Abstract
Nod is a chromokinesin-like protein that plays a critical role in segregating achiasmate chromosomes during female meiosis. The C-terminal half of the Nod protein contains two putative DNA-binding domains. The first of these domains, known as the HMGN domain, consists of three tandemly repeated high-mobility group N motifs. This domain was previously shown to be both necessary and sufficient for binding of the C-terminal half of Nod to mitotic chromosomes in embryos. The second putative DNA-binding domain, denoted HhH(2)/NDD, is a helix-hairpin-helix(2)/Nod-like DNA-binding domain. Although the HhH(2)/NDD domain is not required or sufficient for chromosome binding in embryos, several well-characterized nod mutations have been mapped in this domain. To characterize the role of the HhH(2)/NDD domain in mediating Nod function, we created a series of UAS-driven transgene constructs capable of expressing either a wild-type Nod-GFP fusion protein or proteins in which the HhH(2)/NDD domain had been altered by site-directed mutagenesis. Although wild-type Nod-GFP localizes to the oocyte chromosomes and rescues the segregation defect in nod mutant oocytes, two of three proteins carrying mutants in the HhH(2)/NDD domain fail to either rescue the nod mutant phenotype or bind to oocyte chromosomes. However, these mutant proteins do bind to the polytene chromosomes in nurse-cell nuclei and enter the oocyte nucleus. Thus, even though the HhH(2)/NDD domain is not essential for chromosome binding in other cell types, it is required for chromosome binding in the oocyte. These HhH(2)/NDD mutants also block the localization of Nod to the posterior pole of stage 9–10A oocytes, a process that is thought to facilitate the interaction of Nod with the plus ends of microtubules (Cui et al. 2005). This observation suggests that the Nod HhH2/NDD domain may play other roles in addition to binding Nod to meiotic chromosomes.
THE accurate disjunction of homologs during meiosis is accomplished by a series of highly coordinated processes beginning in meiotic prophase. In most meiotic systems, homologous centromeres are oriented toward opposite poles by chiasmata, which restrict the movement of the chromosomes and serve to orient the centromeres toward the poles (Nicklas 1974; Hawley 1989). However, Drosophila melanogaster females also employ a highly efficient back-up mechanism, known as homologous achiasmate segregation, to ensure the separation of homologous chromosomes that fail to undergo exchange (Hawley and Theurkauf 1993). The basic components of this segregation system are: (1) the maintenance of heterochromatic pairings until metaphase (Dernberg et al. 1996), (2) the ability of those pairings to ensure achiasmate segregations (Hawley et al. 1992; Karpen et al. 1996), and (3) the precocious but controlled separation of nonexchange homologs toward opposite poles of the spindle during prometaphase (Theurkauf and Hawley 1992).
This controlled movement of the achiasmate chromosomes on the prometaphase meiotic spindle is dependent on Nod, a 666-amino-acid chromokinesin-like protein that is localized along the arms of meiotic chromosomes (Zhang et al. 1990; Afshar et al. 1995a,b). In the absence of functional Nod protein, achiasmate chromosomes dissociate from the main chromosome mass immediately after nuclear envelope breakdown (NEB) by simply migrating off the ends of the developing spindle (Theurkauf and Hawley 1992). These meiotic errors result in high levels of chromosome loss and nondisjunction (Carpenter 1973; Zhang and Hawley 1990). Both genetic and cytological studies suggest that Nod functions to hold chromosomes at or near the metaphase plate, opposing the poleward forces exerted by the kinetochores (Theurkauf and Hawley 1992; Matthies et al. 1999). Given that the microtubules (MTs) in the oocyte spindle are arranged with their plus ends at or near the metaphase plate (Riparbelli and Calliani 2005), these results initially suggested that Nod acted as a plus-end-directed motor that pushes chromosomes toward the metaphase plate (Matthies et al. 1999). A function of Nod in pushing chromosome arms toward the metaphase plate has also been demonstrated in mitotic cells by Goshima and Vale (2003). These authors have shown that in Drosophila cells in which Nod function is ablated by RNAi the arms of most chromosome were extended along the spindles axis toward one of the two poles, rather than being held at or near the metaphase plate.
Although several lines of evidence show that the motor-like domain of Nod lacks the capacity for vectorial transport (Matthies et al. 2001), we have recently demonstrated that both full-length Nod protein and a construct containing only the Nod motor-like domain bind primarily to the plus ends of microtubules in vitro and stimulate polymerization of microtubules (Cui et al. 2005). Thus, as noted by Cui et al. (2005), one can imagine a mechanism for Nod function in which Nod binds to the plus end of microtubules (and along the length of chromosome arms). Nod then “opens” or “relaxes” enough to allow the insertion of a tubulin subunit at the plus end of the microtubule, before reclamping on the new plus end of the growing fiber. Reiterations of this mechanism would serve to push the chromosomes away from the poles of the spindle, mimicking the effect observed in cytological experiments without requiring the Nod motor protein to exhibit conventional motor activity.
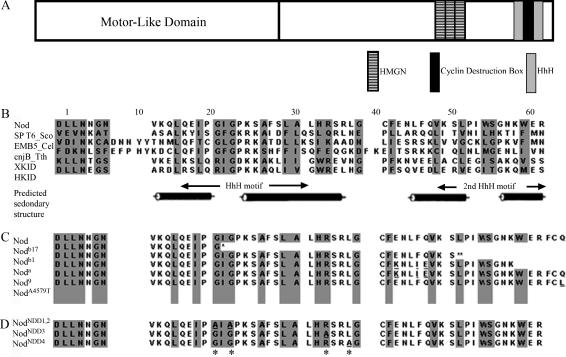
To better understand the mechanism by which Nod functions, we have endeavored to understand the mechanism by which Nod binds to the arms of meiotic chromosomes. Afshar et al. (1995b) demonstrated that Nod fragments that carried only the N-terminal motor-like domain bound only to spindle microtubules, while constructs containing only the C-terminal half of Nod bound to chromosomes and not to microtubules. Accordingly, we have focused on identifying the functional elements within the C-terminal half of Nod that are required for binding to meiotic chromosomes. As shown in Figure 1A, the C terminus of Nod contains two known classes of DNA-binding domains, and the first domain spans residues 522–594 and is composed of three copies of a high-mobility group N (HMGN) motif. Afshar et al. (1995b) demonstrated that this domain was both necessary and sufficient to localize ectopically expressed Nod protein to mitotic chromosomes in embryos, suggesting that this domain plays a critical role in binding to chromosomes.
Figure 1.
The structure of the Drosophila Nod chromokinesin-like protein. (A) A schematic comparison of the structure of the full-length Nod protein. The motor-like domain of Nod falls entirely within the first 318 amino acids at the N terminus. Shaded regions indicate the HMGN domains, the putative D-box cyclin destruction domain (see discussion), and the two HhH motifs. Note that the HhH(2)/NDD domain, as described by Doherty et al. (1996), is composed of two HhH motifs, both of which are encompassed by regions of predicted α-helical structure. (B) Sequence composition of numerous proteins containing the HhH(2)/NDD domain (adapted from Doherty et al. 1996). Listed below the Nod sequence are three of the nine sequences used by Doherty et al. (1996) to define the HhH(2)/NDD domain. The last two sequences are from the chromokinesins XKid and HKid (Funabiki and Murray 2000; Tokai et al. 1996). Cylinder represents α-helix and arrows represent β-strands. (C) The structure of existing mutants in the Nod HhH(2)/NDD domain (Rasooly et al. 1994; Zwick et al. 1999). Underlining denotes the mutations existing in nod alleles, * denotes the breakpoint in nodb17, and ** denotes that the remaining 20 amino acids are altered by a frameshift mutation in nodb1. (D) Mutants in the HhH(2)/NDD domain produced by site-directed in vitro mutagenesis. * denotes the positions of the mutated amino acids, and underlining denotes the mutations.
