Abstract
The orthopoxviruses ectromelia virus (ECTV) and vaccinia virus (VACV) express secreted gamma interferon binding proteins (IFN-γBPs) with homology to the ligand binding domains of the host's IFN-γ receptor (IFN-γR1). Homology between these proteins is limited to the extracellular portions of the IFN-γR1 and the first ∼200 amino acids of the IFN-γBPs. The remaining 60 amino acids at the C termini of the IFN-γBPs contain a single cysteine residue shown to be important in covalent dimerization of the secreted proteins. The function of the remaining C-terminal domain (CTD) has remained elusive, yet this region is conserved within all orthopoxvirus IFN-γBPs. Using a series of C-terminal deletion constructs, we have determined that the CTD is essential for IFN-γ binding despite having no predicted homology to the IFN-γR1. Truncation of the ECTV IFN-γBP by more than two amino acid residues results in a complete loss of binding activity for both murine IFN-γ and human IFN-γ (hIFN-γ), as measured by surface plasmon resonance (SPR) and bioassay. Equivalent truncation of the VACV IFN-γBP resulted in comparable loss of hIFN-γ binding activity by SPR. Full-length IFN-γBPs were observed to form higher-ordered structures larger than the previously reported dimers. Mutants that were unable to bind IFN-γ with high affinity in SPR experiments failed to assemble into these higher-ordered structures and migrated as dimers. We conclude that the unique CTD of orthopoxvirus IFN-γBPs is important for the assembly of covalent homodimers as well as the assembly of higher-ordered structures essential for IFN-γ binding.
Ectromelia virus (ECTV) is an orthopoxvirus closely related to variola virus, the causative agent of smallpox. Like other orthopoxviruses, ECTV encodes secreted homologs of cellular receptors and regulatory cytokine binding proteins that block cytokine action, allowing them to modulate the host immune response and establish infection. The orthopoxviruses have been shown to encode soluble receptors for type I (α/β) and type II (γ) interferons (IFN), interleukin-1, and tumor necrosis factor alpha, as well as multiple chemokine binding domains (4, 21, 27, 29). These viral cytokine binding proteins bind their cognate ligand(s) with affinity comparable to or higher than their cognate cellular receptors and are thought to be important in virus evasion of the immune system. Several of these viral decoy receptors show significant homology to the host cytokine receptor, suggesting they were acquired by the virus and adapted for immunomodulation throughout the course of poxvirus evolution (8, 16, 25, 28). The broad species specificity of the IFN-γ binding proteins (IFN-γBPs) distinguishes them from the IFN-γ cellular receptors (IFN-γR1), which are generally thought to be species specific (i.e., mouse IFN-γ [mIFN-γ] binds IFN-γR1 from mouse and not human cells) (2, 23). Cross-linking assays using IFN-γ from several species have demonstrated that the ECTV IFN-γBPs (EVC4 [8] and EVM158 [9]) have the interesting property of binding mIFN-γ, human IFN-γ (hIFN-γ), and rabbit IFN-γ with high affinity (22). Vaccinia virus (VACV) IFN-γBP (VVB8, VACVCOP236) can be cross-linked to hIFN-γ and mIFN-γ as well as rabbit and bovine IFN-γ; however, competition assays with both cold and radiolabeled IFN-γ revealed that binding of mIFN-γ is at a substantially reduced affinity (1, 22). This finding correlated with the ability of VACV IFN-γBP to neutralize the bioactivity of rat, human, bovine, and rabbit IFN-γ, but not mIFN-γ, in a bioassay (1).
In addition to possessing expanded species specificity, the structure of the secreted IFN-γBPs is clearly unique compared to the host IFN-γR1. All of the poxvirus IFN-γBPs contain a C-terminal domain (CTD) of approximately 60 amino acids without homology to the cellular receptor (19). Previous studies have implicated the CTD in the covalent dimerization of the orthopoxvirus IFN-γBPs. Reducing and nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) has revealed that both ECTV and VACV IFN-γBPs are secreted as disulfide-linked homodimers (ECTV C216 and VACV C217) and are detected in tissue culture medium as early as 4 h after infection (3, 6). Experiments with sucrose gradient density centrifugation have suggested that functional VACV IFN-γBP exists as a covalent homodimer in solution (3); however, our current studies clearly demonstrate that both ECTV and VACV IFN-γBPs form higher-order structures in solution. For ECTV IFN-γBP, we demonstrate that assembly of these higher-ordered structures is dependent upon the full-length CTD, independent of the covalent linkage of homodimers and necessary for high-affinity IFN-γ binding and antagonism.
MATERIALS AND METHODS
Cell and virus culture.
African green monkey kidney cell lines BS-C-1 (ATCC CCL-26) and CV-1 (ATCC CCL-70) were maintained in Dulbecco's modified Eagle's medium containing 50 units/ml penicillin, 50 μg/ml streptomycin, 2 mM l-glutamine, and 10% FetalClone II (HyClone Laboratories, Inc., Logan, UT) at 37°C in a 5% CO2 atmosphere. The Western Reserve strain of VACV and a derivative virus expressing the T7 RNA polymerase were kindly provided by Bernard Moss (15). VACV-t7,b8r− (hereafter VACV-t7) virus was constructed by deleting the VACV IFN-γbp (b8r) open reading frame using the gpt transient-dominant selection system (14). Virus stocks were prepared in HELA-S3 (ATCC CCL-2.2) and stored at −70°C until use. Virus infectivity was measured on BS-C-1 monolayers as described previously (10).
