Abstract
Intersectin 1L is a scaffolding protein involved in endocytosis that also has guanine nucleotide exchange activity for Cdc42. In the context of the full-length protein, the catalytic exchange activity of the DH domain is repressed. Here we use biochemical methods to dissect the mechanism for this inhibition. We demonstrate that the intersectin 1L SH3 domains, which bind endocytic proteins, directly inhibit the activity of the DH domain in assays for both binding and exchange of Cdc42. This inhibitory mechanism seems to act through steric hindrance of Cdc42 binding by an intramolecular interaction between the intersectin 1L SH3 domain region and the adjacent DH domain. Surprisingly, the mode of SH3 domain binding is other than through the proline peptide binding pocket. The dual role of the SH3 domains in endocytosis and repression of exchange activity suggests that the intersectin 1L exchange activity is regulated by endocytosis. We show that the endocytic protein, dynamin, competes for binding to the SH3 domains with the neural Wiskott-Aldrich Syndrome protein, an actin filament nucleation protein that is a substrate for activated Cdc42. Swapping of SH3 domain binding partners might act as a switch controlling the actin nucleation activity of intersectin 1L.
INTRODUCTION
Rho family guanine nucleotide exchange factors (GEFs) are critical regulatory proteins of cellular pathways that require regulation of actin cytoskeleton rearrangements (for review see Zheng, 2001; Hoffman and Cerione, 2002). Their conserved catalytic domain, the Dbl homology (DH) domain (Hart et al., 1991, 1994) catalyzes the release of GDP from Rho GTPases and thus subsequent activation by GTP binding. DH domains are invariably found upstream and adjacent to pleckstrin homology (PH) domains, which are thought to influence their activity (Liu et al., 1998; Das et al., 2000) and membrane localization (Whitehead et al., 1999). The noncatalytic parts of the structurally complex GEFs link the exchange activity to cellular processes and inhibit the DH domain exchange activity (Zheng, 2001; Hoffman and Cerione, 2002). Cellular inputs, such as protein (Hart et al., 1998; Scita et al., 1999; Innocenti et al., 2002) and phospholipid binding to (Han et al., 1998; Nimnual et al., 1998; Crompton et al., 2000; Das et al., 2000; Russo et al., 2001), and phosphorylation of (Crespo et al., 1997; Han et al., 1997; Schuebel et al., 1998; Aghazadeh et al., 2000) these regulatory regions derepress exchange activity. Integration of cellular signals by Rho GEFs can focus GTPase activity to allow temporal and spatially localized activation of actin cytoskeletal rearrangements.
Intersectin 1L, a neuronal splice variant of the endocytic scaffolding protein intersectin 1, is a Rho family GEF that is composed of two N-terminal EH domains (EH1, EH2), a large region of putative coiled-coils, five SH3 domains (SH3A, B, C, D, and E; Roos and Kelly, 1998; Yamabhai et al., 1998; Okamoto et al., 1999; Sengar et al., 1999), followed by the DH and PH domains and a carboxyl-terminal C2 domain. This GEF is unusual in that the DH and PH domains are not fundamental to the cellular role of the protein and are instead alternatively spliced onto a ubiquitously expressed isoform, intersectin 1S (Guipponi et al., 1998; Hussain et al., 1999; Pucharcos et al., 2001). Intersectin 1S, which binds multiple endocytic proteins, is localized to sites of clathrin-mediated endocytosis through protein interactions with its EH domains (Hussain et al., 1999; Sengar et al., 1999). The Drosophila intersectin 1S homologue, Dap160, colocalizes with the endocytic protein, dynamin, to peri-active zone regions in the neuromuscular junction that are sites of synaptic vesicle endocytosis (Roos and Kelly, 1999). Furthermore, overexpression of intersectins or the SH3 domains alone inhibits clathrin-mediated endocytosis of transferrin (Sengar et al., 1999; Simpson et al., 1999; Pucharcos et al., 2000). One attractive mechanism for this inhibition is the binding and sequestration of dynamin by the SH3 domains away from productive endocytic complexes (Roos and Kelly, 1998; Yamabhai et al., 1998; Hussain et al., 1999; Okamoto et al., 1999; Sengar et al., 1999). Based on these studies, intersectin 1 has been proposed to act as a scaffolding protein that holds a pool of the endocytic machinery at specialized zones of the plasma membrane. Because the noncatalytic domains of intersectin 1 are so strongly linked to the endocytic pathway, it is plausible that the GEF activity of intersectin 1L is regulated in some manner by endocytosis.
Several recent studies address the pathways downstream of the intersectin 1L GEF activity. The intersectin 1 SH3 domains interact with not only endocytic proteins, but also with a stimulator of actin filament nucleation (Miki et al., 1996, 1998; Rohatgi et al., 1999), the neural Wiskott-Aldrich syndrome protein (N-WASP; Hussain et al., 2001; McGavin et al., 2001). Microinjection of intersectin 1L or the DH domain alone stimulates filopodia formation in cultured cells, an effect not seen with intersectin 1S (Hussain et al., 2001) despite its ability to interact with N-WASP. Binding of the N-WASP proline-rich domain (PRD) to intersectin 1L stimulates the Cdc42 exchange activity of immunoprecipitates of the full-length protein (Hussain et al., 2001). Additional insight comes from a study of an intersectin 1L homologue, the ubiquitously expressed intersectin 2L (Pucharcos et al., 2000). Intersectin 2L participates with WASP and Cdc42 in the stimulated endocytosis of T-cell antigen receptor (TCR), which is actin dependent. The presence of the DH domain mitigated the inhibitory effects of the rest of intersectin 2L on stimulated TCR endocytosis (McGavin et al., 2001). These studies of intersectin 2L therefore support the suggestion that intersectin 1L acts to link endocytosis and actin cytoskeleton regulation.
To coordinate the processes of endocytosis and actin cytoskeletal rearrangements, intersectin 1L must integrate the various signals it receives and regulate an output through its DH domain exchange activity. We find that the adjacent SH3 domains are likely to be critically responsible in this regulation. We show that the SH3 domain region inhibits exchange activity through direct interaction with the DH domain and blockage of Cdc42 binding. Furthermore, we show that neither dynamin nor N-WASP binding alone is sufficient to activate the intersectin 1L GEF activity and that an additional level of regulation is necessary. Furthermore, dynamin binding competes with N-WASP binding, suggesting a mechanism for the productive regulation of actin cytoskeletal rearrangements at the nerve terminal in response to endocytosis.
MATERIALS AND METHODS
DNA Constructs and Recombinant Proteins
A mouse intersectin 1L (ese1L) clone with Kozak sequence was generated by PCR from cDNA made from a C57 Black mouse brain and ligated into the KpnI and NotI sites in mammalian expression vector pCDNA3.1/Myc-His (+) B (Invitrogen, Carlsbad, CA). The sequence differs from GenBank entry 4378891 at amino acids (aa) 179, Trp to Leu, and 402, Ala to Arg. These substitutions revert the amino acid residues to conserved sequence with the human, rat, and Xenopus homologues. An additional five-aa insert, Val-Lys-Gly-Glu-Trp is present between aa 767 and 768. The DHPH domain expression construct, aa 1214–1570 with Kozak sequence was constructed by PCR cloning into pCDNA3.1/Myc-His (+) B at the KpnI and NotI sites. Glutathione S-transferase (GST) fusion protein constructs were generated by PCR cloning into pGEX5X-1 (Amersham Pharmacia, Piscataway, NJ). GST DHPH, aa 1214–1570, GST DHPHC2, aa 1214–1714, and GST DH, aa 1214–1429, were cloned into the BamHI and NotI sites. GST SH3ABCDE-DHPH, aa 693-1570, GST SH3CDE-DHPH, aa 961-1570, GST SH3DE-DHPH, aa 1049–1570, GST SH3E-DHPH, aa 1128–1570, GST SH3CDE-DH, aa 961-1431, GST SH3A, aa 693–810, GST SH3B, aa 872–999, GST SH3C, aa 961-1065, and GST SH3D, aa 1049–1147, were cloned into the EcoRI and NotI sites. GST SH3ABCDE, aa 693-1214, GST SH3CDE, aa 961-1214, and GST SH3E, aa 1128–1214, were cloned into the EcoRI site. The P1122L and P1198L mutations in the SH3D and SH3E, respectively, were produced using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). GST EH1EH2, aa 1–307, was cloned into the BamHI and EcoRI sites of pGEX2T. Recombinant GST fusion proteins were expressed in Escherichia coli BL21 cells according to standard methods and purified by batch binding from cell lysates to glutathione agarose beads (Sigma-Aldrich, St. Louis, MO). Recombinant SH3ABCDE was produced by first purification as a GST fusion protein and subsequent cleavage by factor XA protease (Pierce, Rockford, IL) and then repurified from GST and the protease as recommended by the manufacturer. GST fusion constructs for GST RhoA, Rac1, and Cdc42 were the generous gift of Dr. D. Kalman (Emory University, Atlanta, GA). Recombinant Cdc42 was cleaved from GST Cdc42 on glutathione agarose beads by thrombin protease (Amersham Pharmacia). 6× His DHPH, aa 1214–1570 was subcloned into pET32c vector using the BamHI and NotI sites. 6× His DH, aa 1214–1429, was cloned into pET32C using the BamHI site. 6× His SH3E-DHPH, aa 1128–1570, was subcloned into pET32a vector using the EcoRI and NotI sites. Recombinant 6× his fusion proteins were expressed in BL21 cells and batch-purified over Ni-NTA Superflow (Qiagen, Valencia, CA). Recombinant HA epitope-tagged dynamin was the generous gift of Ms. A. Jones and Dr. S. Schmid (The Scripps Research Institute, La Jolla, CA). Recombinant N-WASP was expressed from an N-WASP expressing baculovirus, the kind gift from Dr. J. Taunton (University of California, San Francisco, CA). N-WASP was expressed in SF9 cells cultured in suspension and purified as previously published (Miki et al., 1998; Rohatgi et al., 1999).
