Abstract
Tcf-4 is a member of the Tcf/Lef family of transcription factors that interact functionally with β-catenin to mediate Wnt signaling in vertebrates. We have previously demonstrated that the tumor suppressor function of APC in the small intestine is mediated via regulation of Tcf-4/β-catenin transcriptional activity. To gain further insight into the role of Tcf-4 in development and carcinogenesis we have generated several mouse monoclonal antibodies, one of which is specific for Tcf-4 and another of which recognizes both Tcf-3 and Tcf-4. Immunohistochemistry performed with the Tcf 4- specific monoclonal antibody revealed high levels of expression in normal intestinal and mammary epithelium and carcinomas derived therefrom. Additional sites of Tcf-3 expression, as revealed by staining with the Tcf-3/−4 antibody, occurred only within the stomach epithelium, hair follicles, and keratinocytes of the skin. A temporal Tcf-4 expression gradient was observed along the crypt-villus axis of human small intestinal epithelium: strong Tcf-4 expression was present within the crypts of early (week 16) human fetal small intestine, with the villi showing barely detectable Tcf-4 protein levels. Tcf-4 expression levels increased dramatically on the villi of more highly developed (week 22) fetal small intestine. We conclude that Tcf-4 exhibits a highly restricted expression pattern related to the developmental stage of the intestinal epithelium. The high levels of Tcf-4 expression in mammary epithelium and mammary carcinomas may also indicate a role in the development of this tissue and breast carcinoma.
Recent studies have established that members of the Tcf/Lef family of high mobility group box transcription factors function as important downstream effectors of the Wnt/Wingless signal transduction cascades in Xenopus and Drosophila development. 1-4 This signaling function is dependent on the physical interaction with β-catenin, a 92-kd cytoplasmic protein that contains a potent transcriptional activation domain. 4-6 Signaling via the Wnt/Wingless cascades stimulates the nuclear translocation of β-catenin, resulting in the formation of Tcf/β-catenin complexes capable of transactivating target genes. The Tcf/Lef family comprises four members, denoted Tcf-1, Lef-1, Tcf-3, and Tcf-4. 7 Tcf-4 has an expression pattern indicative of an important role in vertebrate development. Tcf-4 expression during murine embryogenesis occurs much later than Tcf-1, Tcf-3, and Lef-1 (embryonic day 10.5) and on initial analysis was revealed to be restricted to the midbrain and intestinal epithelium. 8 The expression pattern of Tcf-4 within the central nervous system largely overlaps with that of three members of the Wnt family: Wnt-1, which is essential for midbrain development, 9 Wnt-3, and Wnt-3a. Tcf-4 is therefore a likely candidate for mediating signaling via these factors within the developing midbrain. A similar Wnt-driven function for Tcf-4 in the embryonic intestine is indicated by its high levels of expression in this tissue. In mice lacking Tcf-4, development of the small intestine is severely impaired as a direct result of an inability to maintain the stem cells within the intervillus regions. 10 We have recently shown that the nuclei of colon carcinoma cell lines contain constitutively active Tcf-4/β-catenin complexes as a direct consequence of either loss of function of the tumor suppressor protein APC or gain of function mutations in β-catenin itself. 11,12 This is believed to result in the uncontrolled transcription of Tcf target genes, leading to transformation of colon epithelial cells and initiation of polyp formation. Regulation of the transcriptional activity of Tcf-4/β-catenin has important implications for embryonic development as well as for carcinogenesis in the intestinal epithelium. It is currently not known if Tcf-4 is expressed in adult tissues other than intestinal epithelium and therefore whether dysregulation of Tcf-4/β-catenin signaling activity is potentially involved in the onset or progression of other forms of human cancer. In this report, we describe the generation of mouse monoclonal antibodies specific for Tcf-4 or cross-reactive with Tcf-3 and Tcf-4 and the results of a detailed immunohistochemical analysis of Tcf-4/Tcf-3 expression and discuss the possible implications of the Tcf-4 expression data in the context of human development and carcinogenesis.
