Abstract
Vaccinia virus (VV) has a complex morphogenetic pathway whose first steps are poorly characterized. We have studied the early phase of VV assembly, when viral factories and spherical immature viruses (IVs) form in the cytoplasm of the infected cell. After freeze-substitution numerous cellular elements are detected around assembling viruses: membranes, ribosomes, microtubules, filaments, and unidentified structures. A double membrane is clearly resolved in the VV envelope for the first time, and freeze fracture reveals groups of tubules interacting laterally on the surface of the viroplasm foci. These data strongly support the hypothesis of a cellular tubulovesicular compartment, related to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), as the origin of the first VV envelope. Moreover, the cytoskeletal vimentin intermediate filaments are found around viral factories and inside the viroplasm foci, where vimentin and the VV core protein p39 colocalize in the areas where crescents protrude. Confocal microscopy showed that ERGIC elements and vimentin filaments concentrate in the viral factories. We propose that modified cellular ERGIC membranes and vimentin intermediate filaments act coordinately in the construction of viral factories and the first VV form through a unique mechanism of viral morphogenesis from cellular elements.
The characterization of the complex relationships established between viruses and cells has traditionally provided unique tools for studying the virus life cycle and, simultaneously, particular aspects of the cellular systems used by the viruses. In this sense, some of the most challenging viruses, due to their complexity, are included in the Poxviridae family, whose best-characterized member is vaccinia virus (VV) (reviewed in reference 33). VV is well-known among molecular biologists as a very useful expression vector and is now being used to design new vaccines against a number of pathogens (34). At the same time, VV has become the focus of cell biologists due to the complex interactions that the virus establishes with cellular systems (10, 38, 62). A detailed characterization of VV structure and morphogenesis would be of considerable help for the manipulation of its assembly in vitro and the construction of viruses with defined characteristics. However, the size and complex organization of this virus makes it a difficult challenge for structural biologists.
Some of the most unknown aspects of the VV morphogenetic pathway are the origin of the viral factories and the formation of the first VV particle (see references 18 and 33 for a general description of VV morphogenesis). The viral factories are large cytoplasmic perinuclear areas defined as the centers of VV replication and assembly. The latter takes place in electron-dense masses within the viral factories, known as viroplasm foci (7). These structures are formed by the recruitment of viral, and most probably, cellular elements as well. By mechanisms still to be defined, membranous elements attach to the surface of the VV foci, acquire a curvature, and form the crescent. The viral crescents represent the first evidence of VV assembly, but how they form as well as how the crescents get to assemble the spherical immature viruses (IVs) is largely unknown. There is still a considerable controversy about the basic structure and origin of the viral crescents. The first proposal (11) of a single membrane for the first VV envelope has been recently recalled (19). This membrane would be synthesized de novo, somehow induced by the virus (11). However, there are experimental data pointing to a cellular origin of the membranes forming the crescents. Specific markers for the transitional elements operating between the endoplasmic reticulum and the Golgi complex (also known as ERGIC, from “endoplasmic reticulum-Golgi intermediate compartment”) label membranes connected with the viral crescents (47, 62). Consistently, the VV proteins p21 (encoded by the A17L gene), p15 (encoded by the A14L gene), and p8 (encoded by the A13L gene), identified as envelope proteins of the first VV infectious form (the intracellular mature virus, or IMV), have been shown to be cotranslationally inserted into the ER to be later transported to and retained in the intermediate compartment of infected cells (25, 52). IMVs originate from IVs through a major reorganization taking place after DNA packaging that renders the first infectious virus. IMVs can use microtubules to move in the cytoplasm (53). Some IMVs become wrapped by a double membrane derived from the trans-Golgi network (56) or tubular endosomes (66) to form the intracellular enveloped virus (IEV). It has been recently reported that IEVs use microtubules to reach the plasma membrane (18, 70, 73), where the controlled polymerization of actin (also used by some bacteria and cellular vesicles) helps them to exit the cell (10, 14). By fusion with the plasma membrane, these virions lose their outermost membrane and are released from the cell as extracellular enveloped virus (EEV). This is, in fact, one of the most exceptional aspects of VV morphogenesis: the production of two different infectious forms, IMVs and EEVs, that seem to have different roles in cell-to-cell spread and disease transmission (6, 69).
Electron microscopy (EM) studies have played a central role in the characterization of viral assembly. Nowadays, structural biology tries to study native structures as much as possible. In this sense, cryomicroscopy has represented a revolution in biology (3, 30). Vitrification of proteins, viruses, and cells is providing completely new information on the organization of macromolecular complexes (9, 28). In the case of large structures, such as whole cells, physical restrictions make the vitrification procedure more difficult to apply successfully. Nevertheless, these procedures have been considerably improved in recent years, giving us unique tools to study the formation of viral particles in their intracellular environment (40).
Among the different techniques available today, freeze-substitution after ultra-rapid freezing is a superior method for preserving cell ultrastructure (22). The procedure is based on the application of a very mild dehydration at low temperature (−90°C) in previously vitrified cells. Under these conditions the water of the sample is mildly replaced by the solvent, with a minimal distortion of fine structures. Under these conditions, preservation of very fine structural details in cells gets close to the results of cryomicroscopy of vitrified proteins and viruses. In addition, transmission EM of metal replicas from surfaces exposed by freeze fracture or freeze fracture followed by freeze etching provides three-dimensional information of the cell surface and structures within the intracellular environment (58). Applied to vitrified, highly preserved cells, these methods can provide valuable three-dimensional information, complementary to the data provided by freeze-substitution (43).
On the other hand, the advances in molecular biology are providing new tools to manipulate viral genomes and thus to engineer new kinds of mutant viruses. Studies on VV morphogenesis have traditionally relied on the characterization of cells infected with wild-type virus in the presence of certain drugs or infected with temperature-sensitive mutants. However, the development in the last decade of the technology for generating conditional lethal mutants, in which the expression of a specific protein can be inducibly regulated, has significantly contributed to investigations of the role of specific gene products in VV morphogenesis. This strategy is based on the use of the Escherichia coli lacI operator/repressor system to control the expression of a target viral gene, providing a way to keep this gene repressed unless the inducer isopropyl-β-d-thiogalactopyranoside (IPTG) is added to the medium of cells infected with the conditional mutant (8, 23, 49, 74, 75, 77, 78). By applying this technology several groups have generated a number of conditional lethal mutants that have facilitated the study of VV morphogenesis. Thus, it has been reported that p21 protein (the product of the A17L gene) plays a key role in the organization of viral crescents (45-47, 77) and p15 (the product of A14L gene) plays a key role in their attachment to the viral factory (48, 68). The role of several VV core proteins has also been explored (8, 16, 23, 74, 75). These mutants have been instrumental tools for the study of VV morphogenesis, since with them assembly can be reversibly blocked and synchronized at a very early stage.
Following the infection of HeLa cells with wild-type VV and with two VV conditional lethal mutants, we have performed a detailed ultrastructural study of the different stages of VV assembly at both early and late postinfection (p.i.) times. The controversy on the one membrane/two membrane organization of the first VV envelope has been definitively resolved due to the superior preservation provided by cryomethods that were not applied in previous studies. New data on some other aspects of VV morphogenesis from cellular elements have been obtained. We propose that deeply modified cellular membranes and cytoskeletal intermediate filaments (IFs) would act coordinately to build the viral factories and the IVs.
MATERIALS AND METHODS
Cells, viruses, and antisera.
HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% newborn bovine serum. VV wild-type strain Western Reserve (WR) was propagated and titrated in BSC40 cells (46). The conditional lethal mutants VVindA17L and VVindA14L, which inducibly express the VVp21 (encoded by the A17L gene) and p15 (encoded by the A14L gene) envelope proteins, respectively, had been previously generated and were grown in BSC40 cells in the presence of 2 mM IPTG, as described previously (45, 48). The rabbit polyclonal antisera against VV proteins p21, p15, and p39 (encoded by the A5L gene) have been previously described (46, 47). Antibodies specific for cytoskeletal proteins (vimentin, tubulin, actin, and cytokeratin) were purchased from Sigma: monoclonal antivimentin antibodies (clones V9 and VIM 13.2), a whole antivimentin antiserum raised in goat (V4630), a monoclonal anti-cytokeratin antibody (clone K8.13), an anti-keratin polyclonal antiserum raised in guinea pig (K4252), a rabbit anti-actin antiserum (A2668), and an anti-tubulin antiserum (T3526). The mouse monoclonal antibody G1193 against human ERGIC-53 protein was kindly provided by Hans P. Hauri (Biozentrum of the University of Basel). Secondary antibodies conjugated with Texas Red or fluorescein were purchased from Molecular Probes. Secondary antibodies-gold conjugates were provided by BioCell (Cardiff, United Kingdom).
Immunofluorescence microscopy.
HeLa cells grown on coverslips were infected at a multiplicity of infection (MOI) of 5 PFU/cell with VV strain WR. At 8 h p.i. cells were washed with phosphate-buffered saline (PBS) and fixed in methanol at −20°C for 5 min. After being washed with PBS, coverslips were blocked for 30 min with a solution of PBS containing 2% bovine serum albumin. Cells were then incubated for 1 h at 37°C with antibodies directed to the VV p21 protein together with antivimentin or anti-ERGIC-53 antibodies. The coverslips were extensively washed with PBS and incubated for 1 h at 37°C with secondary anti-mouse immunoglobulin G conjugated with fluorescein isothiocyanate (FITC) and anti-rabbit immunoglobulin G antibodies conjugated with Texas Red. The DNA staining reagent To-Pro (Molecular Probes) was included in this incubation. After several washings with PBS the coverslips were mounted with Fluoromount-G (Southern Biotecnology Associates, Inc.) on glass slides. Images were obtained using a Bio-Rad Radiance 2000 Confocal Laser microscope.
Fixation of cell cultures in situ for EM studies.
Monolayers of HeLa cells were infected at an MOI of 5 PFU/cell with the WR strain of VV in the absence or presence of 100 μg of rifampin/ml. HeLa cells were also infected at a similar MOI with VVindA17L or VVindA14L in the absence or presence of the inducer IPTG. For ultrastructural studies cells were fixed in situ with a mixture of 2% glutaraldehyde and 1% tannic acid in 0.4 M HEPES buffer (pH 7.5) for 1 h at room temperature. Fixed monolayers were removed from the culture dishes in the fixative and were transferred to Eppendorf tubes. After centrifugation and being washed with HEPES buffer, the cells were stored at 4°C until use.