Antibodies
Polyclonal anti-intersectin 1 EH domain antibody (no. 4396, Alpha Diagnostic International, San Antonio, TX) and polyclonal anti-intersectin 1L DH domain antibody (no. 4199, Alpha Diagnostic International, Inc.) were raised in rabbits against the GST EH1EH2 fusion protein and the GST DH fusion protein, respectively. Affinity purification was done using the original GST fusion proteins after preadsorption of antiserum against GST. Rabbit polyclonal anti-dynamin antibody (no. 2704) was previously described (Roos and Kelly, 1998). Rabbit polyclonal anti–N-WASP antibody was a gift from Dr. J. Taunton (University of California, San Francisco). Antibodies against RhoA (sc-418) and Cdc42 (sc-8401) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against Rac1 (cat no. R56220) was purchased from Transduction Laboratories (San Diego, CA). Anti-6× His Tag antibody was purchased from Novagen (Madison, WI).
Cell Culture and Immunofluorescence
Cos cells were maintained in DME H-21 supplemented with 10% fetal bovine serum and penicillin/streptomycin at 37°C in 5% CO2. Transfection of DHPH constructs in pCDNA3.1/Myc-His (+) B was done with FuGENE 6 transfection reagent (Roche, Indianapolis, IN) in serum-containing media. Forty-eight hours posttransfection cells were fixed with 4% p-formaldehyde, blocked with 2% BSA, and 1% fish skin gelatin and permeabilized with 0.02% saponin in PBS. Actin filaments were visualized with rhodamine-phalloidin (Molecular Probes, Eugene, OR). Photos were taken with Kodak EliteChrome Select Series 100 film on a 35-mm camera on a Zeiss Axioskop microscope through a 100× oil immersion lens. Color slides were developed and scanned into computer files.
GST Pull-downs
Rat brain cytosol was prepared as previously described (Clift-O'Grady et al., 1998) and then dialyzed into binding buffer. GST fusion proteins were bound to glutathione agarose beads (Sigma Chemical Co.) in binding buffer (20 mM Tris, pH 7.4, 100 mM NaCl, 10 mM EDTA, 1 mM DTT) with 1% Triton X-100. MgCl2 binding buffer is binding buffer with 5 mM MgCl2 replacing the 10 mM EDTA. Incubation with rat brain cytosol or recombinant protein was done rotating overnight at 4°C. Proteins pulled down from rat brain cytosol were eluted with 10 mM reduced glutathione in 50 mM Tris, pH 8.0. Pulled down recombinant proteins were eluted as above or by boiling in sample buffer. All eluates were resolved by SDS-PAGE, transferred to Immobilon-P PVDF membrane (Millipore, Billerica, MA) or Protran nitrocellulose (Schleicher and Schuell, Dassel, Germany), and blocked with blotto (5% dry milk, 0.05% Tween 20 in PBS). Western blotting was visualized with horseradish peroxidase–conjugated secondary antibodies in ECL Western detection reagents (Amersham Pharmacia) on Hyperfilm ECL (Amersham Pharmacia.) Any alteration of method is noted in the figure legends.
In Vitro Exchange Assays
In vitro exchange assays were carried out as described in Zheng et al. (1995). GTPase was preloaded with nucleotide by incubating in loading buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 2 mM EDTA, 0.2 mM DTT, 100 μM AMP-PNP, and 10 μM GDP) for 5 min at RT. MgCl2 was added to 5 mM final concentration and incubated at RT for 15 min. Loaded GTPase (20 μl; final concentration of 0.25–1.25 μM as specified in the figure legends) was added to exchange proteins in reaction buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 10 mM MgCl2, 100 μM AMP-PNP, 0.5 mg/ml BSA, and 5 μM [35S]GTPγS; ∼11,000 cpm/pmol) in 80 μl total volume. Any recombinant proteins were preincubated with the exchange proteins for 2 h at 4°C. Reactions were done at RT with 15-μl reaction aliquots removed at time points, diluted in cold termination buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 10 mM MgCl2), and filtered through nitrocellulose filters. When loss of [3H]GDP is followed rather than binding of [35S]GTPγS, ∼10 μM [3H]GDP (10 Ci/mmol) replaces cold GDP in the loading buffer and 1 mM GTP replaces [35S]GTPγS in the reaction buffer. The exchange index at 10-min compares extent of exchange at 10 min using the exchange catalyzed by GST DHPH at 10 min as 100% and the background binding of [35S]GTPγS in the presence of GST as 0. The exchange index was calculated by subtracting out the GST control background counts for each exchange reaction and dividing by the DHPH-positive control counts to determine exchange activity relative to the DHPH domains alone.
RESULTS
The Intersectin 1L DH Domain Interacts with the Actin Cytoskeleton
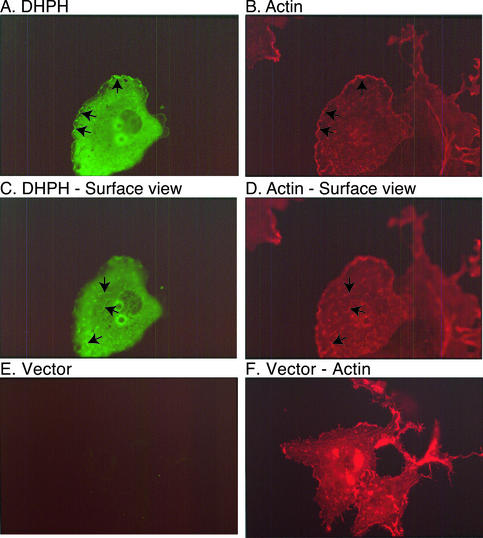
Intersectin 1L has both downstream and upstream partners. Because such partners often colocalize, light microscopy can help to identify them. Full-length intersectin 1L normally associates with clathrin-coated pits at the plasma membrane and not with the actin cytoskeleton (Hussain et al., 1999; Sengar et al., 1999, and unpublished data). To determine if the DH domain can interact with actin structures, we overexpressed DHPH domain constructs in Cos cells by transient transfection. The localization of overexpressed DHPH fragments, which lack the N-terminal EH domains that direct intersectin 1 to sites of endocytosis, is strikingly different from that of the full-length protein. The DHPH fragment strongly colocalizes with actin particularly in ruffles at the cell periphery (Figure 1, A and B, arrows, focal plane through the cell center) and also on the cell surface (Figure 1, C and D, arrows, focal plane on dorsal surface of cells). The anti-DH domain antibody has no background immunoreactivity in vector-transfected cells (Figure 1, E and F). These morphological data suggest that intersectin 1L may link the endocytic machinery to actin turnover perhaps via interaction with Rac1, which is enriched in plasma membrane ruffles.