Nod also carries a second putative DNA-binding motif known as a helix-hairpin-helix(2)/Nod-like DNA-binding domain, denoted as a HhH(2)/NDD domain (Doherty et al. 1996). This domain, which spans residues 614–660, consists of two helix-hairpin-helix (HhH) motifs, which are thought to function as sequence nonspecific DNA-binding domains (Doherty et al. 1996; Zhao and Grishin 2000). Each of these two HhH motifs is encompassed by a region of predicted α-helical coiling (see Figure 1). HhH(2)/NDD domains are found in proteins such as the Caenorhabditis elegans Emb-5 protein, which is thought to play a role in chromatin remodeling (Nishiwaki et al. 1993; Hubbard et al. 1996), the chromokinesin proteins Nod, Hkid, and Xkid (Zhang et al. 1990; Funabiki and Murray 2000; Levesque and Compton 2001; Yajima et al. 2003), the RNA polymerase-interacting protein SPT6 in Saccharomyces cerevisiae (Endoh et al. 2004), and a meiosis-specific protein cnjB in Tetrahymena thermophila (Taylor and Martindale 1993).
Although the deletion analysis of the Nod C terminus performed by Afshar et al. (1995b) revealed no obvious role for the HhH(2)/NDD domain in binding Nod to the mitotic chromosomes of embryos, several lines of evidence suggest an important role for this domain. First, as noted above, the HhH(2)/NDD domain is shared with two other chromokinesins (HKid and XKid), as well as with putative DNA-binding proteins from a variety of organisms (Figure 1B). Second, there are a number of loss-of-function nod mutations that map within the HhH(2)/NDD domain (Figure 1C). These mutations include two frameshift mutants (nodb1 and nodb17) that create null alleles of nod, the null allele noda, a natural haplotype (nod9) composed of three-amino-acid substitutions within this region that behaves as a hypomorphic nod allele, and a naturally occurring polymorphism (nodA4579T) whose presence is correlated with elevated levels of meiotic nondisjunction in wild-type stocks (Rasooly et al. 1994; Zwick et al. 1999). The noda allele carries the same three missense mutations as nod9, as well as a nonsense mutation in position 654 that results in the loss of the last 12 amino acids (Rasooly et al. 1994; Zwick et al. 1999). The nodA4579T allele encodes a protein that carries a glutamine-to-leucine substitution at position 660 (Zwick et al. 1999).
To better understand the roles of the HhH(2)/NDD domain in mediating Nod function, we have generated transgenic flies that express either the full-length Nod protein or proteins bearing missense mutants in the HhH(2)/NDD domains. In each case the Nod protein is fused to GFP at the C terminus. Although the wild-type Nod-GFP construct rescues the nod segregational defect and localizes to the oocyte chromosomes, two of the three HhH(2)/NDD mutants fail to rescue the nod mutant phenotype and fail to localize to the oocyte chromosomes (despite entering the oocyte nucleus). However, the mutant proteins do bind to nurse-cell chromosomes. Thus, although the HhH(2)/NDD domain is not required for chromosome binding in some cell types, such as nurse cells or embryos (Afshar et al. 1995b), it is essential for chromosome binding in the oocyte. These mutants also block the localization of Nod to the posterior pole of stage 9–10A egg chambers that is observed in wild-type oocytes, suggesting that the Nod HhH(2)/NDD domain may play other roles in addition to binding Nod to meiotic chromosomes.
MATERIALS AND METHODS
Fly stocks:
The descriptions of the nodb27 and noda mutations and their molecular characterization were previously reported (Zhang and Hawley 1990; Rasooly et al. 1994). Germline expression of wild-type and mutant Nod-GFP proteins was achieved by expressing UASp:Nod-GFP fusion constructs under the control of the nanos-Gal4∷VP16 driver (Rorth 1998).
Construction of Nod-GFP constructs:
Three point mutations were generated in nod cDNA in pOT2 vector by using the Transformer site-directed mutagenesis method (CLONTECH). The entire coding sequence was sequenced on both strands to verify the identity of the induced mutations. Full-length wild-type and mutant nod cDNAs were amplified by PCR and a SmaI site was added on the 5′-end of the PCR products. Wild-type and mutant nod cDNA were then cloned into the SmaI site of pEGFP-N1 vector (CLONTECH) by blunt-end ligation. Subsequently, wild-type and mutant Nod-GFP were released by digestion with KpnI and BamHI and cloned into pUASP vector (a generous gift from Pernille Rorth) double digested with KpnI and BamHI.
Creation of transgene-bearing stocks:
Wild-type and mutant UASp:Nod-GFP were introduced into Drosophila via P-element-mediated transformation. To introduce p{UASp-Nod-GFP}-carrying chromosomes into the appropriate background for cytological and genetic studies, FM7nod/y+Y males were crossed to either y w; Sp1 Bl1 Lrm Bc1 Pu2/SM6a; spapol or y w; D/TM3; spapol females. Subsequently, FM7, nodb27/y w; +/SM6a; spapol or FM7, nodb27/y w; +/ TM3; spapol females were crossed with males carrying p{UASp-Nod-GFP}. At the same time, y noda/y+Y males were crossed with FM7/FM7; D/TM3; spapol females, and then y noda/FM7; +/TM3; spapol females were crossed to y w/y+Y; p{nos-Gal4:VP16}; spapol males. Finally, FM7, nodb27/y w; p{UAS-Nod-GFP}/TM3 (or SM6); spapol females were crossed to y noda/y+Y; p{nos-Gal4∷VP16}/TM3; spapol males to generate FM7, nodb27/y noda; p{UASp-Nod-GFP}/p{nos-Gal4∷VP16}; spapol females.
Measurement of nondisjunction:
For each nod transgenic line, we crossed at least 15 FM7, nodb27/y noda; p{UAS-Nod-GFP}/p{nos-Gal4:vp16} spapol females individually to attached-XY, v f B; C(4)RM, ci eyR males and assessed the frequency of X and 4th chromosome nondisjunction as previously described (Hawley et al. 1992).
Cytological studies:
Oocytes were prepared and examined as previously described with minor modifications (Theurkauf and Hawley 1992; Matthies et al. 2000). Egg chambers from 3- to 7-day-old females were extracted by quick pulses of a blender using modified Robb's medium. The mixture was passed sequentially through a loose and fine mesh to separate late-stage oocytes. The oocytes were fixed in 4% paraformaldehyde for 20 min (oocytes prior to stage 14) or 8% paraformaldehyde for 5 min (stage 14 oocytes) on a rotator at room temperature in a hypertonic solution, therefore preventing hypotonic activation of the mature oocytes. After removal of the chorion and vitelline membranes, the oocytes were permeabilized with 1% Triton X-100 in phosphate NaCl buffer (PBS). Oocytes were labeled with 1:500 MAS078P (Harlan Sera-Lab) and 1:500 MAB1864 (Chemicon) rat antitubulin monoclonal antibodies and 1:1000 anti-GFP BD living color rabbit polyclonal antibody (CLONTECH). These oocytes were then labeled with Cy3 conjugated anti-rat secondary antibodies (1:250) or Alexa 488 conjugated anti-rabbit secondary antibody (1:1000) purchased from Jackson Immunoresearch and Molecular Probes, respectively. Chromosomes were stained with DAPI at 1 μg/ml. Immunofluorescence data were collected in 0.2-μm Z steps 3–5 μm above and below the subject of interest. The resulting data were deconvolved using the Softworx package (API).