Plasmids and mutagenesis.
The ECTV ifn-γbp gene was cloned downstream of the T7 promoter of the pTM1 vector (15) and expressed as previously described (6). Single-base mutations were carried out using the Gene Tailor site-directed mutagenesis system (Invitrogen, Carlsbad, CA) and a two-step PCR protocol (17). All mutant ifn-γbp genes contained native IFN-γBP signal peptide, and genotypes were confirmed by sequencing.
Transfection.
We have previously expressed and purified ECTV IFN-γBP that retains its biological activity using the VACV-t7 expression system (5, 15). The expression of IFN-γBP in this system has been optimized by comparative studies that varied the multiplicity of infection, time of harvest, and chemically defined medium to a yield of approximately 0.5 μg/106 CV-1 cells. In brief, CV-1 cells were grown in six-well tissue culture plates to ∼80% confluence and were infected with VACV-t7 at a multiplicity of infection of 10 PFU/cell for 1 h at 37°C. The cells were then washed in Opti-MEM and transfected with pTM1 (2 μg; vector control) alone or pTM1 containing wild-type (WT) or mutant ifn-γbp+ genes (2 μg) using Lipofectamine 2000 reagent in Opti-MEM according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). At 24 h posttransfection, supernatant was collected, clarified, and filtered to remove virus using 0.1-μm centrifugal filters (Millipore, Bedford, MA). Transfection supernatants were assayed and quantified via Western blot densitometry using an IFN-γBP standard purified through ion exchange (Q Sepharose) and gel filtration (Superdex 200) chromatography. No further purification of the transfection supernatants was necessary prior to surface plasmon resonance (SPR) or bioassay experiments.
Surface plasmon resonance (Biacore) with mIFN-γ.
Real-time interaction of IFN-γBP with mIFN-γ was measured by SPR on a Biacore 2000 (Biacore Inc., Piscataway, NJ). For mIFN-γ, flow cells of a carboxymethylated dextran (CM5) sensor chip were activated using 50 mM N-hydroxysuccinimide and 200 mM N-ethyl-N′-(dimethylaminopropyl)carbodiimide for 7 min at a flow rate of 5 μl/min. mIFN-γ (R&D Systems, Minneapolis, MN), diluted in 10 mM sodium acetate pH 5.0, was immobilized at a flow rate of 5 μl/min for 5 min. The surface was treated using 1 M ethanolamine hydrochloride at pH 8.5 for 7 min with a flow rate of 5 μl/min to deactivate excess reactive esters and remove noncovalently bound ligand. The mIFN-γ surface was stable following repeated rounds of regeneration and remained so for several weeks. IFN-γBP supernatants from the VACV-t7 system were diluted in Opti-MEM to concentrations from 50 to 0.5 nM and were injected in random order over the mIFN-γ sensor chips. Dissociation occurred in HBS-EP running buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20; Biacore Inc.) for 20 min following injection. To correct for refractive index changes, responses generated with the control (activated and blocked) surface were subtracted from the responses with the mIFN-γ surface. To correct for nonspecific binding, responses generated during the injection of pTM1 control transfection supernatant were subtracted from the data set. The doubly referenced data were analyzed and fit globally to the 1:1 binding model using BIAevaluation 4.1 software.
Human IFN-γ expression, refolding, and surface plasmon resonance (Biacore).
The C-terminal AviTag (7) sequence (Avidity, Denver, CO) was inserted at the C terminus of the hIFN-γ sequence (31) using standard techniques to generate pET21a hifn-γb. BL21(DE3) cells (Invitrogen, Carlsbad, CA) were transformed with pET21a hifn-γb and pACYC-184 containing an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible biotin protein ligase (BirA; Avidity, Denver, CO). Cells were grown in the presence of ampicillin (100 μg/ml) and chloramphenicol (10 μg/ml) to an optical density at 600 nm of 0.6 to 0.8 followed by induction with 0.1 mM IPTG plus 10 μM d-biotin for 3 h. Cells were harvested by centrifugation, and inclusion bodies were isolated using BugBuster reagent (EMD Biosciences, San Diego, CA). Inclusion bodies were solubilized in 100 mM Tris pH 8.0, 2.5 mM EDTA, 6 M guanidine HCl and refolded by drop-wise dilution into 100 mM Tris pH 8.0, 2.5 mM EDTA, 500 mM l-arginine. Refolded protein was dialyzed into 20 mM Tris pH 8.0, 100 mM urea prior to ion-exchange purification on S-Sepharose (Sigma, St. Louis, MO). Gel filtration with Superdex 75 (GE Biosciences, Piscataway, NJ) was used to enrich for properly folded hIFN-γB homodimers and exclude unfolded monomers and protein aggregates. Streptavidin (SA)-coated sensor chips (Biacore, Piscataway, NJ) were used to capture refolded hIFN-γB. The IFN-γBP samples were injected over the hIFN-γB surface for 2 min at a flow rate of 5 μl/min in HBS-EP buffer. To correct for refractive index changes, responses generated with the control surface (SA blocked with 10 μM d-biotin) were subtracted from the responses of the hIFN-γB surfaces. hIFN-γB surfaces were regenerated using a 1-min injection of 100 mM glycine pH 2.8.
Interferon protection (VSV) assay.