For specific detection of proteins, monolayers of infected HeLa cells were submitted to a mild fixation with a solution of 4% paraformaldehyde containing 0.1% glutaraldehyde in PBS (pH 7.4). Fixed cells were removed from the dishes in the fixative, transferred to Eppendorf tubes, washed with PBS, and stored at 4°C until use.
Conventional processing for EM.
For ultrastructural studies, fixed cells were processed for embedding in the epoxy-resin EML-812 (Taab laboratories, Berkshire, United Kingdom) by methods previously described (42). Postfixation of cells was done with a mixture of 1% osmium tetroxide and 0.8% potassium ferricyanide in distilled water for 1 h at 4°C. After four washes with HEPES buffer, samples were treated with 2% uranyl acetate, washed again, and dehydrated in increasing concentrations of acetone (50, 70, 90, and 100%) for 10 min each at 4°C. Infiltration in resin was done at room temperature for 1 day. Polymerization of infiltrated samples was done at 60°C for 3 days. Ultrathin (20- to 30-nm-thick) sections of the samples were stained with saturated uranyl acetate and lead citrate by standard procedures.
For immunolabeling studies, cells submitted to mild fixation were processed for embedding in the acrylic-resin Lowicryl K4M (Taab Laboratories) as described previously (41). After dehydration at −20°C in increasing concentrations of ethanol, samples were infiltrated in the resin at −30°C for 1 day and polymerized with UV light, 2 days at −20°C and 2 more days at room temperature. Ultrathin sections were processed for immunogold detection of VV proteins or cytoskeletal components.
Immunogold labeling.
Immunogold localization of VV proteins and cytoskeletal components was done by placing the ultrathin sections on drops of different solutions. After a 30-min incubation with Tris buffer-gelatin (TBG) (30 mM Tris-HCl, pH 8.0, containing 150 mM NaCl, 0.1% bovine serum albumin, and 1% gelatin), sections were floated for 1 h on a drop of the specific primary antibody diluted in TBG. After jet washing with PBS, grids were floated on 4 drops of TBG and incubated 5 min on the last drop before a 45-min incubation with the secondary antibody conjugated with colloidal gold of 5 or 10 nm. Grids were then jet washed in PBS and distilled water before being stained with a solution of saturated uranyl acetate for 30 min (for Lowicryl sections) followed by 1 min with lead citrate (for EML-812 sections). For double-labeling experiments, representative signals corresponding to both primary antibodies were obtained after testing different combinations of labeling steps, as described previously (44).
Quick freezing and freeze-substitution.
Small pellets of chemically fixed cells were cryoprotected with glycerol, applied to small pieces of filter paper, blotted for 15 s, and quick frozen in liquid propane at an approximate speed of 104°C/s. Frozen specimens were transferred to a Reichert-Jung AFS freeze-substitution unit (Leica, Vienna, Austria) and maintained for 24 h at −90°C in a mixture of pure acetone and 0.5% (wt/vol) osmium tetroxide. This incubation allows a complete substitution of the water of the sample by the solvent (42). Samples were then subjected to a controlled increase of temperature before being embedded in EML-812.
Freeze fracture and freeze-etching.
Frozen samples were processed in a BAF 060 freeze fracture unit (BAL-TEC; Liechtenstein). Regular freeze fracture was performed at −150°C following procedures previously described in detail (43). When freeze-etching was carried out after fracturing, the temperature of samples was switched from −150 to −100°C and was maintained at a pressure of 10−7 mbar for 5 min to sublimate the surface layer of ice. Metal replicas of the exposed surfaces were obtained by evaporating 2 nm of platinum with an electron gun at an angle of 45° and 20 nm of carbon with an electron gun at an angle of 90°. Replicas were floated in commercial bleach and incubated overnight for the digestion of cellular material. After being intensively washed in distilled water, the replicas were picked up in Formvar-coated EM grids and studied by EM.
EM: imaging and measurements.
Regular thin sections were collected on uncoated copper grids of 400 mesh, stained, and studied by EM. Serial sections were collected on nickel grids of 50 mesh or parallel bars coated by formvar films. Ultrathin sections of the samples were either stained by standard procedures, stained with saturated uranyl acetate in 70% ethanol (procedure that improves contrast), or processed for immunogold labeling. Metal replicas were picked up on copper 400-mesh grids. Collection of images and measurements were done with two different microscopes: a JEOL 1200-EX II electron microscope operating at 100 kV and a LEO TEM 812 (Zeiss) operating at 120 kV and equipped with an LaB6 filament, Omega in-column energy filter, and slow-scan CCD camera.
RESULTS
VV assembly areas contain numerous cellular elements.
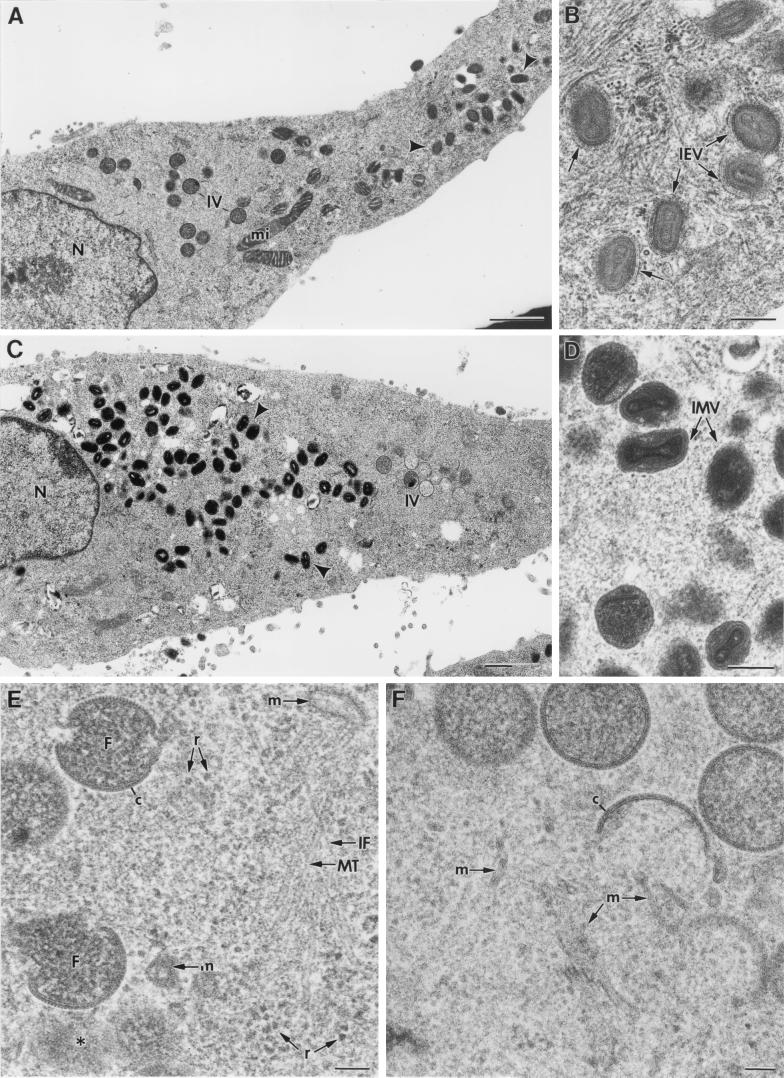
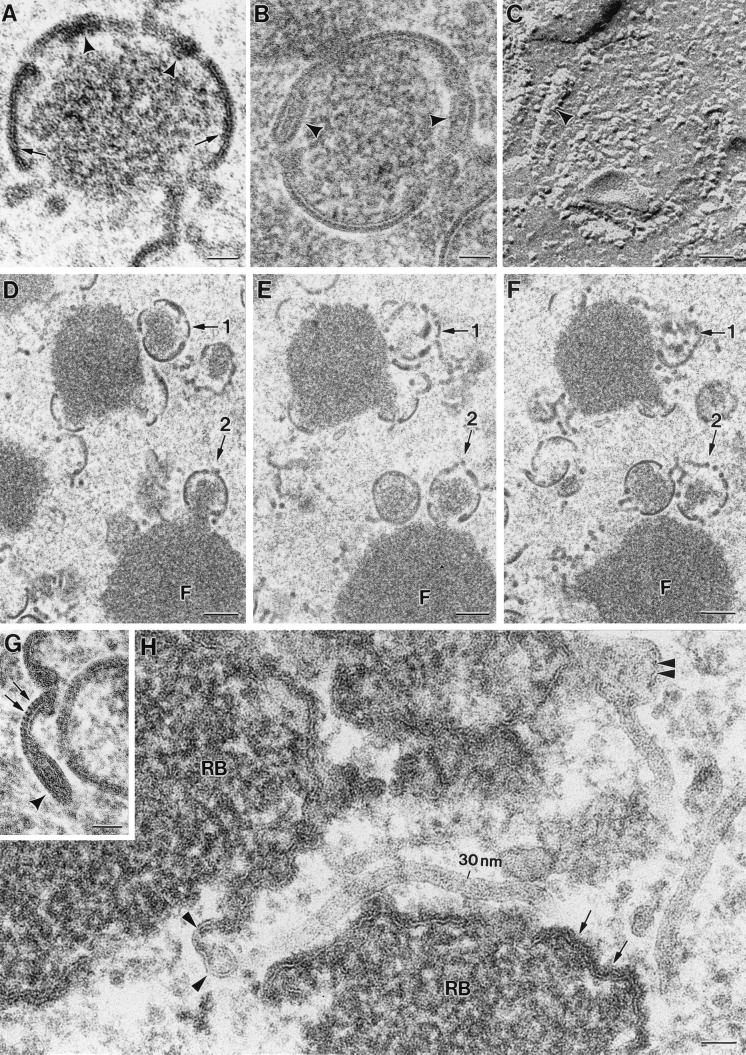
The cytoplasmic regions of VV assembly have been traditionally defined as low-electron-dense areas of organelle exclusion (19, 32). This description is based on the apparent absence of structures around assembling viruses. In fact, this is one of the arguments used to support the hypothesis of a de novo synthesis of membranes originating the viral crescents: the viruses would not have any cellular elements around to be used for their assembly (11, 19). We have addressed this point in studying VV-infected cells (at different p.i. times) by high-preservation procedures, such as freeze-substitution. At low magnification, general structural features are similar to those of the well-known images of VV-infected cells processed by conventional methods (Fig. 1). At 10 h p.i. immature and mature viruses are equally represented (Fig. 1A). Mature virions in the process of taking the additional membrane characteristic of the IEVs are frequent (Fig. 1B). At 24 h p.i. the accumulation of large amounts of IMVs is a general feature (Fig. 1C and D). Apparently, IEVs are scarce at this p.i. time (for a more detailed quantification see below). Higher-magnification fields show clear differences between conventionally processed and freeze-substituted samples (Fig. 1E and F). When freeze-substitution is applied, numerous cellular structures (identified by their characteristic morphology) are distinguished around assembling viruses: membranes, microtubules, cytoskeletal IFs, and ribosomes are seen around the viroplasm foci of the factories and forming IVs (Fig. 1E). An equivalent field from a conventionally processed sample is shown in Fig. 1F: although some membranous elements can still be distinguished around IVs, the cytoplasm has lost part of its content and structural definition. Close proximity between forming IVs and cellular membranes or mitochondria is best appreciated in freeze fracture images (Fig. 1G). IVs are also seen close to rough endoplasmic reticulum (RER) cisternae with peripheral dense spots (Fig. 1H). Some unidentified structures, such as rigid tubules of around 50 to 60 nm in diameter, are frequently seen around assembling IVs (Fig. 1I). These structures are strongly reminiscent of a recently described ER subdomain (2). The presence of structures in the areas of assembly was seen at every time p.i. tested, although their amount clearly decreases at long times p.i.