RESULTS
A full-length Nod-GFP construct functions as a wild-type Nod protein and rescues the segregational defects observed in nod oocytes:
We show here that a transgene construct expressing a wild-type Nod protein fused to GFP at its C terminus (Nod-GFP) behaves similarly to wild-type Nod protein in three critical respects. First, this construct rescues the meiotic defects observed in nod mutant oocytes. Second, the Nod-GFP construct generates a weak increase in achiasmate nondisjunction in oocytes carrying two normal chromosomal nod+ alleles, similar to that observed by Matthies et al. (1999) in flies with three doses of a chromosomally located nod+ gene. Third, as discussed below, Nod-GFP properly localizes to the oocyte chromosomes both prior to and after NEB, as had been shown for wild-type Nod protein by immunolocalization (Afshar et al. 1995a).
We measured the levels of X and 4th chromosome nondisjunction in FM7/X females that are either wild type (nod+) or mutant (nodb27/noda) with respect to their X chromosomal nod genes in the presence or absence of a Nod-GFP transgene construct. As reviewed by Hawley et al. (1992), the FM7 balancer chromosome serves to suppress X chromosomal exchange such that both the X and 4th chromosomes are always achiasmate in these females. The levels of nondisjunction (1.7% for both the X and 4th chromosomes) observed in FM7, nod+/nod+ females bearing one copy of nanos-Gal4∷VP16 driver are similar to the frequencies of achiasmate nondisjunction in FM7/X females observed in previous studies (Hawley and Theurkauf 1993). However, as a consequence of ablating Nod function, the frequencies of X and 4th chromosome nondisjunction observed in FM7, nodb27/noda oocytes are elevated to 53.2 and 78.9%, respectively (Table 1). The majority of apparent 4th chromosome nondisjunction events result from loss of the 4th chromosome.
TABLE 1.
The abilities of wild-type Nod-GFP or mutant Nod-GFP proteins to rescue nod defects in FM7,w/y w; pol/pol females1
| Nod-GFP expression construct
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gamete type
|
Nod+
|
NodNDD1,2
|
NodNDD3: | NodNDD4: | |||||||
| Background nod genotypeb:
|
Nonea
|
Line no.: | 1
|
2
|
1
|
2
|
1
|
1
|
|||
| Mother | Father | nod+ | nod | nod | nod | nod | nod | nod | nod | ||
| Regular | |||||||||||
| X 4 | X̂Y 4̂4 | 765 | 58 | 871 | 463 | 80 | 108 | 249 | 786 | ||
| X 4 | O 4̂4 | 694 | 47 | 627 | 328 | 57 | 66 | 161 | 520 | ||
| X nondisjunction | |||||||||||
| XX 4 | O 4̂4 | 1 | 47 | 68 | 17 | 44 | 47 | 33 | 21 | ||
| O 4 | X̂Y 4̂4 | 3 | 23 | 40 | 13 | 28 | 28 | 51 | 11 | ||
| 4 nondisjunction | |||||||||||
| X 44 | X̂Y O | 4 | 7 | 9 | 4 | 11 | 5 | 29 | 17 | ||
| X 44 | OO | 0 | 8 | 8 | 3 | 8 | 17 | 31 | 8 | ||
| X O | X̂Y 4̂4 | 3 | 166 | 6 | 37 | 361 | 298 | 399 | 92 | ||
| X O | O 4̂4 | 0 | 109 | 5 | 20 | 189 | 171 | 207 | 32 | ||
| X, 4 nondisjunction | |||||||||||
| XX 44 | OO | 6 | 6 | 1 | 1 | 7 | 5 | 31 | 3 | ||
| OO | X̂Y 4̂4 | 3 | 90 | 2 | 8 | 265 | 279 | 187 | 22 | ||
| XX O | O 4̂4 | 0 | 87 | 8 | 8 | 138 | 84 | 67 | 10 | ||
| O 44 | X̂Y O | 0 | 1 | 19 | 2 | 5 | 17 | 6 | 9 | ||
| Total | 147 | 602 | 1664 | 791 | 1193 | 1040 | 1428 | 1553 | |||
| Adjusted total | 1492 | 820 | 1802 | 955 | 1680 | 1491 | 1780 | 1607 | |||
| Nondisjunction | |||||||||||
| X | 1.7% | 53.2% | 15.3% | 10.5% | 58.0% | 60.5% | 39.6% | 9.5% | |||
| 4 | 1.7% | 78.9% | 4.9% | 10.7% | 83.3% | 83.9% | 67.5% | 14.7% | |||
Females were crossed to attached XY, y+ v f B; C(4),ci eyR males and nondisjunction was calculated as described in materials and methods. FM7 w denotes the genotype FM7, y[31d] w v B.
The controls consists of the genotypes of FM7/y w; nanos-Gal4∷VP16/+; pol/pol and FM, nodb27/y noda; pol/pol.
“nod” denotes the genotype FM7, nodb27/ y noda.
As shown in Table 1, expression of either of two separate insertions of a UASp:Nod-GFP construct (driven by the nanos-Gal4∷VP16 germline driver) in FM7,nodb27/noda oocytes reduces the frequency of X chromosomal nondisjunction by three- to fivefold and the frequency of 4th chromosome nondisjunction by 7- to 20-fold. The residual nondisjunctional events that were observed in FM7, nodb27/noda oocytes expressing Nod-GFP suggest either that the amount of Nod-GFP produced by the UASp:Nod-GFP construct may not be sufficient to fully rescue the nod defect or that this construct is not fully functional. In addition to rescuing the meiotic nondisjunction observed in FM7, nodb27/noda oocytes, expression of either of these two separate insertions of a UASp:Nod-GFP construct in FM7, nod+/nod+ oocytes creates a weak increase in meiotic nondisjunction (see Table 2). As noted above, this ability to increase the level of X and 4th chromosome nondisjunction in FM7, nod+/nod+ females parallels the ability of an additional chromosomal copy of nod+, carried by a duplication, to produce a similar increase in the frequency of achiasmate nondisjunction. Both observations suggest that the Nod-GFP protein possesses substantial ability to rescue the nod defect.
TABLE 2.
The effects of overexpressing of wild-type Nod-GFP or mutant Nod-GFP proteins on achiasmate chromosome segregation in FM7,w/yw;pol/pol females
| Nod-GFP expression construct
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gamete type
|
Nod+
|
Nod NDD1,2
|
NodNDD3: | NodNDD4: | |||||
| Mother | Father | Nonea | Line no.: | 1 | 2 | 1 | 2 | 1 | 1 |
| Regular | |||||||||
| X 4 | X̂Y 4̂4 | 765 | 595 | 876 | 1391 | 1051 | 759 | 756 | |
| X 4 | O 4̂4 | 694 | 540 | 751 | 1389 | 833 | 575 | 543 | |
| X nondisjunction | |||||||||
| O 4 | X̂Y 4̂4 | 1 | 29 | 31 | 2 | 2 | 7 | 10 | |
| XX 4 | O 4̂4 | 3 | 15 | 23 | 2 | 2 | 11 | 7 | |
| 4 nondisjunction | |||||||||
| X 44 | X̂Y O | 4 | 3 | 1 | 0 | 0 | 0 | 2 | |
| X 44 | O O | 0 | 2 | 9 | 1 | 0 | 0 | 1 | |
| X O | X̂Y 4̂4 | 3 | 8 | 1 | 0 | 0 | 0 | 2 | |
| X O | O 4̂4 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| X, 4 nondisjunction | |||||||||
| XX 44 | OO | 6 | 1 | 1 | 0 | 0 | 0 | 1 | |
| O O | X̂Y 4̂4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| XX O | O 4̂4 | 0 | 9 | 4 | 0 | 0 | 0 | 3 | |
| O 44 | X̂Y O | 0 | 12 | 15 | 1 | 0 | 0 | 5 | |
| Total | 1479 | 1215 | 1712 | 2780 | 1884 | 1352 | 1330 | ||
| Adjusted total | 1492 | 1281 | 1786 | 2791 | 1892 | 1370 | 1356 | ||
| Nondisjunction | |||||||||
| X | 1.7% | 10.3% | 8.3% | 0.4% | 0.4% | 2.6% | 3.8% | ||
| 4 | 1.7% | 4.5% | 2.9% | 0.1% | 0.0% | 0.0% | 1.7% | ||
The genotype of these females was FM7 /y w; nanos-Gal4∷VP16/+; pol/pol.
