Abstract
Malaria is among the oldest of diseases. In one form or another, it has infected and affected our ancestors since long before the origin of the human line. During our recent evolution, its influence has probably been greater than that of any other infectious agent. Here we attempt to trace the forms and impacts of malaria from a distant past through historical times to the present. In the last sections, we review the current burdens of malaria across the world and discuss present-day approaches to its management. Only by following, or attempting to follow, malaria throughout its evolution and history can we understand its character and so be better prepared for our future management of this ancient ill.
INTRODUCTION
This article is about the nature and impact of malaria; it is not a review of scientific research on malaria. It explores how, when, and where malaria parasites, their mosquito vectors, and humans, may have interacted and with what effect on ourselves, their human hosts. This is not a straightforward task. It is hampered by difficulties of collecting appropriate and reliable data and by problems of their interpretation. Inferences or conclusions may vary from well-founded and widely accepted to tentative or controversial. But, while the picture remains blurred in a number of places, the many faces and features of malaria and its imprint on the human species emerge, usually clearly and unmistakably.
Many published discussions have dealt with the experiences of malaria, its nature, and its effects (see, e.g., references 5, 28, 29, 31, 35, 42-44, 47, 73, 91, 95, 106, 111, 112, 117, 123, 124, 139, 141, 165, 167, 173, 176, 192, 195, 200). They are informative and revealing, especially because each is a product of the medical and human health context, general outlook, and knowledge base of its time. Our present times also offer a unique perspective on this subject. It is only within the past half century that we have experienced malaria against a background of human health and health services which are, for the most part, vastly better than in all previous generations. In the same period, we have witnessed both the achievements of the first globally coordinated health delivery programs and also their problems. Within recent decades, and especially the last, biochemical and molecular genetic technologies to investigate distant events in the evolution and coevolution of malaria parasites and humans have become available.
The following, therefore, is a brief reconstruction of the evolution and history of malaria and its burdens as we may perceive them at the start of the 21st century.
MALARIA PARASITES OF HUMANS
Malaria is due to blood infection by protozoan parasites of the genus Plasmodium, which are transmitted from one human to another by female Anopheles mosquitoes. Four species of malaria parasite infect humans (Table 1). The two which almost certainly achieved the widest global distribution are Plasmodium vivax and Plasmodium malariae. To the Europeans, these have been known and characterized since historically ancient times (5, 29, 95, 106) as the “benign tertian” (P. vivax) and “quartan” (P. malariae) periodic fevers. “Benign tertian” fevers were so named because they were not associated with the severe and often fatal manifestations of the “subtertian, malignant” periodic fevers (P. falciparum). “Tertian” and “quartan” refers to their characteristic feature of an acute febrile episode, or paroxysm, that returns respectively every third (P. vivax) or fourth (P. malariae) day. Tertian and quartan fevers are referred to with similar frequency in writings from northern Europe through much of the past millennium and from around the shores of the Mediterranean Sea from about the 5th century B.C. onward (29, 47, 95, 106).
TABLE 1.
Some representative distributions of the four recognized species of malaria parasites of humans in the world today
| Species | Distribution of species (%) in following area (total no. of cases):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Sub-Saharan Africaa
|
Asiaa (all) (863) | South Central Asiab and Middle East (14,539,081) | Western Pacificb and Southeast Asia (86,461,294) | Western Pacificc (Vanuatu) (1,708) | Central America and Caribbeand (178,242) | South Americaa (859,480) | ||
| West and Central (858) | East and Southern (297) | |||||||
| P. falciparum | 88.2 | 78.8 | 4.2 | 19.8 | 51.4 | 43.0 | 12.9 | 29.2 |
| P. vivax | 1.2 | 9.8 | 95.6 | 80.2e | 48.6e | 56.1 | 87.1 | 70.6 |
| P. malariae | 2.2 | 3.0 | 0.0 | 0.9 | 0.0 | 0.2 | ||
| P. ovale | 8.4 | 8.4 | 0.2 | 0.0 | 0.0 | 0.0 | ||
Data courtesy of the United Kingdom Malaria Reference Laboratory; obtained from cases of malaria diagnosed in travelers returning to the United Kingdom, 1 January to 31 December 1996.
Data courtesy of World Health Organization Regional Offices 1993 to 1996 (unpublished data) analyzed for The World Health Report, 1999 (210).
Data from reference 107; obtained from cases of malaria diagnosed in Vanuatu from 1988 to 1992.
Data courtesy of the Pan American Health Organization, Status of Malaria Programmes in the Americas XLVI Report, 1998; obtained from cases of malaria diagnosed and reported by countries in Central and South America for 1996 or 1997.
Data include all three species P. vivax, P. malariae, and P. ovale.
Today, P. malariae has lost whatever predominance it may once have had and P. vivax and P. falciparum are the most commonly encountered malaria parasites (Table 1). P. vivax is still found sporadically in some temperate regions, where in the past it was widely prevalent. It remains, however, very common throughout much of the tropics and subtropics. Because of the temperature limitations on its transmission by its mosquito vectors, P. falciparum is normally present only in tropical, subtropical, and warm temperate regions. In the tropics today, P. falciparum remains widely prevalent.
The fourth human malaria parasite is Plasmodium ovale, which, like P. vivax, is the agent of a tertian malaria and which, also like P. vivax malaria today, carries a very low risk of fatal outcome. P. ovale has the most limited distribution of all the malaria parasites of humans. While it is prevalent throughout most of sub-Saharan Africa, it is otherwise known to be endemic only in New Guinea and the Philippines (122).
EPIDEMIOLOGY OF THE BURDEN OF HUMAN MALARIA
Before we attempt to trace the passage and effects of malaria through evolutionary and historical time, we will review the principles which determine how malaria affects individuals and populations.
Disease States of Malaria
The experience of malarial infection has many different forms, which we touch on here in outline. The diseases due to all four species of malaria parasite share the characteristic febrile episodes with their tendency to regular periodic paroxyms with chills, rigors, and sweating. They also have many symptoms in common with other infectious illnesses, including body aches, headache and nausea, general weakness, and prostration. Untreated infections of malaria are characterized by enlargement of the spleen. In P. falciparum malaria, severe and life-threatening conditions commonly arise. These cause dysfunction of vital organs, i.e., the lungs, kidneys, liver, and, most famously, the brain during “cerebral malaria.” Severe anaemia can occur. These are the conditions which are associated with most of the mortality of acute malaria. Chronic infection with P. malariae can result in a nephrotic syndrome, and this, too, can eventually be fatal.
Repeated attacks of malaria due to any species of the parasites over several to many years severely debilitate body and mind. Cachexia, a wasting of body tissues, takes place, and splenic enlargement becomes a constant feature. Lethargic and with sunken and sallow features, spindly limbs, and hard swollen belly is the general description of the condition. In this state the affected individual succumbs to diseases or other hardships that would scarcely threaten a person in reasonable health. Under the burden of chronic malaria, both the quality and duration of life are greatly reduced.
An individual's experience of malaria at a particular time is, however, strongly governed by the type and degree of antimalarial immunity that he or she may have attained.
Immunity to Malaria
Protective immunity against malaria can be thought of in different categories. There are two types of clinical immunity, one which reduces the risk of death from malaria and another which reduces the intensity of clinical symptoms. A third type of protective antimalarial immunity is antiparasitic immunity, which directly reduces the numbers of parasites in an infected individual. These are epidemiological definitions of immunity. The actual cellular and molecular mechanisms of immunity may overlap to a considerable degree between these categories. The number of malarial inoculations experienced, and the intervals between them, are all-important to the malaria immune status of an individual. In the case of acute attacks of P. falciparum malaria, it is possible that a degree of immunity to some aspects of severe, life-threatening disease may be achieved after only one or two infections (82). However, clinical immunity to other, non-life-threatening clinical effects of malaria requires more and frequent inoculations of malaria (34, 191). Effective antiparasitic immunity is achieved only after very many and frequent infections (101, 124).
Two other forms of acquired immunity to malaria should be mentioned here. One is immunity which could protect against the parasites ever becoming established in a human host, in other words immunity against the stages which are introduced into the human body by a mosquito bite. These are the sporozoites and also the early, nonpathogenic stages as they develop in the liver. While immunity against sporozoites and liver stages may eventually develop following long exposure to intense malaria transmission, it is probably not very significant in the crucial early years of exposure. The other form of nonprotective immunity against malaria parasites is that against the sexual blood stages of the parasites. These are the stages which are infective to mosquitoes. Antibodies against antigens in gametocytes and gametes of malaria parasites develop readily during the course of natural infections and probably have some effect on the transmission dynamics of malaria in certain endemic situations. Anti-sexual-stage immunity, however, has no direct relevance to the status of an individual with respect to his or her experience of a current malarial infection.
The attainment of protective immunity against the disease-causing asexual blood stages of malaria parasites is a complex process. Not only is such immunity specific to each parasites species, but also there is certainly a major element of parasite “strain” specificity to antimalarial immunity (105, 130, 148, 182). Much of the slow acquisition of antiparasitic immunity occurs because an individual is usually subjected to a succession of inoculations of parasites which are genetically and antigenically distinct from each other. Therefore, to combat each infection, a human host must mount a new and “strain”-specific immune response to each antigenically distinct parasite inoculum. Only when a sufficiently wide spectrum of such parasite “strains” has been experienced is effective immunity achieved against all the parasites within a locality where infection is endemic.
However, not only are the parasites in different inoculations genetically different but also, during the course of each infection, the parasites from a single inoculation undergo clonal “antigenic variation” (130). To control an infection, the host must mount a new specific immune response to each new antigenic variant as it arises. Through this process, a single malarial infection can be prolonged over many months to years.
Because of the time taken to achieve effective immunity to malaria under conditions of endemic infection, antimalarial immunity is often said to be “age dependent.” In the sense intended, however, it would be more accurate to say that it is “duration of exposure dependent.” There are, nevertheless, truly age-dependent aspects both to the attainment of immunity and to the pathologic responses to malarial infection. Very young children appear to have a poor capacity to acquire effective protective antimalarial immunity of any sort, while older children and adults may so do more readily (9, 10). Infants and the very young are more prone to malarial anaemia, while cerebral damage due to P. falciparum malaria predominates in slightly older children. Yet other severe conditions, including renal, hepatic, and pulmonary failure, are most commonly seen in adults (6).
Every individual exposed to endemic malaria thus faces a long and dangerous battle to achieve protective immunity against the diversity of malaria parasites and their antigens to which he or she may become exposed. However, having been achieved at such cost, effective immunity is readily lost again. An interval of perhaps half a year to a year without reinfection appears to be sufficient to leave an individual vulnerable, once more, to the full impact of a malarial infection (158, 182, 186; A. Lukas, personal communication). Direct evidence for this statement is admittedly hard to find, yet it has long been attested by anecdote and expert opinion. Loss of protective immunity within a few months without reinfection also appears to hold in relation to infection-induced protective immunity against malaria in animals (R. Carter, unpublished observations).
If the above statement is indeed correct, then the number, type, and pattern of delivery of malarial inoculations, in other words the type of malarial endemicity, to which an individual is exposed must profoundly affect his or her immune status and hence disease status, as is now discussed.
Malarial Endemicity
Following the approach of MacDonald (124), malarial endemicity may be categorized into three types (Table 2). One is stable endemic malaria. Stable malaria occurs when a population is continuously exposed to a fairly constant rate of malarial inoculation. The next category is unstable endemic malaria, under which a population is subjected to more or less permanent malaria transmission but under circumstances in which there are large fluctuations in the rates at which malarial inoculations are delivered to individuals within the population. In terms of disease and immunity, these fluctuations become especially significant when they cause individuals to experience intervals of a year to several years between inoculations of malaria. The third type is epidemic malaria. This is, in effect, an extreme form of unstable malaria. It occurs when a population, or even a small group of individuals, is subjected to an increase in malaria transmission rates above that previously or normally experienced. When P. falcaparum is involved, malaria epidemics can be among the most lethal forces of nature.
TABLE 2.
Characteristics of the three categories of malaria transmission
| Type of malaria | Geographical location(s)a | Malaria inoculation rates | Protective immunity in the population | Transmission characteristics |
|---|---|---|---|---|
| Stable malaria | Sub-Saharan Africa | Regular, low to very high | High in older age groups; low in children under 5 years | Perennial or seasonal; regular contact between vectors and human hosts |
| Unstable malaria | (Europe) and Mediterranean, Asia and Western Pacific, (North), Central and South America and Caribbean | Irregular, low to medium | Unreliable in older age groups; absent in children under 5 year old | Perennial or seasonal; irregular contact between vectors and human hosts |
| Epidemic malaria | Highland areas of tropical Africa; Central Asia and Caucuses; Asia and Latin America | Rising suddenly, low to medium | Low or absent in all age groups | Very variable, subject to sudden and rapid change |
Malaria is not at present endemic in the geographical locations shown in parenthesis.
It should be noted that the mean rates of malarial inoculation or transmission intensity are not included in the definitions of any of these three types of malarial endemicity. High or low transmission rates can, in principle, occur for stable, unstable, and epidemic malaria (124) (Table 2). Nevertheless, the highest natural malaria inoculation rates, those of hundreds of infectious bites per individual per year, probably occur only under stable endemic conditions. Inoculation rates of as few as one or two infectious bites per year are characteristic of unstable conditions but can also be encountered under conditions of stable endemic malaria (90). Malaria epidemics can, and probably often do, occur under conditions of relatively low to moderate malarial inoculation rates (124).
How Endemicity and Immunity Interact To Determine the Effects of Malaria
Stable malaria.
Under conditions of stable malaria, because of the regularly delivered inoculations, a strong protective immunity against overt illness and risk of death from malaria is acquired, usually by the age of 4 or 5 years. Before this age there is much morbidity and mortality, especially in the presence of P. falciparum malaria. Historically, and still today, such conditions have prevailed mainly in sub-Saharan Africa. The following quotation is characteristic of the historical picture of malaria in tropical Africa. “In East Africa 50% of native children die before the age of 4 years, mostly from malaria” (Brumpt, 1922, quoted in reference 176).
The so-called age-dependent pattern of immunity to malaria in sub-Saharan Africa is often attributed to the intense malaria transmission rates in this region. However, we suggest that it is the stability of malaria transmission in tropical Africa, due to biological features of the Anopheles gambiae group of vectors of malaria, their ecology, and their environment (discussed below), that is the more important feature of African malaria transmission. Because, individuals rarely go more than a few months without a malaria challenge under conditions of stable transmission, there is little risk that immunity, once attained, will be lost again. This remains true even in the setting of quite low annual malarial inoculation rates (128). There is, nevertheless, a clear shift from severe malarial disease in younger children (younger than 5 years) toward relatively increased rates of both mild (191) and severe (179) disease in older age groups at entomological malarial inoculation rates probably below about 10 to 20 infectious bites per year. However, under conditions of stable malaria in Africa, the lifetime risk of severe disease, and hence of direct malaria mortality, due to P. falciparum seems to remain fairly constant across the spectrum of inoculation rates from very high to very low (179).
Unstable and epidemic malaria.
By contrast, where transmission conditions are unstable, low to moderate mean malarial inoculation rates can be highly dangerous. This is because immunity to malaria, while slowly gained, is, as we have suggested, rapidly lost following perhaps half a year to a year without infection. Wherever the rate of delivery of malarial inoculations is both low and highly erratic, extended periods of a year or so without reinfection occur often, while at the same time the risk of eventual reinfection remains high. In these circumstances, individuals are vulnerable throughout life to clinically active malarial infection and are often affected by it. Furthermore, all species of malaria parasite, and not just P. falciparum, become dangerous under these conditions. Recurrent infections with any species of malaria parasite are eventually so debilitating that life expectancy can be reduced to half or less of that in a contemporary malaria-free and otherwise salubrious environment (47, 123). The situation is illustrated by the following quotation which refers to malarious districts around the shores of the Mediterranean in the early 19th century. “The most fertile portions of (Italy) are a prey to (malaria); the labourer wanders… the ghost of a man, a sufferer from his cradle to his grave; aged even in childhood, and laying down in misery that (brief) life which was but one disease… . Such also is Sicily, such Sardinia and such is classic Greece. To live a living death and to be cut off from even half that life” (123).
Wherever antimalarial immunity has declined, has been lost, or was never achieved in the first place, populations at the margins of malaria transmission zones are vulnerable to epidemic malaria. When P. falciparum is involved in an epidemic, death rates can become very high. A single untreated attack of P. falciparum malaria in a nonimmune individual carries a risk of death that may be anywhere from a few percent to at least 20 to 30% according to circumstances (3, 12, 21). These higher rates are the kinds of mortality rate that occur during P. falciparum malaria epidemics (30, 45a, 49, 67, 75, 219). The following quotation (67) is representative of these events. “In each house were… three or four patients who complained of chilling, severe headaches, sweating, pain in back and extremities… After four or five relapses, the headaches and pain became unbearable for many patients who then exhibited a muddling delirium with coma, ending in death. Most… were between the ages of 5 and 20 years. Since they are far away from even the simplest clinic, which means no possibility of saving their lives, they are dying like bees in a smoked hive” (from a Field Report from the 1958 malaria epidemic in Ethiopia [67]). In a season, this very typical malaria epidemic produced about 3.5 million cases of malaria among which both P. vivax and P. falciparum malaria would have been present; it took about 150,000 lives.
Similar and even higher malaria mortality rates were experienced by Europeans entering tropical regions before the introduction of quinine in the mid-19th century (20, 42-44, 156, 197). Indeed, the consequence of the introduction of nonimmune persons into a malaria-endemic region is, in effect, a form of epidemic malaria.
Biological Basis of Stable, Unstable, and Epidemic Malaria
The differences in stability of malaria transmission, notably between tropical Africa and most other malarious regions, are due largely to the behaviors and other biological characteristics of the regional species and subspecies of Anopheles vectors and to their environment. The strong human-biting preferences and highly domestic habits of the tropical African vectors (17, 39) lead to very uniform contact between them and the human blood source in sub-Saharan Africa. The climatic conditions are also highly conducive to malaria transmission, being warm and humid with relatively few fluctuations. This supports longevity of the vector mosquitoes and rapid development of the parasites within them. All of these features combine to a recipe for stable and, indeed, generally intense malaria transmission.
Elsewhere, in the tropical, subtropical, and temperate worlds, the females of most Anopheles species have a preference for animal rather than human contact and have less domestic breeding, resting, and dispersal habits than the African vectors. The climates and especially the microclimates experienced by these vector mosquitoes are usually cooler, drier, and more variable than in tropical Africa, leading to more uncertain survival of the mosquitoes and less reliable development of the parasites within them. These are conditions that lead to highly erratic contact between potential vectors of human malaria and the human hosts and to very irregular delivery of malaria inoculations, in other words, to unstable malaria transmission.
Epidemic malaria arises in any situation in which new or elevated malaria transmission capacity is suddenly introduced into a population with inadequate levels of immunity with which to absorb it. The circumstances under which this can occur are extremely diverse (192). Typically they involve nonimmune populations in otherwise malaria-free locations adjacent to regions of endemic malaria. An epidemic occurs when atypical weather, e.g., drought, excess rainfall, and higher than usual temperatures, create conditions that transiently support malaria transmission. However, warfare, pioneering, and malaria control, among other human activities, have all been responsible for the creation of malaria epidemics.
Indirect Mortality of Malaria
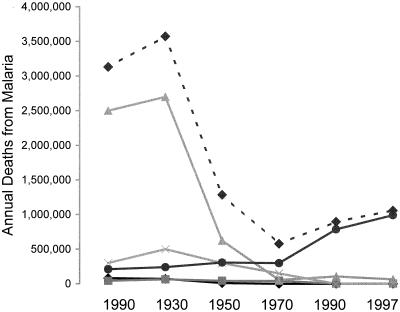
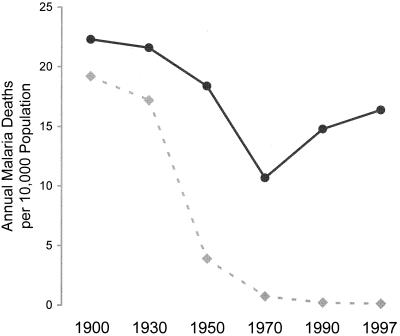
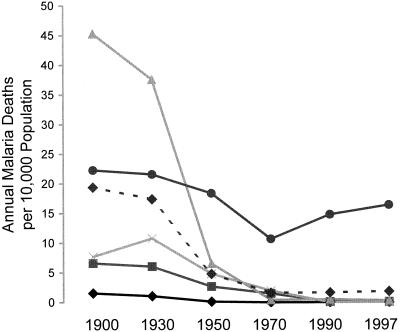
Under whatever condition of endemicity malaria occurs, much, and according to the evidence of a number of analyses, generally most (74, 134, 142, 219), of the malaria-related mortality has in the past been an indirect result of the effects of malarial infection combined with other infections and conditions. Wherever interventions have been conducted for the reduction of malaria transmission, reductions in total mortality rates have been several times greater than the malaria-related death rates as estimated prior to the interventions (3, 134). Some of this discrepancy may be due to failures to detect or account for all the deaths in which malaria would have been an apparent cause. However, most of the excess deaths associated with the presence of malaria, but not obviously due to malaria, can be attributed to delayed or indirect causes. These could include the nephropathy of long-term P. malariae infection (74). However, more generally, the deaths are due to the predisposing effects of malaria to death from other conditions such as respiratory infections (74) and malnutrition (219).
Nonlethal Health Consequences of Endemic Malaria
In historical times, and as recently as the early to mid-20th century in southern Asia, continual malarial infection and reinfection had devastating effects on the mental, physical, social, and economic conditions of the individuals and communities affected. In addition to its toll in death and general morbidity, there are several features of the burden of malaria that warrant specific mention.
Malaria presents particular problems during pregnancy. This is probably due, at least in part, to the “immunosuppressed” status of the pregnant woman. Even in otherwise highly malaria-immune women, the risk of malarial infection in pregnancy is high, with increased risk of low birth weight, miscarriage, infant mortality (14), and morbidity and mortality in the pregnant woman. “Malaria exerts a considerable influence in reducing the births and increasing the number of still births in a community” (Bentley, 1911, quoted in reference 176).
