Abstract
Vesicular trafficking plays an important role in a virulence mechanism of the enteric protozoan parasite Entamoeba histolytica as secreted and lysosomal cysteine protease (CP) contributes to both cytolysis of tissues and degradation of internalized host cells. Despite the primary importance of intracellular sorting in pathogenesis, the molecular mechanism of CP trafficking remains largely unknown. In this report we demonstrate that transport of CP is regulated through a specific interaction of Rab7A small GTPase (EhRab7A) with the retromerlike complex. The amoebic retromerlike complex composed of Vps26, Vps29, and Vps35 was identified as EhRab7A-binding proteins. The amoebic retromerlike complex specifically bound to GTP-EhRab7A, but not GDP-EhRab7A through the direct binding via the carboxy terminus of EhVps26. In erythrophagocytosis the retromerlike complex was recruited to prephagosomal vacuoles, the unique preparatory vacuole of digestive enzymes, and later to phagosomes. This dynamism was indistinguishable from that of EhRab7A, and consistent with the premise that the retromerlike complex is involved in the retrograde transport of putative hydrolase receptor(s) from preparatory vacuoles and phagosomes to the Golgi apparatus. EhRab7A overexpression caused enlargement of lysosomes and decrease of the cellular CP activity. The reduced CP activity was restored by the coexpression of EhVps26, implying that the EhRab7A-mediated transport of CP to phagosomes is regulated by the retromerlike complex.
INTRODUCTION
Rab GTPases play an essential role to regulate intracellular membrane trafficking. The compartmentalization and functions of Rab proteins are modulated at multiple layers of mechanisms including isoprenylation of the carboxy terminus, nucleotide exchange, and binding of specific effector molecules (Stenmark and Olkkonen, 2001; Takai et al., 2001; Tuvim et al., 2001). Among these modulators, effectors play key roles in the control of 7-90 Rab proteins depending on organisms from fission yeast and amoeba to mammals and plants. A variety of effectors have been identified and shown to interact with specific Rab protein. For instance, regulatory secretions of neurotransmitters from neurons or insulin from pancreatic β-cells are regulated by the specific interaction of Rab3 with its effectors (Jahn and Sudhof, 1999). At least four proteins, Rabphilin3, Rim1, Rim2, and Noc2, are known to bind Rab3 and recruit cAMP-GEFII, 14-3-3, and zyxin, respectively, to regulate cAMP responsiveness, phosphoserine-dependent signaling, and the interaction with cytoskeleton (Kotake et al., 1997; Ozaki et al., 2000; Sun et al., 2003). The specificity of the Rab-effector interaction is attributable to primary sequence motifs in only a few cases including exophilins or Slip/Slac2, Rab3, and Rab27 effectors (Izumi et al., 2003). However, the majority of Rab effectors lack recognizable conserved binding motifs. For instance, Rab5 effectors, rabaptin-5, Rabex-5, EEA1, Rabenosyn-5, hVps34/p150, p110β/p35α, rabip4′, and rabankyrin-5, which are known to regulate the early endosomal trafficking through specific binding to Rab5 revealed no conserved binding motif (Zerial and McBride, 2001).
An enteric protozoan parasite Entamoeba histolytica is an etiological agent of amebiasis, which causes 50 million cases of amoebic colitis and liver abscess and leads to 100,000 deaths every year (Haque et al., 2003; Huston, 2004). E. histolytica reveals only two developmental stages, dividing trophozoite and dormant cyst stage, in its simplified life cycle (Ravdin, 1988). Despite being unicellular throughout its life cycle, this protist shows remarkable complexity of Rab proteins; it possesses more than 90 Rab genes in the genome, highlighting E. histolytica as an organism with extremely diverse and complex Rab functions (Loftus et al., 2005; Saito-Nakano et al., 2005). Vesicular trafficking plays an indispensable role for this organisms in nutrient uptake by ingestion and degradation of microorganisms in the large intestine, and also in secretion of cyto- and histolytic degradative proteins including cysteine proteases (CP; Que and Reed, 2000) and pore forming peptides (amoebapore: AP; Zhang et al., 2004), both of which have been implicated for colonization in mammalian tissues and evasion from host immune system. CP, consisting of >30 members of the gene family (Bruchhaus et al., 2003; Tannich, personal communication), is currently considered to be primarily responsible for the pathogenesis of E. histolytica as genes encoding certain isotypes, i.e., CP1 and CP5, are either absent or degenerated in a closely-related but nonpathogenic E. dispar species (Bruchhaus et al., 1996; Willhoeft et al., 1999). In addition, overexpression of CP2 caused augmentation of monolayer destruction, but no change in liver abscess formation (Hellberg et al., 2001). Antisense inhibition of CP5, a putative membrane-bound CP isotype, resulted in a reduced capacity of liver abscess formation (Ankri et al., 1999). These results suggest that both secreted and surface-located CPs are involved in pathogenesis in vitro and in vivo. The specific role of individual CP, except above-mentioned CP2 and CP5, remains totally unknown; neither is the mechanism of targeting to lysosomes and phagosomes nor secretion of CP. In addition to secreted CP, phagocytosis is also closely related to the virulence of E. histolytica. A phagocytosis-deficient mutant made by chemical mutagenesis was shown to be less virulent (Orozco et al., 1985). Interestingly, this mutant cell line was shown to secrete a significantly less amount of CP (Carpeniseanu et al., 2000). These data strongly suggest the shared molecular machinery was hampered in this mutant, which resulted in a defect in phagocytosis and CP secretion, which reinforces significance of vesicular trafficking in pathogenesis of this organism. We previously demonstrated that this parasite reveals an unprecedented vacuolar compartment, which we designated prephagosomal vacuole (PPV; Saito-Nakano et al., 2004). We showed that PPV serves as reservoir for processing, activation, and storage of digestive proteins, e.g., CP2 and amoebapore, before the transport to phagosomes. EhRab5 and one of the Rab7 isotypes, EhRab7A, are sequentially involved in the formation of PPV. EhRab7A, the closest homologue of human Rab7, is specifically involved in the PPV formation among the nine amoebic Rab7 isotypes (Saito-Nakano et al., 2005). The presence of multiple Rab7 isoforms has not been previously shown except for plants (Pereira-Leal and Seabra, 2001), suggesting that the mechanisms of Rab7 isotype-specific regulation must be present in this organism. Here we report that the EhRab7A interacts with a retromerlike complex that consists of three components of the mammalian and yeast retromers, Vps26, Vps29, and Vps35, but lacks the two other components Vps5 and Vps17 (Seaman, 2005). This retromerlike complex binds to EhRab7A in a GTP-dependent manner. Enlargement of lysosomes and reduction of CP activity, caused by the overexpression of EhRab7A, was compensated by overexpression of Vps26, suggesting that cellular transport and secretion of CP are regulated at least in part by specific interaction of EhRab7A with this novel Rab7-effector complex. To our knowledge this is the first demonstration of the retromerlike complex that plays a role in vesicular transport via a specific interaction with Rab small GTPases.
