Abstract
We previously described a human immunodeficiency virus type 1 (HIV-1) envelope mutant that introduces a disulfide bridge between the gp120 surface proteins and gp41 transmembrane proteins (J. M. Binley, R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore, J. Virol. 74:627-643, 2000). Here we produced pseudovirions bearing the mutant envelope and a reporter gene to examine the mutant’s infectious properties. These pseudovirions attach to cells expressing CD4 and coreceptor but infect only when triggered with reducing agent, implying that gp120-gp41 dissociation is necessary for infection. Further studies suggested that virus entry was arrested after CD4 and coreceptor engagement. By measuring the activities of various entry inhibitors against the arrested intermediate, we found that gp120-targeting inhibitors typically act prior to virus attachment, whereas gp41 inhibitors are able to act postattachment. Unexpectedly, a significant fraction of antibodies in HIV-1-positive sera neutralized virus postattachment, suggesting that downstream fusion events and structures figure prominently in the host immune response. Overall, this disulfide-shackled virus is a unique tool with potential utility in vaccine design, drug discovery, and elucidation of the HIV-1 entry process.
Human immunodeficiency virus type 1 (HIV-1) enters susceptible target cells via a complex cascade of receptor-mediated events. A fine characterization of this process is complicated by the transient nature of the lipid and protein rearrangements involved. The envelope glycoprotein (Env) is responsible for viral attachment and fusion. Env consists of noncovalently associated trimers of heterodimers comprising gp120 surface and gp41 transmembrane glycoproteins (29, 39). During infection, gp120 attaches to the CD4 receptor and undergoes conformational changes that enable coreceptor binding (39). This leads to further changes in gp41 (22) to form a six-helix bundle consisting of three alpha-helical hairpins (7, 48) and culminates in lipid mixing and membrane fusion.
The study of HIV-1 entry and the Env conformations involved has provided a rich source of targets for a new generation of antiretroviral therapies (6, 16, 18). The most clinically advanced HIV-1 entry inhibitor, the peptide T-20 (also known as DP178), blocks fusion at nanomolar concentrations (49) by binding to a structure known as the gp41 prehairpin intermediate that becomes available during the fusion process (22).
Env represents the primary target for the neutralizing antibody response. Successful vaccines against many viral infections elicit neutralizing antibodies (4) but have been difficult to elicit against HIV-1. The virus evades host immunity by exposing hypervariable and heavily glycosylated regions on gp120, while the conserved domains that bind its cellular receptors are located in recessed cavities (29, 39). As a result, only a few monoclonal antibodies (MAbs) against Env isolated to date are both potently and broadly neutralizing (5, 9, 33, 34, 39, 41, 44, 45, 52). Since conserved domains and potential targets for neutralizing antibodies may become exposed after receptor binding, fusion intermediates may find utility in vaccine research (12, 13, 20, 26, 33, 37, 51).
Until now, HIV-1 fusion intermediates have been generated by incubating virus or Env-expressing cells with target cells at nonpermissive temperatures or by treatment with chemicals (8, 19, 21, 23-25, 32). These intermediates suffer the drawback that they are stabilized in nonphysiologic conditions. Members of our group previously described a soluble Env mutant, engineered to introduce a disulfide bond between gp120 and gp41 (the SOS mutant [3]) that stabilized gp120-gp41 association while retaining the structural properties of native Env. We reasoned that this mutant might have useful properties in the context of viral fusion. Thus, we generated SOS mutant pseudovirus and found that fusion was arrested midway into the infection process. Rapid fusion could be triggered upon brief exposure of cell-attached pseudovirus to a reducing agent, allowing precise synchronization of fusion events. The unique fusion intermediate we describe may find broad utility in further unraveling aspects of the viral entry process, in antiretroviral drug development, and as a basis for a novel HIV-1 vaccine strategy.
MATERIALS AND METHODS
MAbs, peptides, and sera.
The following anti-gp120 MAbs were used (each a whole immunoglobulin G [IgG], unless specified): CD4 binding site-overlapping (CD4bs) MAb IgG1b12 and its monovalent fragment, Fab b12 (5); CD4-IgG2, a chimera containing four copies of CD4 domains 1 and 2 fused to a IgG Fc domain (35); 2G12, against a unique gp120 epitope formed by terminal residues of N-linked glycans (41, 44); MAb 17b and Fab X5, directed to CD4-induced (CD4i) epitopes (33, 45); and 447-52D, against the V3 loop (9). MAbs against gp41 included 2F5 and 4E10, against a C-terminal region of the gp41 ectodomain (34, 52); 7B2, against the gp41 cluster I region; and 2.2B, against the gp41 cluster II region (3). MAbs 2F5, 4E10, and 2G12 were provided by H. Katinger (Polymun Scientific Inc., Vienna, Austria). MAbs 17b, 7B2, and 2.2B were provided by J. Robinson (Tulane University, Tulane, La.). MAb 447-52D was provided by S. Zolla-Pazner (Veterans Affairs Medical Center, New York, N.Y.) and the AIDS reference reagent program. MAbs IgG1b12, 2G12, 2F5, 4E10, and the CD4-IgG2 chimera (PRO 542) are broadly neutralizing (5, 34, 35, 44, 52). MAb 17b neutralizes diverse strains of HIV-1 in the presence of recombinant soluble CD4 (sCD4) (45). Fab X5 is also broadly neutralizing (33). Purified HIV immune globulin (HIVIG) was obtained from J. Mascola (Vaccine Research Center, National Institutes of Health, Washington, D.C.). Potently neutralizing serum from the HIV-1-infected donor FDA2 (40) was provided by G. Quinnan (Uniformed Services University of the Health Sciences, Bethesda, Md.). Other HIV-1-positive sera were derived from donors DA, CM, MT, and AC (1a).