Materials and Methods
Production and Purification of the Tcf-4 Fusion Protein
A 900-bp SacI-NsiI insert, encoding amino acids 31–331 of the region directly N-terminal to the high mobility group box of hTcf-4, was ligated into a Pet21b plasmid (Novagen, Madison, WI) to generate Pet21b-Tcf 4. The bacterial strain DH5α was transformed with the recombinant plasmid, ampicillin-resistant colonies were isolated, and plasmid minipreps were analyzed for the correct Tcf-4 insert by restriction digestion and sequencing. Pet21b-Tcf 4 was subsequently transformed into BL21 bacteria and cultured in LB-carbenicillin (100μg/ml) at 250 rpm at 37°C to an OD600 of 0.6. Isopropyl-1-thio-β-d-galactopyranoside was subsequently added to a final concentration of 1.0 mmol/L to induce production of the Tcf-4/Histidine fusion protein, and culturing continued for an additional 3 hours. Bacteria were harvested by centrifugation for 10 minutes at 4000× g at 4°C and the pellet resuspended in 8 ml of ice-cold binding buffer (5 mmol/L imidazole, 500 mmol/L NaCl, 160 mmol/L Tris-HCl, pH 7.9). The suspension was sonicated on ice for 10 minutes and subsequently centrifuged at 10,000 rpm for 45 minutes at 4°C. The supernatant was passed over a Ni2+-agarose column at 4°C and the bound Tcf-4/Histidine fusion protein was then eluted with 2 ml of wash buffer (500 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.9, 25 mmol/L imidazole).
Generation of Tcf-4 and Tcf-3/−4 Monoclonal Antibodies
Six-week-old BALB/c mice were immunized by intraperitoneal injection of 200 μg of fusion protein in Freund’s complete adjuvant (Difco, Detroit, MI), with a second injection in Freund’s incomplete adjuvant (Difco) 14 days later. Five additional injections were performed using 200 μg of fusion protein in phosphate-buffered saline (PBS) at weekly intervals. A mouse with an anti-Tcf-4 titer of 1/500 was sacrificed, the spleen isolated, and 1 × 10 8 splenocytes fused to an equal number of NS-1 myeloma cells using a standard polyethylene glycol protocol as described previously. 13 The fused cell population was resuspended in hypoxanthine aminopterin thymidine selection medium (Life Technologies, Breda, The Netherlands) and plated into twenty-five 96-well flat-bottom culture plates. Selection was allowed to occur over a 2-week period and hybridoma supernatants were screened for anti-Tcf 4 and anti-Tcf-3/−4 antibodies. Positive hybridomas were repeatedly subcloned to generate clonal hybridomas secreting monoclonal Tcf-4 and Tcf-3/−4 antibodies.
Cell Culture
African green monkey kidney cells (COS) were routinely cultured in Dulbecco’s modified Eagle medium (Life Technologies) supplemented with 10% fetal calf serum and antibiotics. HT-29 and SW620 cells were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal calf serum and antibiotics.
Hybridoma Screening Assay
Approximately 10 × 10 6 COS cells were transiently transfected with 10 μg of pCDNA vectors expressing hTcf-4, mTcf-3, hTcf-1, or hLef-1 using DEAE-dextran as described previously. 13 Cells were subsequently plated into 96-well flat-bottom culture plates at a concentration of 10 4 per well. The cells were cultured for 48 hours, washed once with PBS, and fixed with 100% methanol for 2 hours at −20°C. Screening the hybridomas for anti-Tcf-4 antibodies was performed by incubating 100 μl of hybridoma supernatant with a well containing fixed COS cell transfectants for 1.5 hours at room temperature. Detection was carried out with a rabbit anti-mouse horseradish peroxidase coupled antibody (DAKO, Glostrup, Denmark) and 0.02% amino ethyl carbonate/0.1% hydrogen peroxide in 0.1 mol/L sodium acetate, pH 4.8, as a color substrate. Individual wells were examined for nuclear staining using an inverted microscope.
