Abstract
Overexpression of cell surface glycoproteins of the CD44 family is an early event in the colorectal adenoma-carcinoma sequence. This suggests a link with disruption of APC tumor suppressor protein-mediated regulation of β-catenin/Tcf-4 signaling, which is crucial in initiating tumorigenesis. To explore this hypothesis, we analyzed CD44 expression in the intestinal mucosa of mice and humans with genetic defects in either APC or Tcf-4, leading to constitutive activation or blockade of the β-catenin/Tcf-4 pathway, respectively. We show that CD44 expression in the non-neoplastic intestinal mucosa of Apc mutant mice is confined to the crypt epithelium but that CD44 is strongly overexpressed in adenomas as well as in invasive carcinomas. This overexpression includes the standard part of the CD44 (CD44s) as well as variant exons (CD44v). Interestingly, deregulated CD44 expression is already present in aberrant crypt foci with dysplasia (ACFs), the earliest detectable lesions of colorectal neoplasia. Like ACFs of Apc-mutant mice, ACFs of familial adenomatous polyposis (FAP) patients also overexpress CD44. In sharp contrast, Tcf-4 mutant mice show a complete absence of CD44 in the epithelium of the small intestine. This loss of CD44 concurs with loss of stem cell characteristics, shared with adenoma cells. Our results indicate that CD44 expression is part of a genetic program controlled by the β-catenin/Tcf-4 signaling pathway and suggest a role for CD44 in the generation and turnover of epithelial cells.
Colorectal cancer is common in the western world and represents the second leading cause of cancer-related death. 1 It evolves through a series of morphologically recognizable stages known as the adenoma-carcinoma sequence. 2 Although complex genetic alterations accumulate along this sequence, mutations involving components of the Wnt-Wingless signaling cascade appear to play a key role in the early transformation of colonic epithelium. Individuals who inherit adenomatous polyposis coli (APC) tumor suppressor gene mutations, familial adenomatous polyposis (FAP) patients, develop thousands of colorectal tumors, consistent with a gatekeeping role of the APC protein in colorectal tumorigenesis. 3,4 The APC protein has been observed to interact with β-catenin, 2,5 originally identified on the basis of its association with cadherin adhesion molecules but now recognized as an essential component of the Wnt-Wingless cascade. 6 In this cascade, β-catenin functions as a transcriptional activator when complexed with members of the Tcf family of DNA-binding proteins. 7,8 In APC−/− colon carcinoma cell lines, transcriptionally active nuclear β-catenin/Tcf-4 complexes are constitutively present. 9 Comparable complexes between β-catenin and Tcf/Lef proteins exist in APC+/+ colon carcinoma 10 and melanoma 11 cells as a result of dominant mutations affecting the amino terminus of β-catenin. Thus, mutation in either APC or in β-catenin can lead to constitutive nuclear complexes between co-activator β-catenin and Tcf-4 in intestinal epithelium. This will result in activated transcription of Tcf-4 target genes in such cells. Thus far, the Tcf-4 target genes relevant for the tumorigenesis process have not been identified.
CD44 is a family of cell-surface glycoproteins generated from a single gene by alternative splicing and differential glycosylation. 12-15 Members of the CD44 family have been implicated in a number of important biological processes, including lymphocyte homing, 12,16,17 hematopoiesis, 18 and tumor progression and metastasis. 14,19-26 In these processes, CD44 is believed to function as a cell adhesion receptor, linking extracellular matrix molecules, specifically hyaluronate, to the cell and the cytoskeleton. 12,27-30 Furthermore, CD44 isoforms decorated with heparan sulfate side chains have been shown to bind growth factors and can promote growth factor receptor-mediated signaling. 31-34 Studies from our own and other laboratories have shown that CD44 glycoproteins, which are normally expressed only in the lower crypt epithelium of the intestinal mucosa, are overexpressed in colorectal cancer and may play a role in the generation and turnover of epithelial cells. 25,35-41 CD44 overexpression is an early event in the colorectal adenoma-carcinoma sequence, 25,37 suggesting that CD44 expression is, directly or indirectly, regulated by β-catenin/Tcf-4-mediated transcription. To explore the latter hypothesis, we analyzed CD44 expression in the normal and neoplastic intestinal mucosa of mice and humans with genetic defects in either APC or Tcf-4.
Materials and Methods
Apc and Tcf-4 Mutant Mice
Normal and neoplastic small-intestinal tissue from C57BL/6JIco-Apc+/Apc1638N mice (Apc+/−) and wild-type C57BL/6 (Apc+/+) control mice was obtained from the Department of Human Genetics, University of Leiden, Leiden, The Netherlands. The Apc+/Apc1638N mice are heterozygous for an Apc chain-terminating mutation in Apc codon 1638, although the expected truncated protein is not detectable by conventional Western analysis. 42 The mice were sacrificed between 6 and 12 month of age, after which the entire intestine was opened longitudinally and inspected for neoplastic lesions. Lesions with surrounding normal tissue were sampled for routine processing and fixed in formalin or Notox (Earth Safe Industries, Bellemead, NJ) and embedded in paraffin. Embryos of Tcf-4−/− and Tcf-4+/− mice 43 were obtained from the Department of Immunology, University Hospital, Utrecht, The Netherlands, embedded in paraffin, and sectioned at 6 μm thickness.