The T-20 peptide (49) is based on a sequence from the gp41 C-terminal helical region and was synthesized and handled as described previously (35). PA14 (PRO140) is a mouse MAb to CCR5 (47).
Plasmids and mutagenesis.
The plasmid pCAGGS (36) was used to express membrane-bound Env of the primary R5 isolate JR-FL. We expressed Env proteins as both full-length gp160 and a mutant, truncated at residue 708, leaving three amino acids of the gp41 cytoplasmic tail, termed ΔCT. Mutations were made to introduce cysteines at residues 501 and 605 (the SOS mutant [3]). A mutation was generated to replace the gp120/gp41 cleavage site REKR by the inefficiently cleaved GEKR, termed UNC. Plasmid pNL4-3.Luc.R-E-, expresses an HIV-1 genome fragment with frameshifts in Env and Vpr and a luciferase reporter gene in place of Nef (10).
Production of pseudovirions and viral infectivity assays.
Pseudoviruses were produced as described previously by transfection of 293T cells with pNL4-3.Luc.R-E- and Env-expressing pCAGGS-based plasmids (10). Single-round infections were performed using U87.CD4.CCR5, HOS.CD4.CCR5, Cf2Th.CD4.CCR5, or Cf2Th.synCCR5 cells (10, 28). Briefly, virus (normalized for p24 content by enzyme-linked immunosorbent assay) was incubated with cells for 2 h. In some experiments, 5 mM dithiothreitol (DTT) was subsequently added for 10 min. Luciferase activity was measured as described previously (10). All assays were performed at least in duplicate and were repeated for a total of at least four replicates to ensure consistency.
In assays where virus was pretreated with DTT, virus was subsequently pelleted in Eppendorf tubes at 21,000 × g for 30 min to remove DTT and then resuspended in fresh medium before proceeding with infection. In general, to normalize for the possible side effects of pelleting on virus infectivity, untreated virus was spun down in parallel and used as a control.
Neutralization assays.
Neutralization was measured in various formats with all incubations at 37°C, unless specified.
(i) Standard neutralization assay.
The SOS virus was incubated with inhibitor for 1 h before being transferred to U87.CD4.CCR5 cells for a further 2 h of incubation. Unbound virus was removed by changing medium, and the culture was incubated for a further 1 h. Cells were subsequently treated with 5 mM DTT for 10 min, and the medium was then replaced. Wild-type (WT) virus was used in neutralization assays using the same procedure and washes but without the DTT incubation. In a modified assay, the wash step was omitted and the inhibitor remained present until the DTT was removed. This is referred to as the standard (no wash) format.
(ii) Preattachment neutralization assay.
As in the standard assay, virus and inhibitor were incubated together for 1 h, but inhibitor was then removed by centrifugation in Eppendorf tubes for 30 min at 21,000 × g and resuspension of the virus pellet in fresh medium. Virus was then added to cells, and the assay was then performed in the same manner as the standard assay. In parallel controls, untreated virus was pelleted as above but before the 1-h incubation of virus with inhibitor and subsequent addition to cells.
(iii) Postattachment neutralization assay.
SOS virus was allowed to attach to cells for 2 h to form an SOS-arrested intermediate (SAI), and medium was replaced to remove any unattached virus. Inhibitors were then added for a further 1 h before DTT treatment (without replacing the medium) for 10 min. In a modified postattachment neutralization protocol, medium was replaced before as well as after the DTT incubation. This was referred to as the postattachment (washout) format.
To generate a temperature-arrested intermediate (TAI) for postattachment neutralization, WT gp160-bearing pseudovirions were allowed to attach to target cells at 14°C for 2 h. The medium was then replaced, and inhibitors were added for a further 1 h at 14°C before washing with phosphate-buffered saline, replacement of medium, and warming to 37°C.
RESULTS
Infectivity of WT and mutant pseudovirions.
We first examined the infectivity of pseudovirions bearing WT Env, SOS disulfide mutant Env (3), and proteolytically unprocessed Env (UNC). As expected, UNC pseudovirions were essentially noninfectious, since Env processing is required for fusion (30) (Fig. 1A). SOS virus was also noninfectious (Fig. 1A), presumably because the gp120-gp41 disulfide bond prevents fusion. Virions bearing WT gp160 with a truncated cytoplasmic tail, ΔCT, infected cells to the same extent as those bearing full-length gp160 (Fig. 1A). It has been previously shown that cytoplasmic tail truncation increases the exposure of the CD4 and coreceptor binding sites, thus rendering the virus more sensitive to neutralization (17). Since this property might allow a somewhat greater sensitivity to titrate the effects of fusion inhibitors, ΔCT Env-bearing viruses were selected for further experiments.
