Abstract
8-Oxoguanine (8-oxoG), a common and mutagenic form of oxidized guanine in DNA, is eliminated mainly through base excision repair. In human cells its repair is initiated by human OGG1 (hOGG1), an 8-oxoG DNA glycosylase. We investigated the effects of an acute cadmium exposure of human lymphoblastoid cells on the activity of hOGG1. We show that coinciding with alteration of the redox cellular status, the 8-oxoG DNA glycosylase activity of hOGG1 was nearly completely inhibited. However, the hOGG1 activity returned to normal levels once the redox cellular status was normalized. In vitro, the activity of purified hOGG1 was abolished by cadmium and could not be recovered by EDTA. In cells, however, the reversible inactivation of OGG1 activity by cadmium was strictly associated with reversible oxidation of the protein. Moreover, the 8-oxoG DNA glycosylase activity of purified OGG1 and that from crude extracts were modulated by cysteine-modifying agents. Oxidation of OGG1 by the thiol oxidant diamide led to inhibition of the activity and a protein migration pattern similar to that seen in cadmium-treated cells. These results suggest that cadmium inhibits hOGG1 activity mainly by indirect oxidation of critical cysteine residues and that excretion of the metal from the cells leads to normalization of the redox cell status and restoration of an active hOGG1. The results presented here unveil a novel redox-dependent mechanism for the regulation of OGG1 activity.
Cadmium is a ubiquitous environmental pollutant that has been classified as a human carcinogen by the International Agency for Research on Cancer (27). Increasing evidence indicates that multifactor mechanisms might be involved in cadmium-induced toxicity. Inhibition of cysteine-sensitive proteins and metal replacement in proteins are the most commonly invoked pathways for toxicity. In particular, the replacement of critical Zn atoms is responsible for the Cd-induced inactivation of several DNA repair pathways (25). However, it is suggested that generation of an oxidative stress in the cell plays a central role (41, 44). Although cadmium is not a Fenton metal and thus does not directly generate reactive oxygen species (ROS) (32), a number of studies show increased ROS production in cadmium-treated cells (19, 38). Consistently, in many cases, free radical scavengers or antioxidants were shown to lessen cadmium toxicity (8, 17, 45). Moreover, the hypersensitivity to Cd of yeast strains defective in either thioredoxin or thioredoxin reductase clearly underscores the importance of the antioxidant defenses of cells for survival when exposed to Cd (43). The exact mechanism by which the heavy metal creates an oxidative stress is not known, but due to its high affinity to sulfhydryl groups, cadmium is thought to create an oxidative stress through depletion of reduced glutathione (9, 15, 16).
Cellular targets for ROS are numerous and include lipids, proteins, and DNA (12, 14, 33). A major product of ROS attack in genomic DNA is the premutagenic lesion 7,8-dihydro-8-oxoguanine (8-oxoG), which causes G-to-T transversions (22, 34). The main defense against the mutagenic effect of 8-oxoG is the base excision repair pathway, which in eukaryotes is initiated by the OGG1 protein, a DNA glycosylase that catalyzes the excision of 8-oxoG from DNA (5). ROS-mediated accumulation of 8-oxoG was reported after cadmium treatment in a number of cell systems (17, 45). Not only could the oxidative stress generated by cadmium exposure yield oxidative DNA damage, but the heavy metal was also described as being an inhibitor of oxidative DNA repair pathways (11). In particular, exposure to cadmium was shown to reduce the 8-oxoG DNA glycosylase activity levels from extracts of both lung tissue from rats and rat lung cell lines (36, 37). These effects were associated with lower levels of protein expression. It was also shown in in vitro assays that cadmium is a direct and irreversible inhibitor of the mouse OGG1 protein (48). However, at present little is known concerning the effect of cadmium on the human OGG1 (hOGG1) protein activity. Only one study recently addressed this question, demonstrating that several hours' exposure of human cells to low cadmium doses reduced hOGG1 activity through a decrease in the hOGG1 gene expression at the transcriptional level (47). The present study aimed at establishing whether an acute exposure of human cells to a high cadmium concentration alters the 8-oxoG DNA glycosylase activity of hOGG1 and at clarifying the underlying mechanisms of Cd genotoxicity.
MATERIALS AND METHODS
Cell culture conditions.
The human Boleth lymphoblastoid cell line, kindly obtained from A. Schmitz (CEA), was established by the French Polymorphism Center (CEPH, Paris, France) after Epstein-Barr virus transformation of lymphocytes from a healthy donor. Cells were grown in suspension in RPMI 1640 supplemented with 15% heat-inactivated fetal bovine serum, 20 mM HEPES, 2.5 mM sodium pyruvate, 4 mM glutamine, and antibiotics (penicillin-streptomycin) (all provided by Gibco, Carlsbad, CA). Cells were maintained in a humidified incubator at 5% CO2 at 37°C and fed with fresh medium at intervals of 48 h.
Cadmium treatment.
Cells were treated at a density of 1 × 106 cells/ml with cadmium chloride in phosphate-buffered saline (PBS) for 30 min. They were washed twice in PBS and returned to the incubator in fresh medium. At different times during and after treatment they were washed in PBS by centrifugation and stored as dried pellets in liquid nitrogen until protein extraction.
Protein extraction.
Cell extracts were obtained by sonication of cell pellets in 20 mM Tris-HCl, pH 8; 250 mM NaCl; and a cocktail of aprotinin, antipain, and leupeptin (0.8 μg/ml each). The homogenate was centrifuged at 20,000 × g for 30 min at 4°C, and the supernatant was aliquoted and stored at −80°C for biochemical assays. Protein content was measured using a Bio-Rad assay kit (Bio-Rad Laboratories, Richmond, CA) with bovine serum albumin as a standard.
Intracellular cadmium determination.
At different times during and after metal exposure cells were washed twice in PBS-EDTA (2 mM) and lysed in lysis buffer (Promega) at 4°C. Samples were treated with ultrapure 65% nitric acid (Normaton quality grade; VWR Prolabo) and diluted in ultrapure water. Cadmium concentration was measured by inductively coupled plasma mass spectrometry (ICP-MS) using an X7 series quadrupole Thermo Elemental apparatus and related to the protein content. The ICP-MS apparatus was calibrated with a SPEX CertiPrep standard (Jobin Yvon, Longjumeau, France). Yttrium was used as an internal standard (1 ppb).