FAP Patients
Colon mucosa biopsies from a 38-year-old male and a 30-year-old female FAP patient, taken at routine colonoscopy, were obtained from the files of the Department of Pathology, Academic Medical Center, University of Amsterdam. The biopsies were embedded in paraffin, and 6-μm sections were prepared.
Monoclonal Antibodies (MAbs)
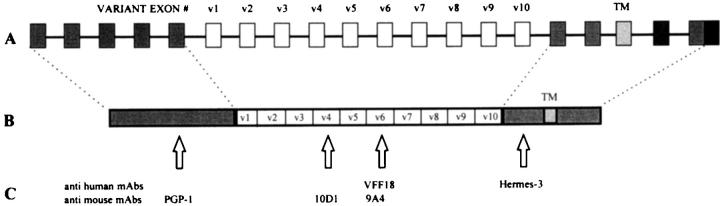
The MAbs used were Hermes-3 against an epitope on the constant part of the human CD44 molecule (CD44s), 16 VFF18 against human CD44v6, 44 PGP-1 against mouse CD44s (Pharmingen, San Diego, CA), 10D1 against mouse CD44v4, 45 9A4 against mouse CD44v6, 45 and PCNA against proliferating cell nuclear antigen (PCNA; Dako, Glostrup, Denmark) (Figure 1) ▶ .
Figure 1.
A: Schematic representation of the CD44 gene. Open boxes indicate exons that can be alternatively spliced. TM, transmembrane region. B and C: Schematic representation of the CD44 protein with localizations of the epitopes that are recognized by the anti-human monoclonal antibodies VFF18 and Hermes-3 and the anti-mouse antibodies PGP-1, 10D1, and 9A4. v1 to v10, domains encoded by variant exons.
Immunohistochemistry
Detection of CD44 and PCNA in mouse and human paraffin-embedded tissue sections was performed as described previously, 35 with the following modifications. In brief, the sections were deparaffinated, rehydrated, and boiled in a citrate buffer (0.01 mol/L, pH 6, Merck 6448) for antigen retrieval. On mouse and human tissue, biotinylated rabbit-anti-rat F(ab′)2 (Dako) and rabbit-anti-mouse F(ab′)2 (Dako) were used as secondary antibodies, respectively. For color development, 3,3-diaminobenzidine tetrachloride (DAB; Sigma, Bornem, Belgium) was used.
The immunohistochemical staining was scored semiquantitatively based on the staining intensity of positively stained tumor cells. The samples were scored as follows: −, negative; −/+, equivocal/very weak; +, weak; ++, moderate; +++, strong.
Reverse Transcription Polymerase Chain Reaction and Southern Blot Analysis
RNA isolation and first-strand cDNA synthesis were performed as described previously. 46 Polymerase chain reaction (PCR) was performed with 1.5 U of Taq DNA polymerase (Gibco BRL/Life Technologies, Gaithersburg, MD), 300 μmol/L dNTPs (Pharmacia Biotech, Uppsala, Sweden), and 2 mmol/L MgCl2 in 1X PCR buffer (both Gibco BRL/Life Technologies). Primers used were M44CU (5′-CCCAGGTAGCTTCCTTAACCC-3′) in combination with M44CD (5′-CGTAGAGAGGACCGTGACCGA-3′). PCR was started with a 5-minutes denaturation step at 95°C, after which amplification was performed at 35 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 1 minute, and elongation at 72°C for 2 minutes. After a final elongation step for 10 minutes at 72°C, samples were cooled on ice. PCR products were resolved in 1.5% agarose/Tris-buffered ethanolamine gel and blotted on Hybond-N+ membranes (Amersham, Little Chalfont, UK).
To generate 32P-labeled exon-specific probes, we used the plasmid pZeo SV mCD44v4-v10, containing the murine CD44 exon v4-v10 (a kind gift from Dr. M. Hofmann from the Institut für Genetics, Forschungszentrum, Karlsruhe, Germany). To generate a 32P-labeled exon-v3 probe, we used DNA from normal mouse skin. For the generation of the CD44s probe we used the plasmid pZeo S mCD44st, containing the murine CD44 standard region. The PCR mixtures for the v3 and v9 exons contained 2 mmol/L MgCl2, 100 μmol/L dATP, dTTP, and dGTP, and 13.2 μmol/L dCTP. The mixtures for the v6 exon contained 1 mmol/L MgCl2, 200 μmol/L dATP, dTTP, and dGTP, and 26.4 μmol/L dCTP. The mixtures for the standard region contained 2 mmol/L MgCl2, 300 μmol/L dATP, dTTP, and dGTP, and 39.6 μmol/L dCTP. In addition, all PCR mixtures contained 0.22 MBq [α-32P]dCTP, 10 pmol of each oligonucleotide primer, and 1.5 U of Taq DNA polymerase. The primers used for v3 were MV3U (5′ GTACGGAGTCAAATACCAAC3′) and MV3D (5′ TGGTACTGGAGATAAAATCT 3′), for v6 were MV6U (5′ CTCCTAATAGTACAGCAGAA 3′) and MV6D (5′ AGTTGTCCCTTCTGTCACAT 3′), and for v9 were MV9U (5′ CACAGAGTCATTCTAGAAC 3′) and MV9D (5′ TGCTAGATGGCAGAATAGAA 3′). Samples were amplified for 35 cycles in a PTC-100 (MJ Research, Watertown, CA). Each cycle consisted of denaturation at 95°C (for CD44s, v3, and v9) or at 96°C (for v6) for 30 seconds, annealing at 55°C (CD44s, v3, and v9) or 50°C (V6) for 1 minute, and extension at 72°C for 2 minutes (for CD44s, v3, and v9) or 1 minute (for V6), followed by a final elongation step for 10 minutes at 72°C. The PCR products resulted in 32P-labeled exon-specific probes and were used to hybridize the membranes according to standard procedures.
