Abstract
Protein phosphatase-directed toxins such as okadaic acid (OA) are general apoptosis inducers. We show that a protein (inhibitor of radiation- and OA-induced apoptosis, Irod/Ian5), belonging to the family of immune-associated nucleotide binding proteins, protected Jurkat T-cells against OA- and γ-radiation-induced apoptosis. Unlike previously described antiapoptotic proteins Irod/Ian5 did not protect against anti-Fas, tumor necrosis factor-α, staurosporine, UV-light, or a number of chemotherapeutic drugs. Irod antagonized a calmodulin-dependent protein kinase II-dependent step upstream of activation of caspase 3. Irod has predicted GTP-binding, coiled-coil, and membrane binding domains. Irod localized to the centrosomal/Golgi/endoplasmic reticulum compartment. Deletion of either the C-terminal membrane binding domain or the N-terminal GTP-binding domain did not affect the antiapoptotic function of Irod, nor the centrosomal localization. The middle part of Irod, containing the coiled-coil domain, was therefore responsible for centrosomal anchoring and resistance toward death. Being widely expressed and able to protect also nonimmune cells, the function of Irod may not be limited to the immune system. The function and localization of Irod indicate that the centrosome and calmodulin-dependent protein kinase II may have important roles in apoptosis signaling.
INTRODUCTION
Toxins such as okadaic acid (OA) can induce apoptosis in most, if not all, animal cells. The death can be caspase dependent or caspase independent, and although enhanced by p53 (Yan et al., 1997), can occur in cells devoid of p53 (Boe et al., 1991; Gjertsen et al., 1994; Fladmark et al., 1999; Morimoto et al., 1999; Sandal et al., 2001). The first step in OA action requires inhibition of both of the major serine/threonine protein phosphatases PP1 and PP2A (Fladmark et al., 1999). An early consequence of PP inhibition is hyperphosphorylation of a number of substrates of the multifunctional calmodulin-dependent protein kinase II (CaMKII). Inhibitors of CaMKII can abrogate OA-induced apoptosis (Fladmark et al., 2002).
To gain more insight into the apoptogenic action of PP inhibitors, a functional cDNA library-based screening was undertaken, by using a Jurkat T-cell cDNA fragment library, to identify cDNAs able to protect against OA-induced death. One resistant cell clone had a cDNA (termed Oar-2) corresponding to part of a human gene (GenBank accession no. AK002158) (Sandal et al., 2001). This gene has sequence similarity to a protein family characterized by GTP-binding and predicted protein–protein interaction domains of type coiled-coil or leucine-rich repeats. The protein family has members in plants mediating the hypersensitivity response to invading pathogens (Staskawicz et al., 1995; Moffett et al., 2002), and members expressed in the mammalian immune system (Ian proteins) with hitherto unknown function (Krucken et al., 1997; Poirier et al., 1999; Daheron et al., 2001; Cambot et al., 2002; Hornum et al., 2002; MacMurray et al., 2002). Little is known about the role of the GTP-binding domain, but it seems to be required for the response to pathogen of a plant resistance protein (Moffett et al., 2002). The high number of genes, 10 human and 11 murine (MacMurray et al., 2002), coding for mammalian Ian proteins, suggests functional diversity. This may be reflected by distinct subcellular localization. The Imap38-1/mIan2 is nuclear (Krucken et al., 1999), mIan4 is mitochondrial (Daheron et al., 2001), and hImap1/hIan2 is associated with the endoplasmic reticulum (ER) (Stamm et al., 2002). Recently, the rat ortholog of AK002158 was identified as a major genetic locus responsible for the lymphopenic autoimmune diabetes type I in the BB rat (Hornum et al., 2002; MacMurray et al., 2002). In the present study, the full-length cDNA of AK002158, cloned from a human spleen cDNA library, was demonstrated to protect specifically against death induced by OA or γ-radiation, and hence named inhibitor of radiation- and OA-induced death, Irod/Ian5, or Irod for short. Full-length Irod was distributed in the centrosome/Golgi/endoplasmic reticulum compartments, which is a novel finding for a member of this protein family. Truncated Irod devoid of its C-terminal putative membrane anchor had full antiapoptotic effect and was confined to the centrosomal area. The centrosome receives increasing attention as being a signaling center, and not merely responsible for microtubule organization (Bornens, 2002; Meraldi and Nigg, 2002). It contains the key enzymes (PP2A, PP1, and CaMKII) involved in the early steps of OA-induced apoptosis (Pietromonaco et al., 1995; Takahashi et al., 1999; Matsumoto and Maller, 2002).
To our knowledge, Irod/Ian5 is the first Ian protein with an experimentally established function, i.e., to inhibit a CaMKII-dependent apoptosis pathway induced in response to ionizing radiation, and mimicked by PP inhibitors such as okadaic acid. An intriguing observation was that the putative GTP-binding domain of Irod was dispensable for the antiapoptotic action. The widespread expression of Irod, and its ability to counteract apoptosis also in nonimmune cells, suggests that its function may not be restricted to the immune system.
MATERIALS AND METHODS
Materials
Okadaic acid (0-8010), biszbenzimide fluorochrome (Hoechst 33342), camptothecin (C-9911), staurosporin (S-4400), daunorubicin (D-8809), doxorubicin (D1515), cycloheximide (C-6255), tumor necrosis factor (TNF)-α (T-0157), and bleomycin were from Sigma-Aldrich (St. Louis, MO). The CaMKII inhibitor KN93 was from Seikagaku America (Ijamsville, MD), and the caspase inhibitor z-val-ala-DL-asp-fluormethylketone (zVAD-fmk) was from Bachem Feinchemikal A.G. (Bubendorf, Switzerland). The transfection reagent DMRIE-C was from Invitrogen (Paisley, United Kingdom).
Irod Cloning and Modification
A human spleen cDNA library (597; http://www.rzpd.de) was screened at the Deutsche Krebsforschungs Zentrum Resource Centre (Heidelberg, Germany), by using as probe the Oar2 cDNA fragment of 419 base pairs, isolated from an OA-resistant cell clone (Sandal et al., 2001). The clone DKFZp597C0168Q3 was sequenced and found to contain a cDNA (in pSPORT1) corresponding to the full-length of a human gene (AK002158) encoding a human protein with unknown function (Irod).
For cell transfections, the Irod cDNA was excised from pSPORT1 with EcoR1/BamH1 for sense ligation and with NotI/EcoR1 for antisense ligation into pcDNA3.1/Myc-His (Invitrogen, San Diego, CA).
To visualize the localization of Irod and enable control that the whole protein sequence was present at a particular locus in the cell, the hemagglutinin (HA) epitope (YPYDVPDYA) of the influenza virus was added in-frame (5′or 3′) with Irod. These constructs were cloned into the BamH1/EcoR1 site in a modified 96J-G (pJim-GFP) retrovirus vector (Lorens et al., 2000), in-frame with green fluorescent protein (GFP), resulting in HA-Irod-GFP and Irod-HA-GFP. Truncated versions of HA-Irod were constructed with deleted GTP/GDP-binding domain (ΔGTP/GDP; deletion of amino acid 1–143) and deleted trans-membrane domain (ΔTM; deletion of amino acid 282–307).
Cell Handling and Scoring of Apoptosis
Human embryonic kidney cells (293T) were cultured in DMEM. Jurkat E-6 T lymphoma cells (American Type Culture Collection, Rockville, MD) and human prostate carcinoma cells (LNCaP; American Type Culture Collection) were grown in RPMI 1640 medium. LnCap cells stably expressing Bcl-2 (Beham et al., 1997) was a kind gift from T.J. McDonnell (The MD Anderson Cancer Center, University of Texas, Houston, TX).
All media were supplemented with 10% heat-inactivated fetal calf serum. For assays of apoptosis induction, the Jurkat cells were grown at a density of 2 × 105/ml. The γ-radiation was 25 Gy by using a Cs137 γ source. 293T cells were seeded at a density of 2 × 104/cm2, transfected ∼18 h later, and subjected to apoptogenic stimuli ∼30 h thereafter. Apoptosis was determined as described and validated previously (Sandal et al., 2001, 2002). The background was generally no more than 5% apoptotic cells, and was subtracted from all individual calculations. Chromatin condensation was judged by microscopy of cells fixed in phosphate-buffered saline containing 2% formaldehyde and 10 μg/ml bisbenzimide (Hoechst 33342; Calbiochem, San Diego, CA).
Jurkat T-cells were transfected, using DMRIE-C, with pcDNA3.1 vector carrying Irod cDNA, antisense Irod cDNA (as-Irod), or the empty pcDNA3.1 vector. Stable transfectants were selected with G418 (400 μg/ml) for 4 wk. LNCap cells and 293T cells were transfected, using DMRIE-C and CaPO4 precipitation, respectively, with the pcDNA3.1 constructs described above, doped (1:8) with a GFP reporter gene in pCMX-SAH/Y145F (Ogawa et al., 1995).