Figure 1.
The DHPH domains of intersectin 1L localize to actin ruffles. The DHPH domains of intersectin 1L are overexpressed in Cos cells and visualized with antibody no. 4199, anti-intersectin DH domain, and FITC-conjugated secondary antibody (A, C, and E). Filamentous actin is visualized with rhodamine-phalloidin (B, D, and F). Although much of the DHPH expression is cytosolic, there is colocalization with actin ruffles at the periphery (arrows in A and B) and on the dorsal surface (arrows in C and D) of the cells. (E) The anti-intersectin DH domain antibody has little background staining in vector-transfected Cos cells at the same photographic exposure.
We did not see induction of filopodia, as has been reported after microinjection of the DH domain and full-length intersectin 1L into Swiss 3T3 cells (Hussain et al., 2001). Expression by transfection may not produce the same effects in cells as acute induction by microinjection. More subtle changes in cell morphology may be present, but were not dramatic in the overall population of transfected cells.
The Intersectin 1L DH Domain Acts as an Exchange Factor In Vitro Specifically for Cdc42
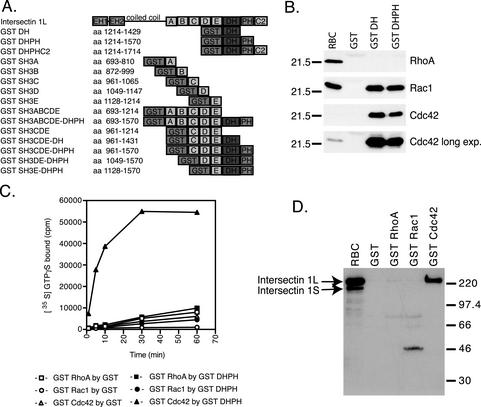
The intersectin 1L DH domain has been shown to catalyze exchange for one GTPase, Cdc42 (Hussain et al., 2001; McGavin et al., 2001). Because many DH domains show exchange activity on multiple Rho GTPase family members (Hart et al., 1994; Olson et al., 1996), we tested DH domain specificity using recombinant GST fusion proteins in several complementary assays. Figure 2A displays the intersectin 1L domain structure and aa position of the recombinant fusion proteins used in this study. GST fusion proteins of the DH domain alone and the DHPH domains together were used in pull-down experiments with rat brain cytosol. As with many DH domains, the intersectin 1L DH domain showed interactions with several GTPases, in this case Rac1 and Cdc42 (Figure 2B). Binding of Rac1 is consistent with the localization of the intersectin 1L DH domain to actin ruffles (Figure 1). However, Cdc42 was far more enriched than Rac1 in the pull-down eluates relative to rat brain cytosol. No RhoA binding was observed to the intersectin 1L DH domain.
Figure 2.
The intersectin 1L DH domain can associate with Cdc42 and Rac1, but its exchange activity is focused on Cdc42. (A) Diagram of the intersectin 1L domain structure. The domain structure and aa position of the recombinant fusion proteins used in this study are displayed. (B) GST pull-downs of small GTPases by the DH domain from rat brain cytosol. GST, GST DH, and GST DHPH, 6 μM, in 5 mg rat brain cytosol in binding buffer. GST alone does not pull-down any small GTPase. GST fusion proteins containing the DH domain pull-down Rac1 and Cdc42, but not RhoA. Overexposure of the Cdc42 samples allows normalization to the starting material and reveals that Cdc42 recovery is significantly higher than that of Rac1. (C) In vitro exchange reaction time course. GST and GST DHPH fusion protein, 750 nM, were assayed for exchange activity using 250 nM GST RhoA, GST Rac1, and GST Cdc42. [35S]GTPγS loading by GST on GST RhoA (□), GST DHPH on GST RhoA (▪), GST on GST Rac1 (○), GST DHPH on GST Rac1 (●), GST on GST Cdc42 (▵), and GST DHPH on GST Cdc42 (▴) as shown. The intersectin 1L DH domain exchange activity is specific for Cdc42. (D) GST pull-downs of intersectin L from rat brain cytosol by GST small GTPases. GST, GST RhoA, GST Rac1, and GST Cdc42, 2.3 μM, were rotated overnight in 5 mg rat brain cytosol at 4°C in binding buffer. After elution from glutathione agarose, the samples were blotted with antibody no. 4396 that recognizes both the long and short forms of intersectin 1. GST Cdc42 pulls down intersectin L, but not intersectin S. GST RhoA and GST Rac1 do not pull down any intersectin.
To determine if binding specificity correlated with exchange specificity, the GST fusion proteins of DHPH (Figure 2C) and DH (unpublished data) were tested for guanine nucleotide exchange activity in vitro by incorporation of [35S]GTPγS into GST fusion proteins of RhoA, Rac1, and Cdc42. Surprisingly, GST DHPH and GST DH exhibited guanine nucleotide exchange activity specifically on Cdc42 and not RhoA or Rac1 despite having some binding activity toward the latter. In the absence of added exchange factor activity, GST RhoA, Rac1, and Cdc42 fusion proteins incorporated [35S]GTPγS to the same degree in binding studies (unpublished data). The same specificity for Cdc42 was found for intersectin 2L using a Pak-Cdc42/Rac interactive-binding domain assay (McGavin et al., 2001). We next asked whether the binding of the DH domain to small GTPases was affected by the remainder of the intersectin 1L protein. GST fusion proteins of RhoA, Rac1, and Cdc42 were used in pull-downs with rat brain cytosol (Figure 2D) and bound intersectin 1 was quantified with an antibody against the EH domains that recognizes both the long and short forms of intersectin 1. GST Cdc42, but not GST RhoA or GST Rac1 pulled down intersectin 1L but not the shorter, ubiquitous intersectin 1S, which lacks the DH and other C-terminal domains, indicating that Cdc42 alone is able to interact with the DH domain in the context of full-length intersectin 1L.
The In Vitro GEF Activity of the Intersectin 1L DH Domain Is Negatively Regulated by Its Adjacent SH3 Domains
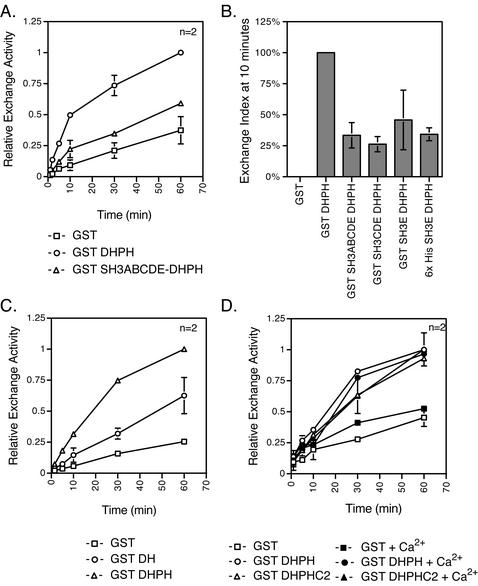
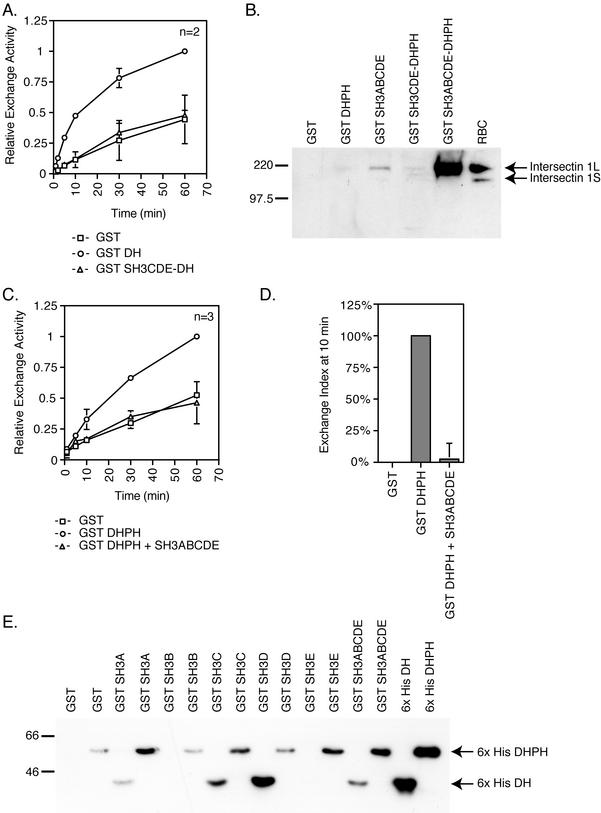
Like many other GEFs, the intersectin 1L DH domain seems to be in a normally repressed state and needs to be activated by binding of other cytoplasmic components (Hussain et al., 2001). Repression of GEF activity could be due to inhibition by the noncatalytic domains of intersectin 1L. To identify such domains, GST DH constructs containing adjacent domains, the upstream SH3 domains or the downstream PH and C2 domains, were assayed for exchange activity in vitro by incorporation of [35S]GTPγS into GST Cdc42. The exchange activity of GST SH3ABCDE-DHPH was inhibited relative to GST DHPH alone (Figure 3A). At the 10-min time point, the extent of exchange by fusion proteins containing one SH3 domain, SH3E, in the context of both GST and 6× His fusion proteins, is reduced relative to the DHPH domains alone. Additional SH3 domains may increase the inhibition (Figure 3B). The consistent and reproducible reduction of exchange activity in constructs containing SH3 domains demonstrates that the SH3 domains are acting to inhibit DH domain exchange activity. When these same intersectin 1L fragments are immunoprecipitated out of transfected cell extracts, the exchange activity of the SH3 domain containing constructs are inhibited relative to the DHPH domains alone (unpublished data). Furthermore, because inhibition is seen when assays were done in vitro with recombinant proteins, the inhibition caused by the SH3 domains is likely to be direct.