Immunohistochemical Staining of Tissue Samples
Fetal tissue was obtained from second trimester abortions according to the guidelines of the University Hospital, Utrecht committee on the use of human subjects in scientific research. Tissue samples (listed in Table 1 ▶ ) were fixed in 4% formaldehyde-PBS, embedded in paraffin, and sectioned at 4 μm thickness. Sections were treated with 1.5% H2O2 in methanol for 20 minutes. The slides were subsequently immersed in 0.01 mol/L citrate buffer, pH 6.0, and incubated for 15 minutes at 90°C in a steam bath. Slides were washed in PBS and incubated with 2% goat nonimmune serum-2% bovine serum albumin for 20 minutes at room temperature to block nonspecific binding. Antibodies against Tcf-4 (6H5) or Tcf-3/−4 (6F12) were used at a final concentration of 10 μg/ml in 4% normal human serum. The primary antibody was detected with rabbit anti-mouse/horseradish peroxidase and amplified with swine anti-rabbit/horseradish peroxidase antibody (DAKO) at a1/250 dilution in PBS.
Table 1.
Summary of Human Tissue Staining Patterns Generated Using 6H5 and 6F12 mAbs
| Tissue | 6F12 | 6H5 |
|---|---|---|
| Thymus | − | − |
| Mammary gland | + | + |
| Mammary carcinoma | + | + |
| Stomach | + | − |
| Lung | + | − |
| Skin | + | − |
| Small Intestine | + | + |
| Colon | + | + |
| Colon carcinoma | + | + |
| Appendix | + | + |
| Pancreas | − | − |
| Tonsil | − | − |
| Adrenal gland | − | − |
| Thyroid gland | − | − |
| Kidney | − | − |
| Spleen | − | − |
| Lymph nodes | − | − |
| Prostate | − | − |
| Bone marrow | − | − |
| Liver | − | − |
+, positive staining; −, negative staining.
Gel Retardation Assays
Assays were performed as described previously. 11 As the optimal Tcf probe, we used a double-stranded oligonucleotide ACTCTGGTACTGGCCCTTTGATCTTTCTGG. The mutant Tcf probe comprised a double-stranded oligonucleotide ACTCTGGTACTGGCCCGGGGATCTTTCTGG. Extracts were prepared from intact nuclei of HT29 colon carcinoma cells. Binding reaction mixtures contained 3 μg nuclear protein, 0.5 ng probe, and 100 ng poly(dI-dC) in 25 μl binding buffer (60 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 10% glycerol). Samples were incubated for 20 minutes at room temperature before addition of 0.25 μg of anti-Tcf-4 antibody (6H5), then incubated for another 20 minutes. The samples were subsequently subjected to nondenaturing polyacrylamide gel electrophoresis (PAGE).
Immunoprecipitations
Approximately 10 × 10 6 SW620 colon carcinoma cells were used as a protein sample. Whole cell lysates were prepared as described previously. 14 The lysates were subsequently incubated with 10 μg of 6H5 anti-Tcf-4 mAb at 4°C for 1 hour and antibodies recovered using Protein A/G plus agarose (Santa Cruz Biotechnology, Santa Cruz, CA). The agarose beads were washed 3 times with 1 ml each of buffer B (20 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, and 0.5% NP-40). Antibody/protein complexes were eluted by adding sodium dodecyl sulfate-PAGE sample buffer and boiling for 5 minutes. The protein samples were then resolved by sodium dodecyl sulfate-PAGE and the protein transferred to PVD immobilon-P membrane (Millipore, Bedford, MA). The blots were blocked in 1% bovine serum albumin in PBS plus 0.1% Tween 20 and incubated in a 1:1000 dilution of β-catenin antibody (Signal Transduction Laboratories, Lexington, KY). Blots were developed using the ECL system (Amersham, Little Chalfont, UK).
