Abstract
Respiratory syncytial virus (RSV) is the foremost respiratory pathogen in newborns and claims millions of lives annually. However, there has been no methodical study of the pathway(s) of entry of RSV or its interaction with nonrespiratory tissues. We and others have recently established a significant association between allergic conjunctivitis and the presence of RSV in the eye. Here we adopt a BALB/c mouse model and demonstrate that when instilled in the live murine eye, RSV not only replicated robustly in the eye but also migrated to the lung and produced a respiratory disease that is indistinguishable from the standard, nasally acquired RSV disease. Ocularly applied synthetic anti-RSV small interfering RNA prevented infection of the eye as well as the lung. RSV infection of the eye activated a plethora of ocular cytokines and chemokines with profound relevance to inflammation of the eye. Anticytokine treatments in the eye reduced ocular inflammation but had no effect on viral growth in both eye and lung, demonstrating a role of the cytokine response in ocular pathology. These results establish the eye as a major gateway of respiratory infection and a respiratory virus as a bona fide eye pathogen, thus offering novel intervention and treatment options.
Respiratory syncytial virus (RSV) is a member of the Pneumovirus genus of the Paramyxoviridae family. Like other viruses of this family, RSV contains a nonsegmented negative-strand (antimessage sense) RNA genome, which is about 15 kb long (13). The RSV disease, often loosely called “croup” in children, is characterized by symptoms that are not unlike those of common cold or flu, i.e., wheezing, bronchiolitis, pneumonia, and asthma. RSV continues to be the leading killer among infectious diseases, with an annual death toll of about a million worldwide (11, 14). To this day, no reliable vaccine or preventive antiviral against RSV exists (11, 14, 29). Therapy with interferon, ribavirin, and human immunoglobulin G (IgG) remains unreliable, controversial, expensive, and mostly supportive (29, 38).
The highly contagious nature of RSV infection makes it important to determine its etiology. Although the lung is undoubtedly a major organ infected in RSV disease, neither the full tissue tropism of the virus nor the identity of the cellular receptor is known. In cell culture, RSV infects cell lines unrelated to the lung, such as the fibroblasts CV-1 and HeLa, in addition to cells of lung epithelial origin such as primary bronchiolar (NHBE) and type II-like alveolar carcinoma (A549) cells. In other words, RSV shows the potential to infect cells other than those of the lung and the respiratory tract, at least in culture.
By the same token, the exact physiological route of entry of RSV in the body needs to be systematically investigated. In a pioneering attempt more than two decades ago (20), live RSV was instilled into a small number of human volunteers through the nose (n = 12), eye (n = 12), or mouth (n = 12). Virus was measured only in the nasal secretion and was detected in roughly one-third of the subjects only when introduced by eye or nose, but not when introduced by mouth. As this was a human trial, pulmonary viral titer could not be determined, and pulmonary function remained unaffected in all subjects. Only mild symptoms of the upper, but not lower, respiratory tract were noted after ocular instillation; however, similar suboptimal infection was also observed with standard nasal instillation of RSV. Thus, it was not clear whether the viral input or infection procedure needed further optimization. Years later (17), the use of disposable eye-nose goggles was shown to be associated with a decrease in nosocomial respiratory infection. Unfortunately, neither of these studies were confirmed or continued further, possibly due to heightened safety concerns with human experiments and the lack of an animal model. In recent times, RSV sequences were recovered in a larger percentage (23%) of human patients with allergic conjunctivitis than in apparently normal controls (16). Our studies of pediatric patients with respiratory infection and conjunctivitis (red eye) showed a significantly (P < 0.05) higher occurrence of RSV in the eye than was seen in healthy people (5). We were also able to infect human corneal epithelial cells in culture with RSV. Thus, while these results suggested an interaction between the ocular tissue and RSV, the potential role of the eye in lung infection remained unexplored.
It is now well established that the immunopathology of a variety of diseases is regulated by key cytokines, such as interleukins (IL) and tumor necrosis factor (TNF), that activate downstream signaling pathways of immunological importance. A special subclass of cytokines comprises chemotactic peptides, known as chemokines, which promote the migration of inflammatory and noninflammatory cells to the appropriate target tissue (12, 34). The chemokines are subdivided into four groups (CXC, CX3C, CC, and C) according to the positioning of the first two closely paired and highly conserved Cys residues of their amino acid sequences (36). The CC chemokines, generally involved in inflammation through monocyte-macrophage activation and recruitment, play particularly important roles in the pathology of the RSV-infected lung and in various ocular diseases and infections (23, 28, 41, 42, 44). The clinical benefit stems from the discovery of proteins, antibodies, and small-molecule antagonists that inhibit chemokine activities and thereby reduce allergy and inflammation (1, 15, 39, 42). We reasoned that development of a rational intervention for the ocular pathology may benefit from an understanding of the cytokines response activated in the RSV-infected eye, which is currently unknown.
In view of the variability and limited scope of human subjects, we set out to examine the eye-to-lung travel of a respiratory virus in an animal model. Traditionally, the BALB/c mouse has served as a well-defined laboratory animal in which pulmonary viral infection and replication are achieved by introduction of RSV via the respiratory tract, such as the nose and trachea (6, 10). The infected mice also exhibit severe respiratory symptoms and pathology characteristic of RSV infection. In this communication, we demonstrate ocular RSV infection and inflammation in the mouse model and provide the first direct evidence of eye-to-lung transmission of the virus, resulting in respiratory disease. We establish the eye as a major gateway of lung infection and offer potential strategies for treatment of ocular inflammation and prevention of the disease.
MATERIALS AND METHODS
Instillation of virus and inhibitors in the eye.