Determination of glutathione content.
An aliquot of the 20,000 × g supernatant from the cell lysate was treated with an equal volume of 10% trichloroacetic acid. After centrifugation at 15,000 × g for 15 min at 4°C, total glutathione content was measured on the supernatant by the Tietze recycling assay (1). Oxidized glutathione (GSSG) was measured by the same method after derivatization of reduced glutathione using 2-vinylpyridine.
Purification of human OGG1.
Glutathione S-transferase-tagged recombinant hOGG1 was expressed in Escherichia coli BL21. After induction, cells from 3 liters of culture (A600 of 2.3) were harvested by centrifugation, resuspended, and sonicated in 120 ml of ice-cold lysis buffer (25 mM phosphate sodium, 10% glycerol, 1 mM EDTA, 500 mM NaCl, pH 7.6) with antipain (2.5 μg/ml), aprotinin (2.5 μg/ml), leupeptin (2.5 μg/ml), and lysozyme (1 mg/ml). The lysate was centrifuged at 100,000 × g for 30 min at 4°C, and the supernatant was incubated with 7 ml of glutathione Sepharose 4B (Amersham Biosciences) for 1 h at room temperature. The mixture was loaded on a column, and the resin was washed with 35 ml of lysis buffer and equilibrated with 35 ml of G0 buffer (25 mM phosphate sodium, 150 mM NaCl, pH 7.6). Twenty-seven milligrams of glutathione S-transferase-OGG1 was eluted in the same buffer with 30 mM glutathione. The protein was dialyzed against G0 and incubated with 250 U of thrombin (Amersham Biosciences) overnight at room temperature. The digestion mix was diluted three times with 25 mM sodium phosphate, pH 7.6, and loaded on a 1-ml column of resource S (Amersham Biosciences). Cleaved hOGG1 was eluted with a linear NaCl gradient in phosphate buffer. Nine milligrams of recovered protein was diluted four times with 25 mM sodium phosphate, pH 7.6, to 50 mM NaCl and loaded on a 1-ml Hitrap heparin column (Amersham Biosciences). hOGG1 was eluted with a linear NaCl gradient and collected at 400 mM NaCl. A 6.5-mg quantity of protein was obtained and conserved in 50% glycerol at −20°C.
8-OxoG DNA glycosylase assay.
A 34-mer oligonucleotide containing an 8-oxoguanine at position 16 was labeled at the 5′ end using [γ-32P]ATP (3,000 Ci/mmol; Amersham Biosciences) and T4 polynucleotide kinase (New England Biolabs). The 32P-labeled strand was hybridized to its complementary oligonucleotide containing a cytosine (C) opposite the lesion yielding the 8-oxo-G:C duplex. In a standard reaction mixture various amount of protein extracts or purified OGG1 (6-μl final volume) were added to a 9-μl reaction mixture containing 25 fmol of the 8-oxo-G:C-labeled duplex in 20 mM Tris-HCl, 1 mM EDTA, pH 6.8. The reaction mixtures were incubated at 37°C for 25 min. NaOH (0.1 N final concentration) was added, and the mixtures were further incubated for 10 min at 37°C and stopped with formamide dye, followed by heating for 5 min at 95°C. The products of the reaction were resolved by denaturing 20% polyacrylamide gel electrophoresis (19:1 acrylamide:bisacrylamide). Gels were scanned, and band intensities were quantified using a Storm PhosphorImager (Amersham Bioscience).
Western blot analysis.
Aliquots from cell extracts (50 μg) were denatured by being heated for 5 min at 95°C in either Laemmli buffer containing 100 mM dithiothreitol (DTT) (reducing Western blot assay) or Laemmli buffer without DTT (nonreducing Western blot assay). After denaturation samples were electrophoresed on a 12.5% sodium dodecyl sulfate-polyacrylamide gel and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with 5% nonfat dry milk in PBS-T (PBS with 0.1% Tween 20) and incubated with rabbit polyclonal anti-human OGG1 (2) in 1% blocking reagent (Roche Diagnostic) for 2 h at room temperature. After three 15-min washes with PBS-T, the membrane was incubated with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibody at a 1/20,000 dilution at room temperature. After three additional washing steps, the protein-antibody complexes were visualized by ECL (Amersham Biosciences).
Analysis of hOGG1 redox conformation.
Purified hOGG1 was incubated for 30 min at 37°C with diamide or cadmium in 20 mM Tris-HCl (pH 6.8), precipitated by addition of 20% trichloroacetic acid (7.5% final concentration) for 15 min on ice, and centrifuged at 4°C at 16,000 × g for 20 min. After being washed with ice-cold acetone, the protein was incubated for 90 min at 30°C in 10 mM AMS (4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid), denatured in Laemmli buffer without DTT, electrophoresed on a 15% sodium dodecyl sulfate-polyacrylamide gel, and revealed using the Imperial Protein staining reagent (Pierce).
RESULTS
Reversible inhibition of cellular 8-oxoG DNA glycosylase activity by cadmium.
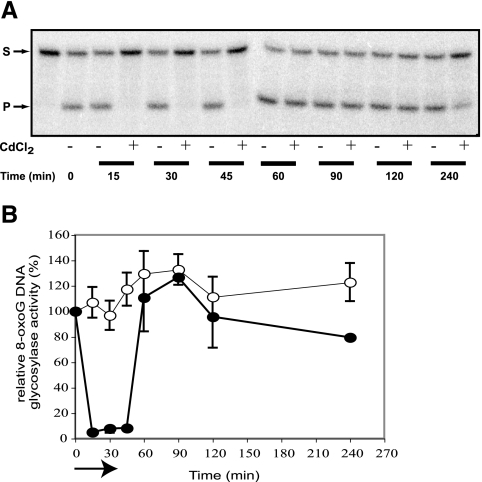
To analyze the consequences of an acute exposure to cadmium on human cells, lymphoblastoid cells were incubated in the presence of 350 μM CdCl2 for 30 min (10). At different times during the treatment and after removal of the Cd solution, cell extracts were analyzed for their 8-oxoG DNA glycosylase activity. Cd exposure led to a rapid and nearly total inhibition of the cellular 8-oxoG DNA glycosylase activity during the treatment and up to 15 min after it. Surprisingly, a complete recovery of the activity was observed starting 15 min after the cells were washed and put back on medium (Fig. 1).
