Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8 (HHV-8) is a γ-herpesvirus consistently identified in Kaposi’s sarcoma (KS), primary effusion lymphoma, and multicentric Castleman’s disease. KSHV infection appears to be necessary, but not be sufficient for development of KS without other co-factors. However, factors that facilitate KSHV to cause KS have not been well defined. Because patients with KS are often immunosuppressed and susceptible to many infectious agents including human herpesvirus 6 (HHV-6), we investigated the potential of HHV-6 to influence the replication of KSHV. By co-culturing HHV-6-infected T cells with KSHV-latent BCBL-1 cell line, infecting BCBL-1 cells with HHV-6 virions, and generating heterokaryons between HHV-6-infected T cells and BCBL-1 cells, we showed that HHV-6 played a critical role in induction of KSHV replication, as determined by production of lytic phase mRNA transcripts and viral proteins. We confirmed and extended the results by using a luciferase reporter assay in which KSHV ORF50 promoter, the first promoter activated during KSHV replication, drove the luciferase expression. Besides HHV-6, we also found that cytokines such as interferon-γ partially contributed to induction of KSHV replication in the co-culture system. These findings suggest that HHV-6 may participate in KS pathogenesis by promoting KSHV replication and increasing KSHV viral load.
Kaposi’s sarcoma-associated herpesvirus (KSHV, also known as human herpesvirus 8 or HHV-8) is the first known member of γ2-herpesviruses (genus Rhadinovirus) to infect humans, which was originally discovered by Chang and colleagues,1 and Moore and colleagues2 in the lesions of acquired immunodeficiency syndrome-related Kaposi’s sarcoma (AIDS-KS). Today, KSHV has been detected in more than 95% of all KS lesions, regardless of the stage or clinical form of the disease.3 KSHV is also known to associate with two lymphoproliferative diseases, multicentric Castleman’s disease and primary effusion lymphomas (PEL, also termed body cavity-based lymphomas or BCBL).4 Although epidemiological evidence strongly implicates KSHV as the etiological agent of KS,5–11 KSHV infection appears to be necessary but not sufficient for the development of KS without the involvement of other factors to reactivate KSHV lytic replication.
KSHV establishes latent infection in their natural host cells. During latent infection the viral genome persists as an episome, and viral gene expression is highly restricted. Pharmaceutically, KSHV can be reactivated by 12-O-tetradecanoylphorbol 13-acetate (TPA) treatment, resulting in the expression of a wide variety of viral-encoded proteins, the production of progeny virions, and eventual death of the host cell. Clinically, lytic cycle replication is consistently detected in a small subset of cells within the lesions of KS as well as PEL, and multicentric Castleman’s disease,12–14 although a vast majority of cells are latently infected with KSHV. Regulation of viral replication is critical to disease progression because the tissue deterioration and infection progression is proportionally related to the percentage of virus-infected cells undergoing reactivation. Indeed, studies have shown that KSHV viral load is higher in KS patients than in KSHV-infected individuals without KS, and KSHV viral load also increases during progression of this disease.15,16 However, who and how to reactivate latent virus are not well defined.
A couple of agents have been considered as the co-factors to reactivate KSHV. One potentially important co-factor is human immunodeficiency virus (HIV). AIDS-KS is known to be more aggressive, disseminated, and resistant to treatment than other forms of KS disease,17–19 suggesting a role of HIV in the disease progression. Previous studies have shown that KS tumor cells themselves are not infected with HIV-1.20 Therefore, HIV-1 may not play a direct oncogenic role in AIDS-KS. Evidence supports a role of HIV-1 in the initiation and progression of KS through mechanisms other than immunosuppression.21,22 It is possible that diffusible factors synthesized by HIV may act on and reactivate KSHV.
Besides HIV, several other viruses, including human herpesvirus 6 (HHV-6), human cytomegalovirus (HCMV), papilloma virus, and BK virus that are commonly found in immunocompromised individuals, have been proposed to play a role in KS.23–27 HHV-6 is a ubiquitous virus that infects the majority of the human population (∼90%). After primary infection, HHV-6 persists in the host in a latent form and has the potential to cause disease on reactivation, particularly in the immunocompromised host, such as AIDS, KS, or AIDS-KS.28–33 In addition, early studies have also shown that HHV-6 can activate Epstein-Barr virus (EBV) replication and contribute to progression of EBV-associated disease.34 Vieira and colleagues35 showed that infection of HCMV increased the production of KSHV in endothelial cells and activated lytic cycle gene expression in keratinocytes, suggesting that HCMV could influence KSHV pathogenesis. The fact is that HHV-6 is most closely related to HCMV as a β-herpesvirus and both EBV and KSHV belong to γ-herpesviruses. Such kinship led us to hypothesize that HHV-6 may participate in the progression of KS by promoting KSHV replication and increasing KSHV viral load.
To explore the possible role of HHV-6 in the pathogenesis of KS or AIDS-KS, in this study, we showed that there was a lytic cycle replication of KSHV by HHV-6 infection. The replication was indicated by an increase of both ORF26 mRNA (encoding minor capsid protein) and viral protein expressions of KSHV. Furthermore, we confirmed and extended these results by using a luciferase reporter assay in which KSHV ORF50 promoter, the first promoter activated during KSHV replication, drove luciferase expression. These novel findings are believed to be the first report on the role of HHV-6 in KSHV lytic replication and sheds light on the understanding of KS pathogenesis.
Materials and Methods
Cell Culture and Viral Infection
The BCBL-1 and BC-3 cells, both of which are EBV-negative and KSHV-positive PEL cell lines, were obtained through the AIDS Research and Reference Reagent Program, National Institutes of Health, Bethesda, MD. The JJhan cells are the human CD4+ T-cell line that was kindly provided by Dr. W.-H. Wu from the University of Hong Kong.36 Both BCBL-1 and JJhan cells were maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 2 mmol/L l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified, 5% CO2 atmosphere. BC-3 cells were grown in RPMI + 20% fetal bovine serum.37
HHV-6 (U1102 strain) was propagated in JJhan cells as described elsewhere.38,39 HHV-6-infected JJhan cells were mixed with uninfected cells at a ratio of 1:10. When 75% of the cells showed cytopathic effects, as determined by light microscope, cell-free culture fluid was harvested and filtered through a 0.45-μm-pore-size filter and the virus was pelleted by centrifugation (25,000 × g) for 90 minutes at 4°C. The virus pellet was suspended in RPMI 1640 and frozen at −80°C until used. The HHV-6 titer expressed as the 50% tissue culture infective dose (TCID50) was determined by scoring the number of JJhan cells exhibiting cytopathic effects. The virus stock used had a titer of 105 TCID50/ml. BCBL-1 cells (2 × 106 to 3 × 106) were pelleted and infected with HHV-6 (104 TCID50/ml for 106 cells) for 2 hours at 37°C and subsequently suspended in 10 ml of culture medium.
Flow Cytometry
The expressions of CD46 antigen on the surface of cell and viral core protein in the cytoplasm were evaluated by flow cytometry analysis. To detect the expression of CD molecules, ∼1 × 106 cells were washed and suspended in fluorescence-activated cell sorting (FACS) buffer [1% bovine serum albumin-0.1% sodium azide in phosphate-buffered saline (PBS)], then incubated with fluorescein isothiocyanate-conjugated anti-human CD46-specific monoclonal antibody (mAb) (anti-human CD46, clone 169-1-E4.3, mouse IgG2a; Dakewe Biotech Co. Ltd., Shenzhen City, Guangdong, China) on ice for 30 minutes. Cells were then washed with PBS, fixed with 1% paraformaldehyde in PBS, and analyzed with a FACScan (Becton-Dickinson). To detect viral core protein expression, intracellular staining was performed. Briefly, cells infected with HHV-6 for 96 hours were harvested and suspended in FACS buffer, and then fixed in 1% paraformaldehyde in FACS buffer for 10 minutes at room temperature. The cells were washed with 0.03% saponin prepared in PBS and incubated with 0.5 μg of anti-gp116/gp64/gp54 (core protein) of HHV-6 mAb (clone 6A5G3, mouse IgG2b; Advanced Biotechnologies Inc., Columbia, MD) in 0.3% saponin on ice for 30 minutes, then washed with FACS buffer. Fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Pierce) was then added, and the mixture was incubated for 1 hour on ice. Cells were washed in FACS buffer without saponin and analyzed with a FACScan.
