Abstract
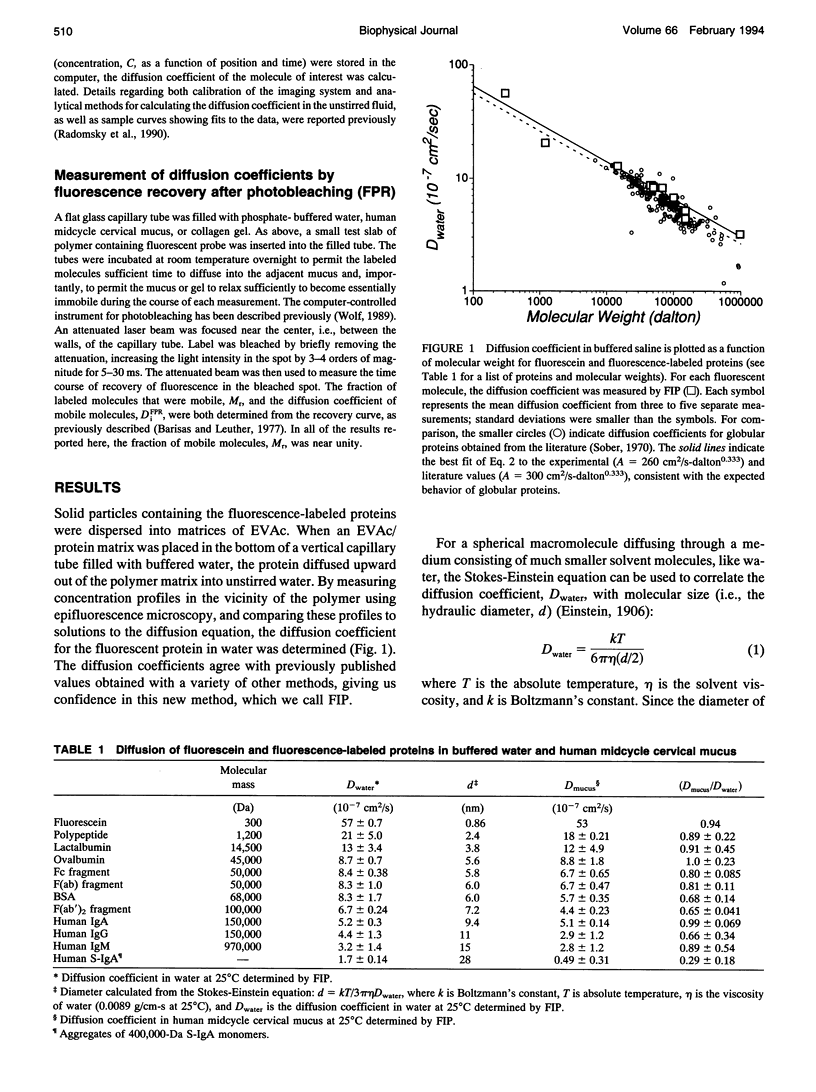
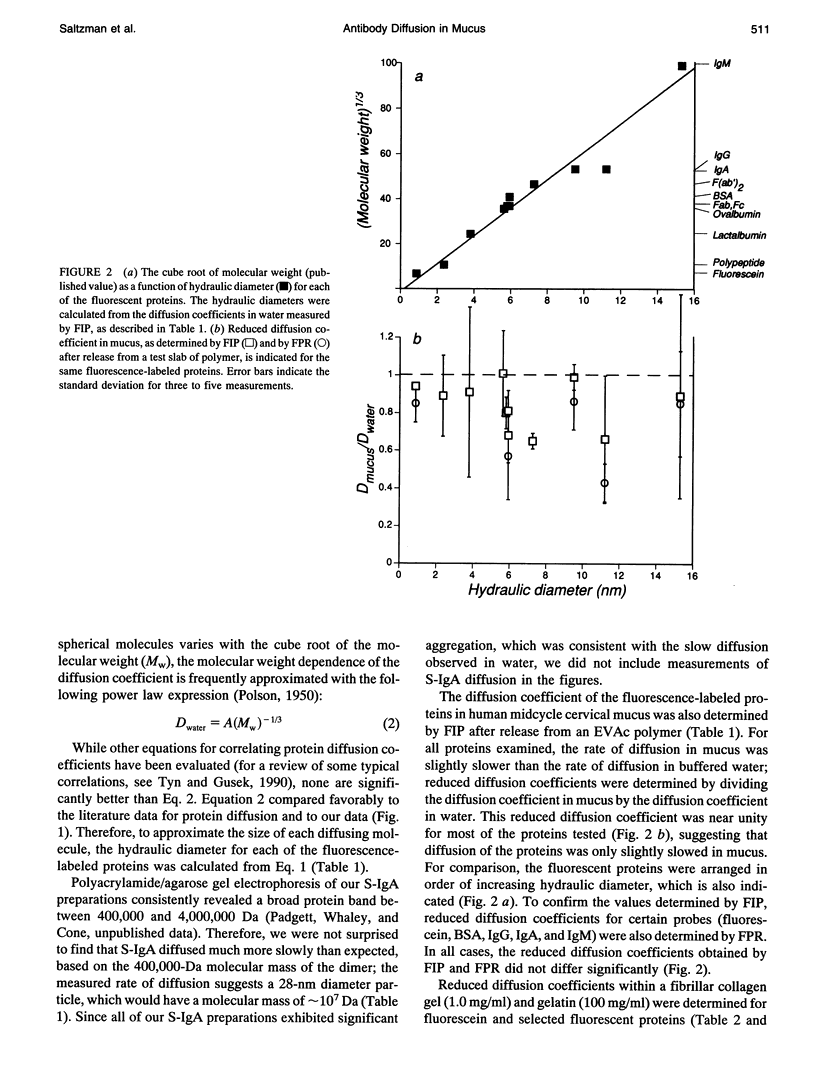
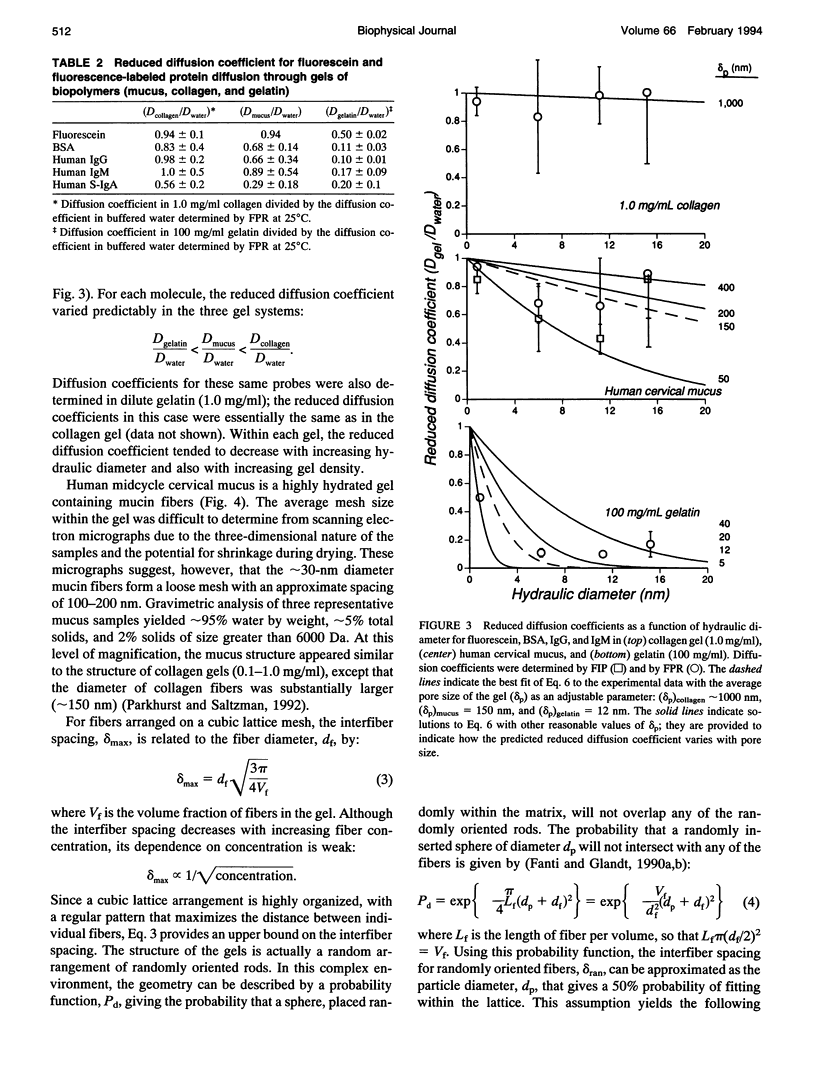
The mucosal immune system actively transports large quantities of antibodies into all mucus secretions, and these secreted antibodies help prevent infectious entry of many pathogens. Mucus is generally thought to protect epithelial cells by forming a diffusional barrier through which only small molecules can pass. However, electron microscopy indicates that the pore size in mucus is approximately 100 nm, which suggests that antibodies as well as other large molecules might also diffuse through mucus. We measured the diffusion coefficients for antibodies and other proteins within human midcycle cervical mucus using two techniques: fluorescence imaging of concentration profiles and fluorescence photobleaching recovery. The two techniques are complementary, since the rates of diffusion are observed over millimeter distances with fluorescence imaging of concentration profiles and micron distances with fluorescence photobleaching recovery. Both methods yielded essentially the same diffusion coefficients. In contrast to previous reports indicating mucus significantly impedes diffusion of small molecules, antibody diffusion in mucus was relatively unimpeded. In our observations IgG, IgG fragments, IgA, and IgM diffused almost as rapidly in cervical mucus as in water (1.0 > Dmucus/Dwater > 0.7). Simple models for diffusion through water-filled pores suggest that the hydrodynamic pore size for cervical mucus is approximately 100 nm, smaller than the approximately 1000 nm pore size of a collagen gel (at 1 mg/ml) and larger than the approximately 10 nm pore size of gelatin (at 100 mg/ml). This estimated pore size is consistent both with electron micrographs and geometric models of interfiber spacing. Based on these results, we predict that particles as large as viruses can diffuse rapidly through human