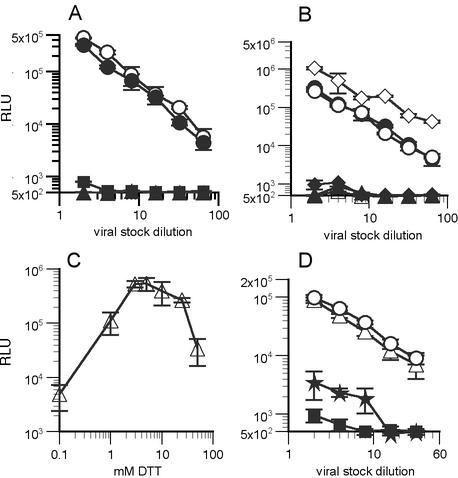
FIG. 1.
Analysis of the functional activity of SOS pseudovirions. Single-round infectivity experiments were performed using U87.CD4.CCR5 cells. (A) Infectivity of pseudovirions bearing gp160 WT (open circles), gp160ΔCT WT (closed circles), gp160ΔCT SOS (squares), and gp160ΔCT UNC (triangles). RLU, relative light units. (B) Effect of adding DTT to cultures after virus incubation with target cells. Symbols denote gp160ΔCT-Env bearing pseudoviruses as follows: circles, WT; diamonds, SOS; and triangles, UNC. Open symbols denote culture wells to which DTT was added, and closed symbols denote wells in which the medium was merely changed. (C) DTT treatment was titrated by adding graded amounts after virus incubation with cells. (D) SOS pseudovirions were exposed to DTT either before (stars) or after (circles) attachment to U87.CD4.CCR5 cells, both before and after (open triangles), or neither (closed squares). Similar results were obtained with Cf2.CD4.CCR5 and HOS.CD4.CCR5 cells (data not shown).
Fusion of the SOS-arrested intermediate by addition of reducing agent.
In a cell-cell fusion assay, SOS fusion was found to be DTT dependent (1). Building on this result, we determined whether infection of SOS pseudovirions could be rescued by reduction of the SOS disulfide bond. Thus, target cells preincubated with SOS pseudovirions were treated with 5 mM DTT. This resulted in infection of SOS virus, similar to WT levels (Fig. 1B), but did not affect the WT or UNC pseudoviruses. Further experiments confirmed 5 mM as the optimal DTT concentration, and this was used subsequently (Fig. 1C).
Reducing agent induces fusion only after virus attachment.
We next examined whether the timing of DTT treatment is important for inducing SOS fusion. When free pseudovirus was pretreated with DTT and then pelleted, resuspended in fresh medium, and added to cells, infection remained inefficient (Fig. 1D). However, DTT treatment of virus both before and after attachment to cells gave results identical to those obtained with DTT posttreatment alone. Thus, DTT pretreatment does not affect virus infectivity. Overall, DTT treatment after virus attachment to cells was necessary and sufficient for SOS fusion (Fig. 1D). Therefore, infection proceeds via a novel, redox-sensitive structure. We refer to this as the SAI. We next explored the nature of the SAI by examining its susceptibility to various classes of HIV-1 entry inhibitors.
The SAI engages both CD4 and coreceptor.
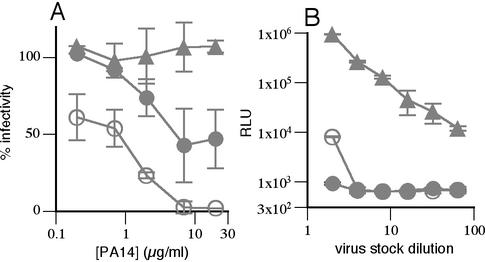
To determine if SOS pseudovirions attach to the CCR5 coreceptor, we used an anti-CCR5 MAb (PA14) that is known to inhibit infection (47). PA14 effectively inhibited SOS infection when added before or concurrently with virus (Fig. 2A). However, if added after the SAI had formed, it had no effect (Fig. 2A). A similar result was found using the anti-CCR5 MAb 2D7 (1). These results suggest that the SAI engages both CD4 and CCR5, but they do not exclude the possibility that virus attachment sterically obstructs anti-CCR5 MAb binding. To further explore this issue, virions were preincubated with 10 μg of sCD4/ml and then incubated with CCR5+ CD4− cells. Infection was observed in the presence but not absence of sCD4, suggesting that the virus binds to CCR5 in a CD4-dependent manner (Fig. 2B). Consistent with this notion, prior studies demonstrated that sCD4 induces exposure of the coreceptor binding site on soluble SOS Env proteins (3).
FIG. 2.
SOS pseudovirions engage both CD4 and CCR5 upon attachment. (A) The ability of the anti-CCR5 MAb PA14 to inhibit SOS-VLP infection before and after the attachment of virus to cells. Symbols are as follows: closed circles, cells pretreated with PA14, followed by washing; open circles, PA14 and virus added to cells concomitantly and coincubated thereafter; triangles, PA14 added after virus attachment to cells. (B) Ability of SOS pseudovirions to attach to CCR5-expressing Cf2.Th.synCCR5 cells in the presence (open circles) or absence (closed triangles) of sCD4. Triangles denote infection of positive control Cf2.Th.CD4.CCR5 cells.
The SAI is sensitive to entry inhibitors that target gp41.
Based on the above findings, SOS pseudovirions offer a unique opportunity to dissect the mechanisms of viral entry inhibitors at physiologic temperature. In turn, this might allow further characterization of the nature of the SAI. Three neutralization assays were developed (see Materials and Methods). In the standard neutralization assay, virus and inhibitors are preincubated prior to their addition to cells. In the preattachment neutralization assay, inhibitors are incubated with virus and then removed by centrifuging virus. In the postattachment neutralization assay, inhibitors are added after the SAI has formed but prior to the addition of reducing agent.