The 293T cells were also transfected with pBabeMN retrovirus constructs containing the 419-base pair cDNA fragment originally isolated from an OA-resistant cell line (Sandal et al., 2001), with the HA-Irod-GFP and Irod-HA-GFP in pJim-GFP vector (see above), with truncated versions of HA-Irod, coexpressed with GFP, using the IRES bicistronic retroviral vector described previously (Sandal et al., 2002), and with Bcl-2 (in pBillNeo expression vector, a gift from T.J. McDonnell). To probe the role of CaMKII 293T cells were transfected with the autocamtide-2-related inhibitory peptide (AIP; KKALRRQEAVDAL) (Ishida et al., 1995), the autocamtide-3 derived inhibitory peptide (AC3-I; KKALHRQEAVDAL) (Braun and Schulman, 1995b), as well as the inactive AC3-I derived inhibitor (AC3-C). The expression vectors (pNP220 [AIP], pNP217 [AC3-I], pNP218 [AC3-C]) were kindly provided by Dr. Ulrich Bayer and Howard Schulman (Stanford University School of Medicine, Stanford, CA). See also Fladmark et al., 2002.
Northern Blot and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
RNA was extracted from cells by using the TRIzol Reagent (Invitrogen, Paisley, United Kingdom). For Northern blots, the RNA (20 μg/lane) was separated on a 1.2% formaldehyde/agarose gel. Irod-specific probes were produced by PCR on pcDNA3.1-Irod vector, resulting in probes specific for either full-length, 5′-end or 3′-end of the cDNA. Multiple tissue expression array (MTE) and multiple tissue Northern array (MTN; BD Biosciences Clontech) was hybridized with different Irod-specific probes according to the manufacturer's protocol.
To identify the RNA originating from the transfected DNA in Jurkat cells, cDNA was synthesized from total RNA samples using random hexamer primers and AMV-reverse transcriptase. The primary cDNA products were amplified by PCR. For detection of Irod expression, we used a T7 forward primer and an Irod-specific reverse primer (SP4): 5′-CATGCTCCATAGACCAC-3′. For detection of Irod-antisense expression a BHG reverse primer and an Irod-specific forward primer (SP5) 5′-GTTGACACGCCCTCCAT-3′. Both reactions were predicted to produce fragments of ∼1400 base pairs. T7 forward and BHG reverse primers, predicted to produce a 240-base pair fragment, were used to detect the expression of control vector.
Western Blot Analysis
Cell extraction, electrophoresis, blotting, and detection of immunoreactive proteins was as described previously (Sandal et al., 2002). The membranes were probed with 0.2% monoclonal mouse anti MDM2 (SMP14; Santa Cruz Biotechnology, Santa Cruz, CA), 0.2% monoclonal mouse anti p53 (Bp53-12; Santa Cruz Biotechnology), 1 μg/ml of polyclonal rabbit anti-caspase3/CPP32 antibody (AHZ0052; Biosource International, Nivelles, Belgium), 0.4% monoclonal mouse anti-Bcl-2 (ZS18-0193), 0.2% polyclonal rabbit anti-HA (Zymed Laboratories, South San Francisco, CA), 1 μg/ml polyclonal rabbit anti-poly(ADP-ribose) polymerase (PARP) (06-557; Upstate Biotechnology, Lace Placid, NY), or 0.1% polyclonal rabbit anti-Bax (N-20; Santa Cruz Biotechnology). Blots were reprobed with 0.01% monoclonal mouse anti β-actin (AC-15-ab6276, Abcam Limited, Cambridge, UK).
Immunofluorescence
293T cells growing on coverslips were transfected with Irod-GFP fusion constructs carrying a C- or N-terminal HA epitope (see above) and fixed 30 h thereafter. They were processed for immunofluorescence as described by Srinivasan et al., (1994), except that the cells were permeabilized with 0.1% Triton X-100, and further incubated overnight at 4°C with 1 mg/ml bovine serum albumin and 0.5% NP-40. Immunostaining was performed using mAb to the HA tag (clone12CA5; Roche Diagnostics, Indianapolis, IN), mAb to the endoplasmic reticulum marker calnexin (a gift from Dr. Ari Helenius, Swiss Federal Institute of Technology, Zurich, Switzerland), or polyclonal antibody to the Golgi marker mannosidase II (a gift from Dr. Kelley Moremen, University of Georgia, Athens, GA). The secondary antibodies (Coulter-Immunotec, Marseille, France) were tetramethylrhodamine B isothiocyanate labeled and used at 20 μg/ml. Mitochondria were visualized by staining with MitoTracker dye on live cells, according to manufacturer's protocol (Molecular Probes, Leiden, The Netherlands). Fluorescence was analyzed using a DM IRBE microscope (Leica, Heidelberg, Germany), equipped with fluorescein isothiocyanate and tetramethylrhodamine B isothiocyanate filter optics (Leica). Photomicrographs and image processing was performed using either Openlab software packages (Improvision, Coventry, United Kingdom) or Leica laser scanning confocal NT by using Leica NT software.
Bioinformatics and Statistical Analysis
The identification of putative GTP-binding domain and putative membrane binding domains of Irod was by SMART analysis (http://smart.embl-heidelberg.de/) and of putative coiled–coil regions by COILS (Lupas et al., 1991, 1996a,b; http://www.ch.embnet.org/software/COILS_form.html. Multiple sequence alignment of human (AK002158) rat (AY055776) and mouse (MacMurray et al., 2002) Irod was performed using the Clustal W multiple sequence alignment package. For determination of statistical significance the Wilcoxon paired signed rank test was used.
RESULTS
Irod Protects Jurkat T-Cells Specifically against Okadaic Acid- and γ-Radiation-induced Apoptosis
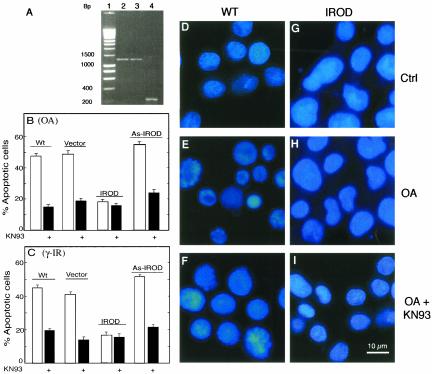
The previously described short cDNA fragment (Oar2) of the gene AK002158 (Irod/Ian5), which in fibroblasts protected against OA-induced death, could act by a number of mechanisms (Sandal et al., 2001). We reasoned that if Oar2 cDNA coded for a dominantly negative peptide counteracting the proapoptotic action of full-length Irod/Ian5, its action should be mimicked by transfection with antisense-Irod cDNA. If it encoded a proapoptotic peptide, its action should be mimicked by overexpression of full-length Irod. To test this experimentally we cloned AK002158 (Irod) from a human spleen cDNA library, by using Oar-2 cDNA as probe, and produced sense and antisense constructs for cell transfection. Stable expression of the transfected genes in Jurkat T-lymphoma cells was confirmed by RT-PCR (Figure 1A). The Irod cells resisted OA-induced apoptosis better than wild-type cells and cells expressing empty vector (Figure 1, B, E, and H). On the other hand, cells transfected with antisense-Irod cDNA had increased sensitivity to OA-induced death compared with wild-type cells or cells transfected with empty vector (p < 0.004 as judged by the Wilcoxon paired comparison test). This suggested that Irod itself had an antiapoptotic function.
Figure 1.
Irod confers resistance selectively to cell death induced by OA and γ-radiation in Jurkat T-cells. (A) Expression, determined by RT-PCR, in stably transfected Jurkat T-cell clones, of pcDNA3.1-Irod (lane 2), pcDNA3.1-as-Irod (lane 3), and pcDNA3.1-empty vector (lane 4). (B) Percentage of apoptotic cells after 6-h treatment with 800 nM OA in the absence (open bars) or presence (filled bars) of the CaMKII inhibitor KN93 (20 μM). (C) Percentage of apoptotic cells 6 h after exposure to γ-radiation (25 Gy). The cells were incubated in the absence (open bars) or presence (filled bars) of KN93 (20 μM). Data in B and C represent the mean of at least four experiments ± SEM. The background, including KN93 treatment alone, was generally not >5% and was subtracted from all calculations. (D–I) Cells stained with the DNA binding fluorescent dye (Hoechst 33342) after 6 h of incubation with vehicle (D and G), 800 nM OA (E and H), or 800 nM OA + 20 μM KN93 (F and I). Wild-type cells are shown in D–F, and Irod-expressing cells in G–I. For further experimental details, see the MATERIALS AND METHODS.
The specificity of the antiapoptotic effect of Irod was explored using different death inducers. We first subjected the cells to γ-radiation, to which lymphoid cells are particularly sensitive (Rudner et al., 2001). The cells overexpressing Irod showed similar resistance toward apoptosis induced by γ-radiation or OA (Table 1 and Figure 1, B and C). The as-Irod–transfected cells had a significantly higher rate of apoptosis in response to γ-radiation than either wild-type cells (p < 0.005) or cells transfected with empty vector (p < 0.004). This suggested that the Jurkat cells expressed enough endogenous Irod to protect against apoptosis.