Results
Expression of CD44 in the Normal and Neoplastic Intestinal Mucosa of Apc+/Apc1638N Mice
CD44 expression in the epithelium of the histologically normal small-intestinal mucosa of Apc+/Apc1638N (Apc+/−) mice and wild-type (Apc+/+) mice was identical and was restricted to the crypts (Figure 2A ▶ ; Table 1 ▶ ). In these crypt areas, CD44 expression was localized to the basolateral membranes of the cells. Epitopes encoded by the constant (standard) part of the CD44 (CD44s) and by the alternatively spliced exons CD44v4 and v6 were expressed in a similar pattern. However, the intensity of staining differed; whereas the MAbs against CD44s and CD44v6 stained with high intensity, staining with the anti-CD44v4 MAb was weak (Table 1) ▶ . In the lamina propria, CD44s, but not CD44v4 or CD44v6 expression, was observed on stromal cells, lymphocytes, and macrophages.
Figure 2.
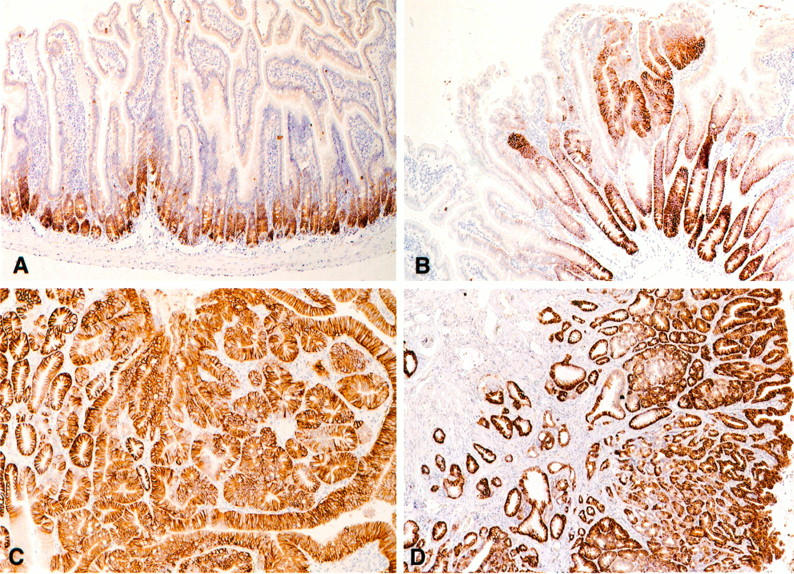
CD44 expression in the normal and neoplastic intestinal epithelium of the Apc+/Apc1638 mice. CD44v6 expression in non-neoplastic intestinal mucosa (A), in dysplastic ACF (B), in an adenomatous polyp (C), and in invasive carcinoma (D) of Apc+/Apc1638 mice.
Table 1.
Summary of CD44 Expression in the Normal and Neoplastic Intestinal Mucosa of Mice and Humans with Genetic Defects in Either APC or Tcf-4
| Model | Tissue | MAbs | ||||
|---|---|---|---|---|---|---|
| CD44s | CD44v4 | CD44v6 | PCNA | |||
| Mouse | ||||||
| Apc+/− | Small bowel | Crypt | ++ | −/+ | ++ | ∼ |
| Villus | − | − | − | ∼ | ||
| ACF | +++ | −/+ | +++ | ∼ | ||
| Adenoma | +++ | + | +++ | ∼ | ||
| Carcinoma | +++ | + | +++ | ∼ | ||
| Colon | Crypt base | ++ | −/+ | ++ | ∼ | |
| Crypt | − | − | − | ∼ | ||
| Tcf-4−/− | Small bowel | Crypt | − | ∼ | − | − |
| Villus | − | ∼ | − | − | ||
| Colon | Crypt base | ++ | ∼ | ++ | ∼ | |
| Crypt | − | ∼ | − | ∼ | ||
| Tcf+/− | Small bowel | Crypt | ++ | ∼ | ++ | +++ |
| Villus | − | ∼ | − | + | ||
| Colon | Crypt base | ++ | ∼ | ++ | ∼ | |
| Crypt | − | ∼ | − | ∼ | ||
| Human | ||||||
| FAP | Small bowel | Crypt | −/+ | ∼ | + | ∼ |
| Villus | − | ∼ | − | ∼ | ||
| Colon | Crypt base | −/+ | ∼ | + | ∼ | |
| Crypt | − | ∼ | − | ∼ | ||
| ACF | + | ∼ | +++ | ∼ |
Staining intensity was scored as follows: −, negative; −/+, equivocal/very weak; +, weak; ++, moderate; +++, strong. ∼, not tested.