Female BALB/c mice, 6 to 8 weeks old, were purchased from Charles River Laboratories. RSV (Long strain, serotype A) was grown on HEp-2 cells and purified on sucrose layers to a concentration of 1011 as described previously (6). Dilutions were done in phosphate-buffered saline (PBS) immediately before use as needed. A similarly diluted sucrose solution was used in sham-infected control mice. We followed the inoculation procedure optimized for other ocular pathogens such as herpes simplex virus (HSV) (42), which results in a consistent infection without causing “blepharitis” (eyelid inflammation). In brief, mice were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg), and the right eye was lightly scarified by two twists of a 2-mm corneal trephine. Various quantities of virus in 2 μl PBS was dropped onto the corneal surface and massaged in with closed eyelids. Control mice were inoculated with the same volume of PBS containing the same concentration of sucrose as the corresponding viral inoculum. The day of the inoculation was considered day 0.
In routine RNA interference (RNAi) experiments, 1 nmol of small interfering RNA (siRNA), complexed with TransIT TKO transfection reagent (Mirus), was applied in the eye at 30 min (unless otherwise stated) before instillation of RSV. The siRNA was based on the NA(N)19NN design, corresponding to the sequence AAGCCCTATAACATCAAATTCAA of the P mRNA of RSV. Each strand of the siRNA was 21 nucleotides long and contained 3′-terminal dTdT extensions. The design followed the “asymmetry rule” (25, 37), with a ΔG difference of −6.7 kcal/mol between the two termini. Fluorescent siRNAs, labeled with Cy3 at the 5′ end of the sense strand, were custom designed and similarly applied into the eye. All siRNAs were commercially synthesized (Dharmacon). Negative control, scrambled siRNA of undisclosed sequence was purchased from Ambion.
When used, monoclonal rat antibody with neutralizing activity towards mouse IL-1α (R&D Systems) or TNF-α (BD Biosciences) was administered subconjunctivally at the same time as RSV. Just before use, the antibody was diluted in sterile PBS such that 25 μg antibody was present in 1 μl of the injected volume. The same amount of nonimmune mouse IgG was used as control.
Assays.
To detect Cy3-labeled siRNA (red), mice were sacrificed at 12 h, 24 h, 36 h, and 48 h after instillation of the liposomal siRNA, and the eyes were enucleated and prepared as 10-μm cryosections for fluorescence microscopy. Gross microscopic examinations were performed in a TE2000-E2 imaging station (Nikon) equipped with epifluorescence illumination and a Photometrics Coolsnap (Sony) image-capturing device.
When the eye or lung was to be used for reverse transcription-PCR immediately after organ collection, the tissue was mechanically homogenized in RNAwiz (Ambion) and RNA was purified according to the manufacturer's protocol. Quantitative real-time PCR was performed using standardized primers as described before (5, 43). In brief, first-strand cDNA was made using the GeneAmp RNA PCR Core kit (Perkin-Elmer-Applied Biosystems), and real-time PCR was performed on the iCycler iQ quantitative PCR system (Bio-Rad Laboratories) using the iQ SYBR green SuperMix. Gene expression measurements were calculated using the manufacturer's software; GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal control. Infectious virus in the lung or eye was determined by serial dilution of the clarified tissue homogenate and plaque assay on HEp-2 monolayer as described previously (6). IL-1α and TNF-α in the homogenate were quantified by standard enzyme-linked immunosorbent assay (ELISA) (R&D Systems) (5, 6).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting (Western blotting) using chemiluminescence-based detection of the horseradish peroxidase-conjugated secondary antibody were performed as described previously (43). Primary antibodies against RSV and mouse actin were from Chemicon International, and goat IgGs against mouse MIG, RANTES, and IP-10 were from R&D Systems.
Ocular and pulmonary pathology.
Ocular disease was evaluated with a slit lamp biomicroscope essentially as described previously (18). Pathology was scored on a scale of 0 to 4 as follows: 0, clear eye; 1, slight redness in the corners; 2, moderate redness and injection; 3, conjunctival and corneal injection with ciliary flush; 4, extensive injection, generally associated with some mucus. Eyes were examined in a coded fashion, with the reader unaware of the treatment given. Pulmonary pathology was enumerated as described before (6). In brief, lungs were perfused and fixed in 10% buffered formalin and embedded in paraffin. Multiple 4-μm-thick sections were stained with hematoxylin and eosin and examined by light microscopy. Inflammatory infiltrates were scored by enumerating the layers of inflammatory cells surrounding the vessels. Finding zero to three layers of inflammatory cells was considered normal. Finding more than three layers of inflammatory cells (surrounding 50% or more of the circumference of the vessel) was considered abnormal. The number of abnormal perivascular and peribronchial spaces divided by the total such spaces was the percentage reported as the pathology score.
RESULTS
Ocularly inoculated RSV infects the eye and the lung.
