Abstract
The infectious cycle of the human polyomavirus JC (JCV) is ultimately regulated in cellular nuclei at the level of viral protein expression and genomic replication. Such activity is prompted by interactions between variant nucleotide sequences within the JCV regulatory region (promoter) and cellular transcription factors that bind specific DNA consensus sites. In previous work we identified an NF-1 class member, NF-1X, as a critical transcription factor affecting the JCV cellular host range. Within variant JCV promoters, as well as other viral and cellular promoters, adjacently located NF-1 and AP-1 consensus sites are often found. The close proximity of these two binding sites suggests the opportunity for interaction between NF-1 and AP-1 proteins. Here, by electrophoretic mobility shift assays, we show temporal and dose-dependent interference by an AP-1 family member, c-Jun, upon NF-1 proteins binding an NF-1 consensus site derived from JCV promoter sequence. Moreover, as demonstrated by protein-protein interaction assays, we identify specific binding affinity independent of DNA binding between NF-1X and c-Jun. Finally, to compare the binding profiles of NF-1X and c-Jun on JCV promoter sequence in parallel with in vivo detection of viral activity levels, we developed an anchored transcriptional promoter (ATP) assay. With use of extracts from JCV-infected cells transfected to overexpress either NF-1X or c-Jun, ATP assays showed concurrent increases in NF-1X binding and viral protein expression. Conversely, increased c-Jun binding accompanied decreases in both NF-1X binding and viral protein expression. Therefore, inhibition of NF-1X binding by c-Jun appears to play a role in regulating levels of JCV activity.
The human polyomavirus JC (JCV) is the etiologic agent of the fatal demyelinating disease progressive multifocal leukoencephalopathy. During the course of trafficking to target oligodendrocytes sequestered in the central nervous system (CNS), JCV is known to also bind, enter, and to some extent infect peripheral cell types, including tonsillar stromal cells (stromal), B-lymphocytes, and kidney cells. While the presence of JCV in these varied cell types is well documented (22), a thorough understanding of how the inherent differences in cellular machinery affect viral activity has yet to be reached.
In part, cellular susceptibility to JCV is governed by events at the transcriptional level. Several cellular transcription factors implicated in the regulation of JCV gene expression include NF-κB (29), Tst-1 (35), Y-box binding protein 1 (16), and Purα (7), as well as members of transcription factor groupings, such as the nuclear factor 1 (NF-1) (2) and activator protein 1 (AP-1) families (1). Consensus binding sites for these and other DNA-binding proteins are centrally located within the JCV regulatory region (promoter) in an approximately 200-bp section of highly variable nucleotide sequence. This variable sequence offers distinct arrangements and assortments of transcription factor binding sites, which, in turn, lend to the diversity of viral activity seen between variant JCV genomes (15). Viral activity, however, is also affected by the unique expression pattern of transcription factors found within each cell type JCV enters (23, 26). Interestingly, cellular transcription factor expression patterns change as cells mature, and variations can be pronounced depending on the pathway of differentiation (26). Moreover, synergistic, competitive, and/or inhibitory interactions occurring between any number of cellular transcription factors (and cis-acting viral proteins) have the potential to further alter net levels of JCV activity. Such parameters suggest that JCV transcription and replication is controlled by multilayered interactions between cellular machinery, viral proteins, and infectious viral promoter sequences.
In humans, the NF-1 family of DNA-binding proteins (NF-1A, -B, -C, and -X) (X is also known as D) is encoded by four discrete genes. Differential splicing of the NF-1 mRNA transcripts results in multiple isoforms of each NF-1 class member (11). First isolated from HeLa cells and found to stimulate adenovirus DNA replication, NF-1 proteins have since been associated with transcriptional regulation of several cellular and viral genes. The variable C-terminal regions of NF-1 proteins convey differences in transcriptional activity and also serve to distinguish the NF-1 class types and splice variants. With highly conserved N-terminal domains shared between the NF-1 class types, homo- and/or heterodimerization bears a variety of protein structures, all capable of binding alpha-helical DNA representative of the consensus sequence 5′-TGG (A/C)N5GCCAA-3′ (6).
Enhanced expression of NF-1 class X has been correlated with the support of JCV activity. Previously, we showed that JCV-susceptible stromal cells express elevated levels of NF-1X compared to nonsusceptible cell types (26). The same relationship between cellular NF-1X expression and JCV susceptibility was observed in permissive SH-EP and nonpermissive SH-SY5Y neuroblastoma cell lines (32). Moreover, we demonstrated that when a JCV-susceptible human hematopoietic progenitor cell line, KG-1, was treated with the mitogen phorbol 12-myristate 13-acetate to induce differentiation to a macrophage-like phenotype, it no longer supported JCV activity. However, transfecting the macrophage-like phenotype with an expression vector encoding NF-1X restored JCV susceptibility. A similar observation was made using nonsusceptible human neuronal cells derived from a human CNS progenitor cell culture system (23). In both cases, the conveyance of JCV susceptibility was specific to NF-1X, as demonstrated by the failure of parallel transfections with expression vectors encoding the other NF-1 class members.
The AP-1 family of transcription factors has also been associated with JC viral activity (1, 17). Many members of this transcription factor family, like the NF-1 proteins, dimerize to facilitate DNA binding. AP-1 dimers are composed of Jun (c-Jun, JunB, and JunD) as well as Fos (c-Fos and FosB) proteins and have affinity for DNA containing the consensus sequence 5′-TGA(G/C)TCA-3′. AP-1 proteins comprise three domains: a C-terminal leucine zipper necessary for dimerization (19, 34), a central basic region responsible for DNA binding, and an N terminus involved in transcriptional activation (4). It has been demonstrated that c-Jun can modulate transcriptional activity of JC virus, as well as the unwinding of DNA mediated by the early T antigen of mouse polyomavirus (12, 13, 17).
Adjacent NF-1 and AP-1 consensus sites are encountered in JCV and BK virus promoters, as well as several brain-specific genes, including those encoding myelin basic protein, neurofilament, human glial fibrillary acidic protein (GFAP), and proenkephalin (1, 18, 33). This juxtaposition of NF-1 and AP-1 consensus sites in various genes associated with the human CNS suggests that interactions between NF-1 and AP-1 proteins play a role in tissue-specific host and viral gene expression. In addition to juxtaposed binding sites, physical interaction between NF-1 and AP-1 proteins has recently been observed in regard to GFAP expression in an astrocytic cell line (10); however, the nature of this interaction is unclear.
Here, we demonstrate interference by c-Jun upon binding of NF-1 proteins to an NF-1 consensus site derived from JCV promoter sequence. We also demonstrate specific protein-protein binding affinity, independent of DNA binding, between NF-1X and c-Jun using a number of techniques, including immunoprecipitation assays, Western blots, in vitro transcription/translation, and glutathione S-transferase (GST) fusion protein capture. Moreover, utilization of a JCV-anchored transcriptional promoter (ATP) assay we have developed reveals differentials of viral activity concurrent with varied profiles of NF-1X and c-Jun binding to JCV promoter sequence. The interactions described here between NF-1X, c-Jun, and JCV promoter sequence likely play a role in the regulation of JCV activity.
