Abstract
1. Changes in ionized calcium in giant axons were followed by recording the light produced by injected aequorin.
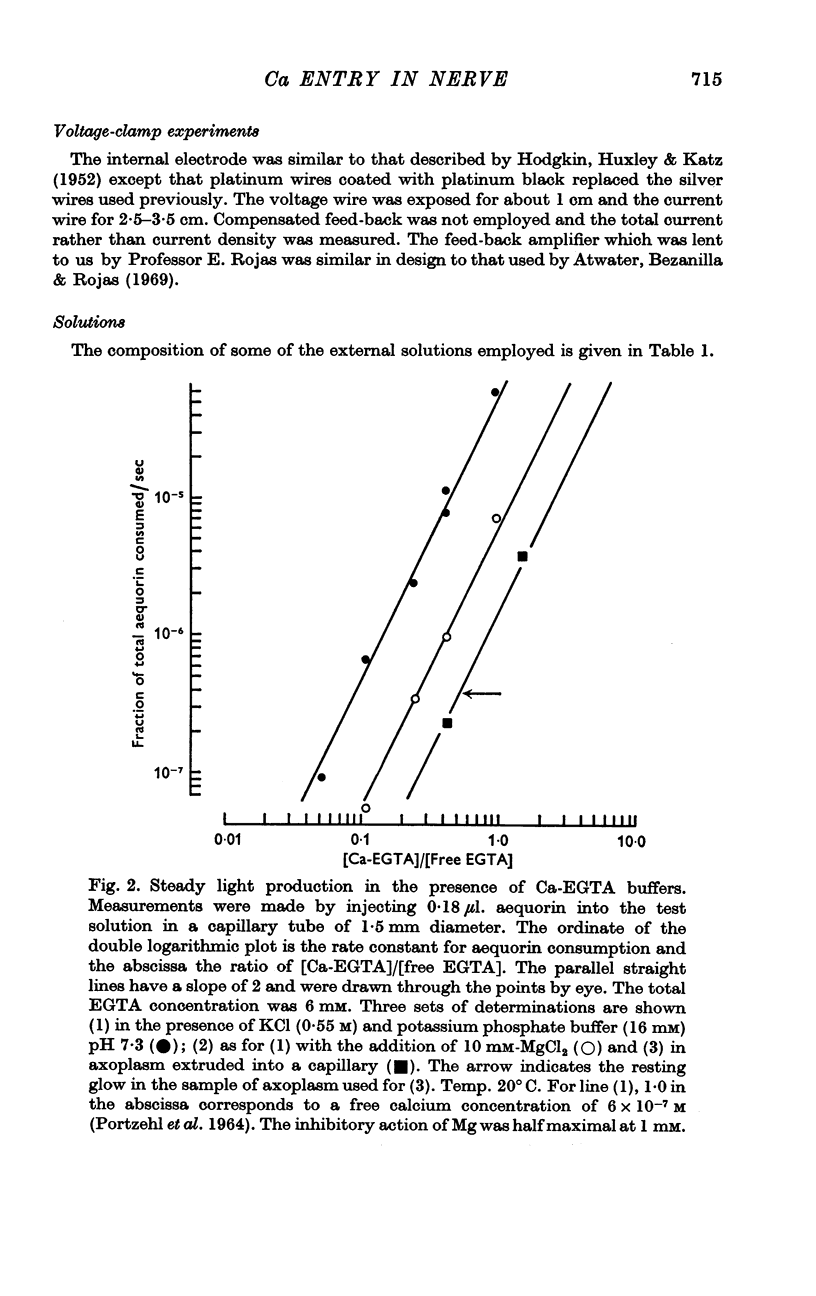
2. From the effect of injecting calcium buffers the internal concentration of ionized calcium was found to be about the same as in a mixture of 45 Ca EGTA:55 free EGTA, i.e. about 0·3 μM.
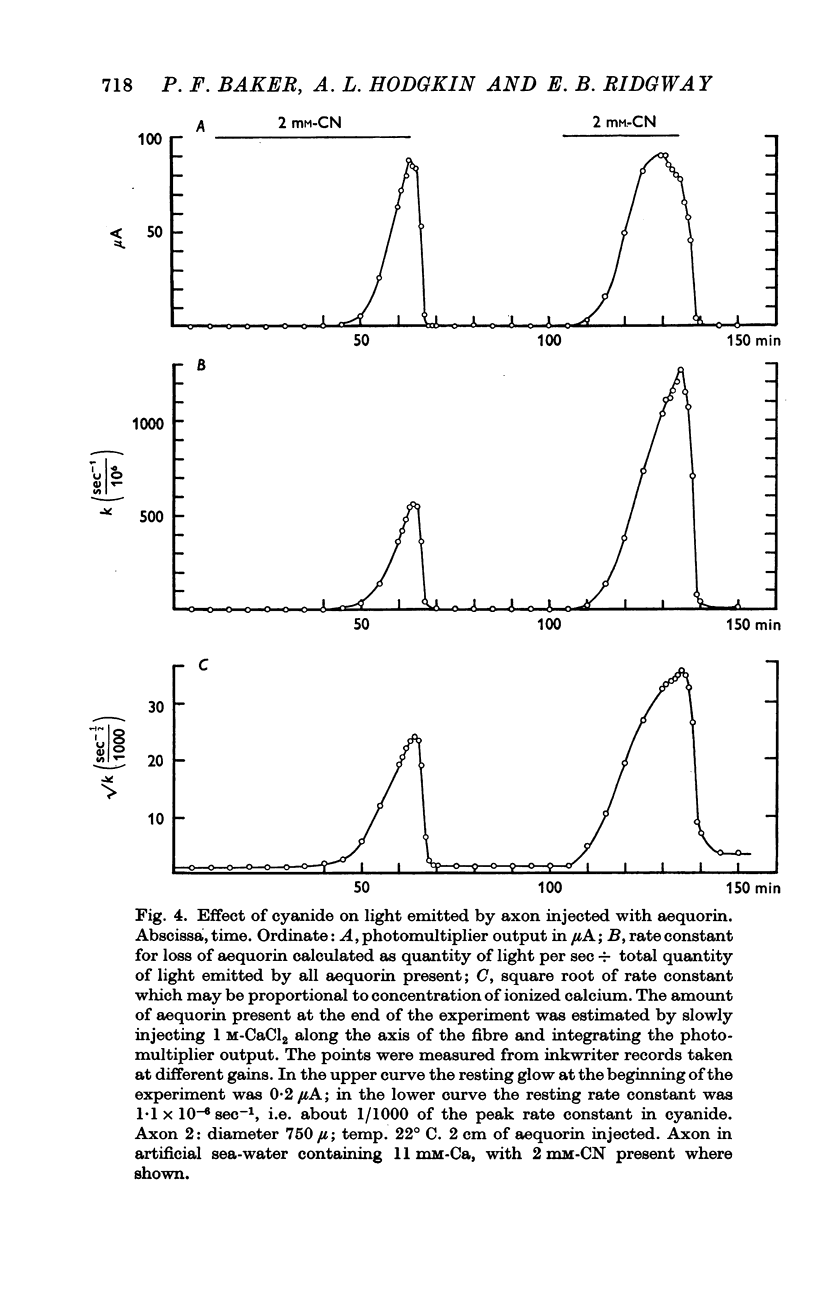
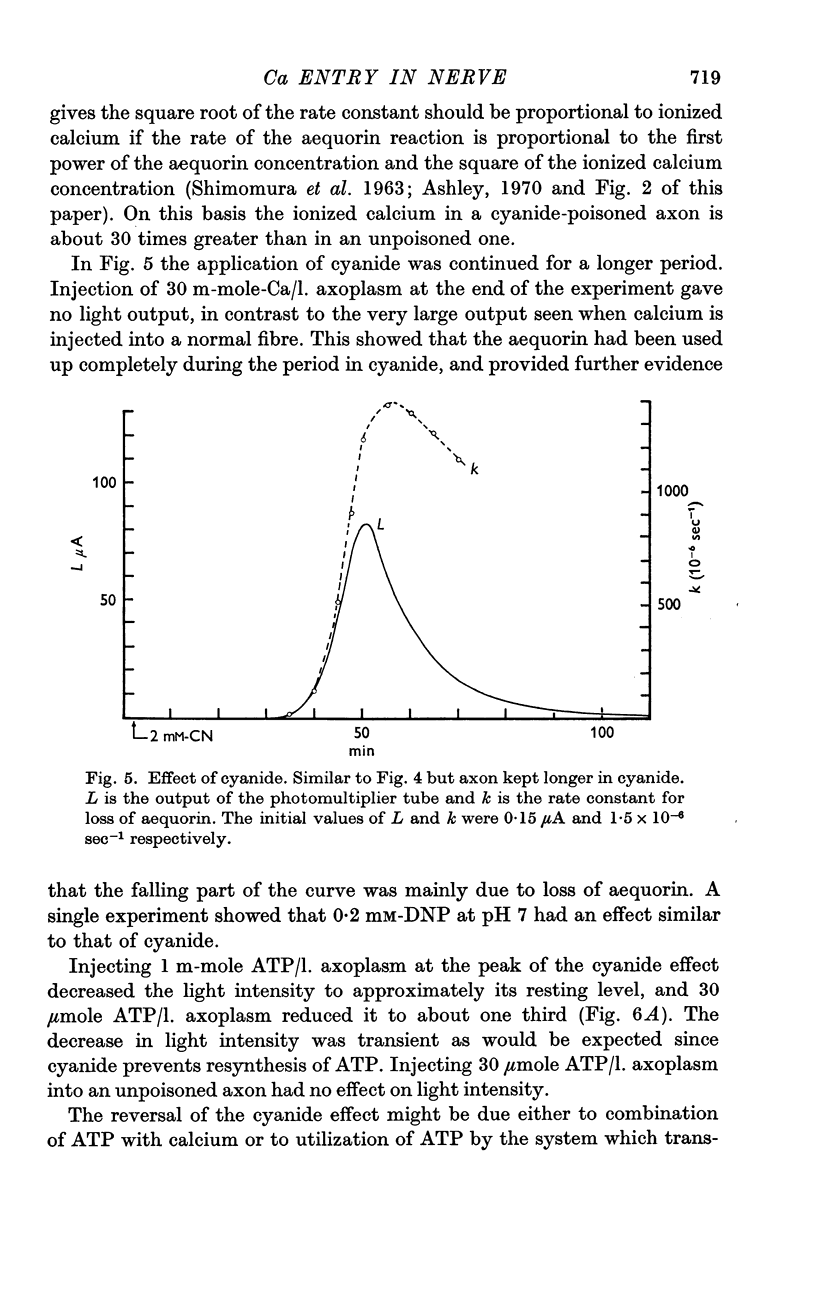
3. After an axon had been exposed to cyanide for 50-100 min the velocity of the aequorin reaction increased about 500 times. This effect, which could be reversed rapidly by removing cyanide, was probably brought about by release of calcium from an internal store.
