Abstract
This study examines the role of the cellular protein hDaxx in controlling human cytomegalovirus (HCMV) immediate-early (IE) gene expression and viral replication. Using permissive cell lines that either overexpress hDaxx or are depleted of hDaxx expression by the use of short hairpin RNA, we demonstrate that hDaxx functions as a repressor of HCMV IE gene expression and replication. In addition, we demonstrate that the impaired growth phenotype associated with the UL82 (pp71) deletion mutant is abolished when hDaxx knockdown cells are infected, suggesting that pp71 functions to relieve hDaxx-mediated repression during HCMV infection.
Human cytomegalovirus (HCMV) transcription is temporally regulated in a coordinated cascade which consists of immediate-early (IE), early (E), and late (L) gene expression. Immediate-early genes are transcribed first and encode critical regulatory proteins that function in part to control the expression of viral early and late genes (26, 29, 30). Certain virion tegument proteins which are delivered to the host cell from the infectious virion have been shown to play an important role in controlling efficient IE gene expression (1, 3, 7, 10, 20, 31). Specifically, we and others have demonstrated that the UL82-encoded tegument protein pp71 is involved in regulating the expression of a number of IE genes (1, 2, 7, 10, 20). In studies using a UL82 (pp71) deletion mutant virus, we demonstrated that pp71 protein delivered from the tegument plays an important role in regulating IE gene expression and viral replication (1, 2).
The mechanism by which pp71 regulates IE gene expression is currently unclear. pp71 has been shown to interact with several cellular proteins, including hDaxx (7, 14). During HCMV infection, pp71 and hDaxx colocalize at specific nuclear structures called nuclear domain 10 (ND10) (2, 7, 14). Previous reports have demonstrated that HCMV and other herpesvirus genomes localize to ND10 domains immediately after infection and that ND10 domains represent sites of active viral gene transcription (5, 7, 11-14, 21, 22, 27). Interestingly, abolishing pp71's ability to interact with hDaxx blocked pp71 localization to ND10 domains (2, 7, 14) and inhibited pp71's ability to transactivate the major immediate-early promoter (MIEP) in transient reporter assays (7). We have also demonstrated that pp71 mutant viruses lacking either of two hDaxx binding domains (7) were severely inhibited in viral replication and IE gene expression at low multiplicities of infection (MOIs) (2). These data suggest that pp71's interaction with the cellular protein hDaxx is important for regulating IE gene expression and viral replication.
hDaxx has been recognized as a regulator of both apoptosis and gene expression (reviewed in reference 23). The mechanisms by which hDaxx regulates these two processes are controversial and not completely understood. hDaxx was originally identified as a proapoptotic protein which could enhance Fas-induced apoptosis (28, 32). However, other reports using small interfering RNA directed against hDaxx have demonstrated that hDaxx functions as an antiapoptotic protein following certain stimuli (4, 24, 25). hDaxx's role in regulating gene expression is also unclear. Although hDaxx has been associated with transcriptional activation, hDaxx is primarily thought to function as a transcriptional repressor (4, 6, 9, 18, 19, 25, 28). Studies using small interfering RNA directed against hDaxx have demonstrated that hDaxx can repress NF-κB-, E2F-1-, Pax3-, and Ets-1-mediated transactivation (25). Additionally, hDaxx has been shown to bind the avian sarcoma virus integrase protein and represses avian sarcoma virus transcription (6). The mechanism by which hDaxx regulates HCMV IE gene expression is currently unclear. Transient transfection assays have demonstrated that cotransfection of pp71 with hDaxx has a synergistic effect on the activation of the HCMV MIEP (7). In addition, HCMV infection of Daxx null mouse cells led to a twofold reduction in the number of IE2 protein-expressing cells (14). Taken together, these results suggest that hDaxx functions as a positive regulator of the MIEP and of IE gene expression. However, preliminary studies by Reeves et al. suggested that overexpression of hDaxx represses HCMV replication (M. Reeves, J. Baillie, R. Greaves, and J. Sinclair., Abstr. 29th Int. Herpesvirus Workshop, abstr. 1.09, 2004). Therefore, given the conflicting data and the multifunctional nature of hDaxx, it is unclear if hDaxx functions as an activator or repressor during HCMV infection. For this study, we used HCMV-permissive cell lines that either overexpress hDaxx or are depleted of hDaxx expression to determine whether hDaxx functions as an activator or repressor of HCMV IE gene expression and replication.