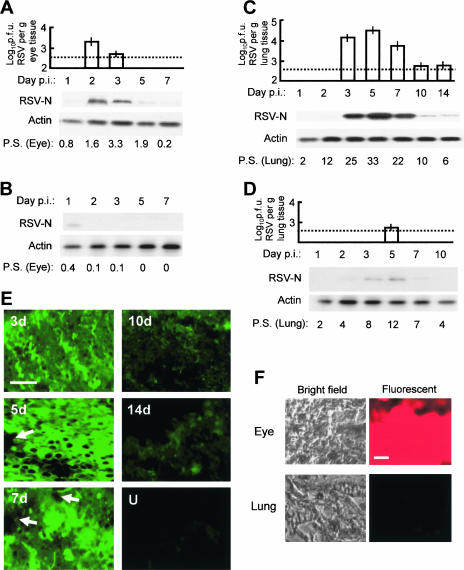
To first test whether RSV can infect the eye, we administered various amounts of purified RSV, ranging from 104 to 108 PFU, into the BALB/c mouse cornea as described in Materials and Methods. We then measured infectious progeny virus in the eye as well as in the lungs at different days afterward. While 104 PFU produced no obvious infection, 108 PFU resulted in severe ocular inflammation, mucus, conjunctivitis, and discomfort, suggestive of an excessive infection plane uncharacteristic of the human disease. With amounts between 104 and 108 PFU, a graded infection was seen. In this paper, we present results obtained using 2 × 106 PFU RSV, which, as shown below, produced optimal lung infection, approximating that caused by nasally introduced RSV (6). Representative results show that infectious RSV titer peaked in the eye on day 2 and decreased subsequently (Fig. 1A). Infectious RSV was appreciable in the lung tissue as well. Pulmonary viral titer was measurable on day 3 after ocular inoculation, reached its peak on day 5, and then slowly subsided to the threshold of detection at around day 14 (Fig. 1C). In both tissues, the viral titer paralleled the viral protein level as measured by the amount of RSV N protein in immunoblots. Since robust protein expression by negative-strand RNA viruses requires de novo viral replication and since the input virus was undetectable on day 1, the results clearly indicate that virus replicated in both the eye and the lung tissues. We had previously shown that when introduced through the traditional nasal route, RSV attains the highest pulmonary growth on day 4 (6). Thus, it appears that the ocular route takes roughly an extra day (day 5) to establish maximal pulmonary growth. We speculate that this is due to two reasons: (i) the time taken for the virus to replicate in the eye, which amplifies the inoculum, and (ii) the travel time from eye to lung. As we show below, inhibition of viral replication in the eye by siRNA significantly reduces lung infection.
FIG. 1.
Replication of ocularly inoculated RSV in the eye and the lung and inhibition by siRNA. RSV was inoculated in BALB/c mouse eyes at day 0. Eye and lung tissues were collected at the indicated days postinoculation (p.i.), and infectious viral titer and viral protein synthesis were determined. The dotted horizontal lines are the lower limits of detection due to serial dilution. (A) RSV growth in the eye. Upper panel, infectious viral titer; lower panel, Western blotting for viral N protein and control cellular actin. P.S., pathology score determined by slit lamp examination (see Materials and Methods). (B) As in panel A, with anti-RSV siRNA administered in the eye 30 min before the virus (2 × 106 PFU per eye). Due to undetectable viral titer, only the Western blot is shown for viral growth. Note the inhibition of the virus, going from panel A to panel B. (C) As in panel A, except that the tissue is lung. (D) Studies of the lung tissue when RSV and anti-RSV siRNA were administered in the eye. All errors bars (standard deviations) are from three experiments. Note that the virus disappears in the eye (A) as it appears in the lung (B), suggesting travel. Also, compare panel A with B and panel C with D to note the drastic inhibition of the virus by siRNA. (E) Immunostaining for RSV in lung sections on different days after RSV infection. U, uninfected (i.e., no virus was put in the eye). The arrows point to vascular spaces. Bar, 400 μm. (F) Bright-field and fluorescent images of eye and lung sections at 24 h after instillation of Cy3-labeled siRNA in the eye. Bar, 400 μm. Brain and liver sections looked essentially like those of the lung (i.e., lacked fluorescence) and hence are not shown.
We then tested whether the ocular route produces ocular or pulmonary pathology. Examination of eye and lung tissue sections indeed revealed significant pathology in both as indicated by the pathology scores (Fig. 1A and C). In slit lamp examination, various degrees of teary, red eyes were observed, with minor ciliary flush and conjunctival injections. Some discharge was noticed in more severe cases at the peak of the inflammatory reaction. In representative examples, RSV proteins could also be detected in situ by indirect immunofluorescence of eye and lung sections (Fig. 1E). The chronology of the pathology scores generally paralleled the viral titer (Fig. 1A and C). Finally, the mice showed the typical outcomes of RSV disease (data not shown), such as rapid respiration, reduced activity, and weight loss (6). Clearly, once the virus reaches the lungs, its history and path become irrelevant; i.e., the nature and course of the pulmonary disease and subsequent clearance of the virus follow the same kinetics regardless of the initial tissue of administration. Together, these results establish that the eye can indeed act as a bona fide route of pulmonary infection in the mouse model.
Early intervention in ocular viral growth inhibits pulmonary infection.
In recent years RNAi has emerged as a key strategy to knock down gene expression (3, 19). We provided proof of concept that properly designed synthetic siRNA could act as a potent antiviral agent in cell culture as well as in the BALB/c mouse model (2, 4, 6). Once we found that pulmonary viral infection can occur through the eye, a logical question was whether this knowledge could be exploited for prevention by using RNAi in the eye. The optimal results, obtained with 1 nmol liposomal siRNA applied 30 min before RSV, revealed that the infectious viral titer was strongly inhibited (Fig. 1, compare panels A and B) and was below the threshold of detection of our assay, i.e., under 6 × 102 PFU per μg eye tissue. Small amounts of viral protein could be detected (Fig. 1B, day 1), some of which likely represent the input virus. Thus, the siRNA caused nearly complete inhibition of RSV replication in the eye.