Table 1.
The effect of various death inducers on Jurkat cell clonesa
|
Percent apoptotic cells after treatment -/+
SEMb
| |||||||
|---|---|---|---|---|---|---|---|
| Treatment (drug, time, concentration) | WT | WT (+ KN93) | pcDNA3.1 | IROD | IROD (+ KN93) | As-IROD | IROD/As-IRODc |
| Okadaic acid, 6 h, 800 nM | 47.8 ± 4.0 | 18.2 ± 3.2 | 45.6 ± 4.0 | 17.3 ± 1.0 | 17.3 ± 2 | 55.2 ± 2.8 | 0.31 |
| γ-radiation (25 Gy) + 6 h postincubation | 41.8 ± 3.2 | 18.4 ± 1.4 | 45.6 ± 3.6 | 18.7 ± 1.4 | 14.2 ± 2.7 | 57.1 ± 3.0 | 0.33 |
| Camptothecin, 5 h, 1 μM | 39.3 ± 2.8 | 35.9 ± 1.5 | 44.5 ± 1.3 | 39.0 ± 1.7 | 38.3 ± 1.5 | 51.3 ± 2.3 | 0.76 |
| Serum deprivation, 24 h | 19.7 ± 2.5 | — | 23.2 ± 2.3 | 17.2 ± 2.1 | — | 21.5 ± 1.2 | 0.80 |
| Bleomycin, 6.5 h, 1000 μg/ml | 35.4 ± 1.8 | 37.3 ± 1.5 | 35.6 ± 2.7 | 32.7 ± 0.5 | 33.8 ± 1.5 | 39.3 ± 1.6 | 0.83 |
| TNFα, 6 h, 100 ng/mld | 44.2 ± 3.5 | 40.6 ± 0.6 | 55.6 ± 2.4 | 45.5 ± 3.4 | 35.7 ± 2 | 51.1 ± 3.5 | 0.89 |
| Daunorubicin, 2 h, 5 μM | 34.7 ± 1.5 | 30.9 ± 1.5 | 44.4 ± 4.5 | 42.0 ± 3 | 34.5 ± 2.9 | 45.7 ± 4.5 | 0.92 |
| Doxorubicin, 3 h, 50 nM | 36.6 ± 1.3 | — | 34.4 ± 1.1 | 30.0 ± 4 | — | 31.8 ± 2.6 | 0.94 |
| Staurosporin, 3 h, 300 nM | 56.6 ± 1.3 | 59.1 ± 0.5 | 55.9 ± 4 | 56.1 ± 1.3 | 58 ± 1.2 | 59.3 ± 0.5 | 0.95 |
| UVC radiation (250 nm, 0.5 h), 24 h postincub. | 62.8 ± 3.8 | — | 62.1 ± 4 | 60.4 ± 4.6 | — | 58.7 ± 1.8 | 1.0 |
| Anti-Fas, 5 h, 50 ng/ml | 27.7 ± 5 | 41.6 ± 1.9 | 36.1 ± 5.5 | 29.5 ± 4.4 | 35.5 ± 1.6 | 22 ± 2.6 | 1.3 |
Cells were treated at a density of 0.3 × 106 cells/ml with different death inducers, for time periods and concentrations indicated, followed by fixation in 4% formaldehyde containing Hoechst DNA staining. Apoptotic cells were scored as described in experimental section.
Data represent the mean ± SEM of at least three separate experiments.
Difference in sensitivity towards the various apoptosis inducers in Irod and as-Irod expressing cells was expressed as the ratio IROD/As-IROD, and death inducers were ranked according to this value.
Cotreated with cyclohexemide, 1 μg/ml, for 5 hours. When present, KN93 (20 μM) was added together with the death inducer.
The cells overexpressing Irod were not protected against UV-C treatment (Table 1). Ionizing radiation induces double strand breaks in DNA, whereas UV-C radiation is believed to induce apoptosis mainly through single-strand DNA damage (Lu et al., 1998; Lakin and Jackson, 1999). It was therefore tested whether cells with enforced Irod expression were protected against bleomycin, which is a radiomimetic agent believed to induce apoptosis mainly via the induction of double-strand breaks in DNA (Benitez-Bribiesca and Sanchez-Suarez, 1999; Tounekti et al., 2001), or camptothecin, which is a topoisomerase inhibitor known to induce double-strand breaks (Wu et al., 2002). Irod overexpression afforded only a minor, if any, protection against any of these DNA damage-inducing agents (Table 1).
In the next series of experiments, the ability of Irod to protect against CD95 (Fas/Apo-1)-mediated apoptosis was tested. Ionizing radiation is believed to activate CD95 (Kasibhatla et al., 1998; Fujimori et al., 2000; Kasibhatla et al., 2000), and apoptosis in Jurkat cells induced by ionizing radiation and CD95-ligation is subject to common regulation downstream of caspase 8 activation (Boesen-de Cock et al., 1999). The Irod-overexpressing cells were not protected against CD95 ligation (Table 1), suggesting that Irod acted at a step in the γ-radiation pathway not shared by CD95-mediated apoptosis. The specificity of Irod was illustrated further by its inability to protect against apoptosis induced by serum withdrawal, daunorubicin, doxorubicin, or staurosporin (Table 1).
OA and γ-Radiation Induce Apoptosis through a CaMKII-dependent Pathway Inhibited by Irod, Upstream of Caspase 3 Cleavage
We have recently shown that CaMKII is activated by PP inhibitors such as OA and that CaMKII activity is required for rapid and efficient PP inhibitor-induced death (Fladmark et al., 2002). If apoptosis induced by γ-radiation and OA shared a common pathway inhibited by Irod, one might expect the CaMKII inhibitor KN93 to protect against γ-radiation as well as against OA. In fact, KN93 protected to similar extent against OA- and γ-radiation-induced apoptosis (Figure 1, B and C, and Table 1). The CaMKII inhibitor and Irod were similar also in not protecting against bleomycin, camptothecin, daunorubicin, TNF-α, staurosporin, and anti-Fas (Table 1). The addition of KN93 did not additionally protect cells overexpressing Irod against either OA (Figure 1, B, F, and I) or γ-radiation (Figure 1C). This suggested that Irod might block a CaMKII-dependent step, possibly common for the death pathway induced by γ-radiation and OA.
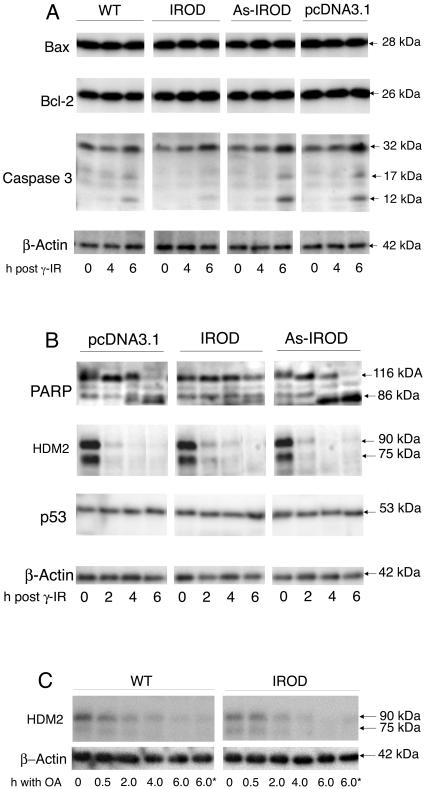
The degradation of the caspase substrate PARP and of procaspase 3 occurred slightly earlier in as-Irod transfectants than wild-type cells. These events were retarded in cells with enforced expression of Irod (Figure 2A). This indicated that Irod acted upstream of caspase 3 cleavage in the death pathway.
Figure 2.
Irod protects against caspase 3 and PARP cleavage, but not HDM2 down-regulation. Jurkat T-cells, wild-type (WT), expressing empty vector (pcDNA3.1), Irod (IROD), or Irod in antisense direction (As-IROD) were irradiated with 25 Gy (A and B) and incubated for up to 6 h post-γ-radiation, or treated with 800 nM OA in the absence or presence (*) of 100 μM caspase inhibitor zVAD-fmk (C). Extracts were processed for Western blotting. The position and size of caspase 3, Bcl-2, Bax, PARP, HDM2, p53, and β-actin is indicated. Note stronger cleavage of caspase 3 (A) and PARP (B) in cells expressing as-Irod, and less cleavage in cells expressing Irod. Also note the rapid down-regulation of HDM2 in response to γ-radiation (B) and OA treatment (C). Similar results were obtained in at least three separate experiments.
To know whether Irod affected the expression level of known apoptosis-modulating proteins, the level of p53, the proapoptotic Bax, and the antiapoptotic Bcl-2 protein was compared in wild-type cells and Jurkat cells stably transfected with Irod or as-Irod cDNA. No difference was noted in basal expression (Figure 2). The Jurkat cell p53 level was stable after irradiation. This suggested either that Jurkat cell p53, which is mutated in the C-terminal domain responsible for transactivation (Cheng and Haas, 1990), was unable to be up-regulated after MDM2 down-regulation, or that MDM2 down-regulation did not occur.