FIG. 1.
Freeze-substitution and freeze fracture of VV-infected cells. Low-magnification fields of freeze-substituted HeLa cells infected with Western Reserve (WR) VV at 10 (A and B) and 24 (C and D) h p.i. shows the characteristic accumulation of spherical IVs (marked IV) and dense brick-shaped mature viruses (arrowheads). As seen in higher-magnification views of selected areas in panels A and C, many mature viruses are IEVs at shorter p.i. times (B) while most of them are IMVs at longer p.i. times (D). Higher-magnification fields show a significant improvement in preservation of fine details in samples processed by freeze-substitution (E) compared to that with conventional processing (F). In freeze-substituted cells, numerous small structures are seen around the viroplasm foci (marked F) of the viral factories (c marks the viral crescents) and assembling IV particles. Microtubules (MT), cytoskeletal IFs, ribosomes (r), and membranes (m) are abundant around assembling IVs at 10 h p.i. (E). (F) Equivalent regions from conventionally processed cultures show few structural details around assembling IVs. Only some membranes (m) are distinguished. (G) Low-magnification views of freeze-fractured infected cells show that IV particles are surrounded by different types of membranes (arrowheads). Asterisks mark the center of cross-fractured IVs. Mitochondria (mi) are frequently located near IVs. (H) Also at 10 h p.i. IVs are frequently close to RER, with dense deposits on their periphery (arrows). (I) Tubular rigid structures (arrows) of around 50 to 60 nm in diameter are frequently seen around VV particles at 10 h p.i. (J to L) Even when assembly is blocked (during 10 h of infection with the recombinant VVindA17L virus in the absence of IPTG), many structural elements are found near the nucleus (J), around the characteristic dense masses representing truncated viral factories (asterisks). IFs are abundant: the arrowhead in panel K points to a longitudinal view, while a cross-section is marked by the arrowhead in panel L. Large areas containing structured material similar to chromatin (single arrow in panel K), and 30-nm-diameter tubular membranes (double arrows in panel L) are also seen. Double arrowheads in panel L point to a cross-section of the 50- to 60-nm-diameter rigid tubes shown in panel I. N, nucleus. Bars, 1 μm in panels A and C, 200 nm in panels B, D, G, H, and J, and 100 nm in panels E, F, I, K, and L.
As previously described (45, 77), VV morphogenesis is blocked at an early stage when expression of the VV p21 protein is prevented by infection with the conditional lethal mutant VVindA17L under nonpermissive conditions (absence of the inducer IPTG). The electron-dense masses representing truncated viroplasm foci, observed in the perinuclear areas under these conditions, are surrounded by numerous tubulovesicular membranes derived from the ERGIC (47). In the present study we have detected many other structural elements around these truncated viroplasm foci: ribosomes, IFs, aggregates similar to nuclear chromatin, and tubular structures of a variety of diameters (Fig. 1J to L).
The envelope of VV IV particles is a double bilayer.
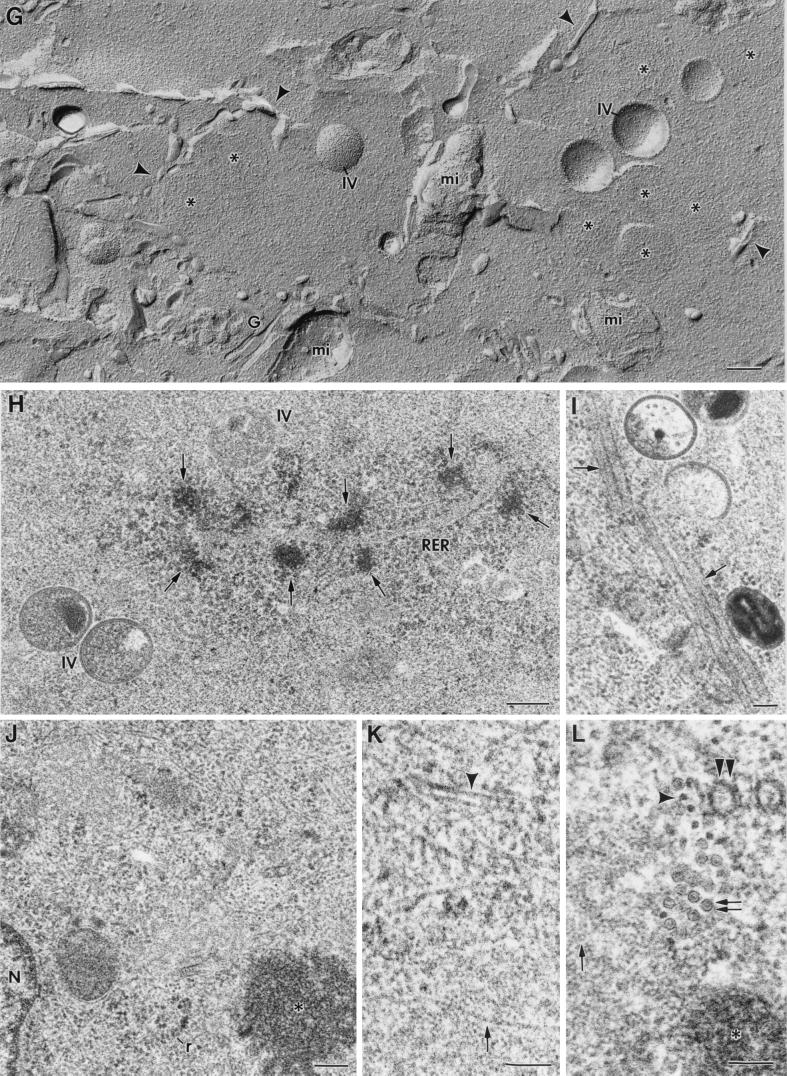
The improved structural preservation obtained in frozen and freeze-substituted infected cells has provided several new data about the organization of the different VV assemblies. A double membrane bilayer is solved in viral crescents and IVs for the first time (Fig. 2A). Depending on the plane of the section, the trilamelar structure of the external membrane can be either clearly distinguished or partially masked by the presence of protrusions (arrows in Fig. 2A), termed spikes or spicules (12). Freeze-substitution shows that the internal membrane of the viral crescents is similar to a conventional cellular membrane, while the external one is deeply modified by the spikes. Examples of cellular membranes within the same cells are shown in Fig. 2B (mitochondrial double membrane) and C (intercellular junction). Thickness and morphology of individual membranes within these structures are similar to the corresponding viral crescents and IVs. These two bilayers are not resolved in viral crescents and IVs from conventionally processed samples, since the external one looks like a fuzzy layer, with spikes in some locations (Fig. 2D). When the section plane goes through the surface of the forming IV, the envelope shows a close-packing-like organization of the spikes (asterisk in Fig. 2E). These images show that when having an equatorial section plane of the envelope, the section can go along the line of spikes or through the space between two lines of spikes. In this case, the profile of the bilayer is seen. For well-preserved VV crescents, the thickness of the whole structure corresponds to almost three times the thickness of a standard plasma membrane: 5 nm for the internal bilayer, 5 nm for the external one, and 3 to 4 nm for the space between them (Fig. 2A).
FIG. 2.
High-magnification fields of freeze-substituted and freeze-fractured samples. (A) Viral crescents have two membranes of 5 nm in thickness, as marked in the image. Depending on the plane of the section, either the three-layer profile or regularly spaced small spikes (arrows) are distinguished in the external membrane, while the internal membrane is always resolved as a typical trilamellar structure. Individual membranes within contiguous cellular double membranes exhibit a similar organization and thickness, for example, in mitochondria (arrows in panel B) or intercellular junctions (arrows in panel C). However, in viroplasm foci from conventionally processed cells (D) the internal membrane of the crescents can be distinguished (arrowhead) but the external bilayer is not preserved. The spikes are seen in some of the crescents (arrows), but the trilamellar profile is lost, probably due to a partial collapse of the structure. (E) When the section plane goes through the surface of the forming IV, a close-packing-like organization for the spikes can be distinguished (asterisk). Both the crescents attached to the viroplasm foci of the factory (c in panel F) and the crescents free in the cytoplasm (c in panel G) have the same structure. (H and I) Freeze fracture also shows the envelope of IVs as double lines in cross-fractured particles (arrows in panel H), like the double membranes of mitochondria (arrows in panel I). Bars, 50 nm.
Viral crescents seem to acquire their final described structure before attaching to the viroplasm foci of the viral factories (Fig. 2F and G). The crescents were confirmed as individual elongated cytoplasmic pieces with one or two visible bilayers at the crescent end, as seen in serial sections (data not shown). The arrow in Fig. 2F points to the external bilayer at one crescent end.
Finally, freeze fracture also reveals a double membrane in viral crescents and IVs: double lines are clearly distinguished in cross-fractured IV particles (Fig. 2H). The double mitochondrial membrane produces very similar images by freeze fracture (Fig. 2I).
Tubular membranes associate with assembling IVs.