Studies of the lungs in the same animals showed that ocularly applied siRNA also led to a significant reduction of pulmonary viral titer (Fig. 1, compare panels C and D). The animals also showed little discomfort or RSV disease as judged by the lack of the clinical correlates described above (data not shown). The small amount of viral growth in the lung (about 100-fold lower than in lungs not treated with siRNA) is explained by the travel of the ocular inoculum without amplification by replication in the eye. Evidently, this amount was too small to establish a significant viral load and pathology. In either case, we can conclude that ocular RNAi treatment can essentially prevent respiratory RSV infection through the eye.
Tissue distribution is a critical issue for a prospective drug because of its bearing on bioavailability and possible toxicity in various organs. Currently, two siRNA drugs are undergoing clinical trials for use in the eye to treat the wet form of age-related macular degeneration. Code-named sirna027 (Sirna Therapeutics) and cand5 (Acuity Pharmaceuticals), they target vascular epidermal growth factor and its receptor, respectively (3). However, no data are available regarding their transit outside the eye. To test the tissue distribution of ocularly applied siRNA, we used fluorescent Cy3-labeled siRNA and investigated its presence in four major organs (eye, lung, brain, and liver) at 12, 24, 36, and 48 h postinstillation. Large amounts of fluorescence were observed in the eye between 12 h and 36 h, which started to decline thereafter. In contrast, no fluorescence could be detected in all other organs tested at all times, and therefore, representative negative results are shown for lung only (Fig. 1F). These results suggest that ocularly applied siRNA does not leave the eye and thus should not cause systemic side effects. Importantly, its absence in the lung specifically suggests that the observed reduction in the lung viral titer was an indirect effect of inhibition of viral growth in the eye, further confirming the eye-to-lung travel.
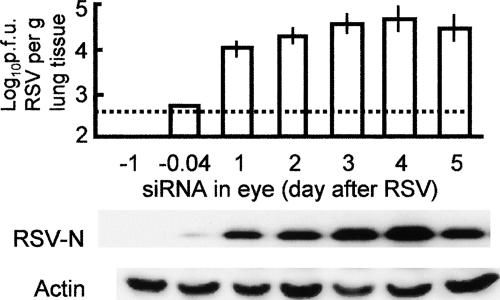
In any therapy, knowledge of the “window of opportunity” is important. To this end, we wanted to know whether and to what extent the ocular siRNA will inhibit pulmonary infection if administered after RSV. In these studies, the anti-RSV siRNA formulation was added to the eye at different times before or after RSV inoculation. The results (Fig. 2) reveal that siRNA, added a day before or at about the same time as the virus, prevents virus migration to the lungs, confirming the results in Fig. 1. In contrast, siRNA added progressively later offered little or no protection. These results additionally confirm eye-to-lung travel of the virus and the preventive role of siRNA in the eye, but only if used promptly.
FIG. 2.
Effect of siRNA administered postinfection. Mouse experiments were performed essentially as for Fig. 1, except that the siRNA was administered in the eye at the following times relative to RSV (x axis, from left): 1 day before RSV; 0.04 day (1 h) before RSV; and then 1, 2, 3, 4, or 5 days after RSV. For titer and protein analyses, lungs were isolated at 5 days after RSV infection because this time point showed maximal titer (e.g., see Fig. 1). Error bars indicate standard deviations. The lower panel shows immunoblots for virus growth (RSV N protein) and an actin control for the same tissue samples.
Activation of proinflammatory cytokines and chemokines in the RSV-infected eye.
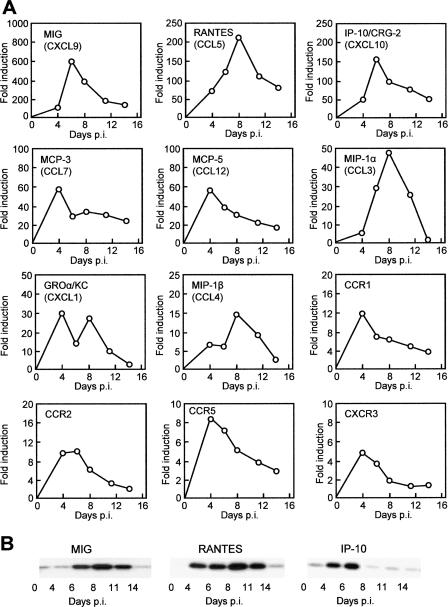
Having shown that the eye can serve as a gateway to lung infection, the next query was whether the eye itself is affected by the virus. Pathogens that are known agents of ocular infection, such as HSV, often cause severely impaired vision and blindness (8). In contrast, there is no report of long-term vision loss or blindness in patients with RSV infection. Thus, we reasoned that the ocular effect, if any, would be short term and perhaps would be manifest as a cytokine-mediated inflammatory response. We carried out a rapid real-time PCR screening of the common cytokines, chemokines, and selected receptors in RSV-infected eye tissue over the full course of infection. These studies led to a number of interesting observations. First, genes activated by at least fourfold (Fig. 3A) were mainly those for CC and CXC chemokines and their receptors. The most strongly activated genes were those for MIG/CXCL9 (monokine induced by gamma interferon), RANTES/CCL5 (regulated upon activation, normal T-cell expressed and secreted), IP-10/CXCL10 (gamma interferon-inducible protein of 10 kDa), MCP-3/CCL7 and -5/CCL12 (monocyte chemotactic protein), and MIP-1α/CCL3 (macrophage inflammatory protein). We confirmed the activation of MIG, RANTES, IP-10, MCP-5, and MIP-1α by ELISA (data not shown); these results were essentially identical to the transcriptional data. Second, although no two genes had exactly identical kinetics of activation, their expression generally peaked between 4 and 8 days after instillation of the virus in the eye and then gradually subsided to basal or near-basal levels by day 14 (the longest time point at which samples were taken). The kinetics of activation (Fig. 3) trailed that of the virus load (Fig. 1) by 1 to 2 days, suggesting that the chemokine profile was a response to viral replication. Expression of a number of chemokine receptors was also up-regulated, suggesting further amplification of the signaling process. The protein levels of the three most highly activated chemokines, namely, MIG, RANTES, and IP-10, were found to be induced; the kinetics of induction were indeed very similar and were comparable to their transcriptional activation (Fig. 3B).