We examined next whether the ubiquitin esterase p90 human MDM2 (HDM2) was down-regulated by irradiation or OA treatment. We also considered the possibility that the antiapoptotic effect of Irod could be explained by increase of the p76 splice variant of HDM2. A high expression of HDM2 is associated with hypersensitivity to ionizing radiation (Dilla et al., 2002), and its 76-kDa splice variant, preferentially expressed in cells with high sensitivity to ionizing radiation, antagonizes p90 HDM2 (Perry et al., 2000; Evans et al., 2001). Enforced expression of Irod did not affect the resting level of either p90 or p76 HDM2. Neither did it modulate the rapid down-regulation of p90 and p76 HDM2 in response to either γ-radiation or OA (Figure 2, B and C), suggesting that Irod might act downstream of this step.
Irod Is Broadly Expressed and Can Confer OA Resistance to Cells Not Related to the Immune System
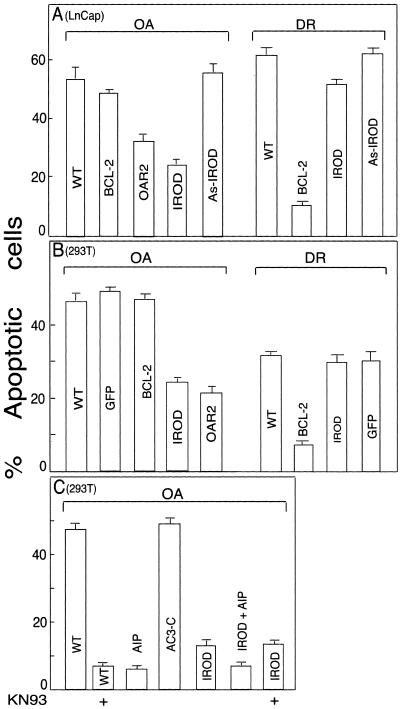
The AK002158 gene was first identified through a general sequencing effort of a human prostate cDNA library, suggesting that Irod could be expressed and have functions outside the immune system. We tested therefore whether stable enforced expression of Irod could protect also LNCaP prostate carcinoma cells against apoptosis. Irod protected the LNCaP cells better against OA-induced than daunorubicin-induced apoptosis. In contrast, LNCaP cells with stable overexpression of Bcl-2 were protected against daunorubicin-induced death and not against OA (Figure 3A). This indicated that Irod acted also on nonimmune cells, and on a death pathway that was completely different from that counteracted by Bcl-2. It could be argued that stable overexpression of Irod or Bcl-2 had led to the selection of cells with secondary properties responsible for the protection against apoptosis. To minimize this risk, we transfected 293T cells transiently with Irod cDNA, and scored them for apoptosis within 48 h after the start of transfection. Such cells were also protected selectively against OA (Figure 3B), suggesting that Irod itself was the antiapoptotic factor. Again, Bcl-2 protected against daunorubin-induced death and failed to protect against OA (Figure 3B).
Figure 3.
Effects of irod, Bcl-2, and specific CaMKII-inhibitors on OA- and daunorubicin-induced apoptosis in nonimmune cells. (A) Apoptotic response to OA (100 nM for 4 h) or 4 μM daunorubicine (DR) for 10 h, of wild-type LNCap cells (WT), or cells stably transfected with Bcl-2, Irod, as-Irod, or cDNA encoding a peptide containing the Irod235–285 sequence (Oar2). (B) Apoptotic response to OA (600 nM for 6 h), or DR (4 μM for 10h), in 293T cells transiently transfected with constructs carrying Bcl-2, Irod, Oar2, or GFP reporter (GFP), compared with WT cells. (C) Effect on OA-induced 293T cell apoptosis of enforced expression of an active CaMKII peptide inhibitor AIP and an inactive control peptide AC3-C, alone or together with Irod. In some experiments of C, indicated on the abscissa, 20 μM KN93 was present together with OA. Data in A–C are shown with SEM (n = 4).
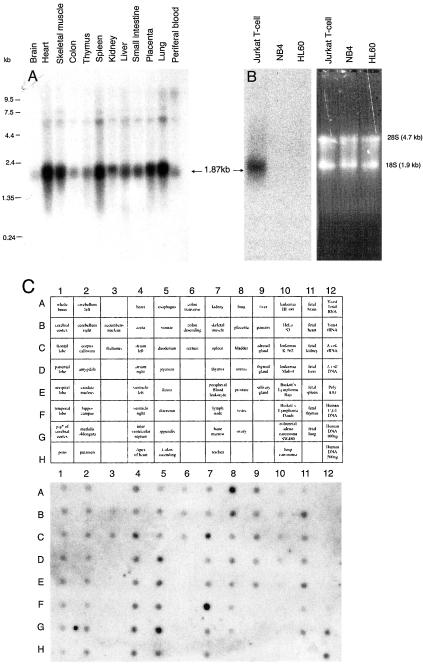
The role of CaMKII activity in OA-induced 293T, cell death was established by using the cell-permeable inhibitor KN93 (for discussions of its specificity, see Fladmark et al., 2002) and expression vectors for specific peptide inhibitors of CaMKII. The specificity of these inhibitors is described by Braun and Schulman (1995a). We observed as efficient inhibition of OA-induced death in cells transfected with the autocamptide-2–related inhibitor KKALRRQEVDAL (AIP) as in cells treated with KN93 (Figure 3C). The related inhibitor KKALHRQEVDAL had similar efficiency (our unpublished data), whereas the negative control peptide (KKALDGEEAVDAL) was inefficient (Figure 3C). Cells transfected with CaMKII inhibitor were not additionally protected by Irod cotransfection (Figure 3C). The ability of Irod to protect also nonimmune cells against apoptosis prompted us to study the tissue distribution of Irod mRNA. A 1.8-kb mRNA band was detected in Jurkat T-cells and a number of human tissues, by using a probe specific for exon 3 of Irod (Figure 4A). A broad tissue distribution of Irod mRNA was revealed also by dot blot analysis (Figure 4, C and D). A probe specific for exon 2 revealed a similar size mRNA and similar tissue distribution (Sandal and Døskeland, unpublished data), suggesting a strict coexpression of exons 2 and 3. We conclude that Irod is expressed in a number of nonimmune tissues, some of which (like skeletal muscle and heart) contain mainly proliferatively quiescent long-lived cells.
Figure 4.
Expression of Irod mRNA in human tissues and selected cell lines. (A) Multiple human tissue Northern blot. (B) Northern blot of Jurkat T-cells and two human AML cell lines. The positions of 28S and 18S rRNA is shown as reference. (C) Multiple human tissue expression array. The origin and position of RNA distributed on the array is indicated in the top subpanel. All blots were hybridized with a probe specific for exon 3 of Irod.
Subcellular Localization of Full-Length and Truncated Irod Suggests the Presence of Separate Anchoring Domains for the Golgi/ER and Centrosomal Compartments
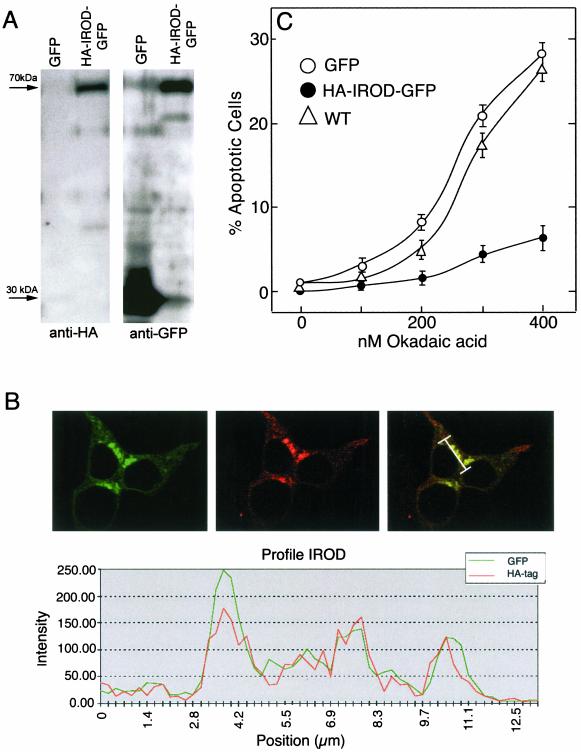
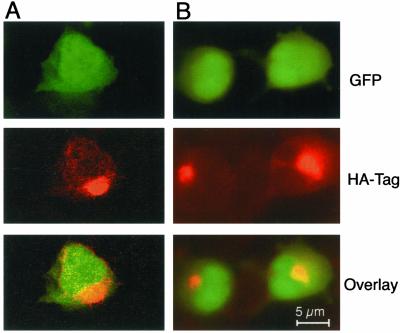
We questioned why Oar-2 cDNA, coding for only a small portion of Irod, could protect against apoptosis as well as full-length Irod cDNA. One explanation could be that Irod was processed in cellulo to a fragment encompassing the Oar-2 coded sequence. To know whether Irod was processed, 293T cells were transfected with cDNA for Irod with an N-terminal HA-tag and a C-terminal GFP marker. Anti-GFP or anti-HA recognized a single peptide band (70 kDa), as predicted for full-length HA-Irod-GFP (Figure 5A). Furthermore, a striking colocalization of GFP and HA was revealed at the subcellular level (Figure 5B). The HA-Irod-GFP–expressing cells were protected against OA-induced apoptosis to similar extent as cells expressing wild-type Irod (Figure 5C), suggesting that the tagged protein has full biological activity and therefore was likely to have folded correctly in the cell. We conclude that Irod is stable and can protect against apoptosis as a full-length protein.