MATERIALS AND METHODS
Cells and Reagents
Trophozoites of E. histolytica strain HM-1:IMSS cl-6 (Diamond et al., 1972) were maintained axenically in Diamond's BI-S-33 medium (Diamond et al., 1978) at 35.5°C. Escherichia coli strain BL21 (DE3) and DH5α were purchased from Novagen (Madison, WI) and Life Technologies (Tokyo, Japan), respectively. All chemicals of analytical grade were purchased from Sigma-Aldrich (Tokyo, Japan) unless otherwise stated.
Plasmid Construction
Standard cloning techniques as previously described (Sambrook and Russell, 2001) were used for routine DNA manipulation, subcloning, and plasmid construction. To produce Escherichia coli recombinant proteins, an entire protein coding region of EhVps26, EhVps29, EhVps35, and the amino-terminal three fourths of EhVps26 (1-295 a.a., ΔC) was amplified from an E. histolytica cDNA library using a pair of appropriate primers, designed on the basis of the nucleotide sequences in the E. histolytica genome database (http://www.tigr.org), with restriction enzyme sites. The PCR products were cloned into appropriate sites of pET15b vector (Novagen, Madison, WI), and resulting plasmids were designated pHisVps26, pHisVps29, pHisVps35, and pHisVps26ΔC, respectively. Plasmids for the production of the amoeba lines expressing engineered EhRab7A or components of the retromerlike complex containing the three tandem c-myc (Myc)-epitopes or green fluorescent protein (GFP)-EhRab7A fusion protein were constructed essentially as previously described for EhRab5 (Saito-Nakano et al., 2004). In brief, the plasmids to express Myc-tagged EhVps29 and EhVps35 were constructed by insertion of the PCR-amplified EhVps29 or EhVps35 fragments into the SmaI-XhoI site of pKT-3M and designated as pKT-M29 or pKT-M35, respectively. A construct to express GFP-EhRab7A fusion protein was produced by inserting the PCR-amplified EhRab7A protein coding region into the SmaI-XhoI site of pKT-MG. To construct a plasmid for double expression, pM7-H was generated by eliminating the EhRab5 protein coding region from pM7-H5 (Saito-Nakano et al., 2004), followed by the insertion of an additional ClaI site downstream of the HA-tag. Plasmids to coexpress EhRab7A and EhVps26 or EhVps26ΔC were constructed by cloning a PCR-amplified EhVps26 or EhVps26ΔC fragment into the ClaI site of pM7-H.
Flow Cytometry
Amoeba trophozoites were adjusted to 1 × 106/ml in BI-S-33 medium with or without 2 μM Lysotracker Green (Molecular Probes, Eugene, OR). After incubation for 30 min at 36°C, trophozoites were washed with cold phosphate-buffered saline (PBS) three times and then analyzed on a FACSCalibur (Becton Dickinson, San Jose, CA) with CellQuest software.
Recombinant Protein Production
Histidine-tagged recombinant EhVps26, EhVps29, and EhVps26ΔC proteins were produced as follows. pHisVps26, pHisVps29, and pHisVps26ΔC were introduced into BL21 (DE3; Novagen, Madison, WI). Expression of these recombinant proteins was induced with 1 mM isopropyl-β-thiogalactoside at 30°C for 5-6 h. The histidine-tagged fusion proteins were purified using a HisTrap kit (Amersham Biosciences, Uppsala, Sweden), according to the manufacturer's instructions, essentially in previously described (Ali et al., 2004a). The HisVps26 and HisVps29 recombinant proteins were further purified using Mono Q anion exchange chromatography as described (Ali et al., 2004b). The recombinant EhRab5 and EhRab7A proteins fused to glutathione S-transferase (GST) at the amino terminus were produced as previously described (Saito-Nakano et al., 2004).
Antibodies
Anti-EhVps26 rabbit antibody was commercially produced at Kitayama Rabes (Nagano, Japan) using purified recombinant histidine-tagged protein. Anti-EhVps35 antibody was made by immunizing a rabbit with a synthetic peptide corresponding to the amino-terminal 22-amino acids (MSRPQRDSVFYSEEEQGNEVKR). Production of the anti-EhRab7A rabbit antibody was previously described (Saito-Nakano et al., 2004). Anti-Myc (9E10) was purchased from Covance (Berkeley, CA). Alexa Fluor anti-mouse and anti-rabbit IgG were obtained from Molecular Probes.
Affinity Purification of GST-Rab7A-binding Proteins
EhRab7A-binding proteins were isolated from the amoebic crude lysate using the GST-EhRab7A fusion protein immobilized on the glutathione 4B-Sepharose resin as described by Christoforidis and Zerial (2000) with some modifications. GST-EhRab5 or GST-EhRab7A were expressed in E. coli and immobilized onto glutathione-Sepharose 4B by incubation of the E. coli lysate with the resin for 1 h at 4°C, in PBS, pH 7.4, containing 200 μM GDP, 5 mM MgCl2, 5 mM 2-mercaptoethanol, Complete Mini EDTA free protease inhibitor cocktail (Roche, Basel, Switzerland), and 10 μg/ml transepoxysuccinyl-l-leucylamido-(4-guanidino)butane (E-64), and 0.1% Triton X-100, followed by extensive washing. The column-bound GST-Rab proteins were loaded with GTPγS by three rounds of guanine nucleotide replacement reactions with nucleotide exchange buffer (20 mM HEPES, pH. 7.5, 100 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol [DTT], 10 mM EDTA, 1 or 0.1 mM GTPγS, 10 μg/ml E-64) and nucleotide stabilization (NS) buffer (identical to nucleotide exchange buffer, without EDTA and containing 1 mM GTPγS). The resulting GST-Rab-immobilized resins were incubated for 2 h at 4°C with the amoebic lysate produced by sonication, centrifugation at 14,000 × g, and filtration in NS buffer with 100 μM GTPγS. After the resins were extensively washed with NS buffer, the GST-Rab-binding proteins were eluted by incubating the resins with 20 mM HEPES (pH. 7.5) containing 1.5 M NaCl, 20 mM EDTA, 1 mM DTT, 0.1 mg/ml E-64, and 5 mM GDP. For controls, the GST-EhRab5 and GST-EhRab7A proteins were loaded and washed with GDP, instead of GTPγS. The eluted proteins were subjected to SDS-PAGE. The putative EhRab7A-specific binding proteins were excised from Coomassie-stained gel, digested with trypsin, and analyzed by liquid chromatography and tandem mass spectrometry as previously described (Okada et al., 2005).