MATERIALS AND METHODS
Human primary tonsillar stromal cells (stromal cells).
Stromal cells were utilized due to their ability to support the complete JCV infectious cycle (24, 25) and high expression levels of NF-1 message (26). Cells were isolated according to protocols described previously (20).
Stromal protein extract.
Stromal cells were washed three times with ice-cold phosphate-buffered saline (PBS), and whole-cell extracts were prepared by a modification of the procedure of Andrews and Faller (3). Cells were scraped from tissue culture flasks, pelleted, and then disrupted by three freeze-thaw cycles on dry ice. Cell pellets were resuspended in two volumes of ice-cold buffer C (20 mM Tris-HCl [pH 7.9], 1.5 mM MgCl2, 420 mM NaCl, 0.2 M EDTA, 25% glycerol) including protease inhibitors (0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 5 μg/μl antipain, 5 μg/μl leupeptin, 5 μg/μl chymostatin, 5 μg/μl aprotinin, 5 μg/μl pepstatin A). Lysates were then centrifuged at 9,000 × g for 5 min at 4°C. Supernatant protein fractions were divided into aliquots and stored at −80°C. Protein concentrations were determined by Bradford assay (5).
Radiolabeled oligonucleotide probes.
Oligonucleotides derived from JCV Mad-1 promoter sequence (nucleotides [nt] 38 to 53) (9) containing either an intact NF-1 binding site (5′-ATGGCTGCCAGCCAAG-3′) or a mutated NF-1 site (mutated sites underlined; 5′-ATTACTGCCAGCTGAG-3′) were synthesized (Invitrogen). Complementary strands were produced and annealed to each corresponding sequence above to form double-stranded oligonucleotides. The above double-stranded oligonucleotides (20 pM) were end labeled with [γ-32P]ATP for 2 h at 37°C, centrifuged through a Microspin G-25 column (Pharmacia/GE Healthcare-Amersham Biosciences) at 735 × g for 2 min, and brought to a working concentration of 0.2 pM with buffer D (10 mM HEPES, 50 mM KCl, 10% glycerol).
Electrophoretic mobility shift assay (EMSA).
Unless otherwise noted, radiolabeled oligonucleotide probes (200,000 cpm) were incubated with 10 μg of stromal protein extract or 0.6 μg of recombinant human c-Jun [rhAP-1(c-Jun); amount recommended by the manufacturer; Promega] in the presence or absence of a 250-fold excess of either unlabeled intact oligonucleotide or unlabeled mutant oligonucleotide. As a binding control, one gel shift unit of the p50 subunit of recombinant human NF-κB was used (amount suggested by the manufacturer; Promega). Unless otherwise noted, incubations were carried out on ice for 30 min. Secondary incubations were conducted, where needed, by adding either cellular protein extract or rhAP-1(c-Jun) to the primary reaction mixture and then placing on ice for an additional 30 min. All incubations were resolved by electrophoresis on 6% polyacrylamide-Tris-glycine gels at 4°C. Gels were dried and exposed to BioMAX MR film (Kodak) for autoradiographical detection of protein/oligonucleotide binding as evidenced by any increase in the molecular weight of the probe (gel shift).
Human progenitor-derived astrocytes (PDA).
Human CNS progenitor cells (progenitors) were isolated from the telencephalon of a human fetal brain at 8 weeks of gestation, obtained in accordance to NIH guidelines and differentiated into an astrocytic lineage (PDA) according to a previously described protocol (26).
Immunoprecipitation (IP) and Western blot.
In these assays we utilized whole-cell extracts. Stromal and PDA cell cultures were lysed in ice-cold lysis buffer E (20 mM HEPES [pH 7.5], 150 mM NaCl, 3% glycerol, 5 mM MgCl2, 1 mM CaCl2, 1 mM EDTA, 1.0% NP-40, and one Complete Mini protease/phosphatase inhibitor tablet [Roche] per 20 ml). Lysates were sonicated on ice three times, 5 s each. After lysates were centrifuged to remove cellular debris, supernatants were precleared by incubation at 4°C for 1 h with 100 μl of protein G slurry (50% protein G agarose beads [Santa Cruz Biotechnology] suspended in 50% lysis buffer E) and 1 μg of a nonspecific rabbit polyclonal antibody (anti-14-3-3; Santa Cruz Biotechnology). Nonspecific complexes were pelleted by centrifugation at 10,000 × g at 4°C for 10 min, and the precleared supernatants were rotated at 4°C with either 1 μg of preimmune rabbit sera (Santa Cruz Biotechnology), anti-NF-1X (Geneka/Active Motif), or anti-c-Jun (Santa Cruz Biotechnology). After 1 h of incubation with these antibodies, a fresh 100 μl of protein G slurry was added for another 1 h of rotation at 4°C. The protein G beads were pelleted by centrifugation at 4°C and washed three times in ice-cold lysis buffer E, one time in ice-cold PBS. Beads were again pelleted by centrifugation at 4°C but then resuspended in 50 μl Laemmli protein loading buffer (PLB) and boiled for 5 min. Supernatants (the immunoprecipitates) from the pelleted beads were resolved on 12% precast sodium dodecyl sulfate (SDS)-polyacrylamide ReadyGels (Bio-Rad) and transblotted onto polyvinylidene difluoride (PVDF) membranes (Millipore). The transblotted membranes were probed with primary antibodies (anti-c-Jun [Santa Cruz Biotechnology] or anti-NF-1X [Geneka/Active Motif]) at 4°C overnight in blocking buffer (25 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20, and 5% nonfat dry milk). Bound primary antibodies were detected with an antirabbit, horseradish peroxidase-conjugated secondary antibody and the Chemiluminescence Luminol reagent kit (Santa Cruz Biotechnology), according to the manufacturer's protocol.
Plasmid constructs.
A GST fusion protein plasmid, pGEX-Jun (21), which encodes GST-c-JUN, was generously provided by James A. Goodrich (University of Colorado). Additionally, pGEX2T, which encodes GST alone, was purchased (Pharmacia/GE Healthcare-Amersham Biosciences) as a suitable control plasmid (vector alone) for experiments conducted with the pGEX-Jun plasmid described above. The pcDNA3NF-1X plasmid was subcloned by forced cloning of the hemagglutinin (HA)-tagged NF-1X2 gene from pCHAmNF-1X2 (described below) into the multicloning site (between the NotI and XbaI restriction sites) of the pcDNA3 vector (Invitrogen). pCHAmNF1-X2 was a generous gift from Richard Gronostajski (The State University of New York at Buffalo) which contains the cDNA coding region of murine NF-1X (with a single amino acid difference, murine NF-1X is >99.7% homologous to human NF-1X). The NF-1X cDNA was subcloned into the pCHA vector to form a fusion protein with an N-terminal hemagglutinin tag. This tag has shown no effect on NF-1X DNA-binding or transactivation functions (6). pCHAmNF-1A1.1 (also from Richard Gronostajski at The State University of New York at Buffalo) is similar to pCHAmNF-1X2, described above, except that it contains the NF-1A1.1 gene rather than the NF-1X2 gene. pM1TC (9) is the entire JCV prototype, Mad-1 genome cloned into the EcoRI restriction site of pBR322.