Intestinal tumors arising in Apc+/− mice invariably showed a strong homogeneous expression of CD44 (Figure 2, C and D ▶ ; Table 1 ▶ ). This overexpression included CD44s as well as CD44v4- and v6-encoded epitopes and, importantly, was already observed in the earliest detectable neoplastic lesions, ie, in ACFs with dysplasia (Figure 2B) ▶ . It remained present in fully developed adenomas (Figure 2C) ▶ and invasive carcinomas (Figure 2D) ▶ . Analysis of CD44 mRNA in tumors versus normal mucosa by reverse transcription PCR and Southern blotting with exon-specific probes showed a preferential up-regulation of high molecular weight CD44 isoforms in tumor tissue (Figure 3) ▶ .
Figure 3.
CD44 mRNA expression in normal and neoplastic intestine of Apc+/Apc1638 mice. RT-PCR amplification products were generated with 5′ and 3′ CD44s primers, from specimens of normal mouse skin, normal mouse small intestine, and mouse intestinal tumor of Apc+/Apc1638 mice, respectively. Amplification products were analyzed on Southern blot by hybridization with 32P-labeled probes, specific for CD44s (A), CD44v3 (B), CD44v6 (C), and CD44v9 (D). *CD44v4-v10 plasmid does not contain exon v3 and therefore does not hybridize with the 32P-labeled exon v3 probe.
ACFs with Dysplasia in FAP Patients Overexpress CD44
Previous studies in human colorectal cancer have shown that CD44 overexpression is present in adenomas as well as in invasive carcinomas. 25,35,37 Based on our present observations in Apc-mutant mice, we explored whether dysplastic ACFs in familial adenomatous polyposis (FAP) patients also overexpress CD44. Indeed, a strong up-regulation of CD44, including both CD44s- and CD44v6-encoded epitopes, was observed in ACFs (Figure 4, B and D ▶ ; Table 1 ▶ ). Hence, as in Apc+/− mice, in FAP patients, deregulation of CD44 expression is present in the earliest detectable neoplastic lesions of colorectal cancer.
Figure 4.
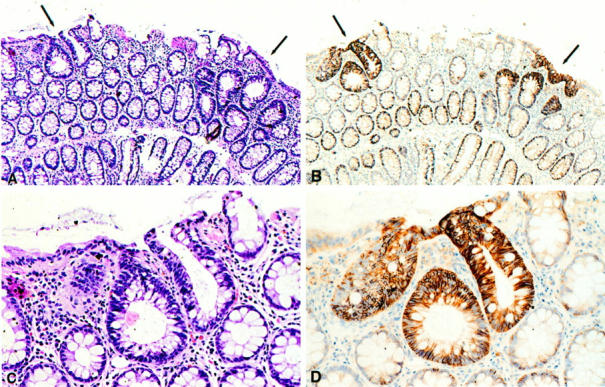
ACF with dysplasia in FAP patients overexpress CD44. Serial sections stained with either hematoxylin and eosin (A and C) or MAb VFF18 against human CD44v6 (B and D) in a dysplastic ACF in the colon of a FAP patient.
Tcf-4 Mutant Mice Lack CD44-Expressing Cells in the Epithelial Lining of the Small Intestine
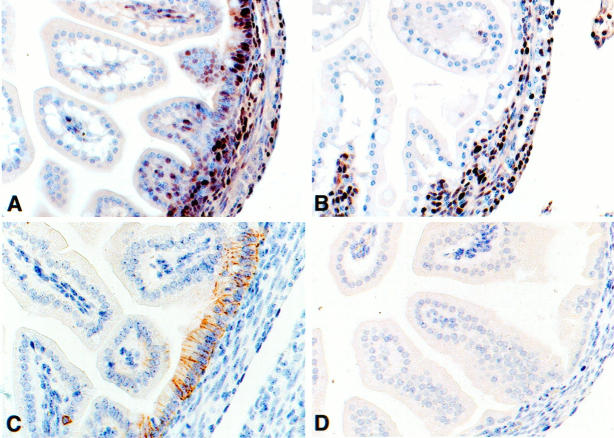
The above data imply an intimate link between loss of Apc tumor suppressor-protein function and CD44 overexpression in the intestinal mucosa and suggest that CD44 expression is directly or indirectly controlled by β-catenin/Tcf-4 mediated transcription. To further explore this hypothesis, we studied the expression of CD44 in the intestinal mucosa of Tcf-4−/− mice. These mice have a striking small-intestinal phenotype with a selective loss of cycling intestinal crypt cells. As a result of tearing of the intestinal epithelial lining and fluid imbalance, this leads to perinatal death. 43 In Figure 5 ▶ , this absence of cycling cells is illustrated by using the proliferation marker PCNA; at day E18, numerous proliferating cells were present in the intervillous epithelium of (Tcf-4+/+ and Tcf-4+/−) control mouse embryos (Figure 5A ▶ ; Table 1 ▶ ). By contrast, the epithelium of the small intestine of the Tcf-4−/− embryos was devoid of proliferating cells (Figure 5B) ▶ . Proliferation of cells in the lamina propria directly underneath the epithelium as well as in other organs (not shown) was not affected by the Tcf-4 disruption. In the (Tcf-4+/+ and Tcf-4+/−) control mice, CD44s (not shown) as well as CD44v6 expression (Figure 5C ▶ ; Table 1 ▶ ) was readily detectable at the basolateral surfaces of the pseudostratified intervillous epithelium. In sharp contrast, the intestinal epithelium of Tcf-4−/− mice did not show any CD44 staining (Figure 5D) ▶ . This lack of CD44 expression was specific for the epithelium of the small intestine, as Tcf-4−/− and control mice showed identical expression of both CD44s and CD44v6 in all other tissues, including the lamina propria of the small and large intestine, the epithelia of the large intestine, stomach, and epidermis and in lymphoid organs (not shown).