FIG. 3.
Induction of cytokines and receptors in RSV-infected eye. (A) mRNA profiles. The RNAs isolated from the RSV-infected (and uninfected control) eyes at different days postinfection (p.i.) were subjected to quantitative real-time RT-PCR as described in Materials and Methods. The relative amounts of RNA were expressed as the ratio to the uninfected control value (fold induction). Each box represents a specific cytokine, with its common name given and the uniform ligand name in parentheses. Each data point is derived from three independent infection experiments; the errors were less than 15% and are omitted for simplicity. (B) Protein profiles of the top three representative cytokines detected by Western blotting at the same time points as the RNA. Actin was present in equal amounts in all lanes (not shown to save space).
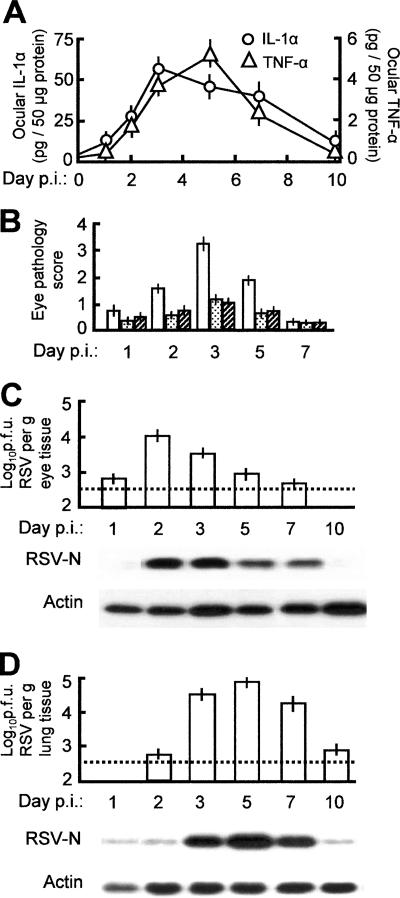
As mentioned earlier, both IL-1 and TNF play important roles in inflammatory responses of various tissues, including the eye. IL-1 is an early-warning cytokine that, together with TNF, activates corneal keratocyte growth factor and promotes neovascularization of the inflamed eye and apoptosis of the corneal stroma at low concentrations (21, 24, 45). Both are activated in essentially all ocular injuries and inflammatory conditions such as conjunctivalized cornea, uveitis, and recurrent herpes stromal keratitis (9, 21, 24, 33, 45). Another commonality is that IL-1β and TNF are activated and/or released by proteolytic processing of their precursors (26); IL-1α, in contrast, is activated transcriptionally. As transcriptional activation did not reveal any significant activation of these genes, we measured the proteins by ELISA and found that both IL-1α and TNF were significantly activated (Fig. 4A) but that IL-1β was not (data not shown).
FIG. 4.
Anticytokine treatment of the RSV-infected eye and its effect. Infection and treatment are described in Materials and Methods. (A) IL-1α and TNF-α levels in the eye assayed by ELISA at different days postinfection (p.i.). (B) Pathology score of the eye and its reduction by neutralizing antibodies against IL-1α and TNF-α (white bars, untreated; stippled bars, IL-1α; hatched bars, TNF-α). The scores are also numerically presented in Fig. 1A. (C and D) RSV growth in the eye and the lung, respectively, when anti-IL-1α antibody was administered in the eye along with RSV. Upper panel, infectious RSV titer; lower panel, Western blot for viral N protein and control cellular actin. Note the relatively greater RSV replication in panels C and D compared to the untreated tissues in panels A and B of Fig. 1. All error bars (standard deviations) are derived from three experiments. In all antibody experiments, nonimmune mouse IgG was used as a control in at least three eyes, and no pathology was seen.
Anticytokine therapy reduces pathology of the RSV-infected eye.
As revealed in Fig. 4A and B, the kinetics of the ocular chemokine response paralleled the ocular pathology, with maximal pathology discernible at around 3 days postinfection. To test the hypothesis that specific cytokine antagonists may provide relief of ocular inflammation in RSV infection, we treated the eye with neutralizing anti-TNF and anti-IL-1α antibodies along with RSV. Both antibodies were highly effective in reducing the ocular pathology (Fig. 4B) to about 50% or less of that in the untreated infected eye.
However, as the inflammatory reaction could be a manifestation of an antiviral response, we tested the effect of anti-inflammatory treatment on viral growth by using IL-1α as a representative target. When applied to the eye, anti-IL-1α did not inhibit viral replication. In fact, the vial titer was slightly higher not only in the eye (Fig. 4C) but in the lung as well (Fig. 4D). Thus, ocular anti-inflammatory treatment relieved inflammation but provided no inhibition of the actual process of infection.
DISCUSSION
We have carried out controlled experiments in a laboratory animal model to provide definitive evidence that RSV, a well-recognized respiratory virus, can infect the eye and use the eye as a gateway to access the lung. In the process, RSV causes substantial pathology of the eye in addition to producing a respiratory disease. Together with previous studies, our results establish RSV as an ocular pathogen as much as a respiratory pathogen and implicate it as a causative agent of viral conjunctivitis of the eye. Although the ocular pathology naturally resolves in about a week in mice (Fig. 4) as well as in human patients (our unpublished results), the long-term ocular damage by RSV has not been carefully evaluated. It is to be noted that childhood RSV infection often predisposes an individual to environmental allergens and asthma later in life (11). It would be interesting to see if RSV infection of the eye plays a role in the genesis of allergic conjunctivitis in the future.