Results
Generation of Tcf-4 and Tcf-3/−4 mAbs
A successful fusion between splenocytes of a mouse immunized with the Pet21b-Tcf-4 recombinant antigen and the myeloma cell line NS-1 yielded > 10,000 hybridomas. Screening of the hybridoma supernatants for Tcf 4-reactive antibodies using COS cells transiently transfected with hTcf-4 and the subsequent subcloning of positive hybridoma populations resulted in 30 clonal hybridomas. These were tested for reactivity against all four mammalian Tcf types (Tcf-1, Lef-1, Tcf-3, and Tcf-4) by screening their supernatants using COS cells expressing the relevant proteins. In this way, hybridomas were selected which fell into two classes, those reactive against Tcf-4 and those reactive against both Tcf-3 and Tcf-4. No cross-reactivity against Tcf-1 or Lef-1 was observed for any of the hybridomas. The hybridoma denoted 6H5 recognized both human and mouse Tcf-4, producing the nuclear staining characteristic of Tcf family members in Tcf-4-transfected COS cells only (Figure 1, a and b) ▶ . In addition, the supernatant of a hybridoma denoted 6F12 was found to recognize human and mouse Tcf-3 and Tcf-4 (Figure 1, c and d) ▶ .
Figure 1.
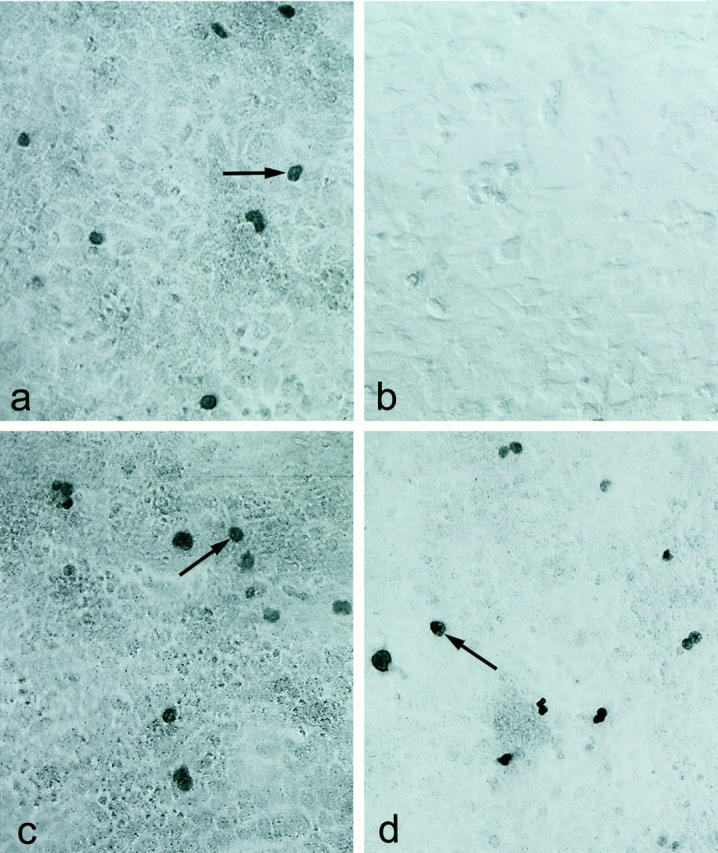
Immunohistochemical staining of hTcf-4 and mTcf-3 in COS cells using the 6H5 and 6F12 mAbs. a: Staining of COS cells expressing hTcf-4 (arrows) by the 6H5 mAb. b: Absence of staining on COS cells expressing mTcf-3 by the 6H5 mAb. Magnification, ×200. c,d: Staining of COS cells expressing hTcf-4 or mTcf-3 (arrows) by the 6F12 mAb. Magnification, ×200.
