Abstract
We have previously shown that, in glioma C6 cells, two nucleotide ADP-sensitive receptors coexist: P2Y1, coupled to PLC and responsible for Ca2+ release, and P2Y12, negatively coupled to adenylate cyclase. In the present study, we examined the effects of the stimulation of these two receptors on ERK1/2 and PI3-K activation, and cell proliferation in either serum-deprived or nonstarved C6 cells.
In response to ADP and its analogues, in serum-starved cells, both p44 ERK1 and p42 ERK2 were activated in a time-dependent manner, as monitored by Western blot analysis using an antiphospho-p42/p44 MAPK antibody. The phosphorylation was reduced both by removal of the extracellular Ca2+ and partially or almost completely by MRS2179 or AR-C69931MX, specific antagonists of the P2Y1 and P2Y12 receptors, respectively. The inhibitory effect of antagonists was additive. These data indicate the involvement of both receptors, P2Y1 and P2Y12, in the ERK1/2 activation, but the P2Y12 receptor contribution predominates.
ERK1/2 activity was positively correlated with cell proliferation of cultured glioma C6 cells.
In nonstarved cells, ADP markedly decreased the PI3-K activity. In contrast, in serum-starved cells, ADP evoked an increase in the PI3-K activity. Blocking of the P2Y1 receptor by MRS2179 additionally increased this ADP response. These results suggest that the P2Y1 receptor has an inhibitory and the P2Y12 receptor a stimulatory effect on PI3-K signalling pathway.
RT–PCR analysis revealed different mRNA expression of both receptors in starved and nonstarved cells. In nonstarved cells, the P2Y1 receptor mRNA predominates, whereas in serum-deprived cells the expression of P2Y12 mRNA becomes more pronounced.
Keywords: P2Y1 and P2Y12 receptors, ADP, ERK1/ERK2, PI3-K, C6 cells proliferation
Introduction
Nucleotide receptors (previously termed ‘purinergic') have been classified as P1 and P2. P1 receptors respond to adenosine, whereas P2 respond to adenine and uridine nucleotides. P2 receptors belong to two families, the intrinsic ion channel P2X receptors, and the seven transmembrane domain, G protein-coupled, P2Y receptors (Burnstock, 1978; Abbracchio & Burnstock, 1994; Communi & Boeynaems, 1997; Boeynaems et al., 2000). Within the family of P2Y, P2Y1 responds selectively to ADP and is partially antagonized by ATP. 2-Methylthio-ADP (2MeSADP), 2-methylthio-ATP (2MeSATP) and 2-chloro-ATP (2ClATP) are also selective agonists of high potency for this receptor, while UTP is not effective. In contrast, P2Y2 receptor responds to ATP and UTP, while 2MeSADP has no effect on its activation. Both cloned types of receptors activate phospholipase C (PLC) and stimulate the increase in inositol 1,4,5-trisphosphate (InsP3). The P2Y1 receptor-generated InsP3 accumulation is competitively blocked by MRS2179 and pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), whereas the P2Y2 receptor is insensitive to these compounds (Boyer et al., 1994; Nicholas et al., 1996; Schachter et al., 1996; Webb et al., 1996; Boarder & Hourani, 1998; King et al., 1998).
The P2Y2 receptor stimulation by both ATP and UTP has been documented in a variety of cells (Boarder & Hourani, 1998; King et al., 1998). It has also been reported in glioma C6 cells (Lin & Chuang, 1994; Sabała et al., 1997; 2001; Tu et al., 2000), while the presence of the P2Y1 receptor in this cell line has been a subject of serious debate (Boyer et al., 1993; 1994; Webb et al., 1996; Boarder & Hourani, 1998). Glioma C6 cells are transformed glial cells with a phenotype similar to astrocytes (Brismar, 1995). The studies on glioma C6 and C6-2B cells revealed that stimulation of an ADP-responding nucleotide receptor results in the inhibition of adenylate cyclase. This receptor inhibited adenylate cyclase in response to agonists of the P2Y1 receptor, but not to its antagonists; it was resistant to PPADS and MRS2179 (Schachter et al., 1996; 1997; Boarder & Hourani, 1998; Jacobson et al., 2001). Most recently, this P2Y receptor subtype, previously termed P2Y1-like (glioma), or P2YTAC (platelets), has been cloned in rat blood platelets and designated as P2Y12 (Hollopeter et al., 2001; Savi et al., 2001). The presence of this receptor has also been indicated in glioma C6-2B (Jin et al., 2001) and in glioma C6 cells (Czajkowski et al., 2002).
Recently, Grobben et al. (2001) have shown the effect of P2YAC (P2Y12) receptor on activation of MAP kinase and on proliferation of glioma C6 cells. On the other hand, our previous studies have indicated that, in glioma C6 cells, ADP acts not only through the P2Y12 receptor, negatively coupled to adenylate cyclase, but also through P2Y1, linked to the stimulation of PLC and Ca2+ release (Sabała et al., 2001; Czajkowski & Barańska, 2002; Czajkowski et al., 2002). Thus, in the present study, we suggest an intriguing possibility that the effect of ADP and its analogues on intracellular signalling pathways involving the Ras/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3-K) may depend on both these receptors. ERK1/2 and PI3-K signal transduction pathways are associated with cell proliferation and differentiation (Seger & Krebs, 1995; Ptasznik et al., 1997; Corvera & Czech, 1998). All our previous investigations were performed on C6 cells growing in the medium supplemented with 10% serum (Sabała et al., 2001; Czajkowski et al., 2002), whereas experiments concerning ERK1/2 and PI3-K activation have been usually performed on serum-deprived cells (Tu et al., 2000; Grobben et al., 2001). Therefore, the aim of this study was to determine whether the ADP-sensitive P2Y1 and P2Y12 nucleotide receptors are similarly involved in the regulation of ERK1/2 and PI3-K activity and cell proliferation, and to examine the possible crosstalk between P2Y1- and P2Y12- triggered signalling pathways in either serum-starved or nonstarved C6 cells.
In this study, we demonstrate that the stimulation of P2Y1 and P2Y12 receptors differentially affects the ERK1/2 and PI3-K signalling pathways. Moreover, we show different mRNA expression levels of both receptors in serum-deprived and nonstarved glioma C6 cells.
Methods
Materials
Minimum essential medium (MEM), newborn calf serum (NCS), antibiotics, Ham's F10 medium, MEM vitamins, MEM nonessential amino acids and phosphate-buffered saline (PBS) were from Gibco BRL (Grand Island, NY, U.S.A.) TRI reagent, ADP, ATP, UTP, 2MeSADP, 2ClATP, BSA, EGTA, cell-dissociation solution, ethidium bromide, agarose, 123b DNA ladder and common chemicals were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.) AR-C69931MX was a kind gift from AstraZeneca (Wilmington, DE, U.S.A.). MRS2179 tetraammonium salt was from Tocris. Expand RT enzyme was purchased from Roche. Taq PCR Core Kit was obtained from QIAGEN.
