
The third European Molecular Biology Organization (EMBO) workshop on the Functional Organization of the Cell Nucleus was held in Prague between 5 and 8 May 2006, and was organized by U. Aebi, I. Raska and W. Earnshaw.
Introduction
The cell nucleus is a highly complex organelle that hosts the chromosomes and many distinct macromolecular domains. Knowing the functional organization of the nucleus is essential for understanding many of the cell functions that are important for health and disease. This field has progressed considerably towards revealing the structure, dynamics and organization of the nucleus, as was evident from the excellent talks and discussions at the third European Molecular Biology Organization (EMBO) workshop on the Functional Organization of the Cell Nucleus held in the beautiful city of Prague. The workshop was attended by 115 participants, and included 31 speakers and 32 shorter talks chosen from the posters.
Order to the chromosomes
The chromosomes form discrete domains within the nucleus. Outside these domains, a compartmentalization activity might dynamically organize nuclear bodies and speckles (Cremer & Cremer, 2001). T. Cremer (Munich, Germany) reported that increased osmolarity caused the condensation of interphase chromosomes and enlargement of the interchromatin compartment. A return to normal osmotic conditions caused chromosomes to occupy their previous positions, indicating a structural ‘memory' of both chromosome positions and the interchromatin compartment containing the nuclear bodies. At present, it is not known which structural elements are engaged in, and necessary for, this remarkable preservation of the topography of the chromatin ‘network'. In particular, detailed structural information on the chromosome surface and the space between individual chromosome territories is missing. Therefore, new optical microscopy and cryo-electron tomography methods are urgently needed to unravel the molecular organization of this part of the nuclear architecture. C. Cremer (Heidelberg, Germany) described an improvement in optical resolution by spatially modulated illumination microscopy, which should allow, for instance, the diameters of transcription complexes as small as 40 nm to be determined, and hence will facilitate better resolution mapping of nuclear organization.
Chromosome position affects gene expression
R. van Driel (Amsterdam, The Netherlands) used fluorescence in situ hybridization to show quantitatively that large genomic regions of highly expressed genes (ridges in the human transcriptome map) and of less-active genes (anti-ridges) on human chromosomes 1 and 11 have different three-dimensional chromatin structures: anti-ridges are 50% more compact, have a more spherical shape and are located closer to the nuclear envelope. W. Bickmore (Edinburgh, UK) followed the spatial localization of genes in the HoxB and HoxD homeobox gene complexes with respect to chromosome territories. Her data point to the existence of two steps in Hox complex activation: first, the opening of chromatin; and second, the looping out of the complex away from the main chromosome territory. The looping out, however, was not sufficient to induce transcription. A. Belmont (Urbana, IL, USA) suggested a potential explanation for the change in loci positions during transcription activation by showing that 1–2 h after targeting a transcriptional activator to a nuclear periphery locus, the latter migrated 1–5 mm roughly perpendicular to the nuclear envelope. The migrating locus recruited the nuclear myosin isoform. This process was inhibited by specific point mutations in actin and myosin I, indicating that the movement of transcriptionally active sites towards the nuclear interior is an active process that is dependent on actin/myosin.
In most eukaryotes, regions of silent chromatin cluster at the nuclear envelope, thereby creating zones that favour gene repression (Hediger & Gasser, 2002). U. Laemmli (Geneva, Switzerland) fused the nucleoporin Nup2, which is located at the nuclear basket of the nuclear pore complex (NPC), to micrococcal nuclease to show that in baker's yeast, active genes associate with NPCs. The interaction was dependent on gene activity and occurred at specific sequences in the promoter regions, but was independent of transcription. E. Hurt (Heidelberg, Germany) and U. Nehrbass (Paris, France) described another mechanism of positioning genes at NPCs that involves Sus1 and Ada2, which are members of the SAGA histone acetyltransferase complex, as well as the messenger RNA export factor Sac3. A possible role for the NPC–promoter interactions is that they facilitate quality control and the transport of mRNA to the cytoplasm. Such a model is, however, not supported in mammalian cells, in which active genes migrate away from the nuclear envelope (see above). In addition, R. Singer (New York, NY, USA) tracked single mRNAs in living mammalian cells to show that their movement in nuclei occurs primarily by diffusion. The ‘corralled' motion of the messenger ribonucleoproteins (mRNPs) was energy-independent and was not directed, indicating the presence of chromatin domains inaccessible to mRNPs and implying that nuclear bodies hinder the movement.