Expression and purification of recombinant GST and GST-Jun proteins.
GST and GST-c-Jun were expressed in competent DH5-α Escherichia coli (Invitrogen) that was transformed with pGEX2T or pGEX-Jun, respectively.
GST pull-down assay.
GST and GST-c-Jun were generated, purified, and immobilized on glutathione-agarose as described above. Cultures of PDA were transiently transfected with pCDNA3NF-1X (2 μg/2 × 106 cells) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. To generate whole-cell extracts, the PDA were disrupted 36 h posttransfection with ice-cold lysis buffer E (20 mM HEPES [pH 7.5], 150 mM NaCl, 3% glycerol, 5 mM MgCl2, 1 mM CaCl2, 1 mM EDTA, 1.0% NP-40, and one Complete Mini protease/phosphatase inhibitor tablet [Roche] per 20 ml). Lysates were sonicated on ice three times, 5 s each, and incubated separately with immobilized GST or GST-c-Jun at 4°C, rotating for 2 h. Resulting complexes were washed four times with 100-bed volumes of binding buffer containing 0.1% NP-40 and once with PBS. Bound proteins were eluted by boiling the complexes for 3 min in 2× PLB, resolved on 12% precast SDS-polyacrylamide ReadyGels (Bio-Rad), transblotted to PVDF membranes (Millipore), and analyzed by anti-NFX-1 (Geneka/Active Motif) chemiluminescent Western blotting.
In vitro binding assay.
GST and GST-c-Jun were generated and purified as described above. Employing pCDNA3NF-1X as a template, [35S]methionine-labeled NF-1X ([35S]NF-1X) was generated using the TNT Coupled in vitro transcription/translation system (Promega) according to the manufacturer's protocol. Approximately 2 μg of GST or GST-c-JUN immobilized on glutathione-agarose was incubated with [35S]NF-1X (to form bead-complexes) in binding buffer (as described in the “Expression” section above) containing 1.0% NP-40 and 1 mg/ml bovine serum albumin. After incubation for 2 h at 4°C with agitation, the bead complexes were washed once in fresh binding buffer as used for the incubation above, twice in binding buffer containing only 0.1% NP-40, twice in binding buffer alone, and once in PBS. The washed bead complexes were disrupted by boiling for 3 min in Laemmli PLB and the components resolved by electrophoresis on 12% precast SDS-polyacrylamide ReadyGels (Bio-Rad). These gels were fixed and stained with Coomassie brilliant blue R-250 in 10% methanol and then dried and exposed to BioMax MR film (Kodak) overnight at −80°C.
PDA nuclear extract.
PDA (5 × 106 cells/162-cm2 flask) were washed with ice-cold Tris-buffered saline (TBS), scraped, pooled, and pelleted by centrifugation at 1,500 × g for 5 min at 4°C. Pellets from each flask were washed in 1 ml ice-cold TBS and resuspended in 400 μl of ice-cold NEB-A (nuclear extract buffer-A) (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, and one Complete Mini protease/phosphatase inhibitor tablet [Roche] per 20 ml) and incubated for 15 min at 4°C to swell the cells. Twenty-five microliters of 10% NP-40 was added to the mixture followed by a brief vortex. The homogenates were centrifuged at 16,000 × g for 30 s at 4°C. The supernatants, containing cytosolic proteins, were collected and stored at −80°C. The nuclear pellets were resuspended in 50 μl ice-cold NEB-C (nuclear extract buffer-C) (20 mM HEPES, pH 7.9, 0.4 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, and one Complete Mini protease/phosphatase inhibitor tablet [Roche] per 20 ml). Samples were rocked for 15 min at 4°C and then centrifuged at 16,000 × g for 5 min at 4°C. Supernatants containing nuclear proteins were stored at −80°C. Protein concentrations were determined by using a Bio-Rad DC protein assay kit according to the manufacturer's protocol. Extracts were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) on 4 to 12% gradient bis-Tris gels (Invitrogen), transferred to a PVDF membrane (Millipore), and analyzed by Western blotting as described in the “IP and Western blot” section but with anti-NF-1A, anti-NF-1B, and anti-NF-1X (Geneka/Active Motif), as well as anti-β-actin (Sigma) and anti-GFAP (Chemicon) primaries. In cases where monoclonal primary antibodies were used, a horseradish peroxidase-conjugated antimouse (Santa Cruz Biotechnology) secondary antibody was employed for detection.
JCV infection in PDA.
PDA were exposed to JCV, Mad-4 variant, at 200 hemagglutination units/106 cells in a minimal covering of serum-free astrocyte medium. Ninety minutes after JCV exposure, the medium was supplemented with fresh complete astrocyte medium. Nuclear and cytosolic fractions of infected PDA were prepared 4 days post-JCV exposure, resolved by SDS-PAGE, and assayed by Western blotting as described in the “PDA nuclear extract” section but with anti-T antigen (Oncogene), a polyclonal rabbit anti-Vp-1 developed in our lab, and anti-β-actin (Sigma).
Overexpression experiments.
PDA were transfected with pCDNA3, pCDNA3NF-1X, or pET-c-Jun (a generous gift from James A. Goodrich, University of Colorado) using 5 μg of DNA/107 cells with Amaxa (Amaxa Inc.) astrocyte nucleofector reagent. At 12 h posttransfection, the media were replaced with fresh medium containing JCV at 200 hemagglutination units/106 cells. After 12 h, medium containing virus was aspirated, cells were washed once, and fresh medium was added. Nuclear fractions of transfected/infected PDA were prepared 4 days post-JCV exposure, resolved by SDS-PAGE, and analyzed by Western blotting as described in the “PDA nuclear extract” section but with anti-NF-1X (Geneka/Active Motif), anti-c-Jun (Santa Cruz Biotechnology), the polyclonal rabbit anti-Vp-1 developed in our lab, and anti-β-actin (Sigma).
ATP binding assay.