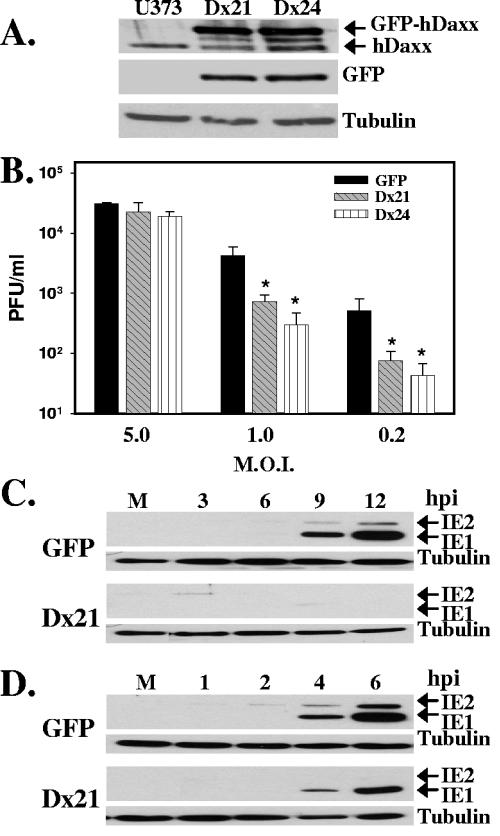
If hDaxx functions to positively regulate viral transcription, then a wild-type virus may replicate more efficiently in cells overexpressing hDaxx. However, if hDaxx functions as a repressor, wild-type virus replication and IE gene expression may be inhibited in cells overexpressing hDaxx. To test these predictions, cell lines overexpressing hDaxx were generated. U373 cells were transfected via electroporation with a plasmid that expresses an hDaxx-green fluorescent protein (GFP) fusion and a neomycin resistance gene (33). As a control, cells were transfected with a GFP plasmid that does not express hDaxx. Cells were selected with G418 (500 μg/ml), and stable clones were isolated. As shown in Fig. 1A, hDaxx overexpression in cell clones was confirmed by Western blot analysis using either an hDaxx or GFP antibody. Endogenous hDaxx levels were constant in all clones and can be differentiated by faster hDaxx migration than GFP-hDaxx fusion protein migration. To determine the effect of hDaxx overexpression on wild-type viral replication, GFP-expressing control cells and hDaxx-overexpressing cells were infected with wild-type virus (2) at an MOI of 5.0, 1.0, or 0.2 PFU/cell. Virus was harvested at 5 or 7 days postinfection and quantified by a plaque assay on human foreskin fibroblasts. As shown in Fig. 1B, wild-type virus replication was inhibited in hDaxx-overexpressing cells in a multiplicity-dependent manner. When hDaxx-overexpressing cells were infected at a multiplicity of 1.0 or 0.2 PFU/cell, wild-type virus replication was inhibited >80% compared with that in control cells. IE gene expression was also examined following infection of hDaxx-overexpressing cells. Control or hDaxx-overexpressing cells were infected with wild-type virus at an MOI of 0.2 (Fig. 1C) or 1.0 (Fig. 1D) PFU/cell. Cell lysates were harvested at various times postinfection and assayed for IE1 and IE2 protein expression by Western blotting. As shown in Fig. 1C and D, the expression of IE1 and IE2 was markedly delayed and reduced following infection of hDaxx-overexpressing cells when compared to infection of control cells. Immunostaining for the pp65 tegument protein in control and hDaxx-overexpressing cells was done to confirm that viral entry was not affected by the overexpression of hDaxx (data not shown). Together, these results demonstrate that overexpression of hDaxx does not enhance HCMV replication and suggest that hDaxx functions to repress HCMV viral replication and IE gene expression.