Initially, we compared WT and SOS pseudoviruses in the standard neutralization assay. WT and SOS viruses showed similar sensitivities to the anti-gp120 MAbs IgG1b12 (5) and 2G12 (41, 44) and to the tetrameric CD4-IgG2 molecule (35) (Table 1). However, the SOS virus was neutralized more strongly by anti-gp41 MAbs 2F5 and 4E10 and the V3 loop-specific MAb 447-52D and more weakly by the T-20 peptide (Table 1).
TABLE 1.
Summary of neutralization of WT and SOS pseudovirions by inhibitorsa
| Inhibitor | Epitope/target | WT
|
SOS
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard
|
Postattachment (TAI)
|
Standard
|
Postattachment (SAI)
|
||||||
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | ||
| IgG1b12 | CD4bs | 0.05 | 0.11 | >30 | >30 | 0.017 | 0.08 | 18 | >30 |
| Fab b12 | CD4bs | ND | ND | ND | ND | 0.03 | 0.3 | >30 | >30 |
| CD4-IgG2 | CD4 | 0.1 | 0.23 | ND | ND | 0.02 | 0.1 | 28 | >30 |
| 2G12 | C3/V4 | 0.21 | 1.8 | ND | ND | 0.14 | 2 | 28 | >30 |
| 447-52D | V3 loop | >30 | >30 | ND | ND | 1.5 | 15 | >30 | >30 |
| 17b | CD4i | >30 | >30 | ND | ND | 28 | >30 | >30 | >30 |
| Fab X5 | CD4i | 25 | >30 | ND | ND | 11 | >30 | >30 | >30 |
| 2F5 | gp41 C-term. ecto. | 0.2 | 1 | 2 | 10 | 0.03 | 0.13 | 0.3 | 0.8 |
| 4E10 | gp41 C-term. ecto. | 2 | 9 | ND | ND | 0.8 | 3 | 3 | 7 |
| T-20 | gp41 prehairpin | 0.05 | 0.4 | 0.005 | 0.03 | 0.2 | 0.7 | 0.03 | 0.1 |
Standard and postattachment neutralization IC50s and IC90s are indicated. Neutralization assays used gp160ΔCT-bearing viruses, except for the postattachment WT neutralization, which used full-length gp160-bearing pseudovirions captured as a 14°C TAI. MAbs 2.2B and 7B2 are not included (see Fig. 3). ND, not done.
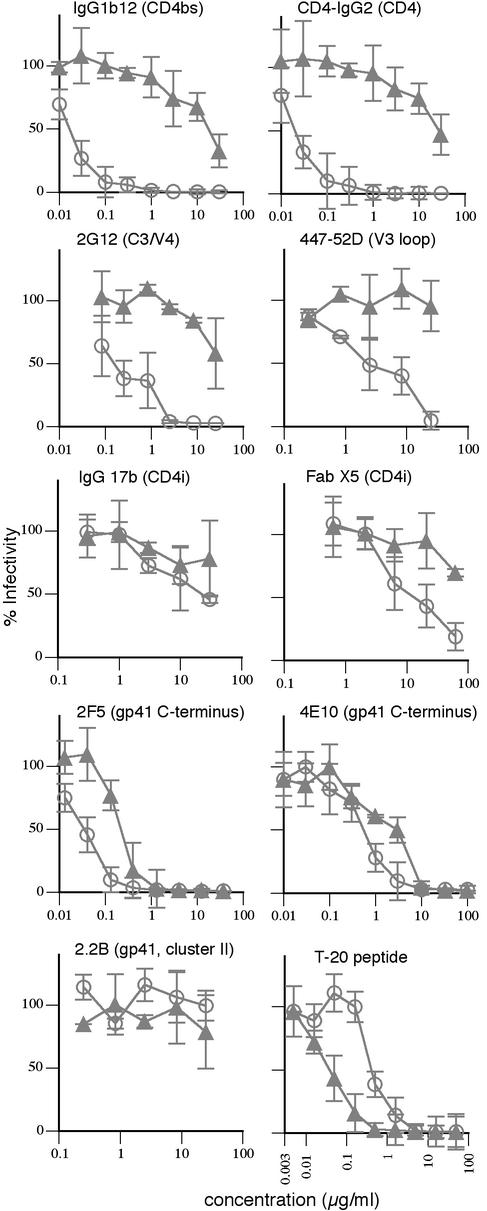
We next compared the efficacy of various reagents to neutralize the SOS virus in standard and postattachment formats. IgG1b12 was active in both standard (Fig. 3; Table 1) and preattachment (Fig. 4A) assays. However, postattachment neutralization was much weaker, consistent with the notion that virus-cell attachment blocks MAb access. In contrast to findings in a previous report, Fab b12 also did not exhibit significant postattachment activity (31) (Table 1). Similarly, MAbs 447-52D and 2G12 did not effectively neutralize postattachment. The lack of postattachment neutralization by the V3 loop-specific antibody 447-52D can be explained by the involvement of the V3 loop in coreceptor engagement (11). We propose that 2G12 binding may be sterically occluded in the SAI or else it may bind to the complex, but it can no longer inhibit fusion. CD4i MAbs that overlap the CCR5 binding site (46, 50) neutralized weakly in all formats (Fig. 3l Table 1). Thus, the postattachment potency of 17b and Fab X5 was similar to that of MAb IgG1b12 in the same assay format (Fig. 3; Table 1). In contrast, 2F5 and another neutralizing anti-gp41 MAb, 4E10, neutralized effectively in the postattachment format (Fig. 3; Table 1). Furthermore, 2F5 performed relatively poorly in a preattachment neutralization assay (Fig. 4A). Previous studies indicated that although MAb 2F5 potently neutralizes HIV-1, it does not bind well to native Env (43). It is possible, then, that 2F5 neutralizes virus after it attaches to cells (42). As expected, nonneutralizing anti-gp41 MAbs 7B2 and 2.2B had no activity in either neutralization assay format (Fig. 3 and data not shown).