ECTV IFN-γBP and mutants were tested for their ability to neutralize the antiviral activity of mIFN-γ on L929 cells. WT and mutant IFN-γBPs in virus-free (0.1-μm-filtered) culture medium from the VACV-t7 transfection system were serially diluted in Opti-MEM (Invitrogen, Carlsbad, CA). Recombinant mIFN-γ (R&D Systems, Minneapolis, MN) was added to each diluted sample to a final concentration of 25 U/ml. Mixtures were incubated at 37°C for 1 h. Incubated mixtures (100 μl/sample) were transferred to 96-well plates previously seeded with 2 × 104 L929 cells/well. After 24 h, cells were challenged for 2 days with ∼50 PFU of vesicular stomatitis virus (VSV) followed by staining with crystal violet solution.
RESULTS
Minimal truncation of IFN-γBP results in loss of ligand binding activity.
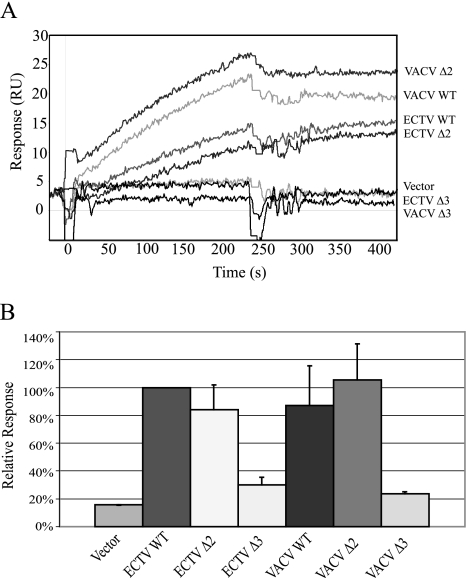
The orthopoxvirus IFN-γBPs have ∼20% identity to the extracellular portion of the host IFN-γ receptors; however, this homology is restricted to the first ∼200 amino acids of the IFN-γBPs. The remaining C-terminal domain of the IFN-γBPs is not homologous to the transmembrane or signaling domains of the receptors but is very well conserved within the orthopoxvirus IFN-γBP family. We previously identified C216 in the ECTV sequence as being important for covalent dimerization of the secreted protein; however, the function of the remaining 60 amino acids still remained unclear. To investigate the functional importance of this region, we constructed a series of truncation mutants in both the ECTV and VACV sequences, beginning at the C terminus and progressing towards the N terminus (Fig. 1). The truncated IFN-γBPs were expressed in the VACV-t7 system as described previously (6) and were expressed at levels comparable to wild-type IFN-γBP (see Fig. S1 in the supplemental material). Expressed proteins were screened for both mIFN-γ (Fig. 2) and hIFN-γ (Fig. 3) binding activity by surface plasmon resonance. Examination of the sensorgrams demonstrated that truncation of the ECTV sequence by two amino acids (Δ2) was well tolerated and this protein retained its ability to bind both mIFN-γ and hIFN-γ, while truncation by an additional amino acid (Δ3) eliminated the ability to bind IFN-γ of either species. Further truncations up to residue 211 resulted in a similar loss of activity (data not shown). Equivalent truncation of the VACV IFN-γBP (identified in Fig. 1) demonstrated a similar loss of binding to hIFN-γ. No binding of VACV IFN-γBP to mIFN-γ could be detected in these experiments.
FIG. 1.
Sequence alignment of ECTV and VACV IFN-γBPs. Predicted signal sequences are boxed. Individual amino acid differences between the proteins are highlighted. The receptor homologous region of the ECTV sequence extends from the signal sequence cleavage site to amino acid 211. The unique orthopoxvirus CTD is underlined. Covalent dimerization of the secreted molecules is mediated by cysteine 216 (ECTV) (6). Sequences were obtained from GenBank (ECTV accession no. AAC99563; VACV accession no. AAA48205) and aligned using CLUSTAL W.
FIG. 2.
Murine IFN-γ binding by IFN-γBP truncation mutants. (A) SPR sensorgrams of full-length and truncated IFN-γBPs binding to mIFN-γ. Expressed proteins were injected over a mIFN-γ sensor surface at a flow rate of 5 μl/min for 2 min. Sensorgrams are typical of those seen over several replicate injections. (B) SPR responses were normalized to wild-type ECTV IFN-γBP and include multiple injections over several IFN-γ surfaces of various densities. Truncation sites for both VACV and ECTV correspond to the C-terminal ending of the ECTV sequence as displayed in Fig. 1.
FIG. 3.
Human IFN-γ binding by IFN-γBP truncation mutants. (A) SPR sensorgrams of full-length and truncated IFN-γBPs binding to hIFN-γ. Expressed proteins were injected over an SA hIFN-γB sensor surface at a flow rate of 5 μl/min for 2 min. Sensorgrams are typical of those seen over several replicate injections. (B) SPR responses were normalized to wild-type ECTV IFN-γBP and include multiple injections over several IFN-γ surfaces of various densities. Truncation sites for both VACV and ECTV correspond to the C-terminal ending of the ECTV sequence as displayed in Fig. 1.
Mutagenesis scanning of the terminal nine amino acids.