Figure 5.
N- and C-Terminally tagged Irod is expressed as an intact fusion protein with antiapoptotic activity. (A) Western blots of 293T cells transiently transfected with pJim-retrovirus vector carrying GFP alone (GFP) or encoding Irod tagged with HA at the N-terminal and fused with GFP at the C-terminal (HA-IROD-GFP), probed with either anti GFP (right lane) or anti HA (left lane). (B) Scanning confocal micrograph of 293T cells transfected with HA-IROD-GFP and illuminated to show GFP expression (left), or the immunofluorescent staining of HA-epitope (middle). The right subpanel is an overlay. The profile (bottom subpanel) represents the fluorescent intensity corresponding to GFP and anti-HA along a section of 13 μm (see bar in the right subpanel). (C) Apoptotic response to OA in 293T cells transiently transfected with HA-IROD-GFP (•), or the empty vector (GFP (○), compared with wild-type cells (▵). Data represents the mean of three separate experiments ± SEM.
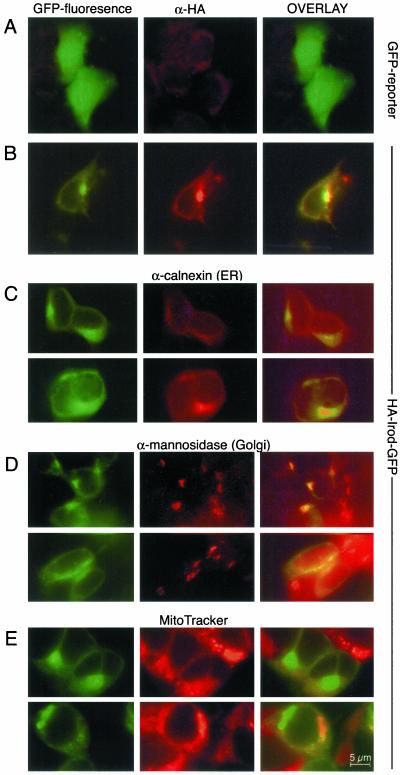
The perinuclear localization of HA-Irod-GFP (Figure 6, B–E, left), suggested a localization of HA-Irod-GFP within centrosomal/Golgi/ER regions. In fact, we observed colocalization of Irod with the ER marker calnexin (Figure 6C), as well as with the Golgi marker mannosidase II (Figure 6D). We did not find any consistent colocalization with a mitochondria-specific dye (Figure 6E). This contrasts with the findings for HA-tagged mIan4, which localized to mitochondria when expressed in 293T cells (Daheron et al., 2001). The possibility remained that Irod could translocate to the mitochondria during apoptogenic stress, but we did not detect any such redistribution of Irod in cells treated with OA (Sandal and Døskeland, unpublished data).
Figure 6.
Subcellular localization of Irod. The top (A) panels show 293T cells transfected with GFP reporter alone. The other panels (B–E) show cells expressing HA-Irod-GFP. The left-hand subpanels show the endogenous GFP fluorescence, the middle subpanels show immunofluorescent staining of the HA-eiptope (A and B), of the ER marker calnexin (C), or the Golgi-marker mannosidase II (D). (E) Distribution of the mitochondrial marker MitoTracker. The right-hand subpanels represent merging of the left and middle images.
Irod has a putative C-terminal transmembrane domain. Irod with this domain deleted (ΔTM-IrodΔ281–307) was mainly associated with the perinuclear, centrosomal region, and had decreased association to the ER and Golgi (Figure 7). This suggested that Irod contained a centrosomal anchoring sequence proximal to the transmembrane domain. Again, Irod behaved differently from mIan4, which assumed a diffuse cytoplasmic distribution when its C-terminal mitochondrial anchor was deleted (Daheron et al., 2001).
Figure 7.
Irod devoid of the predicted transmembrane domain localized to the centrosomal/Golgi region. The experiment was conducted as described in the legend to Figure 5B, except that the 293T cells were transfected with HA-Irod-ΔTM and GFP in a bicistronic vector. Whereas GFP was generally distributed in the cell (top), anti-HA immunostaining of HA-IROD-TM (middle) revealed a distinct, perinuclear labeling, suggesting centrosomal/Golgi localization. Photomicrographs and image processing was performed using either scanning confocal Leica NT software (A) or Openlab (Improvision) software packages (B).
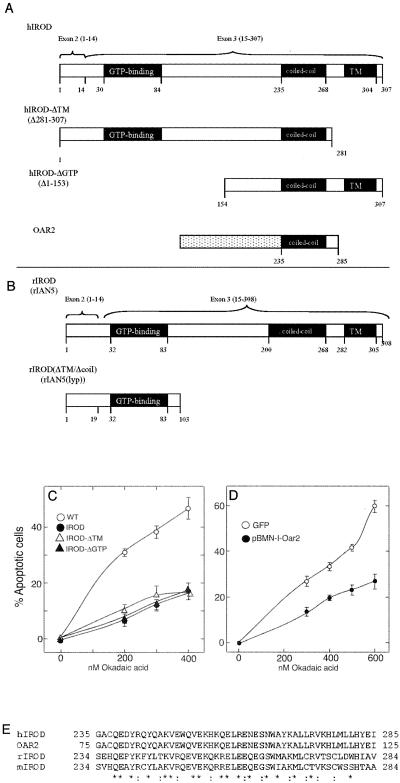
GTP-binding and Golgi/ER-anchoring Domains of Irod Are Dispensable for Its Antiapoptotic Effect
The 307-residue-long Irod has, in addition the hydrophobic C-terminal domain, an N-terminal highly conserved GTP-ase domain (Figure 8A). Cells expressing Irod lacking the GTP-ase domain (HA-IrodΔ1-153) or the transmembrane domain (HA-IrodΔ281-307) were protected from death as well as cells expressing wild-type Irod (Figure 8C). This indicates that residues 153–280 were responsible for the antiapoptotic action of Irod. This region is predicted to contain mainly α-helices, with coiled-coil forming potential from residues 255–280 (Figure 8). The short Oar-2 cDNA fragment (Sandal et al., 2001) was able to attenuate OA-induced apoptosis when stably transfected into prostate carcinoma cells (Figure 3A). It was also efficient when transiently transfected in 293T cells, whether controlled by a cytomegalovirus promoter (Figure 3B) or a long terminal repeat retroviral promoter (Figure 8D). The Oar-2 cDNA encodes for residues 235–285 of Irod, which include the putative coiled-coil region (Figure 8), suggesting that this region may be pivotal for the antiapoptotic effect of Irod.
Figure 8.
Antiapoptotic effect of Irod is confined to its central domain. (A) Exons 2 and 3 and the domain composition of Irod are shown, and the truncated Irod forms coded for by the constructs used in the experiments in C and D. The numbers refers to amino acid residues. The dotted box represents the leading sequence of Oar2. (B) Rat ortholog of Irod (rIrod/Ian5) and the truncated version, rIAN5(lyp) of it, resulting from the frameshift mutation of this gene in the diabetes-prone BB rat. The 19 residue tail of rIROD(lyp) is not encoded by the rIrod gene. (C) Apoptotic response to OA of 293T cells transiently tranfected with a pJIM-IRES-GFP vector for bicistronic expression of GFP and HA-Irod with deleted transmembrane domain (IROD-ΔTM, ▵), Irod with deleted GTP-binding domain (IROD-ΔGTP, ▴), or intact Irod (IROD, •). (D) Response to 4-h treatment with OA in 293T cells transiently transfected with Oar2 cDNA in the pBabeMN-retrovirus construct or with GFP reporter alone. Data represent the mean of three separate experiments ± SEM. (E) Amino acid sequence alignment of human (accession no. AK002158), rat (accession no. AY055776), and mouse (MacMurray et al., 2002) Irod in a predicted coiled-coil region. *, identical amino acids;:, conserved amino acids.