Recombinant Protein-binding Assay
After GST-EhRab7A was immobilized onto glutathione-Sepharose 4B and charged with GTPγS as described above, 1 μg of His-EhVps26FL (full length, 1-413 a.a.) or His-EhVps26ΔC (1-295 a.a.) with or without His-EhVps29 recombinant protein, purified as described above, was mixed with the GST-EhRab7A-bound resin in binding buffer (NS buffer containing 100 μM GTPγS, 10 mM imidazole, Complete Mini EDTA free protease inhibitor cocktail, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 0.1% Triton X-100) for 1 h at 4°C. After extensive washing with binding buffer, bound proteins were eluted with 20 mM HEPES, pH. 7.5, containing 30 mM EDTA, analyzed by SDS-PAGE and immunoblotted with anti-EhVps26 antibody.
Production of E. histolytica Cell Lines Expressing Epitope-tagged and GFP-fused EhRab7A and EhVps26/29/35
Plasmids generated as described above were introduced to the amoeba trophozoites by lipofection as previously described (Nozaki et al., 1999). Geneticin was added at a concentration of 1 μg/ml at 24 h after transfection and gradually increased for ∼2 wk until the geneticin concentration reached 6-10 μg/ml.
Immunoprecipitation and Immunoblotting
The amoeba transfectants transformed with pKT-M29 or pKT-M35 were harvested and lysed with lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM DTT, 1% Triton X-100 and 1 mg/ml E-64). A soluble lysate, after centrifugation at 14,000 × g and filtration, was mixed and incubated with monoclonal and polyclonal antibodies for 1 h at 4°C. After the mixture was further incubated with protein G-Sepharose for 1 h at 4°C, immune complexes bound to the resin were subjected to SDS-PAGE and immunoblot analyses as previously described (Sambrook and Russell, 2001). Primary antibodies were used at a 1:100 or 1:500 dilution in immunoblot analyses.
Anion-Exchange Chromatography
Approximately 2 × 107 E. histolytica trophozoites (250-300 mg wet weight) was resuspended in 1.0 ml of 100 mM Tris-HCl, pH, 9.5, 1.0 mM EDTA, 2.0 mM DTT, and 15% glycerol containing 10 μg/ml E-64 and the protease inhibitor mixture. After sonication, the lysate was centrifuged at 45,000 × g for 15 min at 4°C, filtrated, and dialyzed with the binding buffer (100 mM Tris-HCl, pH, 8.0, 1 mM DTT, 1 mM EDTA, 0.1 mM PMSF, 1 μg/ml E-64, and 10% glycerol). The sample (1 ml) containing 5 mg of soluble lysate protein was applied to a Mono Q 5/5 HR column preequilibrated with the binding buffer on the AKTA Explorer 10S system (Amersham Biosciences). After the column was extensively washed with the binding buffer, bound proteins were eluted with a linear gradient of 0-0.7 M NaCl. All of the fractions (0.3 ml) were subjected to trichloroacetic acid precipitation, and EhVps26 and EhVps35 were detected by immunoblots.
Yeast Two-Hybrid System
The MATCHMAKER GAL4 two hybrid system 3 (BD Biosciences Clontech, Palo Alto, CA) was used to examine interaction between EhRab7A and components of the retromerlike complex. A fragment encoding EhVps26ΔC (a.a. 1-295), EhVps26C (a.a. 296-413), or EhVps29 (full length) was inserted to pGAD vector with appropriate restriction enzyme sites. A fragment encoding EhRab7A (full length), EhRab5 (full length), EhVps29 (full length), EhVps35N (a.a. 1-305), EhVps35I (a.a. 306-582), or EhVps35C (a.a. 583-757) was inserted to pGBK vector. All the pGAD- or pGBK-derived plasmids were introduced into AH109 or Y187 yeast strain by the lithium acetate method. After diploids were produced by mating, positive yeast clones were selected on plates lacking leucine, tryptophan, and histidine. After 3 d, colonies were transferred onto secondary plates for growth monitoring and onto X-gal plates to assay for β-galactosidase activity.
Indirect Immunofluorescence Assay
Indirect immunofluorescence assay was previously described (Saito-Nakano et al., 2004). Briefly, amoeba transformant expressing Myc-EhRab7A was harvested and transferred to 8-mm round wells on glass slides. After amoebae were incubated with gerbil RBC, added to each well at 107 cells/ml, the amoebae were washed and fixed with 3.7% paraformaldehyde for 10 min. Ingested RBC were stained with diaminobenzidine as previously described (Novikoff et al., 1972). After washing, cells were permeabilized with 0.05% Triton X-100 for 10 min and reacted with primary antibody diluted at 1:500 (anti-EhVps26 antiserum) or 1:300 (anti-Myc monoclonal antibody [mAb]) in PBS containing 10% bovine serum albumin. The samples were then reacted with Alexa Fluora 488- or 568-conjugated anti-rabbit or anti-mouse secondary antibody (1:1000) for 1 h. The samples were examined on a Carl-Zeiss LSM 510 confocal laser-scanning microscope (Thornwood, NY). Images were further analyzed using LSM510 software.
CP Assay
Approximately 4 × 105 amoeba transformants were lysed in 100 μl of PBS by three freeze-thaw cycles, and debris was removed by centrifugation at 14,000 × g for 10 min. After preincubation of the lysate in the assay buffer (0.1 M KHPO4, pH 6.1, 1 mM EDTA, 2 mM DTT) at room temperature for 15 min, 200 μl of the preincubated enzyme mixture was mixed with z-Arg-Arg-7-amino-4-trifluorometylcoumarin substrate (ICN, Aurora, OH) at a concentration of 80 μM, and fluorescence emission at 505 nm with excitation of 400 nm was recorded for 15 min. z-Arg-Arg-7-amino-4-trifluorometylcoumarin was utilized as a standard. Specific activities were expressed in mmol z-Arg-Arg-7-amino-4-trifluorometylcoumarin produced per milligram of lysate protein. The significance of the data were evaluated by means of Student's t test.