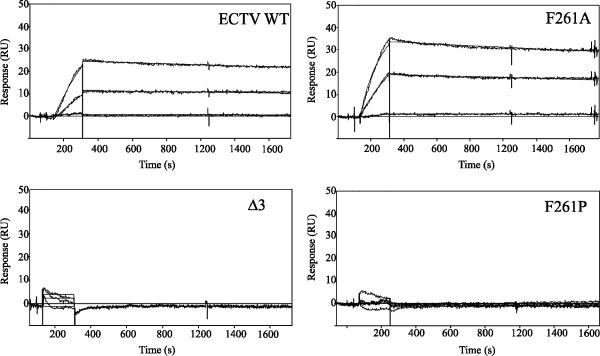
Following the observation that no IFN-γ binding activity was detectable in constructs truncated by more than two amino acids, we constructed a series of alanine scanning mutants from positions 258 to 266 (ECTV) to examine the potential importance of each of the nine C-terminal amino acid side chains on the activity of the protein. Each mutant was expressed, and the kinetics of binding to mIFN-γ were analyzed using SPR (Fig. 4). Examination of the resultant kinetics profiles revealed that no single residue in the C terminus appeared to be responsible for conservation of activity. Alanine substitution at any position resulted in a similar overall affinity (KD); however, in all cases there was a shift in the rate constants to both a faster on-rate (ka) and equivalently faster off-rate (kd).
FIG. 4.
Kinetics analyses of C-terminal mutations for mIFN-γ. Alanine mutagenesis scanning was conducted over the last nine amino acid residues of the ECTV IFN-γBP. Mutants were tested for mIFN-γ binding by SPR, and their affinity and kinetics profiles were determined from the resulting sensorgrams. Kinetics were run in duplicate over three independent mIFN-γ surfaces (n = 6). Representative sensorgrams of concentration series showing the experimental data as well as the fit binding curves calculated using BIAevaluation software are shown. The Δ3 sensorgram is typical of those observed for inactive truncation mutants, and F261A is typical of sensorgrams for the alanine scanning mutants. Constructs for which a very low or background response could be generated have very low to no affinity for mIFN-γ.
Using the PredictProtein algorithm (24), we were able to predict that the C terminus of the IFN-γBP likely adopts an α-helical secondary structure once folded. To test the importance of this local secondary structure on the function of ECTV IFN-γBP, we inserted a proline (F261P) into the middle of this region to disrupt the secondary structure. This alteration resulted in a complete loss of mIFN-γ binding activity as measured by SPR, suggesting an importance of C-terminal secondary structure on the activity of the protein. Conversion of all of the last nine amino acids to alanines (9xAla) also resulted in no mIFN-γ binding activity, even though this sequence is predicted to share a similar α-helical secondary structure (26). Similarly, randomization of the nine terminal amino acids (MLLSTITDF, LTSTLIDFM) resulted in no binding activity, despite a similar predicted secondary structure and secretion into the culture medium (data not shown). To test the effects of extending the C terminus, we constructed ECTV IFN-γBPs containing the naturally occurring VACV C-terminal extension (ECTV+VACV3′) as well as a C-terminal hemagglutinin (HA) tag. The shorter VACV extension (five residues) was not only tolerated but resulted in a slightly slower off-rate than for wild-type ECTV IFN-γBP. Extension with the C-terminal HA tag (nine residues) was not tolerated and resulted in a complete loss of detectable activity. The mIFN-γ binding sensorgrams and kinetics for each construct are presented in Fig. 4 and Table 1, respectively.
TABLE 1.
Summary of kinetics and affinity measurements for ECTV IFN-γBP C-terminal mutants
| Mutant | KD (nM) | ka (105) (M−1 s−1) | kd (10−5) (s−1) |
|---|---|---|---|
| ECTV WT | 0.50 ± 0.05 | 1.16 ± 0.23 | 5.73 ± 0.73 |
| Δ2 | 0.67 ± 0.19 | 2.18 ± 0.02 | 14.55 ± 3.99 |
| Δ3 | BDLa | BDL | BDL |
| Δ8 | BDL | BDL | BDL |
| I258A | 0.29 ± 0.06 | 3.60 ± 0.08 | 10.40 ± 2.23 |
| T259A | 0.29 ± 0.10 | 3.44 ± 0.12 | 9.72 ± 3.11 |
| T260A | 0.28 ± 0.06 | 4.14 ± 0.05 | 11.72 ± 2.71 |
| F261A | 0.39 ± 0.07 | 2.24 ± 0.25 | 8.84 ± 2.49 |
| F261P | BDL | BDL | BDL |
| L262A | 0.45 ± 0.07 | 1.99 ± 0.25 | 9.15 ± 2.58 |
| S263A | 0.36 ± 0.10 | 3.13 ± 0.06 | 11.28 ± 2.85 |
| M264A | 0.30 ± 0.07 | 3.20 ± 0.17 | 9.41 ± 1.81 |
| L265A | 0.26 ± 0.07 | 4.65 ± 0.07 | 12.25 ± 2.88 |
| D266A | 0.25 ± 0.06 | 5.27 ± 0.10 | 13.45 ± 3.18 |
| ECTV+HA | BDL | BDL | BDL |
| ECTV+VACV3′ | 0.53 ± 0.25 | 1.12 ± 0.23 | 3.95 ± 0.78 |
| 9xAla | BDL | BDL | BDL |
| Random 3′ 1 | BDL | BDL | BDL |
| Random 3′ 2 | BDL | BDL | BDL |
BDL, below detection limit.
The C terminus and secondary structure of the IFN-γBP are necessary to neutralize the antiviral effects of IFN-γ.