Site-directed mutants in the HhH(2)/NDD domain fail to rescue the nod defect:
To investigate the role of the HhH(2)/NDD domain in Nod function, we created Nod-GFP constructs that carried mutants in three different components of the HhH(2)/NDD domain by site-directed mutagenesis (see Figure 1D). The first mutation, which is denoted as nodNDD1,2, disrupts the first HhH domain and results in the substitution of alanine for two highly conserved glycine residues at amino acid positions 619 and 621, which are located in the left half of this motif. These two glycine residues within the HhH domains of two bacterial Pol I enzymes were shown to be critical for enzymatic activity (Joyce et al. 1985; Ishino et al. 1995; Doherty et al. 1996). The second mutant, denoted nodNDD3, results in the substitution of an alanine for the arginine residue at position 632. This residue lies outside of the first HhH motif but within a region of predicted α-helical secondary structure that encompasses the first HhH repeat. The third mutant, denoted as nodNDD4, results in the substitution of an alanine for a leucine residue at position 635 that lies within the short region that links the regions of predicted secondary structure.
The nodNDD3 and nodNDD4 mutants were also designed to test the significance of a putative cyclin destruction domain that mapped within the HhH(2)/NDD-binding domain. The D-box cyclin destruction box, RXXLGXXXN, which is shared by the cyclin A, cyclin B, PDS1p, and Cut2 proteins, is necessary for their cell-cycle-dependent proteolysis (Cohen-Fix et al. 1996; King et al. 1996; Funabiki et al. 1997). A homologous sequence (RSRLGCFEN) is encompassed by amino acids 632 to 640 in the carboxy terminal domain of the Nod protein, adjacent to the C-terminal end of the first HhH motif. The potential significance of this domain is suggested by the observation by Castro et al. (2003) that mutation of the arginine residue that begins this domain interferes with the degradation of the Xkid chromokinesin. Both the nodNDD3 and nodNDD4 mutants alter critical conserved residues within this domain.
As described in detail below, the nodNDD1,2 and nodNDD3 mutants ablate Nod function in three respects: (1) they fail to rescue the meiotic defects observed in FM7,nodb27/noda females, (2) they do not increase the level of meiotic nondisjunction observed in FM7/X females carrying two chromosomally normal copies of the nod gene, and (3) as discussed below, they fail to localize to oocyte chromosomes both before and after NEB. Although the nodNDD4 mutation was able to rescue the segregational defects observed in FM7, nodb27/noda females, it failed to increase X and 4th chromosome nondisjunction in FM7, nod+/nod+ females. This protein also exhibited a possible, albeit weak, defect in binding to oocyte chromosomes (see below). Thus, at least the first HhH motif and the surrounding region of predicted secondary structure within the HhH(2)/NDD domain appears to be critical for Nod function.
As shown in Table 1, FM7, nodb27/noda females expressing either NodNDD1,2-GFP or NodNDD3-GFP do not rescue the nod phenotype to any discernable degree. Indeed, these females show levels of X and 4th chromosome nondisjunction and 4th chromosome loss that are similar to those observed in FM7, nodb27/noda controls. These mutant constructs also fail to increase the levels of X and 4th chromosomal nondisjunction observed in FM7, nod+/nod+ females (see Table 2). We do note that only one insert was tested for the NodNDD3-GFP construct. However, as documented below, this construct was clearly expressed as documented by substantial binding to nurse-cell chromosomes.
The NodNDD4 construct demonstrates a substantial ability to rescue the nod defect in FM7, nodb27/noda females. However, it does not increase the levels of X and 4th chromosome nondisjunction in FM7, nod+/nod+ females above that observed in controls (Table 2). This suggests either that this protein retains substantial, but perhaps incomplete, levels of normal function or that the levels of expression of this construct are not fully sufficient to restore full Nod function.
In terms of the putative cyclin destruction domain, we note that the nodNDD4 mutation (which alters a highly conserved leucine residue within the cyclin destruction domain) has only segregational defect and no obvious effect on fertility. Although the nodNDD3 mutation (which replaces the highly conserved first residue of the domain, an arginine) does ablate Nod function, its effects are similar, if not identical, to those of the nodNDD1,2 mutation and appear to be the result of a defect in the binding of Nod to oocyte chromosomes (see below). Thus, the effects of the nodNDD3 mutation seem to be the consequence of altering the HhH(2)/NDD domain rather than altering a domain that controls Nod destruction. For these reasons we consider it unlikely that the RSRLGCFEN domain is critical to Nod function (but see discussion) and will therefore focus our discussion on the HhH(2)/NDD domain.
Localization of wild-type and HhH(2)/NDD mutant Nod-GFP proteins to oocyte chromosomes:
To determine the effects of the HhH(2)/NDD mutants on the ability of Nod to bind chromosomes, we took advantage of the GFP tag to allow us to follow the binding of Nod protein to meiotic and nurse-cell chromosomes. Specifically, we used an anti-GFP antibody to immunolocalize the Nod-GFP fusion proteins during the process of oocyte development.
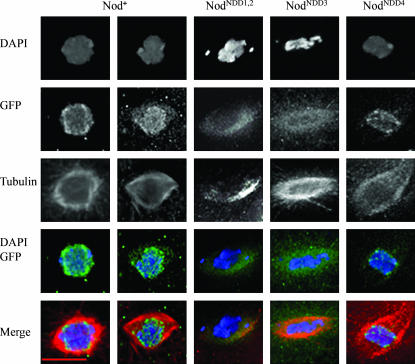
Although low levels of Nod-GFP are observed in multiple germline cell nuclei in the germarium, the expression of UASp:Nod-GFP constructs under the control of a nanos-GAL4∷VP16 driver is suppressed until stage 4–5 (data not shown). Following stage 5, however, high levels of Nod-GFP are observed through stage 14. This pattern of expression parallels that of the endogenous nod gene (Zhang et al. 1990). From stages 4–5 and until NEB at stage 12–13, Nod-GFP binds to the chromosomes of both the oocyte and nurse-cell nuclei. In FM7, nod+/nod+ females, Nod-GFP was bound to the oocyte chromosomes in 62.5% of oocytes (n = 240) and to nurse-cell chromosomes in all of the examined egg chambers (Figure 2). Analysis of prometaphase and metaphase in stage 14 oocytes (Figure 4) revealed that Nod-GFP is localized to the chromosomes in 81.5% of oocytes (n = 27), consistent with the previous observation by Afshar et al. (1995a). In the remaining cases Nod-GFP was localized to only the spindle (14.8%) or both the chromosomes and the spindles (3.7%). Thus, as had been shown for wild-type Nod protein by immunolocalization (Afshar et al. 1995a), Nod-GFP binds to oocyte chromosomes both before and after NEB.