Immunohistochemical Analysis of Tcf-4 and Tcf-3 Expression
To determine the expression patterns of Tcf-4 and Tcf-3 we performed immunohistochemical staining using the Tcf-4 specific mAb 6H5 and the Tcf-3/−4 cross-reactive mAb 6F12 on a panel of human tissues (Table 1) ▶ . We found high levels of nuclear Tcf-4 expression to be present in epithelium of normal small intestine, colon, and colon carcinoma (Figure 2, a-f ▶ ). Tcf-4 expression was also observed in the appendix, but never in stomach epithelium (data not shown). In addition, lobular and ductal epithelium of normal mammary gland and carcinomas derived therefrom exhibited high levels of Tcf-4 expression. (Figure 2, g and h) ▶ . Limited staining of cells within the fibrous tissue immediately adjacent to the epithelium of the intestine and mammary gland was also evident (Figure 2, c, d, g, and h) ▶ . All other tissues tested were negative. The staining patterns of the 6H5 and the 6F12 mAbs were largely overlapping, with exclusive staining of the 6F12 mAb evident only within hair follicles and keratinocytes of the skin. (Figure 2, i and j) ▶ . A lower level of specific staining by the 6F12 mAb was also observed in stomach epithelium (data not shown). A comparison of Tcf-4 protein levels in week 16 and week 22 human fetal small intestinal epithelium revealed a temporal gradient of expression along the crypt-villus axis (Figure 2, e and f) ▶ . At week 16, Tcf-4 expression was high in the crypt regions with barely detectable levels on the villi. However, this situation altered quite dramatically in tissue from a later stage embryo (week 22), with a large increase in Tcf-4 expression on the villi. This expression gradient was also observed along the epithelium lining the crypts of adult colon (Figure 2a) ▶ and along the crypt-villus axis of adult small intestinal epithelium (Figure 2, c and d) ▶ .
Figure 2.
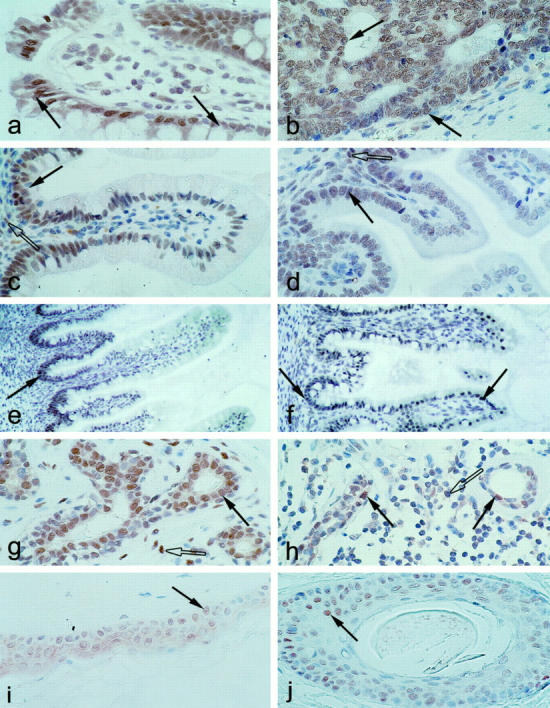
Immunohistochemical analysis of Tcf-4 and Tcf-3 expression in human and mouse tissues. a-h: Immunohistochemical stainings generated using the Tcf-4 specific mAb (6H5). i-j: Immunohistochemical stainings generated using the Tcf-3/−4 mAb (6F12). a,b: Tcf-4 is highly expressed at the tops of the crypts (arrows) of human adult colonic epithelium (a) and human colon carcinoma (b). Magnification, ×150. c,d: Tcf-4 is expressed at high levels in the fibrous tissue (open arrows) and crypts (shaded arrows) of human (c) and mouse (d) adult small intestinal epithelium. Magnification, ×100. e,f: Tcf-4 expression increases along the crypt-villus axis (arrows) during week 16 (e) and week 22 (f) of the development of human small intestinal epithelium. Magnification, ×50. g,h: Tcf-4 is expressed at high levels in the epithelium (shaded arrows) and fibrous tissue (open arrows) of human mammary gland (g) and mammary carcinoma (h). Magnification, ×100. i-j: Tcf-3 is expressed in hair follicles (i) and keratinocytes of skin (j). Magnification, ×100.