How does an ocular virus travel to the respiratory tract? Although we do not have the answer yet, we can offer some thoughts regarding the path and the mechanism of travel. Based on the ocular and nasal anatomy, a highly plausible path is via the lacrimal and nasolacrimal ducts (the tear ducts) and then into nose, trachea, and finally lung. Regarding the mechanism of travel, we entertain two scenarios that are not mutually exclusive. In the first, the virus travels through reinfection and replication of adjoining cells by syncytial contact or by short-range diffusion. In this model, travel is dependent on replication en route. Alternately, the virions may travel in a replication-independent manner, perhaps propelled by the ciliary cells that line the lacrimal and respiratory mucosae. We tentatively prefer the second mechanism for the following reasons. First, inhibition of viral replication in the eye leads to a corresponding reduction in respiratory infection (Fig. 1) but not complete abrogation. Second, the available amount of ocular virus, even when meager (e.g., when ocular replication is inhibited), appears to travel rapidly, reaching the lung in about a day (Fig. 1 and 2). This time is too short for RSV, with its single-burst cycle of roughly 36 h, to infect all the mucosal cells encountered along the way. Thus, ocular viral replication may serve to amplify the viral inoculum that eventually travels to the lung, but the process of travel per se does not involve replication en route. In other words, ocular inhibition reduces the viral load in the lung but does not affect the kinetics of infection or travel. There is another potential pathway that we have not tested. In a recent study, RSV has been shown to infect neuronal cells and processes that innervate the lung (27). In view of the established precedence for infection of the optic nerve by ocular pathogens such as herpes simplex virus (32), it would be interesting to determine whether RSV can access the lung via the optic neuronal system.
We propose that all-round prevention and treatment of the RSV infection during an epidemic would ideally require respiratory (e.g., intranasal) (6) as well as ocular application of the siRNA. Evaluation of the full impact of chemokines activated in the RSV-infected eye will need further research. Nonetheless, the total effect of the five most highly activated CC and CXC chemokines (Fig. 3), namely, MIG, RANTES, IP-10, MCP, and MIP-1α, is weighted towards recruiting monocytes and T cells (12, 34, 36). The CC chemokines (RANTES, MCP, and MIP), in particular, are involved in the pathogenesis of immune-mediated inflammation through monocyte-macrophage activation and recruitment (1). Interesting precedents can be found in RSV-infected respiratory tissues and diseases of the eye. MIG, RANTES, and MIP were highly elevated in bronchoalveloar lavage samples from mice following RSV infection and in the respiratory tracts of children with RSV disease (23, 41). Concentration of these chemokines correlated with airway obstruction and hyperresponsiveness, hallmarks of RSV infection (23). Autoimmune uveitis (AU) of the eye exhibited a very similar chemokine profile, comprising RANTES, IP-10, MCP, MIP-1α, and MIP-1β (1). These chemokines were shown to play a definitive role in AU, and antibody-mediated neutralization of either MIP-1α or MCP delayed the onset and shortened the duration of AU. Studies of mice deficient in MIP-1α established a crucial role of this chemokine in recruiting T cells to the HSV-infected eye and promoting ocular inflammation that frequently results in blindness (42).
It is now established that inactivation of proinflammatory cytokines with specific neutralizing antibodies, soluble receptors, or antagonists ameliorates ocular inflammation, further confirming their role in pathology and providing a therapeutic regimen. TNF antibody preparations (infliximab, etanercept, and adalimumab) similar to the ones used by us are routinely used to reduce arthritic inflammation, and an IL-1 receptor antagonist (anakinra) has shown similar promise (33, 39). In our studies, direct inhibition of these cytokines also reduced ocular inflammation, although it appeared to slightly enhance RSV growth (Fig. 4), which may not be desirable. Overproduction of TNF was also observed in lung tissues following infection by RSV or influenza virus, and as in the eye, TNF depletion reduced the severity of pulmonary illness, likely through reduction of inflammatory T cells, without inhibiting virus growth (22). In either case, these results warrant a more detailed study of chemokine antagonists in ocular RSV infection.
NF-κB is a proinflammatory cytokine known to activate a number of cytokine and chemokine genes, including those for MIG, RANTES, IP-10, MIP-1α, and GROα/KC (mouse homolog of IL-8) (35). We and others have shown that RSV infection leads to early activation of NF-κB) in lung cells (7, 30, 40) and that this was responsible for the activation of specific chemokines such as IL-8. Salicylates (e.g., aspirin) inhibited this activation of NF-κB, which in turn inhibited cytokine induction (7). Subsequently, we have shown that NF-κB is also activated in RSV-infected human corneal epithelial cells in culture (5). NF-κB-dependent transcription of chemokine genes often requires participation of other transcription factors, notably AP-1 (31). Clearly, it is worth testing whether antagonists of any of these transcription factors may be used to treat inflammation of the RSV-infected eye. Various nonsteroidal anti-inflammatory drugs and other compounds that inhibit different steps of the NF-κB activation pathway (such as the salicylates, sulindac, and the proteasome inhibitor PS-341) are already FDA approved for preclinical or clinical use for treatment of inflammation, toxic shock, and cancer (35). Lastly, our preliminary studies also revealed eye-to-lung transmission of human parainfluenza virus in mice (data not shown), thus raising the possibility that the conclusions reported here may well apply to other respiratory pathogens such as adenovirus, influenza virus, rhinovirus, and the severe acute respiratory syndrome virus, all of which are highly important from the public health perspective.