RESULTS AND DISCUSSION
EhRab7A Overexpression Causes Enlargement of Vacuoles that Partially Overlaps the Acidic Compartment
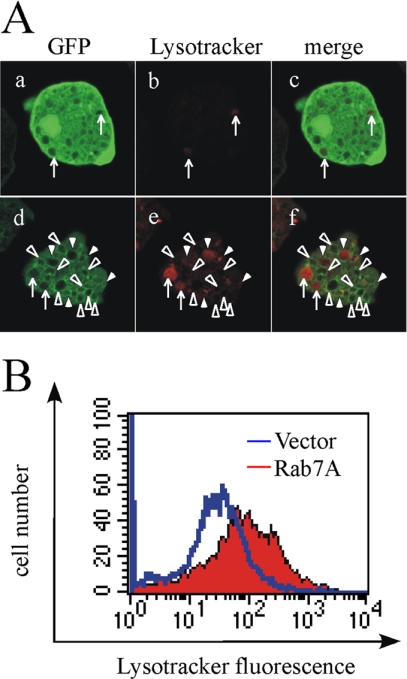
The presence of multiple (≥9) Rab7 isotypes likely infers complexity of lysosomal trafficking in this parasitic protozoon. To better understand the function and regulation of the representative Rab7, EhRab7A, which is the closest homologue of mammalian Rab7 and yeast Ypt7p (54-56% identity) but does not share interchangeable function with these homologues (Saito-Nakano et al., 2004), we first examined phenotypes of EhRab7A overexpression. GFP-EhRab7A overexpressing cells showed multiple enlarged vacuoles, compared with the GFP control transformant (Figure 1A). Because EhRab7A is present throughout the cytoplasm of the wild-type amoeba in steady state (i.e., without interaction with erythrocytes) as previously shown (Saito-Nakano et al., 2004), the formation of these vacuoles were likely attributable to a dominant active effect of GFP-Rab7A overexpression. This is comparable to the case in mammalian cells where overexpression of Rab7 or Rab7 effectors leads to morphological and functional changes of lysosomes. In mammals, overexpression of the dominant active GTP-locked Rab7 caused accumulation of perinuclear lysosomes and the augmented fusion of lysosomes, resulting in the enlargement of lysosomes. In contrast, overexpression of the dominant negative GDP-locked Rab7 caused diffusion of lysosomes to the periphery of the cells and decrease of EGF and LDL degradation (Bucci et al., 2000). Similar effects were also observed in overexpression of Rab7 effectors, i.e., rab-interacting lysosomal protein (RILP; Cantalupo et al., 2001) and Rab7-interacting RING finger protein (Rabring7; Mizuno et al., 2003). In contrast to mammalian Rab7, which is mainly associated with the acidic compartment (Bucci et al., 2000), <50% of GFP-EhRab7A-positive vacuoles were Lysotracker positive (Figure 1A), suggesting that EhRab7A is not primarily localized to late endosomes or lysosomes. The Lysotracker-stained vacuoles were enlarged in the GFP-EhRab7A overexpressor, suggesting that GFP-EhRab7A overexpression facilitates a heterologous fusion of EhRab7A-positive vesicles and late endosomes/lysosomes. To confirm this observation, we examined the acidity of the whole amoebae by measuring fluorescence intensities of the Lysotracker-stained acidic compartment of the EhRab7A-overexpressing and control transformants by FACS. FACS analysis showed a significant increase of the Lysotracker staining (a threefold increase in the peak channels, 32-95) in the EhRab7A-overexpressing transformant, compared with the control transformant (Figure 1B). This was not a consequence of overexpression of small GTPases in general because overexpression of irrelevant small GTPases, e.g., EhRab5 and EhRap2, showed no change in Lysotracker staining (unpublished data). These results suggest that overexpression of EhRab7A specifically resulted in the enlargement of the acidic compartment and the increase in the cell acidity. EhRab7A-overexpression affected neither endocytosis of fluid phase markers nor phagocytosis of 2 μm carboxylated beads (unpublished data).
Figure 1.
Subcellular localization of GFP-EhRab7A and enlargement of the acidic compartment caused by GFP-EhRab7A expression. (A) Subcellular localization of GFP-EhRab7A and Lysotracker in GFP-EhRab7A-overexpressing transformant. GFP- (a-c) or GFP-EhRab7A-overexpressing transformants (d-f) were incubated with 2 μM Lysotracker Red for 30 min. Cells were fixed and subjected to confocal microscopic imaging. Arrows indicate Lysotracker-positive and GFP-EhRab7A-negative vacuoles. Open or filled arrowheads indicate GFP-EhRba7A-positive vacuoles that are not stained with Lysotracker or stained with Lysotracker, respectively. (B) Histograms of Lysotracker fluorescence of Myc-EhRab7A-overexpressing (red) or mock transformants (blue) were shown. The transformants were stained with 2 μM Lysotracker Green for 30 min and then analyzed on a FACS Calibur.
Identification of a Retromerlike Complex as a Novel EhRab7A Effector
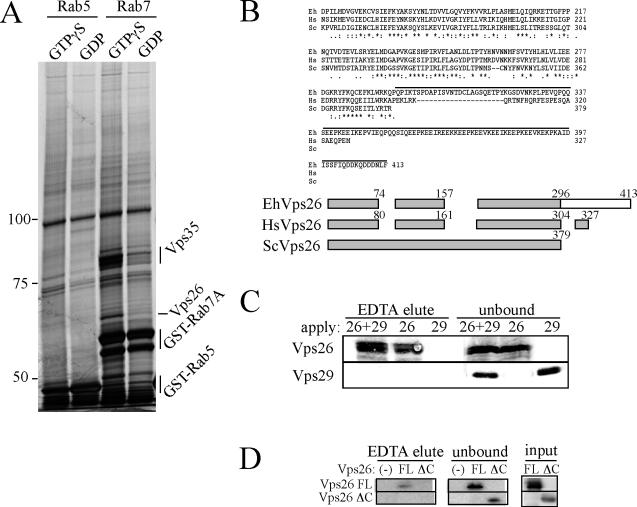
Homology-based genome-wide search of the putative effectors of the amoebic Rab7A using known Rab7 effectors from other organisms, including RILP, Rabring7, hVps34p/p150 (phosphatidylinositol 3-kinase), and proteasome α-subunit (XAPC7) indicated that this organism possesses homologues for Vps34/p150 and XAPC7, but lacks RILP and Rabring7, suggestive of the presence of novel Rab7 effectors in this organism (Cantalupo et al., 2001; Mizuno et al., 2003; Stein et al., 2003; Dong et al., 2004). Thus, we attempted to isolate the effector molecules of EhRab7A by affinity purification to gain an insight in the regulation of the amoebic Rab7A. The approach we took to isolate EhRab7A-specific and GTP-dependent effectors was similar to that previously described (Christoforidis and Zerial, 2000): EhRab7A-binding proteins were specifically bound to and eluted from the GST-EhRab7-immobilized resin by the removal Mg2+ and guanine nucleotide with EDTA. Although many common bands were eluted from both the GTPγS- and GDP-loaded GST-EhRab7A column, the two major bands were eluted significantly more from the GTPγS-loaded GST-EhRab7A column than from the GDP-loaded GST-EhRab7A column (Figure 2A). These two bands were not eluted from either the GTPγS- or GDP-loaded GST-EhRab5 column. Thus, the binding of these proteins appeared to be specific to the GTPγS-loaded EhRab7A. These two major bands were excised from the gel and subjected to de novo sequencing by LC-MS and MS/MS after trypsin digestion as described in Materials and Methods. The top and bottom bands were unequivocally assigned to the amoebic homologue of Vps35p and Vps26p, respectively, as 43 of 113 peptides derived from the top band and 27 of 78 peptides from the bottom band showed identity to the amoebic Vps35 and Vps26 homologues, respectively (39-51% coverage; unpublished data). Vps26 and Vps35 are known as components of retromer complex and are implicated for the retrograde transport of the hydrolase receptor from the late endosomes to the trans-Golgi apparatus (Seaman, 2005).
Figure 2.
