Abstract
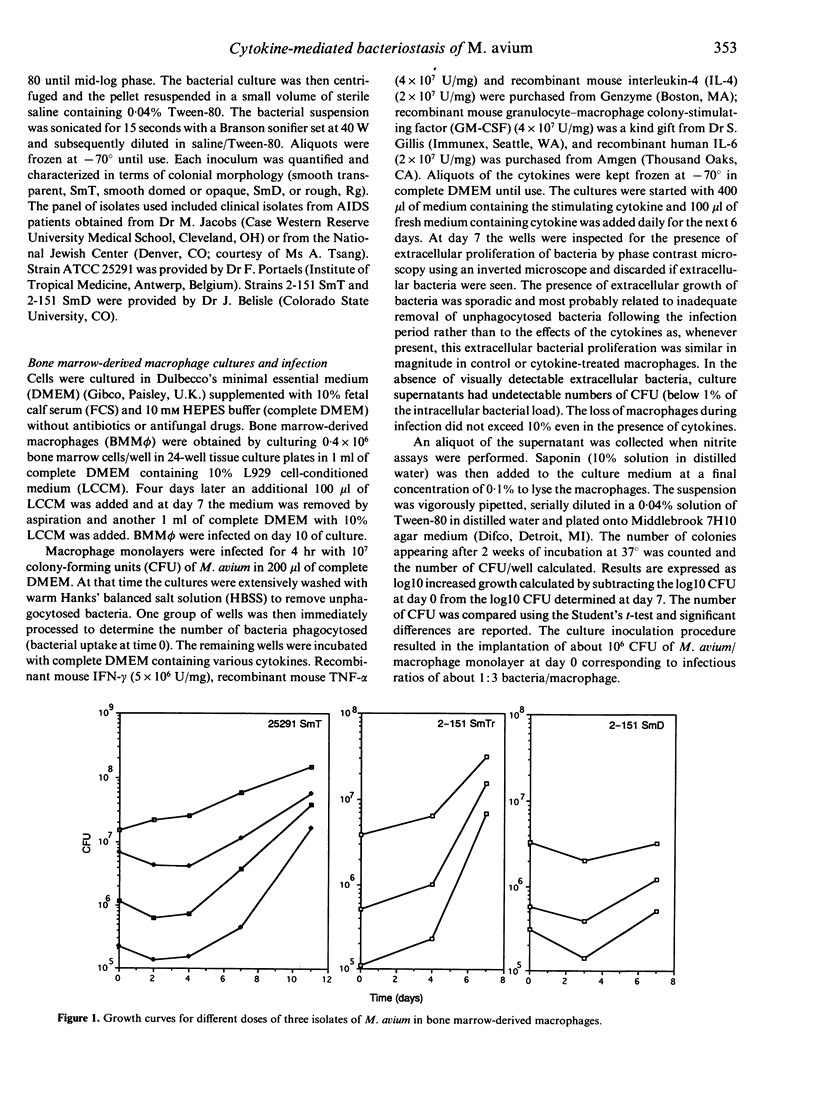
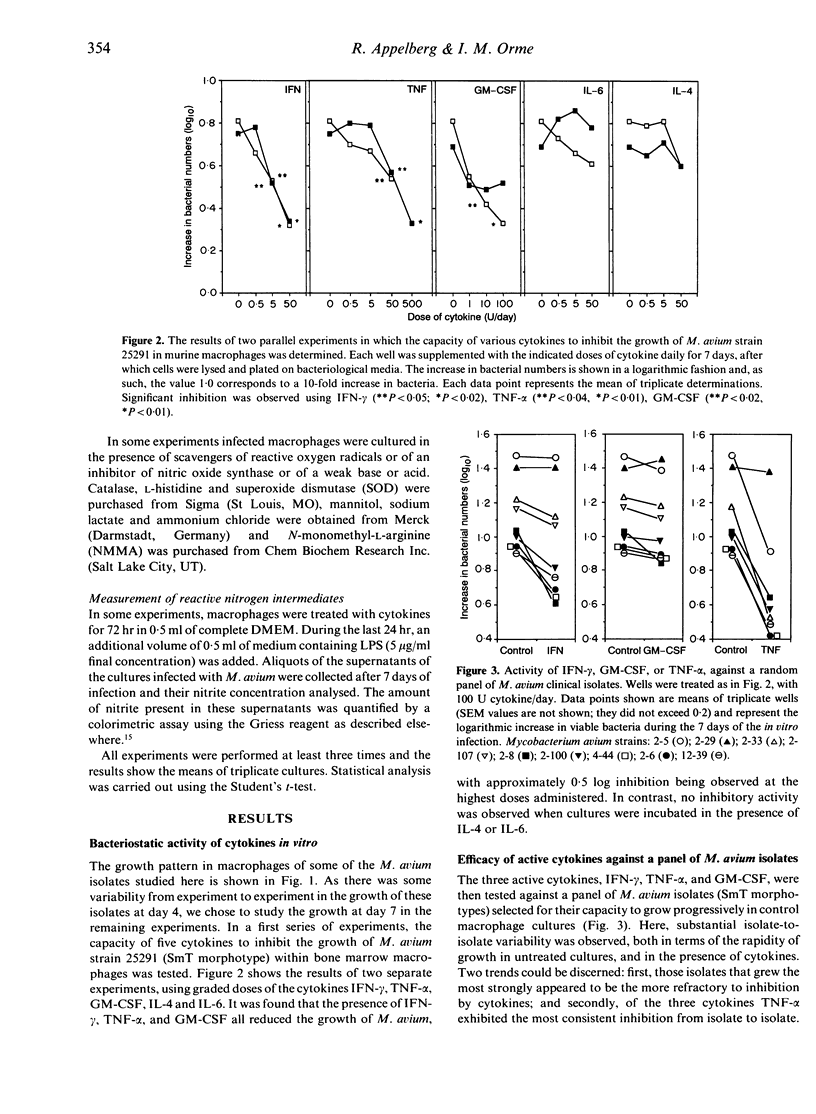
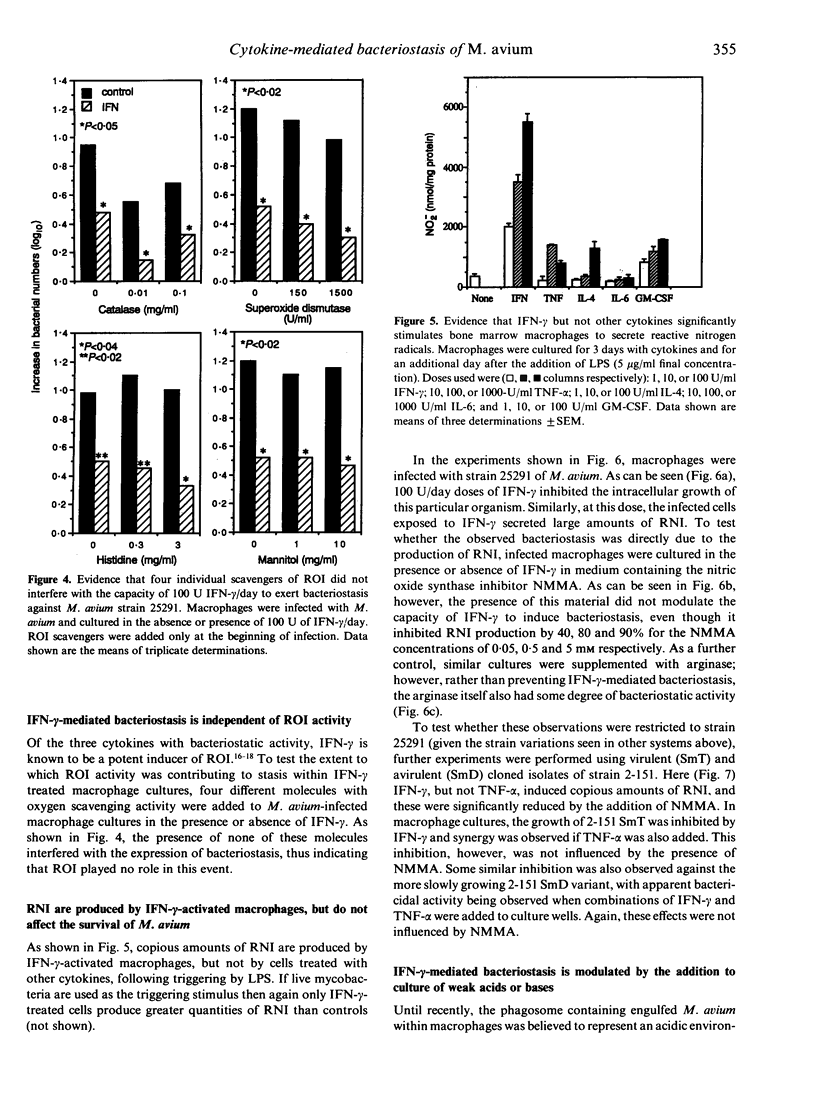
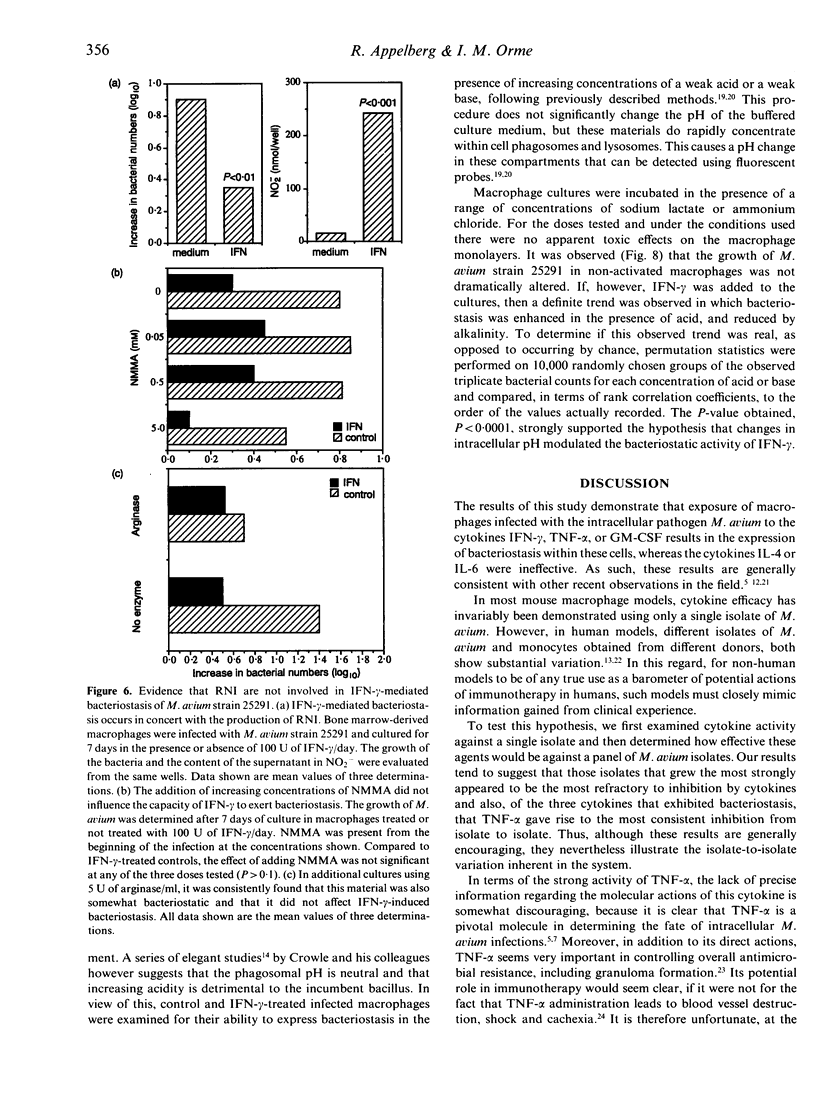
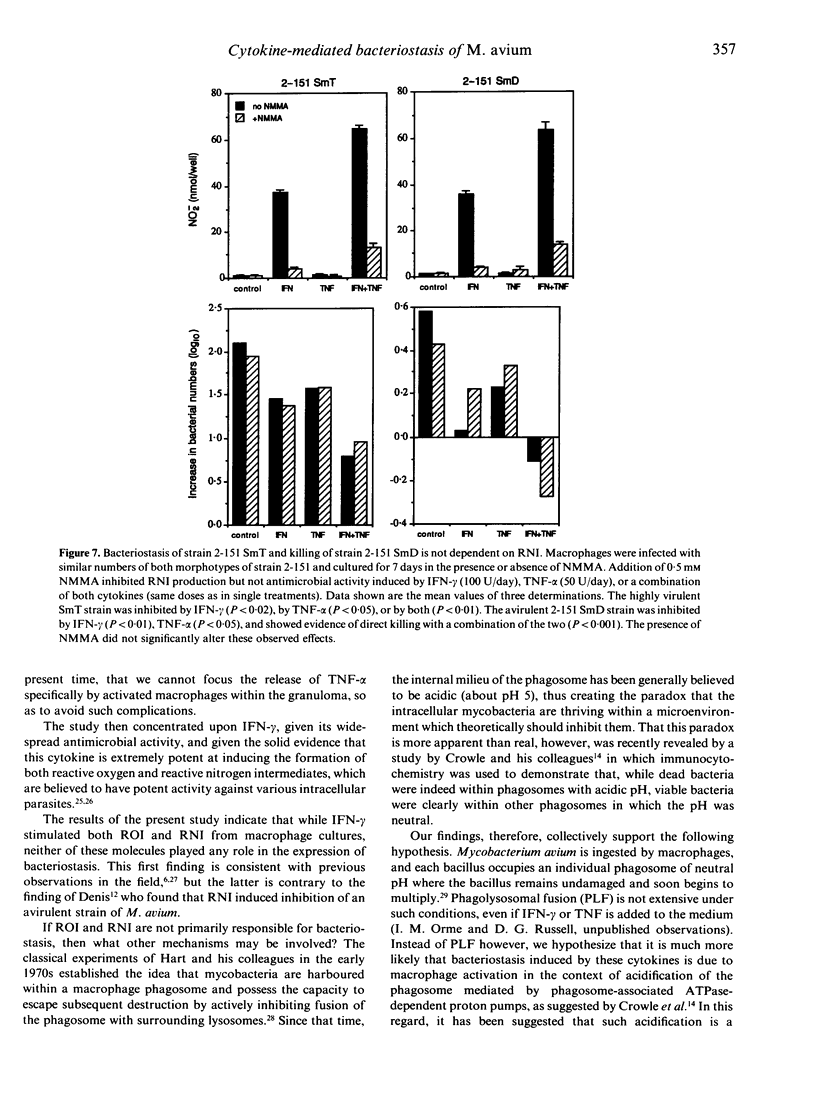
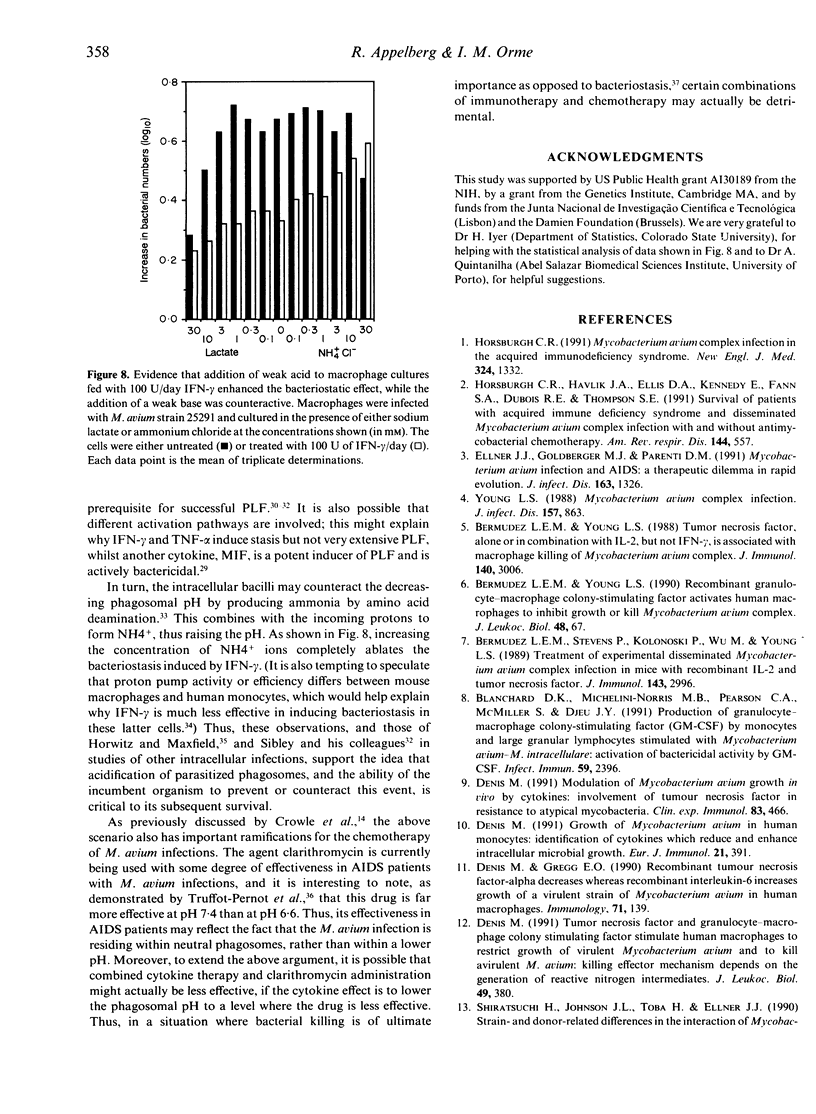
In this study we found that addition of a range of doses of interferon-gamma (IFN-gamma), tumour necrosis factor-alpha (TNF-alpha), or granulocyte-macrophage colony stimulating factor (GM-CSF) to cultures of bone marrow-derived murine macrophages infected with the 25291 strain of Mycobacterium avium gave rise to varying degrees of bacteriostasis. In contrast, similar treatment with interleukin-4 (IL-4) or IL-6 had no effect. However, when similar experiments with the former set of cytokines were performed using a panel of M. avium isolates, substantial isolate-to-isolate variation was observed. In cultures containing IFN-gamma, synthesis of substantial levels of reactive nitrogen intermediates was observed; however, neither these materials, nor reactive oxygen intermediates, were found to be responsible for observed bacteriostasis. In further experiments, in which the culture medium was supplemented