FIG. 1.
Overexpression of hDaxx represses viral replication. (A) Western blot analysis examining expression of hDaxx and GFP in control U373 cells and GFP-hDaxx-overexpressing U373 clones Dx21 and Dx24. Tubulin was included as an internal loading control. (B) Control U373-GFP (black bars) cells or hDaxx-overexpressing cell clones Dx21 (hatched bars) or Dx24 (vertically striped bars) were infected with wild-type virus at an MOI of 5.0, 1.0, or 0.2 PFU/cell. Infectious virus was harvested at 5 days postinfection for cells infected at an MOI of 5.0 PFU/cell and at 7 days postinfection for cells infected at an MOI of 1.0 or 0.2 PFU/cell and quantified by a plaque assay on human foreskin fibroblasts. Asterisks indicate significant differences in viral titers (P < 0.05) between virus produced on hDaxx-overexpressing cells and on control U373-GFP cells. Error bars indicate standard deviations derived from three independent experiments. Control GFP-expressing or hDaxx-overexpressing (Dx21) cells were infected with wild-type virus at an MOI of 0.2 (C) or 1.0 (D) PFU/cell. Cell lysates were prepared at the indicated hours postinfection (hpi) and assayed for IE1 and IE2 expression by Western blotting. Tubulin was included as an internal loading control.
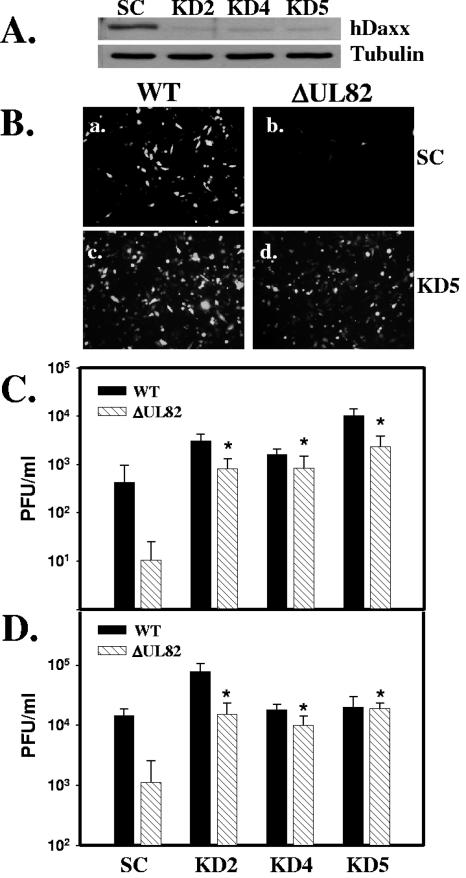
We hypothesized that if hDaxx functions as a repressor during viral infection, then the wild-type virus, and possibly the UL82 deletion mutant virus, would replicate more efficiently in hDaxx knockdown cells. To test this prediction, U373 hDaxx knockdown cell lines were generated using replication-deficient retroviruses carrying short hairpin RNA (shRNA) sequences against hDaxx. A shRNA sequence directed against hDaxx (4) or a control scrambled (Oligoengine) shRNA sequence was inserted into the pSuperRetroPuro vector (Oligoengine) according to the manufacturer's protocol. Infectious retrovirus was then generated, and U373 cells were transduced as previously described (2, 17). Transduced cells were selected with puromycin (1 μg/ml), and individual stable clones were isolated. Western blot analysis was then conducted to examine the hDaxx levels in individual clones. Figure 2A shows a representative blot of hDaxx expression in control cells and hDaxx knockdown cells. These results demonstrate that cells expressing the shRNA directed against hDaxx expressed dramatically reduced levels of hDaxx compared to control cells. The control cells and hDaxx knockdown cells were then infected at an MOI of 0.2 PFU/cell with either wild-type virus or the UL82 deletion mutant virus, termed ADΔUL82 (2). Since both viruses contain a GFP gene within the viral genome, we could easily identify infected cells by looking for GFP expression. As shown in Fig. 2B, there were only a few GFP-positive cells present when control cells were infected with the ADΔUL82 virus (panel b), demonstrating the growth defect of the UL82 deletion mutant virus. However, when the hDaxx knockdown cells were infected with the UL82 deletion mutant, we observed a dramatic increase in the number of GFP-positive cells (Fig. 2B, compare panels b and d). In addition, when hDaxx knockdown cells were infected with the UL82 deletion mutant virus, we observed an approximately equal number of GFP-positive cells to that observed following wild-type infection of control cells (Fig. 2B, compare panels a and d). The replication of wild-type virus and the UL82 deletion mutant virus was then quantified by a plaque assay following infection of either