midcycle cervical mucus, provided the particle forms no adhesive interactions with mucus glycoproteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. L., Quinn J. A. Restricted transport in small pores. A model for steric exclusion and hindered particle motion. Biophys J. 1974 Feb;14(2):130–150. doi: 10.1016/S0006-3495(74)70005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisas B. G., Leuther M. D. Fluorescence photobleaching recovery measurement of protein absolute diffusion constants. Biophys Chem. 1979 Sep;10(2):221–229. doi: 10.1016/0301-4622(79)85044-9. [DOI] [PubMed] [Google Scholar]
- Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesbrock A. R., Reddy M. S., Levine M. J. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991 Oct;59(10):3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M. A., Jain R. K. Interstitial transport of rabbit and sheep antibodies in normal and neoplastic tissues. Cancer Res. 1990 Jun 15;50(12):3487–3492. [PubMed] [Google Scholar]
- Desai M. A., Mutlu M., Vadgama P. A study of macromolecular diffusion through native porcine mucus. Experientia. 1992 Jan 15;48(1):22–26. doi: 10.1007/BF01923598. [DOI] [PubMed] [Google Scholar]
- Desai M. A., Vadgama P. Estimation of effective diffusion coefficients of model solutes through gastric mucus: assessment of a diffusion chamber technique based on spectrophotometric analysis. Analyst. 1991 Nov;116(11):1113–1116. doi: 10.1039/an9911601113. [DOI] [PubMed] [Google Scholar]
- Henry B. T., Adler J., Hibberd S., Cheema M. S., Davis S. S., Rogers T. G. Epi-fluorescence microscopy and image analysis used to measure diffusion coefficients in gel systems. J Pharm Pharmacol. 1992 Jul;44(7):543–549. doi: 10.1111/j.2042-7158.1992.tb05461.x. [DOI] [PubMed] [Google Scholar]
- Lamont J. T. Mucus: the front line of intestinal mucosal defense. Ann N Y Acad Sci. 1992;664:190–201. doi: 10.1111/j.1749-6632.1992.tb39760.x. [DOI] [PubMed] [Google Scholar]