Acknowledgments
This research was supported in part by grants R01 EY013826 and R01 AI59267 from the National Eye Institute and the National Institute of Allergy and Infectious Diseases, respectively.
We thank Solomon Ofori-Aquah for help with microscopy.
Footnotes
Published ahead of print on 18 October 2006.
REFERENCES
- 1.Adamus, G., M. Manczak, and M. Machnicki. 2001. Expression of CC chemokines and their receptors in the eye in autoimmune anterior uveitis associated with EAE. Investig. Ophthalmol. Vis. Sci. 42:2894-2903. [PubMed] [Google Scholar]
- 2.Barik, S. 2004. Control of nonsegmented negative-strand RNA virus replication by siRNA. Virus Res. 102:27-35. [DOI] [PubMed] [Google Scholar]
- 3.Barik, S. 2005. Silence of the transcripts: RNA interference in medicine. J. Mol. Med. 83:764-773. [DOI] [PubMed] [Google Scholar]
- 4.Bitko, V., and S. Barik. 2001. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 1:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitko, V., N. E. Garmon, T. Cao, B. Estrada, J. E. Oakes, R. N. Lausch, and S. Barik. 2004. Activation of cytokines and NF-kappa B in corneal epithelial cells infected by respiratory syncytial virus: potential relevance in ocular inflammation and respiratory infection. BMC Microbiol. 4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitko, V., A. Musiyenko, O. Shulyayeva, and S. Barik. 2005. Inhibition of respiratory viruses by nasally administered siRNA. Nat. Med. 11:50-55. [DOI] [PubMed] [Google Scholar]
- 7.Bitko, V., A. Velazquez, L. Yang, Y. C. Yang, and S. Barik. 1997. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-kappa B and is inhibited by sodium salicylate and aspirin. Virology 232:369-378. [DOI] [PubMed] [Google Scholar]
- 8.Brandt, C. R. 2005. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp. Eye Res. 80:607-621. [DOI] [PubMed] [Google Scholar]
- 9.Brito, B. E., L. M. O'Rourke, Y. Pan, J. Anglin, S. R. Planck, and J. T. Rosenbaum. 1999. IL-1 and TNF receptor-deficient mice show decreased inflammation in an immune complex model of uveitis. Investig. Ophthalmol. Vis. Sci. 40:2583-2589. [PubMed] [Google Scholar]
- 10.Chavez-Bueno, S., A. Mejias, A. M. Gomez, K. D. Olsen, A. M. Rios, M. Fonseca-Aten, O. Ramilo, and H. S. Jafri. 2005. Respiratory syncytial virus-induced acute and chronic airway disease is independent of genetic background: an experimental murine model. Virol. J. 2:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez-Bueno, S., A. Mejias, H. S. Jafri, and O. Ramilo. 2005. Respiratory syncytial virus: old challenges and new approaches. Pediatr. Ann. 34:62-68. [DOI] [PubMed] [Google Scholar]
- 12.Christopherson, K., II, and R. Hromas. 2001. Chemokine regulation of normal and pathologic immune responses. Stem Cells 19:388-396. [DOI] [PubMed] [Google Scholar]
- 13.Collins, P. L., and B. R. Murphy. 2002. Respiratory syncytial virus: reverse genetics and vaccine strategies. Virology 296:204-211. [DOI] [PubMed] [Google Scholar]
- 14.Dakhama, A., Y. M. Lee, and E. W. Gelfand. 2005. Virus-induced airway dysfunction: pathogenesis and biomechanisms. Pediatr. Infect. Dis. J. 24(Suppl. 11):S159-S169. [DOI] [PubMed] [Google Scholar]
- 15.Frieri, M. 2004. Airway epithelial cell release of cytokines: modulation by various therapeutic agents. Allergy Asthma Proc. 25:387-393. [PubMed] [Google Scholar]
- 16.Fujishima, H., Y. Okamoto, L. Saito, and K. Tsubota. 1995. Respiratory syncytial virus and allergic conjunctivitis. J. Allergy Clin. Immunol. 95:663-667. [DOI] [PubMed] [Google Scholar]
- 17.Gala, C. L., C. B. Hall, K. C. Schnabel, P. H. Pincus, P. Blossom, S. W. Hildreth, R. F. Betts, and R. G. Douglas, Jr. 1986. The use of eye-nose goggles to control nosocomial respiratory syncytial virus infection. JAMA 256:2706-2708. [PubMed] [Google Scholar]
- 18.Girgis, D. O., G. D. Sloop, J. M. Reed, and R. J. O'Callaghan. 2003. A new topical model of Staphylococcus corneal infection in the mouse. Investig. Ophthalmol. Vis. Sci. 44:1591-1597. [DOI] [PubMed] [Google Scholar]
- 19.Grunweller, A., and R. K. Hartmann. 2005. RNA interference as a gene-specific approach for molecular medicine. Curr. Med. Chem. 12:3143-3161. [DOI] [PubMed] [Google Scholar]
- 20.Hall, C. B., R. G. Douglas, Jr., K. C. Schnabel, and J. M. Geiman. 1981. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect. Immun. 33:779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong, J. W., J. J. Liu, J. S. Lee, R. R. Mohan, R. R. Mohan, D. J. Woods, Y. G. He, and S. E. Wilson. 2001. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 42:2795-2803. [PubMed] [Google Scholar]
- 22.Hussell, T., A. Pennycook, and P. J. Openshaw. 2001. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31:2566-2573. [DOI] [PubMed] [Google Scholar]