Specific DNA elements find each other over long distances
Polycomb group (PcG) proteins are evolutionarily conserved chromatin modifiers that silence homeotic genes by binding to PcG response elements (PREs; Grimaud et al, 2006). Using fluorescence recovery after photobleaching, D. Arndt-Jovin (Goettingen, Germany) followed PcG proteins in living cells and showed that in Drosophila embryos, PcG proteins-dynamically exchange between a free pool and complexes with a half-life of only a few minutes. In salivary glands, these proteins have different residence times on individual loci, indicating that individual PREs are composed of different PcG complexes. Fab-7 is a well-characterized PRE that is present in the Drosophila bithorax complex. Ectopically expressed Fab-7-containing transgenes recruit PcG proteins, and the additional copies of Fab-7 cooperatively enhance the PcG silencing effect through long-distance interactions between themselves. G. Cavalli (Montpellier, France) described a new role for the RNA-interference (RNAi) machinery in regulating the nuclear organization of PcG chromatin targets. Interestingly, although mutations in components of the RNAi machinery did not change the global level of PcG proteins or their binding to PREs, they affected the maintenance of long-distance contacts between Fab-7 copies. Another role for small RNAs was described by C. Francastel (Paris, France), who showed that 120-nucleotide-long RNAs are transcribed from the mouse centromeric minor satellite. These transcripts accumulate after cell differentiation or in response to stress, and are essential for maintaining the architecture and structure of centromeres.
During cell division, the chromatin is compacted and resolved into discrete mitotic chromosomes, the proper formation of which is essential for the correct distribution of the replicated genome to the daughter cells. W. Earnshaw (Edinburgh, UK) described a novel mechanism involved in the regulation of mitosis. Chicken cells conditionally knocked out for structural maintenance of chromosomes 2 (SMC2) died at anaphase with huge chromatin bridges connecting the segregating nuclei. The sister kinetochores migrated to the poles and separation of loci occurred even though the chromosomes were structurally abnormal. Keeping CDK activity high after anaphase onset rescued the chromosome segregation by suppressing the ability of Repo-Man (a novel protein phosphatase 1 (PP1)-binding protein) to recruit PP1γ and bind chromosomes.
Chromatin organization at origins of replication
DNA replication initiates at origins of replication, which are activated in a specific order and fire only once during each cell cycle (Blow & Dutta, 2005). C. Cardoso (Berlin, Germany) proposed a domino model, in which activation of early replicating origins promotes that of later replicating origins in neighbouring genome domains. Open reading frames (ORFs) are ‘licensed' for replication by loading complexes of mini-chromosome maintenance proteins 2–7 (Mcm2–7). Interestingly, Mcm2–7 complexes are present in a 20-fold excess over the number of ORFs. J. Blow (Dundee, UK) showed that excess Mcm2–7 complexes license sites that do not normally fire during S phase. In vivo, these dormant origins remain suppressed by the caffeine-sensitive checkpoint.