Cell culture
Glioma C6 cells (passages 40–55) were obtained from ATCC and cultured in MEM supplemented with 10% (v v−1) NCS, penicillin (50 IU ml−1), streptomycin (50 μg ml−1) and 2 mM L-glutamine under a humidified atmosphere of 5% CO2 at 37°C. The cells were passaged when confluent, by using nonenzymatic cell dissociation solution, and the medium was changed twice a week. For experiments, cells were cultivated in MEM supplemented with 10% NCS to reach 90% confluence: in 60 mm dishes (for Western blot analysis), in 100 mm dishes (for PI3-K assay), on 24 mm glass coverslips in 35 mm dishes (for calcium measurement), or on 12-well plates (for proliferation assay). In the case of serum-starved cells, the medium was changed to MEM without NCS 48 h before the experiment. At 30 min before the experiment, cells were washed once with PBS and once with the solution containing: 137 mM NaCl, 2.7 mM KCl, 1 mM Na2HPO3, 25 mM glucose, 20 mM HEPES (pH 7.4), 1 mM MgCl2, 1% bovine serum albumin and 2 mM CaCl2 (later referred to as the standard buffer). Only in the experiments performed in the absence of external Ca2+, 500 μM EGTA was added instead of 2 mM CaCl2. All experiments (except [3H]thymidine incorporation) were performed in standard buffer at 37°C. In experiments concerning long-term serum starvation, cells were grown according to Grobben et al. (2001) in a serum-free, chemically defined medium containing Ham's F10/minimal essential medium (MEM, 1 : 1 v v−1), 2 mM L-glutamine, 1% (v v−1), MEM vitamins (100 ×), 1% (v v−1) MEM nonessential amino acids (100 ×), 50 IU ml−1 penicillin, 50 μg ml−1 streptomycin and 30 nM sodium selenite.
Measurement of intracellular calcium
Intracellular Ca2+ concentration ([Ca2+]I) was measured as described previously (Barańska et al., 1995), with the following modifications. Cells on coverslips were washed once with PBS and once with the standard buffer. The cells were incubated at 37°C for 30 min in the standard buffer with 1 μM Fura-2 AM. Thereafter, the coverslips were mounted in a chamber over a Nikon Diaphot inverted-stage microscope equipped with a × 40 oil-immersion fluorescence objective lens. Digital fluorescence microscopy was used to determine the changes in [Ca2+]I. Experiments were carried out on a video imaging system (MagiCal, Applied Imaging Ltd). Data processing and ratio values conversion to [Ca2+]i were carried out using Tardis V8.0 and MS Excel software.
Preparation of cell extracts and Western blot analysis of phospho-ERK1 and ERK2
For experiments cells were plated in 60 mm dishes and made quiescent at confluence by incubating in fresh serum-free MEM for 48 h. Then, the cells were incubated with agonists in the standard buffer at 37°C for various times. When antagonists were used, they were applied 2 min prior to the addition of agonists. The reaction was stopped by aspiration of the medium and addition of sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer (6 mM Tris/HCl, pH 6.8), 0.5% (w v−1) SDS, 10% (v v−1) glycerol, 0.5% (v v−1) 2-mercaptoethanol. After boiling for 2 min, cell lysates were analysed by SDS–PAGE on a 10% (w v−1) polyacrylamide gel. Proteins were blotted onto a nitrocellulose membrane (Hybond-ECL, Amersham Pharmacia Biotech). Immunodetection was performed according to the manufacturer's instructions using rabbit antibodies raised against phosphorylated p44 (ERK1) and p42 (ERK2) (Cell Signalling Technology). Primary antibodies were detected with a horseradish peroxidase conjugated mouse anti-rabbit antibody, and visualized by enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech).
PI3-kinase assay
PI3-K activity was essentially measured as described by Whitman et al. (1985), with the following modifications. Subconfluent serum-starved (48 h) or nonstarved cells grown on 100 mm dishes were incubated with ADP and other compounds in the standard buffer for various intervals at 37°C. Thereafter, cells were washed with ice-cold PBS and the material from two parallel dishes was lysed in 1.4 ml of ice-cold Buffer A (10 mM Tris, pH 7.5, 50 mM NaCl, 50 mM NaF, 5 mM EDTA, 1 mM sodium orthovanadate, 1 mM PMSF, 1% Triton X-100, 10 μg ml−1 of aprotinin and leupeptin, and 1 μg ml−1 of pepstatin A). Cell lysates were kept on ice for 20 min and clarified by centrifugation at 12,000 × g. The resulting supernatants were incubated with a mixture of primary monoclonal antibodies against a regulatory (p85α) subunit of PI3-K (clones 2α and 10α kindly provided by Dr I. Gout from Ludwig Institute for Cancer Research, Royal Free and University College Medical School Branch, London) on a wheel for 2 h at 4°C. Thereafter, protein G-Sepharose was added for another 1 h. The resulting immunoprecipitates containing bound PI3-K were then washed three times in buffer A, once in 50 mM HEPES, pH 7.5, and once in the assay buffer (20 mM Tris HCl pH 7.5, 100 mM NaCl, 0.5 mM EGTA). For the control of the total amount of PI3-K p85 subunit, the immunoprecipitates were suspended in SDS–PAGE sample buffer and subjected to immunoblotting procedure using the same mixture of monoclonal antibodies for detection. For assay PI3-K activity, washed beads were resuspended in 2 × concentrated assay buffer. After 10 min preincubation with the substrate (10 μg of phosphatidylinositol in 5 mM HEPES, pH 7.5, 1 mg ml−1), reactions (50 μl of total volume) were initiated by adding MgCl2/ATP/ATP* (final concentrations: 10 mM MgCl2, 40 μM ATP and 5 μCi of [γ-32P]ATP per reaction). After 15 min at room temperature, reactions were stopped by addition of 100 μl of 1 M HCl and 200 μl of chloroform : methanol (1 : 1, v v−1). Tubes were then vortexed and centrifuged at 3000 × g in a microfuge for 2 min. The lipid organic phase was removed and washed once with 100 μl of HCl : methanol (1 : 1, v v−1), and spotted onto a silica gel 60A TLC plate with linear-K preadsorbent strip (Whatman), previously treated with 1% potassium oxalate. The plate was developed using equilibrated system of propan-1-ol: 2 M acetic acid : 5 M H3PO4 (65 : 35 : 1 v v1). The radioactivity in the spot of phosphatidylinositol 3-phosphate was measured by PhosphoImager (Biorad), using the Quantity One software.
[3H]-thymidine incorporation and cell proliferation
Cells were plated onto 12-well culture plates and grown to confluence. Cells were growth-arrested by incubation in serum-free MEM for 48 h. After 48 h in serum-free medium, agonists and antagonists in proper combinations were added. Simultaneously, cells were labelled with 1 μCi ml−1 of [3H]thymidine for 24 h. The experiments were terminated by washing the cells twice with cold PBS and suspending in 250 μl of cell dissociation solution. Then, the cell solutions were removed into scintillation vials and mixed with 3 ml of dioxan scintillator. The radioactivity was counted using a scintillation counter (LS 6500, Beckman Instr.).
Detection of P2Y1 and P2Y12 mRNA and RT–PCR analysis
Total RNA was extracted from serum-starved (48 h) and nonstarved glioma C6 cells using TRI reagent. In some experiments, long-term starved cells were used. In these experiments, cells were cultured in serum-free, chemically defined medium according to Grobben et al. (2001). Reverse transcription of total RNA from all serum-starved and nonstarved cells was performed using Expand RT enzyme. Specific primers for PCR reaction were designed using ‘DNA Star' software (DNA Star Inc., U.S.A.). P2Y1 primers were based on unique sequences comprising bases 97–120 (5′-AGA ATG CGG CCG GAA GAA GAG TCG-3′, forward) and 686–666 (5′-AGC CCA GGC CAG CCA GGA AGG-3′, backward) of the rat P2Y1 cDNA sequence (Acc. No. U22830). The estimated product length was 590 bp. P2Y12 primers were based on the following unique sequences: 533–554 (5′-AAA CTT CCA GCC CCA GCA ATC T-3′, forward) and 1021–1002 (5′-CAA GGC AGG CGT TCA AGG AC-3′, backward) of the rat P2Y12 cDNA sequence (Acc. No. L46865). The predicted product length was 489 bp. Equal amounts of cDNA were used in parallel experiments. A hot-start PCR protocol was used, involving denaturation at 95°C, annealing at 65°C (P2Y1) or 56°C (P2Y12) and extension at 72°C. A total of 32 cycles were conducted. PCR products were separated on 1% agarose gel, stained with ethidium bromide, and quantified using ImageQuant and MS Excel programs.