DISCUSSION
Irod/Ian5 belongs to a family of proteins with a conserved GTP-binding site, a protein interaction domain, often including a coiled-coil region, and sometimes a transmembrane domain at the C termini (MacMurray et al., 2002; see also Cambot et al., 2002; Stamm et al., 2002) and Figure 8). The wide tissue distribution of Irod, and its ability to protect also nonimmune cells against apoptosis, suggest that its function may not be restricted to the immune system. The present study is the first direct demonstration of a distinct effect on a cellular process, i.e., antagonism of apoptotic death, for this type of protein in a nonplant cell. The antiapoptotic activity of Irod contrasts with the proapoptotic role of the plant members of the protein family. Another difference is that the GTP-binding domain was dispensable for the antiapoptotic Irod action, whereas both the coiled-coil and the nucleotide binding domain must be expressed for plant resistance protein to be functional (Moffett et al., 2002). It seems therefore that Irod has evolved differently from the plant resistance proteins not only with respect to overall function (anti-rather than proapoptotic), but also with respect to the mechanistic role of the domains.
Jurkat T-cells transfected with antisense-Irod cDNA became hypersensitive to γ-radiation and OA, suggesting that the endogenous level of Irod in Jurkat cells can protect against cell death. The level of Irod mRNA in Jurkat cells was comparable to that in many tissues, implying that the basal expression of Irod may be sufficient to protect cells in the intact organism. An in vivo role of Irod is supported by recent observations in the BB rat. In this animal model of autoimmune diabetes type I with lymphopenia, a frameshift mutation creates a stop codon just C-terminal for the GTP-binding domain of rIrod/Ian5 (Hornum et al., 2002; MacMurray et al., 2002). The immune cells of the BB rat had strongly decreased expression of mRNA for rIrod/Ian5 (Hornum et al., 2002), leaving the question open of whether the cells lack rIrod/Ian5 or produce a truncated protein. Our finding that Irod has an antiapoptotic action, depending on its central coiled-coil containing domain, but not its GTP-binding domain, suggest that Irod, if expressed in the BB rat, will be nonfunctional with respect to apoptosis antagonism. It is possible that the subset of T-cells lacking in the BB rat may be particularly sensitive to death inducers sharing the death pathway(s) used by γ-radiation and phosphatase inhibitors such as OA.
In contrast to Irod (Table 1), the members of the IAP family of antiapoptotic proteins protect against a broad spectrum of apoptogens, including anti-CD95 (Fas/Apo-1), TNF-α, staurosporine, and etoposide (reviewed in Deveraux and Reed, 1999). Likewise, the antiapoptotic members of the Bcl-2 family protect T cells and other cells against a number of death inducers, such as staurosporine and daunorubicin (Figure 3, A and B), etoposide, and UV-light (Strasser et al., 1995; Adrian et al., 2001). It is an apparent paradox that autoimmunity can be associated with disrupted expression of the survival protein Irod (see above), as well as with overexpression of the survival protein Bcl-2 (Strasser et al., 1991; Garchon et al., 1994; Mandik-Nayak et al., 2000). This may be related to the difference between Irod and Bcl-2 with respect to the apoptotic death types they protect against.
Irod lacking the C-terminal putative transmembrane domain was mainly localized to the centrosomal area, whereas nontruncated Irod was localized also to Golgi and ER structures (Figures 6 and 7). This suggests that the C-terminal domain was not responsible for the centrosomal localization. The presence of both a centrosomal and a Golgi/ER-anchoring domain is not unprecedented. The coiled-coil scaffold protein CG-NAP/AKAP450/350 contains two separate anchoring domains, one to centrosomes and one to Golgi/ER (Takahashi et al., 1999). The centrosomal/Golgi localization of a member of the Ian protein family is novel. The mIan4 has a C-terminal mitochondrial membrane anchor and assumes a cytoplasmic distribution when this anchor is deleted (Daheron et al., 2001). The hImap1 is localized to the ER, whereas Imap38-1 is nuclear (Krucken et al., 1999). This suggests that the members of this protein family may have evolved to have functions in distinct subcellular compartments.
The centrosome is receiving increasing attention as an important signal transduction center in the cell (Doxsey, 2001; Meraldi and Nigg, 2001; Lange, 2002). Although no precise centrosomal role has been established in apoptosis, aberrant centrosome duplication has been associated with cell death (Sato et al., 2000). The present study serves to further incriminate the centrosome in cell death-relevant signaling. The centrosomal/Golgi location may help explain the specificity of the antiapoptotic action of Irod. The centrosome contains scaffolding proteins, many of which contain coiled-coil domains that link enzymes incriminated in cell cycle regulation, apoptosis, protein phosphorylation, and protein turnover to the centrosome and thereby close to Irod (Pockwinse et al., 1997; Fry et al., 1998; Takahashi et al., 1999; Wigley et al., 1999; Fry et al., 2000; Lorson et al., 2000; Verde et al., 2001; Gergely, 2002). Of particular interest is that the centrosomal/Golgi scaffolding protein CG-NAP/AKAP450/350 binds the OA targets PP2A and PP1 (Takahashi et al., 1999). CaMKII is also associated with centrosomes (Pietromonaco et al., 1995; Matsumoto and Maller, 2002). In the present study, the CaMKII inhibitor KN93 was shown to protect against apoptosis induced by either γ-radiation or OA, suggesting that γ-radiation and OA both have apoptotic pathways involving CaMKII. The CaMKII activity is kept in check by dephosphorylation of an autoactivatory phosphorylation. The dephosphorylation is catalyzed by PP1 and PP2A (Hanson and Schulman, 1992) and autophosphorylation and CaMKII activity is therefore rapidly activated in cells treated with OA (Døskeland et al., 1990; Mellgren et al., 1995). CaMKII can also be activated through proteolytic cleavage (Wright et al., 1997). The involvement of CaMKII in death induced by ionizing radiation is a novel finding.
In fibroblasts transduced with Oar-2 cDNA, which codes for the core coiled-coil domain of Irod, we noted modulation of the OA-induced phosphorylation of a small subset of proteins, including hypophosphorylation of the CaMKII substrate vimentin (Inagaki et al., 1997). The intermediate filament protein vimentin is associated with centrosomes (Maro et al., 1984; Trevor et al., 1995). It is intriguing that the OA-induced vimentin phosphorylation is mimicked in cells depleted of the B55 subunit of PP2A (Turowski et al., 1999) and that the B55/PP2A complex is targeted by ionizing radiation (Guo et al., 2002). We do not know yet whether hypophosphorylation of vimentin is a bystander phenomenon or is involved in the antiapoptotic action of Irod. Presently, experiments are underway to identify the other proteins whose phosphorylation was modulated by Irod/Oar-2. Hopefully, this will give further clues to the components of the CaMKII-dependent apoptotic pathways activated by γ-radiation and OA.
In conclusion, Irod seems to be the first animal member of a phylogenetically ancient family of GTP-binding, coiled-coil proteins with a defined function, i.e., to counteract apoptosis induced by OA and γ-radiation. Irod represents a rare example of a protein with a GTP-binding domain that apparently is dispensable for a major function. Irod was localized to the centrosome via its core and to Golgi/ER via a C-terminal hydrophobic domain, and acted upstream of caspase 3 activation in the apoptotic cascade. Irod provides a link between the apoptotic events induced by γ-radiation and protein phosphatase-directed toxins like OA. Death induced by γ-radiation and OA was counteracted by Irod to a similar extent, and both death pathways depended on CaMKII in a way counteracted by Irod. We propose that the numerous toxins evolved to target PP1 and PP2A may cause apoptosis by mimicking a phylogenetically ancient apoptosis pathway induced by ionizing radiation, and for which the centrosome has a pivotal role.
Acknowledgments
We thank Nina Lied Larsen and Erna Finsås for excellent technical assistance. We also thank Dr. Jaakko Saraste for helpful discussions. This work was supported by grants from the Norwegian Cancer Society (to T.S., S.D., and L.H.), from the Novo Nordic Insulin Foundation (to S.D. and L.H.), and the Norwegian Research Council (to S.D.) and from the foundation of Grethe Harbitz (to L.H.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-10-0700. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-10-0700.
Abbreviations used: As-Irod, antisense-Irod; CaMKII, Ca2+/calmodulin-dependent kinase II, ER, endoplasmic reticulum; GFP, green fluorescence protein; HA, hemagglutinin; HDM2, human MDM2; Irod, inhibitor of radiation- and okadaic acid-induced death; OA, okadaic acid; PP, protein phosphatase.
Footnotes
The gene product of AK002158, which we term Irod/Ian5 or Irod, has been known as full-length Oar-2, hIan5, hImap3, hIan4, and Ian4-like (Daheron et al., 2001; Sandal et al., 2001; Hornum et al., 2002; MacMurray et al., 2002; Stamm et al., 2002). We prefer Irod/Ian5 rather than Irod/Ian4 because there is a human ortholog of mIan4 distinct from Irod, and a mouse ortholog of Irod distinct from mIan4 (MacMurray et al., 2002).