The functional organization of the nuclear periphery
The nuclear lamina is a dense fibrillar network, composed of lamin and lamin-associated proteins, which lines the inner surface of the nuclear envelope. The discovery that point mutations in lamin A cause a complex set of severe human diseases, ranging from muscular dystrophies to premature ageing, has brought the nuclear lamina to the centre stage of scientific interest. Lamin filaments are required for buffering mechanical strength, organizing the chromatin and regulating various nuclear functions (Gruenbaum et al, 2005). Caenorhabditis elegans contains only one lamin protein, and, using this system as a model, H. Herrmann (Heidelberg, Germany) reported that soluble molecules of this protein rapidly assemble into complex fibrillar structures of varying diameters in vitro. Y. Gruenbaum (Jerusalem, Israel) reported that binding of the barrier to autointegration factor (BAF) protein to the C. elegans lamin filaments alters their structure in vitro. He also showed that BAF is required for the normal assembly of lamins in vivo, and is involved in numerous cell activities and developmental decisions. M. Fornerod (Amsterdam, The Netherlands) described the mapping of ∼500 lamin-binding DNA sequences for the B-type lamin (Dm0) in the Drosophila melanogaster genome in vivo. These genes were transcriptionally silent, late replicating, lacked activating histone modifications and were widely spaced, indicating a fractal organization of the Drosophila genome. He concluded that target genes cluster in the genome, and that the clusters are coordinately expressed during development, implying that the nuclear lamina acts as an integrator of genetic and epigenetic chromatin features.
R. Goldman (Chicago, IL, USA) reported data explaining why cells harbouring the lamin A mutant LAΔ50, which causes Hutchison–Gilford progeria syndrome, lose heterochromatin. Not only is the trimethylation of Lys 27 on histone H3 (H3K27me3) lost, but also the methyltransferase for this mark, enhancer of zeste homologue 2 (EZH2), is downregulated (Fig 1). The H3K27me3 modification is present on the extra X chromosome that is silenced in all human female cells. The Xist RNA, which is the key initiator of the silencing of the inactive X chromosome, associates with and accumulates on this chromosome. Most notably, the H3K27me3 mark for inactive X chromosome is lost after ectopic expression of LAΔ50, indicating that the protein directly influences the performance of the Xist RNA in facultative heterochromatic regions.
Figure 1.
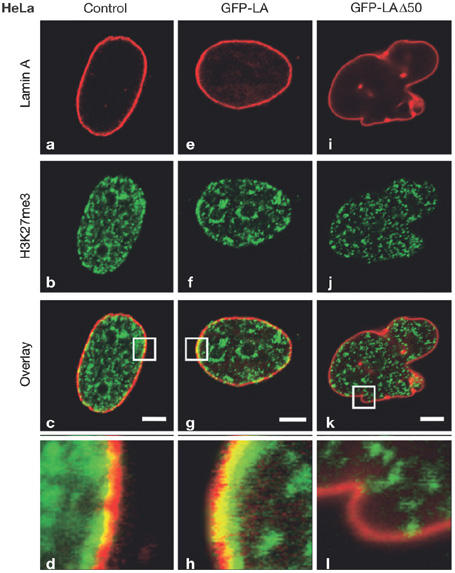
Fluorescence microscopy of HeLa cells labelled for histone H3 Lys 27 trimethylation (H3K27me3) after forced expression of the lamin A-deletion mutant LAΔ50. (a–d) Control cells. (e–h) Cells expressing green fluorescent protein–lamin A (GFP–LA). (i–l) Cells expressing GFP–LAΔ50. GFP–LA-expressing cells displayed a normally shaped nucleus (e) and punctate H3K27me3 staining throughout the nucleus, with prominent arrays at the nuclear periphery (indicated by an asterisk; b,f), where it overlaps with the lamina (g,h). Expression of GFP–LAΔ50 caused lobe formations in the nuclear envelopes of most HeLa cells (i), and there was an overall loss of H3K27me3 staining, especially in the region of the lamina (j,k,l). Enlargements (×7.4) of the overlay areas indicated by white boxes (c,j,k) are shown in (d,h,l). Reproduced from Shumaker et al (2006) with the permission of the National Academy of Sciences.
To exert their functions, lamins are present in a range of protein complexes (Gruenbaum et al, 2005). B. Burke (Gainesville, FL, USA) reported that the nucleoplasmic domain of the inner nuclear membrane protein SUN1 interacts with both NUP153 and the immature, transiently farnesylated form of lamin A (prelamin A). Hence, in addition to its role in linking the nucleoskeleton to the cytoskeleton, proteins that contain the SUN domain might be engaged in lamin assembly. Another lamin-A-associated protein is LAP2α, which lacks a transmembrane domain and associates with both BAF and the retinoblastoma protein. N. Foeger (Vienna, Austria) reported that complexes containing LAP2α and A-type lamins are associated with the E2F-dependent promoters of cyclin D1 and thymidine kinase, and repress transcription, thereby providing further evidence for the role of LAP2α in regulating the cell cycle.