Northern Blot Analysis
Total RNA was isolated from cells by using a phenol/guanidine isothiocyanate/chloroform extraction technique (Trizol; Life Technologies, Inc., Gaithersburg, MD). Twelve μg of total RNA was fractionated on a 1% agarose formaldehyde gel and transferred to a nylon membrane (Zetabind; Cuno Inc., Meriden, CT). Even loading of RNA and efficiency of transfer was confirmed by staining of the 18S and 28S bands on the membrane with methylene blue.40 Membranes were prehybridized with Church’s hybridization buffer and probed with [32P] dCTP-labeled probes. Probes were generated by using gel-purified polymerase chain reaction (PCR) products and a random prime label kit (Roche Molecular Biochemicals, Indianapolis, IN). The membranes were washed with sodium phosphate buffers containing sodium dodecyl sulfate, ethylenediaminetetraacetic acid, and bovine serum albumin and exposed to Kodak film.
Quantitative PCR
Complementary DNA was synthesized from the isolated RNA using TaqMan Gold reverse transcription reagents (Applied Biosystems, Foster City, CA). Reverse transcription was performed by using oligo dT primers at 25°C for 10 minutes, 48°C for 30 minutes, and 95°C for 5 minutes. Quantitative PCR was performed in a GeneAmp 7700 sequence detection machine (Applied Biosystems). The TaqMan PCR Core Reagents kit and primers/probes for β-actin (Applied Biosystems) were used. KSHV ORF26 primer/probe sequences were: forward 5′-AGC CGA AAG GAT TCC ACC AT-3′, reverse 5′-GCT GCG GCA CGA CCA T-3′, and probe 5′-FAM-TGC TCG AAT CCA ACG GAT TTG ACC TC-BHQ1–3′.41 The PCR reaction mixture contained AmpErase Uracil N-glycosylase to destroy any previously amplified product as described.42 Efficiencies of the β-actin and ORF26 amplification were shown to be approximately equal using a validation experiment as described by the sequence detection system manufacturer (Applied Biosystems, User Bulletin 2: Relative Quantitation of Gene Expression).
Transfections and Luciferase Assay
Cells were transfected with Lipofectamine 2000 (Invitrogen, Inc., Carlsbad, CA) following the supplied protocol. Typical transfections of BCBL-1, BC-3, or HHV-6-infected BCBL-1, BC-3 cells involved the introduction of 0.5 μg of reporter plasmid DNA (pORF50 promoter, gift from Y. Yuan, Department of Microbiology, School of Dental Medicine, University of Pennsylvania43) for 105 cells. Cells were harvested at 48 hours after transfection. Luciferase activity was assayed by using the Promega system. All data points were the averages of at least five independent transfections.
Cell-Cell Fusion
HHV-6 latent JJhan cells (5 × 106) was mixed with an equal number of BCBL-1 cells. After washing twice with PBS, cells were resuspended into 1 ml of 100% (w/v) polyethylene glycol 4000 in RPMI 1640 containing 5% (v/v) dimethyl sulfoxide. The cell/polyethylene glycol suspension was centrifuged at 100 × g for 2 minutes, thereafter, 10 ml of RPMI 1640 were added to resuspend the cell pellet. Cells were then centrifuged at 100 × g for 5 minutes, supernatants were removed, and cells were finally resuspended into complete medium.44 To calculate the number of fused cells, DNA staining with propidium iodide solution and flow cytometric analysis were performed as described previously.45
Reverse Transcriptase(RT)-PCR
Complementary DNA was synthesized from isolated RNA using the SuperScript Preamplication System for First Strand cDNA Synthesis (Life Technologies, Inc.) following the manufacturer’s instructions. To ensure no DNA contamination of the RNA, which could lead to false-positive results, the RNA samples were treated with DNase I (Life Technologies, Inc.) before reverse transcription. As an additional control, each sample was also subjected to reverse transcription in the absence of RT. Single-stranded cDNA was then amplified using standard PCR techniques as previously described.46 Primers used for analysis included KSHV ORF26 (also referred to as KS330) primers originally described by Chang and colleagues1 and β-actin by Knipping and colleagues.47
Immunoperoxidase Staining
Cytospin preparations of cultured cells were fixed for 10 minutes in 50:50 acetone:methanol and air-dried. The cells were immunostained to detect two antigens using a highly sensitive avidin-biotin immunoperoxidase technique (Vectastain kit; Vector Laboratories, Burlingame, CA) as previously described.21 The chromogen, 3-amino-4-ethylcarbazole was used, producing a positive red reaction. The panel of mAbs used included KSHV ORF59 (clone 11D1, mouse IgG2b; Advanced Biotechnologies Inc.) and KSHV ORF K8.1 A/B (clone 4A4, mouse IgG1; Advanced Biotechnologies Inc.). Both ORF59 and K8.1 mAbs recognize KSHV lytic cycle proteins and have been previously described.48–50 To calculate the percentage of positive cells, photographs of at least 10 unique fields were taken of every slide, and the number of positive and negative cells counted separately by three individuals, including one who was blinded to the results. Immunostaining was performed on samples from three separate experiments.
Enzyme-Linked Immunosorbent Assay (ELISA)
Production of interferon (IFN)-γ and interleukin (IL)-10 was measured in JJhan, JJhan + HHV-6, BCBL-1, and BCBL-1 + HHV-6 cells before and after co-culture by using ELISA kits (Diacone Research, Fleming, Besancon, France). Undiluted tissue culture supernatants were used as recommended by the supplier. Each sample was assayed in duplicate and a minimum of three times was performed.
Results
Susceptibility of BCBL-1 Cells to HHV-6 Infection
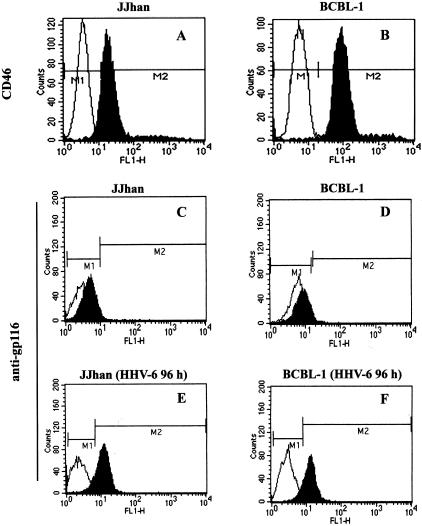
To evaluate whether HHV-6 can affect lytic cycle replication of KSHV in BCBL-1 cells, it was first necessary to determine the susceptibility of BCBL-1 to HHV-6 infection. Flow cytometry performed on normal cell lines showed that CD46 molecule, a cellular receptor for HHV-6,51 was readily expressed on the surface of both JJhan and BCBL-1 cells (Figure 1, top). No detectable levels of HHV-6 gp116 were expressed in JJhan and BCBL-1 cells (Figure 1, middle). However, 89.1% JJhan cells and 86% BCBL-1 cells (the ratio of counts in M2 gate to total events) were positive for HHV-6 gp116 when these cells were infected by HHV-6 for 96 hours. These data indicate that BCBL-1 cells are susceptible to HHV-6 infection.
Figure 1.
Expression of CD46 and HHV-6 gp116 antigens in JJhan and BCBL-1 cells. JJhan (A, C) and BCBL-1 (B, D) cells or HHV-6-infected JJhan (E) and HHV-6-infected BCBL-1 (F) cells were stained with anti-CD46 (top) or with anti-HHV-6 gp116 (middle and bottom) antibodies (fluorescein isothiocyanate-labeled monoclonal antibodies). Fluorescein isothiocyanate-labeled IgG was used as an isotype control antibody. The expressions of CD46 and HHV-6 gp116 were analyzed by FACS. The black shading represents anti-CD46 or anti-gp116-specific antibodies and the white shading represents the isotype control antibody.