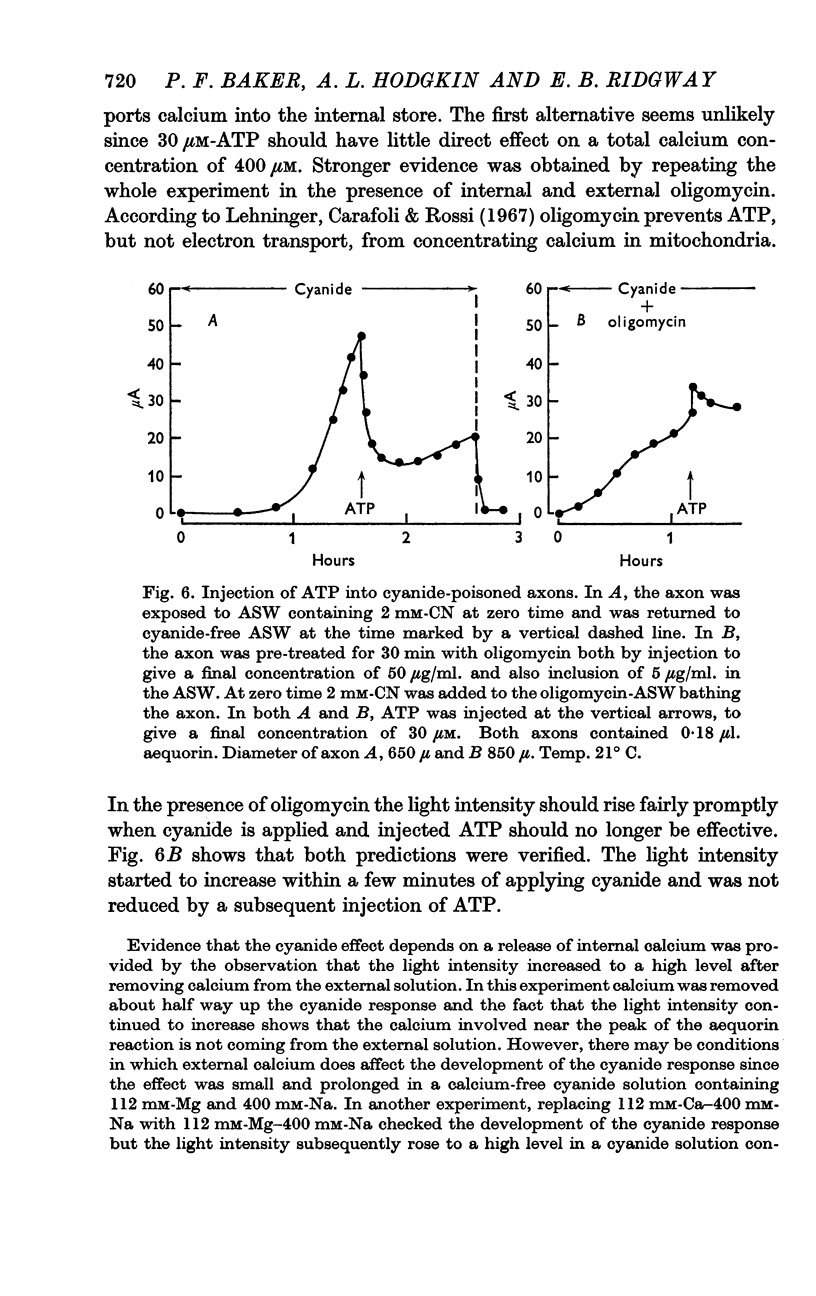
4. Injecting 30 μmole ATP per litre of axoplasm into a cyanide-poisoned axon caused a transient lowering of light intensity; oligomycin blocked the effect.
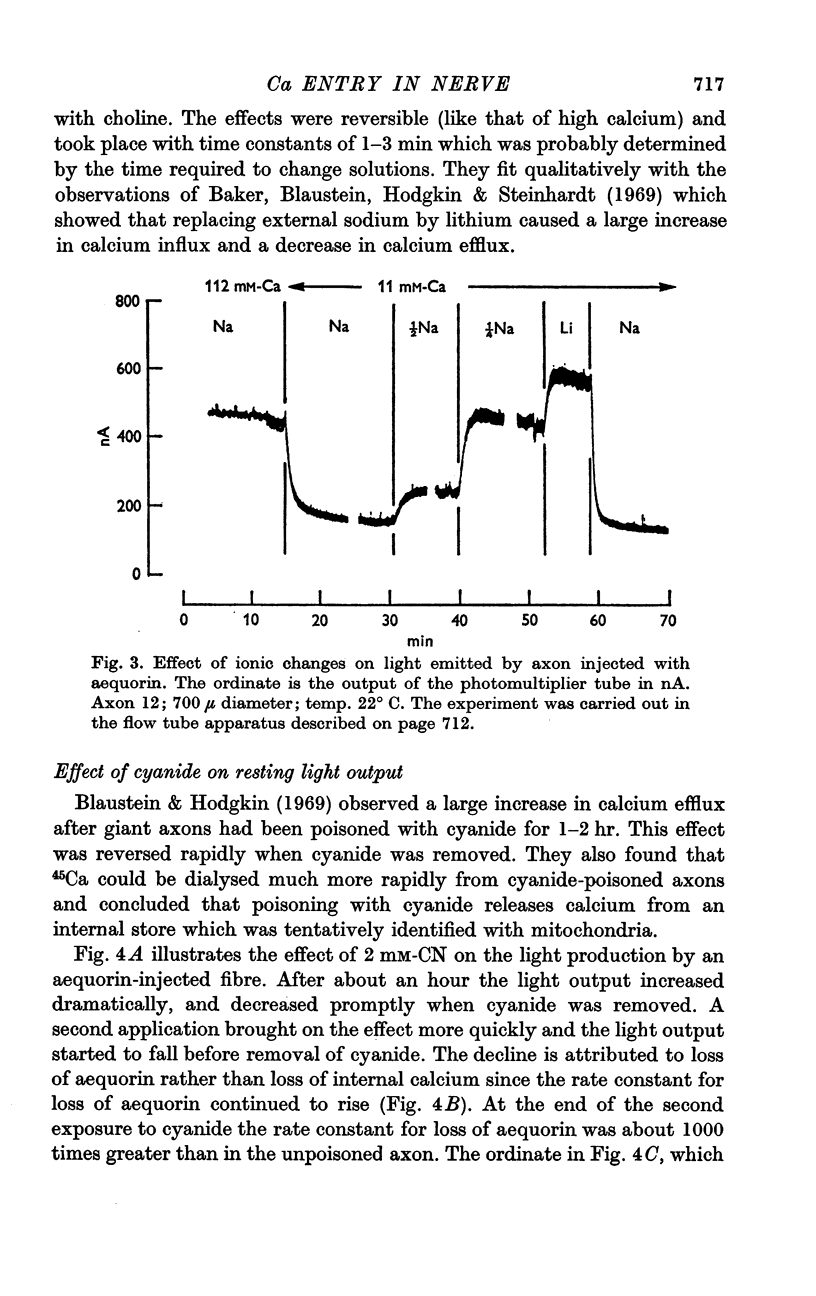
5. Raising external calcium or replacing external sodium by choline or lithium reversibly increased the light produced by axons injected with aequorin.