Results
We have recently characterized P2Y1 and P2Y12 subtypes of nucleotide P2Y receptors as those that respond to ADP and its analogues in glioma C6 cells (Sabała et al., 2001; Czajkowski et al., 2002). These investigations were performed on cells growing in the medium supplemented with 10% serum. However, experiments concerning MAPK and PI3-K activation and cell proliferation are usually performed on serum-deprived, growth-arrested cells that can be synchronized in G0/G1 phase of the cell cycle and, at this stage, minimally incorporate [3H]thymidine, a marker of cell proliferation (Tu et al., 2000). Therefore, to check whether the physiological status of cells may affect ADP-induced signal transduction, we examined the effects of these receptor agonists using nonstarved and 48 h serum-starved cells. In such starved cells, the morphological change could be observed under phase-contrast microscopy. The cells changed from a fibroblast-like flat morphology to a rounded, astrocyte-like shape. However, they remained adherent to dishes, and no apoptotic features or significant loss of viability was observed (data not shown).
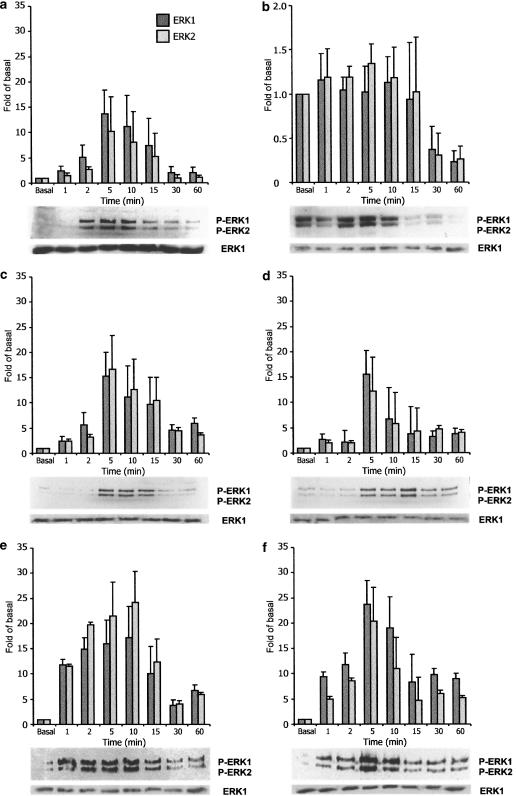
To determine whether ADP-sensitive P2Y1 and P2Y12 receptors are involved in ERK1/2 activation, 48 h serum-starved glioma C6 cells were stimulated with ADP, 2MeSADP and 2ClATP, agonists that in glioma C6 cells activate both of these receptors (Czajkowski & Barańska, 2002). As a control, we used ATP and UTP, the effect of which on ERKs in this cell line was already reported (Tu et al., 2000), and that are known as agonists of the P2Y2 receptor. Tyrosine phosphorylation of p44/ERK1 and p42/ERK2 was monitored by Western blot analysis with an antiphospho-p44/p42 MAPK antibody (see Methods). As shown in Figures 1a, c and d, ADP, 2MeSADP and 2ClATP, respectively, stimulated a rapid increase in the level of p42 and p44 phosphorylation. Densitometric analysis of the blot revealed that, within 5 min, these compounds induced the highest, about 16-fold, increase in the phosphorylation level of the active p44/ERK1 and p42/ERK2. At 30–60 min after agonists addition, phosphorylation strongly declined, in the case of ADP to near control levels. A similar increase in ERK1/2 phosphorylation, visible after 1 min, was observed when the cells were treated with ATP and UTP (Figures 1e and f). Stimulation of cells with these compounds reached the highest, more than 20-fold, increase in activated ERK1/2 after 5–10 min and strongly declined after 30–60 min, just as it was reported by Tu et al. (2000). In nonstarved glioma C6 cells, ADP was not able to additionally stimulate a high basal ERK1/2 activity up to 15 min. The phosphorylation levels of ERK1/2 were reduced starting from 30 min after ADP addition (Figure 1b), similarly as it was observed in starved cells. An alternative pathway, negatively coupled to ERK1/2 activity, could explain this phenomenon. This pathway could be triggered by P2Y1/12 receptors, or by the action of adenosine, the final product of extracellular nucleotides hydrolysis (Grobben et al., 1999; 2001; Claes et al., 2001).
Figure 1.
Time-dependent activation of p44/p42 ERK1/ERK2. C6 cells were grown as described in Methods, and experiments were performed in the standard buffer after 48 h of serum deprivation (a, c–f), or in control, nonstarved cells (b). The cells were stimulated with 10 μM ADP (a, b), 10 μM 2MeSADP (c), 10 μM 2ClATP (d), 100 μM ATP (e) and 100 μM UTP (f) for 1–60 min. The cells were lysed in SDS sample buffer and subjected to standard Western blot analysis using antiphospho-p44/p42 MAPK or total p44 MAPK (as a control) polyclonal antibody. Bands were visualized by ECL method and quantified using ImageQuant and MS Excel programs. The basal level represents ERK1/2 phosphorylation in the absence of receptor agonists. The results are expressed as the means±s.d. from three independent experiments.
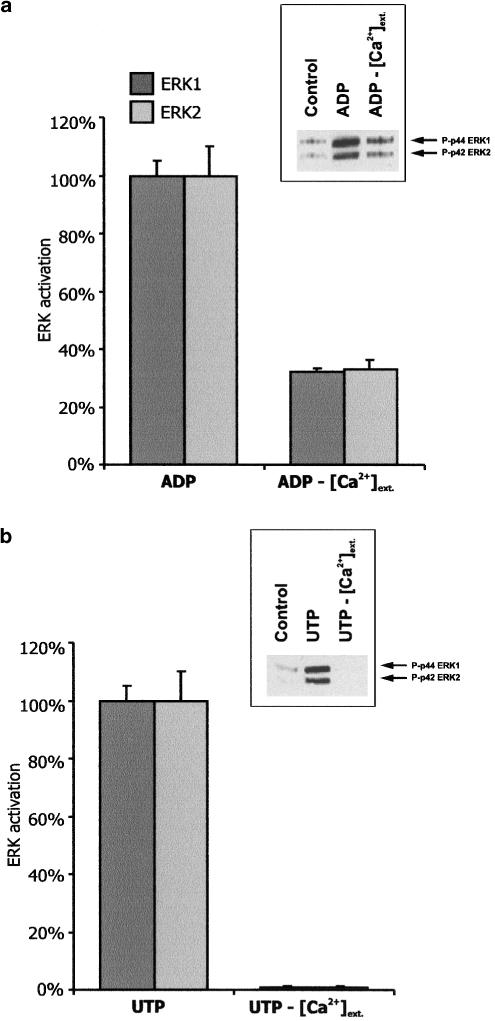
To examine whether the effect of ADP and UTP on ERK1/2 activation was mediated through the activation of nucleotide receptor that could be antagonized with MRS2179, or AR-C69931MX, 48 h serum-starved C6 cells were pretreated for 2 min with these antagonists and then, still in their presence, stimulated for 5 min by nucleotides. As shown in Figure 2, the effects of MRS2179 and AR-C69931MX on ERK1/2 phosphorylation were assessed by Western blot analysis. Figure 2a shows that MRS2179, P2Y1 receptor antagonist, inhibited by approximately 40% ADP-induced ERKs phosphorylation (Figure 2a), whereas it was without effect on UTP-induced ERK1/2 stimulation (Figure 2b). On the other hand, AR-C69931MX, the P2Y12 selective antagonist, inhibited by 85% ADP-induced ERK1/2 phosphorylation. Addition of both antagonists, MRS2179 and AR-C69931MX, resulted in almost complete inhibition of this process (Figure 2a). The increase in [Ca2+]I induced by ADP was significantly reduced by MRS2179 (Figure 2c), whereas this antagonist had no effect on UTP-triggered P2Y2-mediated Ca2+ response (Figure 2d), in agreement with results of our previous studies (Sabała et al., 2001; Czajkowski et al., 2002). Since MRS2179 and AR-C69931MX blocked ERK1/2 activation by 40 and 85%, respectively, and effect of AR-C69931MX was additive to that of MRS2179 (Figure 2a), these results suggested the involvement of P2Y1 and P2Y12, receptors, although the effect of the P2Y12 receptor predominated.