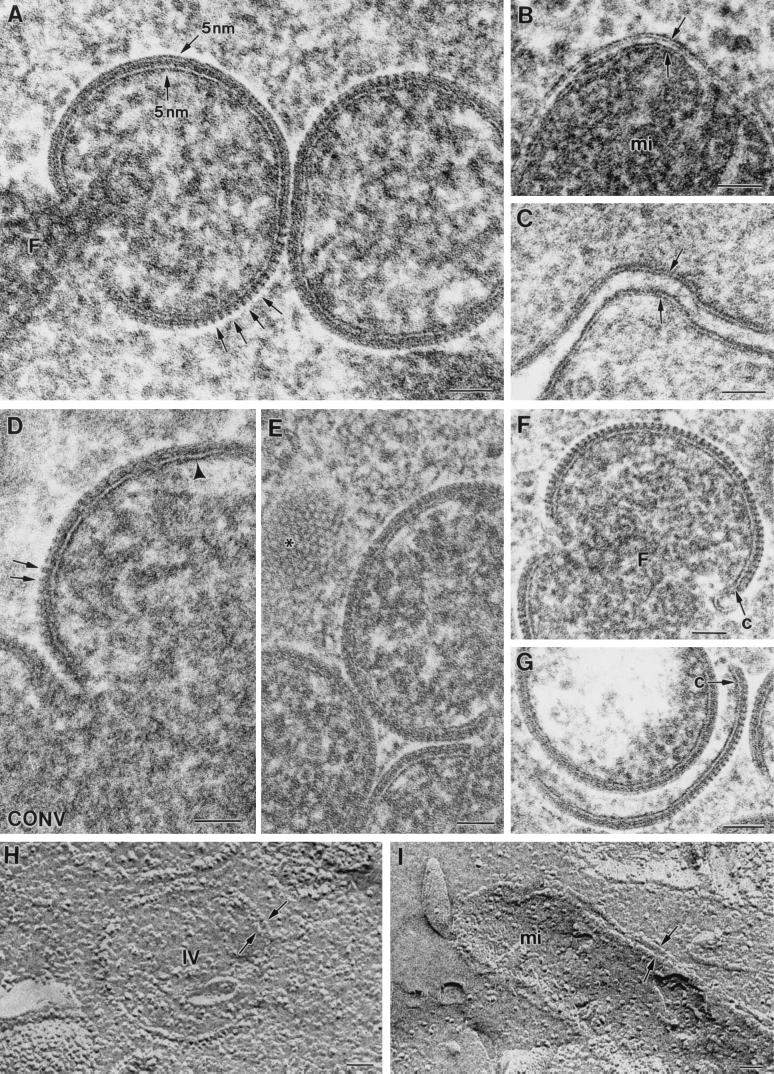
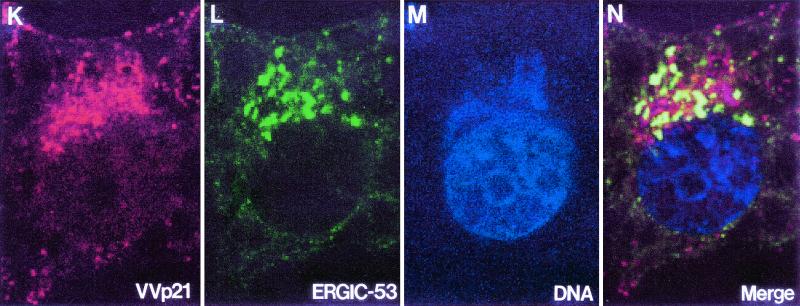
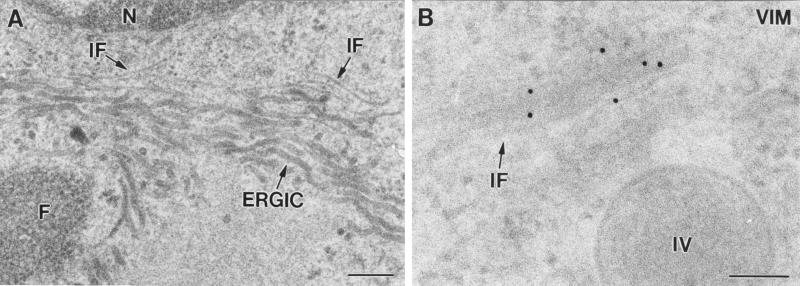
Studying the areas where IVs are forming reveals that many of them have tubular or vesicular membranes associated (Fig. 3A, arrows). It is frequently observed that incomplete IVs with open pores have vesicles associated with them (Fig. 3B and C). Figures 3D to F are three serial sections of the same forming IV, showing tubules and vesicles associated to the structure in different planes. The vesicles are similar to the heads of the 30-nm-thick tubulovesicular elements seen in the areas of VV assembly (Fig. 3G, arrow). These elements are seen in groups around the viroplasm foci, and according to immunolabeling procedures they carry VV envelope proteins (Fig. 3H) and react with a monoclonal antibody specific for the well-characterized marker ERGIC-53 (Fig. 3I). In cells infected with VVindA17L under restrictive conditions (in the absence of VV p21 expression), these tubules attach to the surface of the truncated viroplasm foci, forming a palisade (Fig. 3J). Previous works have also reported the presence of ERGIC-like tubules in the areas of VV assembly. Mohandas and Dales (31) showed images with very prominent tubules, which were described as being continuous with the spherical virion envelopes. The abundance of ERGIC elements carrying VV envelope proteins around and in association with assembling IVs strongly supports the idea that viral crescents form from ERGIC tubules. We then studied the general organization of this compartment in infected cells by confocal microscopy (Fig. 3K to N). In VV-infected cells the areas of assembly are placed in the vicinity of the nucleus and can be clearly visualized by immunofluorescence with specific antibodies against VV proteins (Fig. 3K). Moreover, since these are areas of intensive DNA synthesis, they can also be defined by labeling them with a DNA marker (Fig. 3M). Similar to what is described for other cell types, the ERGIC-53-specific signal mainly concentrates in an area adjacent to the nucleus of HeLa cells, with some peripheral elements, both in uninfected (not shown) and VV-infected cells (Fig. 3L). The position of the viral factory is coincident with the areas of accumulation of ERGIC elements, which are precisely localized within the cytoplasmic mass of VV DNA and proteins (Fig. 3N). Although membranes carrying VV p21 or ERGIC-53 get together near the nucleus, it is clear that strict colocalization is not detected, since green and red colors remain separated in the merge image (Fig. 3N). This is confirmed by analysis of the different planes that compose the whole merge image (data not shown). We think that accumulation of viral proteins could exclude cellular proteins and consequently displace ERGIC-53 to particular subdomains within the membranous compartment.
FIG. 3.
Assembling IVs have vesicles and tubular membranes. (A) Analysis of areas where IVs are forming shows that tubules and vesicles are frequently associated with them (arrows). Arrowheads point to cross-sectioned crescents. Vesicles are frequently seen attached to the pores of uncompleted IVs, as seen in thin sections (arrow in panel B) or after freeze-fracture (arrows in panel C). (D to F) Serial sections of a forming IV. Although in the first section no vesicles or tubules are seen, in the following planes they are associated with the assembling IV. The vesicles are similar to the ends of tubules recruited in the VV assembly areas (arrow in panel G). (H) Groups of tubulovesicular elements are labeled with antibodies specific for VV envelope proteins (here the p21 envelope protein [A17L gene] has been detected) and with the anti-ERGIC-53 antibody (I). (J) The ERGIC tubular elements attach to the surface of the viroplasm foci (asterisk) in a palisade-like arrangement when virus assembly is blocked by infecting cells with VVind A17L in the absence of IPTG. Confocal microscopy shows that viral factories, localized with an antibody against the VV envelope protein p21 (K) and the DNA stain To-Pro (which also stains the cell nucleus) (M), colocalize with ERGIC membranes, detected with an anti-ERGIC-53 monoclonal antibody (L). ERGIC concentrates in intense dots in the region occupied by the factory, as clearly seen in the merge picture (N). Bars, 100 nm.
Other new findings in VV-related structures.
Reexamination of other VV-related structures has shown additional new details. Analysis of cells infected with VVindA14L constitutes a good example. Conventional processing had shown that in the absence of the A14L gene product, p15, viral crescents are unable to interact with the surface of the viroplasm foci within the viral factories (48, 68). These curved membranes were undistinguishable from the normal crescents formed in WR VV-infected cells simultaneously processed by conventional methods. They were seen forming sphere IV-like particles full of holes or interrupted areas (Fig. 4A). However, freeze-substitution and freeze fracture shows that VVindA14L forms IV-like particles with apparently normal crescents together with 30- to 40-nm-diameter tubular structures (Fig. 4B and C). In this mutant the absence of the envelope-associated p15 protein would interfere with the transformation of tubular membranes into normal crescents. Our results strongly suggest that during conventional processing these tubular structures collapse and are no longer distinguishable. Serial sections of HeLa cells infected with the VVindA14L mutant in the absence of p15 protein show that the spherical IV-like particles are formed by individual tubular and crescent-like pieces of membranes in many different orientations (Fig. 4D to F). Some membranous pieces in IV-like particles are able to incorporate the characteristic spikes, which seem to be absent from the tubular structures (Fig. 4G).
FIG. 4.
Redefinition of other VV-related structures. (A) The recombinant VVindA14L forms aberrant IV-like particles in the absence of p15 protein. Conventional processing shows that the envelope of these particles contains individual membranous pieces unable to complete the IV sphere. Some of these pieces are thicker (arrowhead) than the normal crescents (arrows). Freeze-substitution and freeze fracture show that the mentioned thicker pieces are indeed tubes of 30 to 40 nm in diameter (arrowheads in panels B and C). (D to F) Serial sections of the viroplasm foci formed by this virus show that IV-like particles have crescents and curved tubes in many different orientations. (G) Some of these tubular pieces (arrowhead) are connected with pieces of crescents with spikes (arrows). (H) RBs, the truncated viroplasm foci formed in cells infected with VV in the presence of the drug rifampin, exhibit different types of membranes on their periphery: dense membranes of around 18 nm (arrows), twisted dense membranes with vesicular heads (arrowheads), and less dense 30-nm-diameter tubules, some of them with vesicular ends (double arrowhead). Single, 5-nm-thick membrane units are not detected on the surface of RBs. Bars, 50 nm in panels A, B, C, G, and H and 200 nm in panels D, E, and F.