Co-Culture of BCBL-1 Cells with HHV-6-Infected Cells Results in Induction of KSHV Replication
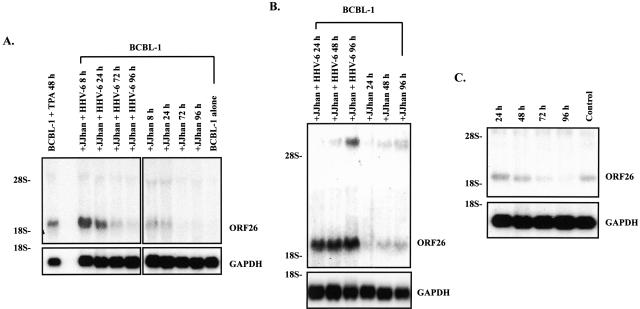
To determine whether HHV-6 can activate KSHV lytic replication, we infected BCBL-1 cells by co-culturing BCBL-1 cells with HHV-6-infected JJhan cells (JJhan + HHV-6). ORF26 mRNA (expressed only during lytic KSHV replication) was analyzed by Northern blot. We found a remarkable increase in KSHV ORF26 mRNA at 8 hours, which gradually decreased with time (Figure 2A). Of interest, ORF26 mRNA also increased after culture with uninfected JJhan cells, but to a lesser extent than those with JJhan + HHV-6 at the same time point (Figure 2A). Analysis of data from five independent experiments demonstrated that, on average, ORF26 expression increased 4.2 ± 0.51-fold at 8 hours, 3.12 ± 0.39-fold at 24 hours, 1.85 ± 0.25-fold at 72 hours, and 1.55 ± 0.13-fold at 96 hours, respectively, when comparing BCBL-1 cells co-cultured with uninfected JJhan cells at same time point (Figure 2A). Analysis of mRNA for the cellular housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH) on the same membrane indicated equal RNA loading (Figure 2A). These data suggest that HHV-6 may activate KHSV lytic replication.
Figure 2.
Northern blot analysis for ORF26 mRNA in BCBL-1 cells. A: ORF26 mRNA expressed in BCBL-1 cells after co-culture with HHV-6-infected JJhan cells by direct cell-cell contact. Total RNA isolated from BCBL-1 cells treated with TPA for 48 hours (positive control, BCBL-1 + TPA 48 hours), BCBL-1 cells co-cultured with HHV-6-infected JJhan cells (JJhan + HHV-6), 8, 24, 72, and 96 hours, respectively (+JJhan + HHV-6 8 hours, 24 hours, 72 hours, 96 hours), BCBL-1 cells co-cultured with uninfected JJhan cells, 8, 24, 72, and 96 hours, respectively (+JJhan 8 hours, 24 hours, 72 hours, 96 hours), BCBL-1 cells alone (negative control, BCBL-1 alone) were transferred to nylon membrane and ORF26 mRNA expression was detected by Northern blot. The same membrane was stripped and reprobed for GAPDH to demonstrate equal loading of the RNA. B: ORF26 mRNA expressed in BCBL-1 cells cultured for 24 to 96 hours in 50% conditioned medium. ORF26 mRNA expression in BCBL-1 cells cultured in 50% conditioned medium from HHV-6-infected JJhan cells for 24, 48, and 96 hours (+JJhan + HHV-6 24 hours, 48 hours, 96 hours) and BCBL-1 cells cultured in 50% conditioned medium from uninfected JJhan cells for 24, 48, and 96 hours (+JJhan 24 hours, 48 hours, 96 hours) was detected by Northern blot. C: ORF26 mRNA expressed in BCBL-1 cells cultured for 24 to 96 hours in KSHV-depleted conditioned medium. ORF26 mRNA expression in BCBL-1 cells cultured in KSHV-depleted conditioned medium for 24, 48, 72, and 96 hours (24 hours, 48 hours, 72 hours, 96 hours) and normal BCBL-1 cells (control) was detected by Northern blot. All results shown are a representative experiment of at least three independent experiments with similar results.
Infection of BCBL-1 Cells with HHV-6 Induces Lytic Cycle Replication of KSHV
Besides HHV-6, many factors may present in the co-culture system and may affect the results. For instance, direct cell-cell contact, soluble factors including HHV-6-related proteins, cytokines and/or growth factors released by HHV-6 infection may all contribute to the increase of KSHV replication. To determine whether soluble factors other than cell-cell contact play a major role in activation of KSHV lytic replication, we cultured BCBL-1 cells in conditional medium that was collected from HHV-6-infected JJhan cells at 96 hours, and filtered to remove contaminating cells. We demonstrated again that ORF26 mRNA drastically increased throughout time. ORF26 expression increased 3.91-fold at 24 hours, 4.55-fold at 48 hours, and 5.82-fold at 96 hours, respectively, compared to BCBL-1 cells cultured in conditioned medium collected from uninfected JJhan cells at same time point (Figure 2B). Such increase, however, began to decline at 120 hours and continually decrease throughout time (data not shown). These data suggest that soluble factors rather than cell-cell contact are responsible for the activation of KSHV lytic replication.
Because the conditional medium from the infected cells may contain infectious HHV-6 virions, which would complicate interpretation of the results, HHV-6 virions were depleted from the cell-free condition medium by ultracentrifuge. HHV-6 was not detected in this virus-depleted medium by PCR whereas it was readily detected in the medium before centrifugation (data not shown). BCBL-1 cells cultured in virion-depleted conditional medium showed less effective induction of ORF26 mRNA expression compared to that of cells cultured in untreated medium (Figure 2, B and C). These results suggest that HHV-6 virions were the likely major factor for the ORF26 mRNA induction by the conditional medium without excluding that other soluble factors may also play a minor role.
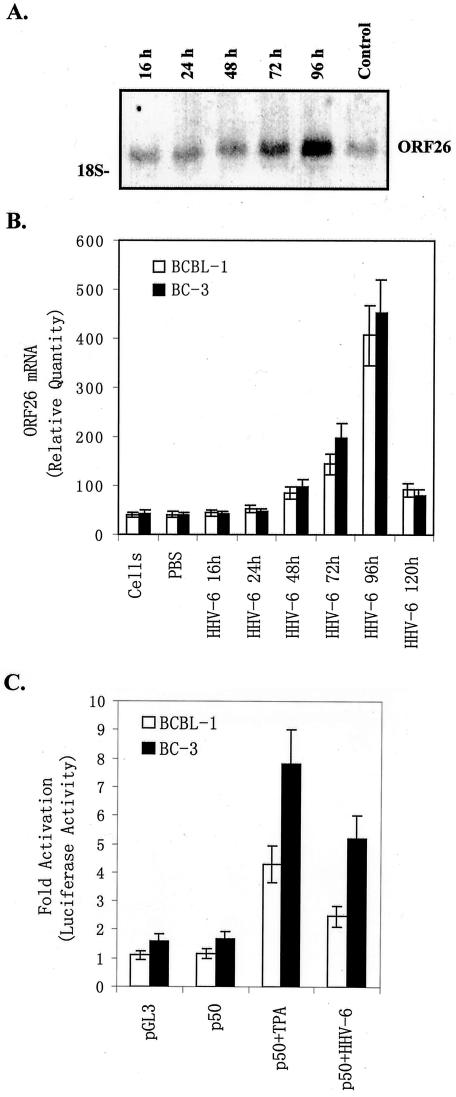
To determine whether the infectious virions of HHV-6 play a major role in induction of KSHV lytic replication, the BCBL-1 cells were directly infected by the HHV-6 virion pelleted from HHV-6-infected JJhan cells supernatants. Analysis of ORF26 mRNA expression demonstrated that ORF26 mRNA increased in BCBL-1 cells with time after infection with HHV-6 virions. Analysis of data from three independent experiments demonstrated that, on average, ORF26 expression increased 1.2 ± 0.29-fold at 16 hours, 1.5 ± 0.3-fold at 24 hours, 1.9 ± 0.32-fold at 48 hours, 3.5 ± 0.51-fold at 72 hours, and 10.6 ± 1.59-fold at 96 hours, respectively, compared to untreated BCBL-1 cells as a control (Figure 3A). ORF26 expression, however, started to decrease at 120 hours, and gradually reached almost the same level as that in untreated BCBL-1 cells at 7 days (data not shown). These data suggest HHV-6 infection alone is insufficient to induce KSHV lytic replication.