Historically, “infantilism” in both sexes and impotence in males were associated with malarious areas (5). “One of the great evils is the impotency so commonly found in waterlogged villages (and) which results from the deterioration of the health produced by constant attacks of fever and the presence of an enlarged spleen” (Dyson, 1895, quoted in reference 176). Together with the problems of malaria in pregnancy and infant mortality, these added up to a large reduction in the fecundity of a population and, in the worst-affected locations, contributed greatly to depopulation under the impact of malaria. “In hyperendemic and severely endemic (for malaria) districts in India the tendency is for the population to decline” (91).
Among the most oppressive and, today, least appreciated of the effects of malaria is that on the mental state of the sufferer. Observers from many different times and places, including Hippocrates (95), Macculloch (123), Jones (106), Anderson (5), and Sinton (176), are remarkably consistent in their portrayals of the condition of the inhabitants of malarious regions especially in Europe or Asia. They depict individuals and communities in states of pronounced mental and psychological distress under the influence of endemic and invariably unstable or low-transmission malaria. The following are typical of these. “Anyone who has observed closely cases of malaria cannot fail to have noticed its effects upon the mentality of the sufferer—mental activity is dulled, irritability of temper is the rule, initiative is lacking, decisions are put off or reached with difficulty, ambition is lost and depression is a prominent symptom” (176); “School children in the Transvaal infected with chronic malaria were mentally classed as “feeble-minded”” (Leipoldt et al., 1921, quoted in reference 176). In today's much healthier world, situations matching their descriptions are conspicuous by their absence. Nevertheless, and not to be confused with the immediate consequences of malarial infection itself on the mental state, a significant proportion of the survivors of malarial infections still carry mental or neurological deficits (97).
Effects of Malaria on Social and Economic Development
An adequate discussion of the economic effects of malaria is beyond the scope of this article. Populations exposed to the unremitting impact of malaria must always have lived and died in destitution of one degree or another. In historical times, economic enterprise has been difficult and often impossible in the presence of malaria (176). Malaria alone has often been sufficient to wreck efforts in pioneering, agriculture, and civil engineering, as illustrated by the following representative quotations. “The hyperendemic areas (for malaria), although sparsely inhabited, are often areas where large plantations and large industrial undertakings are situated and which are, therefore, often the site of a considerable immigrant populations that are quickly mown down.” (Malaria Commission of the League of Nations, 1930, quoted in reference 176); “While there is good land in the Southern United States as in the North, the land in the North sells at about 12 to 20 times the price, the difference being mainly due to malaria” (Carter, 1922, quoted in reference 176); “Railway construction in the tropics is nearly always associated with fulminent epidemics of malaria—“a death a sleeper” is the generalisation on the happenings” (Senior-White, 1928, quoted in reference 176).
Throughout history (176) and to the present day (70), wherever and however it may have manifested itself, malaria has always imposed one of the severest of impediments to social and economic development.
THE “MALARIA HYPOTHESIS” AND RECENT HUMAN EVOLUTION
It can be no surprise, therefore, that malaria parasites should have had a profound impact on recent human evolution (27). This is the proposition of the “malaria hypothesis,” which posits that certain human genetic polymorphisms, especially those affecting red blood cells (RBCs), have been selected to high frequencies because they have protected against the effects of malarial infections. Indeed, a greater number of identified human genetic polymorphisms meet some or all of the expectations of the malaria hypothesis than can be attributed to selection under any other single agent (27).
The first statement of the malaria hypothesis was made in 1948 by J. B. S. Haldane (86), who proposed it as an explanation for the high frequencies of thalassemia around the shores of the Mediterranean Sea, where malaria had long been endemic. Probably unknown to Haldane at the time, a possible association between another inherited hemoglobinopathy, the sickle cell trait, and protection against malaria had already been noted. In 1946, in a study of inpatients at a regional hospital in Northern Rhodesia, now Zambia, E. A. Beet had recorded lower rates of malarial infection among carriers of the sickle cell trait than amongst nonsicklers (11).
In the following, we discuss the evidence for, and implications which arise from, the main candidate human polymorphisms to fall within the terms of the malaria hypothesis.
Thalassemias
The thalassemias are a class of anaemias which are due to abnormalities in the genes coding for hemoglobin. They involve the effective loss of either the alpha (alpha thalassemias) or beta (beta thalassemias) chain of this molecule (92, 185, 198). Many different specific mutations in the genes for the alpha and beta haemoglobin chains are represented among the known thalassemias (65).
In Europe, high frequencies of thalassemias are found around the shores of the Mediterranean Sea, as Haldane had noted (65, 86). Elsewhere, thalassemias occur at elevated frequencies in populations through most of Africa, the Middle East and Central Asia, the Arabian peninsula, the Indian subcontinent, Southeast Asia, southern China, and the islands of the Western Pacific from the Philippines in the north to the Timor Sea in the south and to New Guinea and the islands of Melanesia in the east (27, 65, 119, 198). Up to about 2,000 years ago, these were the probable global limits within which malaria was endemic.
The basis of Haldane's original conjecture (86) has been confirmed and expanded in several studies which show the association of thalassemias with the long-standing exposure of a population to the presence of malaria (see, e.g., references 66 and 215). An approximately 50% reduction in the risk of malarial disease has recently been shown for both heretozygotes and homozygotes for certain alpha+ thalassemias (1).
Glucose-6-Phosphate Dehydrogenase Deficiency
Of broadly similar global distribution to the thalassemias are a group of mutations in the sex-linked gene for glucose-6-phosphate dehydrogenase (G6PD) which give rise to the G6PD deficiencies (119). G6PD deficiencies were originally recognized as a clinical condition known as favism, so called because of the hemolytic crisis that can be caused in those affected, by the consumption of oxidant foods such as fava beans. Today, G6PD deficiency is a recognised hazard in malarious regions because of the associated risk of oxidant stress from taking antimalarial drugs such as primaquine. In heterozygous and hemizygous combinations, G6PD deficiency has now been shown to be associated with a level of protection of about 50% against severe P. falciparum malaria (76, 168). Therefore, high rates of G6PD deficiency in many parts of the world can probably be accounted for as the result of selection by malaria.
Recent evidence indicates that the alleles for G6PD deficiency were selected in African populations between 4,000 and 12,000 years ago (188). This period coincides with some estimates for the time of emergence of P. falciparum as a major human pathogen at around 4,000 years ago (159, 194). Equally, however, the selection for G6PD deficiency may have preceded this event and overlapped a time when P. vivax malaria could still have been a major selective force in Africa (see below). So which parasite could have been responsible for the selection of G6PD deficiency in the African populations?
It may often appear that the malaria hypothesis should be taken to apply mainly, or solely, to P. falciparum. However, as we shall further explore, the hypothesis is equally valid for P. vivax and perhaps for other species of human malaria parasites as well. That P. vivax malaria may represent a force for the selection of G6PD deficiency is consistent with the presence of elevated frequencies of G6PD deficiency in a population in northern Holland (27), where P. vivax malaria, but almost certainly not P. falciparum malaria, was prevalent for at least 500 years. We suggest that either or both of P. falciparum and P. vivax could have supplied the force that first selected for G6PD deficiency in African populations.
Sickle Cell Trait
Neither the thalassemias nor G6PD deficiency is highly protective against death from P. falciparum malaria, with each appearing to reduce the risk by about 50%. There are, however, human mutations which give at least 90% protection against the mortality of malarial infection. These include the gene for sickle cell hemoglobin, or hemoglobin S. The possible protective effects of the sickle cell trait against P. falciparum malaria were, as mentioned above, first noted by Beet in 1946 (11). They were next extensively explored clinically and epidemiologically by Allison (4) and subsequently by many others.
The sickle cell trait is due to a single point mutation in the gene for the beta chain of hemoglobin in which the glutamate at position 6 is replaced with a valine (119). It results in a hemoglobin molecule, hemoglobin S, which, in the homozygote, gives rise to sickle cell anemia. Today in the United States, such individuals commonly reach middle adulthood (198). In the premodern world, however, those affected with sickle cell disease would probably rarely have survived to puberty (4). In reproductive terms, the cost to a hemoglobin S homozygote would have been a loss of fitness of nearly 100%. Such a mutation could have been sustained only if the heterozygotes, those carrying one hemoglobin S gene and one normal, hemoglobin A, gene, had an advantage great enough to balance the cost of the homozygotes to the population. It has been fully confirmed that this advantage is protection against the risk of death from infection with P. falciparum malaria. Children in West Africa who are heterozygous for the sickle cell gene are at approximately 1/10 of the risk of death from P. falciparum malaria as are children who are homozygous for the normal gene (76, 94).
Hemoglobin S clearly fulfils the conditions of the malaria hypothesis. In many parts of Africa, the frequency of the sickle gene exceeds 30% (4, 27, 119, 120). The reproductive losses which these frequencies impose through homozygosity for this gene affect at least 1 birth in 10. They reflect the cost of endemic P. falciparum malaria to a human population.
Hemoglobin C
The gene for hemoglobin C is allelic with that for sickle cell hemoglobin but codes for a lysine instead of a glutamate (hemoglobin A) or a valine (hemoglobin S) at position 6 in the beta chain of hemoglobin. In the homozygous carrier, hemoglobin C confers a loss of fitness which is much less than that associated with the sickle cell trait (homozygous hemoglobin S), being apparently comparable to that of a mild form of thalassemia (119, 120).
Hemoglobin C is found only within certain West African populations, where the frequency of the allele reaches, and sometimes even exceeds, 10 to 20% (119, 120). There is now little doubt that this polymorphism has been, and indeed is probably still being, selected under the effects of P. falciparum malaria in West Africa (133). However, the nature of the protection by hemoglobin C is very different from that associated with hemoglobin S. In the case of hemoglobin S, the heterozygous state confers a high level of protection against death from P. falciparum malaria. In that of hemoglobin C, similar high levels of protection, greater than 90% against infection and therefore against the risk of death by P. falciparum malaria, are achieved only in the homozygote (133). The protective effects of hemoglobin C in a heterozygous combination are, at about 30%, relatively weak.
Now, the rate of selection of an allele whose advantage is expressed mainly in the homogygous state, as is the case for hemoglobin C, is slow compared to that of an allele, such as that for hemoglobin S, which carries a similar advantage but in a heterozygous combination. This principle is discussed more fully in relation to selection for RBC Duffy negativity (see below). Under the influence of the same selective force, therefore, hemoglobin S would be expected at first to achieve higher frequencies than hemoglobin C. However, because the balancing cost of hemoglobin C is low and that of the allelic hemoglobin S is high, hemoglobin C should eventually replace hemoglobin S in populations exposed to selection by P. falciparum. In West Africa this has clearly not yet happened (4, 119, 120), and it suggests that P. falciparum malaria may have arrived only “recently” within the West African population.
When, therefore, might this “recent” arrival of P. falciparum in West Africa have taken place? The relative fitnesses of the different haemoglobin alleles, A, S, and C, in the West African situation can now be given (76, 94, 133), and the relevant calculations can be made (119). We suspect that they would show that the hemoglobin C allele should approach population equilibrium within several thousand years, and almost certainly within less than tens of thousands of years, of selection under P. falciparum malaria, as, indeed, has already been suggested (119, 120). Therefore, by this line of argument, because hemoglobin C has not yet reached its expected equilibrium frequency, P. falciparum has been a selective force in West Africa for less than this time, i.e., probably for less than a few tens of thousands of years.
Hemoglobin E
Populations with high frequencies of the sickle cell gene, the S allele of hemoglobin, extend throughout all but the southernmost malaria-free tip of Africa, through parts of the southern and eastern shores of the Mediterranean, notably in Greece, across the Arabian peninsula and the Indian subcontinent, and as far as the eastern border of present-day Bangladesh (27, 135). Here the prevalence of hemoglobin S ceases. It is immediately replaced, however, by another mutant of hemoglobin, in which the glutamate of position 26 of the beta chain in haemoglobin A is replaced with a lysine (119, 120). This is the gene for hemoglobin E, and it occurs at high frequencies in populations throughout Southeast Asia.
Hemoglobin E has no obvious clinical effect except in combination with certain thalassemia mutations (65, 198). It does not, therefore, impose severe costs on a carrier population. While there appear to be no reports that directly demonstrate protection by hemoglobin E against P. falciparum, there is evidence suggesting that it may protect against P. vivax infection (157). It has also been reported that hemoglobin E trait patients clear infections of P. falciparum more rapidly than do others during treatment with artemisinin derivatives although not during treatment with other antimalarial drugs (100). The geographical region across which hemoglobin E is prevalent is one of the most malarious, both historically and today, and the burden of malaria in this region has therefore long been one of the heaviest in the world. The gene for hemoglobin E is certainly a good candidate to have been selected under the pressure of malaria in accordance with the malaria hypothesis, possibly under the influence of P. vivax as the principal agent of selection prior to the arrival of P. falciparum within these populations.
Ovalocytosis
Beyond the Indonesian and Malay inhabited archipelagos on the Western Pacific rim lies Melanesia, including the island of New Guinea, the Solomon Islands, and the islands of Vanuatu. Outside of tropical Africa, malaria transmission intensities here include the highest in the world. Among the people of this region there occurs a polymorphism which affects one of the main structural proteins of the RBC, known as band 3 (2). A deletion mutation in the gene for this protein has been identified as being at least one genetic determinant of a condition known as ovalocytosis, so named from the abnormal shape of the affected RBCs in the heterozygote (104). Since, as it appears, no homozygotes for this band 3 gene deletion are born (72), the homozygous condition is probably uniformly lethal in utero. The reproductive cost to a homozygote for the ovalocytosis mutation would appear, therefore, to be 100%.
The malaria hypothesis almost certainly explains the high frequency of the ovalocytosis mutation in these populations. Neither the sickle cell gene nor hemoglobin E appears to be present among Melanesian populations. Other than ovalocytosis itself, only the moderately protective G6PD deficiency gene and some thalassemias are found (119). Ovalocytosis occurs at high frequencies (up to 20%) in the highly malarious lowlands of New Guinea but virtually disappears in the malaria-free highlands. Carriers of the trait are at reduced risk of infection with P. falciparum and, especially, P. vivax malaria (26).
Two studies of the frequency of the band 3 gene deletion mutation in children with P. falciparum malaria showed that none who carried the gene (in heterozygous combination) developed cerebral malaria (2, 72). Protection against malarial infection by ovalocytoses of unspecified genotypes has also been demonstrated in populations in nearby Southeast Asia (8, 68). Ovalocytic RBCs resist invasion by P. falciparum (110) and by Plasmodium knowlesi (85), a simian malaria parasite with similar RBC invasion characteristics to P. vivax. It is not known, however, if the ovalocytic conditions which protected against malaria in these studies were due to the same band 3 gene deletion that was shown to protect against cerebral malaria (2, 72). Given the apparent uniform in utero lethality of the homozygote form of the band 3 gene deletion mutation, its high frequency in the lowland populations of New Guinea once again reflects the magnitude of the reproductive cost of malaria to this population.
RBC Duffy Negativity
It is striking and long-recognized fact that most members of indigenous populations of West and Central Africa are completely refractory to infection with P. vivax malaria (71). Almost all members of these populations are also homozygous for an FYnull (RBC Duffy-negative) allele of the gene that controls expression of the Duffy antigen on RBCs (27, 150, 217). The Duffy blood group antigen system is represented mainly by two serologically distinct forms determined by alleles FY*A and FY*B. Each of these alleles also exists in a mutant, unexpressed, or null form as FY*Anull or FY*Bnull (217). The Duffy antigen, which has been identified as a chemokine receptor (98, 150), is also an essential receptor for P. vivax merozoites to be able to enter a host RBC (98, 126, 132). This accounts for the association between complete refractoriness to P. vivax infection in most West and Central Africans and the almost universal homozygous RBC Duffy negativity in these populations.
The molecular genetic basis for RBC Duffy negativity is a single-nucleotide substitution polymorphism (SNP) in the promoter region of the gene for the Duffy antigen. This promoter controls the expression of the Duffy antigen specifically in RBCs. The same SNP is associated with both the FY*A and the FY*B alleles (217), leading to the FY*Anull and FY*Bnull RBC Duffy-negative alleles. Now, the promoter for a structural gene, such as that for the Duffy antigen, governs its expression on its own chromosome strand only. Therefore, in a heterozygous individual, expression of the Duffy antigen in RBCs should be suppressed only on the strand with an FYnull allele, while from the strand with a Duffy-positive allele, the Duffy antigen should be fully expressed.
In accordance with this expectation, Duffy antigen expression on RBCs from individuals from Papua New Guinea who were heterozygous for the FY*Anull allele was approximately half of that on RBCs from homozygous Duffy-positive individuals (217). In the same human population in Papua New Guinea, a 50%, but statistically insignificant, reduction was noted in the prevalence of P. vivax infections among heterozygous FY*A/FY*Anull individuals compared to those who were homozygous Duffy positive (217). The characteristics of human RBCs with genetically reduced RBC Duffy antigen expression (FyxFyx, according to serotype nomenclature [126]) and weak reaction with Duffy antigen antisera was investigated using the simian malaria parasite P. knowlesi, which, like P. vivax, is absolutely dependent on the Duffy antigen for the ability to invade human RBCs. Merozoites of P. knowlesi invaded RBCs from such individuals with about half of the efficiency that they invaded RBCs from individuals with full Duffy antigen expression (126). However, RBCs from heterozygotes for RBC Duffy negativity were invaded as efficiently as were RBCs from normal RBC Duffy-positive individuals (J. Barnwell, personal communication). Susceptibility to infection with P. vivax malaria of FYnull heterozygotes may be partially reduced, but the effect is probably not very strong, certainly by comparison with the total refractoriness to P. vivax malaria of individuals who are homozygous for an FYnull allele.
Throughout West and Central Africa, the frequency of the FY*Bnull allele is, as already noted, close to fixation. In most of these populations, its frequency exceeds 97% (27, 135), leading to almost universal RBC Duffy negativity in these populations. Not surprisingly, P. vivax malaria is very rare throughout the region (71, 131) (Table 1). It might seem natural to conclude that P. vivax malaria must have been the selective agent for the near fixation of the FY*Bnull allele in these African populations, to the point that P. vivax was itself virtually eliminated. While this is our view, it is not universally held (217). Nor is the case, as we will now present it, straightforward.
In contrast to the frequently and directly lethal P. falciparum, it is often assumed that P. vivax exerts little or no selective pressure on a human population. On these grounds, therefore, and notwithstanding that the RBC Duffy-negative condition confers no evident disadvantage on a carrier (87, 121), P. vivax might be discounted as a selective force for the near fixation of RBC Duffy negativity in West and Central Africa. If, on the other hand, P. vivax can, and does, exert a significant selective force, we are confronted with a paradox. Why, in this case, has selection for RBC Duffy negativity not taken place in other parts of the world, in southern Asia and the Western Pacific rim (27, 135), where P. vivax has certainly been present for several thousand years? It has recently been suggested, in a report of the presence of an FY*Anull allele in a single population Papua New Guinea, that such selection may indeed be happening (217). However, no previous studies appear to have detected this allele in Papua New Guinea, where FY*A is otherwise at virtual fixation (27). Moreover, at around 2%, the FY*Anull allele in this single population in Papua New Guinea is still far below the frequencies of the FY*Bnull allele, which, throughout tropical Africa, are rarely less than 50% and more often in excess of 95% (27).
Concerning the first of the points above, there is strong evidence that P. vivax has, in fact, often placed a heavy burden of mortality and loss of fecundity on the populations that it afflicted (47, 176). Its effects are greatest under conditions of relatively low and unstable malaria inoculation rates. These were the conditions that probably prevailed in Africa before 5,000 to 10,000 years ago (118) and possibly throughout much of the preceding 100,000 years. Had P. vivax been prevalent within human populations in West and Central Africa during this period, and we propose that it was, we would expect that the homozygous RBC Duffy-negative condition would have carried a considerable selective advantage and the heterozygous condition a slight one.
We would, however, expect that selection for raised frequencies of an FYnull allele in a population under P. vivax pressure should also take a “long” time. This expectation follows if mainly the FYnull homozygotes are refractory to P. vivax infection but rather little selective advantage is associated with the heterozygous condition. This, as we have just discussed, is quite likely to be the case.
Now, the homozygous combination of a rare allele, as the FY*Bnull gene in Africa would at first have been, is itself almost vanishingly rare. Moreover, even when the homozygous combination of such an allele had arisen, it would, in the next generation, almost invariably have been diluted again to the weakly advantageous heterozygous state in combination with one of the high-frequency FY*B or FY*B RBC Duffy-positive alleles. Thus, a selection which involved mainly the persistently rare FY*Bnull/FY*Bnull homozygotes would inevitably proceed “slowly.” It would proceed slowly, that is, compared, with the speed of selection for a gene such as the allele for hemoglobin S (Hb*S), in which the heterozygous condition, Hb*S/Hb*A, carries the main selective advantage and which, in the early stages of selection, arises as frequently as the Hb*S allele itself. Moreover, the speed of selection for Hb*S would be further increased above that for the FY*Bnull allele, because P. falciparum is probably much more dangerous than P. vivax under any conditions of endemicity. Hb*S has been estimated to approach a balanced equilibrium after around 2,000 years of selection under P. falciparum malaria (119). The “longer” time expected for P. vivax malaria to select for the near fixation of the FY*Bnull allele could, therefore, be in the order of 10,000 to several tens of thousands of years.
While we may infer that the process of selection for near fixation of the FY*Bnull allele in Africa under P. vivax would have taken several tens of thousands of years, we cannot, from the above line of argument, even hazard a guess as to the times when this process may have begun or ended. These may, however, be partly estimated from other evidence.