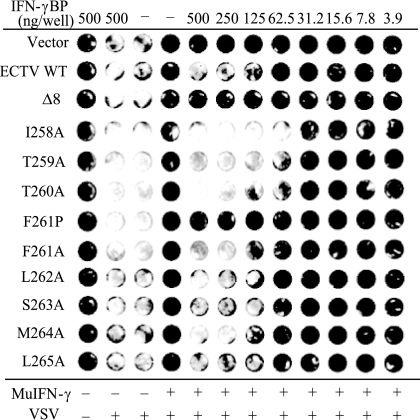
To demonstrate that the effect of IFN-γBP truncation on activity was not confined to detection by SPR, we examined the effect of IFN-γBP truncation on its ability to inhibit mIFN-γ in a bioassay (Fig. 5). The murine fibroblast cell line L929 is responsive to the antiviral effects of interferons. Pretreatment of L929 monolayers with mIFN-γ induces a cellular antiviral state, and the cells are no longer permissive for VSV infection. Inclusion of IFN-γBP in the pretreatment conditions results in sequestration of mIFN-γ and prevents induction of the antiviral state. VSV can then successfully infect cells in the monolayer, and the extent of this infection can be monitored by staining with crystal violet. To examine the role of the C-terminal amino acids in inhibiting the antiviral effects of IFN-γ, equal amounts of WT and mutant ECTV IFN-γBPs were tested for their ability to inhibit the biological activity of mIFN-γ. All of the alanine scanning mutants tested were able to block the antiviral effects of mIFN-γ on L929 cells, consistent with their ability to bind IFN-γ with high affinity in SPR experiments. Neutralizing activity could not be detected from the truncated (Δ8) or F261P constructs, both of which failed to bind mIFN-γ in SPR experiments. Additional experiments demonstrated mIFN-γ inhibition from the Δ2 mutant identical to WT IFN-γBP. No inhibition of mIFN-γ activity could be detected from the Δ3 mutant, consistent with its failure to bind IFN-γ in SPR experiments (data not shown).
FIG. 5.
Bioactivity of IFN-γBP alanine scanning and truncation mutants. Wild-type and C-terminal alanine scanning and truncation mutants of the ECTV IFN-γBP were tested for their ability to neutralize the antiviral effects of IFN-γ. mIFN-γ was preincubated with 0.1-μm-filtered IFN-γBP followed by a 24-h incubation with L929 monolayers. Cells were infected with VSV for 48 h and stained with crystal violet to assess viability. The wild type and all C-terminal alanine mutants were able to effectively neutralize the antiviral effects of IFN-γ. C-terminal truncation (Δ8) or disruption of the local secondary structure (F261P) resulted in an inability to block the antiviral effects of IFN-γ.
Active IFN-γBP forms higher-ordered structures.
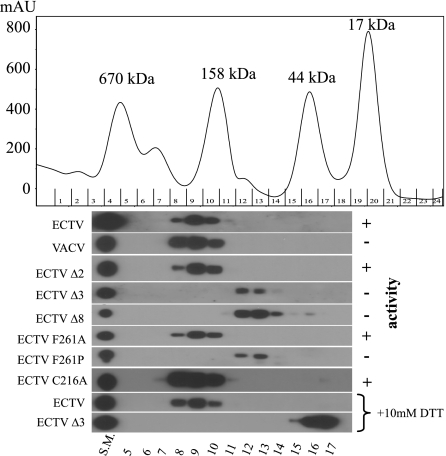
It is clear that truncation of the ECTV IFN-γBP results in a loss of activity in both SPR and a VSV assay. Truncation mutants are expressed at levels similar to wild type, although only ∼40% of the inactive truncation constructs (Δ3 and Δ8) form disulfide-linked dimers by nonreducing SDS-PAGE (see Fig. S1 in the supplemental material). To address the possibility that truncation results in misfolding of the IFN-γBP, we performed gel filtration analyses on supernatants from the VACV-t7 system (Fig. 6). All IFN-γBP constructs that demonstrated activity by SPR and the VSV assay eluted in the range of 163 to 382 kDa with a median of 250 kDa, indicating that the active protein assembles into higher-ordered complexes. Those mutations that resulted in no detectable IFN-γ binding activity eluted in the range of 52 to 122 kDa with a median of 80 kDa, corresponding to the presence of covalently linked dimers. The notable exception to this trend was with VACV IFN-γBP, whose lack of mIFN-γ binding is likely the result of amino acid differences between it and the ECTV IFN-γBP and not the result of differences in the CTD. Assembly of the higher-ordered complexes appears to be independent of the intrachain disulfide bond, as the C216A mutant migrated identically to the WT protein and pretreatment of the IFN-γBP with dithiothreitol (DTT) failed to alter the mobility in the presence of the full-length CTD. Pretreatment of the Δ3 mutant with DTT resulted in a migration pattern consistent with a monomeric form of the IFN-γBP, consistent with failure of the truncated CTD to facilitate oligomerization beyond the covalent homodimer. Gel filtration analysis of purified IFN-γBP demonstrated the same fractionation pattern as the unpurified material from the VACV-t7 system and a single band could be identified by silver staining, indicating that the complexes are assembled from the IFN-γBP alone (see Fig. S2 in the supplemental material).
FIG. 6.
The IFN-γBP C terminus mediates noncovalent oligomerization, as demonstrated in a gel filtration analysis of IFN-γBP mutants. A 2.5-μg aliquot of each construct was injected in a volume of 500 μl over a calibrated Superdex 200 HR column (GE Healthcare, Piscataway, NJ) in 100 mM Tris pH 7.0, 500 mM NaCl, 0.1% Triton X-100. Fractions of 0.5 ml were analyzed by reducing SDS-PAGE and Western blotting for IFN-γBP. Migration of each construct relative to gel filtration standards (Bio-Rad) is indicated. IFN-γ binding activity correlates with migration as a complex with a median size of 250 kDa, whereas the nonactive construct migrates as smaller complexes with a median size of 80 kDa, likely representing covalent dimers. Full-length proteins were resistant to reduction with DTT, whereas truncated IFN-γBP could be reduced to a peak with a median size of 40 kDa, consistent with a monomeric form of the protein. SM, starting material.