Figure 3.
The upstream SH3 domains, but not the downstream PH and C2 domains, inhibit the DH domain exchange activity in vitro. Incorporation of [35S]GTPγS into Cdc42 was measured. (A) In vitro exchange reaction kinetics were measured in experiments using 50 or 75 nM of GST (□), GST DHPH (○), or GST SH3ABCDE-DHPH (▵) and 500 nM recombinant GST Cdc42. The SH3ABCDE domains added in cis with the DHPH domains inhibit the exchange activity of the DH domain. (B) The exchange index at 10 min is a comparison of the exchange activity of the DHPH domains with the SH3ABCDE, SH3CDE, and SH3E domains added in cis relative to the exchange activity of the DHPH domains alone. The GST background cpm have been subtracted out (see MATERIALS AND METHODS). The single SH3E domain inhibits (50%) the DH exchange activity, but additional SH3 domains may increase the inhibitory activity. (C) In vitro exchange reaction kinetics were measured using 75 nM GST (□), GST DH (○), or GST DHPH (▵) on 1 μM Cdc42. The exchange activity of the DH domain alone is reduced relative to the DHPH domain. (D) In vitro exchange reaction time course. Fusion proteins, 75 nM, were assayed for exchange activity on 1.25 μM Cdc42 in the presence or absence of 640 μM Ca2+. GST (□), GST + Ca2+ (▪), GST DHPH (○), GST DHPH + Ca2+ (●), GST DHPHC2 (▵), and GST DHPHC2 + Ca2+ (▴). The C2 domain added in cis does not strongly affect the DH domain activity in either the presence or the absence of Ca2+.
We next tested the role of the PH domain in exchange reactions in vitro. As has been seen for other DH domains (Liu et al., 1998), the exchange activity of the intersectin 1L DH domain alone is reduced relative to the exchange activity of the DHPH domains together (Figure 3C and Hussain et al., 2001). Phosphatidylinositol phosphates have been shown to bind GEF associated PH domains and in some instances this binding regulates the DH domain exchange activity (Han et al., 1998; Nimnual et al., 1998; Crompton et al., 2000; Das et al., 2000; Russo et al., 2001; Snyder et al., 2001). When exchange assays are done with the intersectin 1L DH domain in the presence of phosphatidylinositol-(4,5)-P2 (PIP2) liposomes or soluble phosphatidylinositol phosphates, no effect is observed on the exchange activity (unpublished data), consistent with published results (Snyder et al., 2001). Finally, we tested the role of the downstream C2 domain in regulating DH domain exchange on Cdc42. In contrast to the SH3 domains, the C2 domain did not have a strong effect on the DH domain exchange activity even in the presence of added Ca2+ (Figure 3D), suggesting that the role of the C2 domain in vivo may be other than regulation of exchange activity, perhaps related to membrane binding and localization (Rizo and Sudhof, 1998).
The Intersectin 1L SH3 Domains Inhibit DH Domain Exchange Activity by Reducing Cdc42 Binding
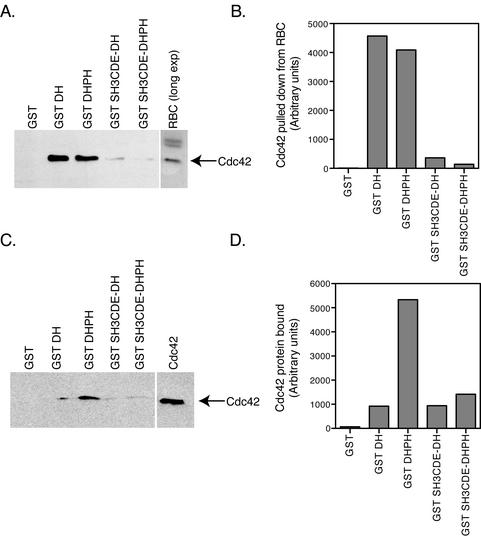
We next explored how the SH3 domains inhibit the activity of the DH domain. The SH3 domains could either be preventing Cdc42 binding, and therefore exchange activity, or they could be inhibiting the enzymatic exchange activity directly. To determine if the SH3 domains affect Cdc42 binding, we carried out GST pull-downs from rat brain cytosol with 2.5 μM GST fusion proteins of the DH and DHPH domains with and without adjacent SH3 domains (Figure 4A). Quantification of the Western immunoreactivity shows a 80–95% inhibition of DH domain-mediated Cdc42 pull-down from rat brain cytosol by the SH3CDE domains (Figure 4B). To see if the reduction in Cdc42 binding was due to a direct effect of the SH3 domains or was through some intermediary protein brought down by the SH3 domains from the rat brain cytosol, we used pure recombinant fusion proteins in an in vitro binding assay, which, however, use much lower concentrations of DH domain. 10 nM GST DH and GST DHPH domains with and without adjacent SH3 domains were bound to recombinant Cdc42 (Figure 4C). Normalization of Cdc42 immunoreactivity relative to the GST fusion protein immunoreactivity (Figure 4D) shows that the SH3CDE domains inhibit binding of Cdc42 to the DHPH domains by 70%. The DH domain alone under these conditions also exhibits less binding to Cdc42 as compared with the DHPH domains. This reduction in Cdc42 binding to the DH domain alone is consistent with the lower exchange activity of the DH domain alone relative to the DHPH domains (Figure 3C). The inconsistency in Cdc42 binding to GST DH between Figure 4A and 4C may be due to the DH domain alone having an intrinsically lower affinity for Cdc42 than the DHPH domains together. This difference in affinity may be more strongly evident in the mid-nanomolar concentration range used for the exchange reactions (Figure 3C) and the binding with recombinant proteins (Figure 4C) than in the low-micromolar concentration range that must be used in the pull-down experiment out of rat brain cytosol (Figure 4A). Unlike in the exchange assay, a further reduction in binding activity is not seen with the addition of the SH3 domains to the DH domain alone (see Figure 5A). Although there are some as yet unexplained differences between using cytosol and pure proteins, the results show that the inhibition of Cdc42 binding is likely due to the direct effect of the SH3 domains blocking the Cdc42 binding site on the DH domain through steric hindrance.
Figure 4.