Direct Demonstration of Nuclear Tcf-4 and Tcf-4/β-Catenin Complexes in Colon Carcinoma Cells
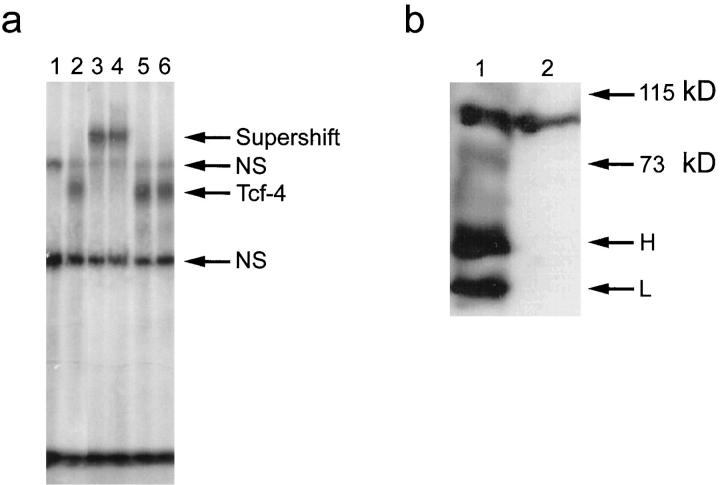
We performed a gel retardation analysis using nuclear extracts prepared from a colon carcinoma cell line, HT-29, and an optimal Tcf binding motif as probe. Specific retardation of the optimal Tcf probe indicated the presence of a Tcf protein in the nuclear extracts (Figure 3a) ▶ . This Tcf/probe complex could be supershifted by addition of either the 6H5 or 6F12 mAb, demonstrating that this complex contains Tcf-4. Addition of irrelevant antibodies did not induce any supershift. To determine the presence of specific Tcf-4/β-catenin complexes in the nuclei of colon carcinoma cells, Tcf-4 was immunoprecipitated using the 6H5 mAb from nuclear extracts prepared from SW620 colon carcinoma cells. Western blot analysis was subsequently performed using a β-catenin mAb. A band of approximately 92 kd, which comigrated with a single band visualized by Western blot analysis of total β-catenin in SW620 cells, was observed in the Tcf-4 immunoprecipitate (Figure 3b) ▶ . This demonstrated the presence of Tcf-4/β-catenin complexes in the nuclei of SW620 colon carcinoma cells.
Figure 3.
a: Gel retardation of Tcf-4 complexes from HT-29 colon carcinoma cells. Tcf-4 complexed to an optimal Tcf binding site probe can be supershifted by addition of the Tcf-4-specific mAb 6H5. Lane 1: Nonspecific (NS) bands generated using a probe comprising a disrupted Tcf binding site. Lane 2 : Specific Tcf-4/probe complex. Lane 3: Supershift of the Tcf-4/probe complex by addition of 6H5 mAb. Lane 4: Supershift of the Tcf-4/probe complex on addition of 6F12 mAb. Lanes 5 and 6: No supershift of the Tcf-4/probe complex induced on addition of control antibodies (anti-APC and anti-Plakoglobin). b: Co-immunoprecipitation of Tcf-4 and β-catenin from SW620 nuclear extracts. Lane 1: A 92-kd band (arrow) visualized by Western blot using an anti-β-catenin mAb after Tcf-4 immunoprecipitation from SW620 nuclear extracts using the 6H5 mAb. H, antibody heavy chain; L, antibody light chain. Lane 2: Western blot analysis of total β-catenin (arrow) in SW620 total cell extracts.