Figure 5.
Tcf-4−/− mice lack CD44 expression in the epithelial lining of the small intestine. Staining with proliferation marker PCNA of small intestinal crypts and villi of a wild-type mouse (A) and of a Tcf-4−/− mouse (B) and CD44v6 staining of small intestinal crypts and villi of a wild-type mouse (C) and of a Tcf-4−/− mouse (D).
Discussion
The development of colorectal cancer is initiated by mutations in either APC or in β-catenin leading to constitutively activated transcription of Tcf-4 target genes in intestinal epithelial cells. 3-10 However, the Tcf-4 target genes that are instrumental in the tumorigenesis process have not been identified yet. Our current studies in mice and humans with genetic defects in either APC or Tcf-4 indicate that expression of CD44, a glycoprotein family involved in cell-matrix adhesion and growth factor presentation, 12,31-34 is controlled by β-catenin/Tcf-4. Activation of β-catenin/Tcf-4 signaling, as present in intestinal tumors arising in Apc-mutant mice and FAP patients, is associated with CD44 overexpression. By contrast, blockade of β-catenin/Tcf-4 signaling by targeted disruption of Tcf-4 leads to a complete absence of CD44-bearing cells from the epithelium of the mouse small intestine.
Previous studies have shown that CD44 is overexpressed in human colorectal tumors. 24,25,35-40 This aberrant expression of CD44 occurs at an early point along the adenoma-carcinoma sequence; CD44 overexpression was already observed in small (<1 cm) adenomas, 25,37 suggesting a possible causal relation with loss of function of the APC tumor suppressor protein. Our present study, for the first time, demonstrates that CD44 is also overexpressed in intestinal tumors arising in Apc-mutant mice (Figures 2 and 3 ▶ ▶ ; Table 1 ▶ ). As in human colorectal cancer, this overexpression was present in adenomas as well as in invasive carcinomas and was accompanied by a deregulated splicing leading to a preferential overexpression of large CD44 isoforms containing variant exon sequences (Figure 3) ▶ . Importantly, in both mice and humans, loss of APC function and deregulation of CD44 were closely linked, as CD44 overexpression was already present in the earliest detectable neoplastic lesions, ie, in ACFs with dysplasia (Figures 2 and 4 ▶ ▶ ; Table 1 ▶ ). In humans, these lesions contain APC but not K-ras mutations. 47 Similarly, the tumors of the Apc-mutant mice used in our current studies showed loss of the wild-type copy of the Apc gene but neither K-, N-, H-ras, nor Tp53 mutations. 48 The precise mechanism by which the Wnt pathway regulates CD44 expression needs further exploration. β-catenin/Tcf-4 might directly interact with promoter/enhancer regions regulating CD44 transcription or, alternatively, involve intermediates, eg, c-Myc, a recently identified Tcf-4 target gene. 49
We observed that Tcf-4 knockout mice lack CD44 expression on the epithelium of the small intestine (Figure 5) ▶ . This loss of CD44 occurred in the context of a phenotype characterized by the absence of a proliferative stem cell compartment in the crypt regions between the villi (Figure 5) ▶ . As a consequence, the epithelium was composed entirely of nondividing cells that lack CD44. Although CD44+ and cycling (PCNA+) cells co-localize at the base of normal crypts, they represent overlapping but distinct cell compartments, indicating that CD44 expression is not directly linked to proliferation. 50 The TCF-4 −/− phenotype, including the loss of CD44, was unique for the small intestine; the crypt epithelium in other parts of the intestine was not affected by the mutation presumably as a result of redundancy with another member of the Tcf family that is expressed in the gut, albeit at lower levels, ie, Tcf-3. 43 The observations in Tcf-4 mutant mice indicate that the genetic program controlled by Tcf-4 establishes the crypt stem cell compartment of the small intestine and suggest that CD44 expression is part of this program. According to this interpretation, overexpression of CD44, as present in colorectal cancer, reflects the persistence of stem cell characteristics by the tumor cells. Indeed, it has been proposed that tumorigenesis in Min-mice is initiated in the multipotent stem cell compartment in the intestinal crypt. 51
Overexpression of CD44 glycoproteins in invasive human colorectal carcinomas is associated with the presence of (occult) metastases and with an unfavorable prognosis. 36,38,39,41 Although the mechanism(s) by which CD44 promotes colorectal tumorigenesis have not yet been defined, the following routes could be involved. First, CD44 is the principle cell-surface receptor for hyaluronan (HA), a ubiquitous glycosaminoglycan (GAG) component of the extracellular and pericellular matrices. 12,28 Interaction between CD44 and HA has been proposed to promote cell motility and, in some systems, enhances tumor growth and metastasis. 12,52 Second, in a recent study by Yu and colleagues, disruption of CD44 in metastatic mammary carcinoma cells was found to induce apoptosis, implying a role for CD44 in the regulation of programmed cell death. 53 CD44 overexpression may counteract apoptosis, leading to enhanced tumor growth and metastasis. Third, CD44 splice variants carrying exon v3 are decorated with heparan sulfate side chains and hence are heparan sulfate proteoglycans (HSPGs). 