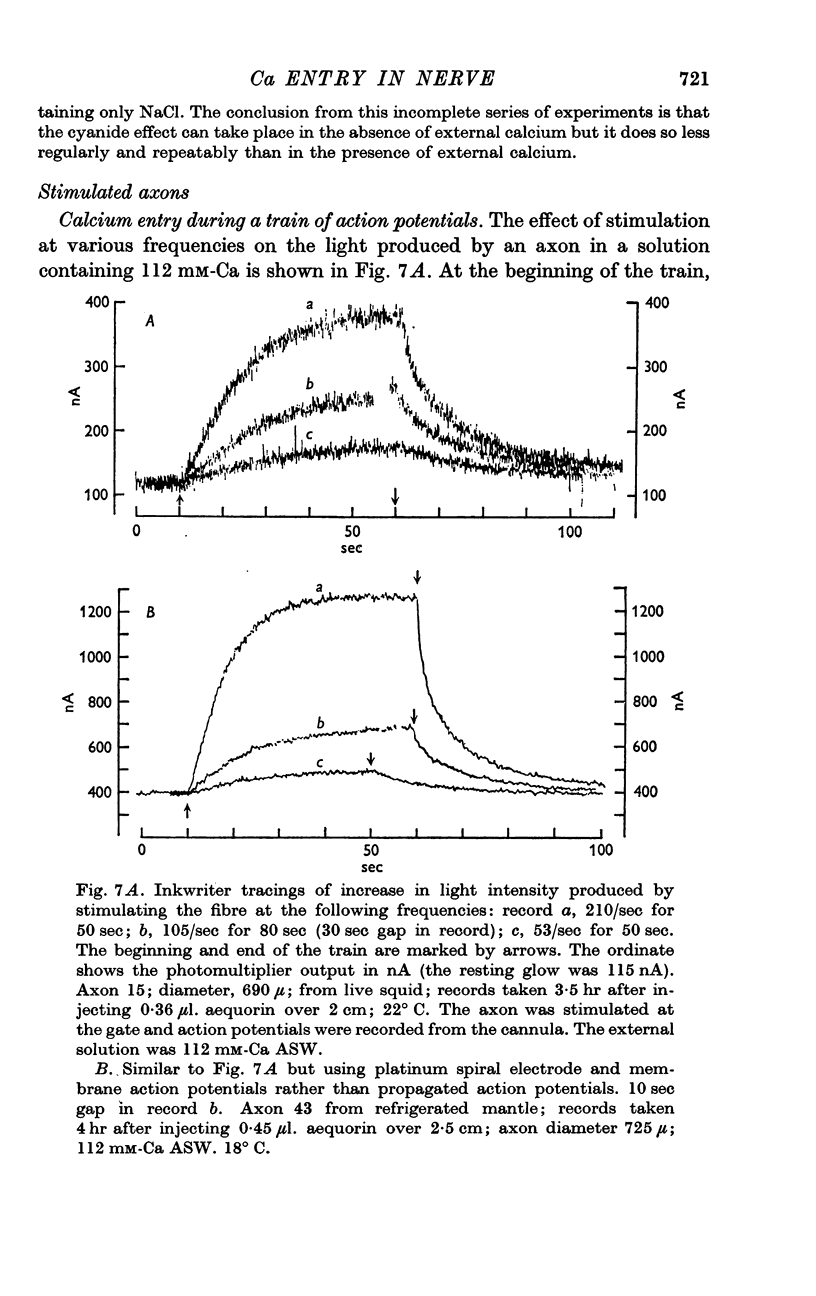
6. Stimulation at 50-200 impulses/sec in a solution containing 112 mM-Ca caused the light intensity to increase to a new steady level; after stimulation the light intensity returned to its original level with a time constant of 10-30 sec. Similar but smaller effects were seen in solutions containing less external calcium. The recovery after stimulation is probably due to uptake of calcium by the internal store.
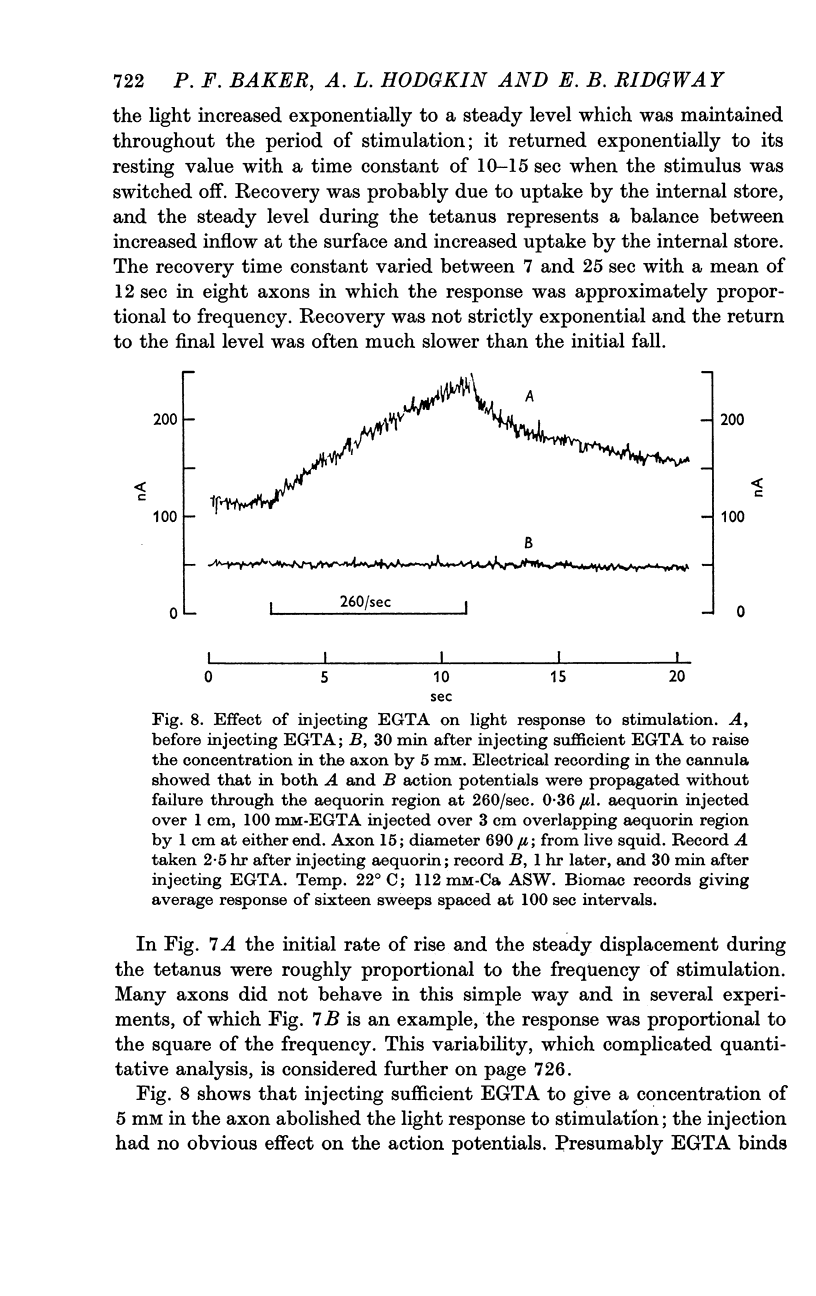