Discussion
We have successfully generated mouse mAbs recognizing human and mouse Tcf-4 alone (6H5) or both human and mouse Tcf-4 and Tcf-3 (6F12). The generation of hybridomas producing mAbs directed against both Tcf-3 and Tcf-4 was not particularly surprising in view of the high overall homology of these proteins in the region used for immunization. 8 No mAbs were found to cross-react with Tcf-1 or Lef-1, which reflects the divergence exhibited between these genes in the N-terminal region selected for use in recombinant antigen production. 15,16 Using the 6H5 mAb, we were able to coimmunoprecipitate β-catenin from the nuclei of a colon carcinoma cell-line, SW620, proving that Tcf-4 is the Tcf/Lef family member generating transcriptionally active complexes with β-catenin in colon carcinoma cells. This also demonstrates the potential use of this antibody as a tool for studying the physical interactions between Tcf-4 and β-catenin or additional factors which may regulate the transcriptional activity of Tcf-4. Immunohistochemical analysis performed with the 6H5 mAb demonstrated a restricted pattern of Tcf-4 expression, with staining evident only within the epithelium of small intestine, colon, appendix, mammary gland, and carcinomas derived therefrom. The limited staining of cells within the fibrous tissue surrounding the epithelium was restricted to the intestine and mammary gland, indicating that this corresponds to specific Tcf-4 expression in this tissue. The absence of any staining within an extensive panel of other tissues including thymus, which exhibits high levels of Tcf-1 and Lef-1 expression, 13,16,17 confirms the specificity of this mAb. The exclusive staining of the Tcf-3/−4 specific mAb 6F12 within hair follicles, stomach epithelium, and keratinocytes of the skin indicates that Tcf-3 is expressed independently of Tcf-4 at these sites. It is interesting to note that this expression of Tcf-3 in the skin and hair follicles coincides with expression of a related high mobility group box transcription factor, Lef-1. 16 There is thought to be some functional redundancy within the Tcf/Lef family, in particular between Tcf-1 and Lef-1 in the thymus and between Tcf-4 and Lef-1 in specific regions of the brain. 11 However, the function of Tcf-3 in the hair follicles would seem to differ from that of Lef-1 because mutant mice lacking Lef-1 yet retaining Tcf-3 expression have a nude phenotype. 18 The specific site and level of expression of Tcf-4 within the intestinal epithelium appears to be related to the stage of development of this tissue. Epithelium of the small intestine taken from an early stage (week 16) fetus shows a high level of Tcf-4 expression within the crypt regions, with little or no expression on the developing villi. This region contains rapidly dividing crypt stem cells, which in normal tissue subsequently migrate along the crypt-villus axis and differentiate into specific villus epithelial cells. 19,20 These expression data support the findings of our recent analysis of mutant mice lacking Tcf-4, which clearly demonstrated an essential role for Tcf-4 in maintaining the crypt stem cell compartments. 11 The observed increase in Tcf-4 expression on the villi of more highly developed (week 22) fetal small intestinal epithelium indicates that Tcf-4 expression is maintained within the migrating crypt cells. Tcf-4 expression thus increases on the villi as a consequence of acquiring differentiated crypt cells expressing Tcf-4, rather than an increase in gene expression per se. There is also a Tcf-4 expression gradient along the crypt-villus axis of human and mouse adult small intestinal epithelium, with higher levels present on the villi relative to the crypt regions. This reduction in Tcf-4 expression in the crypt regions of adult tissue is likely to reflect a concomitant reduction in the proliferation rate after the fetal developmental stages. Blocking this reduction in proliferation via dysregulation of Tcf-4 signaling might therefore be expected to affect development of the intestinal epithelium in such a way as to promote carcinoma formation. Here we also demonstrate high levels of Tcf-4 expression in the epithelium of mammary glands and mammary carcinomas. The Tcf-4-specific and Tcf-3/−4 mAbs will be valuable tools for use in cancer research, in particular for further study of the role of Tcf-4 in colon carcinogenesis and future investigations of possible roles for this transcription factor in mammary gland and skin development and/or carcinogenesis.