Modern human migrations out of Africa are believed to have taken place largely, if not entirely, within the past 100,000 years. Now, RBC Duffy negativity is, as already noted, an apparently harmless genetic condition. Therefore, once an FYnull allele has been selected in a human population, there should be little or no loss of frequency of the gene, especially from a population in which it had become fixed. Outside Africa, RBC Duffy negativity is found in declining frequencies through the Arabian peninsular, across the Middle East, and to the edges of Central Asia, but beyond these areas it is, with rare exceptions, virtually absent (27). Had the high frequencies of the FY*Bnull allele been selected before the main dispersals of modern humans began, this allele should be common in all human populations. Since it is not, its selection in Africa must have been completed only after these dispersals had taken place, i.e., within less than the past 100,000 years. Indeed, since human migrations out of Africa across the Old World and into the Western Pacific have probably continued into much more recent times and have carried almost no trace of the FY*Bnull allele with them, we can probably reduce the time by which selection for African FY*Bnull would have been completed to less than 50,000 years, which is the approximate period of the migrations to Melanesia and Australia. And if, as we do, we take it that the force for the selection of the FY*Bnull allele in Africa was P. vivax malaria, then the fact that at least 5,000 years of exposure to P. vivax in southern Asia and China and the Western Pacific has not led to selection for high levels of RBC Duffy negativity anywhere in this region suggests that the process also takes longer than 5,000 years.
We now have a case that the selection for near fixation of RBC Duffy negativity takes at least 5,000 years and that it was completed in Africa less than 50,000 years ago. Unfortunately, we still cannot place an upper limit upon how long the process takes. The P. vivax pressure could, for example, have begun several hundred thousand years ago and reached completion in a final rapid spurt at any time within the past 50,000 years, or it could have begun in Africa only 6,000 years ago and been completed in the last few hundred years, just in time for us to record the outcome. However, we now have corroborating evidence for the rough period within which the beginning of the selection would have taken place. In a study of haplotypes associated with the FY*Bnull allele in African and European populations, Hamblin and Di Rienzo (87) have proposed that a selective sweep towards the near fixation of RBC Duffy negativity in the African populations began between 97,200 and 6,500 years ago within 95% confidence limits.
We suggest that the most likely period for the selection of RBC Duffy negativity in Africa to have taken place, and therefore for strong selective pressure from P. vivax to have been still active on these populations, lies somewhere between perhaps 10,000 and 5,000 years ago. This falls at the height of the last major glacial period, when equatorial Africa would have been much cooler than today and when it would have been infested with Anopheles mosquitoes, which were at the time relatively inefficient vectors of malaria (39, 118). These are conditions which would have tended to support low levels of unstable, and, therefore, severely life-degrading, P. vivax transmission. They are the conditions which would have strongly favored selection for RBC Duffy negativity in the affected human populations in Africa.
Some Points from the Malaria Hypothesis
How long does it take malaria to select for human polymorphisms?
Human polymorphisms which meet some or all of the expectations of the malaria hypothesis are found in elevated frequencies usually only in populations which have been exposed to malaria for at least several hundred years. Thus, the indigenous populations of northern Europe (with the exception of part of The Netherlands), northern parts of Asia, Australia, and all but the westernmost islands of the Pacific Ocean, and the Americas, from which malaria either has always been absent or has been present for less than this time, are all without these polymorphisms in significant frequencies.
Mutations which are moderately protective against malaria, which protect in heterozygous combination, and which carry a relatively low balancing cost to a population, namely, the thalassemias and G6PD deficiency, are generally the first to reach elevated frequency under selection by malaria. These are the malaria-selected polymorphisms which predominate in the parts of Europe, around the shores of the Mediterranean Sea, where malaria arrived between 2,500 and 2,000 years ago. In northern Europe, where it has arrived more recently, probably mainly between 1,000 and 500 years ago, even these polymorphisms are almost entirely absent.
In regions in which more than 2,000 to 3,000 years of selection by malaria has taken place, namely, in Africa, parts of the Middle East, southern Asia, and Melanesia, mutations are found which are highly protective against malaria in heterozygous combination but which also carry a high balancing cost in homozygous combination. These are the genes for sickle cell hemoglobin and ovalocytosis.
Longest of all, many thousands or tens of thousands of years, may be the selection for alleles whose protective effects against malaria are expressed only, or mainly, when they are present in homozygous combination. Such is the allele for hemoglobin C, which, in homozygous combination, protects strongly against P. falciparum malaria and which has probably not yet reached equilibrium frequencies even after several thousand years of selection in West Africa. Such, also, is the allele for RBC Duffy negativity, which, only in homozygous combination, protects solely and absolutely against P. vivax malaria. High frequencies of RBC Duffy negativity extend from West and Central Africa and, in declining frequency, toward East Africa and southern Africa as well. These regions, and especially West Africa, are the heartlands from which most, if not all, of today's populations of human malaria may have had their origin and in which, therefore, selection under both P. vivax and P. falciparum has been of longest duration.
Did malaria enter most human populations before or after their global dispersal?
In each broadly grouped human population in which they occur, the malaria-protective polymorphisms are usually distinct from each other. Hemoglobin E occurs only in Southeast Asia, and ovalocytosis occurs in Melanesia and also, probably in several different forms, through Southeast Asia. Hemoglobin S is absent from these regions, and hemoglobin C occurs only in West Africa. The variety of different G6PD deficiency mutations found in, and characteristic of, different human populations is large, and for the thalassemias it is even greater (65). The G6PD deficiency mutations are associated with a number of different haplotypes in different human populations (188), and the sickle cell gene occurs in association with several different population haplotypes within Africa and with three distinct haplotypes in the Middle East and India (65). Thus, individual polymorphisms lie within haplotypes which are usually characteristic of the human populations within which the polymorphism is found.
These facts can be most easily explained if each indigenous human population had separated and settled before the selective agents, P. falciparum and P. vivax malaria, arrived within it. This, as will be analyzed in the following sections, is almost undoubtedly the general pattern of events.
Legacy of the impact of malaria on human populations.
Regardless of its implications for our understanding of the evolution and history of malaria, the malaria hypothesis points to the public health reality that our past contacts with malaria have left a large burden of genetic diseases among us. Today this genetic burden, in the form of the thalassemias, sickle cell disease, G6PD deficiencies, and the ovalocytoses, is of the same order as the residual burden of malaria itself. About one-third of a million to half a million babies are born each year with severe forms of these inherited disorders (198).
ORIGINS AND EVOLUTION OF HUMAN MALARIA PARASITES
Origins of Malaria Parasites
If settlement by most human populations preceded the arrival of malaria within them, then when, where, and how did the ancestral malaria parasites arise and when, where, and how did they enter human populations?
The ancestors of the malaria parasites have probably led a parasitic existence almost since there were potential hosts to parasitize, at least half a billion years ago. Of course, the parasitic forms in this lineage must themselves have had a preparasitic ancestor. Molecular genetic evidence strongly suggests that this ancestor was a choroplast-containing, free-living protozoan which become adapted to live in the gut of a group of aquatic invertebrates (204). This single-celled organism probably had obligate sexual reproduction. Indeed, to the present time, all known members of the phylum Apicomplexa, to which the malaria parasites belong, have retained an obligate sexual stage in their life cycles (113). Moreover, the environment where gamete formation and fertilization takes place is still always within the midgut lumen of a host species.
At some relatively early stage in their evolution, these “premalaria parasites” acquired an asexual, and usually intracellular, form of reproduction called schizogony. By evolving this form of “vegetative” reproduction, the parasites greatly increased their proliferative potential. Schizogony may be defined as growth and subsequent nuclear and cellular division into numerous daughter cells from within the body of a single parent cell. It almost invariably takes place during the intracellular phases of parasite growth in the tissues of a host. The completion of each schizogonic event is usually followed by reinvasion of new host cells by the daughter cells or spores (designated sporozoites or merozoites), leading to another round of schizogonic development. In this way, numerous successive rounds of multiplication can take place. It is during schizogony in the RBCs of humans and other vertebrates that certain descendants of these ancestral parasites now cause the disease that we call malaria.
Among the invertebrates to which the ancestors of the malaria parasites became adapted were probably aquatic insect larvae, including those of early Dipterans, the taxonomic order to which mosquitoes and other blood-sucking flies belong. These insects first appeared around 150 million to 200 million years ago. During or following this period, certain lines of the ancestral malaria parasites achieved two-host life cycles which were adapted to the blood-feeding habits of the insect hosts. The malaria parasites of humans are typical products of this line of development since they alternate between human and the blood-feeding female Anopheles mosquito hosts (within whose midguts gamete formation and fertilization still, of course, take place).
In the 150 million years since the appearance of the early Diptera, many different lines of malaria and malaria-like parasites evolved and radiated. They came to parasitize members of most major groups of land vertebrates including reptiles, birds, and mammals. In the mammalian orders today, more species of malaria parasite have been identified among primates than in all other mammals combined (71). Over 25 distinct species of malaria parasites of primates have been named. Four of these are the recognized malaria parasites of humans: P. falciparum, P. vivax, P. malariae, and P. ovale.
Origins of Human Malaria Parasites
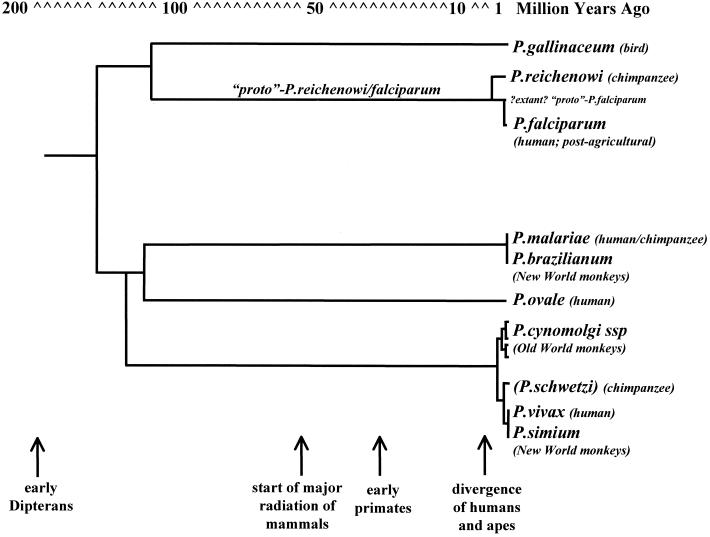
There has been much speculation concerning the evolutionary relationships of the four human malaria parasite species both to each other and to the malaria parasites of other animals. In the past, these discussions depended on observations on the morphology and biology of the parasites and on the evolutionary relationships among, and the geographic locations and origins of, their various host species. More recently, molecular genetic analysis of the parasites themselves has contributed a new dimension to this detective work (Fig. 1).
FIG. 1.
Phylogeny of the malaria parasites of humans and of some other related malaria parasite species. See the text for sources on which this reconstruction is based.
On the basis of such molecular genetic evidence (53, 55, 56), as well as on classical biological grounds (71), P. falciparum is very closely related to, but nevertheless evolutionarily divergent from, a malaria parasites of chimpanzees, P. reichenowi. Curiously, molecular evidence also shows that these two parasite species are more closely related to the malaria parasites of birds than they are to those of other mammals (54, 196) (Fig. 1) as, indeed, had long been suspected by classical biologists (71). The divergence of the lineage of the bird malaria parasites, including P. falciparum and P. reichenowi, from the line that gave rise to all other known and tested malaria parasites of mammals is an ancient event, possibly dating to around 130 million years ago (54). This would place their separation near the very origin of the two-host life cycle involving blood-feeding Dipterans and land vertebrates. By contrast, the separation of the lines that led, on the one hand, to P. falciparum and, on the other, to P. reichenowi has been placed at only 4 million to 10 million years ago (53, 55, 56). This overlaps the period, around 5 million years ago, in which the human line diverged from that of the African great apes (114, 152, 201) (Fig. 1).
The three remaining species of human malaria parasite, P. malariae, P. ovale, and P. vivax, fall within a single clade that includes all the mammalian malaria parasites, other than P. falciparum and P. reichenowi, on which molecular genetic analysis has been done (55, 56, 153) (Fig. 1). Within this clade, all of the primate malaria parasites belong to one or other of the lineages represented by P. malariae, P. ovale, or P. vivax. These three lines appear to have diverged over 100 million years ago (7) (Fig. 1), long before the emergence of the lines leading to the distinct mammalian, let alone primate, orders of today.
Among the primate malaria parasites, P. ovale is known only as an infection in humans and has no genetically confirmed close relative (55, 56, 153). P. ovale is therefore the sole known surviving representative of its line (7).
P. malariae, on the other hand, in addition to infecting humans, is found in apparently indistinguishable form as a natural parasite of chimpanzees in West Africa (71). Moreover, a morphologically indistinguishable parasite, P. brazilianum, infects New World monkeys in Central and South America (71). Molecular genetic analysis has failed to distinguish P. brazilianum and P. malariae (55, 56, 153). There can be no doubt that these are the same or almost the same parasite infecting different primate species, namely, humans and New World monkeys. We take the view that P. brazilianum is, in fact, a zoonotic form of P. malariae which has been introduced recently from humans into the New World monkey populations.
The converse of this, i.e., that P. malariae has been received by lateral transfer from New World monkeys to humans, has also been argued (7). However, a New World origin of this parasite does not reconcile with the common prevalence of P. malariae in the Mediterranean region more than 2,000 years ago (95). Virtually all, if not all, pre-Colombian human migrations of at least the past 50,000 years appear to have been from the Old to the New World, and none have been in the reverse direction. Therefore, had P. malariae originated in the Americas by lateral transfer from monkeys to humans, when, and by what route, could it have entered the Mediterranean region to have become prevalent there at least 2,000 years before any known or likely human traffic with the New World?
Except for P. brazilianum, no other primate malaria parasite is known to belong within the P. malariae lineage. Thus, whether as a human parasite or, in essentially identical form, as a parasite of New World monkeys, P. malariae/P. brazilianum, like P. ovale, is the only confirmed and extant representative of its line.
In contrast to these lonely survivors of ancient lines, P. vivax belongs within a large and closely related body of malaria parasites which today are found mainly infecting monkeys of southern and southeastern Asia and the Western Pacific rim (Fig. 1) (55, 56, 153). Among these parasites, P. cynomolgi and its subspecies have long been considered especially closely related to P. vivax (71). Because these close relatives of P. vivax are today found only infecting monkeys of the southern Asian regions, there is a view that P. vivax itself must have originated from within these regions and from nowhere else (see, e.g., reference 56). However, this conclusion may not necessarily be so simply reached. The most recent estimate for the time of the divergence of P. vivax and P. cynomolgi is in the range of two million to three million years ago (A. Escalante personal communication). At this time, the ancestral hosts of these parasites were probably spread throughout much of the tropical, subtropical, and, indeed, temperate regions of the combined Asian and African landmasses. Therefore, the region(s) where the P. vivax and P. cynomologi lines diverged and hence where P. vivax, in this sense, originated cannot, solely on these grounds, be located more precisely than to somewhere on the continents of the Old World.
There are two other parasites of primates which, like P. cynomolgi, are morphologically indistinguishable from P. vivax. One is a parasite of chimpanzees in West and Central Africa and is named P. schwetzi (71). In contrast to P. cynomolgi, sporozoite infections of which readily induce blood infections in humans, various attempts to infect humans with P. schwetzi have all, apparently, failed (71). It seems appropriate, therefore, to regard P. schwetzi as a separate species from either P. vivax or P. cynomolgi, although it is probably closely related to both. Unfortunately no molecular genetic data yet exist with which to test these relationships.
The other parasite which is morphologically identical to P. vivax is P. simium, a parasite of New World monkeys. Molecular genetic analysis shows that P. simium and P. vivax are, indeed, indistinguishable (55, 56). We take the view that P. simium is almost certainly an enzootic form of P. vivax which was introduced into New World monkeys following the arrival there of humans. Our argument parallels that given above for P. brazilianum and P. malariae. We also draw on the following.
The time of divergence of P. vivax from the closely related parasite of Old World monkeys, P. cynomolgi, is, as already noted, put at two million to three million years ago. Now, South American and Old World simians diverged from each other around 40 million to 50 million years ago (152, 114) and have been geographically isolated from each other probably throughout this time (33). Therefore, the divergence within the past three million years of two lines, one of which, P. cynomlogi, is unquestionably the parasite of an Old World host species, must itself have taken place in the Old World. It follows that P. vivax had its origin in the Old World. P. vivax could not, therefore, have derived from P. simium; the converse, in fact, must be the case.
EXPANSIONS AND DISPERSALS OF HUMAN MALARIA
In spite of the difficulties in trying to identify the region, or even the continent, of origin of the ancestors of extant species of human malaria parasite, the attempt is worthwhile. In the following, we discuss the general proposition that the modern populations of each of the four species of human malaria parasite grew to prominence mostly in Africa and that it is largely, if not entirely, from out of Africa that these populations have dispersed.
Out of Africa
We have argued that P. malariae was introduced from humans into New World monkeys and not vice versa. P. malariae is a natural parasite of African great apes and humans in Africa. It is found in no other Old World primate species within or outside Africa. On these grounds, the simplest hypothesis for the origin of P. malariae is that it was in Africa itself. As a parasite of the ancestor of both humans and African great apes, ancestral P. malariae would have continued to parasitize and cross-infect both host lineages as they diverged around five million years ago. How soon thereafter may P. malariae have been carried by its hominid hosts beyond its African homeland is an open question. P. malariae thrives under both tropical and temperate transmission conditions and is adapted to endemicity in sparse and mobile human populations (see below). Therefore, it could well have been prevalent among hominids migrating from Africa to the warmer regions of Europe and Asia at any time in the past five million years.
P. ovale, by contrast, is a strictly warm-climate parasite. Today it is found commonly throughout tropical Africa and in very limited distribution elsewhere in the tropics, notably in New Guinea and in the Philippines (Table 1) (122). There is no reason not to suppose that it, too, had its origin as a human parasite in Africa.
Because P. falciparum and P. vivax each have a close biological relative (P. reichenowi and P. shwetzi, respectively) which is a parasite of African great apes, an argument, similar to that for P. malariae, can be made for the African origins of both of these species. In contrast to the case for P. malariae, however, the argument concerning these two human-ape parasite pairs requires that, as the human and ape lines diverged, so also did the parasites they carried. In one case, this would have led to P. vivax in the human line and to P. schwetzi in apes, and in the other case, it would have led to P. falciparum in humans and P. reichenowi in apes.
Molecular genetic data on the present-day populations of P. falciparum support the proposition that they did, indeed, spread from an origin in sub-Saharan Africa (40, 41). As already discussed, the evidence from human genetic data imply a West African origin of P. falciparum within the past few thousand years. This is also, according to other population genetic analyses of P. falciparum, the time within which modern populations of the parasites had their origin (159, 194) (see below).
The case for an African origin of most recent P. vivax populations rests entirely on the evidence from the global distributions of RBC Duffy negativity in human populations. The extremely high frequencies of RBC Duffy negativity in African populations and its almost total absence beyond Africa provide strong evidence that the greatest and/or the longest burden of P. vivax malaria was that experienced by African populations (27, 87).
If global populations of all four species of human malaria parasites derive from African sources, how were their characteristics formed there and why, at particular times, did they disperse beyond Africa? Everything that took place was certainly governed by the climatic events of the past 100,000 years. These were dominated by cooling of the planet and by the massive glaciations which affected much of the northern hemisphere. The last glacial period, which was at its coldest about 20,000 years ago, ended with the rapid warming of the planet to approximately present-day temperatures by about 10,000 years ago. The warmer global climate heralded the beginnings of agriculture. According to one appealing line of argument, the entry of agricultural practice into Africa was pivotal to the subsequent evolution and history of human malaria (39, 118).
The Agrarian Revolution in Africa
Around 4,000 to 5,000 years ago, the Neolithic agrarian revolution, which is believed to have begun in the “Fertile Crescent,” southern Turkey and northeastern Iraq, 8,000 years ago, took root in western and Central Africa. In response to the profound changes to the human environment that this engendered, organisms in contact with that environment evolved to adapt. Among these were the Anopheles vectors of human malaria.
Adaptations of the vectors of malaria in Africa.
Outside of Africa, wherever malaria is or has been endemic, the female mosquito vectors of malaria are zoophilic rather than anthropophilic; i.e., they prefer to feed on animals rather than on humans (17). The degree of preference for human blood has important consequences for human malaria transmission (124). All other factors being equal, the probability of a malarial infection being transmitted from one human to another is proportional to the square of the fraction of blood meals that the vector mosquitoes take on humans (124). For example, mosquitoes that take only 1/10 of their blood meals on humans will transmit a malarial infection 100 times less frequently than mosquitoes that take all their blood meals on humans.
In most parts of the world, the anthropophilic index (the probability of a blood meal being on a human) of the vectors of malaria is much less than 50% and often less than 10 to 20% (17). By contrast, in sub-Saharan Africa, the vectors of human malaria usually have an anthropophilic index of 80 to almost 100% (17). This is probably the most important single factor responsible for the stability and intensity of malaria transmission in tropical Africa today. The high human-biting preferences of African Anopheles, as Livingstone (118) was the first to suggest, may have been an adaptation to conditions created by the agrarian revolution in Africa.
In adopting an agricultural way of life, human populations in sub-Saharan Africa changed from a low-density and mobile hunting and gathering life-style to communal living in settlements cleared in the tropical forest. This new, man-made environment had two important consequences for the mosquito populations. One was that the numbers and densities of humans began to increase under the new agricultural economy. The other consequence was that the new life-style generated numerous small water collections close to the human habitations. Those who adopted agriculture thus transformed themselves into large, stable, and accessible sources of blood in the midst of abundant mosquito-breeding sites. The new situation provided a strong selective advantage to mosquito populations which became adapted to breed close to human habitation and to feed primarily on human blood. Livingstone argued (39, 118) that this led to the very high anthropophily of the vectors of African malaria and, in large part, their great vectorial efficiency.
Agricultural village economies had, of course, also developed throughout the tropics and subtropics of Asia and the Middle East up to several thousand years before those in Africa (46). Outside Africa, however, malaria vectors have never acquired the same extraordinary preference for human blood. Why should this be? The answer probably lies in the abundance in Asia of animal species whose domestication was possible, and was achieved, during the rise of agriculture. Because there were none in sub-Saharan Africa (46), malaria vectors with a preference for human blood would have had a strong selective advantage in Africa but nowhere else.
Parasite adaptations before and following the agrarian revolution. (i) P. malariae, the “gypsy” parasite.