Figure 2.
Wild-type or mutant Nod-GFP localization stage 7 nod + oocytes. Oocytes expressing wild-type or mutant UAS-Nod-GFP were stained with DAPI (blue) and anti-GFP antibody (GFP) and analyzed by deconvolution microscopy. Arrowhead indicates the location of oocyte nucleus. Bar, 10 μm.
Figure 4.
Localization of wild-type or mutant Nod-GFP in stage 13–14 nod+/nod+ oocytes. Wild-type or mutant UAS-Nod-GFP expressing oocytes were stained with DAPI (blue), anti-GFP antibody (green), and anti-tubulin antibody (red) and analyzed by deconvolution microscopy. Bar, 10 μm.
Both the NodNDD1,2 and NodNDD3 mutant proteins fail to localize to oocyte chromosomes before and after NEB. As shown in Figure 2, although the NodNDD1,2-GFP fusion protein accumulates along the anterior and posterior cortex of the egg chamber, it does not bind to oocyte chromosomes (n = 0/80) in stage 5–12 egg chambers. Although NodNDD1,2-GFP protein fails to localize to the oocyte chromosomes in stage 5–12 oocytes, it is often observed within the nucleus (see Figure 3) and is therefore closely associated with the chromosomes. Similar observations were made for the NodNDD3-GFP protein (data not shown).
Figure 3.
NodNDD1,2-GFP localizes within the oocyte nuclei envelope but not on the oocyte nuclei. Oocytes expressing NodNDD1,2-GFP were stained with DAPI (blue) and anti-GFP antibody (GFP) and analyzed by deconvolution microscopy. Arrowhead indicates the location of oocyte nucleus. Bar, 10 μm.
Despite its failure to bind to oocyte chromosomes, the NodNDD1,2-GFP protein does bind to the chromosomes in nurse-cell nuclei by stage 5 (Figure 2). Similarly, NodNDD3-GFP binds to polytene nurse-cell nuclei, but does not bind to oocyte chromosomes (n = 0/60) in stage 5–12 egg chambers. The labeling of the nurse-cell chromosomes in these experiments cannot be an artifact of nonspecific binding of the anti-GFP antibody to nurse cells because no staining was observed in egg chambers carrying only nanos-Gal4∷VP16 germline driver (data not shown). Thus the failure of the NodNDD1,2-GFP and NodNDD3-GFP proteins to bind to oocyte chromosomes is not the result of a general inability to bind chromatin, but rather represents a specific defect in the ability of these mutants to bind to meiotic chromosomes.
Perhaps not surprisingly, both the NodNDD1,2-GFP and NodNDD3-GFP proteins also fail to localize to the oocyte chromosomes after NEB. The NodNDD1,2-GFP fusion protein did not bind to the chromatin in prometaphase and metaphase oocytes; instead it exclusively binds to the meiotic spindle (n = 15) (see Figure 4). Similarly NodNDD3-GFP binds exclusively to the meiotic spindle, and not to the meiotic chromosomes, in prometaphase and metaphase oocytes (n = 21) (see Figure 4). These data are consistent with the observation that mutants in the first HhH domain do not rescue the nod defect and suggest that the HhH DNA-binding domain is critical for Nod function.
Unlike the NodNDD1,2-GFP and the NodNDD3-GFP fusion proteins, the NodNDD4-GFP protein, which demonstrates a substantial ability to rescue the nod defect (see above), was shown to bind to the oocyte chromosomes in 50% of stage 5–12 egg chambers examined (n = 90) (Figure 2). Nod NDD4 colocalizes with prometaphase and metaphase chromatin in 84.6% of the oocytes examined and localizes to both chromosomes and spindle in the remaining cases (n = 13) (Figure 4).
The failure of NodNDD1, 2-GFP and NodNDD3-GFP proteins to bind to the oocyte chromosomes is not the result of competition by endogenous Nod protein:
Given their ability to bind to nurse-cell chromosomes, it was possible that the NodNDD1, 2-GFP and NodNDD3-GFP proteins possessed a weak ability to bind oocyte chromosomes and were simply outcompeted by the presence of a large amount of wild-type Nod protein in the oocyte with respect to a small number of potential Nod-binding sites on the oocyte chromosomes. To test this possibility, we repeated the localization studies in FM7, nodb27/noda oocytes that lack functional Nod protein.
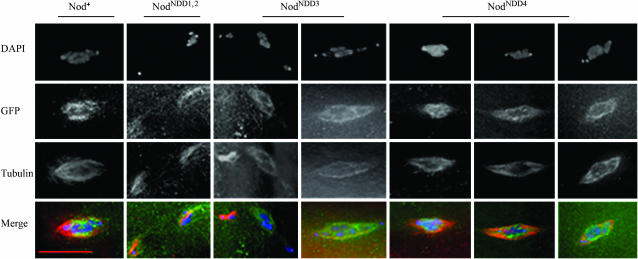
As shown in Figures 5 and 6, Nod-GFP binds to the oocyte chromosomes in 75% of stage 5–12 egg chambers of FM7, nodb27/noda females (n = 32). Nod-GFP also binds to the meiotic chromosomes in 60% of stage 14 oocytes in FM7, nodb27/ noda females (n = 10). While this frequency of Nod binding is somewhat lower than the frequency of Nod binding observed in nod+ females (81.5%), and thus might indicate that the presence of wild-type Nod protein facilitates binding of Nod-GFP, it should be noted that the number of stage 14 oocytes (10) is quite small. In the remaining 40% of stage 14 oocytes examined, Nod-GFP binds either to both the chromosomes and the spindles (20%) or only to the spindles (20%).
Figure 5.
Localization of wild-type or mutant Nod-GFP in stage 7 nod−/nod− oocytes. FM7nodb27/y noda oocytes carrying either wild-type or mutant UAS-Nod-GFP expressing oocytes were stained with DAPI (blue) and anti-GFP antibody (GFP) and analyzed by deconvolution microscopy. Arrowhead indicates the location of oocyte nucleus. Bar, 10 μm.
Figure 6.
Localization of wild-type or mutant Nod-GFP in stage 13–14 nod oocytes. FM7nodb27/y noda;pol/pol oocytes carrying wild-type or mutant UAS-Nod-GFP construct were stained with DAPI (blue), anti-GFP antibody (green), and anti-tubulin antibody (red) and analyzed by deconvolution microscopy. Bar, 10 μm.
However, neither NodNDD1,2-GFP (n = 50) nor NodNDD3-GFP (n = 60) binds to the chromosomes in oocytes of FM7, nodb27/noda females prior to NEB (see Figure 5). In addition, these proteins did not bind to the meiotic chromosomes (n = 8 and n = 18, respectively) in prometaphase oocytes. Indeed, after NEB these two proteins bound only to the meiotic spindle. As shown in Figure 6, the nonexchange X and 4th chromosomes are often well apart from the main chromosome masses in these oocytes, even on relatively short spindles (compare to the image for Nod-GFP). This precocious movement toward or beyond the poles of the spindle in early prometaphase is expected for oocytes that lack Nod function. Note that in the left of the two columns portraying oocytes expressing NodNDD3-GFP, the X and 4th chromosomes (top left) appear separated from the upper pole of the spindle, forming a spur pointing to the left. The right NodNDD3-GFP panel appears more normal; however, even in this oocyte both 4th chromosomes are already at or slightly beyond the poles of the spindle. Similarly, in the column portraying a NodNDD1,2-GFP oocyte a single 4th chromosome (at the bottom left) is well separated from its homolog, which sits just above the main chromosome mass in the upper right of this image.