- 23.Jafri, H. S., S. Chavez-Bueno, A. Mejias, A. M. Gomez, A. M. Rios, S. S. Nassi, M. Yusuf, P. Kapur, R. D. Hardy, J. Hatfield, B. B. Rogers, K. Krisher, and O. Ramilo. 2004. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J. Infect. Dis. 189:1856-1865. [DOI] [PubMed] [Google Scholar]
- 24.Keadle, T. L., N. Usui, K. A. Laycock, J. K. Miller, J. S. Pepose, and P. M. Stuart. 2000. IL-1 and TNF-alpha are important factors in the pathogenesis of murine recurrent herpetic stromal keratitis. Investig. Ophthalmol. Vis. Sci. 41:96-102. [PubMed] [Google Scholar]
- 25.Khvorova, A., A. Reynolds, and S. D. Jayasena. 2003. Functional siRNAs and miRNAs exhibit strand bias. Cell 115:209-216. (Erratum 115:505.) [DOI] [PubMed] [Google Scholar]
- 26.Le, G. T., and G. Abbenante. 2005. Inhibitors of TACE and caspase-1 as anti-inflammatory drugs. Curr. Med. Chem. 12:2963-2977. [DOI] [PubMed] [Google Scholar]
- 27.Li, X. Q., Z. F. Fu, R. Alvarez, C. Henderson, and R. A. Tripp. 2006. Respiratory syncytial virus (RSV) infects neuronal cells and processes that innervate the lung by a process involving RSV G protein. J. Virol. 80:537-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundberg, P., and E. Cantin. 2003. A potential role for CXCR3 chemokines in the response to ocular HSV infection. Curr. Eye Res. 26:137-150. [DOI] [PubMed] [Google Scholar]
- 29.Maggon, K., and S. Barik. 2004. New drugs and treatment for respiratory syncytial virus. Rev. Med. Virol. 14:149-168. [DOI] [PubMed] [Google Scholar]
- 30.Mastronarde, J. G., B. He, M. M. Monick, N. Mukaida, K. Matsushima, and G. W. Hunninghake. 1996. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-kappa B and NF-IL-6. J. Infect. Dis. 174:262-267. [DOI] [PubMed] [Google Scholar]
- 31.Mastronarde, J. G., M. M. Monick, N. Mukaida, K. Matsushima, and G. W. Hunninghake. 1998. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor-kappaB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J. Infect. Dis. 177:1275-1281. [DOI] [PubMed] [Google Scholar]
- 32.Miles, D. H., M. D. Willcox, and S. Athmanathan. 2004. Ocular and neuronal cell apoptosis during HSV-1 infection: a review. Curr. Eye. Res. 29:79-90. [DOI] [PubMed] [Google Scholar]
- 33.Moore, J. E., T. C. McMullen, I. L. Campbell, R. Rohan, Y. Kaji, N. A. Afshari, T. Usui, D. B. Archer, and A. P. Adamis. 2002. The inflammatory milieu associated with conjuctivalized cornea and its alteration with IL-1 RA gene therapy. Investig. Ophthalmol. Vis. Sci. 43:2905-2915. [PubMed] [Google Scholar]
- 34.Murdoch, C., and A. Finn. 2000. Chemokine receptors and their role in inflammation and infectious diseases. Blood 95:3032-3043. [PubMed] [Google Scholar]
- 35.Richmond, A. 2002. NF-kappa B, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217-242. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz, D. S., G. Hutvagner, T. Du, Z. Xu, N. Aronin, and P. D. Zamore. 2003. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115:199-208. [DOI] [PubMed] [Google Scholar]
- 38.Stevens, T. P., and C. B. Hall. 2004. Controversies in palivizumab use. Pediatr. Infect. Dis. J. 23:1051-1052. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, P. C., and M. Feldmann. 2004. New approaches to therapeutic immunomodulation for immune-mediated inflammatory disorders. Curr. Opin. Pharmacol. 4:368-371. [DOI] [PubMed] [Google Scholar]
- 40.Tian, B., Y. Zhang, B. A. Luxon, R. P. Garofalo, A. Casola, M. Sinha, and A. R. Brasier. 2002. Identification of NF-κB-dependent gene networks in respiratory syncytial virus-infected cells. J. Virol. 76:6800-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tripp, R. A., C. Oshansky, and R. Alvarez. 2005. Cytokines and respiratory syncytial virus infection. Proc. Am. Thorac. Soc. 2:147-149. [DOI] [PubMed] [Google Scholar]
- 42.Tumpey, T. M., H. Cheng, D. N. Cook, O. Smithies, J. E. Oakes, and R. N. Lausch. 1998. Absence of macrophage inflammatory protein-1α prevents the development of blinding herpes stromal keratitis. J. Virol. 72:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urnowey, S., T. Ansai, V. Bitko, K. Nakayama, T. Takehara, and S. Barik. 2006. Temporal activation of anti- and pro-apoptotic factors in human gingival fibroblasts infected with the periodontal pathogen, Porphyromonas gingivalis: potential role of bacterial proteases in host signalling. BMC Microbiol. 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Wallace, G. R., S. John Curnow, K. Wloka, M. Salmon, and P. I. Murray. 2004. The role of chemokines and their receptors in ocular disease. Prog. Retin. Eye. Res. 23:435-448. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, S. E., Y. G. He, J. Weng, Q. Li, A. W. McDowall, M. Vital, and E. L. Chwang. 1996. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp. Eye Res. 62:325-327. [DOI] [PubMed] [Google Scholar]