7. Injecting 3 m-mole EGTA per litre axoplasm lowered the resting glow and abolished the aequorin response to stimulation.
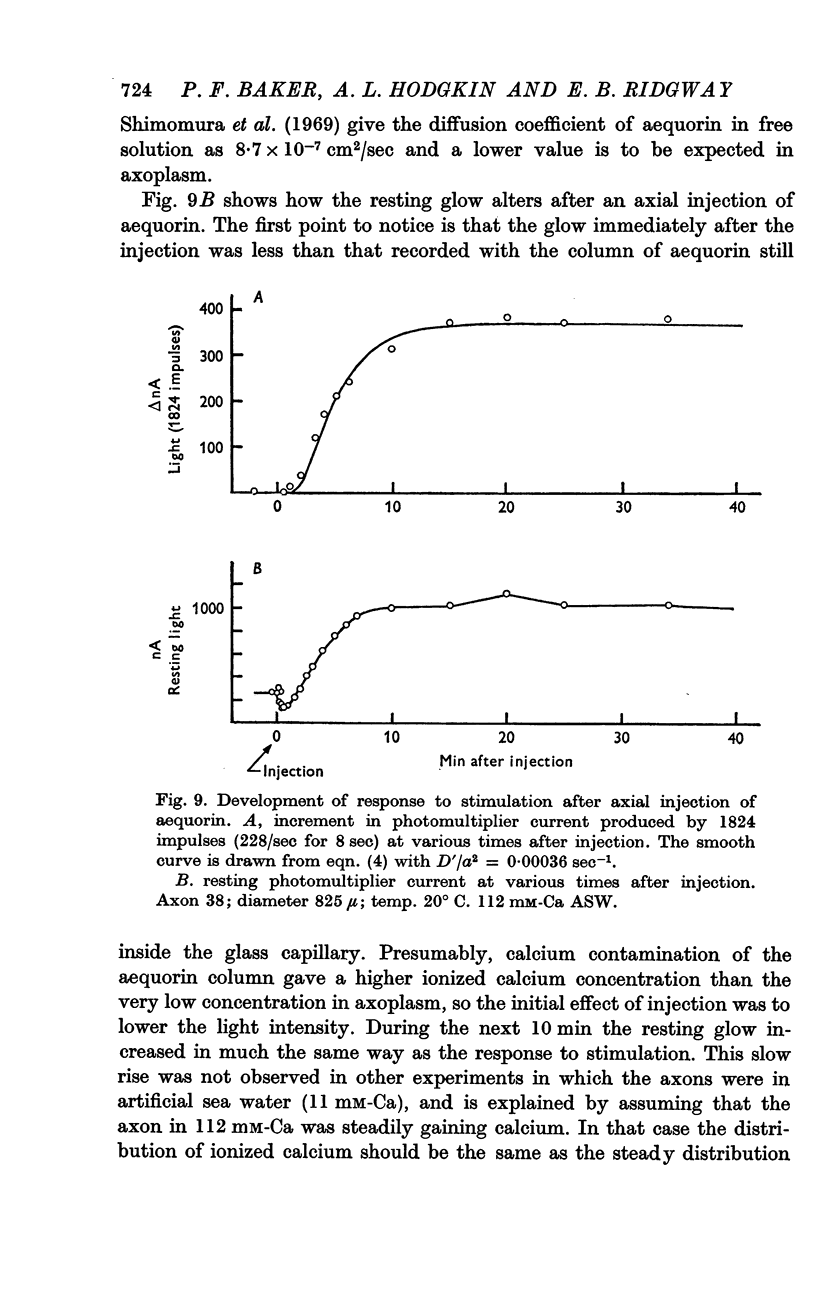
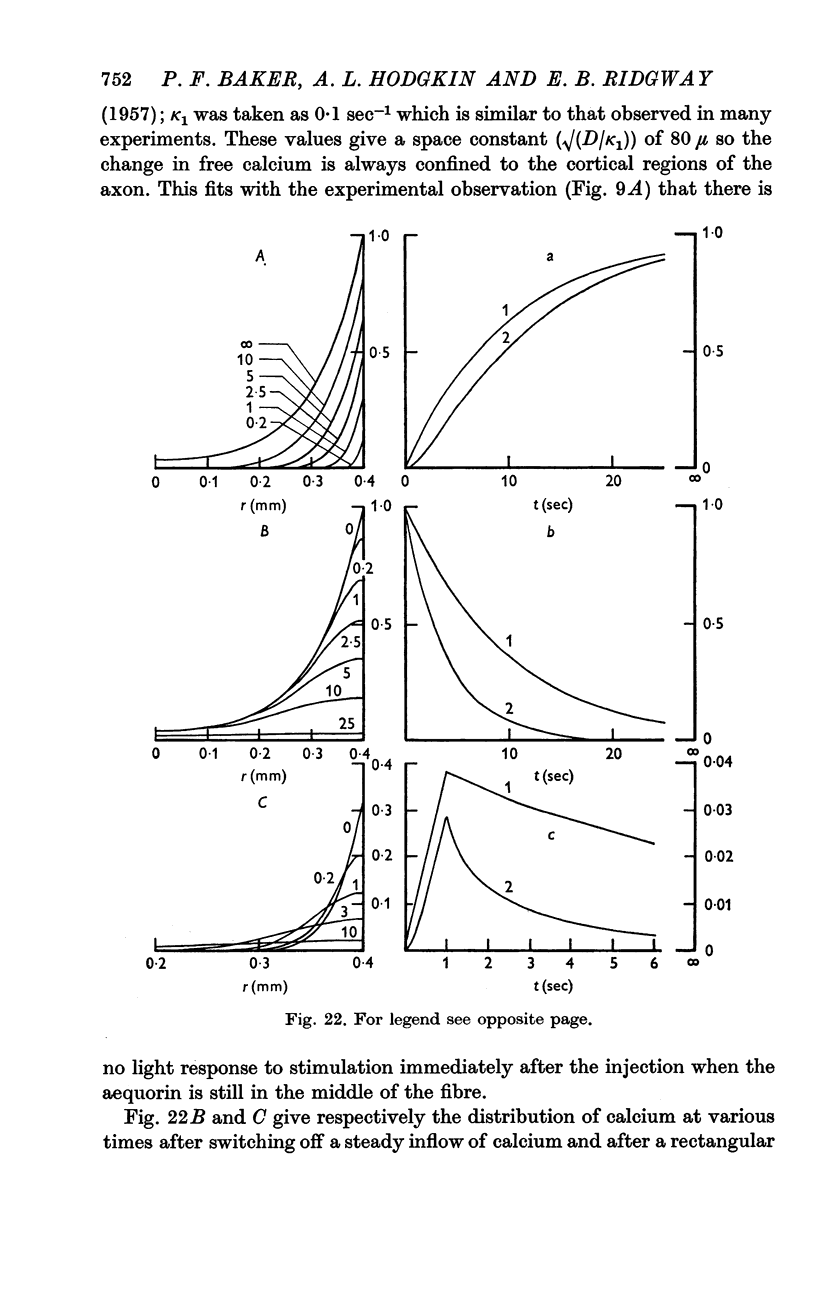
8. There was no light response to stimulation immediately after an axial injection of aequorin and the effect increased to a `steady' level with a half-time of about 5 min. The conclusion is that the rise in calcium concentration resulting from stimulation is confined to the peripheral part of the axon and that the diffusion coefficient of aequorin in axoplasm is about 4 × 10-7 cm2/sec.