control cells or hDaxx knockdown cells. Control cells or three individual clones of hDaxx knockdown cells were infected with wild-type or UL82 deletion mutant virus at an MOI of 0.2 (Fig. 2C) or 1.0 (Fig. 2D) PFU/cell. Infectious virus was harvested at 7 and 5 days postinfection, respectively, and quantified by a plaque assay on UL82-complementing cells (2). UL82 deletion mutant virus production was dramatically reduced (>92% inhibition) at both multiplicities compared with wild-type virus production on control cells. However, when hDaxx knockdown cells were infected with the UL82 deletion mutant virus, the growth defect associated with the virus was completely abolished, and virus production was restored to wild-type levels (Fig. 2C and D). In addition, when cells were infected at a multiplicity of 0.2 PFU/cell, wild-type virus replication was also significantly enhanced on hDaxx knockdown cells. Immunostaining for the pp65 tegument protein in control and hDaxx knockdown cells was done to confirm that viral entry was not affected by the decreased expression of hDaxx (data not shown).
FIG. 2.
Infection of hDaxx knockdown cells abolishes the UL82 deletion mutant growth phenotype. (A) Western blot analysis of hDaxx expression in control cells expressing a scrambled shRNA (SC) and in hDaxx knockdown cell lines KD2, KD4, and KD5. Tubulin was included as an internal loading control. (B) Control (SC) and hDaxx knockdown (KD5) cells were infected with wild-type virus (WT) or the UL82 deletion mutant virus (ΔUL82) at an MOI of 0.2 PFU/cell and examined at 5 days postinfection for GFP-positive cells. Control (SC) and hDaxx knockdown (KD2, KD4, and KD5) cells were infected with either wild-type (black bars) or ΔUL82 (hatched bars) virus at an MOI of 0.2 (C) or 1.0 (D) PFU/cell. Viruses were harvested at 7 and 5 days postinfection, respectively, and infectious virus was quantified by a plaque assay on UL82-complementing cells. Asterisks indicate significant differences in viral titers (P < 0.005) between ΔUL82 virus produced on hDaxx knockdown cells and on control cells. Error bars indicate standard deviations derived from three independent experiments.
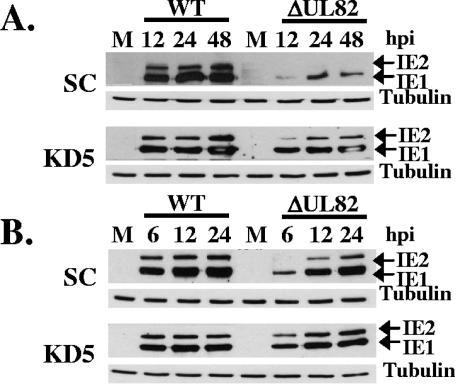
In addition to viral replication, IE gene expression was examined following infection of hDaxx knockdown cells with either wild-type or UL82 deletion mutant virus. Control or hDaxx knockdown cells were infected with wild-type or UL82 deletion mutant virus at an MOI of 0.2 (Fig. 3A) or 1.0 (Fig. 3B) PFU/cell, and cell lysates were harvested at various time points following infection. Western blot analysis was conducted to examine the expression of the immediate-early proteins IE1 and IE2 following infection. As shown in Fig. 3A and B, IE gene expression was inhibited relative to that in wild-type virus following infection of control cells with the UL82 deletion mutant. However, when hDaxx knockdown cells were infected with the UL82 deletion mutant virus, IE gene expression was restored to nearly wild-type levels (Fig. 3A and B). Taken together, these results demonstrate that hDaxx is not required for HCMV replication and that hDaxx functions to repress IE gene expression and HCMV replication. Since knocking down hDaxx expression abolished the UL82 mutant growth phenotype and restored IE gene expression, our results also suggest that the critical function of pp71 required for efficient IE gene expression and viral replication is to relieve hDaxx-mediated repression of IE gene expression.