Figure 3.
Infection of BCBL-1 cells with HHV-6 induces lytic cycle replication of KSHV. A: ORF26 mRNA expressed in BCBL-1 cells infected with pelleted HHV-6. ORF26 mRNA expression in BCBL-1 cells infected with pelleted HHV-6 for 16, 24, 48, 72, and 96 hours (16 hours, 24 hours, 48 hours, 72 hours, 96 hours), and uninfected BCBL-1 (control) cells was detected by Northern blot. A representative experiment is shown; at least three independent experiments were performed with similar results. B: Real-time quantitative PCR for ORF26 mRNA in BCBL-1 and BC-3 cells after infection with pelleted HHV-6. ORF26 mRNA expression in BCBL-1 (white bar) and BC-3 (black bar) cells infected with pelleted HHV-6 for 16, 24, 48, 72, 96, and 120 hours was quantitated by real-time quantitative PCR. Results shown were the statistic of three independent experiments performed in triplicate. C: Infection of HHV-6 promotes induction of KSHV ORF50 promoter activity. BCBL-1 (white bar) and BC-3 (black bar) cells were transfected with pGL-3 basic control or p50 plasmids. p50-transfected cells were either untreated (p50), treated with TPA (p50 + TPA), or infected with HHV-6 (p50 + HHV-6). Luciferase activities were measured as induction (n-fold). Results from a representative experiment performed in triplicate are shown; five independent experiments were performed with similar results.
To more quantitatively measure the level of ORF26 expression, real-time quantitative PCR was performed. In this experiment, the amount of PCR product was measured at each cycle using a fluorescently labeled probe. The results were expressed as Ct values, which corresponded to the cycle at which the fluorescence crosses a particular threshold. The Ct value of the PCR product of interest (KSHV ORF26 in these experiments) and a control mRNA (β-actin) were then used to calculate relative quantities of mRNA between samples. It was found that on average ORF26 expression in BCBL-1 cells infected with pelleted HHV-6 virions was increased 1.07 ± 0.12-fold at 16 hours, 1.26 ± 0.13-fold at 24 hours, 2.04 ± 0.28-fold at 48 hours, 3.5 ± 0.46-fold at 72 hours, 9.9 ± 0.95-fold at 96 hours, and 2.23 ± 0.30-fold at 120 hours compared to BCBL-1 cells treated with PBS (Figure 3B). To determine whether HHV-6-induced KSHV lytic replication is cell type-specific, another KSHV latently infected cell line, BC-3, was used. After HHV-6 infection BC-3 showed similar ORF26 mRNA expression pattern to that of BCBL-1 (Figure 3B). Quantitation showed that HHV-6 virions were increased 1.1 ± 0.13-fold at 16 hours, 1.2 ± 0.14-fold at 24 hours, 2.49 ± 0.33-fold at 48 hours, 5.1 ± 0.56-fold at 72 hours, 11.58 ± 1.12-fold at 96 hours, and 2.08 ± 0.29-fold at 120 hours compared to BC-3 cells treated with PBS. These data suggest that HHV-6-induced KSHV lytic replication is not restricted in BCBL-1 cells and may be a common mechanism that reactivates KSHV in KS.
As a control BCBL-1 cells were also infected with UV-irradiated or heat-inactivated HHV-6 to determine whether inactivated HHV-6 could influence the replication of KSHV. Analysis of ORF26 mRNA expression indicated that both UV-irradiated and heat-inactivated HHV-6 failed to induce KSHV replication (data not shown). To confirm and extend these results, we examined if HHV-6 can enhance KSHV ORF50 promoter activity. KSHV ORF50 encodes a replication and transcription activator homologous to the Epstein-Barr virus Rta, which has been shown to be both necessary and sufficient to activate the KSHV lytic cycle.52,53 In this assay a 1-kb region upstream of the translational start site of ORF50 (KSHV coordinate 71,598) was used to drive luciferase reporter gene expression (p50 construct). Lu, Yuan, and colleagues50 have previously shown that this p50 construct has promoter activity in BCBL-1, JSC1, and 293 cells. Cells transfected with p50 alone showed low baseline levels of luciferase expression (used as a control), which was dramatically enhanced (3.7- and 4.66-fold increase in BCBL-1 and BC-3 cells, respectively) by stimulation with TPA (Figure 3C). Infection of the cells with HHV-6 resulted in a statistically significant increase in luciferase expression (2.15- and 3.1-fold increase in BCBL-1 and BC-3 cells, respectively, compared to the corresponding control, P < 0.05; Figure 3C). Together these data suggest that infection of BCBL-1 cells with HHV-6 is responsible for the induction of ORF26 mRNA and the induction may be partially mediated by ORF50.
Activation of KSHV Transcription by HHV-6 in Heterokaryons
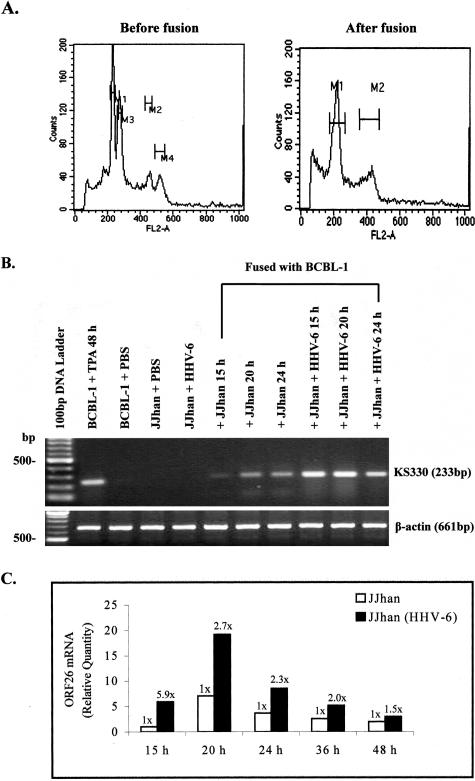
To examine whether HHV-6 can activate replication of the latent KSHV in the same cell, we generated heterokaryons between JJhan and BCBL-1 cells, which are latently infected with HHV-6 and KSHV, respectively. First, DNA staining with propidium iodide solution and subsequent flow cytometric analysis was performed to calculate the number of successfully fused cells. Of the fused cells, 9.23% exhibited DNA contents of tetraploid cells in cell-cell fusion mixture 20 hours after fusion (Figure 4A).
Figure 4.
Expression of ORF26 mRNA in BCBL-1 cells fused with HHV-6-infected JJhan cells. A: Detection of fused cells in the heterokaryon assay by flow cytometry. BCBL-1 cells mixed with HHV-6-infected JJhan cells in the equal number (before fusion) and BCBL-1 cells 20 hours after fusing with HHV-6-infected JJhan cells (after fusion) were fixed, stained with propidium iodide, and analyzed for DNA contents of tetraploid cells (M2 and M4 before fusion, M2 after fusion) by flow cytometry. B: RT-PCR analysis of ORF26 mRNA expression in heterokaryons. ORF26 mRNA expression in BCBL-1 cells fusing with JJhan cells for 15, 20, 24 hours (+JJhan 15 hours, 20 hours, 24 hours) or fusing with HHV-6-infected JJhan cells for 15, 20, 24 hours (+JJhan + HHV-6 15 hours, 20 hours, 24 hours) was detected by RT-PCR. As controls, ORF26 mRNA expression in TPA-treated BCBL-1 (BCBL-1 + TPA 48 hours, positive control) cells, PBS-treated BCBL-1 cells (BCBL-1 + PBS), PBS-treated JJhan cells (JJhan + PBS), or HHV-6-infected JJhan cells (JJhan + HHV-6) were also detected. C: Real-time quantitative PCR analysis for ORF26 mRNA in BCBL-1 cells fused for 15 to 48 hours with HHV-6-infected JJhan cells. ORF26 mRNA expression in BCBL-1 fusing with JJhan cells (white bar, JJhan) or BCBL-1 fusing with HHV-6-infected JJhan cells [black bar, JJhan(HHV-6)] for 15 to 48 hours was quantitated by real-time quantitative PCR. Relative quantities of ORF26 expression are represented on the y axis. *, P < 0.05. Results were the statistic of four independent experiments performed in triplicate.