- Magnusson K. E., Stjernström I. Mucosal barrier mechanisms. Interplay between secretory IgA (SIgA), IgG and mucins on the surface properties and association of salmonellae with intestine and granulocytes. Immunology. 1982 Feb;45(2):239–248. [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Burr D. H., Walker R. I. Intestinal mucus gel and secretory antibody are barriers to Campylobacter jejuni adherence to INT 407 cells. Infect Immun. 1987 Jun;55(6):1431–1435. doi: 10.1128/iai.55.6.1431-1435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge A. C., Protzman W. P. Origin of the antigens in rabbit semen which induce antifertility antibodies. J Reprod Fertil. 1967 Feb;13(1):31–40. doi: 10.1530/jrf.0.0130031. [DOI] [PubMed] [Google Scholar]
- Naz R. K., Saxe J. M., Menge A. C. Inhibition of fertility in rabbits by monoclonal antibodies against sperm. Biol Reprod. 1983 Feb;28(1):249–254. doi: 10.1095/biolreprod28.1.249. [DOI] [PubMed] [Google Scholar]
- O'Rand M. G. Inhibition of fertility and sperm-zona binding by antiserum to the rabbit sperm membrane autoantigen RSA-1. Biol Reprod. 1981 Oct;25(3):621–628. doi: 10.1095/biolreprod25.3.621. [DOI] [PubMed] [Google Scholar]
- Parkhurst M. R., Saltzman W. M. Quantification of human neutrophil motility in three-dimensional collagen gels. Effect of collagen concentration. Biophys J. 1992 Feb;61(2):306–315. doi: 10.1016/S0006-3495(92)81838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Radomsky M. L., Whaley K. J., Cone R. A., Saltzman W. M. Controlled vaginal delivery of antibodies in the mouse. Biol Reprod. 1992 Jul;47(1):133–140. doi: 10.1095/biolreprod47.1.133. [DOI] [PubMed] [Google Scholar]
- Radomsky M. L., Whaley K. J., Cone R. A., Saltzman W. M. Macromolecules released from polymers: diffusion into unstirred fluids. Biomaterials. 1990 Nov;11(9):619–624. doi: 10.1016/0142-9612(90)90018-l. [DOI] [PubMed] [Google Scholar]