References
- Adrian, C., Creagh, E.M., and Martin, S.J. (2001). Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 20, 6627–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Bribiesca, L., and Sanchez-Suarez, P. (1999). Oxidative damage, bleomycin, and gamma radiation induce different types of DNA strand breaks in normal lymphocytes and thymocytes. A comet assay study. Ann. NY Acad. Sci. 887, 133–149. [DOI] [PubMed] [Google Scholar]
- Beham, A., Marin, M.C., Fernandez, A., Herrmann, J., Brisbay, S., Tari, A.M., Lopez-Berestein, G., Lozano, G., Sarkiss, M., and McDonnell, T.J. (1997). Bcl-2 inhibits p53 nuclear import following DNA damage. Oncogene 15, 2767–2772. [DOI] [PubMed] [Google Scholar]
- Boe, R., Gjertsen, B.T., Vintermyr, O.K., Houge, G., Lanotte, M., and Doskeland, S.O. (1991). The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp. Cell Res. 195, 237–246. [DOI] [PubMed] [Google Scholar]
- Boesen-de Cock, J.G., Tepper, A.D., de Vries, E., van Blitterswijk, W.J., and Borst, J. (1999). Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J. Biol. Chem. 274, 14255–14261. [DOI] [PubMed] [Google Scholar]
- Bornens, M. (2002). Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34. [DOI] [PubMed] [Google Scholar]
- Braun, A.P., and Schulman, H. (1995a). The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu. Rev. Physiol. 57, 417–445. [DOI] [PubMed] [Google Scholar]
- Braun, A.P., and Schulman, H. (1995b). A non-selective cation current activated via the multifunctional Ca(2+)-calmodulin-dependent protein kinase in human epithelial cells. J. Physiol. 488, 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambot, M., Aresta, S., Kahn-Perles, B., de Gunzburg, J., and Romeo, P.H. (2002). Human immune associated nucleotide 1: a member of a new guanosine triphosphatase family expressed in resting T and B cells. Blood 99, 3293–3301. [DOI] [PubMed] [Google Scholar]
- Cheng, J., and Haas, M. (1990). Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol. Cell. Biol. 10, 5502–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daheron, L., Zenz, T., Siracusa, L.D., Brenner, C., and Calabretta, B. (2001). Molecular cloning of Ian4: a BCR/ABL-induced gene that encodes an outer membrane mitochondrial protein with GTP-binding activity. Nucleic Acids Res. 29, 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux, Q.L., and Reed, J.C. (1999). IAP family proteins–suppressors of apoptosis. Genes Dev. 13, 239–252. [DOI] [PubMed] [Google Scholar]
- Dilla, T., Romero, J., Sanstisteban, P., and Velasco, J.A. (2002). The mdm2 proto-oncogene sensitizes human medullary thyroid carcinoma cells to ionizing radiation. Oncogene 21, 2376–2386. [DOI] [PubMed] [Google Scholar]
- Døskeland, A.P., Vintermyr, O.K., Flatmark, T., Cotton, R.G., and Doskeland, S.O. (1992). Phenylalanine positively modulates the cAMP-dependent phosphorylation and negatively modulates the vasopressin-induced and okadaic-acid-induced phosphorylation of phenylalanine 4-monooxygenase in intact rat hepatocytes. Eur. J. Biochem. 206, 161–170. [DOI] [PubMed] [Google Scholar]
- Doxsey, S. (2001). Re-evaluating centrosome function. Nat. Rev. Mol. Cell. Biol. 2, 688–698. [DOI] [PubMed] [Google Scholar]
- Evans, S.C., Viswanathan, M., Grier, J.D., Narayana, M., El-Naggar, A.K., and Lozano, G. (2001). An alternatively spliced HDM2 product increases p53 activity by inhibiting HDM2. Oncogene 20, 4041–4049. [DOI] [PubMed] [Google Scholar]
- Fladmark, K.E., Brustugun, O.T., Hovland, R., Boe, R., Gjertsen, B.T., Zhivotovsky, B., and Doskeland, S.O. (1999). Ultrarapid caspase-3 dependent apoptosis induction by serine/threonine phosphatase inhibitors. Cell Death Differ. 6, 1099–1108. [DOI] [PubMed] [Google Scholar]
- Fladmark, K.E., Brustugun, O.T., Mellgren, G., Krakstad, C., Boe, R., Vintermyr, O.K., Schulman, H., and Doskeland, S.O. (2002). Ca2+/calmodulin-dependent protein kinase II is required for microcystin-induced apoptosis. J. Biol. Chem. 277, 2804–2811. [DOI] [PubMed] [Google Scholar]
- Fry, A.M., Mayor, T., Meraldi, P., Stierhof, Y.D., Tanaka, K., and Nigg, E.A. (1998). C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 141, 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry, A.M., Mayor, T., and Nigg, E.A. (2000). Regulating centrosomes by protein phosphorylation. Curr. Top. Dev. Biol. 49, 291–312. [DOI] [PubMed] [Google Scholar]
- Fujimori, Y., Saheki, K., Itoi, H., Okamamoto, T., and Kakishita, E. (2000). Increased expression of Fas (APO-1, CD95) on CD4+ and CD8+ T lymphocytes during total body irradiation. Acta Haematol. 104, 193–196. [DOI] [PubMed] [Google Scholar]
- Garchon, H.J., Luan, J.J., Eloy, L., Bedossa, P., and Bach, J.F. (1994). Genetic analysis of immune dysfunction in non-obese diabetic (NOD) mice: mapping of a susceptibility locus close to the Bcl-2 gene correlates with increased resistance of NOD T cells to apoptosis induction. Eur. J. Immunol. 24, 380–384. [DOI] [PubMed] [Google Scholar]
- Gergely, F. (2002). Centrosomal TACCtics. Bioessays 24, 915–925. [DOI] [PubMed] [Google Scholar]
- Gjertsen, B.T., Cressey, L.I., Ruchaud, S., Houge, G., Lanotte, M., and Doskeland, S.O. (1994). Multiple apoptotic death types triggered through activation of separate pathways by cAMP and inhibitors of protein phosphatases in one (IPC leukemia) cell line. J. Cell Sci. 107, 3363–3377. [DOI] [PubMed] [Google Scholar]
- Guo, C.Y., Brautigan, D.L., and Larner, J.M. (2002). ATM-dependent dissociation of B55 regulatory subunit from nuclear PP2A in response to ionizing radiation. J. Biol. Chem. 277, 4839–4844. [DOI] [PubMed] [Google Scholar]
- Hanson, P.I., and Schulman, H. (1992). Neuronal Ca2+/calmodulin-dependent protein kinases. Annu. Rev. Biochem. 61, 559–601. [DOI] [PubMed] [Google Scholar]
- Hornum, L., Romer, J., and Markholst, H. (2002). The diabetes-prone BB rat carries a frameshift mutation in Ian4, a positional candidate of Iddm1. Diabetes 51, 1972–1979. [DOI] [PubMed] [Google Scholar]
- Inagaki, N., Goto, H., Ogawara, M., Nishi, Y., Ando, S., and Inagaki, M. (1997). Spatial patterns of Ca2+ signals define intracellular distribution of a signaling by Ca2+/Calmodulin-dependent protein kinase II. J. Biol. Chem. 272, 25195–25199. [DOI] [PubMed] [Google Scholar]
- Ishida, A., Kameshita, I., Okuno, S., Kitani, T., and Fujisawa, H. (1995). A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem. Biophys. Res. Commun. 212, 806–812. [DOI] [PubMed] [Google Scholar]
- Kasibhatla, S., Beere, H.M., Brunner, T., Echeverri, F., and Green, D.R. (2000). A `non-canonical' DNA-binding element mediates the response of the Fas-ligand promoter to c-Myc. Curr. Biol. 10, 1205–1208. [DOI] [PubMed] [Google Scholar]
- Kasibhatla, S., Brunner, T., Genestier, L., Echeverri, F., Mahboubi, A., and Green, D.R. (1998). DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol. Cell 1, 543–551. [DOI] [PubMed] [Google Scholar]
- Krucken, J., Schmitt-Wrede, H.P., Markmann-Mulisch, U., and Wunderlich, F. (1997). Novel gene expressed in spleen cells mediating acquired testosterone-resistant immunity to Plasmodium chabaudi malaria. Biochem. Biophys. Res. Commun. 230, 167–170. [DOI] [PubMed] [Google Scholar]
- Krucken, J., Stamm, O., Schmitt-Wrede, H.P., Mincheva, A., Lichter, P., and Wunderlich, F. (1999). Spleen-specific expression of the malaria-inducible intronless mouse gene imap38. J. Biol. Chem. 274, 24383–24391. [DOI] [PubMed] [Google Scholar]
- Lakin, N.D., and Jackson, S.P. (1999). Regulation of p53 in response to DNA damage. Oncogene 18, 7644–7655. [DOI] [PubMed] [Google Scholar]
- Lange, B.M. (2002). Integration of the centrosome in cell cycle control, stress response and signal transduction pathways. Curr. Opin. Cell Biol. 14, 35–43. [DOI] [PubMed] [Google Scholar]
- Lorens, J.B., et al. (2000). Retroviral delivery of peptide modulators of cellular functions. Mol. Ther. 1, 438–447. [DOI] [PubMed] [Google Scholar]