FIG. 1.
Reversible inhibition of the 8-oxoG DNA glycosylase activity in cadmium-treated cells. After a 30-min exposure to 350 μM CdCl2, cells were washed and returned to growth medium at 37°C. Cells were harvested at different times during and after treatment, and the 8-oxoG DNA glycosylase activity was determined on 10 μg of cell extracts as described in Materials and Methods. (A) Representative gel showing the cleavage of the 5′-end-labeled 34-mer duplex containing an 8-oxoG (S). The 16-mer product (P) is indicated by the second arrow. (B) Quantification of hOGG1 activities. Filled and open symbols correspond to extracts from cadmium-treated cells and corresponding control cells, respectively. The arrow indicates the treatment time. Data correspond to at least four experiments.
Cadmium directly and irreversibly inhibits the DNA glycosylase activity of hOGG1 in vitro.
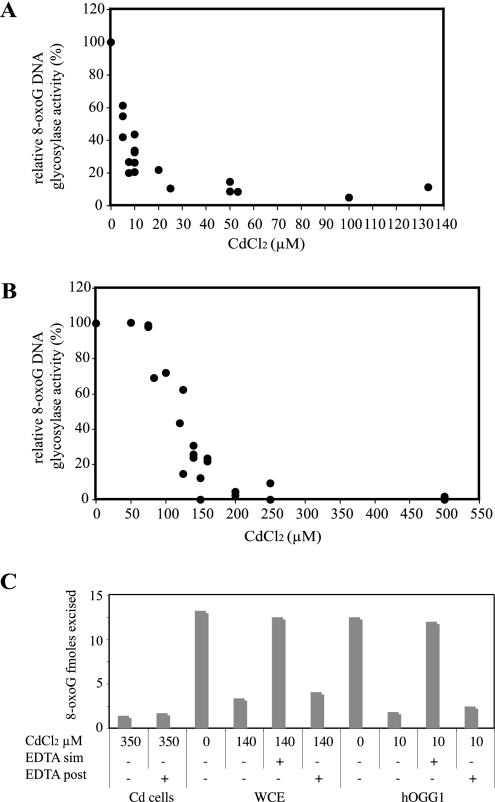
To establish whether cadmium can also directly inhibit the human OGG1 activity, as was shown for the mouse enzyme (48), we tested its effect on purified hOGG1 protein and on crude extracts from untreated Boleth cells. Our results confirmed those reported for the rodent protein by showing that cadmium inhibited 8-oxoG DNA glycosylase activity of purified OGG1 (Fig. 2A). However, doses as low as 5 μM had a potent inhibitory effect whereas concentrations higher than 100 mM were required for the mouse enzyme inactivation. For crude cellular extracts, doses above 50 μM resulted in inhibition of the enzyme (Fig. 2B). In all cases, adding an excess of the metal chelator EDTA simultaneously with Cd addition prevented the inhibitory effect of the metal (Fig. 2C). From these results, it could be suggested that the inhibition observed in cells exposed to Cd is due to a direct effect of the heavy metal on the hOGG1 protein. To test this hypothesis, we tried to reverse the cadmium-induced inactivation of hOGG1 obtained in either Cd-treated cells, Cd-exposed cell extracts, or Cd-exposed purified protein using EDTA. In neither case was EDTA able to revert the cadmium effect (Fig. 2C), thus indicating that in cells the reversible inhibition of OGG1 by cadmium is not simply due to a direct reversible interaction with the metal.
FIG. 2.
Direct and irreversible effect of cadmium on hOGG1 activity. (A and B) Purified hOGG1 (0.5 ng) (A) or extracts from Boleth control cells (10 μg) (B) were incubated for 15 min at 37°C with various amounts of cadmium chloride and then added to the glycosylase reaction mixture as described previously. (C) Effect of 5 mM EDTA on 8-oxoG DNA glycosylase activity in extracts (10 μg) from in vivo cadmium-treated cells (Cd cells), on extracts from in vitro cadmium-treated normal cell extracts (WCE), or on 0.5 ng of purified hOGG1 (hOGG1). In the cases of WCE or hOGG1 EDTA was added either simultaneously with cadmium (sim) or for 15 min after cadmium treatment (post) of the samples.
Cadmium accumulation, oxidative stress, and hOGG1 inhibition in cells.