Rifampin bodies (RBs) are structures formed in VV-infected cells in the presence of the drug rifampin. This drug sequesters the VV p65 membrane protein in cytoplasmic deposits close to the RBs (63). RBs are large structures similar to the viroplasm foci of the viral factories but with peripheral membranes that are not able to form viral crescents. Removal of the drug results in formation of normal crescents within minutes (35). Under these conditions crescents most probably originate from the previously recruited peripheral membranes, whose organization has not been characterized in detail. In our study no single-unit membranes are detected on the surface of RBs. Instead, different types of membranous structures are seen: dense membranes (around 18 nm thick), twisted double membranes with vesicular ends, and tubular elements (30 nm thick), some of them with vesicles at one end (Fig. 4H). Similar 30-nm-thick tubules have been detected before in association with RBs (31).
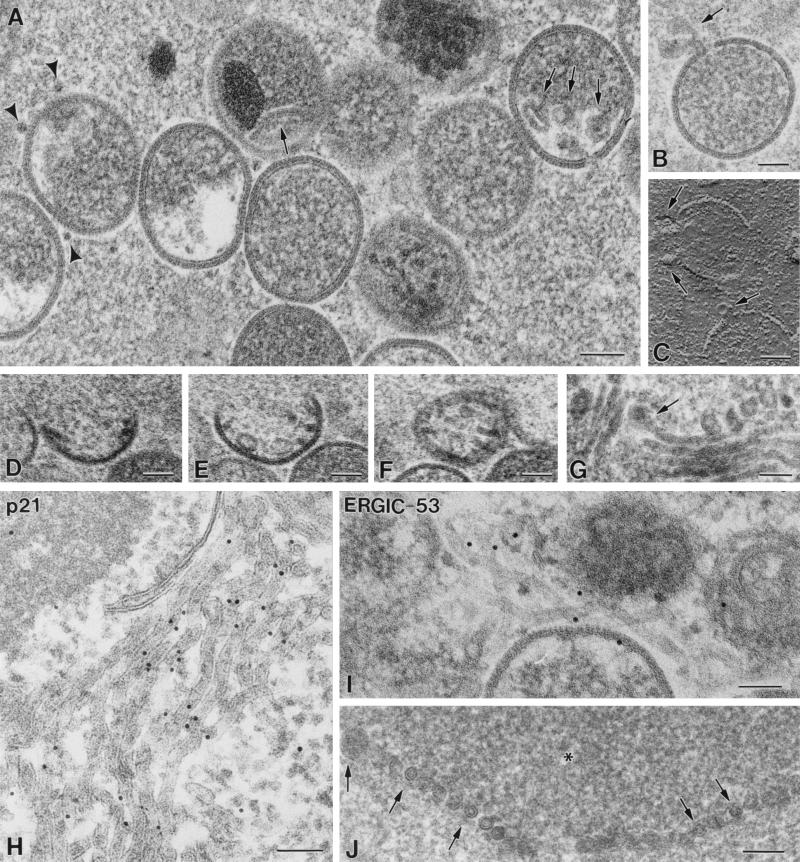
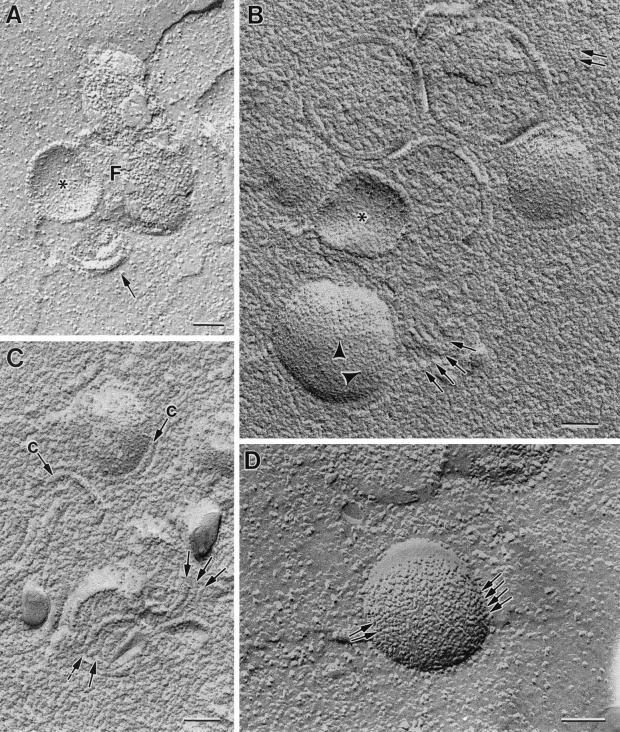
Freeze fracture gives us a three-dimensional view of the arrangement of membranous pieces in viral factories (Fig. 5). Tubular membranes (30 to 40 nm in diameter) can be occasionally seen interacting laterally on the periphery of viroplasm foci within the viral factories (Fig. 5A). When the surface layer of water is eliminated from the foci before making the metal replicas (freeze-etching), these show additional information: linear pieces, whose thickness (15 to 20 nm) is compatible with viral crescents as seen in metal replicas, are frequently seen interacting laterally on the surface of the viroplasm foci (Fig. 5B and C), and spikes organized in close-packing groups are also seen (double arrow in Fig. 5B). IVs fracture along their external surface, as confirmed in thin sections of fractured cells (data not shown). In this external surface, particles arranged in lines are distinguished (arrowheads in Fig. 5B and arrows in Fig. 5D) while on the internal surface no recognizable pattern is distinguished (asterisks in Fig. 5A and B).
FIG. 5.
Freeze-fracture and freeze-etching of cells infected with VVindA17L (A and B), VVindA14L (C), or WR VV (D) show linear pieces interacting laterally on the surface of viroplasm foci. These pieces belong to two categories: occasional 30- to 40-nm-thick structures (arrow in panel A) or frequent 15- to 20-nm-thick structures (arrows in panels B and C). Forming IVs have short linear arrays of particles on their external surface (arrowheads in panel B, arrows in panel D), while their internal surfaces do not have a defined pattern (asterisks in panels A and B). Images in panels A and D correspond to freeze-fractured samples, while panels B and C are replicas of freeze-etched samples. F, viroplasm foci of the viral factory; c, viral crescent. Bars, 100 nm.
Viral polymorphism: potential new VV maturation intermediates.
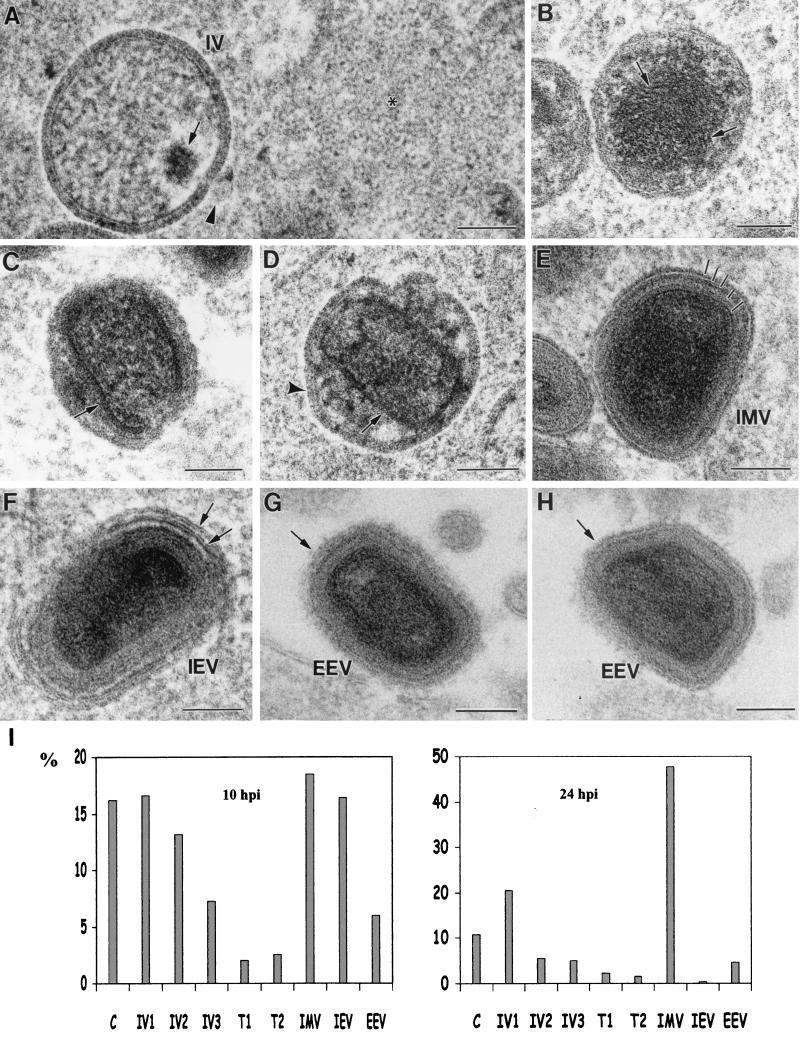
Figure 6 is a collection of images showing the different viral forms detected in freeze-substituted HeLa cells infected with wild-type VV. In Fig. 6A, an IV particle packaging DNA is shown. A large area of structured material (asterisk), which is reminiscent of the organization of nuclear chromatin, is seen nearby. The dense virus shown in Fig. 6B is identical to the structures previously proposed as intermediate maturation stages (64). Serial sections have confirmed that these are independent viral forms and not IMVs sectioned in particular orientations (data not shown). Anti-DNA antibodies confirmed that the dense fiber-like material occupying most of the interior of the particle contains DNA (data not shown). The characteristics of the particles in Fig. 6C and D suggest that they also could be maturation intermediates between IVs and IMVs. These assemblies are round- or ovoid-shaped particles with a variable internal structure, suggesting that the IMV core shell starts to form independently from the double membrane of the envelope (Fig. 6D). Mature VV forms, both intracellular and extracellular, show a complex internal organization (Fig. 6E to H). At least five independent layers (marked in Fig. 6E) are distinguished in IMVs sectioned along an equatorial plane. The interpretation of these profiles is rather complex, since maturation has produced a major reorganization of IV structure. The next viral form is shown in Fig. 6F, where an IEV exhibits a double membrane added around the basic structure of the IMV. Two different section planes of extracellular viral particles (Fig. 6G and H) show that they also have several internal layers and an external fuzzy coat (arrows). The different viral forms shown here are not equally represented at short and long p.i. times (Fig. 6I). At 10 h p.i. forming IVs, IMVs, and IEVs are all abundant. At 24 h p.i., however, although forming IVs are still detected, IMVs are very abundant and IEVs are scarce. Potential transitional maturation intermediates are, in both cases, a minor class and represent less than 5% of the total amount of viral structures. These quantitative data suggest that later in infection assembly and maturation processes are less dynamic and certain events could be taking place at different speeds.