FIG. 3.
IE gene expression following infection of hDaxx knockdown cells. Control (SC) or hDaxx knockdown (KD5) cells were infected with either wild-type (WT) or UL82 deletion mutant (ΔUL82) virus at an MOI of 0.2 (A) or 1.0 (B) PFU/cell. Cell lysates were prepared at the indicated hours postinfection (hpi) and assayed for IE1 and IE2 expression by Western blotting. Tubulin was included as an internal loading control.
Previous studies showed that overexpression of hDaxx together with pp71 enhanced MIEP activity during transient transfection assays, suggesting that hDaxx may function to promote HCMV transcription (7). However, since hDaxx is primarily considered to function as a transcriptional repressor and has been suggested to repress viral infection (6; Reeves et al., Abstr. 29th Int. Herpesvirus Workshop), we wanted to examine the effect of hDaxx expression on IE gene expression and HCMV replication in the context of a viral infection. Using stable cell lines that overexpress hDaxx, we demonstrated that wild-type virus replication and IE gene expression are severely inhibited in hDaxx-overexpressing cells in a multiplicity-dependent manner (Fig. 1B to D). Using shRNA directed against hDaxx, we also demonstrated that wild-type virus replication is enhanced in hDaxx knockdown cells (Fig. 2C). Finally, and most importantly, we have demonstrated that we can abolish the UL82 deletion mutant-associated defects in viral replication and IE gene expression by infecting hDaxx knockdown cells. Taken together, these results indicate that hDaxx functions as a repressor during HCMV infection and that pp71 is responsible for relieving this repression.
The mechanism by which pp71 relieves hDaxx-mediated repression is currently unclear. pp71 has been shown to bind and target hypophosphorylated Rb family member proteins for proteasome-dependent, ubiquitin-independent degradation (15, 16). Recent data suggest that pp71 may also target hDaxx for degradation (27a). Additionally, hDaxx has been shown to interact with a number of cellular proteins that are involved in regulating gene expression, including the Pax-3 (9) and Ets-1 (19) transcription factors, DNA methyltransferase 1 (24), Dek, core histones, and histone deacetylases (8). Therefore, pp71 binding to hDaxx may alter hDaxx's ability to interact with one or more of these proteins or alter the activity associated with these hDaxx complexes. Experiments are currently in progress to determine the mechanism by which pp71 relieves hDaxx-mediated repression of HCMV replication.
Acknowledgments
We are grateful to Garry Nolan for providing Phoenix A cells and to Daiqing Liao for providing the pEGFP-hDaxx construct. We also thank Travis Taylor and Peter Southern for critically reading the manuscript.
This work was supported by NIH grant AI059340 (to W.A.B.).