Another main component of the nuclear envelope is the NPC. Transport of macromolecules through NPCs requires members of the karyopherin family, which are predominantly composed of HEAT repeats (Conti et al, 2006). Karyopherins are arranged in two C-shaped arches, and E. Conti (Heidelberg, Germany) presented an overview of the mechanistic requirements of these large superhelical molecules to bind to cargo or other transport factors. Their inherent flexibility allows the binding of various substrates by a similar type of fold. The mechanistic details of nuclear transport were introduced by B. Fahrenkrog (Basel, Switzerland). She used domain-specific antibodies to show elegantly that the FG-repeat domains of NUP153 and NUP214 alter their location in the pore, depending on the state of the transport process. This indicates that the structure of the NPC is dynamic in response to changes in cell physiology. K. Maeshima (Saitama, Japan) reported on ‘pore-free' islands in HeLa S3 cells early after mitosis. These areas of the envelope become dispersed in the G1-to-S phase transition. Interestingly, pore-free areas are enriched in lamin A and emerin but not in B-type lamins. Hence, A-type lamins seem to have an additional role in regulating NPC distribution.
Dynamic organization of nuclear organelles
Other exciting news came from the field of nucleoli and nuclear bodies. Ribosomal genes are organized in tandem repeats that are confined to chromosomal nucleolar-organizing regions (NORs). The active NORs have less condensed DNA and contain several components of the RNA polymerase I machinery, including the upstream binding factor (UBF). B. McStay (Dundee, UK) assembled pseudo-NORs in mammalian cells, and showed that UBF binds anywhere on ribosomal DNA in a transcription-independent manner and acts as the primary architectural element of active NORs. He showed that even in the absence of transcription, UBF recruits additional components of the transcription machinery, as well as a subset of the U3 protein (UTP) processing factors. In a transcription-dependent manner, further components are recruited, such as pre-ribosomal RNA (pre-rRNA) and U3 small nucleolar RNP (snoRNP). The Treacher Collins syndrome protein is also recruited by UBF to ribosomal chromatin and facilitates recruitment of box C/D snoRNPs to active NORs. I. Grummt (Heidelberg, Germany) found that the nucleolar-remodelling complex (NoRC), which is composed of TIP5 and SNF2h proteins and belongs to the SNF2h-containing family of chromatin-remodelling complexes, silences ribosomal DNA (rDNA) by recruiting histone-modifying enzymes to ribosomal chromatin. This NoRC function requires the presence of a small RNA (100–300 nucleotides) transcribed from a mouse intergenic spacer promoter. A. Beyer (Charlottesville, VA, USA) studied the early steps in ribosome biosynthesis in yeast using a Miller chromatin-spreading electron microscopy method (Fig 2). This approach allowed her to characterize novel intermediate structures associated with 5′ external transcribed spacer and 18S rRNA sequences. These intermediates in nascent rRNA folding were chaperoned by the ribosomal small subunit processome components. Nucleoli disintegrate in mitosis and reform again at the exit of mitosis. D. Hernandez-Verdun (Paris, France) has investigated the mitotic behaviour of both early and late ribosomal processing factors. These factors co-localize around chromosomes in anaphase and are concentrated in pre-nucleolar bodies (PNBs) during telophase. Late factors are recruited to nucleoli as complexes, indicating that PNBs are sites of complex formation at the exit of mitosis. A. Lamond (Dundee, UK) explored two complementary four-dimensional views of the nucleolus: time-lapse green fluorescent protein imaging and time-lapse proteomics. He divided the more than 700 proteins into three distinct classes: those that are mainly in the nucleolus (for example, fibrillarin); those that are in the nucleolus only part of the time (for example, ribosomal proteins); and those that are in the nucleolus depending on time and conditions (for example, protein p68). He focused on ribosomal proteins, and showed that there is a continual synthesis and rapid import of these proteins