RT-PCR analysis for ORF26 transcript in the fused cells demonstrated that expression of ORF26 mRNA could be detected 15 hours after fusion, and it was enhanced throughout time (Figure 4B). To more quantitatively measure the level of ORF26 expression, real-time quantitative PCR was performed. We found that on average ORF26 expression in heterokaryons increased 5.9 ± 0.55-fold at 15 hours, 2.7 ± 0.39-fold at 20 hours, 2.3 ± 0.31-fold at 24 hours, 2.0 ± 0.29-fold at 36 hours, and 1.5 ± 0.16-fold at 48 hours compared to the heterokaryons between BCBL-1 cells and uninfected JJhan cells (Figure 4C). These results suggest that HHV-6 can activate the replication of latent KSHV in heterokaryons.
Induction of KSHV Lytic Cycle RNA Also Results in Induction of Lytic Cycle Protein
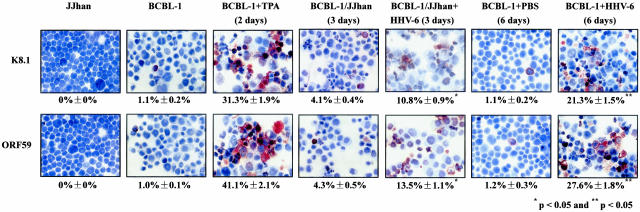
To determine whether induction of KSHV lytic cycle RNA by HHV-6 also resulted in induction of lytic cycle proteins, immunostaining of BCBL-1 cells was performed to detect two KSHV lytic cycle proteins (K8.1 and ORF59). After 3 days of co-culture with JJhan cells infected with HHV-6 (JJhan + HHV-6), 10.8 ± 0.9% of BCBL-1 cells expressed ORF K8.1 compared to 4.1 ± 0.4% of BCBL-1 cells cultured with JJhan cells and 1.1 ± 0.2% of untreated BCBL-1 cells (P < 0.05; Figure 5, the second, fourth, and fifth panel of top row). Similarly, 13.5 ± 1.1% of BCBL-1 cells expressed ORF59 compared to 4.3 ± 0.5% of BCBL-1 cells cultured with JJhan cells and 1.0 ± 0.1% of untreated BCBL-1 cells (P < 0.05; Figure 5, the second, fourth, and fifth panel of bottom row). Furthermore, after 6 days of infection with HHV-6, 21.3 ± 1.5% of BCBL-1 cells expressed ORF K8.1 compared to 1.1 ± 0.2% of BCBL-1 cells cultured with PBS buffer (P < 0.05; Figure 5, the sixth and seventh panel of top row). Similarly, 27.6 ± 1.8% of BCBL-1 cells expressed ORF59 compared to 1.2 ± 0.3% of BCBL-1 cells cultured with PBS buffer (P < 0.05; Figure 5, the sixth and seventh panel of bottom row). This was consistent with the previous report that the expression of ORF59 occurs earlier and more frequently in the lytic cycle, compared to the expression of ORF K8.1.54 As positive controls, it was found that 31.3 ± 1.9% and 41.1 ± 2.1% of BCBL-1 cells expressed ORF K8.1 and ORF59, respectively, after treatment with TPA (Figure 5, the third panel). These results indicate that the induction of KSHV lytic cycle RNA by HHV-6 also results in the induction of lytic cycle proteins.
Figure 5.
Immunohistochemisty staining of BCBL-1 cells co-cultured with HHV-6-infected JJhan cells and infected with pelleted HHV-6. KSHV lytic proteins ORF K8.1 (top) and ORF59 (bottom) expression in JJhan cells, BCBL-1 cells, TPA-treated BCBL-1 cells (BCBL-1 + TPA), PBS-treated BCBL-1 cells (BCBL-1 + PBS), BCBL-1 cell co-cultured with JJhan cells (BCBL-1/JJhan), BCBL-1 cell co-cultured with HHV-6-infected JJhan cells (BCBL-1/JJhan + HHV-6), or HHV-6 infected BCBL-1 cells (BCBL-1 + HHV-6) were detected by immunohistochemistry with ORF K8.1 and ORF59 monoclonal antibodies. * and **, Statistically significant increases in ORF K8.1 and ORF59 expression compared to that of BCBL-1 cells co-cultured with normal JJhan cells for 3 days and cultured in PBS buffer for 6 days, respectively. Original magnifications, ×60.
Th-1 Cytokine, IFN-γ, Partially Contributes to Induction of KSHV Replication
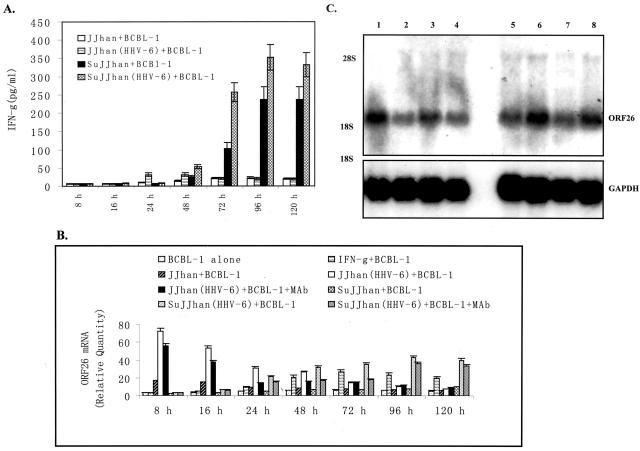
Because there was still a slight increase of ORF26 mRNA in BCBL-1 cells cultured in virus-depleted conditioned medium compared to that of untreated medium at 24 hours (Figure 2, B and C), we reasoned that, besides HHV-6 virions, soluble factors produced by or in response to HHV-6-infected cells, in part, induced KSHV lytic replication. Cytokines released in the co-culture system were the most likely candidates to mediate this response. To identify the cytokines involved in this process and to determine the level of their expression, commercially available ELISA assay for IFN-γ, a representative of Th-1 cytokines, and IL-10, a representative of Th-2 cytokines, were used, respectively. We found that IFN-γ was readily detected in BCBL-1 cells, particularly, after co-culture with JJhan + HHV-6 (threefold increase at 24 hours and 2.1-fold increase at 48 hours compared to that in co-culture with normal JJhan cells) (Figure 6A). Similarly, IFN-γ was also detectable in BCBL-1 cells cultured in the conditional medium from HHV-6-infected JJhan cells. Its expression was increased 2.1-fold at 48 hours and rose to a 2.5-fold increase at 72 hours compared to that of BCBL-1 cells cultured in supernatants from uninfected JJhan cells (Figure 6A).
Figure 6.
IFN-γ partially contributes to induction of KSHV replication. A: Expression of IFN-γ in BCBL-1 cells co-cultured for 8 to 120 hours with HHV-6-infected JJhan cells and cultured in 50% conditioned medium. Supernatants from BCBL-1 cells co-cultured with JJhan (JJhan + BCBL-1), HHV-6-infected JJhan [JJhan(HHV-6) + BCBL-1] or BCBL-1 cells cultured in conditioned medium from JJhan cell culture (SuJJhan + BCBL-1) and HHV-6-infected JJhan cell culture [SuJJhan(HHV-6) + BCBL-1] for various times indicated were collected for detection of IFN-γ by ELISA. Results were the statistic of three independent experiments with duplicate. B: Real-time quantitative PCR analysis for ORF26 mRNA expression in blocking assay by mAb against IFN-γ. Real-time quantitative PCR was used to detected relative quantities of ORF26 mRNA in normal BCBL-1 cells (BCBL-1 alone), IFN-γ-treated BCBL-1 cells (IFN-γ + BCBL-1), BCBL-1 cells co-cultured with JJhan cell (JJhan + BCBL-1), HHV-6-infected JJhan cells [JJhan(HHV-6) + BCBL-1], or HHV-6-infected JJhan cells plus mAb treatment [JJhan(HHV-6) + BCBL-1 + mAb] for various times indicated. The ORF26 mRNA expression in BCBL-1 cells cultured in conditioned medium from JJhan cell (SuJJhan + BCBL-1), HHV-6-infected JJhan cells [SuJJhan(HHV-6) + BCBL-1], or HHV-6-infected JJhan cells plus mAb treatment [SuJJhan(HHV-6) + BCBL-1 + mAb] for various times indicated was also detected by real-time quantitative PCR. The statistic of three independent experiments performed in triplicate was shown. C: Northern blot analysis for ORF26 mRNA expression in blocking assay by mAb against IFN-γ. Northern blot analysis was used to detect ORF26 mRNA in BCBL-1 cells co-cultured with HHV-6-infected JJhan cells at 24 and 48 hours or cultured in conditioned medium at 48 and 72 hours before or after treatment with mAb against IFN-γ, respectively. Lane 1 (from left), BCBL-1 co-cultured with HHV-6-infected JJhan cells (BCBL-1 + JJhan + HHV-6), 24 hours; lane 2, BCBL-1 co-cultured with HHV-6-infected JJhan cells with addition of mAb against IFN-γ (BCBL-1 + JJhan + HHV-6 + mAb), 24 hours; lane 3, BCBL-1 + JJhan + HHV-6, 48 hours; lane 4, BCBL-1 + JJhan + HHV-6 + mAb, 48 hours; lane 5, BCBL-1 cells cultured in conditioned medium from HHV-6-infected JJhan cells with addition of mAb against IFN-γ (BCBL-1 + C.M. + mAb), 72 hours; lane 6, BCBL-1 + C.M., 72 hours; lane 7, BCBL-1 + C.M. + mAb, 48 hours; lane 8, BCBL-1 + C.M., 48 hours. A representative experiment is shown; three independent experiments were run and gave similar results.