In the absence of Nod, the NodNDD4-GFP protein bound to both the oocyte chromosomes prior to NEB and to prometaphase and metaphase chromosomes in stage 14 oocytes. As shown in Figure 6, in the absence of endogenous wild-type Nod protein, NodNDD4-GFP binds to the meiotic chromosomes in 57.1% of stage 14 oocytes, to both the chromosomes and the spindles in 14.3% of the oocytes, and to the spindles only in 28.5% oocytes (n = 21). In Figure 6 (right column), two 4th chromosomes, both bound by NodNDD4-GFP, appear on the same side of the main chromosomal mass as if they are likely to nondisjoin. This was the only case of aberrant segregation observed in 21 oocytes, consistent with the ability of NodNDD4 to largely rescue the nod segregational defect.
Mutants in the HhH(2)/NDD domain also impair a DNA-binding independent function of the Nod protein:
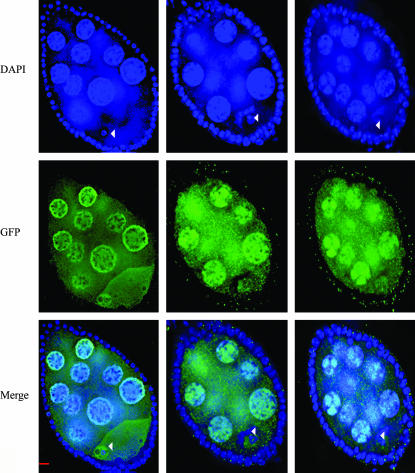
Nod-GFP is detectable in the oocyte cytoplasm concentrating along the anterior and posterior cortex in stage 6–8 egg chambers (Figure 2). However, as demonstrated by Cui et al. (2005) and as shown in Figure 7, by stage 9 of oocyte development the cytoplasmic Nod-GFP protein accumulates at the posterior pole of the oocyte, in a fashion similar to that observed for the plus-end-directed motor kinesin heavy chain (Clark et al. 1997). (The significance of this localization in terms of Nod function is considered in more detail in Cui et al. 2005.) As shown in Figure 7, both the NodNDD1,2-GFP and NodNDD3-GFP fusion proteins fail to localize to the posterior pole in stage 9 oocytes. Consistent with its wild-type behavior with respect to chromosome localization, NodNDD4-GFP does localize to the posterior pole in stage 9 oocytes. As discussed below, the failure of the NodNDD1,2-GFP and NodNDD3-GFP proteins to localize to the posterior pole of stage 9 oocytes suggests that the HhH(2)/NDD domain may mediate protein:protein interactions.
Figure 7.
Expression of wild-type or mutant Nod-GFP construct in stage 9 nod+/nod+ oocytes. Wild-type or mutant UASp:Nod-GFP expressing oocytes were stained with anti-GFP antibody and analyzed by deconvolution microscopy. Bar, 20 μm.
DISCUSSION
Previous molecular and cytological studies have demonstrated the ability of Nod to bind to both DNA in solution (Afshar et al. 1995a) and to meiotic and mitotic chromosomes (Afshar et al. 1995a,b). The observations of Afshar et al. (1995a) that purified Nod protein bound efficiently to AT-rich but not GC-rich satellite sequences in vitro, as well as the genetic studies of Murphy and Karpen (1999), which provided evidence for discrete regions of Nod function (and thus presumably Nod binding) in the heterochromatin, suggest that Nod binding is likely to occur preferentially at certain chromosomal regions. The question then arises as to how Nod binds to meiotic chromosomes and, more specifically, whether that binding in vivo is mediated by direct Nod:DNA interactions or by protein:protein interactions involving Nod and other proteins that themselves are directly bound to DNA. To address these questions, we are attempting to understand the roles of the two putative DNA-binding domains in Nod function. In this study we have demonstrated that the HhH(2)/NDD domain is critical for the binding of Nod to oocyte chromosomes. In the absence of this domain, Nod enters the meiotic nucleus but does not bind to the chromosomes. It would obviously be of some interest to rigorously determine whether the HMGN domain is also critical for Nod function in the oocyte. Unfortunately, none of the existing nod mutants map within this domain, and thus its remains unclear as to whether the HMGN domain is as critical for the meiotic function of Nod as it is for the binding of Nod to mitotic chromosomes (Afshar et al. 1995b).
The role of the HhH(2)/NDD domain in binding to oocyte chromosomes appears to reflect a specific characteristic of chromosomes in the developing oocyte, as evidenced by both the ability of C-terminal Nod fragments that lack this domain to bind to mitotic chromosomes (Afshar et al. 1995b) and the ability of Nod-GFP proteins that lack the HhH(2)/NDD domain to bind to the polytene chromosomes of the nurse cells. One can imagine that the binding to oocyte chromosomes requires specific domains in addition to those required for mitotic or polytene chromosomes. Indeed, as one way of understanding the specific requirement for the HhH(2)/NDD domain in the binding of Nod to oocyte chromosomes, we propose that the remodeling of oocyte chromatin during meiosis creates a specific requirement for the HhH(2)/NDD domain. However, it is also possible that both the HMGN and HhH(2)/NDD domains function to enable Nod to bind to chromosomes. According to this model, the deficit in binding capacity exhibited by proteins lacking the HhH(2)/NDD domain is overcome in the nurse-cell nuclei by the high DNA content of the polytene chromosomes and in the embryonic mitoses studied by Afshar et al. (1995b) as a consequence of the high concentration of Nod fragments produced by expression from a heat-shock promoter. Our data do not allow us to distinguish between these two alternatives.
Although the HhH domain was initially characterized as a sequence nonspecific DNA-binding domain (Doherty et al. 1996), both Mah et al. (2000) and Endoh et al. (2004) have presented evidence that HhH domains may actually mediate protein:protein interactions. Mah et al. (2000) have identified a role for HhH domains in mediating the physical interaction of two HhH domain bearing bacterial proteins (the α-subunit of Escherichia coli RNA polymerase and NusA), and studies by Endoh et al. (2004) have also elucidated a physical interaction between the yeast HhH(2)/NDD domain bearing protein SPT6 and RNA polymerase. Thus, it is at least possible that the Nod HhH(2)/NDD domain can mediate protein:protein interactions. That Nod is a component of at least one protein complex that is targeted to the vicinity of the oocyte nucleus is evidenced by the observation of Arn et al. (2003) that Nod is a part of large protein complex, including the RNA-binding proteins Modulo, PABP, and Smooth, and the known bicoid-localization factor Swallow, which is bound to bicoid mRNA in the oocyte. Thus, it is at least possible that the Nod HhH(2)NDD domain is not involved in direct binding to DNA, but rather that Nod is targeted to the oocyte chromosomes by a Nod:protein interaction that involves the HhH(2)/NDD domain. Toward that end, it would be of significant interest to identify proteins that physically interact with Nod and test such proteins for a role in localizing the Nod protein that enters the nucleus to the oocyte chromosomes. Proposing a role for the HhH(2)/NDD domain in protein:protein interactions might also explain the requirement of the HhH(2)/NDD domain for localizing Nod to the posterior pole of the oocyte in stage 9–10a egg chambers.