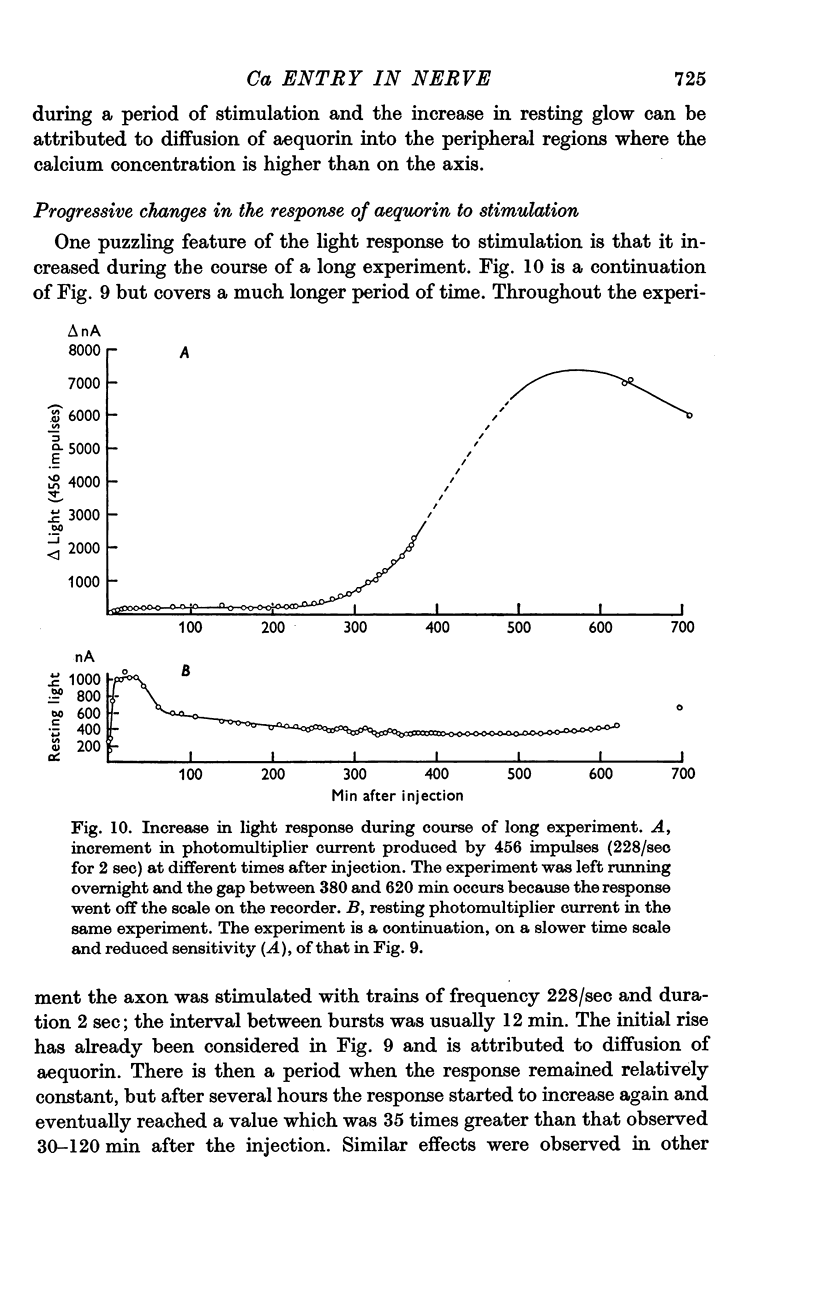
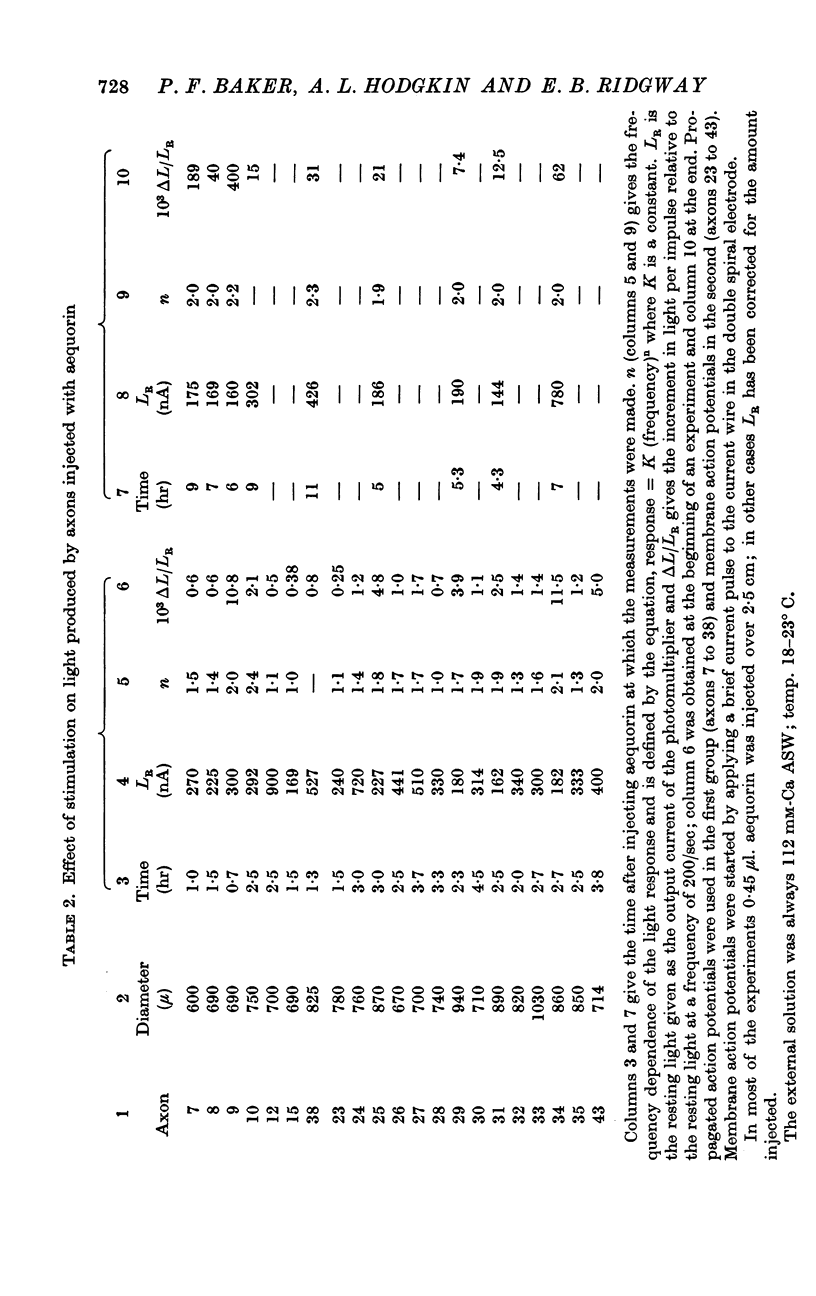
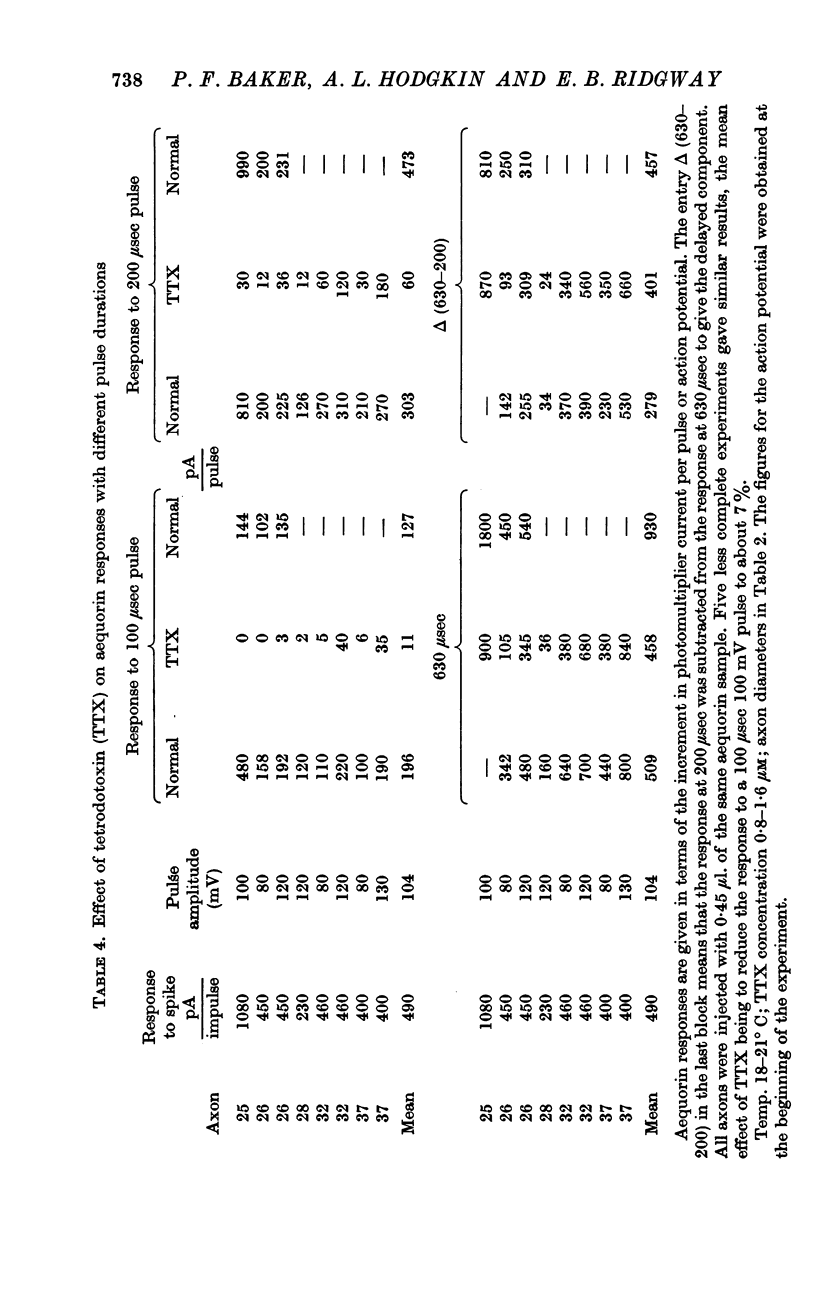
9. The increment in light per impulse often increased markedly during the course of a long experiment and there was also considerable variation between axons.
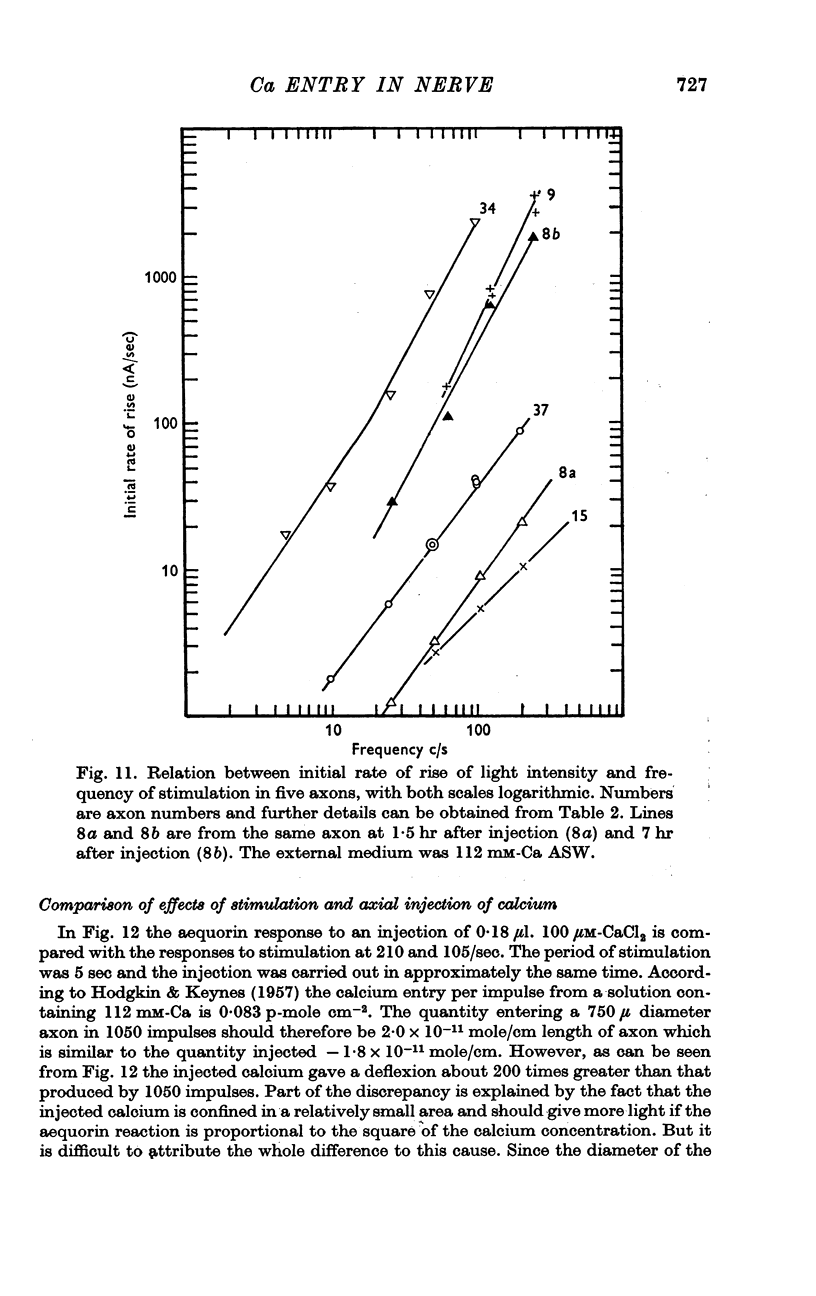
10. If the light response to stimulation was small it was proportional to the frequency of stimulation; if large to the square of the frequency.