Because IFN-γ was detected at the protein level in pg/ml concentrations, experiments were designed to determine whether recombinant cytokines could also induce KSHV lytic replication. BCBL-1 cells were stimulated with recombinant IFN-γ (R&D Systems, Minneapolis, MN) at the final concentration of 50 to 1000 U/ml and KSHV replication was examined. Using real-time quantitative PCR, studies demonstrated that ORF26 mRNA in BCBL-1 cells stimulated with IFN-γ was increased 1.1-fold at 8 hours, 1.3-fold at 16 hours, 1.9-fold at 24 hours, 3.6-fold at 48 hours, 4.3-fold at 72 hours, 4.1-fold at 96 hours, and 3.8-fold at 120 hours, respectively, compared to untreated BCBL-1 cells as a control (Figure 6B), which is consistent with the previous report.21
To test our hypothesis that IFN-γ overexpression may, at least in part, modulate KSHV lytic replication by HHV-6, mAb against IFN-γ blocking assay was performed. As shown in Figure 6B, after addition of mAb to the co-culture of BCBL-1 cells with HHV-6-infected JJhan cells, the expression of ORF26 mRNA markedly decreased. In particular, the expression of ORF26 mRNA decreased 2.2-fold at 24 hours and 1.74-fold at 48 hours compared to the groups without mAb treatment. Similarly, after the addition of mAb to the BCBL-1 cells cultured in conditioned medium from HHV-6-infected JJhan cells, the expression of ORF26 mRNA decreased 1.91-fold at 48 hours and twofold at 72 hours compared to the groups without mAb treatment (Figure 6B). The expression of ORF26 mRNA was not thoroughly blocked by mAb against IFN-γ compared to BCBL-1 cells co-cultured with normal JJhan cells and cultured in the supernatant from normal JJhan cells (Figure 6B). To more visually evaluate the level of ORF26 expression, Northern blot analysis for ORF26 mRNA was performed. As shown in Figure 6C, after the addition of mAb to the co-culture system, the expression of ORF26 mRNA was really inhibited both in co-culture and conditioned medium culture (Figure 6C). These results indicate that IFN-γ partially plays a role in modulating KSHV lytic replication by HHV-6 infection. ELISA assays were also performed for IL-10. Expression of IL-10 in BCBL-1 cells cultured in the conditioned medium and co-cultured with HHV-6-infected JJhan cells were only slightly higher than controls, however, they were not statistically different between the groups and throughout time (data not shown).
Discussion
Co-factors that enhance the lytic replication of KSHV may play an important role in the pathogenesis of KS. Lytic replication is required for the initial spread of KSHV to target B lymphocytes and monocytes after exposure to KSHV. Additional rounds of productive replication are required for KSHV to infect the endothelial cell progenitors to form the spindle cells that are characteristic components of KS tumor. It is likely that high levels of lytic replication will alter the balance between clearance of the infected cells by the host immune system and infection of new cells by virus, favoring the spread of virus to new tissues. Co-factors that are present in the microenvironment of cells infected with KSHV may thus enhance early viral spread and ultimately enhance pathogenicity. Factors enhancing lytic replication may also play a role in the disease process at later time points. At the time that a tumor is first evident, most spindle cells harbor KSHV in the latent phase replication.16 However, a subpopulation of cells undergoing lytic replication are uniformly present in tumors, and these cells have been postulated to play a role in the maintenance of the tumor. Monocytes in KS tumors have been shown to be productively infected with KSHV;55 these cells may be an important reservoir of virus and may be responsible for the high KSHV viral load that is seen in the late stages of KS. The identification of co-factors that enhance KSHV lytic replication at both early and late time points after infection is thus an important goal in understanding KS pathogenesis.
Early studies have shown that HHV-6 can activate EBV replication and contribute to the progression of EBV-associated disease.34 In our current study, we showed that HHV-6 can also activate KHSV in BCBL-1 cell and may be a co-factor of KS disease. Our results reveal several novel points in understanding KS disease progression.
First, our results provide direct experimental evidence that HHV-6 can be a potential co-factor of KS. Several factors have been previously shown to be co-factors of KS.20–27 For instance, Vieira and colleagues24 showed infection of HCMV increased the production of KSHV in endothelial cells and activated lytic cycle gene expression in keratinocytes, suggesting that HCMV could influence KSHV pathogenesis. Here we have demonstrated that infection of BCBL-1 cells with HHV-6 is a critical factor that is responsible for the induction of KSHV lytic replication, suggesting that HHV-6 may promote KS progression by reactivating KSHV lytic replication.
Second, we provide experimental evidence to suggest that co-infection of HHV-6 may promote KSHV lytic replication in KS progression. The molecular switch that controls the transition from KSHV latency to lytic replication is the product of the ORF50 gene, designated KSHV Rta (replication and transcription activator).52,53,56 KSHV ORF50 is an immediate-early KSHV gene product whose expression is detectable before that of early lytic gene products. Expression of ORF50 in PEL cell lines results in activation of expression of early lytic cycle genes such as the polyadenylated nuclear RNA (PAN), viral interleukin 6 (vIL-6), K8, and ORF59, as well as the activation of late lytic gene.52,53,57 As an important and well known co-factor for the development of KS, HIV-1 activated lytic cycle replication of KSHV through induction of KSHV ORF50 promoter activity.58 Here we provided direct evidence that infection of HHV-6 can also initiate induction of KSHV ORF50 promoter activity. Of course, our results did not eliminate the possibility that other immediate-early KSHV genes and their promoters or soluble factors produced by or in response to HHV-6-infected PEL cell lines may also be involved in this process.
Third, we showed that IFN-γ partially contributes to induction of KSHV replication by infection of HHV-6. IFN-γ has long been of particular interest to investigators studying AIDS-KS and KSHV. Previous studies demonstrated that IFN-γ is produced in KS, which is known to promote the growth and proliferation of KS tumor cells in vitro.59–62 In addition, cytokines including IFN-γ have been show to induce normal endothelial cells, the likely precursor cell of the KS tumor cell, to acquire the characteristics typical of KS tumor cells, including spindle-shaped morphology, production of angiogenic factors, and expression of activation markers. Recent studies by Mercader and colleagues21 have indicated that co-culture of HIV-1-infected T cells with KSHV latently infected PEL cell lines induced KSHV reactivation. This induction was the result of soluble factors released by the co-cultured cells, and several cytokines, including IFN-γ, oncostatin M (OSM), and hepatocyte growth factor/scatter factor (HGF/SF).21 In the current study, we demonstrated that IFN-γ produced by the co-culture of BCBL-1 cells with HHV-6-infected T cells is able to play a role in the activation of KSHV lytic replication. These results add additional support to the hypothesis that cytokines play a critical role in the initiation and development of KS by activating KSHV, promoting production of angiogenesis factors, and by providing the necessary growth factors for the tumor cells.63 Whether other factors, such as HHV-6-related proteins or additional cytokines may also be involved in this process is still unknown.