FIG. 6.
Different viral forms detected in VV-infected HeLa cells as processed by freeze-substitution. (A) IV particle packing DNA (arrow). These particles are frequently surrounded by a structured material (asterisk) similar to cellular chromatin. The open pore in the IV particle frequently exhibits a vesicle nearby (arrowhead). (B) Spherical dense particles with fibrous, DNA-like, internal material (arrows). (C and D) Potential intermediate maturation stages in the construction of the internal viral core (arrows) and the IMV. These transitional forms still have the IV envelope (arrowhead in panel D) around the forming core shell. (E) IMV shows a complex organization, with at least five differentiated layers (marked with short lines). (F) IEV with the additional double membrane (arrows). (G and H) Two different section planes of EEV, which have an external fuzzy coat (arrows). (I) Quantification of the relative amounts of the different WR VV assemblies (expressed as percentage of the total population of viral structures) from thin sections of VV-infected HeLa cells at two different p.i. times (10 and 24 h). The structures quantified were named as follows: C, individual viral crescents; IV1, incomplete immature viruses; IV2, apparently completed IVs (closed spheres); IV3, IVs with DNA spot; T1, transitional stage shown in panel B; T2, transitional stages shown in panels C and D. More than 1,000 viral particles were included in the quantification. Bars, 100 nm.
Filaments around and inside the viroplasm foci react with antibodies specific for the cytoskeletal protein vimentin.
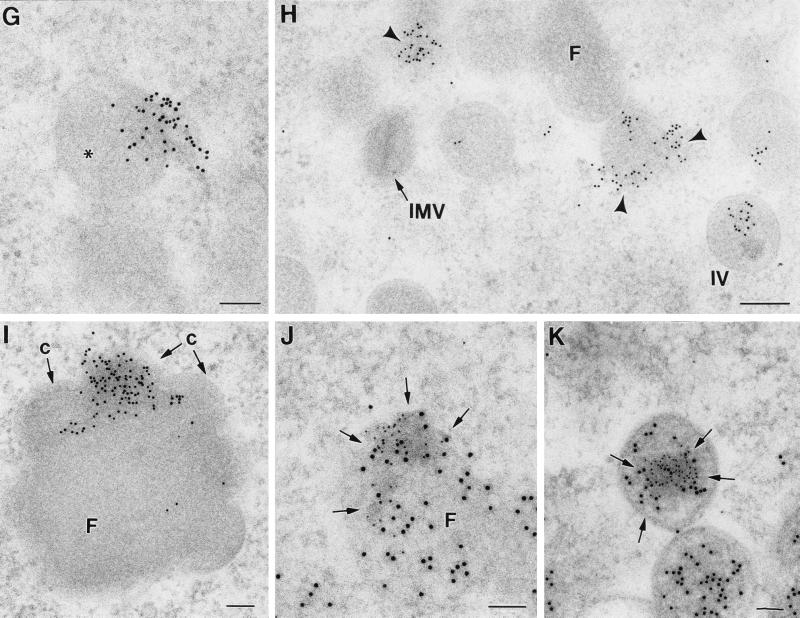
Filaments, whose thickness corresponds to the cytoskeletal IFs, are frequently detected around viroplasm foci of VV factories and forming IVs (Fig. 7A). A fibrous texture can sometimes be distinguished inside the foci, as previously described (44). IFs around viroplasm foci and IVs specifically reacted with three different (monoclonal and polyclonal) vimentin-specific antibodies (Fig. 7B). The three antibodies rendered similar results, with only minor differences in labeling intensity. Confocal microscopy shows that the vimentin-associated signal is mostly concentrated in the perinuclear area of infected cells, surrounds the macrostructures of the viral factories, and appears to be enclosing them (Fig. 7C to F). At the ultrastructural level, vimentin-labeled filaments are also seen inside forming and complete IVs, but IMVs were devoid of labeling (Fig. 7G and H). In cells infected with the recombinant virus VVindA17L, vimentin-associated signal is seen inside small foci formed early after induction of p21 expression by IPTG addition (data not shown) as well as in large foci assembled at long postinduction times (Fig. 7I). In these large foci labeling concentrates in particular areas, mainly where crescents (marked c in Fig. 7I) protrude. Antibodies specific for tubulin, actin, or cytokeratin provided disperse and weak labeling signals around the viroplasm foci or IVs (data not shown). Double-labeling experiments showed that vimentin and the VV core protein p39 (the product of the A4L gene) colocalize in the areas of the viroplasm foci where crescents are coming out (Fig. 7J and K).
FIG. 7.
Identification of filaments recruited around viroplasm foci and IVs in cells infected with VVindA17L (A and I to K) or WR VV (B to H). (A) ERGIC membranes recruited around viroplasm foci (F) are accompanied by filaments (arrows) whose thickness (around 10 nm) corresponds to that of the cytoskeletal IFs. (B) Immunogold shows that these filaments react with antibodies specific for the IF protein vimentin. (C to F) Confocal microscopy shows that vimentin filaments are placed around viral factories, maintaining a close contact with them. In HeLa cells infected for 8 h with WR VV, viral factories were localized with an antibody specific for the VV envelope protein p21 (C) and the DNA stain To-Pro (which also stains the cell nucleus) (E). Detection of vimentin with a specific monoclonal antibody shows its concentration in a perinuclear area (D), coincident with the localization of viral factories. Vimentin filaments appear to enclose the VV factories, as can be observed in the merge picture (F). At the ultrastructural level, labeled vimentin filaments are sometimes seen entering the forming IVs (asterisk in panel G). (H) Labeling concentrates in small viroplasm foci (arrowheads) and inside IVs, while mature viruses (IMVs) are devoid of labeling. (I) In large viroplasm foci formed in HeLa cells infected with VVindA17L at long p.i. times (18 h p.i.), vimentin concentrates in the areas where crescents (c) protrude. In these areas, a VV core protein (p39, the product of the A5L gene) colocalizes with vimentin, as shown by double-labeling experiments. (J and K). The small 5-nm gold particles are associated to vimentin, while 10-nm gold particles are localized the VV core protein p39. Bars, 200 nm in panels A and H and 100 nm in panels B, G, I, J, and K.
These data support the hypothesis that ERGIC cellular membranes and vimentin IFs would act coordinately to build and organize the assembly foci of the viral factories in the first step of VV morphogenesis.
DISCUSSION
The morphogenesis of VV is a complex, multistep process, constituted by an ordered and coordinated recruitment of both viral and cellular components in the cytoplasm of infected cells. The first morphological evidence indicating that VV infection has adequately progressed is provided by the presence of viral factories and crescent-shaped membranes. The assembly foci within the factories, which contain a rather amorphous material in which several VV core proteins have been localized (71), become surrounded by membranous pieces that contain several VV envelope proteins and originate the first viral envelope of the virus. Information about the precise composition of viral factories and IVs is limited, since they have not been isolated from infected cells. The origin of the first VV envelope, present in viral crescents and IVs, has been the subject of recent controversy. Years ago its origin was explained by a hypothetical synthesis de novo induced by the virus (11). However, experimental evidence of this process has not been obtained. When looking for such a mechanism potentially operating for other viruses we found that the de novo synthesis is usually so called when the mechanism of viral envelope acquisition has not been characterized or when the lipidic composition of the viral envelope does not match the average composition of a particular cellular membranous compartment. This is also valid for purified VV IMVs (65). However, it is known today that cellular membranes are much more heterogeneous than previously expected, being a composite of membrane microdomains, also termed rafts (60). Lipid rafts are membrane domains enriched in glycosphingolipids and cholesterol and are involved in signal transduction, and viral components and budding events concentrate in them (37, 55). Since viral assembly seems to take place in particular membrane domains, the final lipidic composition of viral envelopes can markedly differ from the average lipidic composition of the whole membranous compartments from which they originate. Our knowledge in this subject is clearly incomplete.
While the de novo origin of the first VV envelope has not been demonstrated experimentally, there are a number of results supporting the cellular origin of these membranes. In the present work we have detected the accumulation of ERGIC elements in the perinuclear viral factories, as visualized by confocal and electron microscopy. The ERGIC is a system that operates in transport between the endoplasmic reticulum and the cis side of the Golgi complex (1, 54). It seems that early in infection ERGIC membranes concentrate in the factories or that viral elements needed to build the factory migrate to perinuclear regions rich in ERGIC elements. On the other hand, contacts between viral crescents and surrounding ERGIC-like, 30-nm-thick tubular membranes have been observed in VV-infected cells (31, 47, 62). In addition, when HeLa cells are infected with the inducible mutant VVindA17L, numerous ERGIC elements are seen on the periphery of the viroplasm foci. When expression of the protein is allowed, tubular ERGIC elements are seen in contact with the crescents in viroplasm foci (47).
The double membrane resolved in viral crescents and IVs, as well as the tubular pieces in IV-like particles formed when being infected with VVIndA14L, also supports the construction of crescents from tubular membranes, which would condense and curve by the incorporation of VV proteins. Although not proved yet, it has been hypothesized that phosphorylation of a key substrate may initiate the extension of precursor membranes into crescents (67, 72). Interestingly, two VV envelope proteins, p21 and p15, which as mentioned before have both been localized in ERGIC membranes, are phosphorylated (5, 47, 68).
Some other enveloped viruses that assemble intracellularly, such as coronaviruses or flaviviruses, also use the ERGIC membranes as a physical support for particular steps of their life cycle, such as replication and assembly by budding (24, 29, 51). However, VV uses endomembranes in a different way, since whole tubular membranes or cisternae are modified and taken as individual pieces to build large, complex structures. Figure 8 is a working model that explains how this unique mechanism could take place. We do not know how the individual tubular membranous pieces are put together to build the spherical immature viruses, although freeze fracture and freeze-etching replicas of the viroplasm foci suggest that individual crescents could interact laterally in an intermediate step. Interestingly, it has been reported that when IMVs are disrupted with the nonionic detergent NP-40 and β-mercaptoethanol, 30-nm-thick tubular membranes are released (76). It would be interesting to investigate if these tubular pieces come from the basic elements that build the IVs and if their lateral attachment is maintained by disulfide bonds. In this sense it has been reported that disulfide bonds are introduced by VV-specific proteins and that when VV infection is performed under conditions in which disulfide bonding is prevented, IMVs with unstable envelopes are formed (26). This would be in line with the recent finding of specific VV pathways for disulfide-linked formation in viral membrane proteins (57).