into the nucleolus. In parallel, there is a continual degradation of ribosomal proteins in the nucleus, and only a subset of nuclear ribosomal proteins are subsequently assembled into ribosomes and exported. These results indicate that the balance between the production of rRNA and ribosomal proteins is achieved post-transcriptionally and post-translationally. S. Lee (Ottawa, Ontario, Canada) showed that during hypoxia, to lower the energy demands of the cell, the ubiquitin ligase von Hippel–Lindau tumour suppressor protein (VHL) is sequestered to nucleoli, binds to the ribosomal intergenic spacer and silences rDNA genes. On extracellular acidification, nucleolus-sequestered VHL becomes a static player in this molecular network so that the hypoxia-induced factor is no longer degraded through ubiquitination, and cell death is prevented. On the basis of his observations, Lee suggested that both low pH and nucleolar presence of VHL trigger ribosomal chromatin heterochromatization through unknown mechanisms. G. Matera (Cleveland, OH, USA) developed a Drosophila model system to study mutations in the survival motor neuron (smn) gene, which is involved in the biogenesis of the Sm-core particle in vivo. Drosophila SMN localizes at sarcomeres, which implies that it has a function in muscle. The smn hypomorph mutants exhibit a phenotype that, in several aspects, resembles the human disease spinal muscular atrophy.
Figure 2.
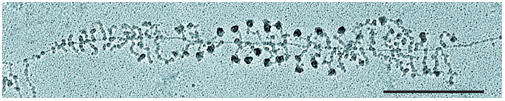
Electron micrograph of a spread preparation of yeast nucleolar chromatin in the form of a Christmas tree. Many ribonucleoprotein structures appearing on nascent transcripts can now be characterized in functional terms. Scale bar, 0.5 mm. Reproduced from Osheim et al (2004) with the permission of Elsevier.
RNA biogenesis and dynamics
Transcription elongation, RNA splicing and transport to the cytoplasm take place after transcription initiation, and all of these steps are subject to regulation. Y. Shav-Tal (Ramat Gan, Israel) presented data obtained using a live-cell imaging system that follows the transcription of a specific mRNA in real time. These data unexpectedly showed that RNA polymerase II pauses during the elongation stage, pointing to a mechanism by which this stage of mRNA production can be controlled. Coupling between transcription and pre-mRNA splicing is a key regulatory mechanism in gene expression. K. Neugebauer (Dresden, Germany) established a chromatin–RNA immunoprecipitation method to show that human c-fos pre-mRNA is co-transcriptionally spliced. Splicing was enhanced when RNA polymerase II elongation was inhibited. R. Sperling (Jerusalem, Israel) described a model of the splicing machine, the supraspliceosome, which is composed of four active (native) spliceosomes connected to each other by pre-mRNA (Fig 3). This model is based on the structure of the native spliceosome—determined at a resolution of 2 nm—and on the successful reconstitution of functional supraspliceosomes from native spliceosomes and synthetic pre-mRNA. J. Beggs (Edinburgh, UK) provided evidence that the biogenesis of U5 and U6 snoRNPs in yeast have many similarities with those in humans, including a cytoplasmic phase in U5 biogenesis and a restriction to the nucleus of U6 snoRNP. B. Daneholt (Stockholm, Sweden) reported an evolutionarily conserved DEAD-box RNA helicase from Chironomus tentans, hrp84, which forms a complex with the Y-box protein YB-1 in the nucleus. This complex is then transported to the cytoplasm where it is required for protein translation, indicating an early programming of translation already at the mRNP assembly stage. Using the same experimental system, L. Wieslander (Stockholm, Sweden) showed that the serine/arginine-rich proteins required for splicing and alternative splicing bind differentially to individual pre-mRNAs, and accompany the mRNPs to the cytoplasm. M. Carmo-Fonseca (Lisbon, Portugal) followed poly(A)+ RNA that is normally degraded in the nucleus by the exosome. In the absence of the exosomal ribonuclease Rrp6, the poly(A)+ RNA accumulated in a discrete subnucleolar region that differed from previously described nucleolar bodies. She suggested that this could form a recognition domain for exosome substrates.