Because the majority of the population is seropositive for HHV-6, reactivation of HHV-6 in immunocompromised patients, such as KS, and AIDS-KS, may further accentuate KSHV replication and thereby contribute to KS and AIDS-KS progression. In addition, HIV-1 Tat, which has also long been of particular interest to investigators studying AIDS-KS pathogenesis has been implied to be able to induce KSHV replication.44,64 On the molecular level, HHV-6 gene products and HHV-6-induced cellular factors can trans-activate the HIV-1 LTR leading to HIV-1 replication.65 On the other hand, HIV-1 Tat can also enhance replication of HHV-6.66 Therefore, further studies are needed to better understand how interactions among three viruses may contribute to the progression of the AIDS-KS disease.
Acknowledgments
We thank Dr. Yan Yuan for plasmid and Dr. Lijun Rong and Ms. Katharina Rothwangl for critical reading of the manuscript.
Footnotes
Address reprint requests to Chun Lu, Ph.D., Department of Microbiology and Immunology, Nanjing Medical University, Nanjing 210029, P. R. China. E-mail: clu@njmu.edu.cn.
Supported by the National Natural Science Foundation of China (grants 30100160 and 30271179 to C.L.).
C.L. and Y.Z. contributed equally to this work.
References
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, Knowles DM, Garber R, Pellett PE, McGeoch DJ, Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcoma. J Virol. 1996;70:549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H, Sata T. Human herpesvirus 8: virology, epidemiology and related diseases. Jpn J Infect Dis. 2000;53:137–155. [PubMed] [Google Scholar]
- Schulz TF. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8). J Gen Virol. 1998;79:1573–1591. doi: 10.1099/0022-1317-79-7-1573. [DOI] [PubMed] [Google Scholar]
- Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Whitby D, Hatziioannou T, Fisher C, van der Walt J, Hatzakis A, Weiss R, Schulz T. Kaposi’s-sarcoma-associated herpesvirus in HIV-negative Kaposi’s sarcoma. Lancet. 1995;345:1043–1044. doi: 10.1016/s0140-6736(95)90780-7. [DOI] [PubMed] [Google Scholar]
- Ambroziak JA, Blackbourn DJ, Herndier BG, Glogau RG, Gullett JH, McDonald AR, Lennette ET, Levy JA. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. doi: 10.1126/science.7725108. [DOI] [PubMed] [Google Scholar]
- Sitas F, Carrara H, Beral V, Newton R, Reeves G, Bull , Jentsch U, Pacella-Norman R, Bourboulia D, Whitby D, Boshoff C, Weiss R. Antibodies against human herpesvirus 8 in black South African patients with cancer. N Engl J Med. 1999;340:1863–1871. doi: 10.1056/NEJM199906173402403. [DOI] [PubMed] [Google Scholar]
- Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- Gao SJ, Kingsley L, Hoover DR, Spira TJ, Rinaldo CR, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore PS. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi’s sarcoma patients. Lancet. 1996;348:858–861. doi: 10.1016/S0140-6736(96)03240-0. [DOI] [PubMed] [Google Scholar]
- Staskus KA, Zhong WD, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson DJ, Ganem D, Hasse AT. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne R, Zhong WD, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- Renne R, Lagunoff M, Zhong W, Ganem D. The size and conformation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J Virol. 1996;70:8151–8154. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker LL, Shankar P, Khan G, Freeman RB, Dezube BJ, Lieberman J, Thorley-Lawson DA. The Kaposi sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med. 1996;184:283–288. doi: 10.1084/jem.184.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzl M, Blasig C, Schreier A, Neipel F, Hohenadl C, Cornali E, Ascherl G, Esser S, Brockmeyer NH, Ekman M, Kaaya EE, Tschachler E, Biberfeld P. Expression of HHV-8 latency-associated T0.7 RNA in spindle cells and endothelial cells of AIDS-associated, classical and African Kaposi’s sarcoma. Int J Cancer. 1997;72:68–71. doi: 10.1002/(sici)1097-0215(19970703)72:1<68::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Friedman-Kien AE, Laubenstein LJ, Rubinstein P, Buimovici-Klein E, Marmor M, Stahl R, Spigland I, Kim KS, Zolla-Pazner S. Disseminated Kaposi’s sarcoma in homosexual men. Ann Intern Med. 1982;96:693–700. doi: 10.7326/0003-4819-96-6-693. [DOI] [PubMed] [Google Scholar]
- Buchbinder A, Friedman-Kien AE. Clinical aspects of Kaposi’s sarcoma. Curr Opin Oncol. 1992;4:867–874. doi: 10.1097/00001622-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Veugelers PJ, Moore PS. The epidemiology of HIV-associated Kaposi’s sarcoma: the unraveling mystery. AIDS. 1996;10(Suppl A):S51–S57. [PubMed] [Google Scholar]
- Delli BP, Donti E, Knowles DM, Friedman-Kien A, Luciw PA, Dina D, Dalla-Favera R, Basilico C. Presence of chromosomal abnormalities and lack of AIDS retrovirus DNA sequences in AIDS-associated Kaposi’s sarcoma. Cancer Res. 1986;46:6333–6338. [PubMed] [Google Scholar]
- Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000;156:1961–1971. doi: 10.1016/S0002-9440(10)65069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyoshi K, Schim van der Loeff M, Cook P, Whitby D, Corrah T, Jaffar S, Cham F, Sabally S, O’Donovan D, Weiss RA, Schulz TF, Whittle H. Kaposi’s sarcoma in the Gambia, West Africa is less frequent in human immunodeficiency virus type 2 than in human immunodeficiency virus type 1 infection despite a high prevalence of human herpesvirus 8. J Hum Virol. 1998;1:193–199. [PubMed] [Google Scholar]
- Giraldo G, Beth E, Huang ES. Kaposi’s sarcoma and its relationship to cytomegalovirus (CMNV). III. CMV DNA and CMV early antigens in Kaposi’s sarcoma. Int J Cancer. 1980;26:23–29. doi: 10.1002/ijc.2910260105. [DOI] [PubMed] [Google Scholar]
- Vieira J, O’hearn P, Kimball L, Chandran B, Corey L. Activation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J Virol. 2001;75:1378–1386. doi: 10.1128/JVI.75.3.1378-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf W, Adams V. Viruses in the pathogenesis of Kaposi’s sarcoma—a review. Biochem Mol Med. 1996;58:1–12. doi: 10.1006/bmme.1996.0025. [DOI] [PubMed] [Google Scholar]
- Adams V, Kempf W, Hassam S, Briner J, Schmid M, Moos R, Pfaltz M. Detection of several types of human papilloma viruses in AIDS-associated Kaposi’s sarcoma. J Med Virol. 1995;46:189–193. doi: 10.1002/jmv.1890460304. [DOI] [PubMed] [Google Scholar]
- Monini P, Rotola A, de Lellis L, Corallini A, Secchiero P, Albini A, Benelli R, Parravicini C, Barbanti Brodano G, Cassai E. Latent BK virus infection and Kaposi’s sarcoma pathogenesis. Int J Cancer. 1996;66:717–722. doi: 10.1002/(SICI)1097-0215(19960611)66:6<717::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Carrigan DR, Asano Y, Benedetti L, Crowley RW, Komaroff AL, Gallo R, Lusso P. Detection of human herpesvirus 6 in plasma of children with primary infection and immunosuppressed patients by polymerase chain reaction. J Infect Dis. 1995;171:273–280. doi: 10.1093/infdis/171.2.273. [DOI] [PubMed] [Google Scholar]
- Bovenzi P, Mirandola P, Secchiero P, Strumia R, Cassai E, Di Luca D. Human herpesvirus 6 (variant A) in Kaposi’s sarcoma. Lancet. 1993;341:1288–1289. doi: 10.1016/0140-6736(93)91198-u. [DOI] [PubMed] [Google Scholar]
- Di Luca D, Dolcetti R, Mirandola P, De Re V, Secchiero P, Carbone A, Boiocchi M, Cassai E. Human herpesvirus 6: a survey of presence and variant distribution in normal peripheral lymphocytes and lymphoproliferative disorders. J Infect Dis. 1994;170:211–215. doi: 10.1093/infdis/170.1.211. [DOI] [PubMed] [Google Scholar]