The Mad-1 JCV promoter was PCR amplified from pM1TC (9) using a modified forward primer (JCV Mad-1) (nt 4992 to 5011) having a biotin group linked to the 5-prime end and a normal reverse primer (JCV Mad-1) (nt 447 to 427). This biotinylated PCR product was coupled to streptavidin agarose beads, as previously described (30), to generate ATP-Mad-1. For preclearing of PDA extracts prior to ATP-Mad-1 exposure, an approximately 1,500-bp segment of NF-1A1.1 coding sequence (locus_ID: NM_010905) was PCR amplified from pCHAmNF-1A1.1 using a modified forward primer (nt 1 to 27) having a biotin group linked to the 5-prime end and a normal reverse primer (nt 1520 to 1500). This PCR product was coupled to streptavidin agarose beads to generate an anchored coding region (ACR-NF-1A1.1).
In an approach similar to antibody-based immunoprecipitations, ATP-Mad-1 slurry was used to pull down DNA binding proteins from aliquots of PDA nuclear extracts (same extracts as those described in “Overexpression experiments”). Briefly, PDA nuclear extracts were precleared by addition to equal volumes of protein binding buffer (1× TBS, pH 7.4, 1% Triton X-100, 1% glycerol, and protease inhibitors) containing 10% ACR-NF-1A1.1 and gently rotated overnight at 4°C. After centrifugation to pellet ACR-NF-1A1.1, the supernatants (precleared nuclear extracts) were added to equal volumes of protein binding buffer containing 10% ATP-Mad-1. After 2 h, at 4°C with gentle rotation, the ATP-Mad-1 was pelleted by centrifugation and washed three times with ATP wash buffer (1× TBS, pH 7.4, 0.5% Triton X-100, 0.5% glycerol, and protease inhibitors) and once with TBS containing protease inhibitors. Bound proteins were eluted by boiling the washed ATP-Mad-1 for 3 min in Laemmli PLB, resolved by SDS-PAGE, and analyzed by Western blotting as described in the “PDA nuclear extract” section but with anti-NF-1X (Geneka/Active Motif) and anti-c-Jun (Santa Cruz Biotechnology).
RESULTS
Inhibition of NF-1 binding by rhAP-1(c-Jun).
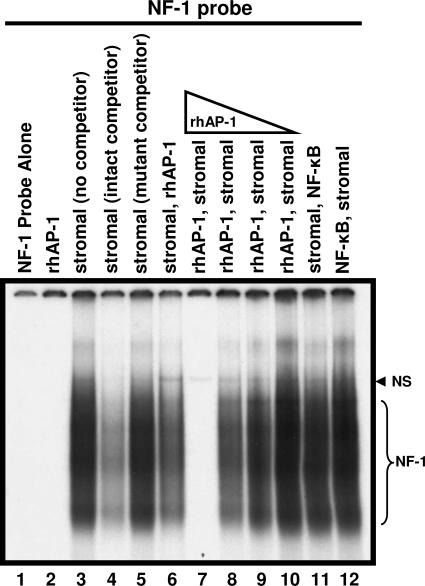
We previously identified the juxtaposition of the NF-1 and c-Jun binding sites in JCV promoters using DNase footprinting analysis (1, 2) and have posed the hypothesis that there is direct protein-protein interaction between NF-1 and c-Jun. To investigate this hypothesis and the effect c-Jun may have on NF-1 binding to a JCV promoter, EMSA assays were performed using a radiolabeled oligonucleotide probe containing the NF-1 consensus site derived from the JCV Mad-1 sequence (NF-1 probe) (Fig. 1). A gel-shifted band specific for binding of NF-1 proteins was observed when stromal cell extract was incubated with the NF-1 probe (Fig. 1, lane 3). The presence of a molar excess of unlabeled NF-1 probe (cold) competed out binding to the NF-1 probe (Fig. 1, lane 4), while an excess of an altered unlabeled probe containing a mutated NF-1 consensus site (mutant) did not reduce binding, demonstrating specificity (Fig. 1, lane 5). A primary 30-min incubation of stromal cell extract with the NF-1 probe, followed by a secondary 30-min incubation after the addition of rhAP-1(c-Jun), reduced binding to the NF-1 probe to some extent (Fig. 1, lane 6). However, reversing the order by first incubating with rhAP-1(c-Jun) and then adding stromal cell nuclear extracts eliminated NF-1 protein binding to the consensus sequence altogether, suggesting a significant inhibitory interaction (Fig. 1, lane 7). This inhibition of NF-1 binding through primary c-Jun incubation was dose dependent. Reducing the concentration of rhAP-1(c-Jun) by 4-, 16-, or 64-fold decreased the inhibitory effect proportionally (Fig. 1, lanes 8 to 10). As a negative control, NF-κB, which like rhAP-1(c-Jun) is not recognized by the NF-1 probe, had no affect on binding to the NF-1 probe (Fig. 1, lanes 11 and 12). These results demonstrate that c-Jun can inhibit binding of the NF-1 protein to the consensus NF-1 site of the JCV Mad-1 promoter if introduced prior to and/or in sufficient quantity with an NF-1 source (e.g., stromal cell extract). Interestingly, the inhibition of NF-1 binding occurs independently of c-Jun binding the NF-1 probe, as demonstrated by the failure of rhAP-1(c-Jun) to shift the NF-1 probe by itself (Fig. 1, lane 2). Free NF-1 probe was routinely run off the bottom the EMSA gels to avoid interference with the clear identification of entire NF-1 smears. In EMSAs where the NF-1 probe was not run off the bottom of the gel (data not shown), addition of rhAP-1 showed no reduction in the free NF-1 probe band compared with lanes having no rhAP-1, ruling out rhAP-1-mediated isotope cleavage from the probe. Therefore, the observed inhibition may be the result of a physical interaction between NF-1 and c-Jun that prevents NF-1 from binding to the JCV promoter and/or an effect of free c-Jun on the NF-1 consensus site, such as electrostatic interactions that have been described by others (27, 28).
FIG. 1.
Competitive gel shift analysis of the binding of proteins (10 μg) from tonsillar stromal-cell extracts to a radiolabeled probe containing an NF-1 binding site. The smeared migration (lane 3) represents specific binding by NF-1 class members (NF-1 bracket). The presence of excess unlabeled, intact oligonucleotide (intact competitor) outcompeted binding to the radiolabeled probe (lane 4), while unlabeled mutant oligonucleotide (mutant competitor) did not (lane 5). The single band observed (lanes 3, 6 to 12) above the NF-1 smear represents nonspecific binding (NS), as evidenced by competition from both intact and mutant competitor oligonucleotides. While NF-1 binding the probe was somewhat reduced when rhAP-1 (0.6 μg) was added after an initial 30-min incubation with stromal extract (lane 6), binding was eliminated when rhAP-1 was incubated with the probe for 30 min before the stromal extract was added (lane 7). Binding to the NF-1 probe was restored as the amount of rhAP-1 present was decreased (0.6, 0.16, 0.04, and 0.01 μg; lanes 7 to 10). This inhibitory effect of rhAP-1 was not due to rhAP-1 binding to the NF-1 probe (lane 2) and was not observed with another DNA-binding protein, NF-κB (lanes 11, 12). In lane 1 the NF-1 probe was free of added protein, showing unbound probe migration off the gel. Free probe was run off the bottom of gels to facilitate the clear identification of NF-1 smears. In EMSA where free probe was not run off (data not shown), rhAP-1 showed no reduction in the free probe band, eliminating concerns of isotope cleavage. Results included are representative of three independent experiments.