P. malariae is the human malaria parasite which would have been best adapted to preagricultural conditions. It could have been, and probably was, sustained at very low infection rates among the sparse and mobile human populations of the time. This is because, unlike other human malaria parasites, P. malariae can remain for decades within a human host and in a state potentially infectious to mosquitoes (71). As a result, infections of P. malariae can be transmitted many years after they have been contracted. It is the perfect adaptation to the parasitization of wandering hosts and mosquito vectors that rarely encounter each other.
Because of low, or very low, infection rates, the burdens of P. malariae on early hunters and gathers may have been relatively light. However, in those who were affected, P. malariae could have imposed a severe long-term burden of morbidity and mortality through the eventually severe and often fatal nephropathy that it can cause (73, 74).
(ii) P. vivax, the great survivor, and P. ovale, the “hothouse plant.”
As human numbers slowly increased in preagricultural Africa, so too would have the prevalence of P. ovale and P. vivax. Both parasites are more dependent than P. malariae on denser and less mobile host populations. Under the still low and unstable preagricultural transmission conditions, both P. vivax and P. ovale malaria would have begun to impose significant burdens of morbidity and mortality on the affected human populations. Gradually, however, the frequency of the mutation for RBC Duffy negativity would have begun to rise until, as it approached fixation, P. vivax malaria itself declined to very low levels in West and Central Africa. However, it was not, and has not been, extinguished there (71, 131). Moreover, P. vivax was to spread beyond Africa and throughout almost the entire habitable zones of the planet. Both within Africa and beyond it, P. vivax has proved itself to be the most tenacious survivor of all the malaria parasites of humans (131).
With no equivalent of homozygous RBC Duffy negativity to repel it, P. ovale remains highly prevalent throughout tropical Africa. Elsewhere, however, it is found only in pockets, all within the tropics. In stark contrast to P. vivax, P. ovale has little resilience beyond its ancestral habitat and none at all outside the tropics and subtropics.
While both P. vivax and P. ovale remain prevalent across Africa, there is little or no trace of the burden they would once have imposed there. Intense and stable transmission induces strongly protective antimalarial immunity in early life. It thereby prevents the devastating experience of lifelong, lingering infection with malaria. Therefore, the arrival of agriculture and the intense transmission rates that this engendered would have largely eliminated the mortality, and most of the morbidity, due to these parasites in Africa.
(iii) P. falciparum, the parasite that “did not exist”.
Wherever transmission conditions are poor, P. falciparum is the least competitive of the four species of human malaria parasite. It can be, and has been, exterminated under conditions which have allowed the far more resilient P. vivax to remain endemic (131). How, therefore, could a parasite as vulnerable as P. falciparum have been sustained at all by inefficient mosquito vectors, among sparse and mobile human populations, and during the climatically cool era that preceded the arrival of agriculture in Africa? The answer, according to some molecular genetic evidence, may be that P. falciparum in its present form had not yet arisen.
Synonymous (not associated with a corresponding amino acid substitution) SNPs have been investigated in protein-coding nuclear genes (159), from intragenic noncoding, nuclear DNA (194), and from mitchondrial genes (40) in a number of independently isolated and geographically separated lines of P. falciparum. According to evolutionary genetic theory (89, 114), the number of synonymous SNPs that may be found between genes from among the members of any species of organism increases linearly with time. From an analysis of the densities of such SNPs within its genome, and with appropriate assumptions, including the absence of selection pressures for or against these mutations, it is possible to extrapolate back to a time when an extant species expanded from a small ancestral population. This has been done in a number of studies of P. falciparum. The results of those referred to above suggest that modern populations of P. falciparum emerged within relatively recent times, and possibly within the past 5,000 to 10,000 years, from a tight population bottleneck (159, 194). According so some (159), the bottleneck could have been so tight as to have been represented by a single clone of the parasites.
Other published molecular genetic evidence points to a longer time since any population bottleneck—up to half a million years (57, 99). It has also been suggested that properties of the P. falciparum DNA sequences used as a source of data may strongly preclude the survival of synonymous mutations and that this, therefore, would lead to considerable underestimations of the age of the present-day global population of these parasites (170). Another line of counterargument has pointed to the existence of the complex alleles of the gene for the MSP1 protein of P. falciparum whose sequences differ at many tens of amino acid positions. Such complex allelic differences in an extant population must be of very ancient origin, it is argued, and would rule out the passage of a haploid organism such as P. falciparum through a recent clonal bottleneck (99).
While a clonal bottleneck may be considered extremely improbable, none of these counterarguments are conclusive in ruling out the possibility that some sort of population bottleneck did occur in the recent history of P. falciparum. There is, moreover, a concordance between the estimates for the more recent emergence of P. falciparum within the past 5,000 to 10,000 years, as offered above, and estimates for the length of time that P. falciparum has exerted selection on human populations in West Africa. As discussed above, this, too, is probably less than 10,000 years. Moreover, the emergence of P. falciparum into the human population in tropical Africa, somewhere within this period, would resolve the enigma of how it could have been sustained under the conditions of preagricultural Africa. In its present form, P. falciparum would simply not have existed.
Where, then, did the present-day parasite come from? It has been argued that there must have been in preagricultural Africa an ancestral population of “proto-P. falciparum,” perhaps as a human parasite or in another host species. Following the advent of agriculture, mutant parasites, increasingly adapted to conditions which had become so favorable to the transmission of human malaria, would have begun to replace, or sidestep, their progenitors in a succession of epidemic bursts (39). In this way, via a series of population bottlenecks that would have purged the rapidly evolving parasites of most genetic diversity except at loci where selection retained it, modern P. falciparum emerged and spread across Africa.
Ironically, and poignantly, had the ancestral material from which modern P. falciparum was to arise not existed, African agriculture should have reduced, rather than increased, the burdens of malaria in Africa. It would have done this by stabilizing malaria transmission rates, thereby eliminating the lethality of chronic malaria through the induction of early protective immunity. But somewhere on that continent, proto-P. falciparum did exist (Figure 1). In the new environment of intense malaria transmission, this parasite transformed to become the agent of an infection that was frequently lethal in the acute, as opposed to the chronic, condition. The intense transmission that induced early immunity simply concentrated the mortality due to P. falciparum malaria into the youngest age groups. P. falciparum had become a killer of human beings which, historically and still today, has few rivals (Tables 2, 3, and 4).
TABLE 3.
Malaria mortality and human population by geographical region for the 20th centurya
| Region, population, and mortality | Population and mortality data for yr:
|
Possible margin of error (fold [×/÷]) | |||||
|---|---|---|---|---|---|---|---|
| 1900 | 1930 | 1950 | 1970 | 1990 | 1997 | ||
| Europe and North America | |||||||
| Population (millions) | 517 | 651 | 767 | 928 | 1,013 | 1,028 | |
| Annual no. of deaths from malaria | 80,000 | 70,000 | 10,000 | 10 | 10 | 20 | 2 |
| Annual no. of deaths from malaria/10,000 | 1.547 | 1.075 | 0.13 | 0.0001 | 0.0001 | 0.0002 | 2 |
| Central America and Caribbean | |||||||
| Population (millions) | 24 | 34 | 51 | 89 | 145 | 161 | |
| Annual no. of deaths from malaria | 40,000 | 60,000 | 40,000 | 40,000 | 1,200 | 500 | 2 |
| Annual no. of deaths from malaria/10,000 | 16.7 | 17.6 | 7.84 | 4.49 | 0.083 | 0.031 | 2 |
| South America | |||||||
| Population (millions) | 40 | 70 | 111 | 189 | 298 | 313 | |
| Annual no. of deaths from malaria | ?2,000 | ?3,000 | 3,500 | 1,800 | 2,500 | 3,500 | 2.5 |
| Annual no. of deaths from malaria/10,000 | ?0.5 | ?0.4 | 0.315 | 0.095 | 0.084 | 0.112 | 2.5 |
| Middle East, South Asia, and Western Pacific | |||||||
| Population (millions) | 552 | 719 | 953 | 1,363 | 2,163 | 2,341 | |
| Annual no. of deaths from malaria | 2,500,000 | 2,700,000 | 625,000 | 57,000 | 107,100 | 65,000 | 2 |
| Annual no. of deaths from malaria/10,000 | 45.3 | 37.6 | 6.56 | 0.418 | 0.495 | 0.278 | 2 |
| China and North East Asia | |||||||
| Population (millions) | 389 | 465 | 620 | 756 | 1,237 | 1,314 | |
| Annual no. of deaths from malaria | 300,000 | 500,000 | 300,000 | 150,000 | 100 | 100 | 2 |
| Annual no. of deaths from malaria/10,000 | 7.71 | 10.8 | 4.85 | 1.98 | 0.001 | 0.001 | 2 |
| Africa, sub-Sahara | |||||||
| Population (millions) | 94 | 111 | 167 | 280 | 532 | 602 | |
| Annual no. of deaths from malaria | 210,000 | 240,000 | 307,000 | 300,000 | 787,000 | 990,000 | 1.5 |
| Annual no. of deaths from malaria/10,000 | 22.3 | 21.6 | 18.4 | 10.7 | 14.8 | 16.4 | 1.5 |
| World | |||||||
| Population (millions) | 1,616 | 2,050 | 2,669 | 3,605 | 5,388 | 5,759 | |
| Annual no. of deaths from malaria | 3,132,000 | 3,573,000 | 1,285,000 | 578,800 | 897,000 | 1,059,000 | 1.5 |
| Annual no. of deaths from malaria/10,000 | 19.4 | 17.4 | 4.82 | 1.61 | 1.66 | 1.84 | 1.5 |
| World minus Africa, sub-Sahara | |||||||
| Population (millions) | 1,522 | 1,939 | 2,502 | 3,325 | 4,856 | 5,157 | |
| Annual no. of deaths from malaria | 2,922,000 | 3,333,000 | 978,000 | 248,000 | 110,900 | 69,000 | 1.5 |
| Annual no. of deaths from malaria/10,000 | 19.2 | 17.2 | 3.91 | 0.748 | 0.228 | 0.134 | 1.5 |
The data are as prepared for The World Health Report, 1999 (210). See the Appendix for further details.
TABLE 4.
Malaria mortality: summary statistics at the beginning and end of the 20th centurya
| Region | Yr | Total no. of deaths from malaria | No. of malaria deaths per 10,000 population | % of all deaths which are due to malaria |
|---|---|---|---|---|
| Europe and North America | 1900 | 80,000 | 1.5 | 0.8 |
| 1997 | 20 | 0.0002 | 0.0001 | |
| Caribbean, Central and South America | 1900 | 42,000 | 6 | 2 |
| 1997 | 4,000 | 0.08 | 0.05 | |
| Asia, China, and Western Pacific | 1900 | 2,800,000 | 30 | 9 |
| 1997 | 65,000 | 0.18 | 0.1 | |
| Sub-Saharan Africa | 1900 | 210,000 | 22 | 6 |
| 1997 | 990,000 | 16 | 9 | |
| World minus sub-Saharan Africa | 1900 | 2,900,000 | 19 | 8 |
| 1997 | 69,000 | 0.13 | 0.08 |
The data presented in this Table are extracted from Table 3. The last column is derived from the same data but is expressed in relation to approximate overall mortality rates in each region for the relevant period.
Beyond Africa
In the Old World.
Prior to the agrarian revolutions, only P. malariae, by virtue of its transmission characteristics, might have been present among the low-density hunter-gatherer populations of the Old World. Here, indeed, P. malariae may already have been present for perhaps hundreds of thousands of years or much, much longer—perhaps since before the origin of the human line.
The failure of RBC Duffy negativity to have reached even moderately high frequencies almost anywhere outside Africa argues against the long presence of P. vivax in human populations beyond this continent. P. vivax may not have existed in Asia or, indeed, anywhere else outside Africa until relatively recently. Indeed, it may not have spread beyond Africa before 10,000 to 30,000 years ago. Perhaps this spread occurred within the 15,000 years since rising global temperatures began the last glacial recession. Perhaps it was not until after the rise of agriculture in the Fertile Crescent around 7,000 to 8,000 years ago. But whenever it was that P. vivax began to spread beyond its African homeland, today, a gradient of declining RBC Duffy negativity, like a serpent's trail in the sand, marks its early contact with Arabia and the Near East (27, 135).
By the time modern P. falciparum emerged into Africa around, as we now conjecture, 4,000 years ago, P. vivax may have already spread far beyond its “native” shores and into the agricultural communities that were expanding in size and numbers throughout the warm southern regions of the Eurasian continent and the Western Pacific. In the same period, P. ovale would also have made its more tentative way beyond Africa into a few other tropical safe havens, such as New Guinea.
These same environments, rich in human settlement and sustaining flourishing populations of Anopheles, were also well prepared for, and receptive to, the arrival and transmission of P. falciparum. As it entered, P. falciparum would have struck each new human settlement in its most lethal form, the epidemic. Thereafter, and depending on the local conditions, P. falciparum would have settled in many areas to a state of more or less stable endemicity while continuing to exact high perennial mortality. This, in turn, would gradually lead to the selection of elevated frequencies of thalassemias and G6PD deficiency and eventually to traits such as sickle cell and ovalocytosis that characterize the descendent populations today.
In the New World.
Two human malaria parasite species, P. malariae and P. vivax, bear a remarkable morphological and molecular genetic similarlity to two malaria parasites of monkeys of Central and South America, respectively: P. brazilianum and P. simium. We have argued that these parasites were almost certainly introduced into the New World monkeys from human infections, and not the reverse. When, therefore, and by what route did human malaria parasites enter the Americas?
The story is well known of how, in the 17th century, native Peruvians introduced Spanish colonists to the effects of Cinchona bark for curing malarial fevers. This story has been offered as evidence that Plasmodium species infecting humans were already present in the Americas before the arrival of Europeans. As evidence for such presence it is, however, far from conclusive. The transfer of the knowledge of the antimalarial properties of Cinchona bark took place more than a century after the first arrival of the Spanish in South America. This is time enough for malaria to have spread far across that continent had it been introduced there by the Europeans. If the bark was already in use among the indigenous people, discovery of its antimalarial properties may have followed rapidly.
The pre-Columbian populations of the New World are almost totally devoid of any of the genetic abnormalities which are associated with long exposure to malarial infection (27). They are therefore unlikely to have had very long experience of P. vivax and almost certainly none of P. falciparum, the two parasite species that we are confident can, and do, select for these polymorphisms. We are unsure whether P. malariae can do this, and we are less confident, on these grounds, that this species was not present in the Americas from pre-Colombian times. P. malariae can persist for up to several decades within the human body. Conceivably it could have survived in individual human carriers arriving from Asia by overland trek or sea voyage of years or decades through the malaria-sterile sub-Arctic north or the Anopheles-free Pacific Ocean. By contrast, entry of P. vivax, let alone P. falciparum, by the northern route must surely have been precluded. Could either of these species have survived a sea voyage or, more probably, a succession of voyages across half of the diameter of the planet from southern Asia or its offshore archipelagos to the eastern coasts of the Americas? It may just be possible.
Thus, while it is at least conceivable that P. malariae may have reached the Americas before, and perhaps long before, the arrival there of European and African peoples, it seems unlikely that P. vivax or, especially, P. falciparum could have done so. The post-Colombian landings of infected Europeans and Africans, on the other hand, through the mediation of the many naturally receptive species of American Anopheles, would readily have sparked first local and then regional epidemics of P. vivax and P. falciparum. Both species would eventually spread and become endemic through most parts of the American continents from the Atlantic to the Pacific and from Canada to Argentina. P. malariae, whether it was first on the scene or not, was to become extremely rare in the New World. P. ovale may never have even gained a foothold (Table 1).
In the Western Pacific.
Until its transient introduction into the northern Australian tropics in the 19th and early 20th centuries, neither the Australian continent nor the islands of the Pacific Ocean beyond the “Buxton line” (22) (south of the equator, eastward of 170° east, and south of 20° south, and north of the equator to the Tropic of Cancer, eastward of 130° east, and north of the Tropic of Cancer, eastwards of 135° east) had probably ever experienced malaria transmission. To the west of the Buxton line lie the highly malarious islands of the Western Pacific Ocean, including New Guinea and Melanesia, Indonesia and the Philippines, and the transiently malarious northern Australia. However, elsewhere across the Pacific Ocean in the huge area beyond the Buxton line up to the west coasts of the Americas, not even the Anopheles vectors of human malaria occur (22, 124). Indeed, the total absence of malaria from these tropical Pacific islands long endowed them with one of the most salubrious environments on Earth (42). As in the Americas, genetic polymorphisms attributed to selection under malaria are virtually absent from indigenous populations throughout this vast region (27).
MALARIA IN HISTORICAL TIMES
On the basis of general scientific evidence, we have tried, in the preceding sections, to portray human malaria as and where it may have been largely in our prehistoric past. In the following, we attempt to trace it, and its effects, through the period of historical record.
From the “Dawn of History” to the Nineteenth Century
Malaria seems to have been known in China for almost 5,000 years. The Nei Ching (The Canon of Medicine) from 4,700 years ago, apparently refers to repeated paroxysmal fevers associated with enlarged spleens and a tendency to epidemic occurrence (96). This suggests P. vivax. P. malariae is unlikely epidemic material. The absence of reference to “deadly effects” of these epidemics argues against the involvement of P. falciparum, and the date precedes the likely time of the emergence of P. falciparum out of Africa. This evidence indicates, therefore, that P. vivax, whether from out of Africa or from elsewhere, was by this time present across the Old World.
From the Indus valley in northern India, Vedic (3,500 to 2,800 years ago) and Brahmanic (2,800 to 1,900 years ago) scriptures contain many references to fevers, some of which are said almost certainly to concern malaria (18). In the Atharva-Veda there is, for example, an incantation to Takman, the fever demon, which runs as follows. “To the cold Takman, to the shaking one, and to the deliriously hot, the glowing, do I render homage. To him that returns on the morrow, to him that returns for two successive days, to the Takman that returns on the third day, shall homage be” (174). Tertian (P. vivax) and quartan (P. malariae) fevers are mentioned in the Vedas (155). They are also said to make reference to autumnal fevers as the “king of diseases” (167). In much later times, autumnal fever is certainly P. falciparum malaria (95, 108). Hence, we may infer that P. falciparum had reached India by around 3,000 years ago.
References to conditions that bear several of the hallmarks of malaria—fevers, intermittent and otherwise, and splenomegaly—have been quoted from Sumerian (169) and Egyptian (52) texts dating from 3,500 to 4,000 years ago and thus from slightly before the earliest Vedic writings. The Sumerian records apparently make frequent reference to deadly epidemic fevers (175). Could these have been the first onslaughts of P. falciparum from out of Africa? Although there seems to be nothing in the descriptions more definitively to identify these outbreaks as malaria, the marshy lands between the Tigris and the Euphrates, where Sumer stood, would have been prime environments for malaria transmission. Its long-term impact, however, could not have been that devastating. Mesopotamian civilizations flourished, rising and falling in vigorous, mutually inflicted succession, throughout historical antiquity. But the region would certainly have remained generally malarious. Over 2,000 years later, in 323 B.C., beyond Mesopotamia on the route to India, Alexander the Great is said to have died of malaria. If true, in a young, fit and healthy man, it would almost certainly have been P. falciparum malaria.
By this time, malaria had already appeared in the writings of the Greeks from around 500 B.C. Characteristically it seems to have coincided with human pioneering and land clearing for agriculture (95, 169). Before this time, references in Greek writings to recurring fevers seem to have been conspicuous by their absence (174). The works attributed to Hippocrates (460 to 377 B.C.)(95), however, leave no doubt about the presence of malaria in Greece in its benign tertian (P. vivax), quartan (P. malariae), and malignant subtertian (P. falciparum) forms.
Malaria seems not to have reached mainland Italy until the second century B.C. (28, 29). For a time, the marsh-ridden countryside around Rome, the Campagna, became a virtual desert, and by early imperial times Sicily and Sardinia had become notorious for summer and autumnal fevers. However, under the prosperity of the Roman Empire (circa 50 B.C. to 400 A.D.), by drainage, husbandry, and building development, malaria was excluded for several centuries from the Roman Campagna itself (28, 29). Then, as the Empire declined and the Campagna fell into ruin, Dark Age and early medieval Rome became surrounded, once more, by the dangerous and largely uninhabitable marshes known and feared by later generations (28, 29). These would have been hotbeds of P. vivax and especially P. falciparum. It is noteworthy, however, that in the one-and-one-half millennia until the present time, periods of several centuries of high malaria incidence on the Campagna seem to have alternated with similar periods when malaria was apparently absent (28, 29). These long episodes of absence and then presence of malaria have been associated with corresponding rising and falling agricultural and economic prosperity (28, 29, 84). They reflect, yet again, the dependence of malaria on prevailing human activity and life-style.
By the beginning of the Christian era, malaria was widespread around the shores of the Mediterranean, in southern Europe, across the Arabian peninsular, and in Central, South, and Southeast Asia, China, Manchuria, Korea, and Japan up to 40° of northern latitude. Malaria probably began to spread into northern Europe in the Dark and Middle Ages. Its path would have lain via France and Britain to the countries of the North Sea seaboard and by this route, and also from the Balkans, into central and eastern Europe. From here it would have crossed into southern Sweden and the Baltic states and thence, and also from the south from the Ukraine, the Caucases, and Central Asia, into Russia to the Volga basin and the Urals. Its progress was, however, probably uneven. In Britain, potential malaria hot spots, such as the English fens, were at first protected by Roman technology (154). Not, it seems, until late medieval times were these and other marshy areas of England, the Somerset levels, and the estuaries of the Rivers Solway and, above all, the Thames and Medway to become severely and enduringly blighted by endemic malaria (47, 154).
Meanwhile the arrival of Europeans and West Africans in the New World at the end of the 15th century A.D. introduced P. vivax, P. falciparum, and, also, perhaps, P. malariae for the first time. At first the malarious heartlands of the Americas were in the Caribbean and parts of Central and South America (13). From the mid-18th century, however, accompanying the economic growth of the Southern States of North America based on slaves brought from West Africa, malaria took firm hold across the North American continent. Throughout the next 100 years, as colonists moved westward across the United States of America and Canada, breaking the ground for agriculture, malaria sprung from the new-claimed land. By around 1850 A.D., it prevailed through the length and breadth of the tropical, subtropical, and temperate regions of the two American continents.