with various concentrations of a weak acid or a weak base in order to influence the pH of macrophage intracellular compartments, it was found that the presence of the weak acid augmented the activity of IFN-gamma, whilst the weak base counteracted this effect. These data support the hypothesis, therefore, that the bacteriostatic effect of IFN-gamma against the growth of M. avium, rather than depending on reactive radical production, is mediated through acidification of the infected phagosome, perhaps through activation of proton pumps in the phagosomal membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez L. E. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993 Feb;91(2):277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Stevens P., Kolonoski P., Wu M., Young L. S. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J Immunol. 1989 Nov 1;143(9):2996–3000. [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Petrofsky M., Young L. S. Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992 Oct;60(10):4245–4252. doi: 10.1128/iai.60.10.4245-4252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J Leukoc Biol. 1990 Jul;48(1):67–73. doi: 10.1002/jlb.48.1.67. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Black C. M., Paliescheskey M., Beaman B. L., Donovan R. M., Goldstein E. Acidification of phagosomes in murine macrophages: blockage by Nocardia asteroides. J Infect Dis. 1986 Dec;154(6):952–958. doi: 10.1093/infdis/154.6.952. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Pearson C. A., McMillen S., Djeu J. Y. Production of granulocyte-macrophage colony-stimulating factor (GM-CSF) by monocytes and large granular lymphocytes stimulated with Mycobacterium avium-M. intracellulare: activation of bactericidal activity by GM-CSF. Infect Immun. 1991 Jul;59(7):2396–2402. doi: 10.1128/iai.59.7.2396-2402.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Dahl R., Ross E., May M. H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991 May;59(5):1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Gregg E. O. Recombinant tumour necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990 Sep;71(1):139–141. [PMC free article] [PubMed] [Google Scholar]
- Denis M. Growth of Mycobacterium avium in human monocytes: identification of cytokines which reduce and enhance intracellular microbial growth. Eur J Immunol. 1991 Feb;21(2):391–395. doi: 10.1002/eji.1830210221. [DOI] [PubMed] [Google Scholar]