IP and Western blot analysis for NF-1 and c-Jun.
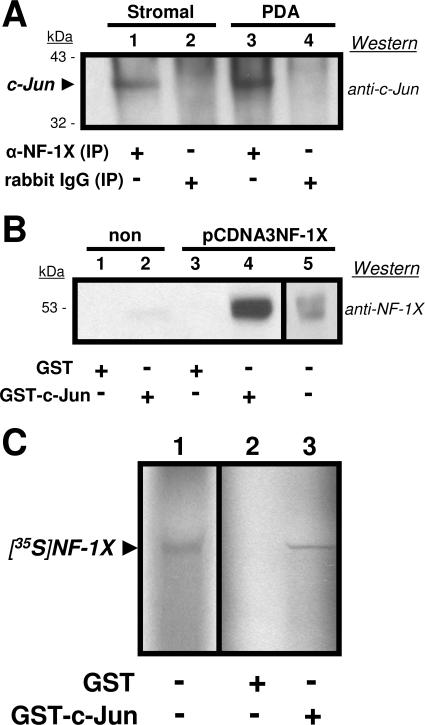
To identify specific interactions between NF-1 class members and c-Jun, we first examined whether these proteins physically bind each other. Western blot analyses were conducted with stromal and PDA total cell lysates subjected to immunoprecipitation (IP) with either anti-NF-1X or an antirabbit immunoglobulin G (IgG) control and then resolved by PAGE and probed with anti-c-Jun. As shown in Fig. 2, c -Jun coprecipitated with NF-1X in both stromal and astrocyte lysates (Fig. 2A, lanes 1 and 3). No binding from the cell lysates was seen with the antirabbit IgG control (Fig. 2A, lanes 2 and 4). These data demonstrate that NF-1 and c-Jun were physically associated in the lysates of both stromal cells and PDA. The detection of NF-1X in reciprocal co-IPs (anti-c-Jun precipitation, followed by anti-NF-1X Western blotting) was problematic, because NF-1X resolves near the 55-kDa size of IgG heavy chains.
FIG. 2.
(A) Western blot utilizing anti-c-Jun versus total cell lysates from human tonsillar-stromal (Stromal; lanes 1 and 2) and astrocytic (PDA; lanes 3 and 4) cell cultures immunoprecipitated (IP) with either anti-NF-1X (lanes 1 and 3) or normal rabbit IgG (lanes 2 and 4). Precipitated proteins were resolved on a 12% gel and transferred to a PVDF membrane for probing with the anti-c-Jun. The arrowhead identifies c-Jun that had been associated with precipitated NF-1X protein. Numbers at left represent mass in kilodaltons of prestained protein standards. Results included are representative of three independent experiments. (B) Western blots utilizing anti-NF-1X versus total cell lysates from astrocytic cell cultures (PDA). Nontransfected (lanes 1 and 2) or pCDNA3NF-1X-transfected (lanes 3 and 4) incubated with either GST (lanes 1 and 3) or GST-c-Jun (lanes 2 and 4) beads. Eluates from beads were resolved on a 12% gel and transferred to a PVDF membrane for probing with anti-NF-1X. The separate reference (lane 5) is lysate from the pCDNA3NF-1X-transfected PDA prior to incubation with beads. Numbers at left represent mass in kilodaltons of prestained protein standards. Results included are representative of three independent experiments. (C) Autoradiograph of an in vitro transcription/translation reaction which utilized pCDNA3NF-1X as a template in the presence of [35S]methionine to express labeled NF-1X protein ([35S]NF-1X). An aliquot of the total reaction product (lane 1) was resolved on a 12% gel in addition to eluates derived from either GST (lane 2) or GST-c-Jun (lane 3) beads incubated with identical aliquots. The resolved gels were dried and then exposed to X-ray film overnight at −80°C. Results included are representative of three independent experiments.
GST pull-down assays.
GST pull-down assays were utilized in which recombinant GST and GST-c-Jun were separately incubated with the total cell lysates of either nontransfected PDA or pcDNA3NF-1X-transfected PDA (Fig. 2B). Proteins dissociated from the isolated (pulled-down) GST and GST-c-Jun were resolved on gels and analyzed in Western blots versus anti-NF-1X. While the constitutively expressed NF-1X in nontransfected PDA lysate showed an association with GST-c-Jun, the overexpression of NF-1X in the transfected PDA more clearly demonstrated this association. These experiments substantiate our findings of a direct binding interaction between NF-1X and c-Jun.
In vitro translation of NF-1X.
An additional GST pull-down assay was performed using the recombinant GST and GST-c-Jun described above, along with in vitro-transcribed/translated 35S-labeled-NF-1X protein ([35S]NF-1X) to rule out possible effects of other cellular proteins on the observed association of NF-1X and c-Jun (Fig. 2C). [35S]NF-1X showed specific association with GST-c-Jun, further demonstrating the specific interaction between NF-1X and c-Jun.
Comparative NF-1 expression in progenitors and PDA.
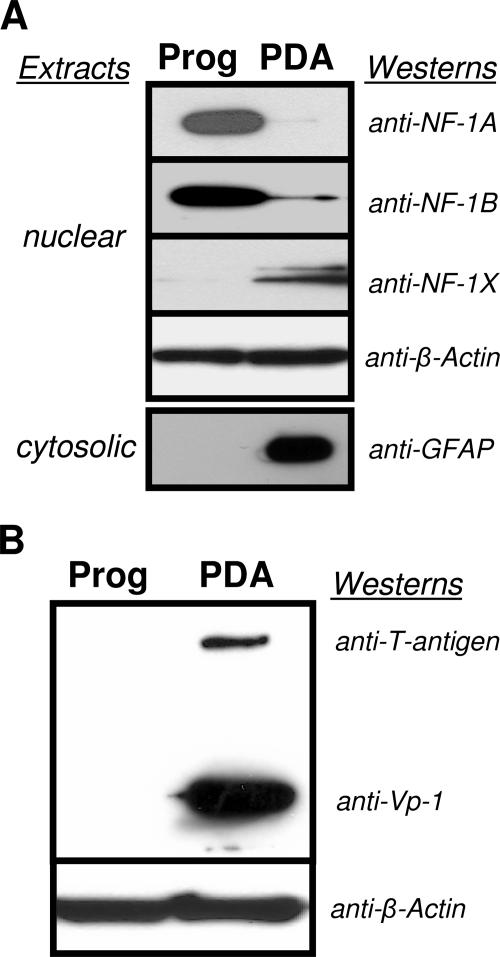
To uncover additional details regarding the control of JCV activity by NF-1X, we analyzed NF-1 protein expression in our model cell culture system of multipotential human CNS progenitors. Progenitors support only low levels of JCV activity compared with that for PDA (23). Our studies here revealed differential protein expression patterns of NF-1 class members, with elevated expression of NF-1X in PDA (Fig. 3A). β-Actin detection served as the control for equivalent total protein loading between progenitor and PDA nuclear extracts. The cytosolic fraction probed with anti-GFAP (a cytoskeletal marker that specifically identifies astrocytes among other CNS cell types) was included to illustrate the complete differentiation from progenitor to PDA.