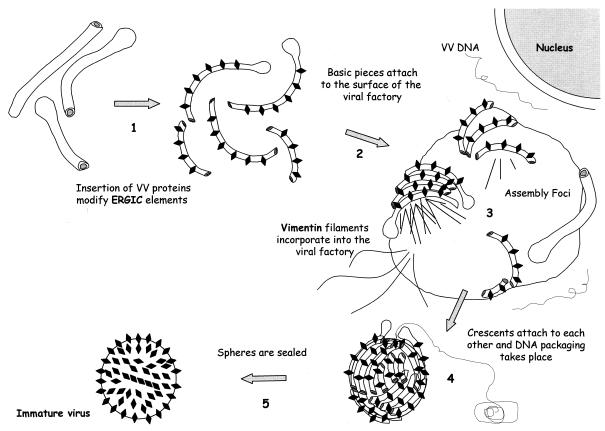
FIG. 8.
Hypothetical steps for the assembly of the first VV form, IV. (Step 1) The ERGIC tubulovesicular structures get deeply modified by the insertion of VV proteins coming from the RER, such as p21 and p15 (encoded by A17L and A14L VV genes, respectively) and the proteins that form the spikes (as-yet unidentified). (Step 2) Modified membranous pieces reach the periphery of viral factories, together with vimentin IFs. (Step 3) These membranes attach to each other on the surface of the viroplasm foci and form the crescents. IFs would participate in the egress of the crescents and the incorporation of VV proteins inside the IVs. (Step 4) The individual pieces originate spherical structures with open pores. DNA packaging would take place through these pores. (Step 5) By unknown mechanisms (lateral fusion of membranous pieces or attachment without fusion?) the IV spheres would finally be sealed.
The membrane pieces that form the first VV envelope interact with the surface of the viroplasm foci. Both confocal and electron microscopy show that vimentin IFs incorporate inside these foci and possibly participate in the egress of crescents, since labeling concentrates in the areas where crescents protrude. The VV p39 core protein colocalizes with vimentin in those regions. Thus, VV would be able to use all three cytoskeletal components during its life cycle, vimentin together with microtubules (18, 70, 73) and actin microfilaments (10, 14). It has been reported that viruses can use microtubules or actin microfilaments for cytoplasmic transport of viral components during entry or egress (61). Interactions between viral components and vimentin IFs have also been documented. The main function of IFs in cells seems to be structural, providing mechanical support for the plasma membrane where it comes into contact with other cells or with the extracellular matrix. It seems that, unlike microtubules and microfilaments, IFs do not participate in cell motility (17). IFs themselves move on microtubular tracks, as described for a nonfilamentous form of vimentin, the vimentin dots (39). A collapse of the IFs around the nucleus has been observed in cells infected with reoviruses, picornaviruses, VV, or human immunodeficiency virus (21, 27, 36, 59). This collapse of vimentin reminds a constitutive cellular process known as the aggresome formation, used by the cells to encapsulate potentially toxic aggregates of misfolded proteins. Protein aggregates are transported to the microtubular organizing center by microtubules, where they become entangled with collapsed IFs (20). VV could take advantage of these mechanisms to initiate the formation of viral factories, as recently suggested for the African swine fever virus (15). In the case of VV, however, our results suggest that, in addition to building a protective cage around the factory, modified vimentin filaments could play a more dynamic role, such as organizing the interior of the viroplasm foci or facilitating the egress of the viral crescents and the incorporation of viral proteins inside immature viral particles.
Structural maturation of IVs renders a very different viral form, the IMV, in which a clear double bilayer similar to the IV envelope is no longer distinguished. Instead, a more complex and multilayered profile is seen, but whose interpretation is not immediate. The IV spikes disappear, and at least five distinct layers are distinguished. As a consequence, behavior of the IMV during entry in cells cannot be predicted without a more detailed characterization of its structure. However, the size of VV constitutes an important obstacle for studying its structure at medium-high resolution. VV particles are so large that the images obtained by cryoelectron microscopy of purified and vitrified IMV virions, although considerably approaching to the native structure of the virus, have not allowed a clear definition of its internal organization (13, 50). New complementary approaches will then be necessary to face the study of this complex virus and to understand some key aspects of VV assembly. How are individual membrane pieces recruited, bound together, and sealed to form ordered three-dimensional structures? How is DNA introduced in the open IV spheres and how do they seal after DNA encapsidation? What is the specific role of vimentin in the construction of the viral factory?
In conclusion, here we describe a novel mechanism for the formation of the VV IV. ERGIC tubular membranes and vimentin IFs seem to be key factors in the construction of the viroplasm foci and egress of crescents. A working model, shown in Fig. 8, is proposed. Whether the viral membrane is formed by a fusion process between adjacent tubules or whether the tubules remain as units linked, for example, by disulfide bonding, remains to be determined. Three-dimensional reconstruction of whole virions (both isolated and within the intracellular environment), although technically challenging, would give us unique information to understand the mechanism of assembly. In this sense, energy filtering and automated electron tomography can be the most adequate method (4), and its application in the study of VV structure and assembly is presently under way.
Acknowledgments
This work was supported by the following grants: 08.2/0042.2/2000 from the Comunidad de Madrid (to C.R. and D.R.), BIO98-0456 and CT98-0225 from the European Union (M.E.), and PB96-0818 from the Comisión Interministerial de Ciencie y Tecnolog|$$|Aa|fia of Spain (to J.L.C.).
We are grateful to Hans Peter Hauri (Biozentrum, Universtity of Basel) for his anti-ERGIC-53 antibody. We are also grateful to Jean Pierre Lechaire and Françoise Gaill (Université Pierre et Marie Curie/CNRS, Paris, France) for their support with the LEO TEM microscope, to David Bellido (University of Barcelona) for his technical assistance with freeze fracture and freeze-etching, and to Carlos Sánchez and M. Angeles Muñoz for their expertise with the use of the confocal microscope.
REFERENCES
- 1.Appenzeller, C., H. Andersson, F. Kappeler, and H.-P. Hauri. 1999. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol. 1:330-334. [DOI] [PubMed] [Google Scholar]
- 2.Aridor, M., K. N. Fish, S. Bannykh, J. Weissman, T. H. Roberts, J. Lippincott-Schwartz, and W. E. Balch. 2001. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol. 152:213-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, T. S., N. H. Olson, and S. D. Fuller. 1999. Adding the third dimension to virus life cycles: three-dimensional reconstruction of icosahedral viruses from cryoelectron micrographs. Microbiol. Mol. Biol. Rev. 63:862-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumeister, W., and A. C. Steven. 2000. Macromolecular electron microscopy in the era of structural genomics. Trends Biochem. Sci. 25:624-631. [DOI] [PubMed] [Google Scholar]
- 5.Betakova, T., E. J. Wolffe, and B. Moss. 1999. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane proteins and C-terminal truncation of the A17L protein are dependent on the F10L kinase. J. Virol. 73:3534-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasco, R., and B. Moss. 1992. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66:4170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns, H. J. F. 1960. The initiation of vaccinia infection. Virology 11:603-623. [DOI] [PubMed] [Google Scholar]
- 8.Cassetti, M. C., M. Merchlinsky, E. J. Wolffe, A. S. Weisberg, and B. Moss. 1998. DNA packaging mutant: repression of the vaccinia virus A32 gene results in noninfectious, DNA-deficient, spherical, enveloped particles. J. Virol. 72:5769-5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castón, J. R., B. L. Trus, F. P. Booy, R. B. Wickner, J. S. Wall, and A. C. Steven. 1997. Structure of L-A virus: a specialized compartment for the transcription and replication of double-stranded RNA. J. Cell Biol. 138:975-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cudmore, S., P. Cossart, G. Griffiths, and M. Way. 1995. Actin-based motility of vaccinia virus. Nature 378:636-638. [DOI] [PubMed] [Google Scholar]
- 11.Dales, S., and E. H. Mosbach. 1968. Vaccinia as a model for membrane biogenesis. Virology 35:564-583. [DOI] [PubMed] [Google Scholar]
- 12.Dales, S., and B. G. T. Pogo. 1981. Biology of poxviruses, p. 54-64. In D. W. Kingsburg and H. Z. Hausen (ed.), Virology monographs. Springer Verlag, Vienna, Austria. [DOI] [PubMed]
- 13.Dubochet, J., M. Adrian, K. Richter, J. Garces, and R. Wittek. 1994. Structure and intracellular mature vaccinia virus observed by cryoelectron microscopy. J. Virol. 68:1935-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frischknecht, F., and M. Way. 2001. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 11:30-38. [DOI] [PubMed] [Google Scholar]
- 15.Heath, C. M., M. Windsor, and T. Wileman. 2001. Aggresomes resemble sites specialized for virus assembly. J. Cell Biol. 153:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heljasvaara, R., D. Rodríguez, C. Risco, J. L. Carrascosa, M. Esteban, and J. R. Rodríguez. 2001. The major core protein P4a (A10L gene) of vaccinia virus is essential for correct assembly of viral DNA into the nucleoprotein complex to form immature viral particles. J. Virol. 75:5778-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann, H., and U. Aebi. 2000. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr. Opin. Cell Biol. 12:79-90. [DOI] [PubMed] [Google Scholar]
- 18.Hollinshead, M., G. Rodger, H. van Eijl, M. Law, R. Hollinshead, D. J. T. Vaux, and G. L. Smith. 2001. Vaccinia virus utilizes microtubules for movement to the cell surface. J. Cell Biol. 154:389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollinshead, M., A. Vanderplasschen, G. L. Smith, and D. J. Vaux. 1999. Vaccinia virus intracellular mature virions contain only one lipid membrane. J. Virol. 73:1503-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston, J. A., C. L. Ward, and R. R. Kopito. 1998. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143:1883-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karczewski, M. K., and K. Strebel. 1996. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J. Virol. 70:494-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellenberger, E. 1991. The potential of cryofixation and freeze-substitution: observations and theoretical considerations. J. Microsc. 161:183-203. [DOI] [PubMed] [Google Scholar]
- 23.Klemperer, N., J. Ward, E. Evans, and P. Traktman. 1997. The vaccinia virus I1 protein is essential for the assembly of mature virions. J. Virol. 71:9285-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krijnse-Locker, J., M. Ericsson, P. J. Rottier, and G. Griffiths. 1994. Characterization of the budding compartment of the mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J. Cell Biol. 124:55-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krijnse-Locker, J., S. Schleich, D. Rodríguez, B. Goud, E. J. Snijder, and G. Griffiths. 1996. The role of a 21-kDa viral membrane protein in the assembly of vaccinia virus from the intermediate compartment. J. Biol. Chem. 271:14950-14958. [DOI] [PubMed] [Google Scholar]
- 26.Krijnse-Locker, J., and G. Griffiths. 1999. An unconventional role for the cytoplasmic disulfide bonds in vaccinia virus proteins. J. Cell Biol. 144:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leao-Ferreira, L. R., N. Moussatché, and V. Moura Neto. 1994. Rearrangement of intermediate filament network of BHK-21 cells infected with vaccinia virus. Arch. Virol. 138:273-285. [DOI] [PubMed] [Google Scholar]
- 28.Llorca, O., E. A. McCormack, G. Hines, J. Gratham, J. Cordell, J. L. Carrascosa, K. R. Willison, J. J. Fernández, and J. M. Valpuesta. 1999. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature 402:693-696. [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie, J. M., M. K. Jones, and E. G. Westway. 1999. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J. Virol. 73:9555-9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntosh, J. R. 2001. Electron microscopy of cells: a new beginning for a new century. J. Cell Biol. 153:F25-F38. [DOI] [PMC free article] [PubMed]
- 31.Mohandas, A. R., and S. Dales. 1995. Involvement of spicules in the formation of vaccinia virus envelopes elucidated by a conditional lethal mutant. Virology 214:494-502. [DOI] [PubMed] [Google Scholar]
- 32.Morgan, C. 1976. Vaccinia virus reexamined: development and release. Virology 73:43-58. [DOI] [PubMed] [Google Scholar]
- 33.Moss, B. 1996. Poxviridae: the viruses and their replication, p. 2637-2761. In. B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fundamental virology. Lippincott-Raven, Philadelphia, Pa.
