 116 μg/ml; IgG, 10 to 980 μg/ml) at levels comparable to those reported by Traggiai et al. (27).
As R5 HIV-1 isolates are the most commonly replicating viruses during primary infection, we infected HIS- Rag2−/−γc−/− mice intraperitoneally with low doses of R5 HIV-1NFN-SX(SL9) (500 or 5,000 TCID50). Despite the presence of very low percentages of CCR5+ cells in the human thymus, we have previously shown that molecularly cloned R5 HIV-1 (HIV-1JR-CSF and HIV-1NFN-SX), as well as pediatric R5 isolates, is able to productively infect human thymocytes in vitro and in vivo (11, 12, 22, 23). All mice infected with 5,000 TCID50 but only two out of five mice infected with 500 TCID50 showed HIV-1 replication. R5 HIV-1 replication was found in cells isolated from thymuses, spleens, and peripheral blood from all five productively infected animals and in bone marrow tested from two of the five animals. Thus, in the HIS- Rag2−/−γc−/− mice, as in the SCID-hu mouse model as we have previously reported (11, 12), R5 HIV-1 was able to infect human thymocytes. Our results with R5 HIV-1 infection in immunodeficient-animal models with reconstituted human CD34+ cell systems are in concordance with those described in several recent publications (2, 4, 10, 29, 30). Watanabe et al. demonstrated the presence of proviral HIV DNA in the thymuses, spleens, and bone marrow of NOD/SCID/IL-2rγ-null mice injected at 6 to 10 weeks of age with cord blood CD34+ cells after intravenous inoculation with high doses (65,000 TCID50) of R5 HIV-1JR-CSF (29). However, they found proviral HIV DNA in only 50% of mice infected with low doses of R5 HIV-1 (200 TCID50) and reported much lower levels of proviral HIV DNA in the thymus than in the spleen. Baenziger et al. generated productive infection in Rag2−/−γc−/− mice with immune systems reconstituted with cord blood CD34+ cells by using intraperitoneal injections with very high doses (2 × 106 TCID50) of R5 HIV-1 but did not test lower TCID50. They did not observe p24 expression in the thymuses of the majority of R5 HIV-infected animals by using immunohistochemistry (2), while we were able to detect R5 HIV-1 in all productively infected animals by coculturing. Moreover, they found a decrease in the CD4/CD8 ratio in only one mouse out of four R5 HIV-1-infected animals more than 17 weeks postinfection, while we observed a decrease in the CD4/CD8 ratio as early as 2 weeks after infection with low-dose R5 HIV-1. High doses of R5 HIV-1 were also used to infect humanized Rag2−/−γc−/− mice in studies reported by Berges et al. (4) and Gorantla et al. (10). Gorantla et al. found a decrease in the CD4/CD8 ratio in mice infected with high doses of one of two R5 HIV-1 strains tested (10). Zhang et al. reported finding RNA in the blood of two Rag2−/−γc−/− mice injected with fetal liver CD34+ cells and intravenously infected with 1 ng of R5 HIV-1JR-CSF but did not observe a decrease in the CD4/CD8 ratio at up to 2 weeks postinfection (30). In conclusion, high doses of R5 HIV-1 (≫500 TCID50) are required to productively infect 100% of the mice, while a lower dose of virus infects only some of the mice with reconstituted HIS.
We found active HIV-1 replication in cells obtained from thymuses (as well as other lymphoid organs and peripheral blood) of two out of five mice infected at 500 TCID50 of R5 HIV-1 and three out of three infected at 5,000 TCID50. Although we did not find a consistent decline in the percentages of human CD4+ T cells in the peripheral blood of the R5 HIV-1-infected mice, we did observe a statistically significant decrease in the CD4+-T-cell/CD8+ T-cell ratios in the peripheral blood of HIS-Rag2−/−γc−/− mice productively infected with R5 HIV-1 compared to those in the peripheral blood of uninfected and nonproductively infected mice at 4 to 6 weeks postinfection, indicative of CD4+-T-cell depletion.
We found that B cells in our HIS-Rag2−/−γc−/− mice were able to produce IgM as well as IgG. The R5 HIV-1-inoculated mice produced IgG and IgM antibodies to an unidentified protein present in the fetal calf serum but we were unable to detect antibodies specific to HIV-1 antigens, similar to the lack of HIV-specific antibodies reported by Gorantla et al. (10). In the NOD/SCID/IL-2rγ-null mouse model, IgM, but no IgG, antibodies to HIV-1 gp120 and p24 were detected in one mouse out of four infected with high doses of R5 HIV-1 resulting in high viral loads in plasma (29). None of four mice infected with low doses of R5 HIV-1 showed HIV-1-specific antibody responses (29). A similarly low incidence of antibody responses (one mouse of six) in X4 HIV-1-infected mice in the NOD/SCID/IL-2rγ-null mouse model was observed (29). Baenziger et al. found only 1 out of 25 Rag2−/−γc−/− mice infected with R5 HIV-1 to produce an IgG, but curiously no IgM, response to p34, gp41, p52, p58, and gp120 proteins (2). The lack of HIV-1-specific antibody in our nonproductively infected HIS-Rag2−/−γc−/− mice is likely due to infection with low doses of HIV-1, although inefficient T-cell help due to selection by both human and murine major histocompatibility molecules may also play a role (16).