FIG. 3.
(A) Anti-NF-1 Western blots demonstrate differential expression levels of three of the four NF-1 class types in comparisons between multipotential hCNS progenitor cell cultures (Prog) and parallel progenitor-derived astrocyte cultures (PDA). The PDA were derived from Prog incubated in astrocyte medium for ≥28 days. Equal amounts from a single stock of Prog nuclear extract were loaded in each lane of the left column. Equal amounts from a single stock of PDA nuclear extract were loaded in lanes of the right column. Loaded nuclear extracts were resolved on 4 to 12% gradient polyacrylamide gels. β-Actin detection was the control for equivalent total protein loading between Prog and PDA nuclear extracts. Detection of cytosolic GFAP, a cytoskeletal marker that specifically identifies astrocytes from other CNS cell types, was assurance of successful differentiation from Prog to PDA. Results included are representative of three independent experiments. (B) Dual anti-T-antigen, anti-Vp-1 Western blots, 4 days post-exposure to JCV, comparing viral protein expression levels in multipotential hCNS progenitor cell cultures (Prog) with those in parallel progenitor derived astrocyte cultures (PDA). The PDA were derived from Prog incubated in astrocyte medium for ≥28 days. β-Actin detection was the control for equivalent total protein loading between Prog and PDA nuclear extracts. Note low-to-no viral-protein expression (infectivity) in Prog compared with PDA. Immunocytochemistry (23) has shown low infectivity in isolated/individual Prog cells 10 days post-JCV exposure; such infectivity may be difficult to demonstrate in a Western blot, as above, which utilizes nuclear extract from cell cultures having only 4 days of viral exposure prior to lysis. Results included are representative of three independent experiments.
JCV infections in the multipotential human CNS progenitor-cell culture system.
JCV activity in human CNS progenitors and PDA was analyzed at 4 days post-JCV exposure (the time interval used for all JCV infection studies presented here) by Western blot assays of nuclear extracts (Fig. 3B). At this time point, PDA showed significant expression of JCV early (T) and late (Vp-1) proteins compared with levels for progenitor cells. In subsequent experiments, levels of Vp-1 expression were used as the measure of JCV activity. This experiment demonstrates the positive role of NF-1X on JCV activity.
Interference of c-Jun on NF-1 binding to JCV promoter.
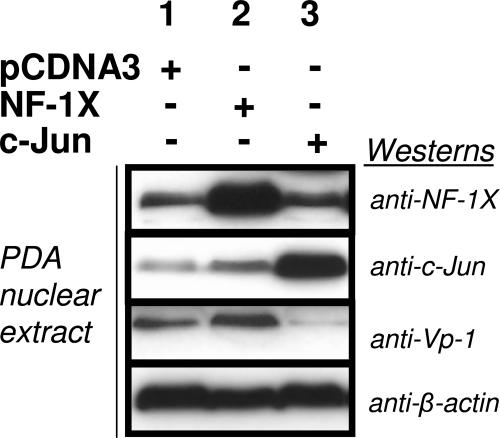
Since some AP-1 binding sites are found adjacent to NF-1 binding sites in JCV promoters, we explored how increased protein expression of either c-Jun or NF-1X might affect protein interactions with JCV promoter sequence (specifically Mad-1) and if interference with NF-1 binding alters JC viral activity. We used ATP binding experiments to compare the binding levels of NF-1X and c-Jun from nuclear extracts of JCV-infected PDA transfected to overexpress NF-1X or c-Jun. Proteins that bound ATP-Mad-1 were eluted and analyzed by Western blotting using anti-NF-1X or anti-c-Jun. PDA transfected to overexpress NF-1X showed a corresponding increase in NF-1X association to ATP-Mad-1 and an increased Vp-1 level compared with control PDA (Fig. 4). On the other hand, the increased association of c-Jun with ATP-Mad-1 in PDA transfected to overexpress c-Jun was partnered with a decrease in Vp-1 expression. This finding suggests that increased c-Jun association with AP-1 binding sites could be responsible for interference with NF-1X binding to adjacent NF-1 sites. Therefore, the inability of NF-1X to bind efficiently to particular NF-1 consensus binding sites in JCV promoters as a result of c-Jun interaction is, in the same measure, playing a role in the observed decrease of JCV activity (Fig. 4 and 5).
FIG. 4.
Western blot assays of nuclear extracts from astrocytic cell cultures (PDA) transfected with pCDNA3 (lane 1), pCDNA3NF-1X (NF-1X; lane 2), or pET-c-Jun (c-Jun; lane 3) and subsequently infected with JCV. Equal amounts of extract were resolved on each lane of a 4 to 12% gradient gel, transferred to a PVDF membrane, and then probed with anti-Vp-1, anti-NF-1X, anti-c-Jun, and anti-β-actin. β-Actin detection served as a control for equivalent total protein loading.
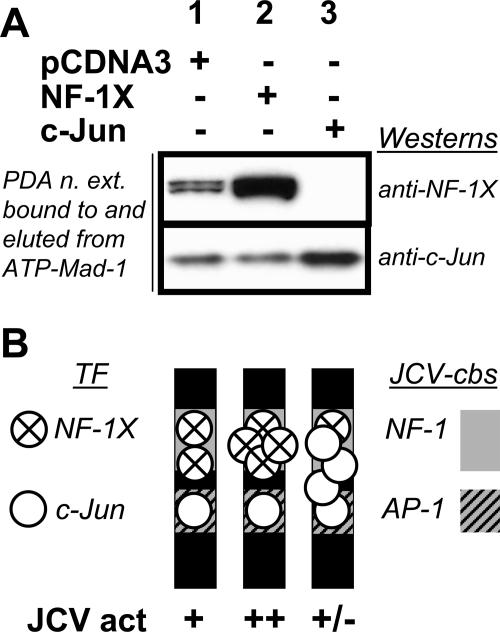
FIG. 5.