REFERENCES
- 1.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 97:14506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantrell, S. R., and W. A. Bresnahan. 2005. Interaction between the human cytomegalovirus UL82 gene product (pp71) and hDaxx regulates immediate-early gene expression and viral replication. J. Virol. 79:7792-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau, N. H., C. D. Vanson, and J. A. Kerry. 1999. Transcriptional regulation of the human cytomegalovirus US11 early gene. J. Virol. 73:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L. Y., and J. D. Chen. 2003. Daxx silencing sensitizes cells to multiple apoptotic pathways. Mol. Cell. Biol. 23:7108-7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doucas, V., M. Tini, D. A. Egan, and R. M. Evans. 1999. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc. Natl. Acad. Sci. USA 96:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greger, J. G., R. A. Katz, A. M. Ishov, G. G. Maul, and A. M. Skalka. 2005. The cellular protein Daxx interacts with avian sarcoma virus integrase and viral DNA to repress viral transcription. J. Virol. 79:4610-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann, H., H. Sindre, and T. Stamminger. 2002. Functional interaction between the pp71 protein of human cytomegalovirus and the PML-interacting protein human Daxx. J. Virol. 76:5769-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenbach, A. D., C. J. McPherson, E. J. Mientjes, R. Iyengar, and G. Grosveld. 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci. 115:3319-3330. [DOI] [PubMed] [Google Scholar]
- 9.Hollenbach, A. D., J. E. Sublett, C. J. McPherson, and G. Grosveld. 1999. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. EMBO J. 18:3702-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homer, E. G., A. Rinaldi, M. J. Nicholl, and C. M. Preston. 1999. Activation of herpesvirus gene expression by the human cytomegalovirus protein pp71. J. Virol. 73:8512-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishov, A. M., and G. G. Maul. 1996. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J. Cell Biol. 134:815-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. Yeh, J. F. Strauss III, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishov, A. M., R. M. Stenberg, and G. G. Maul. 1997. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J. Cell Biol. 138:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishov, A. M., O. V. Vladimirova, and G. G. Maul. 2002. Daxx-mediated accumulation of human cytomegalovirus tegument protein pp71 at ND10 facilitates initiation of viral infection at these nuclear domains. J. Virol. 76:7705-7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalejta, R. F., J. T. Bechtel, and T. Shenk. 2003. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol. Cell. Biol. 23:1885-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalejta, R. F., and T. Shenk. 2003. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc. Natl. Acad. Sci. USA 100:3263-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinsella, T. M., and G. P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405-1413. [DOI] [PubMed] [Google Scholar]
- 18.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E. J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, R., H. Pei, D. K. Watson, and T. S. Papas. 2000. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene 19:745-753. [DOI] [PubMed] [Google Scholar]
- 20.Liu, B., and M. F. Stinski. 1992. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 66:4434-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maul, G. G. 1998. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20:660-667. [DOI] [PubMed] [Google Scholar]
- 22.Maul, G. G., A. M. Ishov, and R. D. Everett. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 217:67-75. [DOI] [PubMed] [Google Scholar]
- 23.Michaelson, J. S. 2000. The Daxx enigma. Apoptosis 5:217-220. [DOI] [PubMed] [Google Scholar]
- 24.Michaelson, J. S., D. Bader, F. Kuo, C. Kozak, and P. Leder. 1999. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 13:1918-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaelson, J. S., and P. Leder. 2003. RNAi reveals anti-apoptotic and transcriptionally repressive activities of DAXX. J. Cell Sci. 116:345-352. [DOI] [PubMed] [Google Scholar]
- 26.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2674. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 27.Rosenke, K., and E. A. Fortunato. 2004. Bromodeoxyuridine-labeled viral particles as a tool for visualization of the immediate-early events of human cytomegalovirus infection. J. Virol. 78:7818-7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Saffert, R. T., and R. F. Kalejta. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 80:3863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torii, S., D. A. Egan, R. A. Evans, and J. C. Reed. 1999. Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs). EMBO J. 18:6037-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wathen, M. W., and M. F. Stinski. 1982. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J. Virol. 41:462-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wathen, M. W., D. R. Thomsen, and M. F. Stinski. 1981. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J. Virol. 38:446-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkler, M., S. A. Rice, and T. Stamminger. 1994. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 68:3943-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang, X., R. Khosravi-Far, H. Y. Chang, and D. Baltimore. 1997. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao, L. Y., A. L. Colosimo, Y. Liu, Y. Wan, and D. Liao. 2003. Adenovirus E1B 55-kilodalton oncoprotein binds to Daxx and eliminates enhancement of p53-dependent transcription by Daxx. J. Virol. 77:11809-11821. [DOI] [PMC free article] [PubMed] [Google Scholar]