Association of EhRab7A with EhVps26, EhVps29, and EhVps35. (A) SDS-PAGE profiles of EhRab7A-binding proteins isolated from the E. histolytica lysate. GST-EhRab7A- or GST-EhRab5-immobilized columns were loaded with GTPγS or GDP and then incubated with the amoeba lysate. Bound proteins were eluted and analyzed by SDS-PAGE, followed by silver staining. Molecular-weight standards are indicated on the left of the gel (kDa). EhVps35 and EhVps26, identified by liquid chromatography and tandem mass spectrometry, as well as costripped GST-EhRab5 and GST-EhRab7, are also indicated. (B) A multiple alignment (top, only the carboxyl half is shown) and a schematic diagram (bottom) of Vps26 from E. histolytica, human, and yeast. The alignment was created by the Neighbor-Joining method using CLUSTAL W version 1.81 with the Blosum matrix. Identical residues are marked with asterisks and conserved amino acid substitutions are marked with either dots or colons. A unique carboxyl-terminal extension present in EhVps26 is overlined (top) or shown as an open box (bottom). (C and D) In vitro binding of recombinant EhRab7A with EhVps26 and EhVps29 (C), and of recombinant EhRab7A with EhVps26FL and EhVps26ΔC (D). The GST-EhRab7A-immobilized column was loaded with GTPγS and incubated with histidine-tagged EhVps26, EhVps29, or both (C), or histidine-tagged EhVps26FL or EhVps26ΔC (D). A fraction eluted from the resin with EDTA and an unbound fraction were analyzed by immunoblotting with anti-EhVps26 (C and D) and EhVps29 (C) antibodies.
The retromer complex is composed of four or five components: Vps5p, Vps17p, Vps26p, Vps29p, and Vps35p in yeast (Reddy and Seaman, 2001) and Vps5p-homologue sorting nexin1 (SNX1), Vps26, Vps29, and Vps35 in mammals (Haft et al., 2000). However, only Vps26, Vps29, and Vps35, but not Vps5, SNX1, or Vps17, are present in the genome database of E. histolytica (Loftus et al., 2005). Because of the lack of these two components, the E. histolytica retromerlike complex likely plays a different role. Size fractionation by gel filtration revealed that the amoebic retromerlike complex showed an apparent molecular weight of 300-500 kDa (unpublished data), which is comparable to that in mammalian cells (Haft et al., 2000). The missing components, sorting nexins and Vps5/17, possess PX domain and BAR domain for the interaction with phosphatidyl inositol 3-phosphate and the curbed membrane (Seaman and Williams, 2002; Habermann, 2004; Seaman, 2005). We found proteins possessing either the BAR domain or the PX domain, but failed to find a protein containing both domains in the genome database. It is conceivable that a combination of these proteins or not-yet-identified accessory proteins play a compensatory role in the complex formation. The interaction between the retromerlike complex and EhRab7A was dependent on the GTP-active state of the Rab protein (Figure 2A), suggesting that this interaction occurs solely to fusion-competent EhRab7A-vesicles/vacuoles.
Identification and Features of Vps26, Vps35, and Vps29 of the Amoebic Retromerlike Complex
It has been shown that retromer complex is localized on endosomes at steady state and functions in the retrograde transport of cation independent mannose-6-phosphate receptor (CI-MPR) from late endosomes to the trans-Golgi network (TGN; Arighi et al., 2004; Seaman, 2004). Predicted homologues of Vps26, Vps35, and Vps29 from E. histolytica (EhVps26, EhVps35, and EhVps29, respectively) were 413-, 757-, and 185-amino acids long with a predicted molecular mass of 48.0, 87.2, and 20.8 kDa and a pI of 4.96, 5.46, and 5.56, respectively. Protein alignments and pair-wise comparisons revealed that the amoebic Vps35 and Vps29 possess 22-27 and 35-56% overall identity throughout proteins to both yeast and mammalian counterparts (unpublished data). The amoebic Vps26 shares 38-44% positional identity to the yeast and mammalian counterparts at the amino-terminal three fourths; however, it possesses an ∼117 a.a.-long carboxyl-terminal extension that is rich in charged amino acids (29 negatively and 14 positively charged a.a. in 75 a.a.) and absent in the yeast and mammalian homologues (Figure 2B). This unique region showed no significant homology to any known domains.
Binding of EhRab7A to the Retromerlike Complex is Mediated by EhVps26
To identify a component of the complex that is responsible for the binding to EhRab7A, we examined the binding of each component to EhRab7A. Because we failed to produce a soluble recombinant EhVps35 protein, we tested if GST-EhRab7A directly binds to histidine-tagged EhVps26 and EhVps29. The GTPγS-loaded GST-EhRab7A was immobilized to the glutathione Sepharose resins, as described above, and mixed with histidine-tagged EhVps26, EhVps29, or both. After the resins were extensively washed, the bound proteins were eluted by EDTA (Figure 2C). The recombinant EhVps26 protein bound to the GST-EhRab7A-immobilized resins in the either presence or absence of EhVps29, whereas EhVps29 did not bind to GST-EhRab7A independent of the presence or absence of EhVps26, suggesting that EhVps26 directly binds to EhRab7A. Furthermore, EhVps26 and EhVps29 do not directly interact.
To further determine the region within EhVps26 responsible for the binding to EhRab7A, the binding between GST-EhRab7A and a full-length His-EhVps26 (EhVps26FL, 1-413 a.a.) or His-EhVps26ΔC (1-295 a.a.) lacking the carboxyl terminal region unique to E. histolytica (Figure 2B) was examined. His-EhVps26FL was found to bind to GTPγS-loaded GST-Rab7A and eluted from the resin by EDTA, whereas His-EhVps26ΔC failed to bind to and be eluted from the resin (Figure 2D). These data suggest that the unique carboxyl-terminal region of EhVps26 is responsible for the binding to EhRab7A, and thus, this interaction may be unique to E. histolytica.
We next examined the binding between EhRab7A and the retromerlike complex and among the components of the retromerlike complex by yeast two-hybrid system. We verified a direct interaction between the amino-terminal three fourths (a.a. 1-295) of EhVps26 (EhVps26ΔC) and the amino-terminal third (a.a. 1-305) of EhVps35 (EhVps35N) and between the carboxyl-terminal fourth (a.a. 583-757) of EhVps35 (EhVps35C) and EhVps29, as well as lack of interaction between EhVps26 and EhVps29 by yeast two-hybrid system (unpublished data). These interactions are similar to those found for the mammalian retromer complex (Haft et al., 2000). On the other hand, we failed to detect the binding between EhRab7A (neither wild-type nor GTP-locked form) and EhVps26 (full-length, amino-, or carboxyl-terminus; unpublished data), which indicates that the interaction is transient and weak in this assay system. Alternatively, only a small fraction of EhRab7A, when recruited to the membrane of PPV and phagosomes, interact with the retromerlike complex.