From the time of the voyages of Columbus until the mid-19th century, European trade and colonization in the tropics were marked by enormous losses of life from infectious disease. More deaths were registered as recognisably due to malaria than to any other disease, although in specific circumstances and locations the tolls from yellow fever and dysentery were often greater (20, 42-44, 156, 197). In the worst locations, as on the coasts of West Africa, mortality rates often exceeding 50% of a company per year of contact were the norm (42). From the mid-19th century onward, a chemically purified antimalarial component in Cinchona bark, quinine, entered widespread use among Europeans in West Africa. Overall mortality rates fell rapidly to less than one-quarter of those before the introduction of quinine. It showed the extent to which the previous mortality had, indeed, been the result mainly of malaria (42).
At some time during the 19th century, malaria reached its global limits. In absolute numbers and in the proportion of the humanity now affected, malaria was exacting its highest ever toll of sickness and death. Well over one-half of the world's population was at significant risk from malaria. Of those directly affected by malaria at least 1 in 10 could expect to die from it. The prosperity and well-being of all who lived within its reach were reduced greatly and usually catastrophically.
Then, from toward the end of the 19th century, throughout North America and Northern and Western Europe, malaria entered an inexorable decline toward its present extinction in these regions. Elsewhere, however, and especially in Asia, malaria's high tide was yet to come.
In the Twentieth Century
The following section is based on material prepared for “The World Health Report, 1999” (210). Details of sources and the approach to the analysis are given in the Appendix to this article. The data analysis itself is presented in Tables 3 and 4 and in Fig. 2 to 5.
FIG. 2.
Malaria mortality in the 20th century. The graph shows the total number of deaths due to malaria per year in Europe and North America (⧫—⧫); the Caribbean and Central and South America (▪——▪); sub-Saharan Africa (•——•); China and Northeast Asia (X—X); the Middle East, South Asia, and the Western Pacific (▴——▴); and worldwide (⧫---⧫) (see Appendix and Table 3).
FIG. 5.
Malaria mortality in the 20th century. The graph shows the number of malaria deaths per 10,000 population per year worldwide minus sub-Saharan Africa (◊--◊) and sub-Saharan Africa (•——•) (see Appendix and Table 3).
The mortality from malaria has, in recent decades, been due almost entirely to P. falciparum. However, in the early 20th century, repeated untreated infections of P. vivax, and prolonged infections of P. malariae, with their associated nephrosis, would have contributed significantly to the mortality due to malaria in Asia and in parts of the Americas and Europe. Throughout the century, global malaria-related deaths may never have fallen below half a million per year; they were probably at least 3 million per year during most of its first half. These figures suggest that somewhere in the region of 150 million to 300 million people have died from the effects of malaria during the past 100 years. If it is taken that around 6,000 million people have died during this period, malaria may be reckoned to have been a factor in between 2 and 5% of all deaths across the planet in the 20th century. It is possible to attribute up to 10% of global deaths to malaria in the early part of the century. In India it probably accounted for over half (31).
Malaria in Europe and the Americas.
Within Europe and North America, the malaria problem at the start of the 20th century was already diminishing. The process of the recession of malaria from Europe from the mid-19th century onward has often been represented as something of a mystery. The circumstances are, however, not difficult to explain. Beginning in northwestern parts of Europe, in England and The Netherlands, human health and rural environments and living conditions, and especially housing, were beginning to improve rapidly, so that contact between humans and vector mosquitoes was in decline. In addition, quinine was becoming cheap and widespread. The conditions for malaria transmission were therefore being steadily eroded, while at the same time the human hosts were becoming increasingly fit to control their infections.
Nevertheless, as the 20th century began, large areas of Europe and North America were still affected. These included the southern and midwestern United States, much of southern Europe, including the Mediterranean and Balkan countries, parts of northern Europe, still including areas of England and The Netherlands, and much of central Europe and southern Russia. Within the global context, however, the numbers of malaria deaths in Europe and North America, at a few tens of thousands per year, were relatively small (Table 3, row 1; Fig. 2 and 3).
FIG. 3.
Malaria mortality in the 20th century. The graph shows the number of malaria deaths per 10,000 population per year in Europe and North America (⧫—⧫); the Caribbean and Central and South America (▪——▪); sub-Saharan Africa (•——•); China and Northeast Asia (X—X); the Middle East, South Asia, and the Western Pacific (▴——▴); and worldwide (⧫—⧫) (see Appendix and Table 3).
Throughout the early part of the century, indigenous human malaria in Europe and North America continued its recession. This, as we have seen, was mainly as a result of the spontaneous decline in contact between human and vector populations, the by-product of rising prosperity. However, it was strongly aided by the vector control measures which began to be implemented from the beginning of the 20th century following the discovery of the role of Anopheles mosquitoes in the transmission of malaria. By the early 1950s, malaria had largely disappeared from North America and from almost all of Europe, including, in large degree, from the European parts of the then Soviet Union (Table 3, row 1). By the end of the 1960s, all these areas had been formally declared malaria free by the World Health Organization.
In Central America and the Caribbean (Table 3, row 2) the malaria problem in the 20th century had, on a per capita basis, been much more significant than that in North America. However, by the last decades of the 20th century, vector control, by house spraying with the residual insecticide DDT, had achieved vast reductions in the numbers of malaria-related deaths, although complete interruption of malaria transmission was not achieved.
In South America, certain countries have long been highly malarious, notably Suriname and the Guianas on the northeastern Atlantic coast. However, most of the population of this continent seem generally to have had little contact with malaria, simply because most people have always lived outside malaria-prone locations. Thus, the absolute amount of malaria-related mortality in the early part of the 20th century appears to have been much less in South America (Table 3, row 3) than in Central America and the Caribbean. Only locally would it have been intense, as in parts of the Amazon and on the northeastern Atlantic coast.
The control of malaria and malaria mortality in the locations of South America with endemic infection remain, however, a considerable problem. This is partly due to the size and remoteness of many of the areas affected. It is also complicated by the development of resistance of the parasites to antimalarial drugs, including chloroquine. Remarkably, chloroquine-resistant malaria parasites had not yet arisen in Central America at the close of the 20th century, in spite of the prevalence of chloroquine-resistant P. falciparum through South America since the early 1960s (145). A major engine of malaria in South America has been, and remains, pioneering and other human activity in previously uninhabited land. Conducted across the vast Amazon basin, these activities have created one of the most difficult problems of malaria control anywhere in the world.
Malaria in Asia and the Western Pacific.
From the beginning of the 20th century to its end, Europe and the Americas carried a small, and eventually a tiny, part of the global burden of malaria. By far the greatest part of this burden has always been in Asia and in Africa. In the first half of the 20th century, the malaria problem in Asia overwhelmed all others (Table 3, rows 4 and 5; Fig. 2 and 3).
On the Indian subcontinent in the early decades of the 20th century, epidemic malaria could kill tens of thousands to hundreds of thousands within a few months in a single region (30-32, 219). However, in spite of the lethality of the epidemics, their death tolls in South Asia were much lower than the malaria-related mortality that was sustained year upon year by the populations of these regions. Working in India at the turn of the century, Ronald Ross named malaria the “million murdering death” (166). Malaria, however, did not kill unassisted. Contemporary commentators often linked famine, a constant specter in Asia in the early part of the 20th century, with the huge malaria-related mortality of the Asian regions (30-32, 219). The frequently poor condition of the affected populations certainly contributed much to their susceptibility to the effects of malaria.
For almost two decades toward the middle of the 20th century, from the early 1930s to the late 1940s, war across Asia and the Western Pacific stirred the cauldrons of malaria. Records from these and other war zones (the American Civil War, the First World War) show large increases in malaria incidences and deaths among the military (13, 47, 64). Under the economic and environmental depredations that war produces, civilian populations would have been no less affected. Then, with the relative political stability which followed the end of the Second World War, the burden of malaria across most of this vast region began a period of remarkable decline (Table 3, row 4; Fig. 2 and 3).
In the late 1940s and early 1950s, national malaria control campaigns were established in almost all of the affected countries of the region, from the Middle East, through the Indian subcontinent and Southeast Asia, to the islands of the Western Pacific, including those of Indonesia and the Philippines. Under the broad direction and encouragement of the newly formed World Health Organization (58), and employing the residual insecticide DDT to spray homes, spectacular reductions in malaria incidence and malaria-related mortality were achieved, especially in India and Ceylon (now Sri Lanka) (142, 172). Crucial to this success was the simultaneous deployment throughout most of the affected areas of health delivery systems providing treatment with highly effective antimalarial drugs, most notably chloroquine. By the mid-1960s, malaria incidence in India and Sri Lanka was so low as to be almost undetectable by the monitors of the now huge national malaria-screening programmes.
However, in spite of this powerfully delivered effort, the anticipated goal, the eradication of malaria, was not achieved in any country of the region. The prohibitive economic and political costs of operating the malaria control campaigns, which from 1954 onward became formally instated by the World Health Organization as Malaria Eradication Campaigns (48), were not sustainable. This, combined with emerging resistance of the parasites and their vectors to the chemicals used to attack them, led, from the early 1970s, to resurgence of malaria transmission throughout southern Asia and the Western Pacific. Most damaging was the emergence of multidrug-resistant P. falciparum, including total resistance to chloroquine. Since the mid-1960s, chloroquine resistance has spread inexorably outward across the tropics of Asia and the Western Pacific and into Africa from a focus of origin in Southeast Asia (145).
These setbacks notwithstanding, however, a return to the previous huge malaria-related mortality rates in this vast section of the human population has never been remotely approached (Table 3, row 4; Fig. 2 and 3). The massive and sustained reduction of malaria-related mortality from the Mediterranean to the Western Pacific in the second half of the 20th century has been a truly outstanding, if precarious, success in the improvement of human health.
China followed its own path in the second half of the 20th century (Table 3, row 5; Fig. 2 and 3). The emergence of strong national government and the absence of major warfare must have contributed significantly to the reductions of its malaria burden in the decades that followed the end of the Second World War. However, political turmoil within China in the late 1960s and early 1970s prevented major advances. Then, in the mid-1970s, a determined antimalaria campaign was initiated in which vector control was integrated with rigorous malaria case detection and treatment (216). Malaria-related deaths in China may now be fewer than several hundred per year, compared with the hundreds of thousands that were sustained through the early decades of the century.
The Chinese antimalaria campaign yielded another outstanding contribution. This was the development of the artemisinin derivatives of Quinhaosu, a traditional Chinese herbal medicine, to combat chloroquine-resistant P. falciparum. In Vietnam, in Southeast Asia, in the early 1990s, artemisinin derivatives contributed dramatically to the reversal of a renewed rise in malaria mortality rates in the region (214). Today these compounds are at the heart of attempts to reformulate global malarial drug policy based on drug combinations which include artemisinin derivatives as a means of combating the emergence and spread of antimalarial drug resistance (212, 213).
Malaria in Africa.
Because of the massive burden of malaria in Asia in the early part of the 20th century, the campaigns against malaria in Asia in its second half were the response to a long-standing crisis. And because of the vulnerability of Asian malaria transmission to vector control measures using DDT and to the relative organizational and political stability that emerged across the region after the Second World War, these campaigns were practical and, as events have shown, to a large extent, if not completely, successful. By contrast, attempts by similar means to control malaria in sub-Saharan Africa had little success, except in its southern and most marginal zones of transmission.
Indeed, the malaria problems of Africa were, and are, of an altogether different type from those confronted anywhere else, both in human terms and in the biological factors that underlie African malaria transmission. Above all, the stability and intensity of malaria transmission in Africa presented two huge, actual or potential, problems. When global campaigns for malaria control were being planned at the World Health Organization in the late 1940s and early 1950s, there were many who doubted that even a tool as powerful as DDT could have a significant impact on African malaria because of the intensity of its transmission (48, 58). Moreover, and by the same token, it was questioned whether it was even safe to try to reduce malaria transmission intensities in tropical Africa because of the eventual loss of immunity in the older population (48, 58, 202, 203) and the consequent risk of epidemic malaria should control measures fail.
National malaria control organizations were nevertheless operational in many African countries by the 1950s. It must soon have become clear, however, that whatever may have been being achieved elsewhere by reducing malaria transmission using DDT, rather little effect was served by this approach in sub-Saharan Africa, except in certain limited circumstances and mainly in its southernmost parts. Nevertheless, a determined optimism reigned among the advocates of “global malaria eradication” and a policy of “intent to tackle the problem in Africa upon an eradication basis” seems to have persisted until at least 1996 (35). In the end, however, and as the goal of “malaria eradication” collapsed in most other malaria-endemic regions of the world (59), this aspiration for Africa also, and inevitably, died (48).
One location, which is effectively a part of the African region and in which malaria control succeeded virtually to the point of eradication, was in the highlands of the island of Madagascar in the Indian Ocean. Here, for around 20 years from the end of the 1960s to the early 1980s, malaria transmission was almost completely suppressed. Then, in 1986, the worst fears of those who had cautioned against malaria eradication campaigns in environments that naturally and/or potentially sustain intense malaria transmission were realised. With all natural immunity against malaria lost in the population and control measures now at a low level, malaria was reintroduced into the Madagascar highlands. For 2 years a severe epidemic raged, with high death rates in all age groups. Probably many tens of thousands lost their lives (45a).
Overall, however, Africa had benefited during the era of “malaria eradication.” It has benefited from a new availability of antimalarial drugs, especially chloroquine. Although drug distribution and access to treatment were relatively poor and largely uncontrolled, the effects during this period were real and evident. Malaria-related deaths in Africa showed evidence of relative (per head of population) decline from the 1950s to the early 1980s (Table 3, row 6; Fig. 3 and 5) (180). But then, from some time in the 1980s, the downward trend in malaria-related mortality appears to have reversed. In relation to total population and in relation to deaths from other causes, the numbers of childhood deaths from malaria in tropical Africa are almost certainly rising again (180, 210)(Table 3, row 6; Table 4, row 4; Fig. 3 and 5). The most likely cause is the spread throughout Africa, from its seedbeds in Southeast Asia, of chloroquine-resistant P. falciparum (180, 181, 190).
Death from Malaria in the Twentieth Century
The foregoing was a sketch of the general picture of malaria through the course of the last century. In the following section, we discuss some quantitative conclusions from this study. The data referred to are summarized in Table 4.
The first half of the twentieth century.
The relatively huge problem of malaria on and around the Asian continent (the Middle and Near East, the Indian subcontinent, Indo-China, the Western Pacific, China, and Northeast Asia) from the beginning to the middle of the 20th century has already been emphasized. As the century began, total malaria deaths stood at around 3 million per year (Table 4, row 3), representing around 30 lives taken by malaria per 10,000 population per year, or approximately 10% of all deaths across these combined regions at this time.
Of course, only a part of this population, perhaps half and perhaps considerably less, would have resided in significantly malarious locations. In these areas, including much of India, much higher proportions, in excess of 50%, of all deaths were probably due to malaria (31, 91, 112). In India, malaria deaths rates could reach 150 per 10,000 population per year across entire provinces, e.g., United Provinces, West Bengal (91, 111, 112, 176). However, because of the highly uneven distribution of malaria across India (91), many localities within these regions would have surpassed even these rates, to a point at which they became uninhabitable through the presence of malaria (91, 176).
In the same period, the early 20th century, in sub-Saharan Africa, with its intense, stable transmission, mainly of P. falciparum, the total malaria death toll was probably around 200,000 to 300,000 per year (Table 3, row 6; Table 4, row 4). This, at the time, represented only about 1/10 of the world malaria death toll (Fig. 4). Across tropical Africa, the rate of malaria deaths would have been relatively uniform and almost all concentrated in the very young. At around 20 to 30 malaria deaths per 10,000 total population per year, between 5 and 10% of all deaths in tropical Africa were due to malaria. In absolute and in relative terms, this was certainly a much lower overall toll than in the most malarious parts of Asia. It supports the proposition that low to medium malaria inoculation rates under unstable transmission conditions can be much more lethal than stable malaria transmission at almost any inoculation rate.
FIG. 4.
Malaria mortality in the 20th century. The graph shows the total number of deaths due to malaria per year worldwide minus sub-Saharan Africa (◊--◊) and in sub-Saharan Africa (•——•) (see Appendix and Table 3).
The annual malaria death toll for the whole of Europe and North America in the first decade of the 20th century was probably around 50,000 to 100,000 per year (Table 4, row 1). Remarkably, this is as much as one-third of the total malaria deaths in sub-Saharan Africa at the time. In the malarious parts of Europe, mainly around the Mediterranean shores and in the Volga basin, and in North America across almost the entire southern states and the Midwest, malaria mortality rates would have been similar to those in many parts of Asia. However, across Europe and North America as a whole, the overall risk of death from malaria was less than 2 per 10,000 total population per year. It contributed to no more than 1% of all the deaths in Europe and North America at the time.
Of all the continental regions, the least populous in the early 20th century were Central and South America, including the Caribbean. Absolute numbers of malaria deaths, at around 40,000 per year, were correspondingly low (Table 4, row 2). However, the relative risk was moderately high. Across the region as a whole, malaria took the lives of 5 to 10 of every 10,000 population per year. The distribution of malaria deaths would have extended through all ages, as in Asia and Europe.
The second half of the twentieth century.
Until the middle of the 20th century, the relative balance of malaria mortality in the different continental regions remained approximately the same (Fig. 2 and 3). From shortly after the Second World War, however, a huge transformation in the global burden of malaria began to take place (Fig. 2 to 5). It is this change which certainly gave rise to the recent common perception that “malaria had gone away.”
In Asia and the Western Pacific rim, the risk of death by malaria plummeted. At around 0.2 per 10,000 population per year across the entire region (Table 4, row 3), the malaria death rate is now less than 1% of what it had been at the beginning of the 20th century. Even in the most malarious countries of the region, such as Cambodia and Laos, no more than 1 to 2% under present circumstances, die from malaria. The situation in these two small countries is, today, held to be a continuing heart of malarial darkness. But even here, the lives culled by malaria are a fraction of those of little more than half a century before.
By the early 1960s, malaria in Europe and North America was extinct. Today, the chances of a European or a North American dying from malaria, other than through involvement in a malarious war zone, when the risk soars, is probably much less than 1 in 100,000 (Table 4, row 1), and it arises only through travel.
For the world as a whole, outside Africa, the chances of dying from malaria are around 1% of what they were at the beginning of the 20th century (Table 4, row 5; Fig. 4 and 5). Thus, from around 1 death in 10 at the century's start, malaria accounted for only about 1 in every 1,000 at its end (Table 4, row 5). Within Africa, however, the proverbial “one million children die of malaria every year” was, by the estimates shown here, finally reached in the last decade of the 20th century (Table 4, row 4) (Fig. 4) (177). Much as they were at the beginning of the 20th century, 5 to 10% of all those born in tropical Africa today are destined to die from malaria before they reach the age of 5 years (Table 4, row 4; Fig. 5).
STRATEGIES TO MANAGE THE BURDEN OF MALARIA TODAY
In most of the world today, therefore, we no longer experience the worst natural impacts of endemic malaria, its massive death tolls, and its otherwise crippling effects on individuals and communities. The reason is partly that the prevalence of malarial infection is now 1/10 or less of what it was at its height. However, in addition to its lower prevalence, malaria case fatality rates have also been reduced to around 1/10 of previous levels. This is because even in most of the world's poorest countries, access to treatment of malarial infections is sufficiently reliable that mortality from acute P. falciparum infection is usually prevented and prolonged, untreated malarial infection is rarely experienced.
But this applies mostly outside tropical Africa. Here, where improvements were, in any case, relatively small, the situation is once again deteriorating (Fig. 4 and 5). Elsewhere, across many tropical and subtropical regions, the problems of and created by malaria are also far from over. In this final section, we examine current global approaches to managing the malaria problem today.
Lessons from the Past
With the exceptions mainly of Europe and North America, elsewhere in the world the mid-20th century goal of malaria eradication was never realised. What was achieved, however, was an unprecedented reduction in the morbidity, and especially the mortality, due to malaria across vast regions of the tropical and subtropical world. For the most part, the types of tool that are available and are used for malaria control today are the same as those which were available during the “eradication” area. From this experience, both in its successes and in its failures, there are important lessons.
The first is that the tools of the last century were, and remain, very effective in reducing the malaria burden. These tools are (i) drugs with which to treat malaria, which not only reduce its immediate morbidity and mortality but also help to contract the human reservoir of malarial infection and hence its transmission, and (ii) methods to reduce human-mosquito contact and thus the transmission of infections, which include house spraying with chemical insecticides, the use of insecticide-treated bed nets and other materials, and other forms of vector control by nonchemical means such as those involving environmental and biological approaches.
The malaria eradication campaigns of the 1950s and 1960s were aimed at total interruption of malaria transmission by a strategy which entailed aggressive antimalarial activity over large geographical areas (117). By their very nature, these campaigns had to be “vertical” in administration and disease specific in operation. The second lesson learnt from that experience is that such highly disease-specific and intensive operations also have major inherent drawbacks. Not the least of these was the difficulty, and eventually the impossibility, of sustaining such efforts in the poorly resourced environments that malaria-endemic countries almost invariably are. With few exceptions, the goal of eradication was never reached, and most of those countries which were involved have been left with continuing burdens of malaria. These have been contained by “malaria control” programs ever since the unsustainable efforts toward “malaria eradication” were abandoned.
Another problem of the large, vertical programs was their effects on general health service delivery, which they tended to bypass and, in some respects, to disrupt. However, the malaria eradication campaigns also delivered some great and lasting benefits. These campaigns were the means of setting up countrywide infrastructures, including large numbers of field clinics for the diagnosis and treatment of malaria and offering many poor rural communities their first access to health services of any kind. These are still the mainstay of health delivery in many remote parts of these countries and are an invaluable legacy to the countries which participated in the malaria eradication campaigns.
The Malaria Situation Today
From “global eradication” to global gloom.
During the 1970s and 1980s, the health sectors of countries where malaria eradication had been attempted, in Asia and the Americas, went through substantial reforms. They moved from a vertical, disease-specific approach to one of supporting the delivery of a more integrated health service. During this period, countries also devolved many of their systems of administration, including the operation of their health systems, to provinces and districts and adopted primary health care as a key strategy (127, 193).