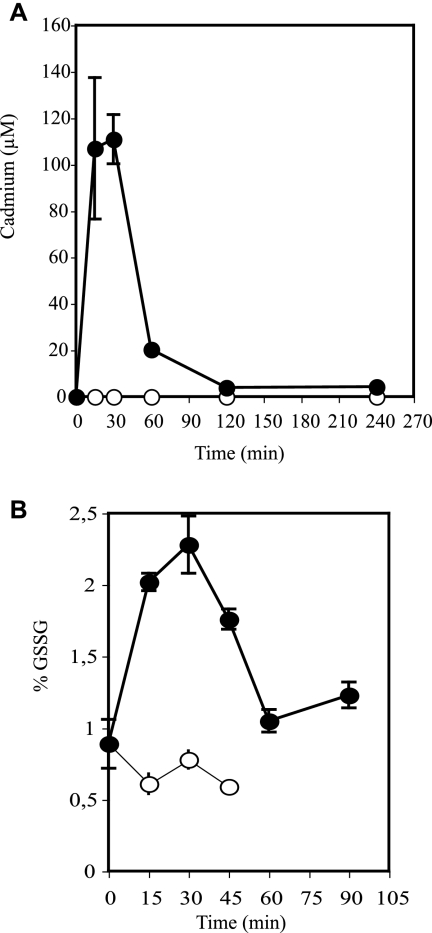
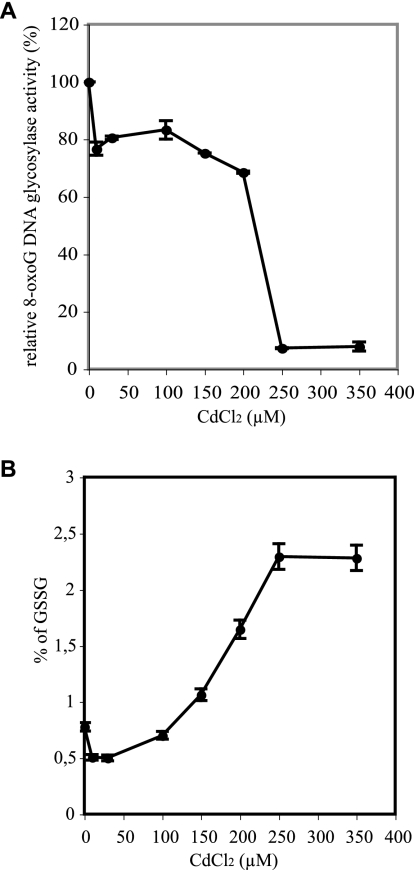
In order to investigate the mechanism of cellular hOGG1 inhibition by Cd, we determined the intracellular concentration of the metal during and after treatment. As shown in Fig. 3A, Cd rapidly accumulated inside cells but was very efficiently eliminated from them after washing. Paralleling the kinetics of metal accumulation in and excretion from the cells, a sharp increase in oxidized glutathione was also observed, an indication of an intense oxidative stress (Fig. 3B). Dose-response curves using the same protocol of exposure indicate that hOGG1 DNA glycosylase activity was slightly inhibited with cadmium doses between 10 and 200 μM whereas complete inhibition was obtained with doses above 200 μM (Fig. 4A). This response correlates well with the induction of oxidative stress. Indeed, the fraction of oxidized glutathione progressively increased with cadmium doses, reaching a plateau at cadmium concentrations inducing complete inhibition of hOGG1 activity (Fig. 4B). Taken together, these results suggest the possibility of an oxidation mechanism for the inactivation of hOGG1 in Cd-treated cells rather than direct inactivation of the enzyme by the metal.
FIG. 3.
Kinetics of metal accumulation and of alteration of the glutathione balance in cadmium-exposed cells. Cells treated and collected as described for Fig. 1 were analyzed for intracellular cadmium concentrations by ICP-MS (A) and level of oxidized glutathione expressed as a percentage of the total glutathione content (B). Filled and open symbols correspond to extracts from cadmium-treated and control cells, respectively. Each point represents the average of at least four independent experiments ± standard deviation. Total glutathione content varied less than 20% between control and Cd-treated cells.
FIG. 4.
Dose effect of cadmium on cellular 8-oxoG DNA glycosylase activity and glutathione balance. Cells were harvested after 30 min of incubation at 37°C with various CdCl2 concentrations and analyzed for 8-oxoG DNA glycosylase activity as described for Fig. 1 (A) and content in oxidized glutathione (B). Data points represent the average of three independent determinations and their standard deviations.
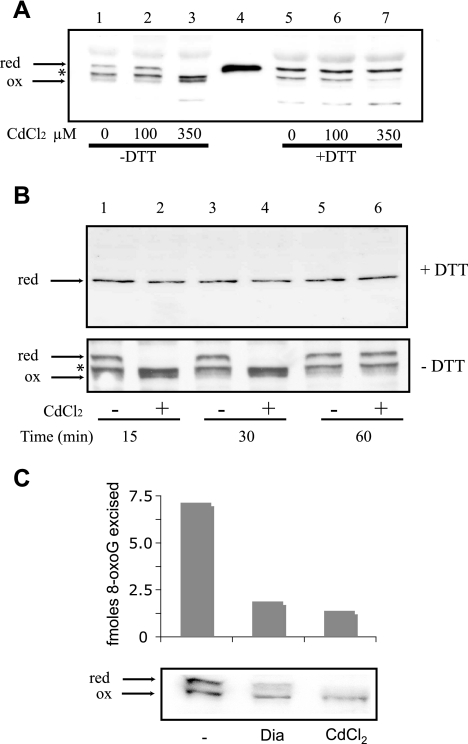
Alteration of hOGG1 activity in cadmium-treated cells is associated with changes in its redox state.
In Boleth cells cadmium exposure leads to a strong oxidative stress through inhibition of the pentose phosphate cycle enzymes and of glutathione reductase with a consequent alteration of the oxidized/reduced glutathione balance (A. Bravard et al., unpublished data). We therefore looked for a possible change in hOGG1 redox status associated with its inhibition and subsequent reactivation. Western blot analysis under nonreducing conditions of extracts from control cells or from cells treated with a noninhibitory Cd concentration (Fig. 5A, lanes 1 and 2) reveals three bands for hOGG1. In extracts from cells treated with the inhibitory concentration of Cd the upper hOGG1 band disappears at the expense of the faster-migrating form of the protein (Fig. 5A, lane 3). When the same extracts were loaded into the gel with a reducing buffer (Fig. 5A, lanes 5 to 7), the upper band was recovered in all extracts including the one from cells treated with 350 mM CdCl2 with the corresponding loss of the faster-migrating band. Analysis of the purified protein under reducing conditions (lane 4) confirms that the upper band observed in the extracts corresponds to the reduced form of hOGG1 and indicates that the lower band enriched in the Cd-treated cells represents an oxidized form of the protein. The middle band either is a nonspecific band or reflects a nonredox modification of hOGG1 present in the extracts.
FIG. 5.
Cadmium-induced reversible oxidation of the cellular hOGG1 protein. (A) Comparison between nonreductive (without DTT) and reductive (with 100 mM DTT) hOGG1 Western blot assays for protein extracts from cells exposed for 30 min at 100 μM and 350 μM compared to control cells. Lane 4 corresponds to 10 ng of a fully reduced form of hOGG1 obtained by denaturation of the protein in Laemmli buffer with 100 mM DTT. The middle band (*) either is a nonspecific band or reflects a nonredox modification of hOGG1 present in the extracts. (B) (Upper panel) Western blot assays for hOGG1 performed under standard (reducing) conditions on 50 μg of protein extracts from cells exposed for 30 min to 350 μM cadmium chloride and corresponding control cells, obtained at 15 min and 30 min during the metal treatment and 30 min thereafter. (Lower panel) The same extracts were analyzed for the presence of hOGG1 by Western blotting under nonreducing conditions, showing the reduced (upper band) and oxidized (lower band) forms of the protein. (C) (Upper panel) 8-OxoG DNA glycosylase activity on 12 μg of protein extracts from control cells (−) or cells treated for 30 min with either 2 mM diamide (Dia) or 350 μM CdCl2. (Lower panel) Western blot assay for hOGG1 under nonreducing conditions on 50 μg of protein from the same extracts.