- Marchette NJ, Melish ME, Hicks R, Kihara S, Sam E, Ching D. Epstein-Barr virus and other herpesvirus infections in Kawasaki syndrome. J Infect Dis. 1990;161:680–684. doi: 10.1093/infdis/161.4.680. [DOI] [PubMed] [Google Scholar]
- Kempf W, Adams V, Hassam S, Schmid M, Moos R, Briner J, Pfaltz M. Detection of human herpesvirus type 6, human herpesvirus type 7, cytomegalovirus and human papillomavirus in cutaneous AIDS-associated Kaposi’s sarcoma. Verh Dtsch Ges Pathol. 1994;78:260–264. [PubMed] [Google Scholar]
- Kempf W, Adams V, Pfaltz M, Briner J, Schmid M, Moos R, Hassam S. Human herpesvirus type 6 and cytomegalovirus in AIDS-associated Kaposi’s sarcoma: no evidence for an etiological association. Hum Pathol. 1995;26:914–919. doi: 10.1016/0046-8177(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Flamand L, Stefanescu I, Ablashi DV, Menezes J. Activation of the Epstein-Barr virus replicative cycle by human herpesvirus 6. J Virol. 1993;67:6768–6777. doi: 10.1128/jvi.67.11.6768-6777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, O’hearn P, Kimball L, Chandran B, Corey L. Activation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J Virol. 2001;75:1378–1386. doi: 10.1128/JVI.75.3.1378-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder RS, Briggs M, Cameron CH, Honess R, Robertson D, Whittle H. A novel lymphotropic herpesvirus. Lancet. 1987;2:390–392. doi: 10.1016/s0140-6736(87)92404-4. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- Downing RG, Sewankambo N, Serwadda D, Honess R, Crawford D, Jarrett R, Griffin BE. Isolation of human lymphotropic herpesviruses from Uganda. Lancet. 1987;2:390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Wyatt LS, Yamanishi K, Rodriguez WJ, Frenkel N. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc Natl Acad Sci USA. 1991;88:5922–5926. doi: 10.1073/pnas.88.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin DL, Schmidt GW. Rapid, reversible staining of Northern blots prior to hybridization. Biotechniques. 1988;6:196–200. [PubMed] [Google Scholar]
- Lu C, Gordon GM, Chandran B, Nickoloff BJ, Foreman KE. Human herpesvirus 8 reactivation and human immunodeficiency virus type 1 gp120. Arch Pathol Lab Med. 2002;126:941–946. doi: 10.5858/2002-126-0941-HHRAHI. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Friborg J, Kong W, Woffendin C, Polverini PJ, Nickoloff BJ, Nabel GJ. Propagation of a human herpesvirus from AIDS-associated Kaposi’s sarcoma. N Engl J Med. 1997;336:163–171. doi: 10.1056/NEJM199701163360302. [DOI] [PubMed] [Google Scholar]
- Lu F, Zhou J, Wiedmer A, Madden K, Yuan Y, Lieberman PM. Chromatin remodeling of the Kaposi’s sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J Virol. 2003;77:11425–11435. doi: 10.1128/JVI.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LM, Chao MF, Chen MY, Shih HM, Chiang YP, Chuang CY, Lee CY. Reciprocal regulatory interaction between human herpesvirus 8 and human immunodeficiency virus type 1. J Biol Chem. 2001;276:13427–13432. doi: 10.1074/jbc.M011314200. [DOI] [PubMed] [Google Scholar]
- Wunschmann S, Stapleton JT. Fluorescence-based quantitative methods for detecting human immunodeficiency virus type 1-induced syncytia. J Clin Microbiol. 2000;38:3055–3060. doi: 10.1128/jcm.38.8.3055-3060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman KE, Alkan S, Krueger AE, Panella JR, Swinnen LJ, Nickoloff BJ. Geographically distinct HHV-8 DNA sequences in Saudi Arabian iatrogenic Kaposi’s sarcoma lesions. Am J Pathol. 1998;153:1001–1004. doi: 10.1016/S0002-9440(10)65642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipping E, Krammer PH, Onel KB, Lehman TJ, Mysler E, Elkon KB. Levels of soluble Fas/APO-1/CD95 in systemic lupus erythematosus and juvenile rheumatoid arthritis. Arthritis Rheum. 1995;38:1735–1737. doi: 10.1002/art.1780381205. [DOI] [PubMed] [Google Scholar]
- Chandran B, Bloomer C, Chan SR, Zhu L, Goldstein E, Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140–149. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- Chan SR, Bloomer C, Chandran B. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF59 protein and the encoding cDNA by monoclonal antibody. Virology. 1998;240:118–126. doi: 10.1006/viro.1997.8911. [DOI] [PubMed] [Google Scholar]
- Zhu L, Puri V, Chandran B. Characterization of human herpesvirus-8 K8.1 A/B glycoproteins by monoclonal antibodies. Virology. 1999;262:237–249. doi: 10.1006/viro.1999.9900. [DOI] [PubMed] [Google Scholar]
- Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Renne R, Kirshner JR, Ganem D. Reactivation of Kaposi’s sarcoma-associated herpesvirus infection from latency by expression of the ORF50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- Zoeteweij JP, Eyes ST, Orenstein JM, Kawamura T, Wu L, Chandran B, Forghani B, Blauvelt A. Identification and rapid quantification of early and late lytic human herpesvirus 8 infection in single cells by flow cytometric analysis: characterization of antiherpesvirus agents. J Virol. 1999;73:5894–5902. doi: 10.1128/jvi.73.7.5894-5902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer NH, Tschachler E, Colombini S, Ensoli B, Sturzl M. Monocytes in Kaposi’s sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradoville L, Gerlach J, Grogan E, Shedd D, Nikiforow S, Metroka C, Miller G. Kaposi’s sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J Virol. 2000;74:6207–6212. doi: 10.1128/jvi.74.13.6207-6212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, Moore PS, Chang Y. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus infected diseases: Kaposi’s sarcoma, primary effusion lymphoma and multicentric Castleman’s disease. Am J Pathol. 2000;156:743–749. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varthakavi V, Smith RM, Deng H, Sun R, Spearman P. Human immunodeficiency virus type-1 activates lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus through induction of KSHV Rta. Virology. 2002;297:270–280. doi: 10.1006/viro.2002.1434. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Buonaguro L, Gallo RC. Molecular mechanisms in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Adv Exp Med Biol. 1991;303:27–38. doi: 10.1007/978-1-4684-6000-1_4. [DOI] [PubMed] [Google Scholar]
- Fiorelli V, Gendelman R, Samaniego F, Markham PD, Ensoli B. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi’s sarcoma spindle cells. J Clin Invest. 1995;95:1723–1734. doi: 10.1172/JCI117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Nakamura S, Salahuddin SZ, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo RC. AIDS-Kaposi’s sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989;243:223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Gallo RC. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Immunol Rev. 1992;127:147–155. doi: 10.1111/j.1600-065x.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Cafaro A. HIV-1 and Kaposi’s sarcoma. Eur J Cancer Prev. 1996;5:410–412. [PubMed] [Google Scholar]
- Harrington W, Sieczkowski L, Sosa C, Chan-a-Sue S, Cai JP, Cabrao L, Wood C. Activation of HHV-8 by HIV-1 tat. Lancet. 1997;349:774–775. doi: 10.1016/s0140-6736(05)60199-7. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Chang CK, Qian G, Chandran B, Wood C. trans-Activation of the HIV promoter by a cDNA and its genomic clones of human herpesvirus 6. Virology. 1994;199:311–322. doi: 10.1006/viro.1994.1129. [DOI] [PubMed] [Google Scholar]
- Sieczkowski L, Chandran B, Wood C. The human immunodeficiency virus tat gene enhances replication of human herpesvirus-6. Virology. 1995;211:544–553. doi: 10.1006/viro.1995.1436. [DOI] [PubMed] [Google Scholar]