In parallel with these changes, however, the 1970s also marked the beginning of a period of trauma in the history of the struggle against malaria. The objective of malaria “eradication” had been abandoned (59). Some disarray followed as new strategies of malaria control and management were attempted. The difficulties were compounded by technical failures resulting from the development of resistance of the parasites to the antimalarial drug chloroquine and of the mosquito vectors to the insecticide DDT, the two “wonder tools” through which the recent previous success had been won (16, 78, 83, 125). Where, not long before, malaria had been reduced, in some countries almost to the point of extinction, renewed outbreaks began to occur.
Although its scale was relatively small compared to that in the first half of the century, the resurgence of malaria, and increasingly in drug-resistant forms, spread global despondency at the failure to resolve this major health issue.
The problem at hand.
In Asia and in most of the Americas, as we have thoroughly discussed, the malaria burden of today is a residue of what once prevailed. It is worst in remote, rural areas and in situations where there is civil unrest or other conflict. Thus, in Asia, some of the areas of highest malaria burden are in the Mekong region along international borders and in other conflict-stricken parts of the countries of the region (211). Similarly, in South America in the Amazon basin, malaria is brought to indigenous people by the incursions of commercial and pioneering activities (144). Elsewhere, for example in the Central Americas, in India and Sri Lanka, and in the islands of the Western Pacific, the burden of malaria is, by historical standards, not yet excessively high, but it persists at unacceptable levels (210). With the constant threat of unmanageable drug-resistant malaria, especially P. falciparum malaria, situations fluctuate between worse and better. Should antimalarial drugs fail completely, the global malaria situation would become catastrophic.
In tropical Africa, the geographical areas of malaria endemicity have remained largely unchanged for at least the past 100, and most probably for the past several thousand, years. It carries by far the greatest burden of malaria today. A prominent aspect of this situation is a lack of adequate health systems to effectively deliver antimalarial drugs or other interventions to those at risk. Africa is highly vulnerable to international failure to ensure the continuing supply of effective antimalarial drugs.
Current Global Approach to Controlling Malaria
The old vertical programs against malaria have been, largely if not completely abandoned. If there is a theme, it is to harness resources, and institutions—local, national, regional, and international—to use whatever means there are that can be brought to bear to reduce, or even contain, the burden of malaria. Rather than as separate and free-standing programs, however, these means are, wherever possible, integrated into the general health systems of countries. The focus is now on the areas of the world most in need and not, as in the past, on those which were susceptible to a particular method of control. These areas are the entire tropical region of Africa and, elsewhere in the world, the most improverished sectors of the populations affected by malaria. A key challenge is to achieve the sure sustainability of antimalarial efforts, however they may be formulated.
In striking contrast to the outlook in the mid-20th century, there is an implicit assumption that malaria will remain with us indefinitely. This is, for the time being at least, a realistic and a constructive attitude. There are, as there will always be in these circumstances, two goals in the management of malaria. These are (i) to treat those who are infected and sick with malaria as quickly and as effectively as possible and (ii) to reduce to the minimum the numbers of those who are at risk of becoming infected and ill with malaria (209).
Treating the sick.
The first goal, treating the sick, is entirely dependent on the effective use of antimalarial drugs delivered to malaria patients in a timely manner. In areas of intense transmission, this will include regular, intermittent prophylactic treatment of high-risk groups such as infants and pregnant mothers. To achieve this, health delivery systems will have to be vastly improved, especially in most of tropical Africa (77), and this will involve fundamental rethinking at many levels. The issues of near-the-home versus health facility-based treatment, public versus private channels of health care delivery, and interactions between governments and funding bodies, are all under active discussion between national governments and international and other agencies interested in health care delivery (137).
At present, in most parts of remote, rural Africa, formal health care systems can rarely be accessed in time when a child's life is at risk. Often this is as a result of malarial infection. Saving these lives will entail drug distribution systems operating near the home, through either community resource persons or drug vendors and other informal health care providers. Governments will have to assume a much greater stewardship role, coordinating delivery, ensuring quality of service through regulation and control, and creating an informed public demand for health care through education and societal movements (136, 138). Such responses to the malaria problem will also help greatly to strengthen general health care delivery systems in these regions of Africa.
At an entirely different level is the issue of the development, production, and availability of effective antimalarial drugs at prices affordable to those in need of them in the countries with endemic infection. Drug resistance, including multidrug resistance, has eliminated, or is rapidly eliminating, the cheap and previously very effective “wonder drug,” chloroquine (163, 164), and its increasingly more costly successors. The solution to this problem requires finding ways to engage the expertise and commitment of the research-based but market-driven global pharmaceutical industry, which holds the key to drug development. Until very recently, commercial interest in the development of new and effective antimalarial drugs for highly impoverished markets has been minimal. However, intensive dialogue between global health institutions and drug companies has resulted in public-private venture capital funds being established for drug discovery and development for malaria (161, 162; R. G. Ridley and W. E. Gutteridge, Proc. Méd. Front. Symp., 1999). Still further financial resources will be needed if such drugs are to be effectively deployed at prices affordable to the target populations.
Among the future tools against malaria, mention must be made of the immense efforts toward the development of protective vaccines against malaria. In spite of the duration of this struggle toward malaria vaccine development (perhaps 20 years of seriously goal-oriented effort to this point), progress has been inexorably forward. The number of clinically testable malaria vaccine candidates and formulations increases yearly. It is the aspiration that a practical blood stage vaccine would protect an individual against the clinical effects of malarial infection for at least several years. This would reduce the dependence of the individual on the health systems for treatment and care. It would also greatly relieve the burden on the health systems themselves.
Reducing the risk of malaria.
The second goal in the management of malaria, reducing the number of individuals at risk of malarial infection and illness, involves reducing human-mosquito contact by whatever means are practical. These means will certainly include, especially in Africa, the expanded deployment and use of insecticide-treated materials, bed nets, and curtains (81, 116, 143) for those at highest risk, namely, infants, young children, and pregnant women. Included in these approaches may be prophylactic interventions, such as can be linked to the Expanded Programme for Immunization initiative. It has been shown in an African setting that intermittent treatment with an antimalarial drug in the first year of life can greatly reduce the numbers of clinical episodes of malaria in infancy without appearing to increase the subsequent risk of infection through loss of early immunity (171).
Vaccines could also play a role in transmission reduction in the future by preventing the infection of mosquitoes (anti-sexual or mosquito stage malaria vaccines) and by preventing vaccinated individuals from becoming infected by mosquitoes (preerythrocytic stage malaria vaccines). Together with insecticide-treated materials and mosquito control measures, these effects of vaccination could greatly impede malaria transmission in a locality.
Other examples of reducing human-mosquito contact, which may be relevant especially in locations of low endemicity, mostly non-African locations, involve the appropriate construction and siting of housing and local environmental improvement. Inexpensive, high-technology approaches will apply computerized information management and Geographic Information Systems to identify locations where targeted attack on malaria transmission is called for. Monitoring, including by satellite, of all aspects and features of a malarious situation will be important to the timing and targeting of antimalarial interventions (25, 187).
Complications may arise, however, as a result of reduction of malaria inoculation rates. The relationship of stability and instability of malaria transmission to acquired immunity to malaria will be critical to how this turns out. Under conditions of stable malaria, immunity is both acquired and retained from high to low malaria inoculation rates. However, at very low inoculation rates under conditions of stable malaria, immunity is poor and older age groups become increasingly vulnerable to severe disease. Under conditions of unstable malaria transmission, except at very high malaria inoculation rates, there is generally little acquired immunity anyway, so that reductions in inoculation rate lead mainly to reduced incidence of severe disease. Potential loss of immunity accompanying transmission reduction must nevertheless be constantly monitored in all situations. Should it occur, it will show up as increasing numbers of cases of clinical malaria in older ages groups. It must be managed by ensuring adequate access to treatment with antimalarial drugs.
Managing epidemic malaria.
Epidemic malaria will remain a constant threat in regions which border on endemic malaria transmission zones. This is especially the case in highland areas adjacent to such zones and in lowland areas, at risk, for example, of widespread flooding, or, indeed, drought. In these locations, extreme weather and climatic fluctuations will periodically create the conditions for malaria epidemics. It should be noted that wherever malaria control in high-transmission areas begins to succeed, those populations will become vulnerable to epidemic malaria, as occurred in Madagascar in 1986. Monitoring and reliable communications, integrated with regional and global capacity to mount rapid distribution of drugs and other relief, must be ensured (140).
Global role in malaria management.
The global institutions which support the management of malaria remain at the heart of this enterprise. It is through them that those technical, economic, and political initiatives can be developed and implemented by which malaria-endemic countries can be enabled to operate, and to be supplied with the resources and materials needed for, their antimalarial efforts. To this end, international efforts are, for example, engaging the private, for-profit sector in setting up processes to ensure the continued development of effective and affordable antimalarial drugs and eventually of vaccines (161, 162; Ridley and Gutteridge, Proc. Méd. Front. Symp., 1999). Civil society and voluntary organisations are being encouraged to work with national government-run programs and the public sector health system. In their malaria control planning, governments are being encouraged to involve and coordinate a wide range of national initiatives. These include government economic and environmental policy planning, as well as enlisting communications, transport, and civil construction programs to the cause of managing malaria. Many of these are aspects in which current efforts to control malaria differ strategically from previous campaigns (136-138).
The resources required to sustain this multifaceted campaign against malaria can only come from international sources. They are much greater than are currently on-line. Nevertheless, through the publicity already mounted in support of global malaria control, the responses from some of world's largest economies to contribute to this effort through bilateral and multilateral arrangements are already encouraging (19).
CONCLUSIONS
How then, can we sum up the lessons of the impact of malaria, past and present, and how can they help us to deal with the disease today? Overall, we can say that the impact of malaria on our species has been very great. Where and how this has occurred has been, nevertheless, very variable. In one form or another, the burden of malaria continues to this day at an unacceptable level. This burden is composed not only of the direct effects of malaria but also of the great legacy of debilitating, and sometimes lethal, inherited diseases that have been selected under its impact in the past. These inherited illnesses will probably be among us for many generations to come.
In the future management of malaria, the tools available, drugs, insecticides, insecticide-treated materials, etc., will be of great importance. They are, individually and collectively, very effective instruments, although they are under constant threat from drug-resistant parasites and insecticide-resistant vectors. Even better malaria control would be achievable if new tools, such as vaccines, were to become available. However, even to maintain old tools, let alone to bring in the new, will require sustained investment of effort and resources from the wealthy nations of the world and on a much greater scale than is taking place at present.
Malaria is now one of the great diseases of poverty. Today no wealthy nation is affected by its endemic presence. In Europe, in the United States of America, and parts of China, there are whole regions which could not have achieved their current degree of prosperity had they been unable first to banish malaria. By contrast, in many other countries, and they include the poorest in the world, the magnitude of the malaria problem is simply overwhelming. The level of material support available for the management of malaria, even with the tools available today, is far below what is needed or what could be provided. And yet malaria-endemic countries are, by and large, better prepared than for many decades past to absorb, and to use to good effect, the resources that are needed to manage their malaria problems. Given the necessary global political will, and there is a tide running in its favor, much can be achieved by greater financial and organizational commitment to the battle against malaria.
Of the malaria problems in India at the start of the 20th century, Rickard Christophers wrote, “At the back of such colossal manifestations as the (malaria) epidemic of 1908… we shall do well to bear in mind the magnitude of the influence against which we are pitting ourselves, and not be led in foolish vein to reduce the remedy of the whole matter to trifling proportions.” The “magnitude of the influence” remained gargantuan for another half century. Yet today, throughout India, that “influence” has been reduced, by comparison, to a small remnant.
Thus, in the longer run of historical time, real improvements have taken place where once they may have seemed almost impossible. And, of course, long-term changes have, at other times, been for the worse. Nevertheless, and in final conclusion, it may be worth noting that we could, in principle, be truly rid of malaria. This possibility arises from the basic realities of malaria transmission. It could happen if, and when, life-styles and environments in all the currently malaria-endemic regions have reduced human mosquito contact below the levels that sustain malaria transmission under their prevailing climatic conditions. This, indeed, is the only basis for the permanent elimination of malaria. However, it could happen only in the presence of the necessary economic, political, and social development in all of the affected countries.
Limits of Uncertainty of the Estimates of Malaria-Related Mortality
The degrees of uncertainty associated with the estimates of malaria-related mortality as presented here are hard to assess. The estimates of malaria mortality rates in tropical Africa, especially for the second half of the 20th century, have been made from considerations of individual examples of recorded mortality rates. These estimates take into account the age distribution of malaria mortality in tropical Africa and assessments of the locations and sizes of the populations at risk. Given the relatively constant malaria mortality rates that these studies reveal within spans of a decade, the mean values that we have given are probably accurate to within less than a factor of 2. For the first half of the century, the mortality estimates for sub-Saharan Africa are absolute expectations from the recorded frequencies of the sickle cell gene. These compare well with recorded estimates of malaria mortality rates from the period covering the 1930s and 1940s (51).
Outside sub-Saharan Africa, the estimates for the second half of the 20th century use malaria reports of the World Health Organization Regional Offices. The estimated mortality data for each country for this period take account of human population size as well as malariological information. We used internal cross-referencing between computed numerical outcomes for different parameters, e.g., malaria incidence rates, mortality rates, and case fatality rates, in order to retain all of them within the limits of biological plausibility, as we have described previously (131). The outcomes are probably accurate within a factor of 2 or less.
The estimates for Europe and North America for the first half of the 20th century are all from national records of causes of mortality and could be expected to have a similar level of accuracy. For the rest of the Americas for the first half of the century, the situation is difficult to assess; the numbers given here are guesses extrapolated from recorded information from the 1940s and 1950s.
For the first half of the 20th century there are few national records of malaria statistics from Asia, including China. The values provided here are extrapolations from extensive and well-documented studies or statements by expert observers (see, e.g. references 30-32, 75, 91, and 176) and from authoritative analyses (see, e.g., references 111, 112, and 219). From the population sizes at risk and from representative malaria mortality rates within this region at different periods, the figures for Asian malaria-related mortality for the early part of the century seem unlikely to be in error by factors of more than 2 or 3 at most.
This analysis has used published statistics for human population sizes at each of the time points represented (87a, 91a, 173a, 205). Relative to the estimates of malaria mortality rates, it is assumed that population statistics have a high degree of accuracy and do not, therefore, contribute significantly to uncertainties associated with the analysis.
Country Composition of the Six Global Regions
The country names listed below do not always represent their current political designations but are used for convenience to encompass human populations wherever they have been located throughout the century.
Europe and North America. Albania, Andorra, Austria, Belgium, Bulgaria, Czechoslovakia, Denmark, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Spain, Sweden, Switzerland, Turkey (in Europe), Russia (in Europe), Yugoslavia, United Kingdom, small European states, Greenland, Canada, United States of America.
Central America and the Caribbean. Belize, Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama; Antigua, Bahamas, Barbados, Bermuda, Cayman Islands, Cuba, Dominican Republic, Haiti, Grenada, Jamaica, Martinique, St Vincent & Grenadines, Puerto Rico, Trinidad & Tobago, other Caribbean islands.
South America. Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Suriname, Venezuela, Peru, Paraguay, Uraguay, Chile, Argentina.
South Asia, Western Pacific, and Middle East. Afganistan, Bahrain, Iran, Iraq, Israel, West Bank, Jordan, Lebanon, Syria, Oman, Qatar, United Arab Emirates, Saudi Arabia, Kuwait, Yemen, Turkey (in Asia), Cyprus, Russia (in Asia)and Republics of the former USSR in Asia, Morocco, Algeria, Tunisia, Libya, Egypt, small states of the region; Pakistan, Bhutan, India, Nepal, Bangladesh, Burma, Sri Lanka, Thailand, Laos, Cambodia, Vietnam, Indonesia including Irian Jaya, Malaysia, Brunei, Philippines, Singapore, Japan, Papua New Guinea, Solomon Islands, Vanuatu, New Caledonia, Australia, New Zealand, Fiji, other Pacific islands.
China and North East Asia. People's Republic of China including the Autonomous Region of Tibet, Mongolia, North Korea, South Korea, Taiwan.
Africa, sub-Sahara. Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo, Djibouti, Equatorial Guinea, Ethiopia, Eritrea, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, Somalia, South Africa, Sudan, Swaziland, Tanzania, Togo, Uganda, Zaire, Zambia, Zimbabwe.
Sources used. (i) Malaria mortality. Europe and North America (13, 38, 62, 63, 64, 84, 184); Central America and Caribbean (50, 63, 79, 141, 167; World Health Organization, unpublished data); South America (69, 73, 74, 79, 141, 142, 208; World Health Organization, unpublished data); South Asia, Western Pacific, and the Middle East (3, 23, 30-32, 60, 61, 75, 79, 88, 91, 111, 112, 149, 176, 213, 219; World Health Organization, unpublished data); China and North East Asia (102, 109, 183); Africa, sub-Sahara; (3, 15, 24, 36, 45, 51, 80, 93, 103, 115, 120, 129, 141, 147, 151, 178, 181, 189, 190, 199; (Robert W. Snow, personal collection).
(ii) World and regional population sizes.
ADDENDUM IN PROOF
Since the final version of this manuscript was submitted for publication, three relevant studies have been published. In one (S. Tavare, C. R. Marshall, O. Will, C. Soligo, and R. D. Martin, Nature 416:726-729, 2002), the fossil record of primates is reviewed. This report shows that primate species occurred throughout the African and Eurasian continents, including present-day western Europe, from the late Oligocene (30 million years ago) to the late Pleistocene (a few hundred thousand years ago). It covers the period of 10 to 2 million years ago, at some time within which (55, 56, A. Escarante, personal communication), P. vivax and the primate malaria parasites most closely related to it probably diverged. Therefore, an hypothesis that this divergence took place in southern and/or southeast Asia would have no prima face basis if it depended solely upon the fact that most of the P. vivax-related malaria parasites are found in primates of these regions today. From the evidence of the primate fossil record, the parasite speciations which have led to today’s human P. vivax malaria could as likely have occurred in Europe or in Africa, as we and others (153) suggest.
The second report (J. Mu, J. Duan, K. D. Makova, D. A. Joy, C. Q. Huynh, O. H. Branch, W.-H. Li, and X.-Z. Su, Nature 418:323-326, 2002) concerns the antiquity of P. falciparum. Following the molecular evolutionary approach of previous workers (159, 194) to estimate the time of the most recent common ancestor of modern populations of P. falciparum, the report by Mu et al. involves the largest amount of P. falciparum DNA sequence examined in this context so far. Their results show higher rates of single nucleotide synonymous substitutions among P. falciparum genomes than were found in previous studies (159, 194). They imply that passage of the ancestors of modern P. falciparum through a tight population bottle neck, if indeed there ever was one, could not have occurred more recently than at least 100,000 years ago. This conclusion is not, however, in conflict with a view that significant recent adaptations may have occurred in populations ancestral to today’s P. falciparum. A small number of mutations selected under the influence of the profound environmental and ecological changes that were taking place in the period between 15 and 4 thousand years ago could have swept through the ancestral populations and led to the formation of modern P. falciparum and, moreover, could have done so without perturbing other features of its genome. In the companion article to that by Mu et al., evidence for selective sweeps of haplotypes in very small regions of the P. falciparum genome was demonstrated by Wootton et al. (J. C. Wootton, X. Feng, M. Ferdig, R. A. Cooper, J. Mu, D. I. Baruch, A. J. Magill, and X. Z. Su, Nature 418:320-323, 2002). These haplotype sweeps were in response to the very recent (within the past 50 years) pressures imposed upon P. falciparum by human use of the antimalarial drug chloroquine.
Acknowledgments
We thank Irwin Sherman, Hamza Babiker, and David Walliker for discussion and comment during the preparation of the manuscript, and we thank P. R. Arbani, Kevin Baird, James Banda, John Barnwell, Ananias Escalante, Renato Gusmao, Adetekumbo Lukas, Emily Lyons, Louis Miller, Guido Sabatinelli, Alan Saul, Allan Schapira, Robert Snow, Thomas Teuscher, and Peter Zimmerman for discussion, scientific comment, and advice.
APPENDIX
Sources of Data and Approaches to Their Analysis
The data presented in Tables 3 and 4 represent an attempt to quantify malaria-related mortality within six major geographical zones at different periods during the 20th century. The time points have been chosen to encompass intervals within which major historical changes have occurred and/or between which there have been significant developments in the malariological situation. The geographical divisions represent ones within which a broad, sometimes a very broad, unity of human and environmental conditions apply and between which clear distinctions can generally be made on the same grounds.
The sources and types of relevant data drawn upon in this synthesis are very variable. For all regions outside sub-Saharan Africa, they derive (i) from individual research reports and statements of “expert opinion” concerning malariological situations in different times and regions, (ii) from records of offices of national governments, and (iii) for the second half of the 20th century, from reports of the World Health Organization Regional Offices. For sub-Saharan Africa, estimates of malaria mortality rates in the earlier part of the century were made from the mortality rates implicit in the frequencies of the sickle cell gene in African populations and from a limited number of reports of malaria mortality rates in African for the period. The estimates for this region for the second half of the century depend entirely on contemporary published scientific reports of malaria mortality in children in tropical Africa. These include a number of such reports, including unpublished data, assembled by Robert W. Snow and used during the preparation of the “World Health Report, 1999” (177, 210). We are grateful for permission to include these data in the present analysis.
REFERENCES
- 1.Allen, S. J., A. O'Donnell, N. D. Alexander, M. P. Alpers, T. E. A. Peto, J. B. Clegg and D. J. Weatherall. 1997. Alpha +-thalassaemia protects children against disease caused by other infections as well as malaria. Proc. Natl. Acad. Sci. USA 94: 14736-14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, S. J., A. O'Donnell, N. D. Alexander, C. S. Mgone, T. E. Peto, J. B. Clegg, M. P. Alpers, and D. J. Weatherall. 1999. Prevention of cerebral malaria in children in Papua New Guinea by southeast Asian ovalocytosis band 3. Am. J. Trop. Med. Hyg. 60:1056-1060. [DOI] [PubMed] [Google Scholar]
- 3.Alles, H. K., K. N. Mendis, and R. Carter. 1998. Malaria mortality rates in South Asia and in Africa: implications for malaria control. Parasitol. Today 14:369-337. [DOI] [PubMed] [Google Scholar]
- 4.Allison, A. C. 1965. Population genetics of abnormal haemoglobins and glucose-6-phosphate dehydrogenase deficiency, p 365-391. In J. H. P. Jonxis (ed.), Abnormal haemoglobins in africa. Blackwell, London, United Kingdom.