Figure 5B shows the analysis of the redox status of hOGG1 at different times in control cells or cells exposed to the inhibitory concentration of CdCl2. At 15 and 30 min, when the DNA glycosylase activity is completely inhibited in the treated cells, the reduced form of hOGG1 cannot be detected (lanes 2 and 4), while it is present again at the 60-min point. This point corresponds to 30 min after the washing of the cells, when their redox status is normalized (Fig. 3) and the DNA glycosylase activity of hOGG1 is completely recovered (Fig. 1). The inactivation of the protein is paralleled by the enrichment of the lower band (Fig. 5B, lower panel, lanes 2 and 4) corresponding to the oxidized form of hOGG1. The oxidized nature of the lower band was confirmed by the presence of a single band of the same intensity when the samples were analyzed under reducing conditions (Fig. 5B, upper panel), ruling out the possibility of the lower band being a degradation product of the protein. Interestingly, incubating cells in 2 mM diamide [1,1′-azobis(N,N-dimethylformamide)], a thiol-oxidizing agent, also leads to the inhibition of the 8-oxoG DNA glycosylase activity correlated with a similar change in the migration pattern for hOGG1 (Fig. 5C). Taken together, these results suggest that the reversible inhibition of the hOGG1 protein activity induced in cells exposed to an acute cadmium treatment is mediated by reversible oxidation of the protein. Moreover, this inactivation of hOGG1 is not specific to the Cd treatment but rather a response to oxidative cellular stress.
Cysteine modifications in hOGG1 modulate its activity.
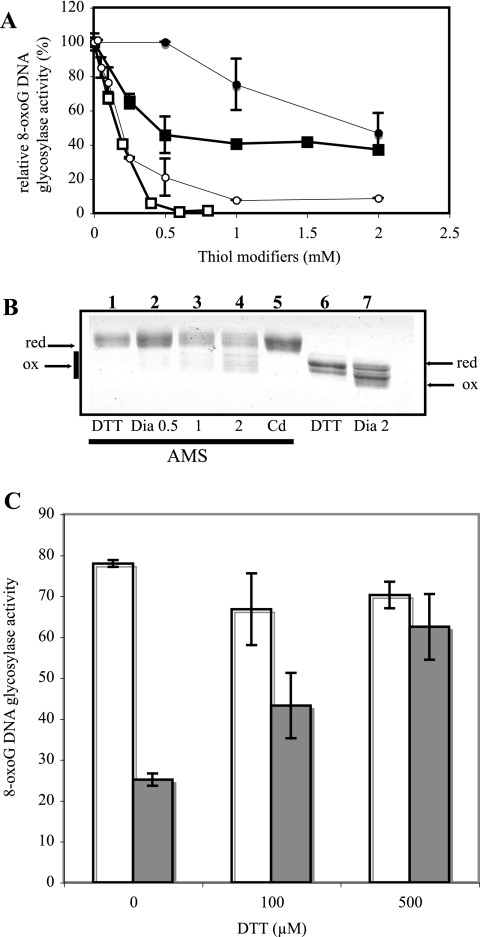
The above hypothesis implies that the 8-oxoG DNA glycosylase activity of hOGG1 can be modulated by oxidative alterations of the protein in response to the redox environment of the cell. In particular, cysteine residues, of which eight are present in hOGG1, are potential targets for oxidative modifications. We therefore examined whether OGG1 activity could be modulated by cysteine-modifying agents. The cysteine-blocking reagent N-ethylmaleimide (NEM) strongly inhibited the 8-oxoG DNA glycosylase activity of either purified hOGG1 or whole untreated cell extracts in a dose-dependent manner (Fig. 6A). Moreover, while hydrogen peroxide had no effect, other oxidants such as nitric oxide donors (data not shown) and diamide (Fig. 6A) were also able to inhibit the enzymatic activity of hOGG1 both in purified material and in cell extracts. In the case of diamide the inhibition of the extract activity was stronger than that of the pure protein. This might reflect the amplification of the oxidative response by secondary oxidative chain reactions involving other cellular components such as lipids. Interestingly, the migration pattern of purified OGG1 protein after oxidation by diamide was similar to that found in cadmium-treated cells (compare lanes 6 and 7 from Fig. 6B with Fig. 5). To test for the redox status of the thiol groups, the purified protein treated with either diamide or Cd was incubated in the presence of AMS before being loaded in the gel. AMS modifies proteins by the addition of a bulky adduct on reduced cysteine residues, causing a shift towards higher-molecular-weight forms. Figure 6B shows that exposure to diamide previous to AMS treatment blocks adduct formation, resulting in a protein with a lower molecular weight (lanes 2 to 4) compared to the completely reduced protein (lane 1). This confirms the oxidation of cysteine moieties by diamide. More important, Cd treatment of purified hOGG1 did not block the modification of cysteines by AMS (lane 5), implying that the metal does not directly oxidize the protein.
FIG. 6.
Effects of thiol-modifying agents on hOGG1 activity and migration pattern. (A) Protein extracts (10 μg) from normal cells (squares) or purified hOGG1 (0.5 ng) (circles) were incubated for 15 min in 20 mM Tris-HCl, pH 6.8, containing various concentrations of NEM (open symbols) or diamide (filled symbols) and then analyzed for their 8-oxoG DNA glycosylase activity. Results are expressed as percentages of the control untreated samples. (B) Migration pattern of hOGG1 (750 ng) after 15 min of incubation with the following reagents: DTT (100 mM), diamide (Dia; 0.5, 1, or 2 mM), and Cd (0.5 mM). On the left of the gel (lanes 1 to 5) samples were incubated after treatment with 10 mM AMS to label free sulfhydryls. Lanes 6 and 7 show reactions under nonreducing conditions. The gel was color stained. (C) Effect of 100 and 500 μM DTT on the 8-oxoG DNA glycosylase activity of purified hOGG1 (5 ng) previously oxidized by 15 min of incubation in the presence of 2 mM diamide in 20 mM Tris-HCl, pH 6.8. The excess diamide was removed before DTT was added through two passages on 10K Microcon columns (Amicon). Open bars correspond to control experiments where the protein was not exposed to diamide.