The SH3 domains directly inhibit the binding of Cdc42 to the DH domain. (A) GST pull-downs of Cdc42 from rat brain cytosol by GST DH domain fusion proteins. GST fusion proteins, 2.5 μM, containing the DH were incubated in 1.5 mg rat brain cytosol in binding buffer. Glutathione agarose beads were added to pull-down the protein after overnight incubation. Cdc42 is pulled down by all the DH domain-containing proteins. However, the SH3CDE domains in cis strongly inhibit the binding of Cdc42 to the DH domain. (B) Quantification of Cdc42 pull-down results. The Western blot in A was quantified using the NIH Image 1.61 program. The SH3CDE domains inhibit Cdc42 binding from rat brain cytosol by 80–95%. GST pull-down using recombinant Cdc42 instead of cytosol. (C) GST DH domain containing fusion proteins, 10 nM, and 160 nM Cdc42 were incubated in binding buffer + 0.1% Triton X-100. Recombinant Cdc42 bound to all the DH domain-containing fusion proteins. The SH3CDE domains again inhibit binding of Cdc42 to the DHPH domains. (D) Cdc42 binding was normalized to GST fusion protein immunoreactivity using NIH Image 1.6 program. The ratio of Cdc42 binding to GST fusion protein is expressed in arbitrary units. Binding of Cdc42 to the DHPH domains is inhibited 70% by the SH3CDE domains. Binding of Cdc42 to the DH domain alone is also 70% lower than to the DHPH domains. No further reduction in Cdc42 binding to the DH domain alone is seen by addition of the SH3CDE domains.
Figure 5.
Evidence for an intramolecular interaction between the intersectin 1L SH3 and DH domains. (A) In vitro exchange reaction kinetics were measured using 250 nM GST (□), GST DH (○), or GST SH3CDE-DH (▵) on 533 nM GST Cdc42. The exchange activity of the DH domain in the absence of the PH domain is reduced by the addition of the SH3CDE domains in cis. (B) GST pull-downs of intersectin 1 from rat brain cytosol by the intersectin 1L SH3 domains. GST SH3, 5 μM, containing fusion in 1.5 mg rat brain cytosol in MgCl2 binding buffer. Western blotting of glutathione agarose eluates was done with antibody no. 4396. The GST SH3ABCDE fusion protein binds intersectin 1L protein. The SH3ABCDE-DHPH fusion protein pulls down larger amounts of intersectin 1L. (C) Time course of an in vitro exchange reaction, where SH3 domains are added in trans. Incorporation of [35S]GTPγS into Cdc42 was followed. Data compiled from several experiments using 25 nM GST + 10–20 μM GST (□), 25 nM GST DHPH + 10–20 μM GST (○), or 25 nM GST DHPH + 10–20 μM (depending on the experiment) recombinant SH3ABCDE in trans (▵) were assayed for exchange activity on 1.25 μM Cdc42. The SH3ABCDE domains added in trans inhibit the activity of the DHPH domains using pure recombinant proteins. (D) The exchange index at 10 min compares fusion protein exchange activity to that of the DHPH domains. The SH3ABCDE domains in trans completely inhibited the exchange activity of the DHPH domains. (E) The SH3 domains bind the DH domain. Binding of recombinant 6× His DH (5 μM) and 6× His DHPH (5 μM) domains by individual and tandem GST SH3 domains (15 μM) in MgCl2 binding buffer. Western blotting of glutathione agarose eluates was done with anti-6× His Tag antibody. The individual SH3A, C, and D domains and the SH3ABCDE domains in tandem bind the DH domain. The SH3E domain binds to the DHPH domains.
The Intersectin 1 SH3 Domains Bind to Intersectin 1L DH Domain
We next further investigated the mechanism for the inhibition of the DH domain. One possibility is that the SH3 domains are acting directly through the DH domain. In this case, the SH3 domains should have inhibitory activity even in the absence of the PH domain. Therefore, we compared e exchange activity of the DH domain alone in cis with the SH3CDE domains with the DH domain alone. Addition of the SH3 domains to the DH domain alone inhibits exchange activity in the absence of the PH domain (Figure 5A), suggesting that the mechanism for SH3 inhibition of exchange activity is through the DH domain, and not the PH domain. Because the intersectin 1L SH3 domains are able to directly inhibit the Cdc42 binding and exchange activity of the DH domain, they might be binding the DH domain itself. We tested this prediction by asking if the SH3 domains could bind to intersectin 1L itself. GST SH3 domain-containing fusion proteins were used in GST pull-down experiments out of rat brain cytosol (Figure 5B). The SH3ABCDE domains in tandem pull down intersectin 1L. Fusion protein that contains both the SH3 and DH interaction domains binds larger amounts of intersectin 1L from rat brain cytosol, implying that both domains can participate in intermolecular interactions. Longer exposure of the Western blot reveals a small amount of intersectin 1L binding to the GST DHPH and GST SH3CDE-DHPH fusion proteins (unpublished data), suggesting that multiple SH3 domains increase the affinity of the interaction.
Further support for a direct interaction between the intersectin 1L SH3 domains and the DH domain and a role for this interaction in regulation of exchange came from in vitro exchange assays with GST DHPH in trans with the SH3ABCDE domains (Figure 5C). Quantification of the extent of exchange at 10 min shows that GST DHPH exchange activity is abolished with the addition of recombinant SH3ABCDE to the exchange reaction (Figure 5D). This inhibition of exchange by the SH3 domains in trans as well as the intermolecular interaction seen with GST pull-downs (Figure 5B) requires concentrations of SH3 domains in the low-micromolar range. However, inhibition of exchange by the SH3 domains in cis to the DH domain is constant down to mid-nanomolar concentrations (Figure 3, A and B, and 5A). Because SH3 inhibition of exchange in cis is not concentration dependent, we favor the idea that the interaction is normally intramolecular. We further show that recombinant SH3 domains, specifically SH3A, B and D, directly bind the recombinant DH domain (Figure 5E). The SH3D domain binds especially strongly. The PH domain may influence this association, supporting an interaction with the SH3E domain but possibly inhibiting the DH domain interaction with other SH3 domains. These results demonstrate a role for a direct interaction between the adjacent SH3 domains and the DH domain in the repression of exchange activity.
SH3–PxxP Binding Pocket Is Not Involved in Binding and Inhibition of DH Domain Activity
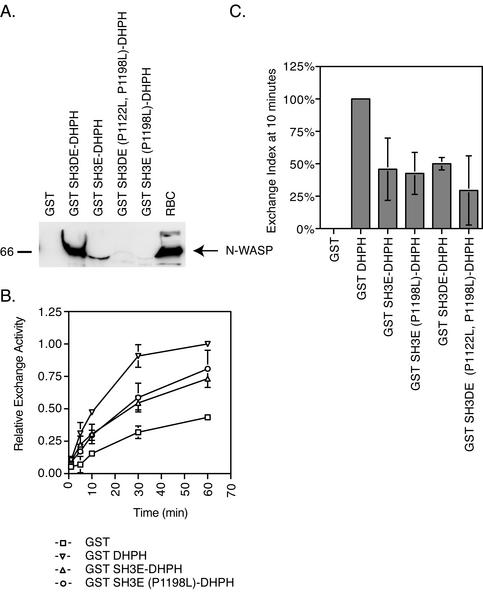
The SH3 domain region directly binds to and inhibits the DH domain in vitro. We next asked whether an SH3 domain interaction with a proline peptide structure (Cicchetti et al., 1992; Ren et al., 1993) is responsible for the inhibitory activity. Although there are no PxxP consensus sequences in the DH domain, it has been shown that other sequences are also able to bind the SH3 domain binding pocket (Mongiovi et al., 1999; Kang et al., 2000). We made mutations that disrupt SH3-PxxP binding (Clark et al., 1992; Qualmann et al., 2000) in the SH3 domains of GST SH3E-DHPH and GST SH3DE-DHPH and tested the in vitro exchange activities of the mutant fusion proteins on Cdc42. The P1198L mutation in GST SH3E-DHPH and the P1122L, P1198L double SH3 mutation in GST SH3DE-DHPH abolish binding of the fusion proteins to N-WASP (Figure 6A) in pull-downs from rat brain cytosol. If the PxxP binding pocket is used for the inhibitory interaction with the DH domain, then the GST SH3E (P1198L)-DHPH and the GST SH3DE (P1122L, P1198L)-DHPH mutant proteins should no longer be inhibited in in vitro exchange assays. Interestingly, the exchange activities of the SH3 domain mutants of the GST SH3E-DHPH and GST SH3DE-DHPH proteins are still inhibited relative to the GST DHPH domain alone (Figure 6B). The exchange index at 10 min is ∼50% for both GST SH3E-DHPH and GST SH3E (P1198L)-DHPH as well as for both GST SH3DE-DHPH and GST SH3DE (P1122L, P1198L)-DHPH relative to the DHPH domains alone (Figure 6C). This suggests that although the SH3 domains are necessary and sufficient for inhibiting exchange activity, it is unlikely that binding and inhibition is through a DH domain interaction with the PxxP binding pockets of the SH3 domains.