FIG. 3.
Standard neutralization and postattachment neutralization using SOS pseudovirions. Various MAbs, CD4-IgG2, and the T-20 peptide were assayed for neutralization of gp160ΔCT SOS pseudovirions using the standard (open circles) or postattachment (closed triangles) assay format.
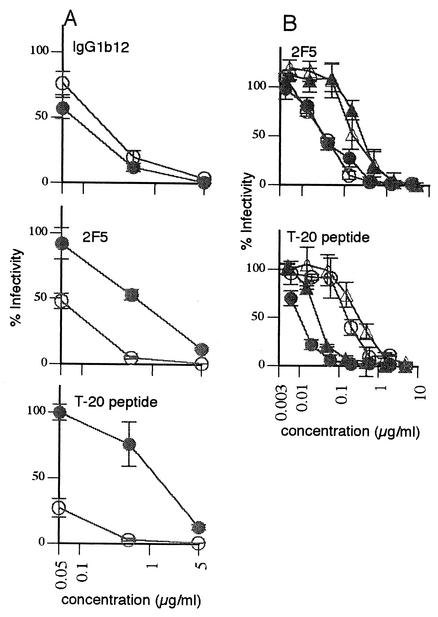
FIG. 4.
Reversibility of neutralization by MAb 2F5 and the T-20 peptide. (A) Comparison of IgG1b12, 2F5, and T-20 in standard (open circles) and preattachment (closed circles) neutralization assays. (B) The T-20 peptide and 2F5 MAb were compared in neutralization assays with modified wash steps. Symbols are as follows: open circles, standard assay; closed triangles, postattachment assay; closed circles, standard assay without a pre-DTT treatment wash step; open triangles, postattachment assay with an additional wash before the DTT incubation.
The peptide T-20 (22) was the only reagent to perform better in the postattachment format than the standard format (Fig. 3, Table 1). In agreement with this, it performed poorly in the preattachment neutralization assay (Fig. 4A). Compared with MAb 2F5, T-20 activity was more sensitive to increased wash steps (Fig. 4B). Remarkably, T-20 appears to exert its potent inhibitory effect largely during the short DTT incubation period, suggesting that fusion proceeds rapidly upon triggering. T-20 might bind to virus more effectively (and perhaps irreversibly) after the fusion potential of the SAI is released, in agreement with separate studies (1).
Comparison of the SAI and temperature-arrested fusion intermediates.
Traditionally, fusion intermediates have been studied in the form of TAIs (24, 32). To compare these to the SAI, we produced a full-length gp160 WT TAI at 14°C. The TAI was inhibited more potently by the T-20 peptide but more weakly by 2F5 and not at all by IgG1b12 (Table 1). This indicates that the TAI had progressed further towards full fusion by the time the inhibitors were added. The decreased 2F5 activity can be partially explained by the low temperature, since 2F5 neutralization of SOS virus was also weaker in these conditions (data not shown). In contrast to the TAI described here, a WT TAI (1) produced at 18°C appeared to be arrested at an earlier stage in the fusion cascade than the SAI. We do not have a direct explanation, but the difference probably stems from the use of different cell lines and the precise assay details. Certainly, this argues that while the SAI behaves quite predictably in different assays, TAIs appear to be somewhat less predictable.
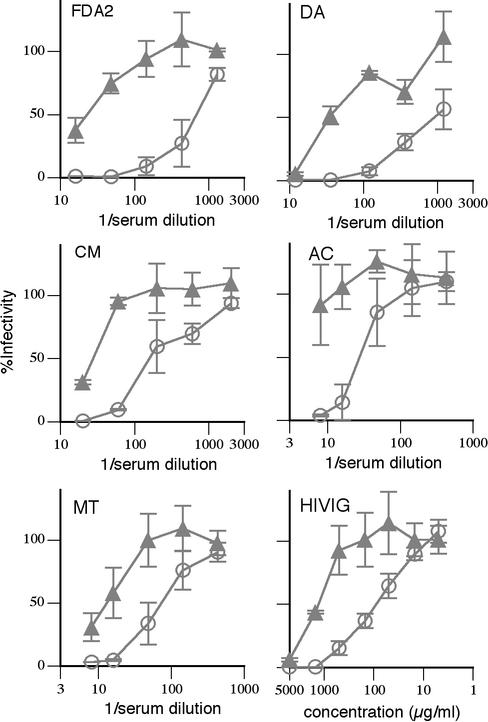
Antibodies capable of postattachment neutralization constitute a significant fraction of the total anti-Env antibody in HIV-positive sera.