31 HSPGs are believed to play an important regulatory role in cell growth and motility by binding growth factors and by presenting these factors to their high-affinity receptors. 54,55 Indeed, CD44-HSPG has been shown to bind fibroblast growth factor (FGF)-2, 32 heparin-binding epidermal growth factor, 32 and hepatocyte growth factor/scatter factor (HGF/SF). 33 The latter interaction is of great potential interest, as HGF/SF functions as a growth and motility factor and promotes metastasis. 56-58 We recently demonstrated that binding of HGF/SF to CD44-HSPG strongly enhances signaling through the c-Met receptor tyrosine kinase, 33 the high-affinity receptor for HGF/SF. This collaboration between CD44-HSPG and c-Met might be an important factor in tumorigenesis. By overexpressing CD44-HSPG tumor cells would acquire an increased sensitivity to HGF/SF-mediated signals, leading to a growth advantage and promoting metastasis. This scenario is supported by the fact that c-Met and HGF/SF are overexpressed in conjunction with CD44 in several tumors, including colorectal cancer. 59-61 Except for binding of HGF/SF, crypt epithelial cells and tumor cells may also use CD44-HSPG to gather and present other heparin-binding growth factors. Interesting candidates are the ligands of the Wnt-Wingless pathway themselves, ie, the Wnt-like growth factors. In studies by Reichsman and co-workers, Wingless signaling was shown to be inhibited by removal of GAGs from cells, 62 and recent studies by Binari et al 63 and Haerry et al 64 have provided genetic evidence for a role of heparan sulfate in wingless signaling. It is tempting to speculate that binding of mesenchymally derived growth factors, including HGF/SF and WNT factors, to CD44 might contribute to the conversion of embryonic intestinal cells into crypt stem cells, which takes place during intestinal morphogenesis. 65,66
In conclusion, our data imply that CD44 expression in normal and malignant intestinal epithelium is regulated by the WNT pathway and suggest that CD44 expression is part of a genetic program controlled by the β-catenin/Tcf-4 signaling pathway and plays a role in the generation and turnover of epithelial cells.
Acknowledgments
We thank Dr. S. Jalkanen for MAb Hermes-3 and Dr. J. Sleeman for MAbs 9A4 and 10D1.
Footnotes
Address reprint requests to Dr. Steven T. Pals, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands. E-mail: s.t.pals@amc.uva.nl.
Supported by grants from the Praeventiefonds (project 28–2575), the Dutch Cancer Society (project UVA 98–1712 and RUL 94–817), and the NWO (project 901–01-166).
References
- 1.American Cancer Society: Cancer Facts and Figures—1994. Atlanta, GA, American Cancer Society, 1994
- 2.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AMM, Bos JL: Genetic alterations during colorectal-tumor development. N Engl J Med 1988, 319:525-532 [DOI] [PubMed] [Google Scholar]
- 3.Nagase H, Nakamura Y: Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat 1993, 2:425-434 [DOI] [PubMed] [Google Scholar]
- 4.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 5.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsius S, Polakis P: Association of the APC gene product with β-catenin. Science 1993, 262:1731-1734 [DOI] [PubMed] [Google Scholar]
- 6.Gumbiner B: Generation and maintenance of epithelial cell polarity. Curr Opin Cell Biol 1990, 2:881-887 [DOI] [PubMed] [Google Scholar]
- 7.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedll R, Birchmeier W: Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 8.Molenaar M, van de Wetering M, Oosterwegel H, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H: Xtcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 1996, 86:391-399 [DOI] [PubMed] [Google Scholar]
- 9.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 10.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 11.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfini E, Polakis P: Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 1997, 275:1790-1792 [DOI] [PubMed] [Google Scholar]
- 12.Lesley J, Hyman R, Kincade PW: CD44 and its interactions with the extracellular matrix. Adv Immunol 1993, 4:271-335 [DOI] [PubMed] [Google Scholar]
- 13.Stamenkovic I, Amiot M, Pesando JM, Seed B: A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell 1989, 56:1057-1063 [DOI] [PubMed] [Google Scholar]
- 14.Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P: A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cell lines. Cell 1991, 65:13-24 [DOI] [PubMed] [Google Scholar]
- 15.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth K, Bell JL: Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA 1992, 89:12160-12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalkanen S, Bargatze RF, de los Toyos J, Butcher EC: Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85–95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol 1987, 105:983-990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degrendle HC, Estress P, Siegelman MH: Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 1997, 278:672-675 [DOI] [PubMed] [Google Scholar]