- 34.Moss, B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA 93:11341-11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss, B., E. N. Rosemblum, and E. Katz. 1969. Rifampin: a specific inhibitor of vaccinia virus assembly. Nature 224:1280-1284. [DOI] [PubMed] [Google Scholar]
- 36.Nédellec, P., P. Vicart, C. Laurent-Winter, C. Martinat, M. C. Prévost, and M. Brahic. 1998. Interaction of Theiler's virus with intermediate filaments of infected cells. J. Virol. 72:9553-9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ploubidou, A., V. Moreau, K. Ashman, I. Reckmann, C. González, and M. Way. 2000. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 19:3932-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prahlad, V., M. Yoon, R. D. Moir, R. D. Vale, and R. D. Goldman. 1998. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143:159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Risco, C., and J. L. Carrascosa. 1999. Visualization of viral assembly in the infected cell. Histol. Histopathol. 14:905-926. [DOI] [PubMed] [Google Scholar]
- 41.Risco, C., L. Menéndez-Arias, T. D. Copeland, P. Pinto da Silva, and S. Oroszlan. 1995. Intracellular transport of the murine leukemia virus during acute infection of NIH 3T3 cells: nuclear import of nucleocapsid protein and integrase. J. Cell Sci. 108:3039-3050. [DOI] [PubMed] [Google Scholar]
- 42.Risco, C., M. Muntión, L. Enjuanes, and J. L. Carrascosa. 1998. Two types of virus-related particles are found during transmissible gastroenteritis virus morphogenesis. J. Virol. 72:4022-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Risco, C., and P. Pinto da Silva. 1998. The fracture-flip technique reveals new structural features of the Escherichia coli cell wall. J. Microsc. 189:213-218. [DOI] [PubMed] [Google Scholar]
- 44.Risco, C., J. R. Rodríguez, W. Demkovicz, R. Heljasvaara, J. L. Carrascosa, M. Esteban, and D. Rodríguez. 1999. The vaccinia virus 39-kDa protein forms a stable complex with the p4a/4a major core protein early in morphogenesis. Virology 265:375-386. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez, D., M. Esteban, and J. R. Rodríguez. 1995. Vaccinia virus A17L gene product is essential for an early step in virion morphogenesis. J. Virol. 69:4640-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez, D., C. Risco, J. R. Rodríguez, J. L. Carrascosa, and M. Esteban. 1996. Inducible expression of the vaccinia virus A17L gene provides a synchronized system to monitor sorting of viral proteins during morphogenesis. J. Virol. 70:7641-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez, J. R., C. Risco, J. L. Carrascosa, M. Esteban, and D. Rodríguez. 1997. Characterization of early stages in vaccinia virus membrane biogenesis: implication of the 21-kilodalton and a newly identified 15-kilodalton envelope protein. J. Virol. 71:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez, J. R., C. Risco, J. L. Carrascosa, M. Esteban, and D. Rodríguez. 1998. Vaccinia virus 15-kilodalton (A14L) protein is essential for assembly and attachment of viral crescents to virosomes. J. Virol. 72:1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodríguez, J. F., and G. L. Smith. 1990. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 18:5347-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roos, N., M. Cyrklaff, S. Cudmore, R. Blasco, J. Krijnse-Locker, and G. Griffiths. 1996. A novel immunogold cryoelectron microscopic approach to investigate the structure of the intracellular and extracellular forms of vaccinia virus. EMBO J. 15:2343-2355. [PMC free article] [PubMed] [Google Scholar]
- 51.Salanueva, I. J., J. L. Carrascosa, and C. Risco. 1999. Structural maturation of the transmissible gastroenteritis coronavirus. J. Virol. 73:7952-7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salmons, T., A. Kuhn, F. Wylie, S. Schleich, J. R. Rodríguez, D. Rodríguez, M. Esteban, G. Griffiths, and J. Krijnse-Locker. 1997. Vaccinia virus membrane proteins p8 and p16 are cotranslationally inserted into the rough endoplasmic reticulum and retained in the intermediate compartment. J. Virol. 71:7404-7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanderson, C. M., M. Hollinshead, and G. L. Smith. 2000. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particles. J. Gen. Virol. 81:47-58. [DOI] [PubMed] [Google Scholar]
- 54.Saraste, J., and E. Kuismanen. 1992. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin. Cell Biol. 3:343-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheiffele, P., A. Rietreld, T. Wilk, and K. Simons. 1997. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 56.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J. Virol. 68:130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Senkevich, T. G., C. L. White, E. U. Kooning, and B. Moss. 2000. A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation. Proc. Natl. Acad. Sci. USA 97:12068-12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Severs, N. J. 1991. Freeze-fracture cytochemistry: a simplified guide and update of developments. J. Microsc. 161:109-134. [DOI] [PubMed] [Google Scholar]
- 59.Sharpe, A. H., L. B. Chen, and B. N. Fields. 1982. The interaction of mammalian reoviruses with the cytoskeleton of monkey kidney CV-1 cells. Virology 120:399-411. [DOI] [PubMed] [Google Scholar]
- 60.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 61.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465-472. [DOI] [PubMed] [Google Scholar]
- 62.Sodeik, B., R. W. Doms, M. Ericsson, G. Hiller, C. E. Machamer, W. van't Hof, G. van Meer, B. Moss, and G. Griffiths. 1993. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J. Cell Biol. 121:521-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sodeik, B., G. Griffiths, M. Ericsson, B. Moss, and R. W. Doms. 1994. Assembly of vaccinia virus: effects of rifampin on the intracellular distribution of viral protein p65. J. Virol. 68:1103-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sodeik, B., S. Cudmore, M. Ericsson, M. Esteban, E. G. Niles, and G. Griffiths. 1995. Assembly of vaccinia virus: incorporation of p14 and p32 into the membrane of the intracellular mature virus. J. Virol. 69:3560-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stern, W., and S. Dales. 1974. Biogenesis of vaccinia: concerning the origin of the envelope phospholipids. Virology 62:293-306. [DOI] [PubMed] [Google Scholar]
- 66.Tooze, J., M. Hollinshead, B. Reis, K. Radsak, and H. Kern. 1993. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 60:163-178. [PubMed] [Google Scholar]
- 67.Traktman, P., A. Caligiuri, A. Jesty, K. Liu, and U. Sankar. 1995. Temperature-sensitive mutants with lesions in the vaccinia virus F10 kinase undergo arrest at the earliest stage of virion morphogenesis. J. Virol. 69:6581-6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Traktman, P., K. Liu, J. DeMasi, R. Rollins, S. Jesty, and B. Unger. 2000. Elucidating the essential role of the A14 phosphoprotein in vaccinia virus morphogenesis: construction and characterization of a tetracycline-inducible recombinant. J. Virol. 74:3682-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1998. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79:877-887. [DOI] [PubMed] [Google Scholar]
- 70.Van Eijl, H., M. Hollinshead, and G. L. Smith. 2000. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology 271:26-36. [DOI] [PubMed] [Google Scholar]
- 71.Vanslyke, J. K., and D. E. Hruby. 1994. Immunolocalization of vaccinia virus structural proteins during virion formation. Virology 198:624-635. [DOI] [PubMed] [Google Scholar]
- 72.Wang, S., and S. Shuman. 1995. Vaccinia virus morphogenesis is blocked by temperature-sensitive mutations in the F10 gene, which encodes protein kinase 2. J. Virol. 69:6376-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ward, B. M., and B. Moss. 2001. Visualization of intracellular movement of vaccinia virus virions containing a green fluorescence protein-B5R membrane protein chimera. J. Virol. 75:4802-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilcock, D., and G. L. Smith. 1996. Vaccinia virions lacking core protein VP8 are decifient in early transcription. J. Virol. 70:934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams, O., E. J. Wolffe, A. S. Weisberg, and M. Merchlinsky. 1999. Vaccinia virus WR gene A5L is required for morphogenesis of mature virions. J. Virol. 73:4590-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilton, S., A. R. Mohandas, and S. Dales. 1995. Organization of the vaccinia envelope and relationship to the structure of intracellular mature virions. Virology 214:503-511. [DOI] [PubMed] [Google Scholar]
- 77.Wolffe, E. J., D. M. Moore, P. J. Peters, and B. Moss. 1996. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that is required to initiate morphogenesis. J. Virol. 70:2797-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, Y., and B. Moss. 1991. Inducer-dependent conditional-lethal mutant animal viruses. Proc. Natl. Acad. Sci. USA 88:1511-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]