A) Western blots from ATP-Mad-1 binding assays demonstrate differential association of NF-1X and c-Jun. ATP assays were performed with PDA nuclear extracts (PDA n. ext.) as used for Fig. 4. The ATP-Mad-1 bound proteins were resolved by PAGE on 4 to 12% gradient gels, transferred to a PVDF membrane, and then probed with anti-c-Jun and anti-NF-1X. Due to gel exposure, NF-1X binding is not visible in lane 3, although some level of binding is expected. (B) Schematic representation of possible NF-1X (circle with X) and c-Jun (unshaded circle) transcription factor (TF) dimers in associations with the adjacent NF-1X (lightly shaded) and AP-1 (hatched) consensus binding sites (cbs) within JCV promoter sequence (dark shading), as well as JCV activity levels (JCV act) relative to that (lane 1) (+) found in PDA transfected with control vector (pCDNA3). Overexpression of NF-1X (lane 2) increases association of NF-1X to the NF-1 cbs without interference of c-Jun binding to the AP-1 cbs and increasing (++) JCV activity. However, overexpression of c-Jun (lane 3) leads to increased c-Jun association with the AP-1 cbs that masks the neighboring NF-1 cbs, thereby decreasing (+/−) JCV activity through decreased NF-1 binding to the NF-1 cbs.
DISCUSSION
JCV transcription and replication depends on a complex and organized sequence of molecular events involving both the virus and the host cell (17). The variant JCV promoter region contains consensus binding sites for a number of cellular transcription factors, including c-Jun (1), NF-1 (2), NF-κB (25), Y-box binding protein 1 (17), Tst-1 (35), and Purα (8). We have previously demonstrated that of the NF-1 family of transcription factors, NF-1X has a significant role in the regulation of JCV expression (26). NF-1X is highly expressed in cell types known to support JCV expression. These include human glial cells, B lymphocytes, tonsillar stromal cells, some established permissive human cell lines, and the astrocytic lineage (PDA) derived from a model cell culture system of human CNS progenitor cells. Neuronal cultures derived from this multipotential cell culture system, which are not susceptible to JCV infection, have been rendered susceptible by transfection-induced overexpression of NF-1X (23). In the same manner, differentiated KG-1 cells, which have lost JCV susceptibility present in the undifferentiated state, have had susceptibility restored due to NF-1X overexpression (26). HeLa cells, which normally do not support activation of the JCV early promoter, also demonstrate the ability to do so after transfection of an NF-1X-expressing plasmid (32).
Our EMSA experiments suggest that interactions between NF-1 and c-Jun may have functional consequences for JCV activity. The interactions observed in our EMSAs are modulated by concentrations of each transcription factor, as well as the order and timing of transcription factor addition to the binding complex. Furthermore, our EMSA strongly supports the idea that NF-1 and c-Jun can physically bind one another (protein to protein) either in the absence of DNA or in the presence of JCV promoter sequence.
In our studies here, the range of susceptibility to JCV infection observed between progenitor and PDA cultures was again linked to differential levels of NF-1X protein expression. Our examinations of NF-1A, NF-1B, and NF-1X proteins in various cell types lead us to believe that expression levels of each NF-1 family member play a distinct role in the control of JCV cellular tropism.
The close proximity of the NF-1 and AP-1 binding sites in the JCV promoter suggest that interactions between NF-1X and the AP-1 proteins are important in the modulation of JCV expression (14). Recently, interaction between NF-1 and AP-1 proteins has been proposed to have an effect on GFAP expression in an astrocytic cell line (10), with the conclusion that the basal expression of GFAP specific to astrocytes depends on both AP-1 and NF-1. Our experiments here show that NF-1X and c-Jun undergo direct, physical interactions, independently of DNA binding, that alter associations of these transcription factors with JCV promoters. Here we used immunoprecipitation, in vitro transcription/translation, and GST-c-Jun pull-down assays to demonstrate binding between the c-Jun and NF-1X proteins. Our in vitro binding experiments demonstrate that only c-Jun and NF-1 are required for such interaction, free of DNA and/or other cellular proteins. The experiments reported here provide evidence that DNA-independent mechanisms affecting interactions between nuclear transcription factors have relevance to aspects of viral activity.
Having established that NF-1X is a critical cellular component conveying transcriptional support of JCV infection and that the association of NF-1X and c-Jun occurs with or without DNA binding, we have set out to better understand what role c-Jun and NF-1X play in affecting viral activity. Our experiments utilizing overexpression of NF-1X showed concurrent increases in expression of JC Vp-1 and binding of NF-1X to ATP-Mad-1, without affecting c-Jun binding. However, overexpression of c-Jun showed increases in c-Jun binding, along with decreases in not only the association of NF-1X to ATP-Mad-1 but also the expression of JC Vp-1 (Fig. 5A). These observations strongly suggest that increased binding of c-Jun on JCV promoters interferes with NF-1X binding by masking the binding site, which may be, in part, responsible for decreased JCV activity (Fig. 5B).
In addition to c-Jun masking NF-1X binding on JCV promoters, the decreased viral activity in cells that overexpress c-Jun may be partly due to “quenching” of NF-1X through protein-protein interaction. The in vivo interaction between NF-1X and c-Jun described here could be functionally significant for JCV propagation and for the regulation of cellular gene expression.
The positive regulation of JCV expression is complex and is limited to cell types that support virus replication (31). Nuclear transcription factors certainly have a significant role in JC virus expression and host cell restriction (14, 16, 24-26). The experiments described here suggest that interactions between transcription factors from different families (NF-1 and AP-1) not only occur independently of DNA binding (protein to protein) but also in the context of associating with JCV promoters (protein to promoter), offering multilevel regulation of JCV expression. These findings support the hypothesis that binding of NF-1X to JCV promoters is a critical mechanism supporting JCV propagation, while c-Jun may also play a role in the regulation of JCV activity by the inhibition of NF-1X binding.
Acknowledgments
This research was supported (in part) by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke. B.F.S. was supported partially by the National Institutes of Health Undergraduate Scholarship Program.
We thank Richard Gronostajski (The State University of New York at Buffalo) and James A. Goodrich (University of Colorado) for supplying the plasmids and Jean Hou for critical reading of the manuscript.