Acknowledgments
We thank Dr. J. Van Es for critically reading the manuscript and M. E. J. Schipper for her expert opinion regarding tissue pathology.
Footnotes
Address reprint requests to Nick Barker, Department of Immunology, University Hospital Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands. E-mail: n.barker@lab.azu.nl.
References
- 1.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H: Xtcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 1996, 86:391-399 [DOI] [PubMed] [Google Scholar]
- 2.Riese J, Yu X, Munnerlyn A, Eresh S, Hsu SC, Grosschedl R, Bienz M: LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell 1997, 88:777-787 [DOI] [PubMed] [Google Scholar]
- 3.Brunner E, Peter O, Schweizer L, Basler K: Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 1997, 385:829-833 [DOI] [PubMed] [Google Scholar]
- 4.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro A, Ypma D, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H: Armadillo co-activates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 1997, 88:789-799 [DOI] [PubMed] [Google Scholar]
- 5.Behrens J, van Kries JP, Kuehl M, Bruhn D, Wedlich R, Grosschedl R, Birchmeier W: Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 6.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R: Nuclear translocation of β-catenin by interaction with transcription factor LEF-1. Mech Dev 1996, 59:3-10 [DOI] [PubMed] [Google Scholar]
- 7.Clevers H, van de Wetering M: TCF/LEF factors earn their wings. Trends Genet 1997, 13:485-489 [DOI] [PubMed] [Google Scholar]
- 8.Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H: Two members of the Tcf family implicated in Wnt/β-catenin signaling during embryogenesis in the mouse. Mol Cell Biol 1998, 18:1248-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMahon AP, Bradley A: The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 1990, 62:1073-1085 [DOI] [PubMed] [Google Scholar]
- 10.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H: Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Gen 1998, 19:379-383 [DOI] [PubMed] [Google Scholar]
- 11.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 12.Morin PJ, Sparks A, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 13.Castrop J, van Wichen D, Koomans-Bitter M, van de Wetering M, de Weger R, van Dongen J, Clevers H: The human TCF-1 gene encodes a nuclear DNA-binding protein, uniquely expressed in normal and neoplastic T lineage lymphocytes. Blood 1995, 86:3050-3059 [PubMed] [Google Scholar]
- 14.Rubinfield B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P: Stabilization of β-catenin by genetic defects in melanoma cell-lines. Science 1997, 275:1790-1792 [DOI] [PubMed] [Google Scholar]
- 15.van de Wetering M, Oosterwegel M, Dooijes D, Clevers H: Identification and cloning of TCF-1, a T-cell specific transcription factor containing a sequence-specific HMG box. EMBO J 1991, 10:123-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travis A, Amsterdam A, Belanger C, Grosschedl R: Lef-1, a gene encoding a lymphoid-specific protein, with an HMG domain, regulates T-cell receptor α enhancer function. Genes Dev 1991, 5:880-894 [DOI] [PubMed] [Google Scholar]
- 17.Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H: Differential expression of the HMG box factors TCF-1 and LEF-1. Development 1993, 118:439-448 [DOI] [PubMed] [Google Scholar]
- 18.Van Genderen V, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R: Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1 deficient mice. Genes Dev 1994, 8:2691-2704 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt GH, Winton DJ, Ponder BA: Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development 1988, 103:785-790 [DOI] [PubMed] [Google Scholar]
- 20.Calvert R, Pothier P: Migration of fetal intestinal intervillous cells in neonatal mice. Anat Rec 1990, 227:199-206 [DOI] [PubMed] [Google Scholar]