Figure 6.
The SH3 domains do not interact with and inhibit the DH domain through their PxxP binding pocket. (A) Inhibition of SH3 domain pull-down of N-WASP from rat brain cytosol by PxxP binding pocket mutations. 5 μM GST fusion proteins in 1.5 mg rat brain cytosol in MgCl2 binding buffer. The GST SH3E-DHPH and GST SH3DE-DPH fusion proteins pull down N-WASP. The binding pocket mutations in the SH3D and SH3E domains abolish binding to N-WASP. Note that the GST SH3E DHPH fusion protein distorts the N-WASP band by running on top of it. (B) Time course of an in vitro exchange reaction with the SH3 mutants. GST (□), GST DHPH (▿), GST SH3E-DHPH (▵) and GST SH3E (P1198L)-DHPH (○), 75 nM, were assayed for exchange activity on 1.25 μM Cdc42. (C) The exchange index at 10 min compares fusion protein exchange activity to that of the DHPH domains. The GST SH3E-DHPH and GST SH3DE-DHPH fusion protein exchange activities are inhibited 50% relative to GST DHPH alone. The P1122L and P1198L mutations in the SH3D and SH3E domains, respectively, that abolish binding to N-WASP do not effect this inhibition.
Binding to SH3 Domains Does Not Interfere with the SH3 Domain–mediated Inhibition of Cdc42 Exchange
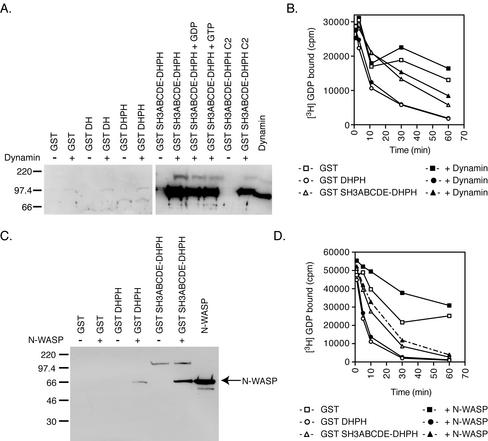
The exchange activity of overexpressed intersectin 1L immunoprecipitated from cell extracts is stimulated by adding proline-rich domain fragments of N-WASP (Hussain et al., 2001). We asked if dynamin, an endocytic protein that also binds the intersectin SH3 domains through a proline peptide interaction, could play a similar role in vitro. Dynamin binds with high affinity specifically to GST constructs containing the SH3 domains, and its binding state is not strongly affected by its nucleotide state or the presence of the C2 domain (Figure 7A). Because dynamin is a GTPase and will therefore bind guanine nucleotides, we altered the exchange reaction assay to follow loss of [3H]GDP from Cdc42 rather than binding of [35S]GTPγS. GST SH3ABCDE-DHPH or DHPH alone was preincubated with recombinant dynamin and then assayed for exchange activity in the continued presence of dynamin. However, dynamin binding to the SH3 domains had no effect on the inhibitory activity of the SH3 domains in the exchange assay (Figure 7B). Stimulation of exchange activity in vivo used the proline-rich domain of N-WASP, not dynamin (Hussain et al., 2001). Therefore, we tested the ability of recombinant N-WASP binding to GST SH3CDE-DHPH to relieve SH3 repression of DH domain exchange activity in vitro. Despite extensive binding of recombinant full-length N-WASP to the GST SH3CDE-DHPH fusion protein (Figure 7C), N-WASP was unable to derepress the DH domain in vitro (Figure 7D). This is consistent with the retention of inhibitory activity by the mutant SH3 domain that cannot bind PRD containing proteins (Figure 6). Addition of PIP2 to these assays increases the binding of N-WASP to the SH3 domains, but does not affect the outcome of the exchange reaction (unpublished data). Unlike what has been reported for the N-WASP PRD in vivo (Hussain et al., 2001), neither dynamin nor N-WASP binding to SH3 domains in vitro has any effect on the ability of the SH3 domains to inhibit the exchange activity of the DH domain. This suggests that some unidentified component in cell extracts may be necessary for the in vivo derepression of DH domain exchange activity.
Figure 7.
Binding of protein to the SH3 domains does not relieve the inhibition of the DH domain in vitro. (A) GST pull-down of recombinant dynamin with DH domain-containing fusion proteins is independent of GTP. GST fusion proteins, 50 nM, containing the DH domain in MgCl2 binding buffer + 1 μM BSA or 1 μM dynamin rotated 4 h at 4°C. Approximately half of the GST SH3ABCDE-DHPH protein is bound by dynamin as quantified by Western blotting signal relative to known dynamin using NIH Image 1.61. The addition of guanine nucleotides or the C2 domain had no effect on dynamin binding. (B) Time course of in vitro exchange reactions with dynamin. The following were assayed for exchange activity on 750 nM GST Cdc42 after the loss of [3H]GDP from Cdc42: 75 nM GST + 2.5 μM BSA (□), 75 nM GST + 1.5 μM dynamin (▪), GST DHPH 75 nM + 2.5 μM BSA (○), 75 nM GST DHPH + 1.5 μM dynamin (●), 75 M GST SH3ABCDE-DHPH + 2.5 μM BSA (▵) and 75 nM GST SH3ABCDE-DHPH + 1.5 μM dynamin (▴) . Addition of dynamin to the exchange reaction does not effect the inhibition by the SH3 domains. (C) GST pull-downs of recombinant N-WASP with DH domain-containing fusion proteins. GST, GST DHPH, and GST SH3ABCDE-DHPH, 50 nM, in MgCl2 binding buffer + 0.5 mg/ml BSA and 450 nM N-WASP was rotated at 4°C for 4 h. Under these conditions approximately half of the GST SH3ABCDE-DHPH is bound to N-WASP as quantified by comparing the Western blotting signal to known N-WASP using NIH Image 1.61. (D) Time course of in vitro exchange reactions with N-WASP. The following were assayed for exchange of cold GTP on 1.25 μM Cdc42 for [3H]GDP: 75 nM GST + BSA (□), GST + 4.5 μM N-WASP (▪), GST DHPH + BSA (○), GST DHPH + 4.5 μM N-WASP (●), GST SH3ABCDE-DHPH + BSA (▵), and GST SH3ABCDE-DHPH + 4.5 μM N-WASP (▴). Addition of N-WASP to the exchange reaction does not effect the inhibition by the SH3 domains.
Dynamin Competes with N-WASP for Binding to the Intersectin 1L SH3 Domains
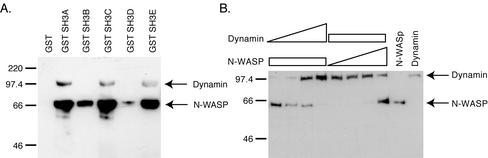
Because the intersectin 1L SH3 domains are responsible for the repression of exchange activity, and they bind proteins involved in two processes, endocytosis and actin cytoskeleton nucleation, we investigated the relationship between these interactions. Proteins involved in divergent pathways could be binding a separate subset of SH3 domains or they could be competing with each other for association with intersectin 1L. First we examined the binding specificity of dynamin and N-WASP for each of the SH3 domains. Using recombinant GST fusion proteins of individual SH3 domains, we carried out pull-downs from rat brain cytosol (Figure 8A). Neither dynamin nor N-WASP showed strong specificity for any one SH3 domain. Dynamin bound strongly to the SH3A, C, and E domains, whereas N-WASP bound strongly to the SH3A, C, and E domains, but also weakly to the SH3B and D domains. Because dynamin and N-WASP are capable of binding similar sets of SH3 domains, we wondered whether they would compete with each other for binding. We therefore used recombinant dynamin and recombinant N-WASP in an in vitro binding competition assay for binding to GST SH3ABCDE-DHPH (Figure 8B). When equimolar amounts of dynamin and N-WASP proteins are added to GST SH3ABCDE-DHPH (Figure 8B, lane 7), dynamin preferentially binds, despite strong binding of both proteins to the SH3 domains at low nM concentrations (unpublished data). Increasing amounts of dynamin successfully compete with moderate amounts of N-WASP for binding to the intersectin 1L SH3 domains (Figure 8B, lanes 1–4). Only at high concentration does N-WASP successfully compete with dynamin for binding (Figure 8B, lanes 5–8), suggesting that dynamin is preferentially binding to the SH3 domains when both proteins are present.