T-cell responses to HIV-1 Gag and Nef peptide antigens measured in gamma interferon ELISPOT assays were not present in our productively infected or HIV-1-exposed HIS-Rag2−/−γc−/− mice. Our observations are in agreement with findings for the HIS-Rag2−/−γc−/− mice infected with very high doses of R5 or X4 HIV-1 (2). Thus, specific T-cell responses to HIV-1 do not develop in the present model despite high levels of productive infection, in contrast to what is observed in HIV-1 infection in humans. A possible explanation for the absence of HIV-1-specific T-cell responses may be the impaired development of lymph nodes in the HIS- Rag2−/−γc−/− mouse model (16).
In conclusion, although we have not been able to observe HIV-1-specific immune responses in the HIS-Rag2−/−γc−/− mouse model, this model does provide a convenient small-animal model that can be used to investigate HIV-1 pathogenesis and HIV-1 therapies. Efforts to improve the model are presently being investigated. Studies with the HIS- Rag2−/−γc−/− mouse model may replace some long and costly studies with nonhuman primates.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI052002, AI39975, AI28697, UCLA CFAR, and the Stein Oppenheimer Endowment Award).
We thank M. Centlivre and N. Legrand for their critical review of the manuscript and K. Grovit-Ferbas, J. F. Hsu, H. Ng, O. Yang, A. Colantonio, and A. M. Aubertin for reagents and assistance.
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Y. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 2.Baenziger, S., R. Tussiwand, E. Schlaepfer, L. Mazzucchelli, M. Heikenwalder, M. O. Kurrer, S. Behnke, J. Frey, A. Oxenius, H. Joller, A. Aguzzi, M. G. Manz, and R. F. Speck. 2006. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice. Proc. Natl. Acad. Sci. USA 103:15951-15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, J. G., D. Unutmaz, M. D. Miller, S. K. Breun, S. M. Grill, J. Mirro, D. R. Littman, A. Rein, and V. N. KewalRamani. 2004. Murine T cells potently restrict human immunodeficiency virus infection. J. Virol. 78:12537-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berges, B. K., W. H. Wheat, B. E. Palmer, E. Connick, and R. Akkina. 2006. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gamma c−/− (RAG-hu) mouse model. Retrovirology 3:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonyhadi, M. L., L. Rabin, S. Salimi, D. A. Brown, J. Kosek, J. M. McCune, and H. Kaneshima. 1993. HIV induces thymus depletion in vivo. Nature 363:728-732. [DOI] [PubMed] [Google Scholar]
- 6.Browning, J., J. W. Horner, M. Pettoello-Mantovani, C. Raker, S. Yurasov, R. A. DePinho, and H. Goldstein. 1997. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. USA 94:14637-14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fultz, P. N. 1993. Nonhuman primate models for AIDS. Clin. Infect. Dis. 17(Suppl. 1):S230-S235. [DOI] [PubMed] [Google Scholar]
- 8.Gardner, M. B. 1993. The importance of nonhuman primate research in the battle against AIDS: a historical perspective. J. Med. Primatol. 22:86-91. [PubMed] [Google Scholar]
- 9.Gimeno, R., K. Weijer, A. Voordouw, C. H. Uittenbogaart, N. Legrand, N. L. Alves, E. Wijnands, B. Blom, and H. Spits. 2004. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− γc−/− mice: functional inactivation of p53 in developing T cells. Blood 104:3886-3893. [DOI] [PubMed] [Google Scholar]
- 10.Gorantla, S., H. Sneller, L. Walters, J. G. Sharp, S. J. Pirruccello, J. T. West, C. Wood, S. Dewhurst, H. E. Gendelman, and L. Poluektova. 20 December 2006. HIV-1 pathobiology studied in humanized BALB/c-Rag2−/−γc−/− mice. J. Virol. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed]
- 11.Gurney, K. B., and C. H. Uittenbogaart. 2006. Human immunodeficiency viral persistence and production in T cell development. Clin. Vaccine Immunol. 13:1237-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurney, K. B., O. O. Yang, S. B. Wilson, and C. H. Uittenbogaart. 2002. TCRgammadelta(+) and CD161(+) thymocytes express HIV-1 in the SCID-hu mouse, potentially contributing to immune dysfunction in HIV infection. J. Immunol. 169:5338-5346. [DOI] [PubMed] [Google Scholar]