Association of EhRab7A, EhVps26, EhVps29, and EhVps35 in the Amoeba
We attempted to verify by immunoprecipitation that EhRab7A interacts with the retromerlike complex containing EhVps26 and EhVps35 and also examined if another ubiquitous component EhVps29 is associated with this Vps26/Vps35 complex in the amoeba. We first attempted to coprecipitate the complex with anti-Myc antibody from the transformants expressing Myc-tagged EhVps29 or EhVps35. Immunoprecipitation of Myc-tagged EhVps29 from Myc-EhVps29-expressing transformants by anti-Myc antibody coprecipitated EhVps26 and EhVps35 (Figure 3A, top panel, lane 6). Similarly, anti-Myc antibody pulled down EhVps35 and EhVps26 from Myc-EhVps35-expressing transformants (Figure 3A, bottom panel, lane 6). Conversely, anti-EhVps26 antibody coprecipitated Myc-EhVps29 (Figure 3A, top panel, lane 4) and Myc-EhVps35 (Figure 3A, bottom panel, lane 4), whereas these proteins were not coprecipitated by a preimmune serum from either of these two transformants (Figure 3A, top and bottom panels, lane 1). In addition, the interaction between Myc-EhVps35 and endogenous EhVps29 was also confirmed by the mass spectrometric analysis of 20-kDa protein coprecipitated with Myc-EhVps35 (unpublished data). These results clearly showed that EhVps26, EhVps29, and EhVps35 form a complex in vivo. Although we failed to detect EhRab7A in the complex in these experiments, we were able to confirm the association of EhRab7A with the retromerlike complex in a converse way. Anti-EhRab7A antibody coprecipitated Myc-tagged EhVps29, EhVps35 (Figure 3A, top and bottom panels, lane 3), and EhVps26 (unpublished data).
Figure 3.
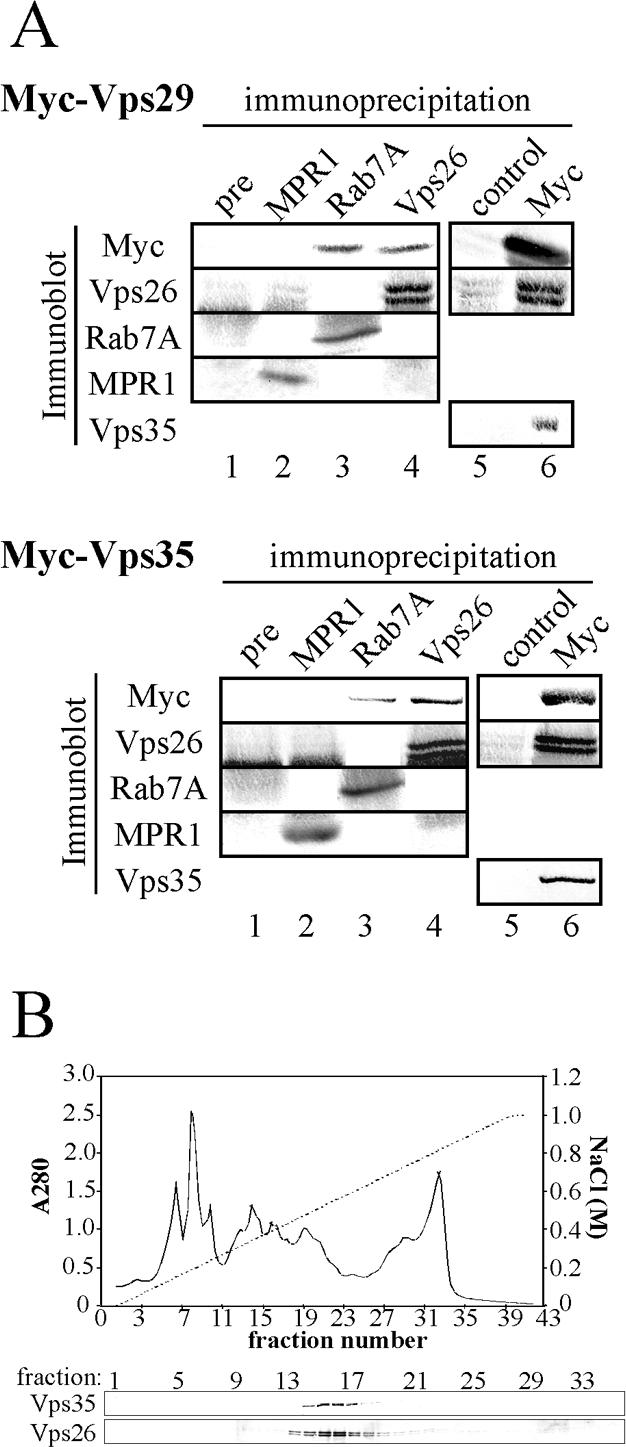
Immunocoprecipitation and coelution on an anion-exchange chromatography of EhRab7A and the retromerlike complex from the E. histolytica lysate. (A) Immunoprecipitation of EhRab7A and the retromerlike complex from the E. histolytica lysate. The lysates derived from the transformants expressing either Myc-EhVps29 (top) or Myc-EhVps35 (bottom) were subjected to immunoprecipitation with the antibodies indicated above the panels. Immunoprecipitated proteins were separated by SDS-PAGE and subjected to immunoblot analysis with antibodies indicated on the left of the panels. Antibodies used are as follows: pre., rabbit preimmune sera; MPR1, anti-EhMPR1 antiserum; Rab7A, anti-EhRab7A; Vps26, anti-EhVps26; Vps35, anti-EhVps35; control, irrelevant mouse IgG; Myc, anti-Myc mAb. (B) Chromatography separation of the native EhVps26 and EhVps35 from the amoeba lysate with a Mono Q anion-exchange column. A top panel shows a profile of optical absorbance at 280 nm (A280, unbroken line) of the amoebic lysate eluted at pH. 9.5 with a linear gradient of 0-1.0 M NaCl (a broken line) on a Mono Q column. Two bottom panels show immunoblots of each fraction with anti-EhVps26 and anti-EhVps35 antibodies.
We also verified the complex formation between EhVps26 and EhVps35 by chromatographic coseparation on a Mono Q anion-exchange column. Although EhVps26 and EhVps35 have different predicted pI values, i.e., 4.96 and 5.46, respectively, these proteins were eluted in identical fractions (Figure 3B). Taken together, these results suggest that EhRab7A binds to the retromerlike complex consisting of EhVps26, EhVps29, and EhVps35. Although it was previously shown that retromer complex was associated with CI-MPR or Vps10p, in mammals or yeast, respectively (Nothwehr et al., 2000; Arighi et al., 2004), E. histolytica lacks a homologue of CI-MPR or Vps10p. Alternatively, E. histolytica has two homologues to cation dependent (CD)-MPR. However, an antibody we raised against the amino-terminal region of one of these putative amoebic MPR (EhMPR1) did not coimmunoprecipitate any of the retromerlike component; neither did antibodies against the amoebic retromerlike complex coprecipitate EhMPR1 (Figure 3A, top and bottom panels, lane 2). Lack of interaction between MPR1 and the retromerlike complex in the amoeba does not necessarily conflict with the previous finding that mammalian and yeast retromer complex binds to CI-MPR as CI- and CD-MPR shares no mutual homology at the protein level.