Figure 8.
Dynamin and N-WASP compete for binding to the intersectin 1L SH3 domains. (A) GST pull-downs of dynamin and N-WASP from rat brain cytosol by individual SH3 domains. GST SH3 domains, 5 μM, in 1.5 mg rat brain cytosol in MgCl2 binding buffer. Dynamin binds to the SH3A, SH3C, and SH3E domains. N-WASP also binds to these domains, but also has some binding to SH3B and SH3D. (B) Dynamin competes with N-WASP for binding to the SH3 domains. 75 nM GST SH3ABCDE-DHPH in MgCl2 binding buffer. In lanes 1–4, 300 nM N-WASP binding in the presence of 55 nM, 113 nM, 750 nM, and 4.5 μM dynamin. In lanes 5–8, 450 nM dynamin binding in the presence of 37.5 nM, 75 nM, 500 nM, and 3 μM N-WASP. Lane 9 contains 3.75 pmol N-WASP. Lane 10 contains 5.6 pmol dynamin protein. When equimolar dynamin and N-WASP are present, lane 7, 450 nM dynamin and 500 nM N-WASP, dynamin preferentially binds. Increasing amounts of dynamin compete with N-WASP binding to the SH3 domains. Only at very high concentrations does N-WASP compete with dynamin for binding to the SH3 domains.
DISCUSSION
Guanine nucleotide exchange factors are critical regulators of the timing and localization of the activation of small GTPases. Their complex domain structure enables them to bind cellular signals that activate their repressed exchange activity (Zheng, 2001; Hoffman and Cerione, 2002). We have used biochemical techniques to investigate the repression and activation mechanism of intersectin 1L, a neuronal GEF specific for Cdc42 that is involved in endocytosis. Here we show by assaying exchange activity in vitro that one mechanism of inhibition is through the direct binding of the upstream SH3 domains to the DH domain. The binding of the SH3 domains to the DH domain blocks the binding of Cdc42 to the catalytic DH domain and therefore blocks exchange. Although there are five SH3 domains directly upstream of the DH domain, one SH3 domain is sufficient to partially inhibit exchange and mediate an interaction with the intersectin 1L DH domain. However, additional domains strengthen both the inhibitory activity and affinity of the interaction with full-length intersectin 1L. This inhibitory mechanism is similar to the inhibitory mechanism of the Vav proto-oncogene (Bustelo, 2002), whose DH domain binding and exchange of Rac1 is blocked by the interaction of an upstream α-helix with the Rac1 binding surface of the DH domain (Aghazadeh et al., 2000). Surprisingly, the mechanism of the inhibitory activity of the SH3 domains is not through their PxxP binding pockets. Instead, another conserved element in their structure is likely to be responsible. Because there is no indication from sequence comparison between the SH3 domains as to what this conserved structure might be, mutation analysis is needed to determine the DH domain binding site in the SH3 domains. However, it is possible, given that all the exchange reactions included the SH3E domain, that an interaction surface in SH3E is critically responsible for the inhibition and that the SH3E interaction positions the other domains to provide additional steric hindrance of Cdc42 binding.
The exchange activity of intersectin 1L, immunoprecipitated from transfected cell extracts, is derepressed by the binding of the proline-rich domain of N-WASP (Hussain et al., 2001). This is similar to the mechanism of stimulation of Sos exchange activity. The Sos DH domain is active for Rac1 exchange only when Sos is in a ternary complex with Eps8 and E3b1 (Scita et al., 1999, 2001; Innocenti et al., 2002). We investigated whether binding of interacting proteins to the SH3 domains can derepress the exchange activity in vitro. Surprisingly, neither binding of dynamin nor binding of full-length N-WASP was able to stimulate exchange activity in a fully pure system. This lack of stimulation shows that simply binding to the SH3 domains is not sufficient to induce DH domain exchange activity and suggests that an additional alteration to the state of intersectin 1L in vivo that we are not reproducing in vitro is necessary for exchange activation. Our finding that the PxxP binding pocket of the SH3 domain is not involved in the inhibition of exchange supports this possibility. Because intersectin 1L is localized to the neuronal synapse (Roos and Kelly, 1999), changes that occur during synapse stimulation, such as alterations in kinase/phosphatase activities, Ca2+ concentration, and phospholipid content of membranes, may predispose intersectin 1L to stimulation by N-WASP binding. As in the case for the Vav GEF, intersectin 1L may need to be phosphorylated in order to derepress the DH domain (Crespo et al., 1997; Han et al., 1997; Schuebel et al., 1998; Aghazadeh et al., 2000). Similarly, binding of an additional protein in vivo may be necessary, as in the case of activation of Sos Rac1 exchange activity by formation of a ternary complex (Scita et al., 1999, 2001; Innocenti et al., 2002). Because our studies have utilized recombinant proteins that are missing the amino-terminal domains, a third possibility is that a missing amino-terminal component of intersectin 1L is involved in the regulation of exchange activity.
Intersectin 1L is an example of a GEF, whose physiological role has been relatively well characterized. Here we have shown that the SH3 domains involved in endocytosis are the critical inhibitory regions of the DH domain exchange activity. This suggests that input from endocytic pathways is important in determining the regulatory state of the DH domain. Because control of actin polymerization is downstream of GEF activity, intersectin 1L may act as a switch that links actin polymerization to an input from the endocytic pathway. Although we do not yet know the exact role played by actin polymerization during endocytosis (Qualmann et al., 2000), there is remarkable colocalization of endocytic events and actin polymerization at the synapse (Dunaevsky and Connor, 2000), and both are tightly regulated by phosphatidylinositol biosynthesis (Cremona and De Camilli, 2001). Intersectin 1L, localized to peri-active zones (Hussain et al., 1999; Roos and Kelly, 1999), is thus well positioned to switch on actin polymerization in endocytic regions only after a burst of exocytosis. Intersectins promote actin polymerization (Hussain et al., 2001; McGavin et al., 2001) but are inhibitors of endocytosis (Sengar et al., 1999; Simpson et al., 1999; Pucharcos et al., 2000), perhaps because they sequester proteins such as dynamin that are needed for endocytosis. Because dynamin and N-WASP compete with each other for binding to the same SH3 domains of intersectin 1L, inhibition of endocytosis through sequestration of dynamin would be associated with inhibition of N-WASP binding. During endocytosis, dynamin must be released from its sites of sequestration to bind other SH3-containing proteins (Liu et al., 1994; Slepnev et al., 1998), and specifically amphiphysins, in order to productively participate in clathrin-mediated endocytosis (David et al., 1996; Shupliakov et al., 1997; Wigge et al., 1997). This would allow N-WASP to bind to the newly available SH3 domains and therefore to stimulate GEF activity specifically when required to promote endocytosis of synaptic vesicles by an actin-mediated process. Intersectin 1L, by localizing both N-WASP and Cdc42-GTP only when needed, would exert precise control over the timing and localization of actin cytoskeletal rearrangements in response to the cellular stimulus of endocytosis.
ACKNOWLEDGMENTS
We thank Dr. J. Taunton for the gifts of N-WASP antibody and baculovirus; A. Jones and Dr. S. Schmid for the gift of dynamin protein; and Dr. N. Jarousse and Dr. S. Dasgupta for critical reading of the manuscript. This work was funded by National Institute of Health grants NIH-NS-15927 and NIH-DA-10154.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–08–0494. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–08–0494.