We showed above that the CD4i MAbs X5 and 17b and the gp41 MAbs 2F5 and 4E10 are able to neutralize virus in the postattachment format. Conversely, MAbs IgG1b12, CD4-IgG2, 447-52D, and 2G12 neutralize at least 1,000-fold less efficiently in the postattachment format than in the standard neutralization format. Although not proven, it is generally considered that CD4bs and V3 loop antibodies constitute a dominant fraction of the neutralizing activity in HIV-positive sera and antibodies like 2F5 and 4E10 are extremely rare (15, 52). We sought to reevaluate the strength of the postattachment neutralizing antibody component of HIV-positive sera and HIVIG using the SAI. Surprisingly, HIV-positive sera neutralized SOS virus only 4- to 38-fold more weakly (mean = 19) in the postattachment format than in the standard format (Fig. 5). This stands in stark contrast to MAb IgG1b12, which neutralizes approximately 1,000-fold more weakly in the postattachment format and more closely mimics the behavior of MAb 2F5, which neutralizes only about 10-fold less effectively in this format. This result suggests that the neutralizing activity of the HIV-positive sera does not derive solely from V3 loop and CD4bs antibodies. The sera appear to contain a significant component of 2F5-like or 17b-like antibodies, or antibodies to as yet uncharacterized epitopes that are able to mediate postattachment neutralization. In fact, several MAbs to the 2F5 and CD4i epitope clusters have been isolated from a phage-displayed antibody library prepared from the FDA2 donor (33, 52).
FIG. 5.
Serum neutralization in pre- and postattachment neutralization assays. Sera from several HIV-positive donors and HIVIG were assayed for neutralization of gp160ΔCT SOS pseudovirus using standard (open circles) and postattachment (closed triangles) neutralization assays.
The above findings provide a means of estimating concentrations of particular MAbs in sera that would be needed to obtain the postattachment neutralization observed. Postattachment 50% inhibitory concentration (IC50) of sera is, on average, approximately a 1:26 serum dilution (Fig. 5). Postattachment IC50 of 17b is approximately 30 μg/ml. If we assume that serum postattachment neutralization is comprised entirely of neutralizing antibodies similar to 17b, then 780 μg/ml would be required. Since the total concentration of anti-Env antibody has been previously estimated at between 100 μg/ml and 1 mg/ml (2), it appears unlikely that such a large proportion of anti-Env antibody would recognize the CD4i epitope (78 to 100%). However, it should be noted that the JR-FL isolate is particularly resistant to CD4i MAbs and that other CD4i antibodies may be more potent than 17b (33). Using a similar calculation, serum postattachment neutralization is equivalent to 7.8 μg of 2F5/ml or 78 μg of 4E10/ml. This translates as 0.78 to 7.8% of the total anti-Env fraction of sera for 2F5 or 7.8 to 78% for 4E10.
DISCUSSION
We describe a mutant HIV-1 pseudovirus in which the gp120 and gp41 envelope proteins are covalently linked by a disulfide bond. This virus appears to engage both CD4 and coreceptor on susceptible cells and then becomes arrested at an intermediate stage of infection, termed the SAI. At this point, treatment with reducing agent unshackles the disulfide bond and triggers infection. Related studies using a cell-to-cell fusion assay also describe redox-regulated fusion using SOS Env (1). Since the fusion of SOS and WT viruses are similar in magnitude, are dependent on CD4 and coreceptor, and are specifically inhibited by different classes of entry blockers, the SAI appears to faithfully represent an intermediate stage of the virus fusion process. In fact, functional disulfide-mutant viral envelopes are not without a historical precedent. Indeed, a disulfide-mutant influenza envelope protein was previously shown to be fusogenic in a redox-regulated manner (27).
The ability of the SOS mutation to regulate virus infection with reducing agent allowed us to probe the mechanisms of HIV-1 entry inhibitors and antibody neutralization. The T-20 peptide recognizes a fusion intermediate known as the gp41 prehairpin. This intermediate appears to be at least partially formed in the SAI, since T-20 activity is inefficiently removed by washing, as has been reported for other intermediates (32). T-20 gains full access to the gp41 prehairpin only after addition of a reducing agent, as evidenced by its more potent inhibitory activity. This might reflect an increased exposure and/or formation of the prehairpin intermediate following gp120 dissociation. In experiments to determine the mechanism of action of neutralizing antibodies, those directed to gp120 in general neutralized virus before or during its attachment to cells, while antibodies directed to gp41 were able to also act postattachment. In contrast to a previous report (38), CD4bs MAb neutralization appeared not to require gp120-gp41 dissociation. This possibility is eliminated in SOS virus neutralization assays because gp120 and gp41 are covalently linked. Surprisingly, we found that several HIV-positive sera were capable of effective postattachment neutralization, reminiscent of neutralizing MAbs to gp41. Some studies have suggested that neutralizing antibodies to gp41 are very rare in HIV-positive sera (15, 52), raising the possibility that the postattachment neutralization observed in sera might arise from other, as yet uncharacterized antibody specificities.