- 18.Miyake K, Medina KL, Hayashi S, Ono S, Hamaoka T, Kincade PW: Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med 1990, 171:477-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horst E, Meijer CJLM, Radaszkiewicz T, Ossekoppele GJ, van Krieken JHJM, Pals ST: Adhesion molecules in the prognosis of diffuse large-cell lymphoma: expression of a lymphocyte homing receptor (CD44), LFA-1 (CD11a/18), and ICAM (CD54). Leukemia 1990, 4:595-599 [PubMed] [Google Scholar]
- 20.Jalkanen S, Joensuu H, Söderström KO, Klemi P: Lymphocyte homing and clinical behaviour of non-Hodgkin’s lymphoma. J Clin Invest 1991, 87:1835-1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sy MS, Guo YJ, Stamenkovic I: Inhibition of tumor growth in vivo with a soluble CD44-immunoglobulin fusion protein. J Exp Med 1992, 176:623-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koopman G, Heider KH, Horst E, Adolf GR, van den Berg F, Ponta H, Herrlich P, Pals ST: Activated human lymphocytes and aggressive non-Hodgkin’s lymphomas express a homologue of the rat metastasis-associated variant of CD44. J Exp Med 1993, 177:897-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stauder R, Eisterer W, Thaler J, Günthert U: CD44 variant isoforms in non-Hodgkin’s lymphomas: a new independent prognostic factor. Blood 1995, 85:2885-2899 [PubMed] [Google Scholar]
- 24.Matsumara Y, Tarin D: Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet 1992, 340:1053-1058 [DOI] [PubMed] [Google Scholar]
- 25.Wielenga VJM, Heider KH, Offerhaus GJA, Adolf GR, van den Berg F, Ponta H, Herrlich P, Pals ST: Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res 1993, 53:4754-4756 [PubMed] [Google Scholar]
- 26.Kauffmann M, Heider KH, Sinn HP, von Minckwitz G, Ponta H, Herrlich P: CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet 1995, 345:615-619 [DOI] [PubMed] [Google Scholar]
- 27.Lacy BE, Underhill CB: The hyaluronate receptor is associated with actin filaments. J Cell Biol 1987, 105:1395-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B: CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61:1303-1313 [DOI] [PubMed] [Google Scholar]
- 29.Kalomiris EL, Bourguignon LY: Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J Cell Biol 1988, 106:319-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukita SK, Oishi N, Sato J, Sagara A, Kawai Tsukita S: ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 1994, 126:391-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson D, Bell JI, Dickinson R, Timans J, Shields J, Whittle N: Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol 1995, 128:673-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett KL, Jackson DG, Simon JC, Tanczos E, Peach R, Modrell B, Stamenkovic I, Plowman G, Aruffo A: CD44 isoforms containing exon v3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol 1995, 128:687-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Voort R, Taher TEI, Wielenga VJM, Prevo R, Smit C, David G, Hartmann G, Gherhardi E, Pals ST: Hepatocyte growth factor/scatter factor presentation by a CD44 splice variant promotes signaling through c-Met receptor tyrosine kinase in tumor cells. J Biol Chem 1999 (in press) [DOI] [PubMed]
- 34.Tanaka Y, Adams DH, Hubscher S, Shaw S: T-cell adhesion induced by proteoglycan immobilized MIP-1b. Nature 1993, 361:69-72 [DOI] [PubMed] [Google Scholar]
- 35.Heider KH, Hofmann M, Horst E, van den Berg F, Ponta H, Herrlich P, Pals ST: A human homologue of the rat metastasis-associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol 1993, 120:227-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulder JWR, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, Offerhaus GJA, Pals ST: Colorectal cancer prognosis and expression of exon-v6 containing CD44 proteins. Lancet 1995, 344:1470-1472 [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Yang XL, Rosada C, Hamilton S, August T: CD44 expression in colorectal adenomas is an early event occurring prior to K-ras and p53 mutation. Arch Biochem Biophys 1994, 310:504-507 [DOI] [PubMed] [Google Scholar]
- 38.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM: Expression of CD44 and variant proteins in human colorectal cancer and its relevance for prognosis. Scand J Gastroenterol 1998, 33:303-309 [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi A, Urano T, Goi T, Saito M, Takeuchi K, Hirose K, Nakagawara G, Shiku H, Furukawa K: Expression of a CD44 variant containing exons 8 to 10 is a useful independent factor for the prediction of prognosis in colorectal cancer patients. J Clin Oncol 1996, 14:1122-1127 [DOI] [PubMed] [Google Scholar]
- 40.Imazeki F, Yokosuka O, Yamaguchi T, Ohto M, Isono K, Omata M: Expression of variant CD44-messenger RNA in colorectal adenocarcinomas and adenomatous polyps in humans. Gastroenterology 1996, 110:362-368 [DOI] [PubMed] [Google Scholar]
- 41.Wielenga VJM, van der Voort R, Mulder JWR, Kruyt PM, Weidema WF, Oosting J, Selderijk CA, van Krimpen C, Offerhaus GJA, Pals ST: CD44 splice variants as prognostic markers in colorectal cancer. Scand J Gastroenterol 1998, 33:82-87 [DOI] [PubMed] [Google Scholar]