- 5.Anderson, W. K. 1927. Malarial psychoses and neuroses. Oxford University Press, London, United Kingdom.
- 6.Anonymous. 1990. Severe and complicated malaria. Trans. R. Soc. trop. Med. Hyg. 84(Suppl. 2):1-65. [PubMed] [Google Scholar]
- 7.Ayala, F. J., A. A. Escalante, A. A. Lal and S. M. Rich. 1998. Evolutionary relationships of human malaria parasites, p. 285-300. In I. W. Sherman (ed.) Malaria: parasite biology, pathogenesis and protection. ASM Press, Washington, D.C.
- 8.Baer, A., E. Lie-Injo, Q. B. Welch, and A. N. Lewis. 1976. Genetic factors and malaria in the Temuan. Am. J. Hum. Genet. 28:179-188. [PMC free article] [PubMed] [Google Scholar]
- 9.Baird, J. K. 1995. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol. Today 11:105-111. [DOI] [PubMed] [Google Scholar]
- 10.Baird, J. K., T. R. Jones, E. W. Danudirgo, B. A. Annis, M. J. Bangs, H. Basri, Purnomo, and S. Masbar. 1991. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am. J. Trop. Med. Hyg. 45:65-76. [DOI] [PubMed] [Google Scholar]
- 11.Beet, E. A. 1946. Sickle cell disease in the Balovale district of Northern Rhodesia. East Afr. Med. J. 23:75-86. [PubMed] [Google Scholar]
- 12.Behrens, R. H., and C. F. Curtis. 1993. Malaria in travellers: epidemiology and prevention. Br. Med. Bull. 49:363-381. [DOI] [PubMed] [Google Scholar]
- 13.Boyd, M. F. 1941. An historical sketch of the prevalence of malaria in North America. Am. J. Trop. Med. 21:223-244. [Google Scholar]
- 14.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 15.Bruce-Chwatt, L. J. 1952. Malaria in African infants and children in southern Nigeria. Ann. Trop. Med. Parasitol. 46:173-200. [DOI] [PubMed] [Google Scholar]
- 16.Bruce-Chwatt, L. J. 1987. Malaria and its control: present situation and future prospects. Annu. Rev. Public Health 8:75-110. [DOI] [PubMed] [Google Scholar]
- 17.Bruce-Chwatt, L. J., C. Garret-Jones, and B. Weitz. 1966. Ten year study (1955-64) of host selection by Anopheline mosquitoes. Bull. W. H. O. 35:405-439. [PMC free article] [PubMed] [Google Scholar]
- 18.Bruce-Chwatt, L. J. 1965. Paleogenesis and paleoepidemiology of primate malaria. Bull. W. H. O. 32:363-387. [PMC free article] [PubMed] [Google Scholar]
- 19.Brugha, R., and G. Walt. 2001. A global health fund: a leap of faith? 323:152-154. [DOI] [PMC free article] [PubMed]
- 20.Bryson, A. 1847. Report on the climate and the principal disease of the African station. William Clowes and Son, London, United Kingdom.
- 21.Buck, R. A. J., and D. Eichenlaub. 1994. Prognostische Faktoren der Malaria tropica—Ergebnisse einer Evaluationsstudis in der Bundesrepublik deutschland 1963-1988. Gesundheitswesen 56:29-32. [PubMed] [Google Scholar]
- 22.Buxton, P. A., and G. H. E. Hopkins. 1927. Researches in Polynesia and Melanesia. (Parts I-IV), p. 67. London School of Hygiene and Tropical Medicine, London, United Kingdom.
- 23.Bynum, W. F. 1998. “Reasons for contentment”: malaria in India, 1900-1920. Parassitologia 40:19-27. [PubMed] [Google Scholar]
- 24.Carme, B., J. C. Bouquety, and H. Plassart. 1993. Mortality and sequelae due to cerebral malaria in African children in Brazzaville. Am. J. Trop. Med. Hyg. 48:216-221. [DOI] [PubMed] [Google Scholar]
- 25.Carter, R., K. Mendis, and D. Roberts. 2000. Spatial targeting of interventions against malaria. Bull. W. H. O. 78:1401-1411. [PMC free article] [PubMed] [Google Scholar]
- 26.Cattani, J. A., F. D. Gibson, M. P. Alpers, and G. G. Crane. 1987. Hereditary ovalocytosis and reduced susceptibility to malaria in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81:705-709 [DOI] [PubMed] [Google Scholar]
- 27.Cavalli-Sforza, L. L., P. Menozzi, and A. Piazza. 1994. The history and geography of human genes. Princeton University Press, Princeton, N.J.
- 28.Celli, A. 1925. Storia della malaria nell'agro Romano. Citta di Castello, Accademia del Lincei, Rome, Italy.
- 29.Celli, A. 1933. The history of Malaria in the Roman Campagna from ancient times. John Bales, Danielsson, London, United Kingdom.
- 30.Christophers, S. R. 1911. Malaria in the Punjab. Sci. Mem. Off. Med. Sanit. Dept. Gov. India (New Series) no. 46. Superintendent Government Printing, Calcutta, Inda.
- 31.Christophers, S. R. 1924. What disease costs India. Indian Med. Gazette 59:196-200. [PMC free article] [PubMed] [Google Scholar]
- 32.Christophers, S. R. 1949. Endemic and epidemic prevalence, p 698-721. In M. F. Boyd (ed.), Malariology. The W. B. Saunders Co., Philadelphia, Pa.
- 33.Ciochon, R. L., and A. B. Chiarelli (ed.). 1980. Evolutionary biology and continental drift. Plenum Press, New York, N.Y.
- 34.Ciuca, M., L. Ballif, and M. Chelarescu-Vieru. 1934. Immunity in malaria. Trans. R. Soc. Trop. Med. Hyg. 27:619-622. [Google Scholar]
- 35.Colbourne, M. J. 1966. Malaria in Africa. Oxford University Press, London, United Kingdom.
- 36.Colbourne, M. J., and G. M. Edington. 1954. Mortality from malaria in Accra. J. Trop. Med. Hyg. 57:203-210. [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Coluzzi, A. 1961. L'eradicazione della malaria. Una sfida al mondo. Ann. Sanita Pubblica 22:241-253. [PubMed] [Google Scholar]
- 39.Coluzzi, M. 1999. The clay feet of the malaria giant and its African roots: hypothesis and inferences about origin, spread and control of Plasmodium falciparum. Parassitologia 41:277-283. [PubMed] [Google Scholar]
- 40.Conway, D. J., C. Fanello, J. M. Lloyd, M. A.-S. Ban, A. H. Baloch, S. D. Somanath, C. Roper, A. M. J. Oduola, B. Mulder, M. M. Povoa, B. Singh, and A. W. Thomas. 2000. Origin of Plasmodium falciparum malaria is traced by mitochondrial DNA Mol. Biochem. Parasitol. 111:163-171. [DOI] [PubMed] [Google Scholar]
- 41.Conway, D. J., R. L. Machado, B. Singh, P. Dessert, Z. S. Mikes, M. M. Povoa, A. M. Oduola, and C. Roper. 2001. Extreme geographical fixation of variation in the Plasmodium falciparum gamete surface protein gene Pfs48/45 compared with microsatellite loci. Mol. Biochem. Parasitol. 115:145-156. [DOI] [PubMed] [Google Scholar]
- 42.Curtin, P. D. 1989. Death by migration: Europe's encounter with the Tropics in the nineteenth century. Cambridge University Press, New York, N.Y.
- 43.Curtin, P. D. 1990. The end of the “White Man's Grave”? Nineteenth-century mortality in West Africa. J. Interdisciplinary Hist. 21:63-88. [Google Scholar]
- 44.Curtin, P. D. 1994. Malarial immunities in nineteenth-century West Africa and the Caribbean. Parassitologia 36:69-82. [PubMed] [Google Scholar]
- 45.Delacollette. C., P. Van der Stuyft, K. Molima, C. Delacollette-Lebrun, and M. Wery. 1989. Etude de la mortalité globale et de la mortalité liée au paludisme dans le Kivu montagneux, Zaire. Rev. Epidem. Sante Publ. 37:161-166. [PubMed] [Google Scholar]
- 45a.de Zulueta, J. 1988. Report on a field mission in Madagascar, July 28-Sept 13, 1988. World Health Organization, Geneva, Switzerland.
- 46.Diamond, J. 1998. Guns, germs and steel. Vintage, London, United Kingdom.
- 47.Dobson, M. J. 1994. Malaria in England: a geographical and historical perspective. Parassitologia 36:35-60. [PubMed] [Google Scholar]
- 48.Dobson, M. J., M. Malowany, and R. W. Snow. 2000. Malaria control in East Africa: the Kampala Conference and the Pare-Tavete Scheme: a meeting of common and high ground. Parassitologia 42:149-166. [PubMed] [Google Scholar]
- 49.Dowling, M. A. C. 1951. The malaria eradication scheme in Mauritius. Br. Med. Bull. 8:72-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Downs, W. G., H. P. S. Gillette, and R. C. Shannon. 1943. A malaria survey of Trinidad and Tobago, British West Indies. J. Natl Malaria Soc. 2(Suppl.):1-44. [Google Scholar]
- 51.Duren, A. N. 1951. Essai d'etude sur l'importance du paludisme dans la mortalite au Congo Belge. Ann. Soc. Belge Med. Trop. 31:129-147. [PubMed] [Google Scholar]
- 52.Ebbell, B. 1937. The papyrus Ebers: the greatest Egyptian medical document. Copenhagen, Denmark.
- 53.Escalante, A. A., and F. J. Ayala. 1994. Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences. Proc. Natl Acad. Sci. USA 91:11373-11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escalante, A. A., and F. J. Ayala. 1995. Evolutionary origin of Plasmodium and other Apicomplexa based on rRNA genes. Proc. Natl Acad. Sci. USA 92:5793-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Escalante, A. A., E. Barrio, and F. J. Ayala. 1995. Evolutionary origin of human and primate malarias: evidence from the circumsporozoite gene. Mol. Biol. Evol. 12:616-626. [DOI] [PubMed] [Google Scholar]
- 56.Escalante, A. A., D. E. Freeland, W. E. Collins, and A. A. Lal. 1998. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc. Natl Acad. Sci. USA 95:8124-8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Escalante, A. A., H. M. Grebert, S. C. Chaiyaroj, M. Magris, S. Biswas, B. L. Nahlen, and A. A. Lal. 2001. Polymorphism in the gene encoding the apical membrane antigen-1 (AMA-1) of Plasmodium falciparum. X. Asembo Bay Cohort Project. Mol. Biochem. Parasitol. 113:279-287. [DOI] [PubMed] [Google Scholar]
- 58.Expert Committee on Malaria. 1948. Report of the Second Session. Malarial control survey and recommendations. Bull. W. H. O. 1:213-252. [Google Scholar]
- 59.Expert Committee on Malaria. 1974. Sixteenth report. W. H. O. Tech. Rep. Ser. 549. World Health Organization, Geneva, Switzerland. [PubMed]
- 60.Farinaud, M. E., J. Royer, and R. Choumara. 1950. Essai de demographie statistique, p. 25. In Haut Commissariat de France en Indochine, Infestation palustre et demographie dans les populations montagnards du Sud-Indochinois, Siagon, Suppl. Bull. Economique de l'Indochine, no. 22.
- 61.Farinaud, M. E., and R. Choumara. 1954. La prophylaxie du paludisme dans les pays Montagnards du Sud Vietnam. Bull. W. H. O. 11:793-837. [PMC free article] [PubMed] [Google Scholar]
- 62.Faust, E. C. 1939. Malaria mortality in the southern United States for the year 1937. Am. J. Trop. Med. 19:447-455. [Google Scholar]
- 63.Faust, E. C. 1941. The distribution of malaria in North America, Mexico, Central America and the West Indies, p. 8-18. In F. R. Moulton (ed.), A symposium on human malaria with special reference to North America and the Caribbean Region. American Association for the Advancement of Science, Washington, D.C.
- 64.Faust, E. C. 1949. Malaria incidence in North America, p. 749-763. In M. F. Boyd (ed.), Malariology. The W. B. Saunders Co., Philadelphia, Pa.
- 65.Flint, J., R. M. Harding, A. J. Boyce, and J. B. Clegg. 1993. The population genetics of haemoglobinopathies. Bailliere's Clin. Haematol. 6:215-262. [DOI] [PubMed] [Google Scholar]
- 66.Flint, J., A. V. S. Hill, D. K. Bowden, S. J. Oppenheimer, P. R. Sill, S. W. Serjeantson, J. Bana-Koiri, K. Bhatia, M. P. Alpers, A. J. Boyce, D. J. Weatherall, and J. B. Clegg. 1986. High frequencies of alpha-thalassaemia are the result of natural selection by malaria. Nature 321:744-750. [DOI] [PubMed] [Google Scholar]
- 67.Fontaine, R. E., A. E. Najjar, and J. S. Prince. 1961. The 1958 malaria epidemic in Ethiopia. Am. J. Trop. Med. Hyg. 10:795-803. [DOI] [PubMed] [Google Scholar]
- 68.Foo, L. C., V. Rekhraj, G. L. Chiang, and J. W. Mak. 1992. Ovalocytosis protects against severe malaria parasitaemia in the Malay aborigines. Am. J. Trop. Med. Hyg. 47:271-275. [DOI] [PubMed] [Google Scholar]
- 69.Gabaldon, A. 1969. Global eradication of malaria: changes of strategy and future outlook. Am. J. Trop. Med. Hyg. 18:641-656. [DOI] [PubMed] [Google Scholar]
- 70.Gallup, J. L., and J. D. Sachs. 2001. The economic burden of malaria. Am. J. Trop. Med. Hyg. 64(Suppl.):85-96. [DOI] [PubMed] [Google Scholar]
- 71.Garnham, P. C. C. 1996. Malaria parasites and other haemosporidia. Blackwell, London, United Kingdom.
- 72.Genton, B., F. Al-Yaman, C. S. Mgone, N. Alexander, M. M. Paniu, and M. P. Alpers. 1995. Ovalocytosis and cerebral malaria. Nature 378:564-565. [DOI] [PubMed] [Google Scholar]
- 73.Giglioli, G. 1930. Malarial nephritis: epidemiological and clinical notes on malaria, blackwater fever, albuminuria and nephritis in the interior of British Guiana. Churchill, London, United Kingdom.
- 74.Giglioli, G. 1972. Changes in the pattern of mortality following the eradication of hyperendemic malaria from a highly susceptible community. Bull. W. H. O. 46:181-202. [PMC free article] [PubMed] [Google Scholar]
- 75.Gill, C. E. 1936. Some points in the epidemiology of malaria arising out of the study of the malaria epidemic in Ceylon in 1934-35. Trans. R. Soc. Trop. Med. Hyg. 29:427-480. [Google Scholar]
- 76.Gilles, H. M., K. A. Fletcher, R. G. Hendrickse, R. Lindner, S. Reddy, and N. Allan. 1967. Glucose 6 phosphate dehydrogenase deficiency, sickling and malaria in African children in South Western Nigeria. Lancet i:138-140. [DOI] [PubMed] [Google Scholar]
- 77.Gilson, L., and A. Mills. 1995. Health sector reforms in sub-Saharan Africa: lessons of the last 10 years. Health Policy 32:215-243. [DOI] [PubMed] [Google Scholar]
- 78.Gish, O. 1992. Malaria eradication and the selective approach to health care: some lessons from Ethiopia. Int. J. Health Serv. 22:179-192. [DOI] [PubMed] [Google Scholar]
- 79.Gramiccia, G., and J. Hempel. 1972. Mortality and morbidity from malaria in countries where malaria eradication is not making satisfactory progress. J. Trop. Med. Hyg. 75:187-192. [PubMed] [Google Scholar]
- 80.Greenwood, B. M., A. K. Bradley, A. M. Greenwood, P. Byass, K. Jammeh, K. Marsh, S. Tulloch, F. S. J. Oldfield, and R. Hayes. 1989. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R. Soc. Trop. Med. Hyg. 81:478-486. [DOI] [PubMed] [Google Scholar]
- 81.Guillet, P., D. Alnwick, M. K. Cham, M. Neira, K. Mukelabai, M. Zaim, and D. Heymann. 2001. Long lasting treated mosquito nets: a break through in malaria prevention. Bull. W. H. O. 79:998. [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 83.Gusmao, R. 1999. Overview of malaria control in the Americas. Parassitologia 41:355-360. [PubMed] [Google Scholar]
- 84.Hackett, L. W. 1937. Malaria in Europe: an ecological study. Oxford University Press, London, United Kingdom.
- 85.Hadley, T., A. Saul, G. Lamont, D. E. Hudson, L. H. Miller, and C. Kidson. 1983. Resistance of Melanesian elliptocytes (ovalocytes) to invasion by Plasmodium knowlesi and Plasmodium falciparum malaria parasites in vitro. J. Clin. Investig. 71:780-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haldane, J. B. S. 1948. The rate of mutation of human genes. Hereditas 35(Suppl.):267-273. [Google Scholar]
- 87.Hamblin, M. T., and A. Di Rienzo. 2000. Detection of the signature of natural selection in humans: evidence from the Duffy blood group locus. Am. J. Hum. Genet. 66:1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87a.Harper Collins. 1997. Collins world atlas. Harper Collins, London, United Kingdom.
- 88.Harrison, M. 1998. “Hot beds of disease”: malaria and civilization in nineteenth century British India. Parassitologia 40:11-18. [PubMed] [Google Scholar]
- 89.Hartl, D. L., and A. G. Clark. 1997. Principles of population genetics. Sinauer, Sunderland, Mass.
- 90.Hay, S. I., D. J. Rogers, J. F. Toomer, and R. W. Snow. 2000. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: a literature survey, internet access and review. Trans. R. Soc. Trop. Med. Hyg. 94:113-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hehir, P. 1927. Malaria in India. Oxford University Press, London, United Kingdom.
- 91a.Heinemann Publishing. 1996-1997. Phillips geographical digest. Heine-mann Publishing, New York, N.Y. (Source: UN demographic yearbook.)
- 92.Higgs, D. R. 1993. α-Thalassaemia. Bailliere's Clin. Haematol. 6:117-150. [DOI] [PubMed] [Google Scholar]
- 93.Hill, A. 1992. Trends in childhood mortality in sub-Saharan mainland Africa, p. 10-31. In E. van de Walle, G. Pison, and M. Sala-Diakanda (ed.), Mortality and society in sub-Saharan Africa. Oxford University Press, Oxford, United Kingdom.
- 94.Hill, A. V. S., E. M. Catherine, D. Kwiatkowski, N. T. M. Anstey, P. Twumasi, P. A. Rowe, S. Bennett, D. Brewster, A. J. McMichael, and B. M. Greenwood. 1991. Common West African HLA antigens are associated with protection against severe malaria. Nature 352:595-600. [DOI] [PubMed] [Google Scholar]
- 95.Hippocrates. (English translation by W. H. S. Jones). 1923. Airs, waters and places VII, p. 83-85; XXIV, p. 133,-137; Epidemics I, p. 181,-211; Epidemics III, p. 251,-257; vol. IV, Aphorisms II, p. 115,; Aphorisms III, p. 125-153. Heinemann, London, United Kingdom; Putnam, New York, N.Y.
- 96.Hoeppli, R. 1959. Parasites and parasitic infections in early medicine and science. University of Malaya, Singapore, Singapore.
- 97.Holding, P. A., and R. W. Snow. 2001. Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence. Am. J. Trop. Med. Hyg. 64(Suppl.):68-75. [DOI] [PubMed] [Google Scholar]
- 98.Horuk, R., C. E. Chitnis, W. C. Darbonne, T. J. Colby, A. Rybicki, T. J. Hadley, and L. H. Miller. 1993. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science 261:1182-1184. [DOI] [PubMed] [Google Scholar]
- 99.Hughes, A. L., and F. Verra. 1998. Ancient polymorphisms and the hypothesis of a recent bottleneck in the malaria parasites Plasmodium falciparum. Genetics 150:511-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hutagalung, R., P. Wilairatana, S. Looareesuwan, G. M. Brittenham, and V. R. Gordeuk. 2000. Influence of hemoglobin E trait on the antimalarial effect of artemisinin derivatives. J. Infect. Dis. 181:1513-1516. [DOI] [PubMed] [Google Scholar]
- 101.James, S. P. 1920. Malaria at home and abroad. Bale and Danielson, London, United Kingdom.
- 102.Jamison, D. T., J. R. Evans, T. King, I. Porter, N. Prescott, and A. Prost. 1984. China. The health sector. The World Bank, Washington, D.C.
- 103.Janssens, P. G., I. H. Vincke, and J. Bafort. 1966. Le paludisme d'Afrique Centrale: son influence sur la morbidité et la mortalité des enfants. Bull. Soc. Pathol. Exot. 59:665. [Google Scholar]
- 104.Jarolim, P., J. Palek, D. Amato, K. Hassan, P. Sapak, G. T. Nurse, H. L. Rubin, S. Zhai, K. E. Sahr, and S. C. Liu. 1991. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc. Natl. Acad. Sci. USA 88:11022-11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeffrey, G. M. 1966. Epidemiological significance of repeated infections with homologous and heterologous strains and species of Plasmodium. Bull. W. H. O. 35:873-872. [PMC free article] [PubMed] [Google Scholar]
- 106.Jones, W. H. S. 1909. Malaria and Greek history. Manchester University Press, Manchester, United Kingdom.