- Rhine W. D., Hsieh D. S., Langer R. Polymers for sustained macromolecule release: procedures to fabricate reproducible delivery systems and control release kinetics. J Pharm Sci. 1980 May;69(3):265–270. doi: 10.1002/jps.2600690305. [DOI] [PubMed] [Google Scholar]
- Saltzman W. M., Langer R. Transport rates of proteins in porous materials with known microgeometry. Biophys J. 1989 Jan;55(1):163–171. doi: 10.1016/S0006-3495(89)82788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W. M., Parkhurst M. R., Parsons-Wingerter P., Zhu W. H. Three-dimensional cell cultures mimic tissues. Ann N Y Acad Sci. 1992 Oct 13;665:259–273. doi: 10.1111/j.1749-6632.1992.tb42590.x. [DOI] [PubMed] [Google Scholar]
- Sherwood J. K., Dause R. B., Saltzman W. M. Controlled antibody delivery systems. Biotechnology (N Y) 1992 Nov;10(11):1446–1449. doi: 10.1038/nbt1192-1446. [DOI] [PubMed] [Google Scholar]
- Smithson K. W., Millar D. B., Jacobs L. R., Gray G. M. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981 Dec 11;214(4526):1241–1244. doi: 10.1126/science.7302593. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Losonsky G., Link H., Hoang Y., Guesry P., Hilpert H., Levine M. M. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N Engl J Med. 1988 May 12;318(19):1240–1243. doi: 10.1056/NEJM198805123181904. [DOI] [PubMed] [Google Scholar]
- Tse S. K., Chadee K. The interaction between intestinal mucus glycoproteins and enteric infections. Parasitol Today. 1991 Jul;7(7):163–172. doi: 10.1016/0169-4758(91)90121-4. [DOI] [PubMed] [Google Scholar]
- Wolf D. E. Designing, building, and using a fluorescence recovery after photobleaching instrument. Methods Cell Biol. 1989;30:271–306. doi: 10.1016/s0091-679x(08)60983-8. [DOI] [PubMed] [Google Scholar]
- Yudin A. I., Hanson F. W., Katz D. F. Human cervical mucus and its interaction with sperm: a fine-structural view. Biol Reprod. 1989 Mar;40(3):661–671. doi: 10.1095/biolreprod40.3.661. [DOI] [PubMed] [Google Scholar]