Figure 2.
Effect of P2Y1 and P2Y12 receptor antagonists on ADP-evoked ERK1/ERK2 activation in glioma C6 cells. The 48 h starved cells were preincubated for 2 min with 30 μM MRS2179 (P2Y1 receptor antagonist) or with 10 μM AR-C69931MX (P2Y12 receptor antagonist), and, still in their presence, stimulated for 5 min with 10 μM ADP. UTP (100 μM) was used as a negative control for MRS2179 action and intracellular Ca2+ concentration was measured to demonstrate the effect of this antagonist on Ca2+ signals. (a) Effect of antagonists on ADP-evoked p44/p42 ERK1/2 activation. (b) Effect of MRS2179 on UTP-evoked p44/p42 ERK1/2 activation. The cells (a, b) were stimulated for 5 min, lysed in SDS sample buffer and subjected to Western blot procedure. Bands were visualized by the ECL method and quantified using ImageQuant and MS Excel programs. The results are expressed as the means±s.d. from three independent experiments. (c) Effect of MRS2179 on ADP-induced changes in [Ca2+]i. (d) Effect of MRS2179 on UTP-induced changes in [Ca2+]i. (c, d) Each bar represents the mean value of the initial calcium response peak corresponding to the emptying of intracellular calcium stores. Data are means±s.d. from 24 cells recorded on three separate occasions. Each trace in this figure represents the mean ratio value of the Ca2+ response of 16 cells recorded in two experiments.
In order to determine whether the effect of ADP and UTP on the p44/p42 ERK1/ERK2 activation is Ca2+ dependent, serum-starved cells were placed in calcium-free medium, and ERK1 and ERK2 activity was measured 5 min after treatment of cells with ADP (Figure 3a) or UTP (Figure 3b). As shown, extracellular Ca2+ removal inhibited the ADP-induced ERK1/2 activation by about 70%, whereas that induced by UTP was almost completely blocked. We have previously indicated that, in glioma C6 cells, ADP initiated a biphasic Ca2+ response, consistent with the PLC-mediated Ca2+ release from the endoplasmic reticulum (ER) calcium stores (the first phase), and Ca2+ enter from the extracellular space (the second phase). In the absence of extracellular Ca2+, ADP resulted only in the initial, transient rise in [Ca2+]I (the first phase), which declined to the basal level during 5 min incubation with the agonist, indicating that this cytosolic Ca2+ elevation was caused only by PLC-mediated release of Ca2+ from the ER stores (Barańska et al., 1999; Sabała et al., 2001; Czajkowski & Barańska, 2002). In the experiment presented in Figure 3a, intracellular Ca2+ depletion was ADP-induced. This ADP treatment, performed in a calcium-free medium, led to a greater decrease in ERK1/2 phosphorylation (70%, Figure 3a) than MRS2179 (40%, Figure 2a). Furthermore, we found that the 30 min preincubation of the cells with 500 μM EGTA in a calcium-free medium, simultaneously with the [Ca2+]i depletion, led to a complete (100%) inhibition of ADP-induced ERK1/2 phosphorylation (not shown). Thus, these data pointed out the significant role of Ca2+ in ERK1/2 activation, triggered not only by PLC-, but also by adenylate cyclase-coupled receptor.
Figure 3.
Effect of extracellular calcium on ADP- and UTP-stimulated ERK1/ERK2 activation. The 48 h serum-starved cells were stimulated for 5 min in the standard buffer containing 2 mM CaCl2 or 500 μM EGTA (calcium free buffer) with 10 μM ADP (a) or with 100 μM UTP (b), lysed in SDS sample buffer and subjected to Western blot procedure. Bands were visualized by ECL method and quantified using ImageQuant and MS Excel programs. Mean values±s.d. of three independent experiments are shown.
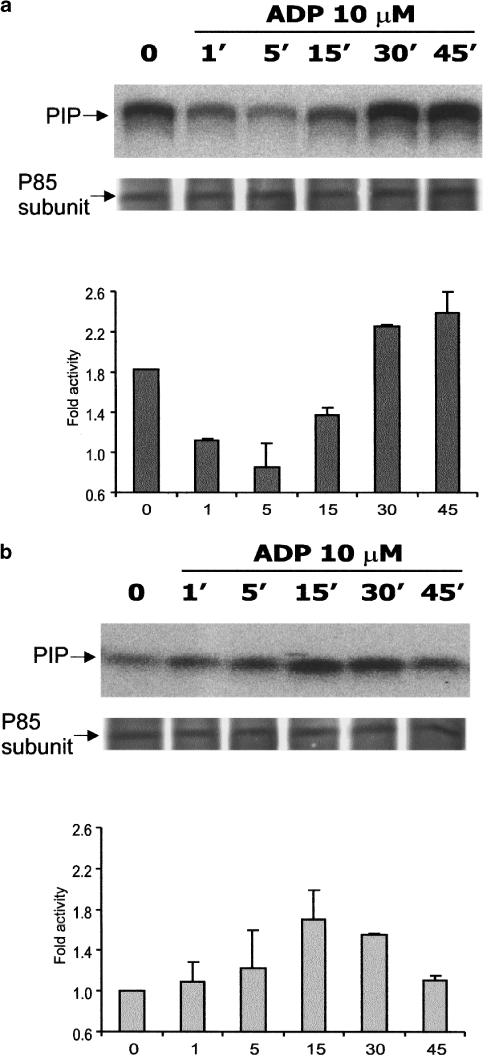
Figure 4 shows changes in PI3-K activation induced by ADP as a function of time. As shown, the modulatory effect of extracellular ADP on PI3-K activation is distinctly dependent on the physiological status of the cells. The nonstarved cells were characterized by higher constitutive activity of the enzyme than the serum-starved ones. Moreover, in nonstarved cells, ADP, within the first 5 min of incubation, markedly decreased PI3-K activation, and only after 30 min it reached the control level again (Figure 4a). On the contrary, in the cells made quiescent at confluence by incubation for 48 h in a serum-free medium, ADP evoked a gradual increase in PI3-K activity that reached the maximal level after 15 min (Figure 4b).
Figure 4.
Time course of ADP-evoked PI3-kinase activity. C6 cells were grown as described in Methods, and experiments were performed on nonstarved cells (a) or on the cells after 48 h of serum deprivation (b). The cells were stimulated with 10 μM ADP for 1–45 min, lysed and subjected to immunoprecipitation and kinase assay. Phosphatidylinositol 3-phosphate (PIP) bands were visualized with PhosphoImager using the Quantity One software. In each sample, the amount of immunoprecipitated p85 subunit is presented. The results are expressed as the means±s.d. from three independent experiments.