Colocalization of EhRab7A and EhVps26 by Immunofluorescence Assay
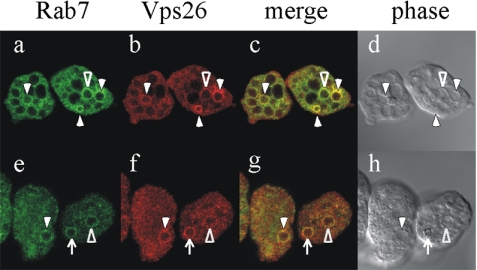
To confirm association of EhRab7A and retromerlike complex in the amoeba and also to demonstrate kinetics of these proteins during phagocytosis of erythrocytes, we examined intracellular localization of EhRab7A and EhVps26 in the transformant expressing Myc-tagged EhRab7A by immunofluorescence imaging using anti-Myc or anti-EhVps26 antibody to probe Myc-tagged EhRab7A or EhVps26, respectively (Figure 4). Localization of EhRab7A and EhVps26 dramatically changes during phagocytosis of erythrocytes. 1) EhRab7A and EhVps26 were scattered throughout the cytoplasm and most likely localized to small vesicles before incubation with erythrocytes (unpublished data). 2) On interaction with an erythrocyte EhRab7A is recruited to the amoeba-specific vacuoles, designated as “prephagosomal vacuoles (PPV),” together with EhRab5 (Saito-Nakano et al., 2004). We examined trophozoites that attached to, but not yet ingested erythrocytes at 5 min after the addition of erythrocytes (Figure 4, a-d). EhRab7A and EhVps26 were well colocalized on the PPV membrane. 3) After 30 min, when the amoeba contained one or more erythrocytes, EhRab7A was associated with both PPV and phagosomes, on both of which EhVps26 colocalized with EhRab7A (Figure 4, e-h). These results are consistent with the notion that EhRab7A and EhVps26 interact when recruited to PPV and phagosomes during erythrophagocytosis. Similar results were obtained when the transformant expressing Myc-EhVps29 or Myc-EhVps35 was stained with anti-Myc and anti-EhRab7A antibodies (unpublished data). The dynamics of retromerlike complex and EhRab7A from PPV to phagosomes during erythrophagocytosis resembles that of CP and AP, two major digestive proteins implicated for the pathogenesis of this parasite (unpublished data; also see Saito-Nakano et al., 2004). We also examined the colocalization of GTP- or GDP-locked EhRab7A and EhVps26 to verify in vivo the nucleotide dependence of the binding. As expected, EhVps26 colocalized with GTP-locked EhRab7A but failed to colocalize with GDP-locked EhRab7A on phagosomes and PPV (unpublished data).
Figure 4.
Colocalization of EhRab7A and EhVps26. Dynamism of EhRab7A and EhVps26 during erythrophagocytosis. Amoeba trophozoites expressing Myc-EhRab7A were incubated with gerbil erythrocytes for 5 (a-d) or 30 min (e-h). After removing the uningested erythrocytes, amoebae were fixed and the localization of EhRab7A and EhVps26 were examined by immunofluorescence assay with anti-Myc mAb (green) and anti-EhVps26 antibody (red). Merged fluorescence images and phase-contrast images on bright field are also shown. Solid arrowheads indicate EhRab7A/EhVps26-double-positive vacuoles. Open arrowheads indicate EhRab7A-positive vacuoles not associated with EhVps26. Arrows depict erythrocyte-containing phagosomes where both EhRab7A and EhVps26 are localized.
The intracellular dynamics of the complex is consistent with the presumption that the amoebic retromerlike complex is involved in the retrograde transport of the hydrolase receptor as proposed for the yeast and mammalian counterparts (Seaman, 2005). Verges et al. (2004) have recently reported that the transcytosis of the polymeric immunoglobulin (Ig) receptor (pIgR) is regulated by retromer complex in pIgR-expressing MDCK cells. Interestingly, this retromer complex that interacted with pIgR did not associate with SNX 1 or 2, mammalian homologues of Vps5p, similar to the amoebic retromerlike complex. Furthermore, they showed that Vps35, but not Vps26, bound to pIgR, which is in good contrast to a case of the amoebic retromerlike complex, EhVps26 of which binds to EhRab7A. Vps35 is also known to directly interact with CI-MPR or Vps10p (Nothwehr et al., 2000; Arighi et al., 2004). Thus, the retromer function is not restricted to the retrograde transport of CI-MPR or Vps10. It is also conceivable that Vps35 is capable of binding to various regulatory and scaffold proteins not yet identified. We propose that the amoebic retromerlike complex is recruited to PPV and phagosomes by the specific interaction to GTP-form EhRab7A, and involved, upon dissociation from EhRab7A, in the retrograde transport of not-yet-identified hydrolase receptor(s) from PPV and phagosomes to the Golgi apparatus. It has been shown in the mammalian system that trafficking of CI-MPR is also regulated by various proteins, i.e., AP-1, AP-2, GGA, PACS-1, and TIP47 (Ghosh et al., 2003). Among these adaptor proteins, TIP47 is involved in the retrieval of CI-MPR from late endosomes to the Golgi together with Rab9 (Carroll et al., 2001). It has also been shown that AP-1 together with Rab4 is involved in the regulation of the vesicle formation from endosomes (Pagano et al., 2004). Our genome-wide survey of E. histolytica revealed that this organism possesses large, intermediate, and small subunits of AP complex and clathrin heavy chain, but lacks Rab4, Rab9 (Saito-Nakano et al., 2005), TIP47 (unpublished results), and CI-MPR (see above). It is conceivable that the amebic retromerlike complex regulates the retrieval of not-yet-identified hydrolase receptor(s) in a coordinated interaction with other Rab and adaptor proteins.