Vaccine candidates based on recombinant Env proteins have so far failed to reliably elicit neutralizing antibodies in animal models (4). Since Env dramatically refolds after receptor binding, exposing erstwhile cryptic but potentially vulnerable sites, there is significant interest in fusion intermediates as a possible means of eliciting neutralizing antibodies (12, 13, 20, 26, 33, 37, 51). A better understanding of the target(s) of neutralizing antibodies, assisted by the work described here, might enable us to design immunogens able to elicit such antibodies. The SAI might provide a way to focus antibody responses on stably exposed postattachment neutralizing epitopes, while other epitope clusters are blocked. Since the SAI is a functional fusion intermediate that is stable at physiological temperatures without any additional treatments, it might have advantages as an immunogen.
The SAI has several other potential applications. It might be useful for the development or optimization of antiviral drugs that preferentially act postattachment like the T-20 peptide (16, 18). It should also be of value to assess the synergy of drugs that inhibit various stages of viral entry (14, 35). In addition, it may provide the basis for lentiviral vectors able to impart synchronized gene delivery. Finally, it could be used to distinguish gene expression events in target cells during viral attachment and fusion, thereby increasing our understanding of virus-host interactions. In summary, the novel reducing agent-regulated HIV-1 we describe provides a new tool for investigation of the process and inhibition of viral entry and may provide new opportunities in antiretroviral drug development and vaccine design.
Acknowledgments
This work was supported by NIH grants AI49566 (J.M.B.) and AI33292 (D.R.B.) and the International AIDS Vaccine Initiative.
We thank G. Melikyan for guidance and M. Muesing, D. Montefiori, P. Poignard, M. Franti, M. Zwick, R. Pantophlet, R. Sanders, and D. Schiller for helpful discussions. We thank N. Landau for advice and for providing the HOS cell lines, J. Sodroski for the Cf2 cell lines, and J. Mascola for HIVIG. We thank H. Katinger, J. Robinson, S. Zolla-Pazner, G. Quinnan, and J. Mascola for providing antibodies and sera.
REFERENCES
- 1.Abrahamyan, L. G., R. M. Markosyan, J. P. Moore, F. S. Cohen, and G. B. Melikyan. 2003. Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion. J. Virol. 77:5829-5836. [DOI] [PMC free article] [PubMed]
- 1a.Binley, J. M., H. J. Ditzel, C. F. Barbas III, N. Sullivan, J. Sodroski, P. W. Parren, and D. R. Burton. 1996. Human antibody responses to HIV type 1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved antibody motifs in antigen binding. AIDS Res. Hum. Retrovir. 12:911-924. [DOI] [PubMed] [Google Scholar]
- 2.Binley, J. M., P. J. Klasse, Y. Cao, I. Jones, M. Markowitz, D. D. Ho, and J. P. Moore. 1997. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J. Virol. 71:2799-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton, D. R. 2002. Opinion: Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2:706-713. [DOI] [PubMed] [Google Scholar]
- 5.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 6.Chan, D. C., C. T. Chutkowski, and P. S. Kim. 1998. Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target. Proc. Natl. Acad. Sci. USA 95:15613-15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 8.Chernomordik, L. V., V. A. Frolov, E. Leikina, P. Bronk, and J. Zimmerberg. 1998. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 140:1369-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conley, A. J., M. K. Gorny, J. A. Kessler II, L. J. Boots, M. Ossorio-Castro, S. Koenig, D. W. Lineberger, E. A. Emini, C. Williams, and S. Zolla-Pazner. 1994. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J. Virol. 68:6994-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 11.Cormier, E. G., and T. Dragic. 2002. The crown and stem of the V3 loop play distinct roles in human immunodeficiency virus type 1 envelope glycoprotein interactions with the CCR5 coreceptor. J. Virol. 76:8953-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Rosny, E., R. Vassell, P. T. Wingfield, C. T. Wild, and C. D. Weiss. 2001. Peptides corresponding to the heptad repeat motifs in the transmembrane protein (gp41) of human immunodeficiency virus type 1 elicit antibodies to receptor-activated conformations of the envelope glycoprotein. J. Virol. 75:8859-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devico, A., A. Silver, A. M. Thronton, M. G. Sarngadharan, and R. Pal. 1996. Covalently crosslinked complexes of human immunodeficiency virus type 1 (HIV-1) gp120 and CD4 receptor elicit a neutralizing immune response that includes antibodies selective for primary virus isolates. Virology 218:258-263. [DOI] [PubMed] [Google Scholar]
- 14.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151:F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71:2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert, D. M., V. N. Malashkevich, L. H. Hong, P. A. Carr, and P. S. Kim. 1999. Inhibiting HIV-1 entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell 99:103-115. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, T. G., T. L. Hoffman, F. Baribaud, S. Wyss, C. C. LaBranche, J. Romano, J. Adkinson, M. Sharron, J. A. Hoxie, and R. W. Doms. 2001. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J. Virol. 75:5230-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer, M., T. M. Kapoor, T. Strassmaier, W. Weissenhorn, J. J. Skehel, D. Oprian, S. L. Schreiber, D. C. Wiley, and S. C. Harrison. 1999. Selection of gp41-mediated HIV-1 cell entry inhibitors from biased combinatorial libraries of non-natural binding elements. Nat. Struct. Biol. 6:953-960. [DOI] [PubMed] [Google Scholar]