To specify whether the P2Y1, or the P2Y12 receptor, is responsible for the increase in the PI3-K activity, the starved cells were preincubated with MRS2179, the specific antagonist of P2Y1 receptor for 2 min, and then, still in the presence of this antagonist, stimulated with ADP for 15 min. Figure 5 shows that, in the presence of MRS2179, the PI3-K activation was markedly increased, indicating that blocking of the P2Y1 receptor activity had a stimulatory effect on the kinase. On the other hand, preincubation of the cells with protein kinase C inhibitor, GF 109203X, that enhanced the Ca2+ signal triggered by P2Y1 receptor (not shown), abolished PI3-K activation (Figure 5).
Figure 5.
Effect of MRS2179 and GF109203X on ADP-stimulated PI3-K activity in serum-starved glioma C6 cells. The cells were grown as described in Methods, and experiments were performed in the standard buffer after 48 h of serum deprivation. MRS2179 (30 μM) and GF109203X (1 μM) were added 2 min prior to addition of ADP. After 15 min of stimulation with 10 μM ADP, the cells were lysed and subjected to immunoprecipitation and kinase assay. PI3-K activity was measured in vitro as described. Bands were visualized with PhosphoImager, using the Quantity One software. The results are expressed as the means±s.d. from three experiments.
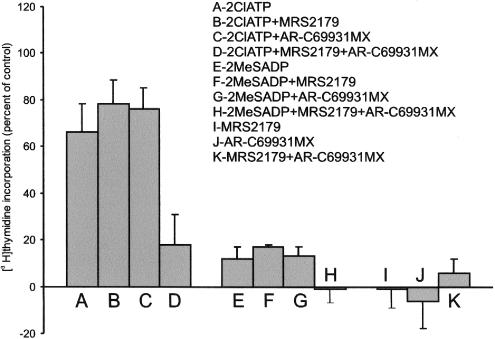
In the subsequent experiments, the influence of P2Y receptors on cell proliferation, estimated by changes in the DNA synthesis, was investigated (Figure 6). In these experiments, agonists and antagonists of the P2Y1 and P2Y12 receptors were added to the medium for 24 h (see Methods). 2MeSADP and 2ClATP modestly decreased (statistically insignificant) proliferation of cells incubated in the medium supplemented with 10% serum. Antagonists, MRS2179 and AR-C69931MX were without effect on this process (not shown). In starved cells, 2ClATP distinctly stimulated the incorporation of [3H]thymidine above the control level, whereas the effect of 2MeSADP was much smaller (Figure 6). MRS2179 and AR-C69931MX added separately to the cells stimulated with agonists were without effect on [3H]thymidine uptake. However, when both antagonists were added together to the cells, not only 2ClATP- but also 2MeSADP-induced [3H]thymidine incorporation was strongly inhibited (Figure 6). These trends were confirmed using different detection methods including MTT assay and 5BrdU incorporation (not shown). Thus, similarly as in the case of ERK1/2 activation, these data suggested that, in starved glioma C6 cells, proliferation could be enhanced by both P2Y1 and P2Y12, receptors. The enhanced proliferation of C6 cells due to a P2YAC (P2Y12) receptor-mediated activation of p42/p44 MAPK was previously reported by Claes et al. (2001). The difference in the response of the cells to 2MeSADP and 2ClATP found in this study was probably due to the hydrolysis of the first agonist caused by ectonucleotidases, similarly as it was previously found for ATP and other nucleotides (Grobben et al., 1999; 2001). It is worth adding that ADP treatment for 5 or 15 min, applied in experiments presented herein in Figures 2, 3 and 5, was too short to produce detectable hydrolysis of ADP, and such a possibility in the case of these experiments should not be taken into consideration.
Figure 6.
Effect of agonists and antagonists of P2Y receptors on proliferation of serum-deprived glioma C6 cells, estimated by changes in DNA synthesis. Cells were grown in 12-well plates in serum-free medium for 48 h, as described in Methods. Agonists, 2MeSADP and 2ClATP, and antagonists, MRS2179 and AR-C69931MX, were added at concentrations used in experiments shown in Figures 1 and 2. Cells were labelled with 1 μCi ml−1 [3H]thymidine and incubated in the presence of agonists or antagonists for 24 h. For other details, see Methods. The number of cells grown without any addition (Control) was taken as the baseline. The results are expressed as the means±s.d. from 3–5 experiments.
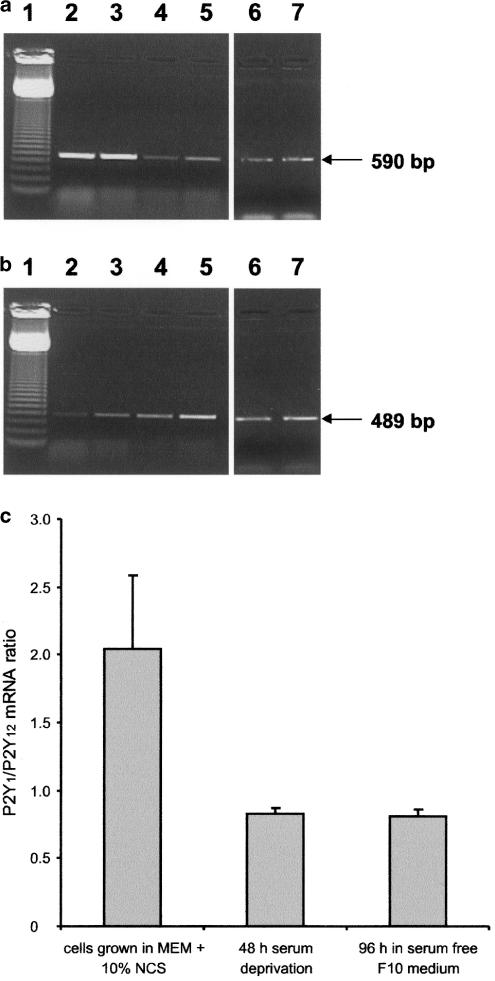
In order to find out whether culturing conditions may have an impact on the expression of P2Y1 and P2Y12 receptors, the changes in mRNA level of both receptors upon serum starvation were measured using RT–PCR method. In the subsequent experiments, the cells were starved for 48 h in serum-free MEM, exactly at the same conditions as used for experiments concerning ERK1/2 and PI3-K activation as well as proliferation. Figure 7a shows that, upon such treatment, the level of P2Y1 mRNA expression was decreased (lanes 4 and 5 versus lanes 2 and 3), whereas the level of expression of P2Y12 receptor was slightly increased (Figure 7b). To further elucidate whether culturing conditions may affect the expression of P2Y1 and P2Y12 receptors, glioma C6 cells were cultured for 4 days in serum-free, chemically defined Ham's F10 medium, as described by Grobben et al. (2001) (see Methods), and the changes in both receptors' mRNA levels were measured. Figure 7a shows that, upon such treatment, the level of P2Y1 mRNA expression is also strongly decreased (lanes 6 and 7 versus lanes 2 and 3). The changes in relative amount of both mRNAs are shown in Figure 7c. When the cells were cultured in MEM supplemented with 10% NCS, the ratio of P2Y1 versus P2Y12 level was 2.05±0.54. After 48 h starvation, the relative level of both receptors fell to 0.83±0.04 and in cells cultured in serum-free Ham's F10 medium to 0.81±0.05.
Figure 7.