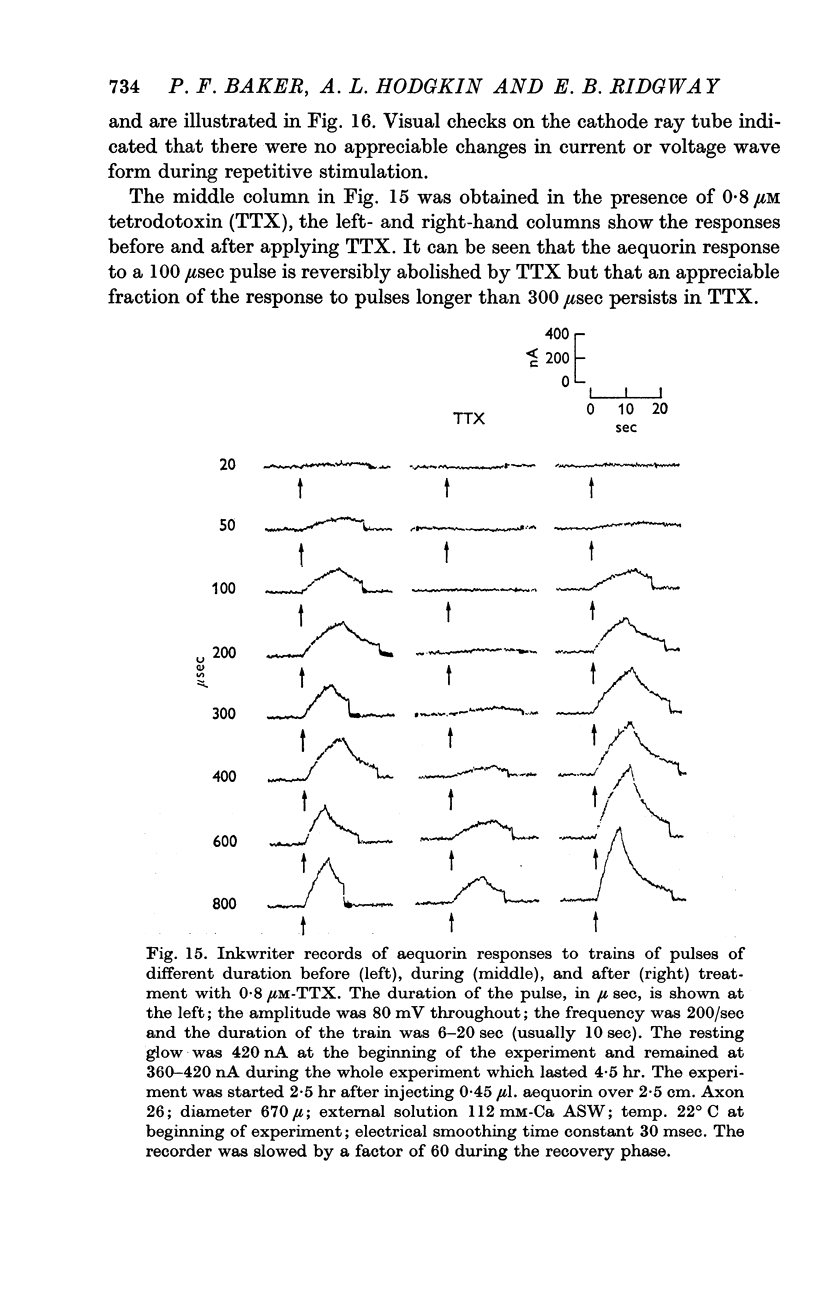
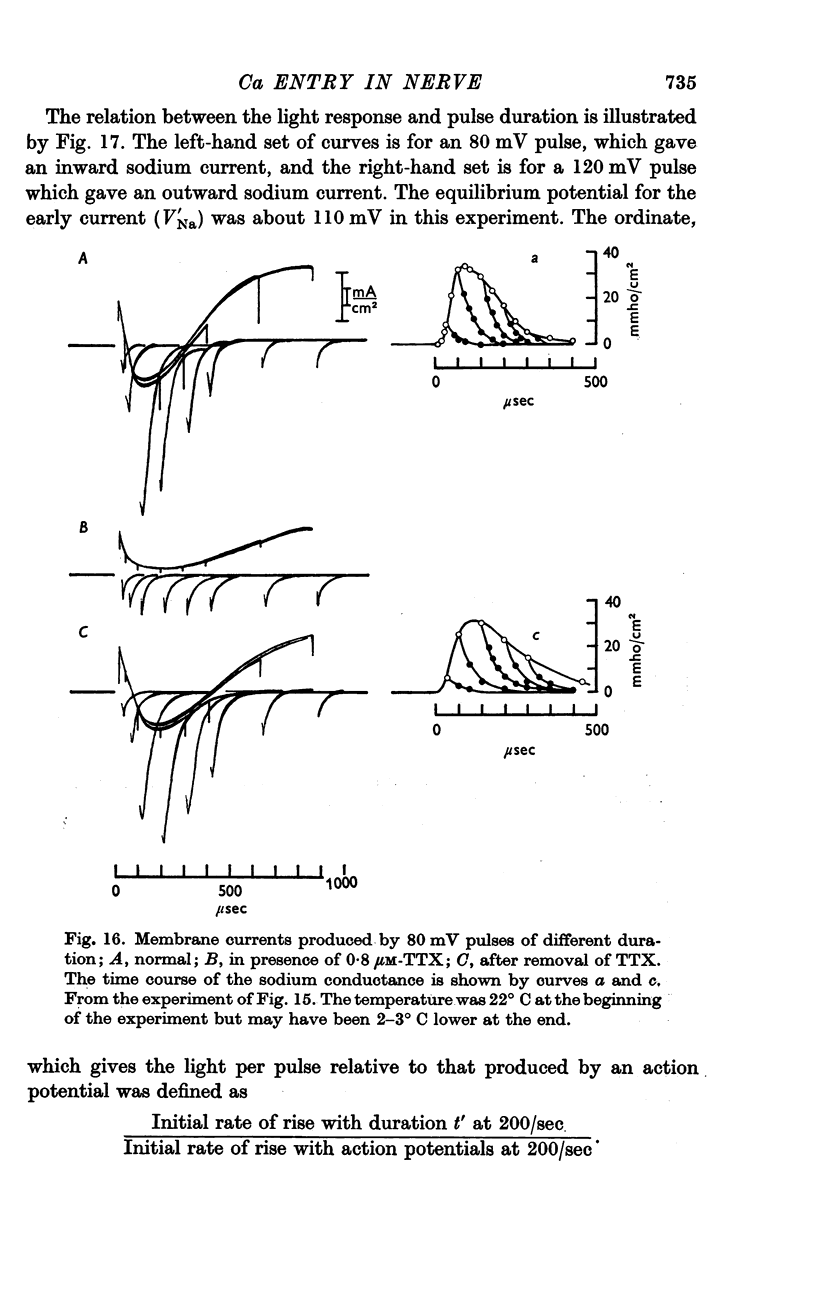
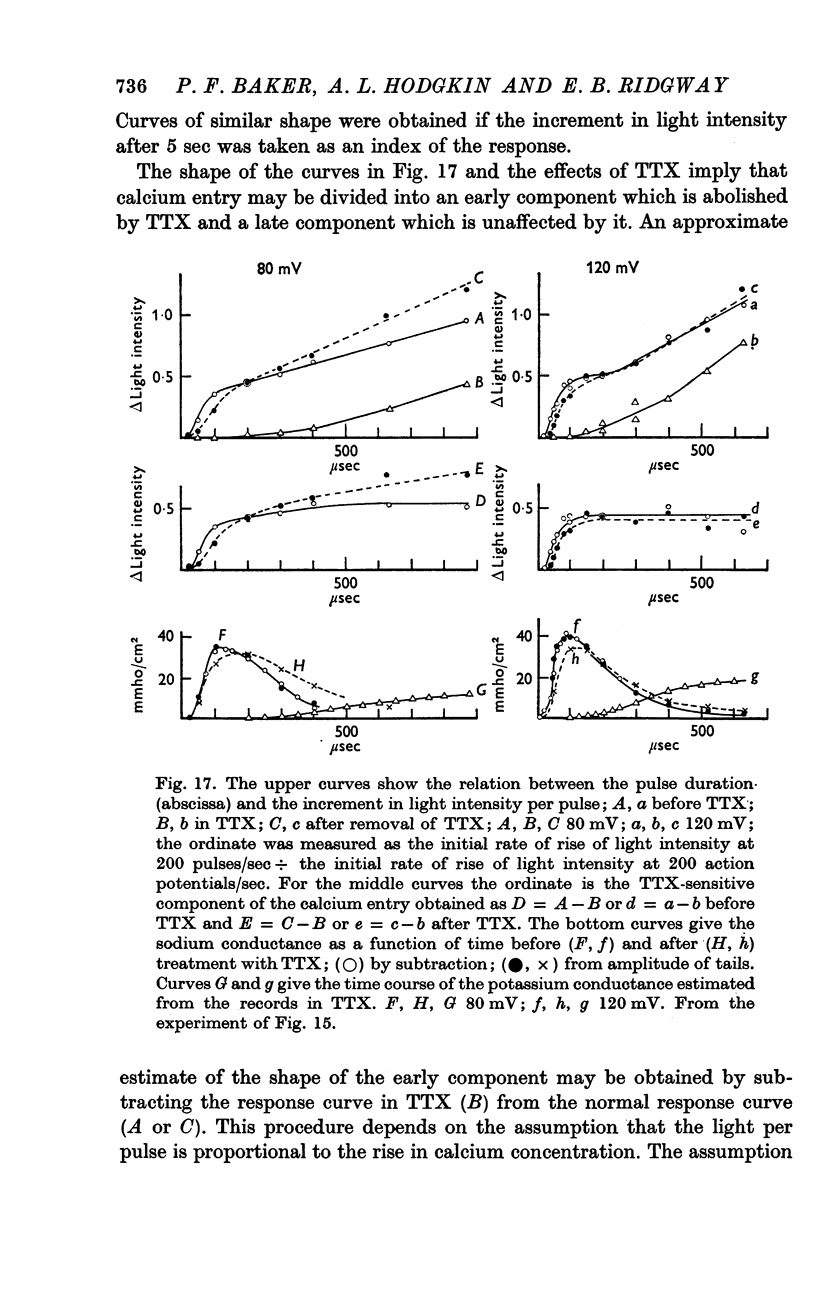
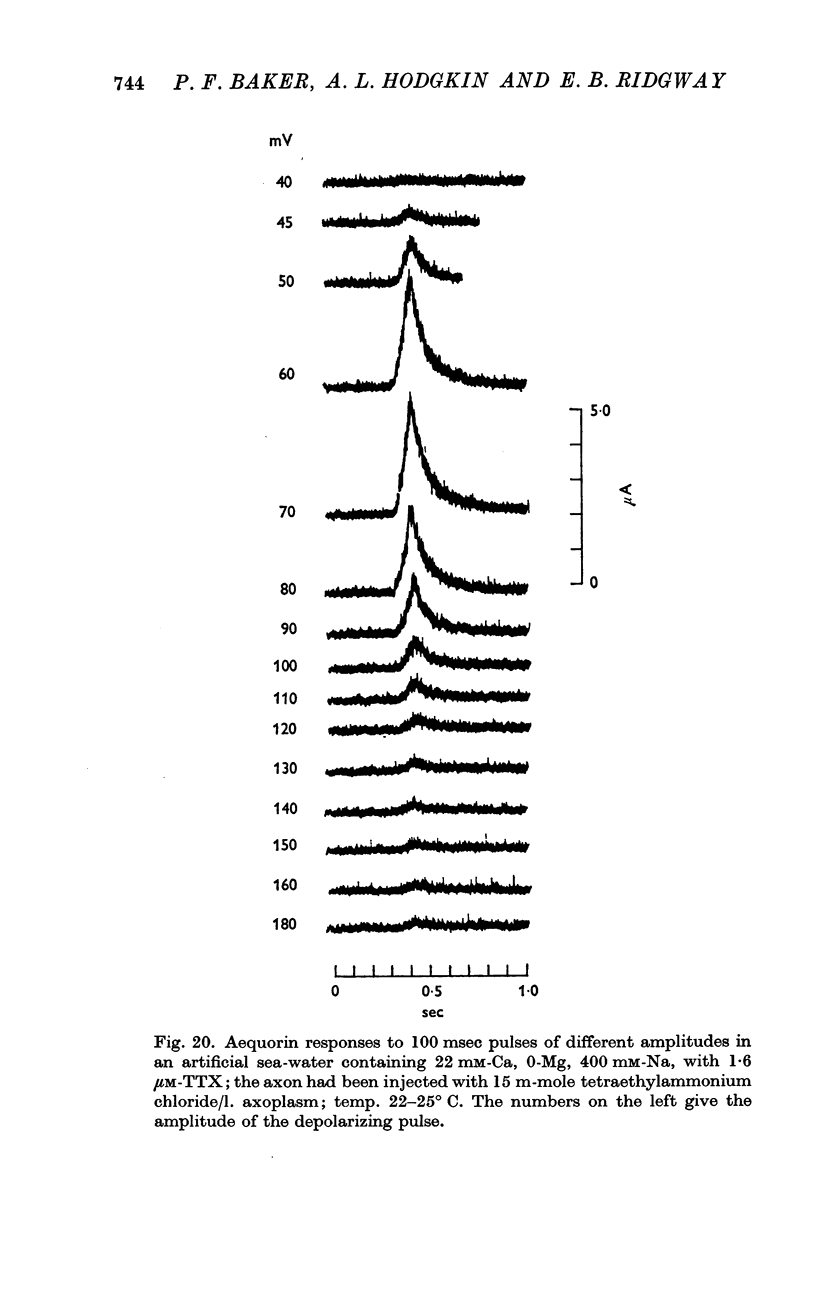
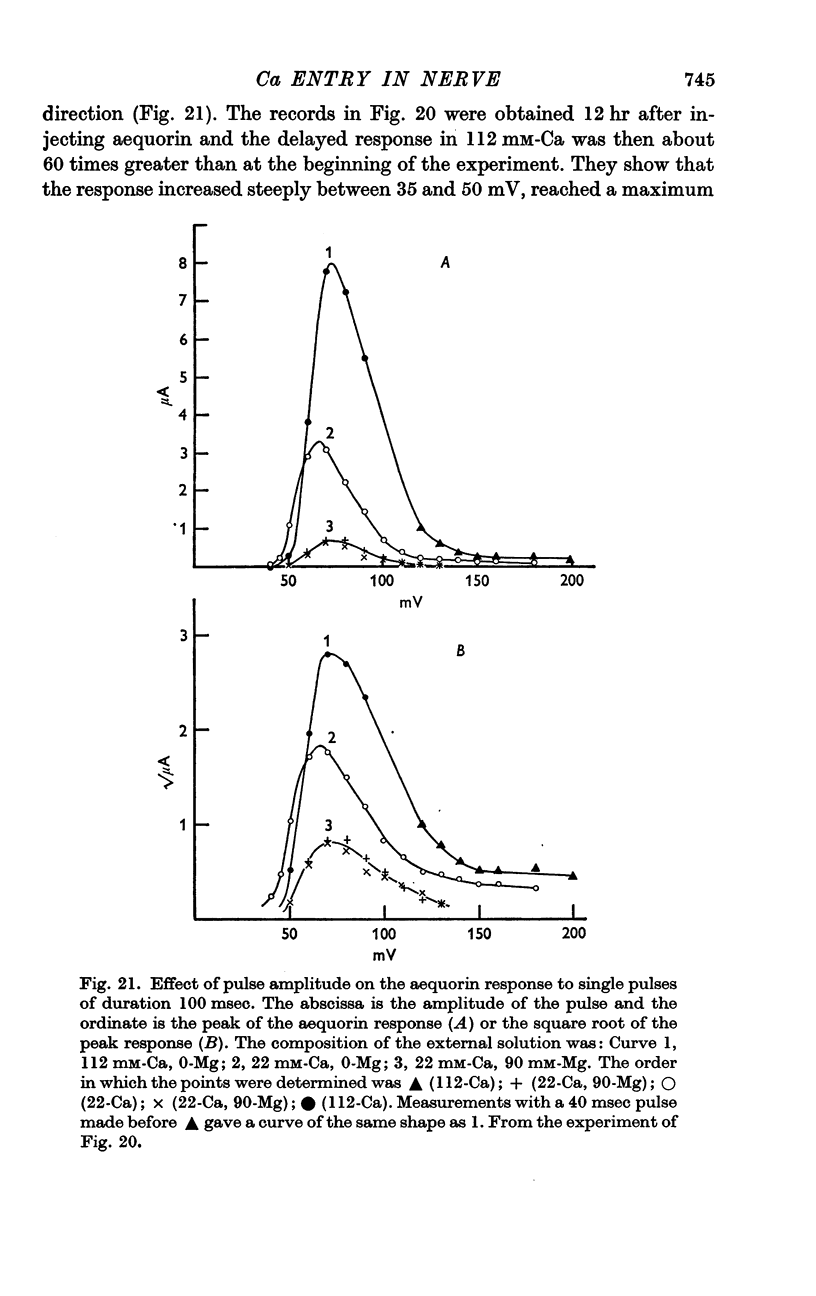
11. Voltage-clamp experiments showed that the calcium entry associated with a depolarizing pulse could be divided into an early component which was abolished by tetrodotoxin (TTX), and a late component which was unaffected by this inhibitor.
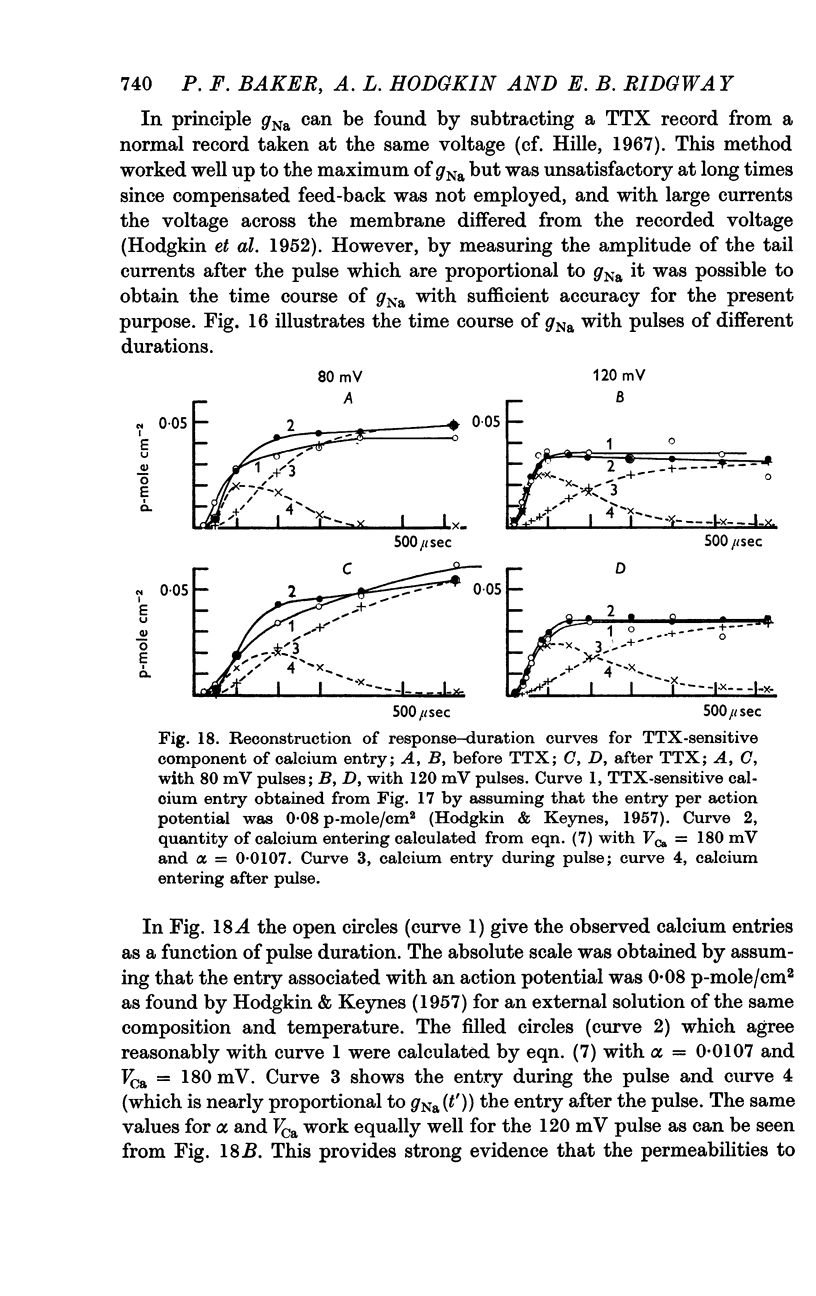
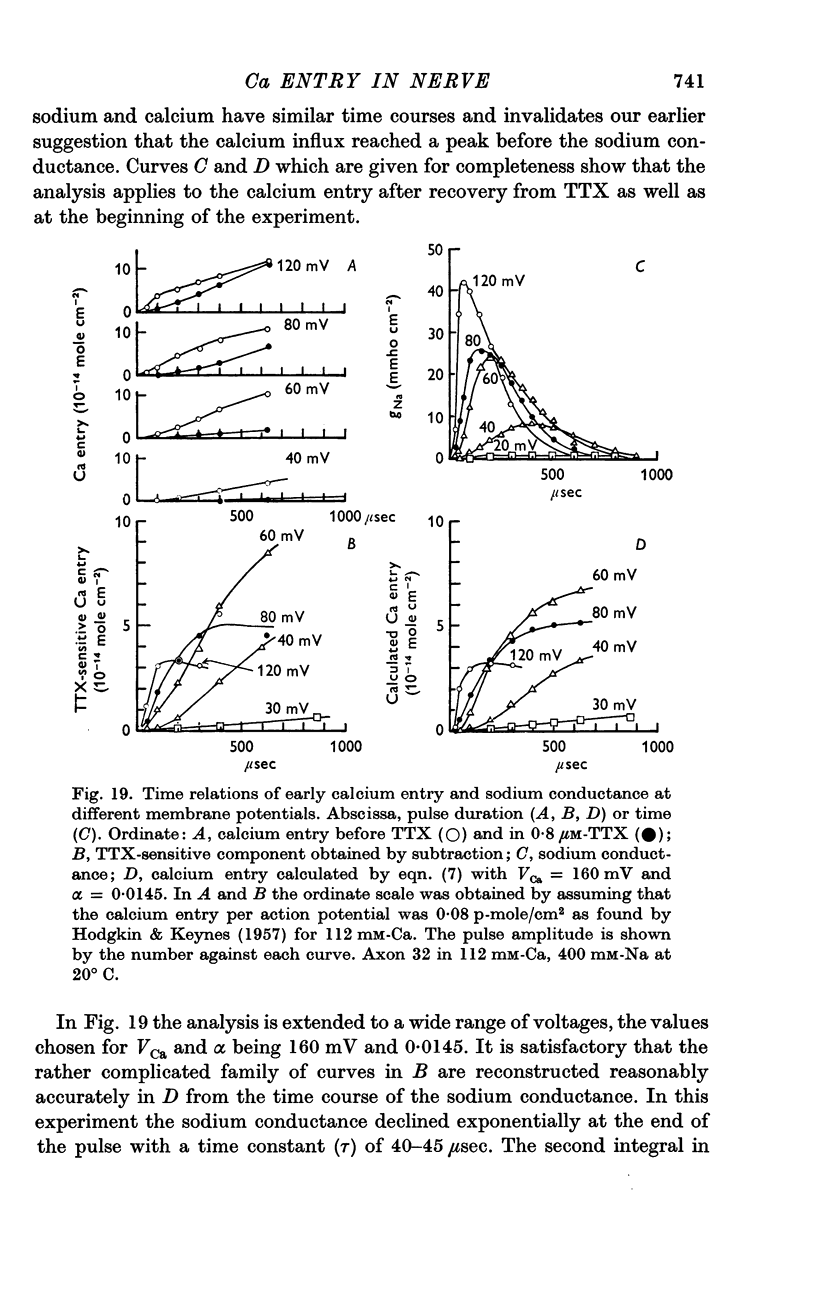
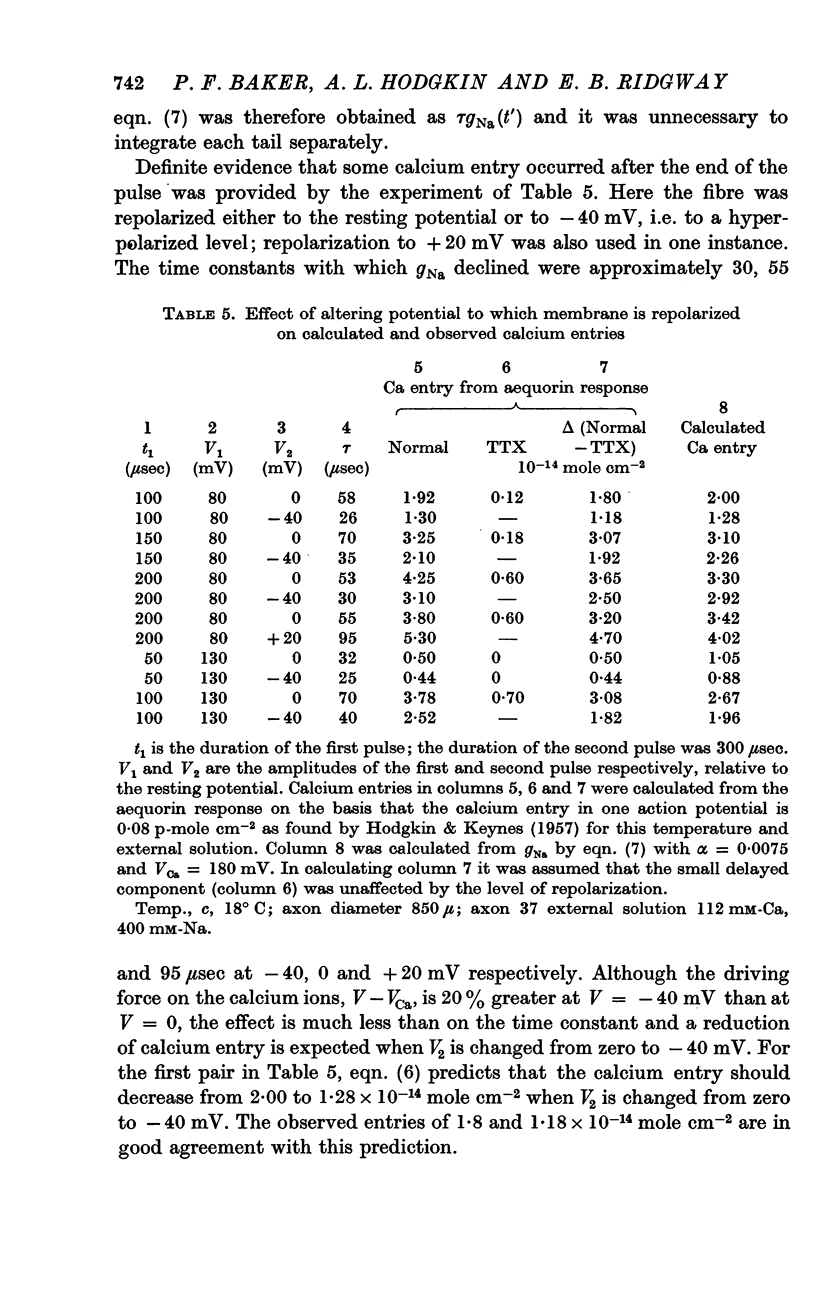
12. The time relations of the early calcium entry were consistent with its being a leak of calcium ions through the sodium channel; the permeability of the sodium channel to calcium was about 1% of the permeability to sodium.
13. The late entry of calcium was little changed by injecting enough tetraethylammonium (TEA) to block the outward potassium current; it was greatly reduced by external concentrations of manganese which had little effect on the maximum potassium conductance.
14. The voltage—response curve for the late entry of calcium had a well defined maximum and was similar in shape to the curve relating calcium entry to depolarization at the presynaptic ending (Katz & Miledi, 1969, 1970).