- 19.Finnegan, C. M., W. Berg, G. K. Lewis, and A. L. DeVico. 2001. Antigenic properties of the human immunodeficiency virus envelope during cell-cell fusion. J. Virol. 75:11096-11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouts, T., K. Godfrey, K. Bobb, D. Montefiori, C. V. Hanson, V. S. Kalyanaraman, A. DeVico, and R. Pal. 2002. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc. Natl. Acad. Sci. USA 99:11842-11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frey, S., M. Marsh, S. Gunther, A. Pelchen-Matthews, P. Stephens, S. Ortlepp, and T. Stegmann. 1995. Temperature dependence of cell-cell fusion induced by the envelope glycoprotein of human immunodeficiency virus type 1. J. Virol. 69:1462-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 23.Gallo, S. A., A. Puri, and R. Blumenthal. 2001. HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process. Biochemistry 40:12231-12236. [DOI] [PubMed] [Google Scholar]
- 24.Golding, H., M. Zaitseva, E. de Rosny, L. R. King, J. Manischewitz, I. Sidorov, M. K. Gorny, S. Zolla-Pazner, D. S. Dimitrov, and C. D. Weiss. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 76:6780-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart, T. K., A. Truneh, and P. J. Bugelski. 1996. Characterization of CD4-gp120 activation intermediates during human immunodeficiency virus type 1 syncytium formation. AIDS Res. Hum. Retrovir. 12:1305-1313. [DOI] [PubMed] [Google Scholar]
- 26.Kang, C. Y., K. Hariharan, P. L. Nara, J. Sodroski, and J. P. Moore. 1994. Immunization with a soluble CD4-gp120 complex preferentially induces neutralizing anti-human immunodeficiency virus type 1 antibodies directed to conformation-dependent epitopes of gp120. J. Virol. 68:5854-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemble, G. W., D. L. Bodian, J. Rose, I. A. Wilson, and J. M. White. 1992. Intermonomer disulfide bonds impair the fusion activity of influenza virus hemagglutinin. J. Virol. 66:4940-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolchinsky, P., E. Kiprilov, and J. Sodroski. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J. Virol. 75:2041-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCune, J. M., L. B. Rabin, M. B. Feinberg, M. Lieberman, J. C. Kosek, G. R. Reyes, and I. L. Weissman. 1988. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 53:55-67. [DOI] [PubMed] [Google Scholar]
- 31.McInerney, T. L., L. McLain, S. J. Armstrong, and N. J. Dimmock. 1997. A human IgG1 (b12) specific for the CD4 binding site of HIV-1 neutralizes by inhibiting the virus fusion entry process, but b12 Fab neutralizes by inhibiting a postfusion event. Virology 233:313-326. [DOI] [PubMed] [Google Scholar]
- 32.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moulard, M., S. K. Phogat, Y. Shu, A. F. Labrijn, X. Xiao, J. M. Binley, M. Y. Zhang, I. A. Sidorov, C. C. Broder, J. Robinson, P. W. Parren, D. R. Burton, and D. S. Dimitrov. 2002. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc. Natl. Acad. Sci. USA 99:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagashima, K. A., D. A. Thompson, S. I. Rosenfield, P. J. Maddon, T. Dragic, and W. C. Olson. 2001. Human immunodeficiency virus type 1 entry inhibitors PRO 542 and T-20 are potently synergistic in blocking virus-cell and cell-cell fusion. J. Infect. Dis. 183:1121-1125. [DOI] [PubMed] [Google Scholar]
- 36.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 37.Nunberg, J. H., K. E. Follis, M. Trahey, and R. A. LaCasse. 2000. Turning a corner on HIV neutralization? Microbes Infect. 2:213-221. [DOI] [PubMed] [Google Scholar]
- 38.Poignard, P., T. Fouts, D. Naniche, J. P. Moore, and Q. J. Sattentau. 1996. Neutralizing antibodies to human immunodeficiency virus type-1 gp120 induce envelope glycoprotein subunit dissociation. J. Exp. Med. 183:473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poignard, P., E. O. Saphire, P. W. Parren, and D. R. Burton. 2001. gp120: biologic aspects of structural features. Annu. Rev. Immunol. 19:253-274. [DOI] [PubMed] [Google Scholar]
- 40.Quinnan, G. V., Jr., P. F. Zhang, D. W. Fu, M. Dong, and H. J. Alter. 1999. Expression and characterization of HIV type 1 envelope protein associated with a broadly reactive neutralizing antibody response. AIDS Res. Hum. Retrovir. 15:561-570. [DOI] [PubMed] [Google Scholar]
- 41.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sattentau, Q. J., M. Moulard, B. Brivet, F. Botto, J. C. Guillemot, I. Mondor, P. Poignard, and S. Ugolini. 1999. Antibody neutralization of HIV-1 and the potential for vaccine design. Immunol. Lett. 66:143-149. [DOI] [PubMed] [Google Scholar]
- 43.Sattentau, Q. J., S. Zolla-Pazner, and P. Poignard. 1995. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology 206:713-717. [DOI] [PubMed] [Google Scholar]
- 44.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 47.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 49.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 51.Xiang, S. H., P. D. Kwong, R. Gupta, C. D. Rizzuto, D. J. Casper, R. Wyatt, L. Wang, W. A. Hendrickson, M. L. Doyle, and J. Sodroski. 2002. Mutagenic Stabilization and/or Disruption of a CD4-Bound State Reveals Distinct Conformations of the Human Immunodeficiency Virus Type 1 gp120 Envelope Glycoprotein. J. Virol 76:9888-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]