DISCUSSION
Although the orthopoxvirus IFN-γBPs appear to be structurally related to, and perhaps derived from, a host cellular IFN-γR1, there are features unique to the viral proteins that make them attractive to study. Foremost, the viral IFN-γBPs show the unique ability to bind IFN-γ from several species with high affinity, whereas the cellular receptors are regarded as being species specific in their ligand binding. All of the orthopoxvirus IFN-γBPs share a unique CTD of ∼60 amino acids with no homology to the ligand binding or transmembrane domains of the cellular receptor. This region has previously been identified as important for covalent dimerization of the secreted proteins (3, 6); however, a functional role of this unique region has not yet been described.
In this study, we investigated the functional role of the CTD through a series of truncations of the ECTV IFN-γBP beginning at the C terminus and progressing towards the N terminus of the protein. Truncation of the protein by two amino acids (Δ2) had no effect on the binding of IFN-γ as assayed by SPR. Truncation by one additional amino acid (Δ3) or more resulted in a complete loss of IFN-γ binding. A similar result was observed in the VACV IFN-γBP when truncated to the corresponding position as the ECTV Δ3, despite the naturally occurring extension on the VACV C terminus.
Following the observation that the last few C-terminal amino acids of the IFN-γBP are essential to the function of the protein, we constructed a series of alanine scanning mutants throughout the last nine amino acids of the protein. Substitution of alanine for any of the C-terminal amino acids had little effect on the overall affinity (KD) of the protein for mIFN-γ; however, there were notable changes in the rate constants of the constructs. Several of the constructs had a two- to threefold increase in the association rate (ka), and this was generally accompanied by a corresponding two to three times faster dissociation rate (kd). Calculation of the ka in Biacore experiments is dependent upon the active concentration of the injected analyte, in this case, the IFN-γBP. Because of the inability of densitometry to accurately assess the specific activity of the protein, it is unclear whether the observed faster association rates for each construct represent a true shift or merely a higher specific activity of the expressed protein. By contrast, calculations of the dissociation rate (kd) are independent of active analyte concentration and likely represent true shifts in the dissociation rate of the protein complex. All of the alanine scanning mutants demonstrated a two- to threefold shift in the dissociation rate of the protein; however, all of the mutants retained mIFN-γ binding activity, suggesting that no single amino acid side chain is essential for binding. Rather, it is likely that there is a small contribution from several or all of the side chains in this region on the activity of the protein. We employed in silico analysis to help provide insight into the structure of this region (24). Secondary structure prediction revealed that the C terminus of the protein likely adopts an α-helical conformation. To test the hypothesis that the secondary structure of the region is important for ligand binding, we substituted a proline residue, a strong disruptor of secondary structure (11), into the middle of the C-terminal region (F261P). Disruption of the regional secondary structure in the C terminus eliminated IFN-γ binding activity. Taken together, these results suggest that the local secondary structure of the IFN-γBP C terminus is essential for the protein's activity and that individual amino acid side chains are not responsible for the activity of the protein. All of the constructs were expressed at levels comparable to that of wild-type IFN-γBP, and all mutants migrated as homodimers under nonreducing SDS-PAGE, suggesting that the substitutions did not disrupt this portion of the assembly process.
The human immunoglobulin G1 Fc fragment has been used extensively as a method to add a high-affinity tag to a protein. This system has been used in our lab for the evaluation of viral interleukin-18 binding proteins (12, 13) as well as by others for the evaluation of the VACV IFN-γBP (30). The latter study is of interest because of the homology of the ECTV IFN-γBP and VACV IFN-γBP. Attempts in our lab to construct a C-terminal Fc fusion of ECTV IFN-γBP have resulted in an inactive protein (data not shown). It is possible that the short extension that naturally occurs on the VACV sequence acts as a linker region allowing the tagged VACV IFN-γBP to retain some degree of function. In this study, addition of the naturally occurring VACV IFN-γBP C-terminal extension (five amino acids) to the ECTV IFN-γBP was well tolerated and did not disrupt binding of mIFN-γ by the ECTV protein. Extension with a longer nine-amino-acid HA tag resulted in complete loss of activity, perhaps indicating that there is a steric limitation within the folded molecule limiting the allowable length of the extension.
It is unclear whether the importance of the IFN-γBP CTD is in direct ligand binding or in aiding in the correct folding and assembly of the binding protein itself. To investigate these possibilities, we constructed an IFN-γBP with an FXa cleavage site between the receptor homologous regions and the CTD (TCAIRSK to TCAIEGRSK). While this cleavable construct was expressed normally and cleaved as expected, there was no detectable IFN-γ binding activity prior to cleavage, indicating that the introduction of two amino acids for the cleavage site was sufficient to disrupt the structure and activity of the molecule. Likewise, attempts to express the CTD independently have been unsuccessful in both insect cells and the VACV-t7 system, prohibiting studies to rescue the truncated proteins with soluble or coexpressed CTD. Addition of a 10-fold molar excess of soluble peptide, corresponding to the terminal nine amino acids, had no rescue effect on the Δ3 or Δ8 truncation mutants, nor did it have an inhibitory effect on the binding of WT or the Δ2 mutant to mIFN-γ in SPR studies (data not shown).