Figure 3.
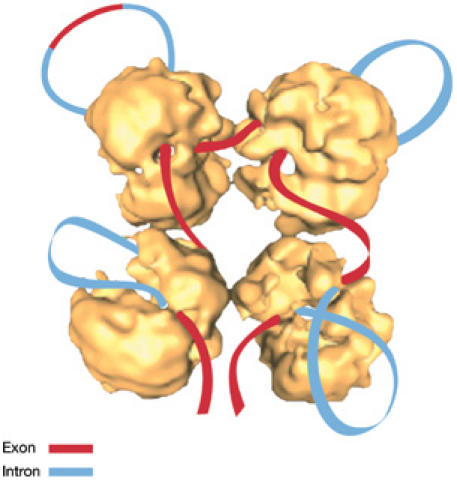
A schematic model of the supraspliceosome in which the pre-messenger RNA connects four native spliceosomes. Introns are shown in blue and exons are shown in red. The supraspliceosome presents a platform onto which the exons can be aligned and splice junctions can be checked before splicing occurs. Adapted from Azubel et al (2006).
Conclusion
This meeting documented exciting news about nuclear complexity and dynamics. These rapid advances in our understanding of nuclear processes, and in the development of novel microscopic, genomic and proteomic techniques, promise that at the next meeting, we will be able to view the nucleus at a higher resolution, allowing an even better understanding of its organization and functions.

Yosef Gruenbaum

Ivan Raska

Harald Herrmann
Acknowledgments
We gratefully acknowledge support from the European Union FP6 Life Science, Genomics and Biotechnology for Health area (LSHM-CT-2005-018690), the Cooperation Program in Cancer Research of the German Cancer Research Center (DKFZ), the Israel Ministry of Science and Technology (MOST) and the Israel Ministry of Health (MOH) to Y.G. and H.H, and grants from the Czech Grant Agency (304/04/0692 and 304/06/1691), the Czech Academy (AVOZ50110509) and the Ministry of Education (MSM0021620806 and LC535) to I.R. We thank the speakers whose work we have cited, and apologize to those colleagues who gave excellent talks but were not mentioned in this report due to space limitations. We are also grateful to R. Sperling and U. Aebi for critical comments.
References
- Azubel M, Habib N, Sperling R, Sperling J (2006) Native spliceosomes assemble with pre-mRNA to form supraspliceosomes. J Mol Biol 356: 955–966 [DOI] [PubMed] [Google Scholar]
- Blow JJ, Dutta A (2005) Preventing re-replication of chromosomal DNA. Nat Rev Cell Mol Biol 6: 476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Muller CW, Stewart M (2006) Karyopherin flexibility in nucleocytoplasmic transport. Curr Opin Struct Biol 16: 237–244 [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer C (2001) Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2: 292–301 [DOI] [PubMed] [Google Scholar]
- Grimaud C, Negre N, Cavalli G (2006) From genetics to epigenetics: the tale of Polycomb group and trithorax group genes. Chromosome Res 14: 363–375 [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y, Margalit A, Shumaker DK, Wilson KL (2005) The nuclear lamina comes of age. Nat Rev Mol Cell Biol 6: 21–31 [DOI] [PubMed] [Google Scholar]
- Hediger F, Gasser SM (2002) Nuclear organization and silencing: putting things in their place. Nat Cell Biol 4: E53–E55 [DOI] [PubMed] [Google Scholar]
- Osheim YN, French SL, Keck KM, Champion EA, Spasov K, Dragon F, Baserga SJ, Beyer AL (2004) Pre-18S ribosomal RNA is structurally compacted into the SSU processome prior to being cleaved from nascent transcripts in Saccharomyces cerevisiae. Mol Cell 16: 943–954 [DOI] [PubMed] [Google Scholar]
- Shumaker DK, Dechat T, Kohlmaier A, Adam DA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, Jenuwein T, Goldman RE (2006) Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci USA 103: 8703–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]