Since the hOGG1 inhibition in Cd-treated cells is reversible, we tested whether the enzyme inhibited by oxidation with diamide could be reactivated by reduction of the protein. As shown in Fig. 6C, diamide-induced inactivation of hOGG1 can be completely reversed by the reducing agent DTT. In the case of the Cd-inactivated protein shown in Fig. 6B, lane 5, treatment with DTT did not allow recovery of the activity (data not shown), supporting the notion of a distinct inactivation mechanism by direct exposure of the protein to the metal.
DISCUSSION
Protein modification by oxidation/reduction is emerging as a critical mechanism for the modulation of protein activities. Here we show that the oxidative stress induced by a short exposure of human cells to a toxic concentration of cadmium results in nearly complete but reversible inactivation of the 8-oxoG DNA glycosylase activity of hOGG1. Chronic exposure to low doses of cadmium lowers the cellular activity of OGG1 in animal models (36, 37) or in human cells (47). In the former cases, the cadmium-induced reduction of OGG1 activity was paralleled by lower enzyme levels most likely resulting from inhibition of gene expression at the transcriptional level. In human cells, this reduction was the consequence of decreased binding of transcription factor Sp1 to the hOGG1 promoter (47). Our results indicate that exposure of cells to a toxic cadmium concentration inhibits hOGG1 activity very rapidly through posttranslational modifications. More importantly, the inactivation of the protein is completely reversed in the cell after removal of the metal, unveiling a novel mechanism for modulating hOGG1 activity.
As is the case for the mouse enzyme (48), Cd can directly inactivate hOGG1 8-oxoG DNA glycosylase activity in vitro (Fig. 2). It has been proposed elsewhere (48) that this direct effect of Cd on the OGG1 activity is the result of its capacity to replace the Ca atom identified in the crystal structure of the protein (6). However, also as described for the mouse model, this direct inhibition appears irreversible, suggesting that in cadmium-treated cells the mechanism underlying the reversible inhibition of hOGG1 is mainly indirect. With the balance of glutathione and GSSG providing a dynamic indicator of oxidative stress (30, 35, 39), our data confirmed that cadmium compromises the cellular redox state, inducing a temporary shift towards a strongly oxidizing environment. The alteration of the glutathione balance characterized by an increase in the oxidized form, GSSG, when the heavy metal accumulated in cells was paralleled by an inhibition of the cellular DNA glycosylase activity. Therefore, the dramatic change induced by Cd in the redox status of the cell could produce reversible oxidative modifications of critical residues in the OGG1 protein. Disruption of the intracellular homeostasis by cadmium-induced oxidative stress leading to protein thiolation (16) and alteration of thiol transferases has been reported previously (9, 23). The human OGG1 protein, possessing eight cysteine residues, with two of them, C253 and C255, in the active site (4, 6), is a good candidate for regulation through oxidative modifications. Another characteristic of these particular cysteines is that they are surrounded by positively charged amino acids, making them more susceptible to oxidation by the stabilization of the thiolate anion (Cys-S−) (31). Also arguing for this hypothesis, the activity of OGG1 immunoprecipitated from a human cell line overexpressing hOGG1 was found to be inhibited by nitric oxide treatment of the cells (29). Using nonreducing Western blot assays, we have established that the modifications of cellular 8-oxoG DNA glycosylase activity in cadmium-treated cells clearly coincided with changes in the redox state of the OGG1 protein. Similar results showing a redox-induced modification and regulation of hOGG1 after Cd treatment, albeit with different kinetics probably due to the different permeability of cell membranes to Cd, were obtained with two human epithelial cell lines (data not shown). Although we cannot rule out the possibility that other posttranslational modifications induced by Cd treatment of the cells could have minor effects on the activity of hOGG1, our results support the hypothesis that cadmium-induced alterations of the cellular hOGG1 activity resulted mainly from an indirect effect mediated through changes in the oxidizing environment that in turn lead to reversible modifications of the redox state of the hOGG1 protein. Cysteine residues can be modified reversibly in different ways that can affect the activities of proteins. Such modifications include S-nitrosylation, S-glutathionylation, and formation of sulfenic acids and intra- or intermolecular disulfides. They are thought to protect proteins from further irreversible oxidation to sulfonic acid. Once the redox cellular environment is normalized, these oxidized forms can revert to yield a nonmodified protein. In many cases the reversion reaction is carried out by specific redox regulatory proteins such as thioredoxin, glutaredoxin, sulfidoredoxin, or APE1/ref-1 (3, 18, 21). Examples of redox-regulated proteins include a number of transcription factors binding to DNA such as AP-1 (26), p53 (40), and NF-κB (20). Confirming that hOGG1 activity can be regulated by redox modification of the protein, we showed here that hOGG1 activity can be inhibited using the cysteine-modifying agent NEM or the thiol oxidant diamide, indicating that modification of sulfhydryl moieties within the hOGG1 protein can impair its activity. Furthermore, diamide-induced inhibition of purified OGG1 is reversible by using a reducing agent.
It is tempting to speculate that the redox state of hOGG1 can be modulated by a reductive protein partner in human cells allowing recovery of a functional enzyme after its inactivation by an oxidative burst. As mentioned above, there are at the moment a few proteins, TRx, GRx, SRx, and APE1/ref-1, whose roles in controlling protein thiol oxidation have been demonstrated. Among them, APE1/ref-1 appears as an interesting candidate for a modulator of the hOGG1 activity: it is a bifunctional protein which is involved in activation of several transcription factors through its redox domain (7, 21, 24, 40, 46) and also the second enzyme of the base excision repair pathway through its endonuclease domain cleaving the abasic sites resulting from DNA glycosylase activity (13, 28, 42). Further work is obviously required for sustain this hypothesis.