Gel filtration was used to investigate the influences of the CTD on assembly of the IFN-γBP oligomeric structures. Although this technique possesses low resolution for defining the molecular mass of folded proteins, there was a distinct observable difference between the retention range of IFN-γBP constructs that showed binding to IFN-γ (163 to 382 kDa) versus those in which activity could not be detected (52 to 122 kDa). The latter complexes correspond to the covalent homodimers observable by nonreducing SDS-PAGE, while the larger complexes represent oligomers of the dimeric subunits. Gel filtration of purified IFN-γBP was consistent with the larger migrating form, indicating that the oligomers are composed of IFN-γBP subunits and do not likely contain other unidentified proteins. The assembly of IFN-γBP complexes is dependent upon the full-length CTD but appears to be independent of the covalent homodimer, as assembly is unaffected by mutation of C216 or chemical reduction in the presence of the full-length CTD. Limited proteolysis revealed increased susceptibility of the Δ3 mutant to carboxypeptidase A digestion, whereas the WT and Δ2 mutant were not sensitive to carboxypeptidase (data not shown). These results suggest that truncation has resulted in accessibility of the C terminus, which is sterically inaccessible in the WT IFN-γBP and involved in assembly of the higher-ordered multimeric structures that bind IFN-γ.
Together, these data suggest that the IFN-γBP is assembled into higher-ordered structures that are dependent upon the full-length CTD and its α-helical secondary structure. The assembly of these oligomers is independent of the formation of covalent homodimers, and we have shown previously that covalent dimerization of ECTV IFN-γBP is not required for activity (6). Analysis of the myxoma virus IFN-γBP, M-T7, demonstrated that this protein exists in solution as higher-ordered structures (trimers). M-T7 oligomerization is likely to be independent of disulfide bonding, as a homologous residue to ECTV IFN-γBP C216 is not present, and the protein migrates as monomers during denaturing gel filtration (18). The oligomeric status of insect-expressed VACV IFN-γBP has been analyzed previously using gradient density centrifugation, revealing a homodimer (3). We observed no difference in the mobility of virally expressed VACV versus ECTV IFN-γBP using gel filtration and concluded that, like the ECTV IFN-γBP, the VACV IFN-γBP forms oligomers from the dimeric subunits. A subset of the orthopoxviruses contain a 5-amino-acid extension at their C terminus (see Fig. S3 in the supplemental material). The VACV C-terminal extension exists on all VACV isolates and one CPXV isolate (GRI), as well as RPXV (a VACV derivative [20]). This extension appears to be the result of a five-nucleotide insertion/deletion, although the parental sequence cannot be determined. Inclusion of the VACV C-terminal extension on the ECTV IFN-γBP did not adversely affect mIFN-γ binding; therefore, we find it unlikely that the difference at the C termini between these proteins offers an explanation for their differing species specificities. We conclude that, like the myxoma homolog, the orthopoxvirus IFN-γBPs exist as oligomers. Higher-resolution experiments to address the assembly of the orthopoxvirus IFN-γBPs as well as the stoichiometry of complexes with IFN-γ are ongoing.
Homology between the orthopoxvirus IFN-γBPs and the cellular IFN-γR1 led to the discovery of these viral immunomodulators. One would predict that the portions of the viral IFN-γBPs that are homologous to the receptor would be sufficient to bind IFN-γ. In this study, we demonstrate that the unique CTD is essential to the activity of both the ECTV and VACV IFN-γBPs. Mutational analysis revealed that the α-helical structure of the C terminus is essential for both activity as well as assembly of high-molecular-weight complexes. Mutants that failed to bind IFN-γ also failed to form these high-molecular-weight complexes. It is still unclear, however, if the results presented here indicate a direct role for the CTD in IFN-γ binding or if the function of this domain is primarily in oligomerization. All together, these data indicate that the poxvirus IFN-γBPs require their unique CTD and the oligomerization it provides in order to bind IFN-γ with high affinity.
Supplementary Material
Acknowledgments
We thank Joe Baldassare, David Esteban, Michael Green, Dan Hoft, Sergey Korolev, and Abdul Waheed for their training and suggestions. We also thank Sixue Chen of the Donald Danforth Plant Sciences Center (St. Louis, MO) for the generous use of their Biacore 2000, without which this work would not have been possible.
A.A.N. was supported in part by an American Heart Association predoctoral fellowship. R.M.L.B. was supported by NIH/NIAID/CMB/MSC grant N01 AI15436. M.R.W. was supported by NIH grant R01 AI47300.