- 42.Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt E, Lipkin M, Khan PM, Kucherlapati R: A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA 1994, 91:8969-8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H: Absence of epithelial stem cell compartments in Tcf-4−/− small intestine. Nature Genet 1998, 19:379-383 [DOI] [PubMed] [Google Scholar]
- 44.Heider KH, Sproll M, Susani S, Patzelt E, Beaumier P, Ostermann E, Ahorn H, Adolf GR: Characterization of a high-affinity monoclonal antibody specific for CD44v6 as candidate for immunotherapy of squamous cell carcinomas. Cancer Immunol Immunother 1996, 43:245-253 [DOI] [PubMed] [Google Scholar]
- 45.Weiss JM, Sleeman J, Renkl AC, Dittmar H, Termeer CC, Taxis S, Howells N, Hofmann M, Kohler G, Schopf E, Ponta H, Herrlich P, Simon JC: An essential role for CD44 variant isoforms in epidermal Langerhans cell and blood dendritic cell function. J Cell Biol 1997, 137:1137-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Voort R, Taher TEI, Keehnen RMJ, Smit L, Groenink M, Pals ST: Paracrine regulation of germinal center B cell adhesion through the c-Met-hepatocyte growth factor/scatter factor pathway. J Exp Med 1997, 185:2121-2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jen J, Powell SM, Papadopoulos N, Smith K, Hamilton SR, Vogelstein B, Kinzler KW: Molecular determinants of dysplasia in colorectal lesions. Cancer Res 1994, 54:5523-5526 [PubMed] [Google Scholar]
- 48.Smits R, Kartheuser A, Jagmohan-Changur S, Leblanc V, Breukel C, de Vries A, van Kranen H, van Krieken JH, Williamson S, Edelmann W, Kucherlapati R, Khan PM, Fodde R: Loss of Apc and the entire chromosome 18 but absence of mutations at the Ras and Tp53 genes in intestinal tumors from Apc1638N, a mouse model for Apc-driven carcinogenesis. Carcinogenesis 1997, 18:321-327 [DOI] [PubMed] [Google Scholar]
- 49.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-Myc as a target of the APC pathway. Science 1998, 281:1509-1512 [DOI] [PubMed] [Google Scholar]
- 50.Furuta K, Zahurak M, Yang XL, Rosada C, Goodman SN, August JT, Hamilton SR: Relationship between CD44 expression and cell proliferation in epithelium and stroma of colorectal neoplasms. Am J Pathol 1996, 149:1147-1155 [PMC free article] [PubMed] [Google Scholar]
- 51.Moser AR, Dove WF, Roth KA, Gordon JI: The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol 1992, 116:1517-1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I: Interaction between CD44 and hyaluronate is directely implicated in the regulation of tumor development. J Exp Med 1994, 180:53-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu Q, Toole BP, Stamenkovic I: Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med 1997, 186:1985-1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruoslahti E, Yamaguchi Y: Proteoglycans as modulators of growth factor activities. Cell 1991, 64:867-869 [DOI] [PubMed] [Google Scholar]
- 55.Schlessinger J, Lax I, Lemmon M: Regulation of growth factor activation by proteoglycans. What is the role of the low affinity receptors? Cell 1995, 83:357-360 [DOI] [PubMed] [Google Scholar]
- 56.Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W: Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol 1990, 111:2097-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giordano S, Zhen Z, Medico E, Gaudino G, Galimi F, Comoglio PM: Transfer of motogenic and invasive response to scatter factor/hepatocyte growth factor by transfection of human MET protooncogene. Proc Natl Acad Sci USA 1993, 90:649-653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rong S, Segal S, Anver M, Resau JH, Vande Woude GF: Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA 1994, 91:4731-4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Renzo MF, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L, Nordlinger B, Bretti S, Bottardi S, Giordano S, Plebani M, Gespach C, Comoglio PM: Overexpression and amplification of the Met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1995, 1:147-154 [PubMed] [Google Scholar]
- 60.Liu C, Park M, Tsao MS: Overexpression of c-met proto-oncogene but not epidermal growth factor receptor or c-erbB-2 in primary human colorectal carcinomas. Oncogene 1992, 7:181-185 [PubMed] [Google Scholar]
- 61.Prat M, Narishman RP, Crepaldi T, Nicotra MR, Natali PG, Comoglio PM: The receptor encoded by the human c-met oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer 1991, 49:323-328 [DOI] [PubMed] [Google Scholar]
- 62.Reichsman F, Smith L, Cumberledge S: Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J Cell Biol 1996, 135:819-827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Binari RC, Staveley BE, Johnson WA, Godavarti A, Sasisekharan R, Manoukian AS: Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development 1997, 124:2623-2632 [DOI] [PubMed] [Google Scholar]
- 64.Haerry TE, Heslip TR, Marsh JL, O’Connor MB: Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila. Development 1997, 124:3055-3064 [DOI] [PubMed] [Google Scholar]
- 65.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C: Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 1993, 123:223-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kediger M: Growth and development of intestinal mucosa. Campbell FC eds. Small Bowel Enterocyte Culture and Transplantation. 1994, :pp 1-30 RG Landes Co, Austin, TX, [Google Scholar]