- Lorson, M.A., Horvitz, H.R., and van den Heuvel, S. (2000). LIN-5 is a novel component of the spindle apparatus required for chromosome segregation and cleavage plane specification in Caenorhabditis elegans. J. Cell Biol. 148, 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H., Taya, Y., Ikeda, M., and Levine, A.J. (1998). Ultraviolet radiation, but not gamma radiation or etoposide-induced DNA damage, results in the phosphorylation of the murine p53 protein at serine-389. Proc. Natl. Acad. Sci. USA 95, 6399–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas, A. (1996a). Coiled coils: new structures and new functions. Trends Biochem. Sci. 21, 375–382. [PubMed] [Google Scholar]
- Lupas, A. (1996b). Prediction and analysis of coiled-coil structures. Methods Enzymol. 266, 513–525. [DOI] [PubMed] [Google Scholar]
- Lupas, A., Van Dyke, M., and Stock, J. (1991). Predicting coiled coils from protein sequences. Science 252, 1162–1164. [DOI] [PubMed] [Google Scholar]
- MacMurray, A.J., et al. (2002). Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res. 12, 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandik-Nayak, L., Nayak, S., Sokol, C., Eaton-Bassiri, A., Madaio, M.P., Caton, A.J., and Erikson, J. (2000). The origin of anti-nuclear antibodies in bcl-2 transgenic mice. Int. Immunol. 12, 353–364. [DOI] [PubMed] [Google Scholar]
- Maro, B., Paintrand, M., Sauron, M.E., Paulin, D., and Bornens, M. (1984). Vimentin filaments and centrosomes. Are they associated? Exp. Cell Res. 150, 452–458. [DOI] [PubMed] [Google Scholar]
- Matsumoto, Y., and Maller, J.L. (2002). Calcium, calmodulin, and CaMKII requirement for initiation of centrosome duplication in Xenopus egg extracts. Science 295, 499–502. [DOI] [PubMed] [Google Scholar]
- Mellgren, G., Vintermyr, O.K., and Doskeland, S.O. (1995). Okadaic acid, cAMP, and selected nutrients inhibit hepatocyte proliferation at different stages in G 1, modulation of the cAMP effect by phosphatase inhibitors and nutrients. J. Cell. Physiol. 163, 232–240. [DOI] [PubMed] [Google Scholar]
- Meraldi, P., and Nigg, E.A. (2001). Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J. Cell Sci. 114, 3749–3757. [DOI] [PubMed] [Google Scholar]
- Meraldi, P., and Nigg, E.A. (2002). The centrosome cycle. FEBS Lett. 521, 9–13. [DOI] [PubMed] [Google Scholar]
- Moffett, P., Farnham, G., Peart, J., and Baulcombe, D.C. (2002). Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 21, 4511–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, Y., Morimoto, H., Kobayashi, S., Ohba, T., and Haneji, T. (1999). The protein phosphatase inhibitors, okadaic acid and calyculin A, induce apoptosis in human submandibular gland ductal cell line HSG cells. Oral Dis. 5, 104–110. [DOI] [PubMed] [Google Scholar]
- Ogawa, H., Inouye, S., Tsuji, F.I., Yasuda, K., and Umesono, K. (1995). Localization, trafficking, and temperature-dependence of the Aequorea green fluorescent protein in cultured vertebrate cells. Proc. Natl. Acad. Sci. USA 92, 11899–11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, M.E., Mendrysa, S.M., Saucedo, L.J., Tannous, P., and Holubar, M. (2000). p76(MDM2) inhibits the ability of p90(MDM2) to destabilize p53. J. Biol. Chem. 275, 5733–5738. [DOI] [PubMed] [Google Scholar]
- Pietromonaco, S.F., Seluja, G.A., and Elias, L. (1995). Identification of enzymatically active Ca2+/calmodulin-dependent protein kinase in centrosomes of hemopoietic cells. Blood Cells Mol. Dis. 21, 34–41. [DOI] [PubMed] [Google Scholar]
- Pockwinse, S.M., Krockmalnic, G., Doxsey, S.J., Nickerson, J., Lian, J.B., van Wijnen, A.J., Stein, J.L., Stein, G.S., and Penman, S. (1997). Cell cycle independent interaction of CDC2 with the centrosome, which is associated with the nuclear matrix-intermediate filament scaffold. Proc. Natl. Acad. Sci. USA 94, 3022–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier, G.M., Anderson, G., Huvar, A., Wagaman, P.C., Shuttleworth, J., Jenkinson, E., Jackson, M.R., Peterson, P.A., and Erlander, M.G. (1999). Immune-associated nucleotide-1 (IAN-1) is a thymic selection marker and defines a novel gene family conserved in plants. J. Immunol. 163, 4960–4969. [PubMed] [Google Scholar]
- Rudner, J., Belka, C., Marini, P., Wagner, R.J., Faltin, H., Lepple-Wienhues, A., Bamberg, M., and Budach, W. (2001). Radiation sensitivity and apoptosis in human lymphoma cells. Int. J. Radiat. Biol. 77, 1–11. [DOI] [PubMed] [Google Scholar]
- Sandal, T., Ahlgren, R., Lillehaug, J., and Doskeland, S.O. (2001). Establishment of okadaic acid resistant cell clones using a cDNA expression library. Cell Death Differ. 8, 754–766. [DOI] [PubMed] [Google Scholar]
- Sandal, T., Stapnes, C., Kleivdal, H., Hedin, L., and Doskeland, S.O. (2002). A novel, extraneuronal role for cyclin-dependent protein kinase 5 (CDK5). Modulation of cAMP-induced apoptosis in rat leukemia cells. J. Biol. Chem. 277, 20783–20793. [DOI] [PubMed] [Google Scholar]
- Sato, N., Mizumoto, K., Nakamura, M., Ueno, H., Minamishima, Y.A., Farber, J.L., and Tanaka, M. (2000). A possible role for centrosome overduplication in radiation-induced cell death. Oncogene 19, 5281–5290. [DOI] [PubMed] [Google Scholar]
- Srinivasan, M., Edman, C.F., and Schulman, H. (1994). Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J. Cell Biol. 126, 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm, O., Krucken, J., Schmitt-Wrede, H.P., Benten, W.P., and Wunderlich, F. (2002). Human ortholog to mouse gene imap38 encoding an ER-localizable G-protein belongs to a gene family clustered on chromosome 7q32–36. Gene 282, 159–167. [DOI] [PubMed] [Google Scholar]
- Staskawicz, B.J., Ausubel, F.M., Baker, B.J., Ellis, J.G., and Jones, J.D. (1995). Molecular genetics of plant disease resistance. Science 268, 661–667. [DOI] [PubMed] [Google Scholar]
- Strasser, A., Harris, A.W., Huang, D.C., Krammer, P.H., and Cory, S. (1995). Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 14, 6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser, A., Whittingham, S., Vaux, D.L., Bath, M.L., Adams, J.M., Cory, S., and Harris, A.W. (1991). Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. USA 88, 8661–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M., Shibata, H., Shimakawa, M., Miyamoto, M., Mukai, H., and Ono, Y. (1999). Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the Golgi apparatus. J. Biol. Chem. 274, 17267–17274. [DOI] [PubMed] [Google Scholar]
- Tounekti, O., Kenani, A., Foray, N., Orlowski, S., and Mir, L.M. (2001). The ratio of single- to double-strand DNA breaks and their absolute values determine cell death pathway. Br. J. Cancer 84, 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevor, K.T., McGuire, J.G., and Leonova, E.V. (1995). Association of vimentin intermediate filaments with the centrosome. J. Cell Sci. 108, 343–356. [DOI] [PubMed] [Google Scholar]
- Turowski, P., Myles, T., Hemmings, B.A., Fernandez, A., and Lamb, N.J. (1999). Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell 10, 1997–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde, I., Pahlke, G., Salanova, M., Zhang, G., Wang, S., Coletti, D., Onuffer, J., Jin, S.L., and Conti, M. (2001). Myomegalin is a novel protein of the Golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J. Biol. Chem. 276, 11189–11198. [DOI] [PubMed] [Google Scholar]
- Wigley, W.C., Fabunmi, R.P., Lee, M.G., Marino, C.R., Muallem, S., DeMartino, G.N., and Thomas, P.J. (1999). Dynamic association of proteasomal machinery with the centrosome. J. Cell Biol. 145, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S.C., Schellenberger, U., Ji, L., Wang, H., and Larrick, J.W. (1997). Calmodulin-dependent protein kinase II mediates signal transduction in apoptosis. FASEB J. 11, 843–849. [DOI] [PubMed] [Google Scholar]
- Wu, J., Yin, M.B., Hapke, G., Toth, K., and Rustum, Y.M. (2002). Induction of biphasic DNA double strand breaks and activation of multiple repair protein complexes by DNA topoisomerase I drug 7-ethyl-10-hydroxy-camptothecin. Mol. Pharmacol. 61, 742–748. [DOI] [PubMed] [Google Scholar]
- Yan, Y., Shay, J.W., Wright, W.E., and Mumby, M.C. (1997). Inhibition of protein phosphatase activity induces p53-dependent apoptosis in the absence of p53 transactivation. J. Biol. Chem. 272, 15220–15226. [DOI] [PubMed] [Google Scholar]