- Denis M. Modulation of Mycobacterium avium growth in vivo by cytokines: involvement of tumour necrosis factor in resistance to atypical mycobacteria. Clin Exp Immunol. 1991 Mar;83(3):466–471. doi: 10.1111/j.1365-2249.1991.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Goldberger M. J., Parenti D. M. Mycobacterium avium infection and AIDS: a therapeutic dilemma in rapid evolution. J Infect Dis. 1991 Jun;163(6):1326–1335. doi: 10.1093/infdis/163.6.1326. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A. Strain virulence and the lysosomal response in macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1974 Oct;10(4):742–746. doi: 10.1128/iai.10.4.742-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Havlik J. A., Ellis D. A., Kennedy E., Fann S. A., Dubois R. E., Thompson S. E. Survival of patients with acquired immune deficiency syndrome and disseminated Mycobacterium avium complex infection with and without antimycobacterial chemotherapy. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):557–559. doi: 10.1164/ajrccm/144.3_Pt_1.557. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Ley V., Robbins E. S., Nussenzweig V., Andrews N. W. The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med. 1990 Feb 1;171(2):401–413. doi: 10.1084/jem.171.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Furney S. K., Skinner P. S., Roberts A. D., Brennan P. J., Russell D. G., Shiratsuchi H., Ellner J. J., Weiser W. Y. Inhibition of growth of Mycobacterium avium in murine and human mononuclear phagocytes by migration inhibitory factor. Infect Immun. 1993 Jan;61(1):338–342. doi: 10.1128/iai.61.1.338-342.1993. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. K., Banerjee D. K. Effect of gamma interferon on hydrogen peroxide production by cultured mouse peritoneal macrophages. Infect Immun. 1986 Nov;54(2):597–599. doi: 10.1128/iai.54.2.597-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Ellner J. J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991 May 1;146(9):3165–3170. [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Toba H., Ellner J. J. Strain- and donor-related differences in the interaction of Mycobacterium avium with human monocytes and its modulation by interferon-gamma. J Infect Dis. 1990 Oct;162(4):932–938. doi: 10.1093/infdis/162.4.932. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Weidner E., Krahenbuhl J. L. Phagosome acidification blocked by intracellular Toxoplasma gondii. 1985 May 30-Jun 5Nature. 315(6018):416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K. W., Gallati H., Sinigaglia F., Walz A., Garotta G. Identity between human interferon-gamma and "macrophage-activating factor" produced by human T lymphocytes. Eur J Immunol. 1986 Dec;16(12):1471–1477. doi: 10.1002/eji.1830161202. [DOI] [PubMed] [Google Scholar]
- Truffot-Pernot C., Ji B., Grosset J. Effect of pH on the in vitro potency of clarithromycin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991 Aug;35(8):1677–1678. doi: 10.1128/aac.35.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M. In vitro activity of antimicrobial agents against the Mycobacterium avium complex inside macrophages from HIV1-infected individuals: the link to clinical response to treatment? Res Microbiol. 1992 May;143(4):411–419. doi: 10.1016/0923-2508(92)90055-s. [DOI] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]