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Amemiya, K., R. Traub, L. Durham, and E. O. Major. 1992. Adjacent nuclear factor-1 and activator protein binding sites in the enhancer of the neurotropic JC virus. A common characteristic of many brain-specific genes. J. Biol. Chem. 267:14204-14211. [PubMed] [Google Scholar]
- 2.Amemiya, K., R. Traub, L. Durham, and E. O. Major. 1989. Interaction of a nuclear factor-1-like protein with the regulatory region of the human polyomavirus JC virus. J. Biol. Chem. 264:7025-7032. [PubMed] [Google Scholar]
- 3.Andrews, N. C., and D. V. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohmann, D., and R. Tjian. 1989. Biochemical analysis of transcriptional activation by Jun: differential activity of c- and v-Jun. Cell 59:709-717. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry, A. Z., G. E. Lyons, and R. M. Gronostajski. 1997. Expression patterns of the four nuclear factor I genes during mouse embryogenesis indicate a potential role in development. Dev. Dyn. 208:313-325. [DOI] [PubMed] [Google Scholar]
- 7.Chen, N. N., C. F. Chang, G. L. Gallia, D. A. Kerr, E. M. Johnson, C. P. Krachmarov, S. M. Barr, R. J. Frisque, B. Bollag, and K. Khalili. 1995. Cooperative action of cellular proteins YB-1 and Pur alpha with the tumor antigen of the human JC polyomavirus determines their interaction with the viral lytic control element. Proc. Natl. Acad. Sci. USA 92:1087-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, N. N., and K. Khalili. 1995. Transcriptional regulation of human JC polyomavirus promoters by cellular proteins YB-1 and Pur α in glial cells. J. Virol. 69:5843-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisque, R. J., G. L. Bream, and M. T. Cannella. 1984. Human polyomavirus JC virus genome. J. Virol. 51:458-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopalan, S. M., K. M. Wilczynska, B. S. Konik, L. Bryan, and T. Kordula. 2006. Astrocyte-specific expression of the alpha1-antichymotrypsin and glial fibrillary acidic protein genes requires activator protein-1. J. Biol. Chem. 281:1956-1963. [DOI] [PubMed] [Google Scholar]
- 11.Gronostajski, R. M. 2000. Roles of the NFI/CTF gene family in transcription and development. Gene 249:31-45. [DOI] [PubMed] [Google Scholar]
- 12.Guo, J., T. Kitamura, H. Ebihara, C. Sugimoto, T. Kunitake, J. Takehisa, Y. Q. Na, M. N. Al-Ahdal, A. Hallin, K. Kawabe, F. Taguchi, and Y. Yogo. 1996. Geographical distribution of the human polyomavirus JC virus type A and B and isolation of a new type from Ghana. J Gen. Virol. 77:919-927. [DOI] [PubMed] [Google Scholar]
- 13.Guo, W., W. J. Tang, X. Bu, V. Bermudez, M. Martin, and W. R. Folk. 1996. AP1 enhances polyomavirus DNA replication by promoting T-antigen-mediated unwinding of DNA. J. Virol. 70:4914-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen, P. N., and E. O. Major. 2001. A classification scheme for human polyomavirus JCV variants based on the nucleotide sequence of the noncoding regulatory region. J. Neurovirol. 7:280-287. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, P. N., and E. O. Major. 1999. Viral variant nucleotide sequences help expose leukocytic positioning in the JC virus pathway to the CNS. J. Leukoc. Biol. 65:428-438. [DOI] [PubMed] [Google Scholar]
- 16.Kerr, D., C. F. Chang, N. Chen, G. Gallia, G. Raj, B. Schwartz, and K. Khalili. 1994. Transcription of a human neurotropic virus promoter in glial cells: effect of YB-1 on expression of the JC virus late gene. J. Virol. 68:7637-7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, J., S. Woolridge, R. Biffi, E. Borghi, A. Lassak, P. Ferrante, S. Amini, K. Khalili, and M. Safak. 2003. Members of the AP-1 family, c-Jun and c-Fos, functionally interact with JC virus early regulatory protein large T antigen. J. Virol. 77:5241-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus, R. J., L. Shadley, and J. E. Mertz. 2001. Nuclear factor 1 family members mediate repression of the BK virus late promoter. Virology 287:89-104. [DOI] [PubMed] [Google Scholar]
- 19.Landschulz, W. H., P. F. Johnson, and S. L. McKnight. 1989. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science 243:1681-1688. [DOI] [PubMed] [Google Scholar]
- 20.Lisignoli, G., M. C. Monaco, A. Facchini, S. Toneguzzi, L. Cattini, D. M. Hilbert, S. Lavaroni, O. Belvedere, and A. Degrassi. 1996. In vitro cultured stromal cells from human tonsils display a distinct phenotype and induce B cell adhesion and proliferation. Eur. J. Immunol. 26:17-27. [DOI] [PubMed] [Google Scholar]
- 21.Lively, T. N., H. A. Ferguson, S. K. Galasinski, A. G. Seto, and J. A. Goodrich. 2001. c-Jun binds the N terminus of human TAF(II)250 to derepress RNA polymerase II transcription in vitro. J. Biol. Chem. 276:25582-25588. [DOI] [PubMed] [Google Scholar]
- 22.Major, E. O. 2001. Human polyomaviruses. In D. M. Knipe, B. N. Fields, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus, (ed.), Field's virology. Lippincott Williams & Wilkins, New York, N.Y.
- 23.Messam, C. A., J. Hou, R. M. Gronostajski, and E. O. Major. 2003. Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann. Neurol. 53:636-646. [DOI] [PubMed] [Google Scholar]
- 24.Monaco, M. C., W. J. Atwood, M. Gravell, C. S. Tornatore, and E. O. Major. 1996. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J. Virol. 70:7004-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monaco, M. C., P. N. Jensen, J. Hou, L. C. Durham, and E. O. Major. 1998. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J. Virol. 72:9918-9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monaco, M. C., B. F. Sabath, L. C. Durham, and E. O. Major. 2001. JC virus multiplication in human hematopoietic progenitor cells requires the NF-1 class D transcription factor. J. Virol. 75:9687-9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Carrozzi, V. R., and T. K. Kerppola. 2001. Control of the orientation of Fos-Jun binding and the transcriptional cooperativity of Fos-Jun-NFAT1 complexes. J. Biol. Chem. 276:21797-21808. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez-Carrozzi, V. R., and T. K. Kerppola. 2001. Long-range electrostatic interactions influence the orientation of Fos-Jun binding at AP-1 sites. J. Mol. Biol. 305:411-427. [DOI] [PubMed] [Google Scholar]
- 29.Ranganathan, P. N., and K. Khalili. 1993. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 21:1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravichandran, V., and E. O. Major. Viral proteomics: a promising approach for understanding JV virus tropism. Proteomics, in press. [DOI] [PubMed]
- 31.Seth, P., F. Diaz, and E. O. Major. 2003. Advances in the biology of JC virus and induction of progressive multifocal leukoencephalopathy. J. Neurovirol. 9:236-246. [DOI] [PubMed] [Google Scholar]
- 32.Shinohara, T., K. Nagashima, and E. O. Major. 1997. Propagation of the human polyomavirus, JCV, in human neuroblastoma cell lines. Virology 228:269-277. [DOI] [PubMed] [Google Scholar]
- 33.Sundsfjord, A., T. Johansen, T. Flaegstad, U. Moens, P. Villand, S. Subramani, and T. Traavik. 1990. At least two types of control regions can be found among naturally occurring BK virus strains. J. Virol. 64:3864-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner, R., and R. Tjian. 1989. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science 243:1689-1694. [DOI] [PubMed] [Google Scholar]
- 35.Wegner, M., D. W. Drolet, and M. G. Rosenfeld. 1993. Regulation of JC virus by the POU-domain transcription factor Tst-1: implications for progressive multifocal leukoencephalopathy. Proc. Natl. Acad. Sci. USA 90:4743-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]