The observation by Cui et al. (2005) that full-length Nod-GFP localizes to the posterior end of stage 9 oocytes is contrary to Clark et al.'s (1997) observation that a Nod-KHC-β-gal fusion construct, which contains the Nod motor-like domain, the coiled-coil domain of kinesin heavy chain (KHC), and the β-gal moiety, is an effective marker for the minus ends of microtubules. Given that the binding of Nod to posterior poles requires the HhH(2)/NDD domain, it is not surprising that the Nod-KHC-β-gal fusion protein does not bind to the posterior pole of the oocyte, but the well-documented capacity of this protein and its GFP derivatives to bind to regions containing the minus ends of microtubules (Grieder et al. 2000; Clegg et al. 2001) remains a mystery. We can, however, say that the binding of the Nod-KhC-β gal fusion protein to the minus ends of microtubules is unlikely to reflect an innate activity of the Nod motor-like domain. This is evidenced by the observation of Cui et al. (2005) that both full-length Nod and the N-terminal Nod motor domain alone bind to the ends of microtubules and stimulate microtubule polymerization in vitro. The observation of Nod-stimulated polymerization suggests that the observed end binding is likely preferential, or restricted, to plus ends of microtubules. Thus it seems reasonable to suggest that the ability of the Nod-KHC-β-gal fusion protein to localize to minus ends of microtubule arrays might well be the function of some other component of this construct, perhaps the coiled-coil domain derived from KHC. That microtubule localization domains outside the motor domain do exist for at least some chromokinesin proteins has been demonstrated by Shiroguchi et al. (2003), who mapped a second microtubule-binding domain in the C-terminal (nonmotor) domain of Kid. Alternatively, as appears to be the case in terms of the ability of Nod to bind to posterior pole in stage 9–10 oocytes, this localization of the Nod-KHC-β-gal construct to minus ends of microtubules may not reflect an innate affinity of this protein for minus ends of microtubules, but rather an interaction with some other protein that does move to or bind the minus ends.
Taken together all of these data support a model in which Nod binds to the arms of oocyte chromosomes by a process that involves the HhH(2)/NDD domain and perhaps the HMGN domain as well. Nod also interacts with the plus ends of microtubules in a fashion that stimulates microtubule polymerization (Cui et al. 2005). Such a model explains the observations of Matthies et al. (1999), who demonstrated that Nod serves to push the chromosomes toward the metaphase plate and thus counteracts the poleward forces acting at the kinetochore. Indeed, Cui et al. (2005) have proposed a model for Nod function that is based on a mechanism proposed by Dickinson and Purich (2002) for actin-based motors. This model, known as the clamped-filament elongation model, proposes a “lock, load, and fire” mechanism in which the motor binds to the growing filament end (lock), the second step (load) allows the addition of a new filament subunit, and the third step (fire) reattaches the clamp to the new extended end of the fiber. In a similar fashion, Cui et al. (2005) proposed a mechanism for Nod function in which Nod binds to the plus end of microtubules by its motor-like domain and along the length of chromosome arms via the HhH(2)/NDD domain. The Nod motor-like domain then opens or relaxes enough to allow the insertion of a tubulin subunit at the plus end of the microtubule, before reclamping on the new plus end of the growing fiber. Reiterations of this mechanism would serve to push the chromosomes away from the poles of the spindle, mimicking the effect observed in cytological experiments (Matthies et al. 1999), without requiring the Nod motor protein to exhibit conventional motor activity in standard in vitro assays. If this model is correct, it provides a reasonable model for both the function of Nod and the so-called “polar ejection force” (Rieder et al. 1986; Hays and Salmon 1990; Marshall et al. 2001).
Finally, we should note that a putative cyclin destruction domain was also mapped within the HhH(2)/NDD-binding domain. The D-box cyclin destruction box, RXXLGXXXN, which is shared by the cyclin A, cyclin B, PDS1p, and Cut2 proteins, is necessary for the cell-cycle-dependent proteolysis of cyclin (Cohen-Fix et al. 1996; King et al. 1996; Funabiki et al. 1997). A homologous sequence (RSRLGCFEN) is encompassed by amino acids 632–640 of the Nod protein, adjacent to the C-terminal end of the first HhH motif. We had wondered if the function of Nod was also dependent on cell-cycle-dependent proteolysis, in a manner that required this site. As noted above, both the nodNDD3 and nodNDD4 mutants alter critical conserved residues within this domain. However, the nodNDD4 mutation has only weak discernible effects on segregation and no obvious effect on fertility. Although the nodNDD3 mutation does ablate Nod function, its effects are similar, if not identical, to those of the nodNDD1,2 mutation and thus seem likely to be the result of disrupting the HhH(2)/NDD domain. Thus our data fail to find any obvious role of this domain in mediating Nod function. However, we have not yet examined anaphase I meiosis and thus it is at least possible that these mutants impair this process in some subtle fashion that does not disrupt chromosome segregation.
Acknowledgments
The authors are grateful to Heiner Matthies, whose prior work and ideas were critical to the development of this project. We thank Susan Gustafson for technical assistance, Joe Kramer for helpful advice and consultation, and Robert Cohen and Bill Saxton for comments on an early draft of this manuscript. We thank Scott Page, Cathy Lake, Jennifer Jeffress, Wei Gong, William Gilliland, Susan Gustafson, Heiner Matthies, and Kim McKim for helpful comments on the manuscript. We also thank Jerry Workman and Michael Bustin for helpful discussions. The research was supported both by funds from the Stowers Institute for Medical Research and by a grant to R.S.H. from the National Science Foundation. R.S.H. gratefully acknowledges the National Science Foundation, and particularly the late DeLill Nasser, for nearly 2 decades of support for the “nod project.”