REFERENCES
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Bustelo XR. Regulation of Vav proteins by intramolecular events. Front Biosci. 2002;7:d24–d30. doi: 10.2741/A766. [DOI] [PubMed] [Google Scholar]
- Cicchetti P, Mayer BJ, Thiel G, Baltimore D. Identification of a protein that binds to the SH3 region of Abl and is similar to Bcr and GAP-rho. Science. 1992;257:803–806. doi: 10.1126/science.1379745. [DOI] [PubMed] [Google Scholar]
- Clark SG, Stern MJ, Horvitz HR. C. elegans cell-signaling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Clift-O'Grady L, Desnos C, Lichtenstein Y, Faundez V, Horng JT, Kelly RB. Reconstitution of synaptic vesicle biogenesis from PC12 cell membranes. Methods. 1998;16:150–159. doi: 10.1006/meth.1998.0662. [DOI] [PubMed] [Google Scholar]
- Cremona O, De Camilli P. Phosphoinositides in membrane traffic at the synapse. J Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Crompton AM, Foley LH, Wood A, Roscoe W, Stokoe D, McCormick F, Symons M, Bollag G. Regulation of Tiam1 nucleotide exchange activity by pleckstrin domain binding ligands. J Biol Chem. 2000;275:25751–25759. doi: 10.1074/jbc.M002050200. [DOI] [PubMed] [Google Scholar]
- Das B, Shu X, Day GJ, Han J, Krishna UM, Falck JR, Broek D. Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J Biol Chem. 2000;275:15074–150781. doi: 10.1074/jbc.M907269199. [DOI] [PubMed] [Google Scholar]
- David C, McPherson PS, Mundigl O, de Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaevsky A, Connor EA. F-actin is concentrated in nonrelease domains at frog neuromuscular junctions. J Neurosci. 2000;20:6007–6012. doi: 10.1523/JNEUROSCI.20-16-06007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guipponi M, Scott HS, Chen H, Schebesta A, Rossier C, Antonarakis SE. Two isoforms of a human intersectin (ITSN) protein are produced by brain-specific alternative splicing in a stop codon. Genomics. 1998;53:369–376. doi: 10.1006/geno.1998.5521. [DOI] [PubMed] [Google Scholar]
- Han J, Das B, Wei W, Van Aelst L, Mosteller RD, Khosravi-Far R, Westwick JK, Der CJ, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, et al. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Eva A, Evans T, Aaronson SA, Cerione RA. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991;354:311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Eva A, Zangrilli D, Aaronson SA, Evans T, Cerione RA, Zheng Y. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J Biol Chem. 1994;269:62–65. [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Hoffman GR, Cerione RA. Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett. 2002;513:85–91. doi: 10.1016/s0014-5793(01)03310-5. [DOI] [PubMed] [Google Scholar]
- Hussain NK, et al. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- Hussain NK, Yamabhai M, Ramjaun AR, Guy AM, Baranes D, O'Bryan JP, Der CJ, Kay BK, McPherson PS. Splice variants of intersectin are components of the endocytic machinery in neurons and nonneuronal cells. J Biol Chem. 1999;274:15671–15677. doi: 10.1074/jbc.274.22.15671. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Tenca P, Frittoli E, Faretta M, Tocchetti A, Di Fiore PP, Scita G. Mechanisms through which Sos-1 coordinates the activation of Ras and Rac. J Cell Biol. 2002;156:125–136. doi: 10.1083/jcb.200108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Freund C, Duke-Cohan JS, Musacchio A, Wagner G, Rudd CE. SH3 domain recognition of a proline-independent tyrosine-based RKxxYxxY motif in immune cell adaptor SKAP55. EMBO J. 2000;19:2889–2899. doi: 10.1093/emboj/19.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Powell KA, Sudhof TC, Robinson PJ. Dynamin I is a Ca(2+)-sensitive phospholipid-binding protein with very high affinity for protein kinase C. J Biol Chem. 1994;269:21043–21050. [PubMed] [Google Scholar]
- Liu X, et al. NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell. 1998;95:269–77. doi: 10.1016/s0092-8674(00)81757-2. [DOI] [PubMed] [Google Scholar]
- McGavin MK, Badour K, Hardy LA, Kubiseski TJ, Zhang J, Siminovitch KA. The intersectin 2 adaptor links Wiskott Aldrich Syndrome protein (WASp)-mediated actin polymerization to T cell antigen receptor endocytosis. J Exp Med. 2001;194:1777–1787. doi: 10.1084/jem.194.12.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin- depolymerizing protein N-WASP. Nature. 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- Mongiovi AM, Romano PR, Panni S, Mendoza M, Wong WT, Musacchio A, Cesareni G, Di Fiore PP. A novel peptide-SH3 interaction. EMBO J. 1999;18:5300–5309. doi: 10.1093/emboj/18.19.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Schoch S, Sudhof TC. EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J Biol Chem. 1999;274:18446–18454. doi: 10.1074/jbc.274.26.18446. [DOI] [PubMed] [Google Scholar]
- Olson MF, Pasteris NG, Gorski JL, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- Pucharcos C, Casas C, Nadal M, Estivill X, de la Luna S. The human intersectin genes and their spliced variants are differentially expressed. Biochim Biophys Acta. 2001;1521:1–11. doi: 10.1016/s0167-4781(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Pucharcos C, Estivill X, de la Luna S. Intersectin 2, a new multimodular protein involved in clathrin-mediated endocytosis. FEBS Lett. 2000;478:43–51. doi: 10.1016/s0014-5793(00)01793-2. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Kessels MM, Kelly RB. Molecular links between endocytosis and the actin cytoskeleton. J Cell Biol. 2000;150:F111–F116. doi: 10.1083/jcb.150.5.f111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Roos J, Kelly RB. Dap160, a neural-specific Eps15 homology and multiple SH3 domain- containing protein that interacts with Drosophila dynamin. J Biol Chem. 1998;273:19108–19119. doi: 10.1074/jbc.273.30.19108. [DOI] [PubMed] [Google Scholar]
- Roos J, Kelly RB. The endocytic machinery in nerve terminals surrounds sites of exocytosis. Curr Biol. 1999;9:1411–1414. doi: 10.1016/s0960-9822(00)80087-1. [DOI] [PubMed] [Google Scholar]
- Russo C, Gao Y, Mancini P, Vanni C, Porotto M, Falasca M, Torrisi MR, Zheng Y, Eva A. Modulation of oncogenic DBL activity by phosphoinositol phosphate binding to pleckstrin homology domain. J Biol Chem. 2001;276:19524–19531. doi: 10.1074/jbc.M009742200. [DOI] [PubMed] [Google Scholar]
- Schuebel KE, Movilla N, Rosa JL, Bustelo XR. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore PP. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- Scita G, et al. An effector region in Eps8 is responsible for the activation of the Rac-specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J Cell Biol. 2001;154:1031–1044. doi: 10.1083/jcb.200103146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengar AS, Wang W, Bishay J, Cohen S, Egan SE. The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J. 1999;18:1159–1171. doi: 10.1093/emboj/18.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Simpson F, Hussain NK, Qualmann B, Kelly RB, Kay BK, McPherson PS, Schmid SL. SH3-domain-containing proteins function at distinct steps in clathrin- coated vesicle formation. Nat Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa GC, Butler MH, Grabs D, Camilli PD. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- Snyder JT, Rossman KL, Baumeister MA, Pruitt WM, Siderovski DP, Der CJ, Lemmon MA, Sondek J. Quantitative analysis of the effect of phosphoinositide interactions on the function of Dbl family proteins. J Biol Chem. 2001;276:45868–45875. doi: 10.1074/jbc.M106731200. [DOI] [PubMed] [Google Scholar]
- Whitehead IP, et al. Dependence of Dbl and Dbs transformation on MEK and NF-kappaB activation. Mol Cell Biol. 1999;19:7759–7770. doi: 10.1128/mcb.19.11.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P, Vallis Y, McMahon HT. Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr Biol. 1997;7:554–560. doi: 10.1016/s0960-9822(06)00254-5. [DOI] [PubMed] [Google Scholar]
- Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesareni G, Kay BK. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J Biol Chem. 1998;273:31401–31407. doi: 10.1074/jbc.273.47.31401. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Hart MJ, Cerione RA. Guanine nucleotide exchange catalyzed by dbl oncogene product. Methods Enzymol. 1995;256:77–84. doi: 10.1016/0076-6879(95)56011-4. [DOI] [PubMed] [Google Scholar]