Footnotes
Published ahead of print on 23 August 2006.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alcami, A., and G. L. Smith. 1995. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J. Virol. 69:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcami, A., and G. L. Smith. 1996. Soluble interferon-gamma receptors encoded by poxviruses. Comp. Immunol. Microbiol. Infect. Dis. 19:305-317. [DOI] [PubMed] [Google Scholar]
- 3.Alcami, A., and G. L. Smith. 2002. The vaccinia virus soluble interferon-gamma receptor is a homodimer. J. Gen. Virol. 83:545-549. [DOI] [PubMed] [Google Scholar]
- 4.Alejo, A., M. B. Ruiz-Arguello, Y. Ho, V. P. Smith, M. Saraiva, and A. Alcami. 2006. A chemokine-binding domain in the tumor necrosis factor receptor from variola (smallpox) virus. Proc. Natl. Acad. Sci. USA 103:5995-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander, W. A., B. Moss, and T. R. Fuerst. 1992. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J. Virol. 66:2934-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai, H., R. M. Buller, N. Chen, M. Green, and A. A. Nuara. 2005. Biosynthesis of the IFN-gamma binding protein of ectromelia virus, the causative agent of mousepox. Virology 334:41-50. [DOI] [PubMed] [Google Scholar]
- 7.Beckett, D., E. Kovaleva, and P. J. Schatz. 1999. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, N., R. M. Buller, E. M. Wall, and C. Upton. 2000. Analysis of host response modifier ORFs of ectromelia virus, the causative agent of mousepox. Virus Res. 66:155-173. [DOI] [PubMed] [Google Scholar]
- 9.Chen, N., M. I. Danila, Z. Feng, R. M. Buller, C. Wang, X. Han, E. J. Lefkowitz, and C. Upton. 2003. The genomic sequence of ectromelia virus, the causative agent of mousepox. Virology 317:165-186. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W., R. Drillien, D. Spehner, and R. M. Buller. 1992. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology 187:433-442. [DOI] [PubMed] [Google Scholar]
- 11.Chou, P. Y., and G. D. Fasman. 1978. Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. Relat Areas Mol. Biol. 47:45-148. [DOI] [PubMed] [Google Scholar]
- 12.Esteban, D. J., and R. M. Buller. 2004. Identification of residues in an orthopoxvirus interleukin-18 binding protein involved in ligand binding and species specificity. Virology 323:197-207. [DOI] [PubMed] [Google Scholar]
- 13.Esteban, D. J., A. A. Nuara, and R. M. Buller. 2004. Interleukin-18 and glycosaminoglycan binding by a protein encoded by variola virus. J. Gen. Virol. 85:1291-1299. [DOI] [PubMed] [Google Scholar]
- 14.Falkner, F. G., and B. Moss. 1990. Transient dominant selection of recombinant vaccinia viruses. J. Virol. 64:3108-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuerst, T. R., P. L. Earl, and B. Moss. 1987. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol. Cell. Biol. 7:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston, J. B., and G. McFadden. 2003. Poxvirus immunomodulatory strategies: current perspectives. J. Virol. 77:6093-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kammann, M., J. Laufs, J. Schell, and B. Gronenborn. 1989. Rapid insertional mutagenesis of DNA by polymerase chain reaction (PCR). Nucleic Acids Res. 17:5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalani, A. S., K. Graham, K. Mossman, K. Rajarathnam, I. Clark-Lewis, D. Kelvin, and G. McFadden. 1997. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J. Virol. 71:4356-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefkowitz, E. J., C. Upton, S. S. Changayil, C. Buck, P. Traktman, and R. M. Buller. 2005. Poxvirus Bioinformatics Resource Center: a comprehensive Poxviridae informational and analytical resource. Nucleic Acids Res. 33:D311-D316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, G., N. Chen, R. L. Roper, Z. Feng, A. Hunter, M. Danila, E. J. Lefkowitz, R. M. Buller, and C. Upton. 2005. Complete coding sequences of the rabbitpox virus genome. J. Gen. Virol. 86:2969-2977. [DOI] [PubMed] [Google Scholar]
- 21.Loparev, V. N., J. M. Parsons, J. C. Knight, J. F. Panus, C. A. Ray, R. M. Buller, D. J. Pickup, and J. J. Esposito. 1998. A third distinct tumor necrosis factor receptor of orthopoxviruses. Proc. Natl. Acad. Sci. USA 95:3786-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossman, K., C. Upton, R. M. Buller, and G. McFadden. 1995. Species specificity of ectromelia virus and vaccinia virus interferon-gamma binding proteins. Virology 208:762-769. [DOI] [PubMed] [Google Scholar]
- 23.Pestka, S., C. D. Krause, and M. R. Walter. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8-32. [DOI] [PubMed] [Google Scholar]
- 24.Rost, B., G. Yachdav, and J. Liu. 2004. The PredictProtein server. Nucleic Acids Res. 32:W321-W326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 26.Shental-Bechor, D., S. Kirca, N. Ben Tal, and T. Haliloglu. 2005. Monte Carlo studies of folding, dynamics, and stability in alpha-helices. Biophys. J. 88:2391-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, C. A., T. D. Smith, P. J. Smolak, D. Friend, H. Hagen, M. Gerhart, L. Park, D. J. Pickup, D. Torrance, K. Mohler, K. Schooley, and R. G. Goodwin. 1997. Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits beta chemokine activity yet lacks sequence homology to known chemokine receptors. Virology 236:316-327. [DOI] [PubMed] [Google Scholar]
- 28.Smith, G. L., J. A. Symons, A. Khanna, A. Vanderplasschen, and A. Alcami. 1997. Vaccinia virus immune evasion. Immunol. Rev. 159:137-154. [DOI] [PubMed] [Google Scholar]
- 29.Smith, V. P., and A. Alcami. 2000. Expression of secreted cytokine and chemokine inhibitors by ectromelia virus. J. Virol. 74:8460-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Symons, J. A., D. C. Tscharke, N. Price, and G. L. Smith. 2002. A study of the vaccinia virus interferon-gamma receptor and its contribution to virus virulence. J. Gen. Virol. 83:1953-1964. [DOI] [PubMed] [Google Scholar]
- 31.Walter, M. R., W. T. Windsor, T. L. Nagabhushan, D. J. Lundell, C. A. Lunn, P. J. Zauodny, and S. K. Narula. 1995. Crystal structure of a complex between interferon-gamma and its soluble high-affinity receptor. Nature 376:230-235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.