In summary, we have shown that an acute exposure of human cells to cadmium leads to the reversible inactivation of hOGG1 activity through an indirect mechanism resulting from the cadmium-induced oxidative stress. Our results indicate that hOGG1 must possess critical redox-sensitive residues whose reduced state is important for the 8-oxoG DNA glycosylase activity. This finding confirms the central importance of oxidative stress in cadmium effect, and particular in its carcinogenic potential, leading to oxidative DNA damages but also to inhibition of a critical enzyme involved in the repair of oxidized bases. More largely, the fact that hOGG1 activity is sensitive to alterations in the cellular redox equilibrium opens interesting questions about its behavior during oxidative stresses known to occur in numerous physiological or pathological situations such as inflammation, cancer, or neurodegenerative diseases.
Acknowledgments
We thank Clarisse Mariet (CEA, Saclay) for the ICP-MS analysis and Jean-Baptiste Charbonnier (CEA, Saclay) and Anna Campalans (CEA, Fontenay aux Roses) for helpful discussions. We also thank Jérôme Lebeau and Kathy Ory (CEA, Fontenay aux Roses) for providing cell pellets.
This work was supported by the CEA, the CNRS, and grants from the Programme de Toxicologie Nucléaire et Environmentale and the Association pour la Recherche sur le Cancer (ARC 3836).
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Anderson, M. E. 1985. Tissue glutathione, p. 317-323. In R. A. Greenwald (ed.), Handbook of methods for oxygen radical research. CRC Press, Inc., Boca Raton, Fla.
- 2.Audebert, M., J. B. Charbonnier, S. Boiteux, and J. P. Radicella. 2002. Mitochondrial targeting of human 8-oxoguanine DNA glycosylase hOGG1 is impaired by a somatic mutation found in kidney cancer. DNA Repair (Amsterdam) 1:497-505. [DOI] [PubMed] [Google Scholar]
- 3.Biswas, S., A. S. Chida, and I. Rahman. 2006. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem. Pharmacol. 71:551-564. [DOI] [PubMed] [Google Scholar]
- 4.Bjoras, M., L. Luna, B. Johnsen, E. Hoff, T. Haug, T. Rognes, and E. Seeberg. 1997. Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7,8-dihydro-8-oxoguanine and abasic sites. EMBO J. 16:6314-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boiteux, S., and J. P. Radicella. 2000. The human OGG1 gene: structure, functions, and its implication in the process of carcinogenesis. Arch. Biochem. Biophys. 377:1-8. [DOI] [PubMed] [Google Scholar]
- 6.Bruner, S. D., D. P. G. Norman, and G. L. Verdine. 2000. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 403:859-866. [DOI] [PubMed] [Google Scholar]
- 7.Cao, X., F. Kambe, X. Lu, N. Kobayashi, S. Ohmori, and H. Seo. 2005. Glutathionylation of two cysteine residues in paired domain regulates DNA binding activity of Pax-8. J. Biol. Chem. 280:25901-25906. [DOI] [PubMed] [Google Scholar]
- 8.Chao, J. I., and J. L. Yang. 2001. Alteration of cadmium-induced mutational spectrum by catalase depletion in Chinese hamster ovary-K1 cells. Mutat. Res. 498:7-18. [DOI] [PubMed] [Google Scholar]
- 9.Chrestensen, C. A., D. W. Starke, and J. J. Mieyal. 2000. Acute cadmium exposure inactivates thioltransferase (glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J. Biol. Chem. 275:26556-26565. [DOI] [PubMed] [Google Scholar]
- 10.Coutant, A., N. Bidon-Wagner, C. Levalois, B. Lectard, J. Lebeau, and S. Chevillard. Biochimie, in press. [DOI] [PubMed]
- 11.Dally, H., and A. Hartwig. 1997. Induction and repair inhibition of oxidative DNA damage by nickel(II) and cadmium(II) in mammalian cells. Carcinogenesis 18:1021-1026. [DOI] [PubMed] [Google Scholar]
- 12.Davies, K. J. 1987. Protein damage and degradation by oxygen radicals. I. General aspects. J. Biol. Chem. 262:9895-9901. [PubMed] [Google Scholar]
- 13.Demple, B., and L. Harrison. 1994. Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem. 63:915-948. [DOI] [PubMed] [Google Scholar]
- 14.de Zwart, L. L., J. H. Meerman, J. N. Commandeur, and N. P. Vermeulen. 1999. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med. 26:202-226. [DOI] [PubMed] [Google Scholar]
- 15.Ercal, N., H. Gurer-Orhan, and N. Aykin-Burns. 2001. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 1:529-539. [DOI] [PubMed] [Google Scholar]
- 16.Figueiredo-Pereira, M. E., S. Yakushin, and G. Cohen. 1998. Disruption of the intracellular sulfhydryl homeostasis by cadmium-induced oxidative stress leads to protein thiolation and ubiquitination in neuronal cells. J. Biol. Chem. 273:12703-12709. [DOI] [PubMed] [Google Scholar]
- 17.Filipic, M., and T. K. Hei. 2004. Mutagenicity of cadmium in mammalian cells: implication of oxidative DNA damage. Mutat. Res. 546:81-91. [DOI] [PubMed] [Google Scholar]
- 18.Findlay, V. J., H. Tapiero, and D. M. Townsend. 2005. Sulfiredoxin: a potential therapeutic agent? Biomed. Pharmacother. 59:374-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fotakis, G., E. Cemeli, D. Anderson, and J. A. Timbrell. 2005. Cadmium chloride-induced DNA and lysosomal damage in a hepatoma cell line. Toxicol. In Vitro 19:481-489. [DOI] [PubMed] [Google Scholar]
- 20.Freemerman, A. J., A. Gallegos, and G. Powis. 1999. Nuclear factor κB transactivation is increased but is not involved in the proliferative effects of thioredoxin overexpression in MCF-7 breast cancer cells. Cancer Res. 59:4090-4094. [PubMed] [Google Scholar]
- 21.Fritz, G., S. Grosch, M. Tomicic, and B. Kaina. 2003. APE/Ref-1 and the mammalian response to genotoxic stress. Toxicology 193:67-78. [DOI] [PubMed] [Google Scholar]