References
- Afshar, K., N. Barton, R. S. Hawley and L. S. B. Goldstein, 1995. a DNA binding and meiotic chromosome localization of the Drosophila NOD kinesin-like protein. Cell 81: 129–138. [DOI] [PubMed] [Google Scholar]
- Afshar, K., J. Scholey and R. S. Hawley, 1995. b Identification of the chromosome localization domain of the Drosophila Nod kinesin-like protein. J. Cell Biol. 131: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arn, E. A., B. J. Cha, W. E. Theurkauf and P. M. Macdonald, 2003. Recognition of a bicoid mRNA localization signal by a protein complex containing Swallow, Nod, and RNA binding proteins. Dev. Cell 4: 41–51. [DOI] [PubMed] [Google Scholar]
- Carpenter, A. T. C., 1973. A meiotic mutant defective in distributive disjunction in Drosophila melanogaster. Genetics 73: 393–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, A., S. Vigneronl, C. Bernis, J. C. Labbe and T. Lorca, 2003. Xkid is degraded in a D-box, KEN-box, and A-box-independent pathway. Mol. Cell. Biol. 23: 4126–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, I. E., L. Y. Jan and Y. N. Jan, 1997. Reciprocal localization of Nod and kinesin fusion proteins indicates microtubule polarity in the Drosophila oocyte, epithelium, neuron and muscle. Development 124: 461–470. [DOI] [PubMed] [Google Scholar]
- Clegg, N. J., S. D. Findley, A. P. Mahowald and H. Ruohola-Baker, 2001. Maelstrom is required to position the MTOC in stage 2–6 Drosophila oocytes. Dev. Genes Evol. 211: 44–48. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix, O., J. M. Peters, M. W. Kirschner and D. Koshland, 1996. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 10: 3081–3093. [DOI] [PubMed] [Google Scholar]
- Cui, W., L. R. Sproul, S. M. Gustafson, H. J. Matthies, S. P. Gilbert et al., 2005. Drosophila Nod protein binds preferentially to the ends of microtubules and promotes microtubule polymerization in vitro. Mol. Biol. Cell 16: 5400–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernberg, A. F., J. W. Sedat and R. S. Hawley, 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Dickinson, R. B., and D. L. Purich, 2002. Clamped-filament elongation model for actin-based motors. Biophys. J. 82: 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, A. J., L. C. Serpell and C. P. Ponting, 1996. The helix-hairpin-helix DNA-binding motif: a structural basis for non-sequence-specific recognition of DNA. Nucleic Acids Res. 24: 2488–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh, M., W. Zhu, J. Hasegawa, H. Watanabe, D. K. Kim et al., 2004. Human Spt6 stimulates transcription elongation by RNA polymerase II in vitro. Mol. Cell Biol. 24: 3324–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki, H., and A. W. Murray, 2000. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102: 411–424. [DOI] [PubMed] [Google Scholar]
- Funabiki, H., H. Yaman, K. Nagao, H. Tanaka, H. Yasuda et al., 1997. Fission yeast Cut2 required for anaphase has two destruction boxes. EMBO J. 16: 5977–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., and R. D. Vale, 2003. The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J. Cell Biol. 162: 1003–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder, N. C., M. De Cuevas and A. C. Spradling, 2000. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development 127: 4253–4264. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., 1989. Genetic and molecular analysis of a simple disjunctional system in Drosophila melanogaster. Prog. Clin. Biol. Res. 311: 277–302. [PubMed] [Google Scholar]
- Hawley, R. S., and W. Theurkauf, 1993. Requiem for distributive segregation: achiasmate segregation in Drosophila melanogaster. Trends Genet. 9: 310–317. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., H. Irick, A. E. Zitron, D. A. Haddox, A. Lohe et al., 1992. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13: 440–467. [DOI] [PubMed] [Google Scholar]
- Hays, T. S., and E. D. Salmon, 1990. Poleward force at the kinetochore in metaphase depends on the number of kinetochore microtubules. J. Cell Biol. 110: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, E. J., Q. Dong and I. Greenwald, 1996. Evidence for physical and functional association between EMB-5 and LIN-12 in Caenorhabditis elegans. Science 273: 112–115. [DOI] [PubMed] [Google Scholar]
- Ishino, Y., A. Takahashi-Fujii, T. Uemori, M. Imamura, I. Kato et al., 1995. The amino acid sequence required for 5′ → 3′ exonuclease activity of Bacillus caldotenax DNA polymerase. Protein Eng. 8: 1171–1175. [DOI] [PubMed] [Google Scholar]
- Joyce, C. M., D. M. Fujii, H. S. Laks, C. M. Hughes and N. D. Grindley, 1985. Genetic mapping and DNA sequence analysis of mutations in the polA gene of Escherichia coli. J. Mol. Biol. 186: 283–293. [DOI] [PubMed] [Google Scholar]
- Karpen, G. H., M. H. Le and H. Le, 1996. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 273: 118–122. [DOI] [PubMed] [Google Scholar]
- King, R. W., M. Glotzer and M. W. Kirschner, 1996. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell. 7: 1343–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque, A. A., and D. A. Compton, 2001. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol. 154: 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah, T. F., K. Kuznedelov, A. Mushegian, K. Severinov and J. Greenblatt, 2000. The alpha subunit of E. coli RNA polymerase activates RNA binding by NusA. Gene Dev. 14: 2664–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, W. F., J. F. Marko, D. A. Agard and J. W. Sedat, 2001. Chromosome elasticity and mitotic polar ejection force measured in living Drosophila embryos by four-dimensional microscopy-based motion analysis. Curr. Biol. 11: 569–578. [DOI] [PubMed] [Google Scholar]
- Matthies, H. J., L. G. Messina, R. Namba, K. J. Greer, M. Y. Walker et al., 1999. Mutations in the alpha-tubulin 67C gene specifically impair achiasmate segregation in Drosophila melanogaster. J. Cell Biol. 147: 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies, H. J., M. Clarkson, R. B. Saint, R. Namba and R. S. Hawley, 2000. Analysis of meiosis in fixed and live oocytes by light microscopy, pp. 67–85 in Drosophila: Protocols, edited by W. S. Sullivan, M. Ashburner and R. S. Hawley. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Matthies, H. J., R. J. Baskin and R. S. Hawley, 2001. Orphan kinesin NOD lacks motile properties but does possess a microtubule-stimulated ATPase activity. Mol. Biol. Cell. 12: 4000–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, T. D., and G. H. Karpen, 1999. Interactions between the nod+ kinesin-like gene and extracentromeric sequences are required for transmission of a Drosophila minichromosome. Cell 81: 139–148. [DOI] [PubMed] [Google Scholar]
- Nicklas, R. B., 1974. Chromosome segregation mechanisms. Genetics 78: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki, K., T. Sano and J. Miwa, 1993. emb-5, a gene required for the correct timing of gut precursor cell division during gastrulation in Caenorhabditis elegans, encodes a protein similar to the yeast nuclear protein SPT6. Mol. Gen. Genet. 239: 313–322. [DOI] [PubMed] [Google Scholar]
- Rasooly, R. S., P. Zhang, A. K. Tibolla and R. S. Hawley, 1994. A structure-function analysis of NOD, a kinesin-like protein from Drosophila melanogaster. Mol. Gen. Genet. 242: 145–151. [DOI] [PubMed] [Google Scholar]
- Rieder, C. L., E. A. Davison, L. C. Jensen, L. Cassimeris and E. D. Salmon, 1986. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J. Cell Biol. 103: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli, M. G., and G. Callaini, 2005. The meiotic spindle of the Drosophila oocyte: the role of Centrosomin and the central aster. J. Cell Sci. 118(13): 2827–2836. [DOI] [PubMed] [Google Scholar]
- Rorth, P., 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78: 113–118. [DOI] [PubMed] [Google Scholar]
- Shiroguchi, K., M. Ohsugi, M. Edamatsu, T. Yamamoto and Y. Y. Toyoshima, 2003. The second microtubule-binding site of monomeric kid enhances the microtubule affinity. J. Biol. Chem. 278: 22460–22465. [DOI] [PubMed] [Google Scholar]
- Taylor, F. M., and D. W. Martindale, 1993. Retroviral-type zinc fingers and glycine-rich repeats in a protein encoded by cnjB, a Tetrahymena gene active during meiosis. Nucleic Acids Res. 21: 4610–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf, W. E., and R. S. Hawley, 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116: 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokai, N., A. Fujimoto-Nishiyama, Y. Toyoshima, S. Yonemura, S. Tsukita et al., 1996. Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J. 15: 457–467. [PMC free article] [PubMed] [Google Scholar]
- Yajima, J., M. Edamatsu, J. Watai-Nishii, N. Tokai-Nishizumi, T. Yamamoto et al., 2003. The human chromokinesin Kid is a plus end-directed microtubule-based motor. EMBO J. 22: 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., and R. S. Hawley, 1990. The genetic analysis of distributive segregation in Drosophila melanogaster. II. Further genetic analysis of the nod locus. Genetics 125: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., B. A. Knowles, L. S. B. Goldstein and R. S. Hawley, 1990. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell 62: 1053–1062. [DOI] [PubMed] [Google Scholar]
- Zhao, X., and N. V. Grishin, 2000. Common fold in helix-hairpin-helix proteins. Nucleic Acids Res. 28: 2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick, M. E., J. L. Salstrom and C. H. Langley, 1999. Genetic variation in rates of nondisjunction: association of two naturally occurring polymorphisms in the chromokinesin nod with increased rates of nondisjunction in Drosophila melanogaster. Genetics 152: 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]