Effect of serum deprivation on the relative expression levels of P2Y1 and P2Y12 receptors in glioma C6 cells. (a) 590 bp RT–PCR product (corresponding to P2Y1 mRNA) and (b) 489 bp RT–PCR product (corresponding to P2Y12 mRNA) were analysed on 1% agarose gel and visualized by ethidium bromide staining. All parallel PCR reactions contained the same amount of cDNA. Lane 1, 123 bp DNA ladder; lanes 2, 3, cells grown in medium supplemented with 10% serum; lanes 4, 5, cells after 48 h serum deprivation; lanes 6, 7, cells grown 4 days in the serum-free medium. (c) Summary of a relative expression of P2Y1 versus P2Y12 receptors in nonstarved, 48 h serum-starved cells and cells cultured in serum-free medium for 4 days. Each bar represents the mean ratio±s.d. from three independent experiments.
Discussion
We show here that mRNA of P2Y1 and P2Y12 receptors are differentially expressed in starved and nonstarved glioma C6 cells. In nonstarved C6 cells, the P2Y1 receptor, linked to PLC and responsible for Ca2+ mobilization, strongly predominates. On the other hand, in serum-deprived, 48 h-starved cells, the level of P2Y1 mRNA is decreased and, in consequence, the expression of P2Y12 becomes more pronounced. Furthermore, in our hands, in long-term starved cells, cultured in the serum-free medium, the P2Y12 mRNA also predominates. These data shed a new light on the problem of the presence of P2Y1 in glioma C6 cells and explain why Grobben et al. (2001), using long-term starved cells, have found that ADP and its analogues stimulate the P2Y12 receptor, referred to P2YAC by them. However, the present study shows that both ADP-sensitive receptors, P2Y1 and P2Y12, may be involved in ERK1/2 activation. The inhibitory effect of Ca2+ removal, MRS2179 or AR-C69931MX, P2Y1 and P2Y12 selective antagonists, respectively, point out the participation of both receptors in this activation, although in starved cells the effect of ADP seems to be primarily mediated by the P2Y12 receptor.
In our previous study, we characterized the P2Y1 receptor in glioma C6 cells using direct visualization of PIP2, RT–PCR and knockdown of P2Y1 receptor expression with antisense oligonucleotide (Czajkowski et al., 2002). We have also shown that the stimulation of P2Y1 by ADP and its analogues initiates a typical, biphasic Ca2+ response, compatible with the capacitative model of Ca2+ influx (Putney & Bird, 1993; Barańska et al., 1999; Berridge, 1999). The presence of external Ca2+ is not necessary to elevate [Ca2+]I (Sabała et al., 2001). However, the kinetics of ADP-evoked [Ca2+]i changes is different from the kinetics of ATP and UTP response mediated by the P2Y2 receptor. The ATP- and UTP-induced [Ca2+]I elevation starts with an initial peak response and is followed by a long sustained plateau phase, whereas the response to ADP is more transient (Sabała et al., 2001). Therefore, we suggest that P2Y1-mediated ERK1/2 activation is initiated by the increase in [Ca2+]i and is additive to that mediated by P2Y12.
Tu et al. (2000) have proposed that, in glioma C6 cells, the mitogenic effect of ATP and UTP, mediated by the P2Y2 receptor, involves the activation of a Ras/Raf/MEK/ERK in a PLC- and Ca2+-dependent pathway. However, Grobben et al. (2001) have postulated that agonists of P2YAC (P2Y12) receptor activate ERK1/2 through a Gi/RhoA/PKC/Raf/MEK-dependent, but Ras-independent cascade. The P2Y12 receptor is negatively coupled to adenylate cyclase. Stimulation of this receptor decreases intracellular cyclic AMP (cAMP) level (Czajkowski & Barańska, 2002) and inhibits cAMP-dependent protein kinase A (PKA) activity. On the other hand, it has been reported that the increase in intracellular cAMP inhibits ERK1/2 activity and C6 cells growth. These results have been obtained both in primary astrocytes and C6 glioma cells (Kurino et al., 1996; Dugan et al., 1999; Qiu et al., 2000; Wang et al., 2001). Wang et al. (2001) have reported that, in glioma C6 cells, cAMP inhibits not only ERK1/2 but also PI3-K/Akt pathways, and both these effects occur by inhibiting Rap1. Rap1 belongs to the Ras family of small GTP-binding proteins, and its activity may be regulated by a variety of specific GTPase-activating proteins, guanine nucleotide exchange factors and adaptor proteins (Zwartkruis & Bos, 1999). Ras proteins regulate activities of the Raf family of protein kinases that phosphorylate and activate MEK1/2, which in turn phosphorylate and activate ERK1/2 (Hagemann & Rapp, 1999). Thus, Wang et al. (2001) have proposed that cAMP inhibits ERK1/2 by inhibiting a Rap1/B-Raf/MEK/ERK signalling. Moreover, the inactivation of ERK1/2 and PI3-K/Akt pathways contributes to a regulation of gene expression, inhibits cellular proliferation and promotes differentiation in glioma C6 cells (Kim et al., 2001; Wang et al., 2001). On the contrary, Roymans et al. (2001) suggested that the enzymatic activity of PI3-K is necessary for cAMP-dependent induction of differentiation in C6 cells. It is worth adding that both second messengers generated by PLC activation, that is, Ca2+ and diacylglycerol, are able to induce Rap1 activation (Zwartkruis & Bos, 1999). On the other hand, PKA inhibitor, HA120, is able to reverse the inhibition of ERK1/2 activity in astrocytes (Dugan et al., 1999).
In nonstarved C6 cells, we herein observed a high, constitutively active PI3-K. This high PI3-K activity, characteristic for glioma and other tumour cells (Furnari et al., 1998), is usually explained as a result of a loss of a lipid phosphatase (PTEN) that dephosphorylates the 3′ position of phosphoinositides, and as such acts as an antagonist of PI3-K. As a consequence, the loss of PTEN function results in constitutive activation of protein kinase B/Akt pathway that regulates cell growth and survival and leads to the development of neoplasia (Cantley & Neel, 1999; Maehama & Dixon, 1999). However, the present study shows that, in nonstarved cells, ADP distinctly inhibits high PI3-K activity. On the contrary, in starved cells where the constitutive activity of PI3-K is much lower, ADP has an opposite function and increases PI3-K activity. The presence of MRS2179, a specific antagonist of P2Y1, potentiates this ADP effect. Thus, P2Y12, expression of which in starved cells is higher than that of P2Y1, seems to be responsible for both ERK1/2 and PI3-K activation.
One can speculate that P2Y12-stimulatory effect on ERK1/2 activity and cellular growth, associated with the decrease in intracellular cAMP level, may involve a similar, but antagonistically acting signalling cascade, as that described for receptors increasing cAMP (Wang et al., 2001). The P2Y1 receptor has a similar stimulatory effect on ERK1/2 activity and cell proliferation. The mechanism by which the P2Y1 receptor stimulates ERK1/2 activity most probably involves a PLC-dependent pathway, similar to that described for the P2Y2 receptor by Tu et al. (2000). On the contrary, the detailed molecular mechanism involved in the inhibition of PI3-K activity by the P2Y1 receptor remains poorly understood. Recently, an adaptor protein, Ruk, has been described as a negative regulator of PI3-K/Akt pathway in cultured primary neurons (Gout et al., 2000). This protein inhibits PI3-K activity by a specific binding to the p85α regulatory subunit of PI3-K.