- 22.Grollman, A. P., and M. Moriya. 1993. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 9:246-249. [DOI] [PubMed] [Google Scholar]
- 23.Hansen, J. M., H. Zhang, and D. P. Jones. 2006. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic. Biol. Med. 40:138-145. [DOI] [PubMed] [Google Scholar]
- 24.Hanson, S., E. Kim, and W. Deppert. 2005. Redox factor 1 (Ref-1) enhances specific DNA binding of p53 by promoting p53 tetramerization. Oncogene 24:1641-1647. [DOI] [PubMed] [Google Scholar]
- 25.Hartwig, A., M. Asmuss, H. Blessing, S. Hoffmann, G. Jahnke, S. Khandelwal, A. Pelzer, and A. Burkle. 2002. Interference by toxic metal ions with zinc-dependent proteins involved in maintaining genomic stability. Food Chem. Toxicol. 40:1179-1184. [DOI] [PubMed] [Google Scholar]
- 26.Hirota, K., M. Matsui, S. Iwata, A. Nishiyama, K. Mori, and J. Yodoi. 1997. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA 94:3633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Agency for Research on Cancer. 1993. Cadmium and cadmium components. IARC Monogr. Eval. Carcinog. Risks Hum. 58:119-237. [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi, T., T. K. Hazra, I. Boldogh, A. E. Tomkinson, M. S. Park, S. Ikeda, and S. Mitra. 2000. Requirement for human AP endonuclease 1 for repair of 3′-blocking damage at DNA single-strand breaks induced by reactive oxygen species. Carcinogenesis 21:1329-1334. [PubMed] [Google Scholar]
- 29.Jaiswal, M., N. F. LaRusso, N. Nishioka, Y. Nakabeppu, and G. J. Gores. 2001. Human Ogg1, a protein involved in the repair of 8-oxoguanine, is inhibited by nitric oxide. Cancer Res. 61:6388-6393. [PubMed] [Google Scholar]
- 30.Jones, D. P. 2002. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 348:93-112. [DOI] [PubMed] [Google Scholar]
- 31.Kim, J. R., H. W. Yoon, K. S. Kwon, S. R. Lee, and S. G. Rhee. 2000. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal. Biochem. 283:214-221. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd, D. R., P. L. Carmichael, and D. H. Phillips. 1998. Comparison of the formation of 8-hydroxy-2′-deoxyguanosine and single- and double-strand breaks in DNA mediated by Fenton reactions. Chem. Res. Toxicol. 11:420-427. [DOI] [PubMed] [Google Scholar]
- 33.Marnett, L. J. 2000. Oxyradicals and DNA damage. Carcinogenesis 21:361-370. [DOI] [PubMed] [Google Scholar]
- 34.Michaels, M. L., and J. H. Miller. 1992. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J. Bacteriol. 174:6321-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pias, E. K., and T. Y. Aw. 2002. Apoptosis in mitotic competent undifferentiated cells is induced by cellular redox imbalance independent of reactive oxygen species production. FASEB J. 16:781-790. [DOI] [PubMed] [Google Scholar]
- 36.Potts, R. J., I. A. Bespalov, S. S. Wallace, R. J. Melamede, and B. A. Hart. 2001. Inhibition of oxidative DNA repair in cadmium-adapted alveolar epithelial cells and the potential involvement of metallothionein. Toxicology 161:25-38. [DOI] [PubMed] [Google Scholar]
- 37.Potts, R. J., R. D. Watkin, and B. A. Hart. 2003. Cadmium exposure down-regulates 8-oxoguanine DNA glycosylase expression in rat lung and alveolar epithelial cells. Toxicology 184:189-202. [DOI] [PubMed] [Google Scholar]
- 38.Pourahmad, J., P. J. O'Brien, F. Jokar, and B. Daraei. 2003. Carcinogenic metal induced sites of reactive oxygen species formation in hepatocytes. Toxicol. In Vitro 17:803-810. [DOI] [PubMed] [Google Scholar]
- 39.Schafer, F. Q., and G. R. Buettner. 2001. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30:1191-1212. [DOI] [PubMed] [Google Scholar]
- 40.Seemann, S., and P. Hainaut. 2005. Roles of thioredoxin reductase 1 and APE/Ref-1 in the control of basal p53 stability and activity. Oncogene 24:3853-3863. [DOI] [PubMed] [Google Scholar]
- 41.Stohs, S. J., D. Bagchi, E. Hassoun, and M. Bagchi. 2001. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 20:77-88. [PubMed] [Google Scholar]
- 42.Vidal, A. E., I. D. Hickson, S. Boiteux, and J. P. Radicella. 2001. Mechanism of stimulation of the DNA glycosylase activity of hOGG1 by the major human AP endonuclease: bypass of the AP lyase activity step. Nucleic Acids Res. 29:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vido, K., D. Spector, G. Lagniel, S. Lopez, M. B. Toledano, and J. Labarre. 2001. A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J. Biol. Chem. 276:8469-8474. [DOI] [PubMed] [Google Scholar]
- 44.Waisberg, M., P. Joseph, B. Hale, and D. Beyersmann. 2003. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192:95-117. [DOI] [PubMed] [Google Scholar]
- 45.Watjen, W., and D. Beyersmann. 2004. Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals 17:65-78. [DOI] [PubMed] [Google Scholar]
- 46.Xanthoudakis, S., G. Miao, F. Wang, Y. C. Pan, and T. Curran. 1992. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 11:3323-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youn, C. K., S. H. Kim, Y. Lee do, S. H. Song, I. Y. Chang, J. W. Hyun, M. H. Chung, and H. J. You. 2005. Cadmium down-regulates human OGG1 through suppression of Sp1 activity. J. Biol. Chem. 280:25185-25195. [DOI] [PubMed] [Google Scholar]
- 48.Zharkov, D. O., and T. A. Rosenquist. 2002. Inactivation of mammalian 8-oxoguanine-DNA glycosylase by cadmium(II): implications for cadmium genotoxicity. DNA Repair (Amsterdam) 1:661-670. [DOI] [PubMed] [Google Scholar]