- 107.Kaneko, A., G. Taleo, M. Kalkoa, J. Yaviong, P. A. Reeve, M. Ganczakowski, C. Shirakawa, K. Palmer, T. Kobayakawa, and A. Bjorkman. 1998. Malaria epidemiology, glucose-6-phosphate dehydrogenase deficiency and human settlement in the Vanuatu Archipelago. Acta Trop. 70:285-302. [DOI] [PubMed] [Google Scholar]
- 108.Kean, B. H., and J. A. Smith. 1944. Death due to estivo-autumnal malaria. Am. J. Trop. Med. Hyg. 24:317-322. [Google Scholar]
- 109.Kidson, C., and K. Indaratna. 1998. Ecology, economics and political will: the vicissitudes of malaria strategies in Asia. Parassitologia 40:39-46. [PubMed] [Google Scholar]
- 110.Kidson, C., G. Lamont, A. Saul, and G. T. Nurse. 1981. Ovalocytic erythrocytes from Melanesians are resistant to invasion by malaria parasites in culture. Proc. Natl. Acad. Sci. USA 78:5829-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klein, I. 1972. Malaria mortality in Bengal, 1840-1921. Indian Econ. Soc. Hist. Rev.9ii:132-160.
- 112.Klein, I. 1973. Death in India, 1871-1921. J. Asian Stud. 22iv:639-659. [PubMed] [Google Scholar]
- 113.Kudo, R. R. 1971. Protozoology. Charles C. Thomas, Springfield, Ill.
- 114.Kumar, S., and S. Blair Hedges. 1998. A molecular timescale for vertebrate evolution. Nature 392:917-920. [DOI] [PubMed] [Google Scholar]
- 115.Lamb, W. H., F. A. Foord, C. M. Lamb, and R. G. Whitehead. 1984. Changes in maternal and child mortality rates in three isolated Gambian villages over ten years. Lancet i:912-914. [DOI] [PubMed] [Google Scholar]
- 116.Lengler, C. 2000. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Database Syst. Rev. 2:CD000363. [DOI] [PubMed]
- 117.Litsios, S. 1996. The tomorrow of malaria. Pacific Press, Wellington, New Zealand.
- 118.Livingstone, F. B. 1958. Anthropological implications of sickle cell gene distribution in West Africa. Am. Anthropol. 60:533-562. [Google Scholar]
- 119.Livingstone, F. B. 1967. Abnormal hemoglobins in human populations. Aldine, Chicago, Ill.
- 120.Livingstone, F. B. 1971. Malaria and human polymorphisms. Annu. Rev. Genet. 5:33-64. [DOI] [PubMed] [Google Scholar]
- 121.Luo, H., A. Chauduri, V. Zbrzezna, Y. He, and A. O. Pogo. 2000. Deletion of the murine Duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Mol. Cell. Biol. 20:3097-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lysenko, A. J., and A. E. Beljaev. 1969. An analysis of the global distribution of Plasmodium ovale. Bull. W. H. O. 40:383-394. [PMC free article] [PubMed] [Google Scholar]
- 123.Macculloch, J. 1827. Malaria. Longman, Rees, Orme, Brown, and Green, London, United Kingdom.
- 124.MacDonald, G. 1957. The epidemiology and control of malaria. Oxford University Press, London, United Kingdom.
- 125.Manfredi, C. 1999. Can the resurgence of malaria be partially attributed to structural adjustment programmes? Parassitologia 41:389-390. [PubMed] [Google Scholar]
- 126.Mason, S. J., L. H. Miller, T. Shiroishi, J. A. Dvorak, and M. H. McGinniss. 1977. The Duffy blood group determinants: their role in the susceptibility of human and animal erythrocytes to Plasmodium knowlesi malaria. Br. J. Haematol. 36:327-335. [DOI] [PubMed] [Google Scholar]
- 127.Mayhew, S. 1996. Integrating MCH/FP and STD/HIV services: current debates and future directions. Health Policy Planning 11:339-353. [DOI] [PubMed] [Google Scholar]
- 128.Mbogo, C. N., R. W. Snow, C. P. Khamala, E. W. Kabiru, J. H. Ouma, J. I. Githure, K. Marsh, and J. C. Beier. 1995. Relationship between Plasmodium falciparum transmission by vector populations and the incidences of severe disease at nine sites on the Kenyan coast. Am. J. Trop. Med. Hyg. 52:201-206. [DOI] [PubMed] [Google Scholar]
- 129.McGregor, I. A., H. M. Gilles, J. H. Walters, A. H. Davies, and F. A. Pearson. 1956. Effects of heavy repeated malarial infections on Gambian infants and children. Br. Med. J. 2:686-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mendis, K. N., P. H. David, and R. Carter. 1991. Antigenic polymorphism in malaria: is it an important mechanism of immune evasion?” Immunol. Today 12:A34-A37 and & Parasitol. Today 7: A34-A37. [DOI] [PubMed] [Google Scholar]
- 131.Mendis, K. N., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64(Suppl.):97-106. [DOI] [PubMed] [Google Scholar]
- 132.Miller, L. H., S. J. Mason, D. F. Clyde, and M. H. McGuiness. 1976. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N. Engl J. Med. 295:302-304. [DOI] [PubMed] [Google Scholar]
- 133.Modiano, D., G. Luoni, B. S. Sirima, F. V. Simpores, A. Konate, E. Rastrelli, A. Olivieri, C. Calissano, G. M. Paganotti, L. D'Urbano, I. Sanou, A. Sawdogo, G. Modiano, and M. Coluzzi. 2001. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature 414:305-308. [DOI] [PubMed] [Google Scholar]
- 134.Molineaux, L. 1988. The epidemiology of human malaria as an explanation of its distribution, including some implications for its control, p 913-998. In W. H. Wernsdorfer and I. A. McGregor (ed.), Malaria. Churchill Livingstone, Edinburgh, United Kingdom.
- 135.Mourant, A. E., A. C. Kopec, and K. Domaniewska-Sobczak. 1976. The distribution of the human blood groups and other polymorphisms. Oxford University Press, London, United Kingdom.
- 136.Nabarro, D. N. 1999. Roll back malaria. Parassitologia 41:501-504. [PubMed] [Google Scholar]
- 137.Nabarro, D. N., and K. N. Mendis. 2000. Roll back malaria is unarguably both necessary and possible. Bull. W. H. O. 78:1454-1455. [PMC free article] [PubMed] [Google Scholar]
- 138.Nabarro, D. N., and E. M. Tayler. 1998. The “roll back malaria” campaign. Science 280:2067-2068. [DOI] [PubMed] [Google Scholar]
- 139.Najera, J. A. 1999. Prevention and control of malaria epidemics. Parassitologia 41:339-347. [PubMed] [Google Scholar]
- 140.Najera, J. A. 1994. Tropical diseases and socioeconomic development. Parassitologia 36:17-33. [PubMed] [Google Scholar]
- 141.Najera, J. A., and J. Hempel. 1996. The burden of malaria. Malaria Unit, Division of Control of Tropical Diseases, World Health Organisation, Geneva, Switzerland.
- 142.Newman, P. 1965. Malaria eradication and population growth: with special reference to Ceylon and British Guiana. Research series no. 10. Bureau of Public Health Economics, School of Public Health, University of Michigan, Ann Arbor.
- 143.N'Guessan, R., F. Darriet, J. M. Doannio, F. Chandre, and P. Carnevale. 2001. Olyset Net efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus after 3 years' field use in Cote d'Ivoire. Med. Vet. Entomol. 15:97-104. [DOI] [PubMed] [Google Scholar]
- 144.Pan American Health Organization and World Health Organization. 1999. Report on the meeting on “roll back malaria” initiative in the rain forest region of South America. PAHO/HCP/HCT/159/00. Pan American Health Organization, Washington, D.C.
- 145.Peters, W. 1987. Chemotherapy and drug resistance in malaria, vol. I and II. Academic Press, Ltd., London, United Kingdom.
- 146.Reference deleted.
- 147.Pison, G., J.-F. Trape, M. Lefebvre, and C. Enell. 1993. Rapid decline in child mortality in rural area in Senegal. Int. J. Epidemiol. 22:72-80. [DOI] [PubMed] [Google Scholar]
- 148.Powell, R. D., J. V. McNamara, and K. H. Rieckman. 1972. Clinical aspects of acquisition of immunity to Falciparum malaria. Proc. Helminthol. Soc. Wash. 39:51-68. [Google Scholar]
- 149.Powers, H. J. 1998. The role of chemotherapy in early malaria control and eradication programmes in Thailand. Parassitologia 40:47-53. [PubMed] [Google Scholar]
- 150.Pogo, A. O., and A. Chaudhuri. 2000. The Duffy protein: a malarial and chemokine receptor. Semin. Hematol. 2:122-129. [DOI] [PubMed] [Google Scholar]
- 151.Pringle, G. 1969. Experimental malaria control and demography in a rural East African community: a retrospect. Joint meeting of the Royal Society of Tropical Medicine and Hygiene and the Societe de Pathologie Exotique. Trans. R. Soc. Trop. Med. Hyg. 63(Suppl.):2-18. [Google Scholar]
- 152.Purvis, A. 1995. A composite estimate of primate phylogeny. Philos. Trans. R. Soc. London Ser. B 348:405-421. [DOI] [PubMed] [Google Scholar]
- 153.Qari, S. H., Y. P. Shi, N. J. Pieniazek, W. E. Collins, and A. A. Lal. 1996. Phylogenetic relationship among the malaria parasites based on small subunit rRNA gene sequences: monophyletic nature of the human malaria parasite Plasmodium falciparum. Mol. Phylogenet. Evol. 6:157-165. [DOI] [PubMed] [Google Scholar]
- 154.Rackham, O. 1994. The illustrated history of the countryside, p. 184-197. Phoenix Illustrated, London, United Kingdom.
- 155.Raina, B. L. 1951. Introduction to malaria problem in India. Bombay, India.
- 156.Ranford, O. 1983. Bid the sickness cease. John Murray, London, United Kingdom.
- 157.Ray, R. N., J. B. Chatterjea, and R. N. Chaudhuri. 1964. Observations on the resistance in HbE thalassaemia disease to induced infection with Plasmodium vivax. Bull. W. H. O. 30:51-55. [PMC free article] [PubMed] [Google Scholar]
- 158.Redmond, W. B. 1941. Immunity to human malaria: characteristics of immunity. p. 231-238. In F. R. Moulton (ed.) A symposium on human malaria with special reference to North America and the Caribbean Region, no. 15. American Association for the Advancement of Science, Washington, D.C.
- 159.Rich, S. M., M. C. Licht, R. R. Hudson, and F. J. Ayala. 1998. Malaria's Eve: evidence of a recent population bottleneck throughout the world populations of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 95:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reference deleted.
- 161.Ridley, R. G. 2001. A role for public-private partnerships in controlling neglected diseases? Bull. W. H. O. 79:771. [PMC free article] [PubMed] [Google Scholar]
- 162.Ridley, R. G. 2001. Putting the partnership into public-private partnerships. Bull. W. H. O. 79:694. [PMC free article] [PubMed] [Google Scholar]
- 163.Ringwald, P. 2001. Resistant malaria in children. Indian Paediatr. 38:9-14. [PubMed] [Google Scholar]
- 164.Ringwald, P., and R. Williams. 2001. Drug resistance in malaria. Pharmacol. News 8:15-20. [Google Scholar]
- 165.Ross, R. 1911. The prevention of malaria. John Murray, London, United Kingdom.
- 166.Ross, R. 1923. Memoires. John Murray, London, United Kingdom.
- 167.Russell, P. F. 1955. Man's mastery of malaria. Oxford University Press, London, United Kingdom.
- 168.Ruwende, C., S. C. Khoo, R. W. Snow, S. N. Yates, D. Kwiatkowski, S. Gupta, P. Warn, C. E. Allsopp, S. C. Gilbert, N. Peschu, C. I. Newbold, B. M. Greenwood, K. Marsh, and A. V. S. Hill. 1995. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 376:246-249. [DOI] [PubMed] [Google Scholar]
- 169.Sarton, G. 1959. A history of science. Harvard University Press, Cambridge, Mass.
- 170.Saul, A. 1999. Circumsporozoite polymorphisms, silent mutations and the evolution of Plasmodium falciparum. Parasitol. Today 15:38-40. [DOI] [PubMed] [Google Scholar]
- 171.Schellenberg, D., C. Menendez, E. Kahigwa, J. Aponte, J. Vidal, M. Tanner, H. Mshinda, and P. Alonso. 2001. Intermittent treatment for malaria and anaemia control at time of routine vaccination in Tanzanian infants: a randomised, placebo-controlled trial. Lancet 357:1471-1477. [DOI] [PubMed] [Google Scholar]
- 172.Shama, V. P. 1996. Re-emergence of malaria in India. Indian J. Med. Res. 103:26-45. [PubMed] [Google Scholar]
- 173.Sherman, I. W. (ed). 1998. Malaria, parasite biology, pathogenesis and protection. ASM Press, Washington, D.C.
- 173a.Showers, V. (ed.). 1989. World facts and figures, 3rd ed. John Wiley & Sons, Inc., New York, N.Y.
- 174.Sigerist, H. E. 1951. A history of medicine, vol. 1. Primitive and archaic medicine. Oxford University Press, New York, N.Y.
- 175.Sigerist, H. E. 1960. Problems of historical-geographical pathology, p. 299. In M. I. Roemer (ed.), Henry E. Sigerist on the sociology of medicine. MD Publications, New York, N.Y.
- 176.Sinton, J. A. 1935-1936. What malaria costs India, nationally, socially and economically. Rec. Mal. Surv. India 5:223-264, 413,-489 6: 92-169. [Google Scholar]
- 177.Snow, R. W., M. Craig, U. Deichmann, and K. Marsh. 1999. Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bull. W. H. O. 77:624-640. [PMC free article] [PubMed] [Google Scholar]
- 178.Snow, R. W., and K. Marsh. 1995. Will reducing Plasmodium falciparum transmission alter malaria mortality among African children? Parasitol. Today 11:188-190. [DOI] [PubMed] [Google Scholar]
- 179.Snow, R. W., J. A. Omumbo, B. Lowe, C. S. Molyneux, J. O. Obiero, A. Palmer, M. W. Weber, M. Pinder, B. Nahlen, C. Obonyo, C. Newbold, S. Gupta, and K. Marsh. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349:1650-1654. [DOI] [PubMed] [Google Scholar]
- 180.Snow, R. W., J.-F. Trape and K. Marsh. 2001. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 17:593-597. [DOI] [PubMed] [Google Scholar]
- 181.Sokhna, C. S., J. F. Molez, P. Ndiaye, B. Sane, and J.-F. Trape. 1997. Tests in vivo de chimiosensibilite de Plasmodium falciparum a la chloroquine au Senegal: evolution de la resistance et estimation de l'efficacite therapeutique. Bull. Soc. Pathol. Exot. 90:83-89. [PubMed] [Google Scholar]
- 182.Taliaferro, W. H. 1949. Immunity to the malarial infections, p. 941. In M. F. Boyd (ed.), Malariology. The W. B. Saunders Co., Philadelphia, Pa.
- 183.Tang, L. H., H. L. Qian, and S. H. Xu. 1991. Review: Malaria and its control in the Peoples Republic of China. S. E. Asia J. Trop. Med. Public Health 22:467-467. [PubMed] [Google Scholar]
- 184.Tchesnova, L. 1998. Socio-economic and scientific premises for forming the strategies against malaria in Russia under Soviet power. Parassitologia 40:103-108. [PubMed] [Google Scholar]
- 185.Thein, S. L. 1993. β-Thalassaemia. Bailliere's Clin. Haematol. 6:151-175. [DOI] [PubMed] [Google Scholar]
- 186.Thomson, J. G. 1933. Immunity in malaria. Trans R. Soc. Trop Med. Hyg. 26:483-514. [Google Scholar]
- 187.Thomson, M. C., and S. J. Connor. 2001. The development of malaria early warning systems for Africa. Trends Parasitol. 17:438-445. [DOI] [PubMed] [Google Scholar]
- 188.Tishkoff, S. A., R. Varkonyi, N. Cahinhinan, S. Abbes, G. Argyropoulos, G. Destro-Bisol, A. Drousiotou, B. Dangerfield, G. Lefranc, J. Loiselet, A. Piro, M. Stoneking, A. Tagarelli, G. Tagarelli, E. H. Touma, S. M. Williams, and A. G. Clark. 2001. Haplotype diversity and linkage disequilibrium at human G6PD: recent origin of alleles that confer malarial resistance. Science 293:455-462. [DOI] [PubMed] [Google Scholar]
- 189.Trape, J.-F, M. C. Quinet, S. Nzingoula, P. Senga, F. Tchichelle, B. Carme, D. Candito, H. Mayanda, and A. Zoulani. 1987. Malaria and urbanization in Central Africa: the example of Brazzaville. V. Pernicious attacks and mortality. Trans. R. Soc. Trop. Med. Hyg. 81(Suppl. 2):34-42. [DOI] [PubMed] [Google Scholar]
- 190.Trape, J.-F. G. Pison, M.-P. Preziosi, C. Enel, A. D. du Lou, V. Delaunay, B. Samb, E. Lagarde, J.-F. Molez, and F. Simondon. 1998. Impact of chloroquine resistance on malaria mortality. C. R. Acad. Sci. Paris 321:689-687. [DOI] [PubMed] [Google Scholar]
- 191.Trape, J.-F., and C. Rogier. 1996. Combating malaria morbidity and mortality by reducing transmission. Parasitol. Today 12:236-240. [DOI] [PubMed] [Google Scholar]
- 192.Trigg, P. I., and A. V. Kondrachine. 1998. The current global malaria situation, p. 11-22.In I. W. Sherman (ed.), Malaria: Parasite Biology, Pathogenesis and Protection. ASM Press, Washington, D.C.
- 193.Venediktov, D. 1998. Alma-Ata and after. World Health Forum 19:79-86. [PubMed] [Google Scholar]
- 194.Volkman, S. K., A. E. Barry, E. J. Lyons, K. M. Nielsen, S. M. Thomas, M. Choi, S. S. Thakore, K. P. Day, D. F. Wirth, and D. L. Hartl. 2001. Recent origin of Plasmodium falciparum from a single progenitor. Science 293:482-484. [DOI] [PubMed] [Google Scholar]
- 195.Wahlgren, M., and P. Perlmann (ed.). 1999. Malaria, molecular and clinical Aspects. Harwood Academic, Amsterdam, The Netherlands.
- 196.Waters, A. P., D. G. Higgins, and T. F. McCutchan. 1991. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc. Natl Acad. Sci. USA 88:3140-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Watt, J. 1979. Medical aspects and consequences of Captain Cook’s voyages, p. 129-157. In R. Fisher and H. Johnson (ed.), Captain Cook and his times. Douglas & Macintyre, London, United Kingdom.
- 198.Weatherall, D. J., and J. B. Clegg. 2001. Inherited haemoglobin disorders: an increasing global health problem. Bull. W. H. O. 79:704-712. [PMC free article] [PubMed] [Google Scholar]
- 199.Wernsdorfer, G., and W. Wernsdorfer. 1967. Malaria im mileren Nilbecken und dessen Randgebieten. Z. Tropenmed. Parasitol. 18:17-44. [PubMed] [Google Scholar]
- 200.Wernsdorfer, G., and W. H. Wernsdorfer. 1988. Social and economic aspects of malaria and its control, p. 1421-1471. In W. H. Wernsdorfer and I. A. McGregor (ed.), Malaria. Churchill Livingstone, Edinburgh, United Kingdom.
- 201.Whitefield, P. 1998. Evolution: the greatest story ever told. Marshall, London, United Kingdom.
- 202.Wilson, D. B. 1949. Malaria incidence in Central and Southern Africa, p. 800-809.In M. F. Boyd (ed.), Malariology. The W. B. Saunders Co., Philadelphia, Pa.
- 203.Wilson, D. B., P. C. C. Garnham, and N. H. Swellengrabel. 1950. A review of hyperendemic malaria. Trop. Dis. Bull. 47:677-698. [PubMed] [Google Scholar]
- 204.Wilson, R. J. M., and D. H. Williamson. 1997. Extrachromosomal DNA in the Apicomplexa. Microbiol. Mol. Biol. Rev. 61:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.World Bank. 1993. World development report 1993. Investing in health. Oxford University Press, New York, N.Y.
- 206.Reference deleted.
- 207.Reference deleted.
- 208.World Health Organization. 1992. World malaria situation in 1996. WHO Wkly. Epidemiol. Rec. 22:161-167, 23: 169-174. [Google Scholar]
- 209.World Health Organization. 1993. A global strategy for malaria. World Health Organization, Geneva, Switzerland.
- 210.World Health Organization. 1999. The world health report 1999. Rolling back malaria, p 49-63. World Health Organization, Geneva, Switzerland.
- 211.World Health Organization. 2000. Implementation of Roll Back Malaria in the six Mekong countries: report on the planning meeting. WHO/MAL/2000.1092. World Health Organization, Geneva, Switzerland.
- 212.World Health Organization. 2000. The use of antimalarial drugs. Report of a WHO informal consultation. WHO/CDS/RBM/2001.33. World Health Organization, Geneva, Switzerland.
- 213.World Health Organization. 2001. Antimalarial drug combination therapy. Report of a WHO technical consultation. WHO/CDS/RBM/2001.35. World Health Organization, Geneva, Switzerland.
- 214.World Health Organization, Western Pacific Regional Office. A message to share: the fight against malaria in Vietnam, in press. Prepared by C. Schuftan. World Health Organization, Geneva, Switzerland.
- 215.Yenchitsomanus, P., K. M. Summers, P. G. Board, K. Bhatia, G. L. Jones, K. Johnston, and G. T. Nurse. 1986. Alpha thalassaemia in Papua New Guinea. Hum. Genet. 74:432-437. [DOI] [PubMed] [Google Scholar]
- 216.Yip, K. 1998. Antimalarial work in China: a historical perspective. Parassitologia 40:29-38. [PubMed] [Google Scholar]
- 217.Zimmerman, P. A., I. Woolley, G. L. Masinde, S. M. Miller, D. T. McNamara, F. Hazlett, C. S. Mgone, M. P. Alpers, B. Genton, B. A. Boatin, and J. W. Kazura. 1999. Emergence of FY*A(null) in a Plasmodium vivax-endemic region of Papua New Guinea. Proc. Natl. Acad. Sci. USA 96:13973-13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Reference deleted.
- 219.Zurbrigg, S. 1994. Re-thinking the “human factor” in malaria mortality: the case of Punjab, 1868-1940. Parassitologia 36:121-135. [PubMed] [Google Scholar]