Class 1A PI3-Ks have been usually activated through tyrosine kinase-dependent mechanisms. However, in the present study, we measured the activation of a class 1A PI3-K downstream of ADP-induced G protein-coupled receptors. Data regarding the potential link between these receptors and p85/p110 PI3-kinase are often controversial. However, it has been most recently reported that agonist binding to Gq-coupled receptors blocks Akt/PKB pathway via the release of active Gαq subunits that inhibit PI3-K. The inhibitory mechanism seems to be independent of PLC, and might involve a protein–protein inhibitory interaction between Gαq and 110α PI3-K (Ballou et al., 2003). On the other hand, receptors that couple to proteins in the Gi/o family can increase PI3-K activity. This effect is mediated by Giβ/γ heterodimers, which activate both the p85 and p110β and p110γ PI3-Ks (Stoyanov et al., 1995; Kurosu et al., 1997; Stephens et al., 1997; Huwiler et al., 2002). Thus, since our study shows that the P2Y1 receptor coupled to Gq is active mainly in nonstarved cells, ADP at such cellular condition should inhibit PI3-K activation. In contrast, in serum-starved cells, where the P2Y12 receptor coupled to Gi predominates, ADP should have the opposite effect. Such regularity is herein observed.
In conclusion, our results demonstrate that both ADP-sensitive receptors, P2Y1 and P2Y12, are involved in ERK1/2 activation and cell proliferation, but in serum-starved cells the effect of ADP on ERK1/2 is primarily mediated by the P2Y12 receptor. The activation of P2Y12 receptor has stimulatory, and the activation of P2Y1, inhibitory effect on PI3-K signalling. Moreover, these responses are strongly dependent on the physiological status of glioma C6 cells. The present study indicates that serum deprivation strongly decreases the expression of the P2Y1 receptor, and therefore may have an impact on intracellular signalling pathways triggered by these two receptors, modulate their physiological effects, and be important for maintaining cellular homeostasis.
Acknowledgments
This study was supported by Grants No. 6PO5A01220 and 3PO5A11922 from the State Committee for Scientific Research (KBN, Poland).
Abbreviations
- cAMP
cyclic AMP
- [Ca2+]I
intracellular Ca2+ concentration
- 2ClATP
2-chloro-ATP
- ER
endoplasmic reticulum
- ERK
Ras/extracellular signal-regulated kinase
- InsP3
inositol 1,4,5-trisphosphate
- MEM
minimum essential medium
- 2MeSADP
2-methylthio-ADP
- 2MeSATP
2-methylthio-ATP
- NCS
newborn calf serum
- PI3-K
phosphatidylinositol 3-kinase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKA
protein kinase A
- PLC
phospholipase C
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- BALLOU L.M., LIN H.-Y., FAN G., JIANG Y.-P., LIN R.Z. Activated Gαq inhibits p110α phosphatidylinositol 3-kinase and Akt. J. Biol. Chem. 2003;278:23472–23479. doi: 10.1074/jbc.M212232200. [DOI] [PubMed] [Google Scholar]
- BARAŃSKA J., CHABAN V., CZARNY M., SABAŁA P. Changes in Ca2+ concentration in phorbol ester and thapsigargin treated glioma C6 cells. The role of protein kinase C in regulation of Ca2+ entry. Cell Calcium. 1995;17:207–215. doi: 10.1016/0143-4160(95)90035-7. [DOI] [PubMed] [Google Scholar]
- BARAŃSKA J., PRZYBYŁEK K., SABAŁA P. Capacitative calcium entry. Glioma C6 cells as a model of nonexcitable cells. Polish J. Pharmacol. 1999;51:153–162. [PubMed] [Google Scholar]
- BERRIDGE M.J. Capacitative calcium entry. Biochem. J. 1999;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M.O. The regulation of vascular function by P2 receptor: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOEYNAEMS J.-M., COMMUNI D., SAVI P., HERBERT J.M. P2Y receptors: in the middle of the road. Trends Pharmacol. Sci. 2000;21:1–3. doi: 10.1016/s0165-6147(99)01415-7. [DOI] [PubMed] [Google Scholar]
- BOYER J.L., LAZAROWSKI E.R., CHEN X.-H., HARDEN T.K. Identification of a P2Y-purinergic receptor that inhibits adenylyl cyclase. J. Pharmacol. Exp. Ther. 1993;267:1140–1146. [PubMed] [Google Scholar]
- BOYER J.L., ZOHN L.E., JACOBSON K.A., HARDEN T.K. Differential effects of P2-purinoceptor antagonists on phospholipase C- and adenylate cyclase-coupled P2Y-purinoceptors. Br. J. Pharmacol. 1994;113:614–620. doi: 10.1111/j.1476-5381.1994.tb17034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRISMAR T. Physiology of transformed glial cells. Glia. 1995;15:231–243. doi: 10.1002/glia.440150305. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.A basis for distinguishing two types of purinergic receptor Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach 1978New York: Raven Press; 107–118.ed. Straub, R.W. & Bolis, L. pp [Google Scholar]
- CANTLEY L.C., NEEL B.G. New insight into tumor suppression: PTEN supresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAES P., GROBBEN B., VAN KOLEN K., ROYMANS D., SLEGERS H. P2YAC-receptor agonists enhance the proliferation of rat C6 glioma cells through activation of the p42/44 mitogen-activated protein kinase. Br. J. Pharmacol. 2001;134:402–408. doi: 10.1038/sj.bjp.0704271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., BOEYNAEMS J.-M. Receptors responsive to extracellular pyrimidine nucleotides. Trends Pharmacol. Sci. 1997;18:83–86. doi: 10.1016/s0165-6147(96)01035-8. [DOI] [PubMed] [Google Scholar]
- CORVERA S., CZECH M.P. Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 1998;8:442–446. doi: 10.1016/s0962-8924(98)01366-x. [DOI] [PubMed] [Google Scholar]
- CZAJKOWSKI R., BARAŃSKA J. Cross-talk between the ATP and ADP nucleotide receptor signalling pathways in glioma C6 cells. Acta Biochim. Polon. 2002;49:877–889. [PubMed] [Google Scholar]
- CZAJKOWSKI R., LEI L., SABAŁA P., BARAÑSKA J. ADP-evoked phospholipase C stimulation and adenylyl cyclase inhibition in glioma C6 cells occurs through two distinct nucleotide receptors, P2Y1 and P2Y12. FEBS Lett. 2002;513:179–183. doi: 10.1016/s0014-5793(02)02255-x. [DOI] [PubMed] [Google Scholar]
- DUGAN L.L., KIM J.S., ZHANG Y., BART R.D., SUN Y., HOLTZMAN D.M., GUTMANN D.H. Differential effects of cAMP in neurons and astrocytes. J. Biol. Chem. 1999;274:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- FURNARI F.B., HUANG H.J., CAVENEE W.K. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–5008. [PubMed] [Google Scholar]
- GOUT I., MIDDLETON G., ADU J., NINKINA N.N., DROBOT L.B., FILONENKO V., MATSUKA G., DAVIES A.M., WATERFIELD M., BUCHMAN V.L. Negative regulation of PI 3-kinase by Ruk, a novel adaptor protein. EMBO J. 2000;19:4015–4025. doi: 10.1093/emboj/19.15.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROBBEN B., ANCIAUX K., ROYMANS D., STEFAN C., BOLLEN M., ESMANS E.L., SLEGERS H. An ecto-nucleotide pyrophosphate is one of the main enzymes involved in the extracellular metabolism of ATP in rat C6 glioma. J. Neurochem. 1999;72:826–834. doi: 10.1046/j.1471-4159.1999.0720826.x. [DOI] [PubMed] [Google Scholar]