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C. An estimate of calcium concentration changes during the contraction of single muscle fibres. J Physiol. 1970 Sep;210(2):133P–134P. [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Bezanilla F., Rojas E. Sodium influxes in internally perfused squid giant axon during voltage clamp. J Physiol. 1969 May;201(3):657–664. doi: 10.1113/jphysiol.1969.sp008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. The early phase of calcium entry in giant axons of Loligo. J Physiol. 1971;214 (Suppl):33P–34P. [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Two phases of calcium entry during the action potential in giant axons of Loligo. J Physiol. 1970 Jun;208(2):80P–82P. [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Phasic entry of calcium in response to depolarization of giant axons of Loligo forbesi. J Physiol. 1971 Jul;216(2):70P–71P. [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W., Mitchell G., Mattingly P. H., Blinks J. R., Van Leeuwen M. Response of aequorin bioluminescence to rapid changes in calcium concentration. Nature. 1969 Jun 14;222(5198):1047–1050. doi: 10.1038/2221047a0. [DOI] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The intracellular calcium contents of some invertebrate nerves. J Physiol. 1956 Nov 28;134(2):399–407. doi: 10.1113/jphysiol.1956.sp005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Lucas K. On a mechanical method of correcting photographic records obtained from the capillary electrometer. J Physiol. 1912 May 6;44(3):225–242. doi: 10.1113/jphysiol.1912.sp001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Ridgway E. B., Ashley C. C. Calcium transients in single muscle fibers. Biochem Biophys Res Commun. 1967 Oct 26;29(2):229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Calcium binding, quantum yield, and emitting molecule in aequorin bioluminescence. Nature. 1970 Sep 26;227(5265):1356–1357. doi: 10.1038/2271356a0. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Properties of the bioluminescent protein aequorin. Biochemistry. 1969 Oct;8(10):3991–3997. doi: 10.1021/bi00838a015. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Saiga Y. Microdetermination of Calcium by Aequorin Luminescence. Science. 1963 Jun 21;140(3573):1339–1340. doi: 10.1126/science.140.3573.1339. [DOI] [PubMed] [Google Scholar]