- 13.Helms, T., B. O. Boehm, R. J. Asaad, R. P. Trezza, P. V. Lehmann, and M. Tary-Lehmann. 2000. Direct visualization of cytokine-producing recall antigen-specific CD4 memory T cells in healthy individuals and HIV patients. J. Immunol. 164:3723-3732. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa, F., M. Yasukawa, B. Lyons, S. Yoshida, T. Miyamoto, G. Yoshimoto, T. Watanabe, K. Akashi, L. D. Shultz, and M. Harada. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood 106:1565-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, H., P. Krimpenfort, M. Haks, J. Allen, B. Blom, C. Demolliere, A. Kruisbeek, H. Spits, and A. Berns. 1999. PIM1 reconstitutes thymus cellularity in interleukin 7- and common gamma chain-mutant mice and permits thymocyte maturation in Rag- but not CD3gamma-deficient mice. J. Exp. Med. 190:1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legrand, N., K. Weijer, and H. Spits. 2006. Experimental models to study development and function of the human immune system in vivo. J. Immunol. 176:2053-2058. [DOI] [PubMed] [Google Scholar]
- 17.Levy, J. A. 1996. The value of primate models for studying human immunodeficiency virus pathogenesis. J. Med. Primatol. 25:163-174. [DOI] [PubMed] [Google Scholar]
- 18.McCune, J. M., R. Namikawa, H. Kaneshima, L. D. Shultz, M. Lieberman, and I. L. Weissman. 1988. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241:1632-1639. [DOI] [PubMed] [Google Scholar]
- 19.Morner, A., A. Bjorndal, J. Albert, V. N. Kewalramani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyo, and E. Bjorling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosier, D. E., R. J. Gulizia, P. D. MacIsaac, B. E. Torbett, and J. A. Levy. 1993. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science 260:689-692. [DOI] [PubMed] [Google Scholar]
- 21.Namikawa, R., K. N. Weilbaecher, H. Kaneshima, E. J. Yee, and J. M. McCune. 1990. Long-term human hematopoiesis in the SCID-hu mouse. J. Exp. Med. 172:1055-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedroza-Martins, L., W. J. Boscardin, D. J. Anisman, D. Schols, Y. J. Bryson, and C. H. Uittenbogaart. 2002. Impact of cytokines on replication in the thymus of primary human immunodeficiency virus type 1 isolates from infants. J. Virol. 76:6929-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedroza-Martins, L., K. B. Gurney, B. E. Torbett, and C. H. Uittenbogaart. 1998. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J. Virol. 72:9441-9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon, B., J. F. Hsu, V. Gudeman, I. S. Chen, and K. Grovit-Ferbas. 2005. Formaldehyde-treated, heat-inactivated virions with increased human immunodeficiency virus type 1 Env can be used to induce high-titer neutralizing antibody responses. J. Virol. 79:10210-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawada, S., K. Gowrishankar, R. Kitamura, M. Suzuki, G. Suzuki, S. Tahara, and A. Koito. 1998. Disturbed CD4+ T cell homeostasis and in vitro HIV-1 susceptibility in transgenic mice expressing T cell line-tropic HIV-1 receptors. J. Exp. Med. 187:1439-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid, I., W. J. Krall, C. H. Uittenbogaart, J. Braun, and J. V. Giorgi. 1992. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry 13:204-208. [DOI] [PubMed] [Google Scholar]
- 27.Traggiai, E., L. Chicha, L. Mazzucchelli, L. Bronz, J. C. Piffaretti, A. Lanzavecchia, and M. G. Manz. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304:104-107. [DOI] [PubMed] [Google Scholar]
- 28.Van Rompay, K. K. 2005. Antiretroviral drug studies in nonhuman primates: a valid animal model for innovative drug efficacy and pathogenesis experiments. AIDS Rev. 7:67-83. [PubMed] [Google Scholar]
- 29.Watanabe, S., K. Terashima, S. Ohta, S. Horibata, M. Yajima, Y. Shiozawa, M. Z. Dewan, Z. Yu, M. Ito, T. Morio, N. Shimizu, M. Honda, and N. Yamamoto. 2007. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109:212-218. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, L., G. I. Kovalev, and L. Su. 28 November 2006, posting date. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. doi: 10.1182/blood-2006-07-033159. [DOI] [PMC free article] [PubMed]