- GROBBEN B., CLAES P., VAN KOLEN K., ROYMANS D., FRANSEN P., SYS S.U., SLEGERS H. Agonists of the P2YAC-receptor activate MAP kinase by a ras-independent pathway in rat C6 glioma. J. Neurochem. 2001;78:1325–1338. doi: 10.1046/j.1471-4159.2001.00524.x. [DOI] [PubMed] [Google Scholar]
- HAGEMANN C., RAPP U.R. Isotype-specific functions of Raf kinases. Exp. Cell Res. 1999;253:34–46. doi: 10.1006/excr.1999.4689. [DOI] [PubMed] [Google Scholar]
- HOLLOPETER G., JANTZEN H.M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R.B., NURDEN P., NURDEN A., JULIUS D., CONLEY P.B. Identification of the platelet ADP-receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- HUWILER A., ROLZ W., DORSCH S., REN S., PFEITSCHIFTER J. Extracellular ATP and UTP activate the protein kinase B/Akt cascade via the P2Y2 purinoceptor in renal mesengial cells. Br. J. Pharmacol. 2002;136:520–529. doi: 10.1038/sj.bjp.0704748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., MORO S., HOFFMANN C., KIM Y.-C., KIM H.S., RAVI R.G., HARDEN T.K., BOYER J.L. Structurally related nucleotides as selective agonists and antagonists at P2Y1 receptor. Il Farmaco. 2001;56:71–75. doi: 10.1016/s0014-827x(01)01023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN J., TOMLINSON W., KIRK I.P., KIM Y.B., HUMPHRIES R.G., KUNAPULI S.P. The C6-2B glioma cell P2YAC receptor is pharmacologically and molecularly identical to the platelet P2Y12 receptor. Br. J. Pharmacol. 2001;133:521–528. doi: 10.1038/sj.bjp.0704114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S., JEE K., KIM D., KOH H., CHUNG J. Cyclic AMP inhibits Akt activity by blocking the membrane localization of PDK1. J. Biol. Chem. 2001;276:12864–12870. doi: 10.1074/jbc.M001492200. [DOI] [PubMed] [Google Scholar]
- KING B.F., TOWNSEND-NICHOLSON A., BURNSTOCK G. Metabotropic receptors for ATP and UTP: exploring the correspondence between native and recombinant nucleotide receptors. Trends Pharmacol. Sci. 1998;19:506–514. doi: 10.1016/s0165-6147(98)01271-1. [DOI] [PubMed] [Google Scholar]
- KURINO M., FUKUNAGA K., USHIO Y., MIYAMOTO E. Cyclic AMP inhibits activation of mitogen-activated protein kinase and cell proliferation in response to growth factors in cultured rat cortical astrocytes. J. Neurochem. 1996;67:2246–2255. doi: 10.1046/j.1471-4159.1996.67062246.x. [DOI] [PubMed] [Google Scholar]
- KUROSU H., MAEHAMA T., OKADA T., YAMAMOTO T., HOSHINO S., FUKUI Y., UI M., HAZEKI O., KATADA T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βγ subunits of G proteins and phosphotyrosyl peptide. J. Biol. Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- LIN W.-W., CHUANG D.-M. Different signal transduction pathways are coupled to the nucleotide receptor and the P2Y receptor in C6 glioma cells. J. Pharmacol. Exp. Therap. 1994;269:926–931. [PubMed] [Google Scholar]
- MAEHAMA T., DIXON J.E. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., LI Q., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- PTASZNIK A., BEATIE G.M., MALLY M.I., CIRULLI V., LOPEZ A., HAYEK A. Phosphatidylinositol 3-kinase is a negative regulator of cellular differentiation. J. Cell Biol. 1997;137:1127–1136. doi: 10.1083/jcb.137.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUTNEY J.W., JR, BIRD G.St.J. The inositol phosphate-calcium signalling system in nonexcitable cells. Endocrine Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- QIU W., ZHUANG S., VON LINTIG F.C., BOSS G.R., PILZ R.B. Cell type-specific regulation of B-Raf kinase by cAMP and 14-3-3 proteins. J. Biol. Chem. 2000;275:31921–31929. doi: 10.1074/jbc.M003327200. [DOI] [PubMed] [Google Scholar]
- ROYMANS D., VISSENBERG K., DE JONGHE C., GROBBEN B., CLAES P., VERBELEN J.-P., Van BROECKHOVEN C., SLEGERS H. Phosphatidylinositol 3-kinase activity is required for the expression of glial fibrillary acidic proteins upon cAMP-dependent induction of differentiation in rat C6 glioma. J. Neurochem. 2001;76:610–618. doi: 10.1046/j.1471-4159.2001.00077.x. [DOI] [PubMed] [Google Scholar]
- SABAŁA P., AMLER E., BARAŃSKA J. Intracellular Ca2+ signals induced by ATP and thapsigargin in glioma C6 cells. Neurochem. Int. 1997;31:55–64. doi: 10.1016/s0197-0186(96)00135-0. [DOI] [PubMed] [Google Scholar]
- SABAŁA P., CZAJKOWSKI R., PRZYBYŁEK K., KALITA K., KACZMAREK L., BARAŃSKA J. Two subtypes of G protein-coupled nucleotide receptors, P2Y1 and P2Y2 are involved in calcium signalling in glioma C6 cells. Br. J. Pharmacol. 2001;132:393–402. doi: 10.1038/sj.bjp.0703843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVI P., LABOURET C., DELESQUE N., GUETTE F., LUPKER J., HERBERT J.M. P2Y12, a new platelet ADP receptor, target of clopidogrel. Biochem. Biophys. Res. Commun. 2001;283:379–383. doi: 10.1006/bbrc.2001.4816. [DOI] [PubMed] [Google Scholar]
- SCHACHTER J.B., LI Q., BOYER J.L., NICHOLAS R.A., HARDEN T.K. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br. J. Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER J.B., BOYER J.L., LI Q., NICHOLAS R.A., HARDEN T.K. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br. J. Pharmacol. 1997;122:1021–1024. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGER R., KREBS E.G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- STEPHENS L.R., EGUINOA A., ERDJUMENT-BROMAGE H., LUI M., COOKE F., CADWELL J., SMRCKA A.S., THELEN M., CADWALLADER K., TEMPST P., HAWKINS P.T. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- STOYANOV B., VOLINIA S., HANCK T., RUBIO I., LOUBTCHENKOV M., MALEK D., STOYANOVA S., VANHAESEBROECK B., DHAND R., NURNBERG B., GIERSCHIK P., SEEDORF K., HSUAN J.J., WATERFIELD M.D., WETZKER R. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- TU M.-T., LUO S.-F., WANG C.-C., CHIEN C.-S., CHIU C.-T., LIN C.-C., YANG C.-M. P2Y2 receptor-mediated proliferation of C6 glioma cells via activation of Ras/Raf/MEK/MAPK pathway. Br. J. Pharmacol. 2000;129:1481–1489. doi: 10.1038/sj.bjp.0703182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG L., LIU F., ADAMO M.L. Cyclic AMP inhibits extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways by inhibiting Rap1. J. Biol. Chem. 2001;276:37242–37249. doi: 10.1074/jbc.M105089200. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., FEOLDE E., VIGNE P., NEARY J.T., RUNBERG A., FRELIN C., BARNARD E.A. The P2Y purinoceptor in rat brain microvascular endothelial cells couple to inhibition of adenylate cyclase. Br. J. Pharmacol. 1996;119:1385–1392. doi: 10.1111/j.1476-5381.1996.tb16050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITMAN M., KAPLAN D.R., SCHAFFHAUSEN B., CANTLEY L., ROBERTS T.M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- ZWARTKRUIS F.J.T., BOS J.L. Ras and Rap 1: two highly related small GTPases with distinct function. Exp. Cell Res. 1999;253:157–165. doi: 10.1006/excr.1999.4695. [DOI] [PubMed] [Google Scholar]