EhRab7A Overexpression Caused a Decrease of CP Activity, Which Was Rescued by Coexpression of Vps26
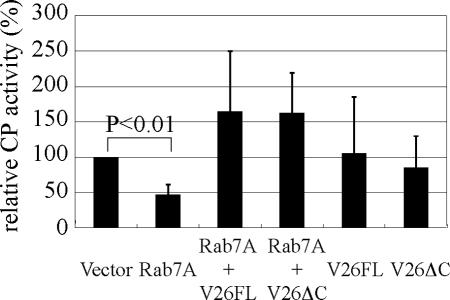
To gain an insight into the biological significance of the interaction between EhRab7A and the retromerlike complex in lysosome and phagosome functions, we examined the total CP activity in the transformants overexpressing EhRab7A or EhVps26. We examined whether the amoebic retromerlike complex is, by analogy to mammalian and yeast retromer complex, involved in the transport of one of the major hydrolases in the amoeba CP2, which was previously shown to be targeted to phagosomes via PPV (Saito-Nakano et al., 2004). EhRab7A overexpression, which also caused a dominant active phenotype in acidification of lysosomes and late endosomes as shown above (Figure 1), resulted in a marked (50%) decrease of the total CP activity (p < 0.01; Figure 5). However, EhRab7A overexpression caused no significant changes in the percentage of secreted CP (unpublished data), suggesting that the decrease of CP activity is not a consequence of missecretion. Furthermore, coexpression of EhVps26 compensated the reduction of CP activity caused by EhRab7A overexpression. This EhRab7A/EhVps26-expressing transformant showed normal morphology of lysosomes and a total acidity as measured with a Lysotracker staining (unpublished data). In mammalian cells, the GTP-Rab7 overexpression did not influence the EGF degradation, whereas RILP and Rabring7 caused accumulation of lysosomes but showed reverse effects on EGF degradation (Bucci et al., 2000; Cantalupo et al., 2001; Mizuno et al., 2003). Quantitative immunoblot analysis of CP1, 2, and 5 in these transformants with specific antibodies showed that the amount of these CP proteins was slightly, but not significantly, reduced in EhRab7A-overexpressing transformant (unpublished data). These results are consistent with the hypothesis that EhRab7A is involved in the transport and further inactivation and partial degradation of CP in lysosomes and this function is negatively regulated through the interaction with EhVps26 of the retromerlike complex.
Figure 5.
Effects of overexpression of EhRab7A, full-length EhVps26 (V26FL), truncated EhVps26 (V26ΔC), or coexpression of EhRab7A and EhVps26 on the total CP activity. Total CP activity was measured by using z-Arg-Arg-7-amino-4-trifluorometylcoumarin as a substrate. CP activities are shown in percentages relative to that of the mock vector transformant. Error bars represent SEs from three independent experiments. Statistical significance of the data were evaluated by Student's t test.
Interestingly, coexpression of EhVps26ΔC also compensated the reduction of CP activity (Figure 5) and total cell acidity (unpublished data). As predicted from yeast two-hybrid studies, EhVps26ΔC retains an ability to bind EhVps35 to form a complex. Thus, EhVps26ΔC may still function in the recycling of the putative hydrolase receptor(s) (see below). Overexpression of only EhVps26FL or EhVps26ΔC did not affect either CP activity (Figure 5) or total acidity (unpublished data), suggesting that the recycling of putative hydrolase receptor(s) via the interaction with the retromerlike complex is saturated, or alternatively, excessive EhVps26 may not form a functional complex.
There are two possible explanations of these data: the reduced CP activity caused by EhRab7A overexpression was restored by coexpression of EhVps26 as a consequence of direct titration of the overexpressed EhRab7A, or, alternatively, overexpression of EhVps26 recruited other components of the retromerlike complex to form more functional complex to activate the recycling of the hydrolase receptor(s), which resulted in the increase of CP activity. A line of evidence indicates that the former possibility is likely the case. First, the fact that immunoprecipitation of EhVps26 from the lysate of the wild-type amoeba with anti-EhVps26 antibody depleted not only EhVps26 but also EhVps35 (unpublished data) indicates that individual components of the retromerlike complex exist in a complex, but not in a free unassociated form. Second, immunoprecipitation of Myc-EhVps35 with anti-Myc antibody from the Myc-EhVps35-expressing transformant coprecipitated endogenous EhVps35, suggesting that at least two EhVps35 molecules interact. Third, size fractionation of the retromerlike complex on gel filtration chromatography, followed by immunoblots with anti-EhVps35 antibody (unpublished data), suggests that most, if not all, of EhVps35 is present as a complex. The fact that not only full-length but truncated EhVps26 restored the reduced CP activity caused by EhRab7A overexpression, indicates that some proportion of EhVps26ΔC replaces endogenous EhVps26 from the retromerlike complex, resulting in the free intact EhVps26, which titrates overexpressed EhRab7A. Because the retromerlike complex form a high-molecular-weight multisubunit complex as indicated by gel filtration chromatography, a retromerlike complex containing EhVps26ΔC is likely functional by interacting with the other components of the complex.
Although these two possibilities may be tested by reverse genetic approaches including siRNA and antisense, our attempt to knockdown Vps26 or EhRab7A by antisense in E. histolytica failed (unpublished data). One should be cautious to interpret data of knockdown and overexpression experiments because they often contradict. It was shown by siRNA that knockdown of SNX1 showed no effect on EGF receptor degradation (Gullapalli et al., 2004; Carlton et al., 2004) or on transferrin receptor trafficking (Carlton et al., 2004), whereas SNX1 is required for proper endosome retrieval of the CI-MPR (Carlton et al., 2005). This contrasts with the findings of previous studies using overexpression showing that overexpression of SNX1 enhances EGF receptor degradation (Kurten et al., 1996; Chin et al., 2001; Zheng et al., 2001), whereas other groups were unable to demonstrate that overexpression of SNX1 or SNX2, 3, or 4 increases the turnover of insulin or EGF receptors (Haft et al., 1998).
Significance of the Interaction between EhRab7A and the Retromerlike Complex in E. histolytica
One of the most striking peculiarities of the amoebic retromerlike complex shown in the present study is its interaction with Rab7: the interaction of the retromer with small GT-Pases has no precedent as described above. This interaction is EhRab7A specific: the retromerlike complex associates with none of EhRab5, EhRab11, and other isotypes of EhRab7 including EhRab7B and EhRab7D (unpublished data). In addition, the interaction between the retromerlike complex and EhRab7A is dependent on the GTP-active state of the Rab protein (Figures 2A and 4B), suggesting that this interaction occurs solely to fusion-competent EhRab7A-vesicles/vacuoles.
In this study, we failed to identify all known Rab7 effectors, RILP, Rabring7, XAPC7, and hVps34/p150. RILP is a soluble protein containing two coiled-coil regions and recruited on late endosomal and lysosomal membranes by Rab7-GTP and then in turn bridges phagosomes with dynein-dinactin, a microtubule-associated motor complex to associate with late endosomes and lysosomes (Harrison et al., 2003). Rabring7 is a soluble, ring finger-containing protein and also contributes to microtubule-mediated lysosomal movement (Mizuno et al., 2003). E. histolytica apparently lacks these two effectors, which is consistent with the fact that tubulins and microtubules in E. histolytica are significantly different from other organisms (Roy and Lohia, 2004; Vayssie et al., 2004), and may suggest that vesicular trafficking in E. histolytica depends on microtubule-independent mechanisms or use unique molecule(s) to interact with microtubules. Two other putative Rab7 effectors, hVps34/p150 and XAPC7, might have been overlooked in our affinity screening because hVps34/p150 generally shows the highest affinity to nucleotide-free Rab7 (Stein et al., 2003), and the molecular mass of XAPC7 is 27 kDa, which was below the limit of our detection due to contamination of GST-fusion proteins.
Acknowledgments
We thank Yasuo Shigeta, Mai Nudejima, Fumie Tokumaru, and Brian Hayama for technical assistance. The nucleotide sequences reported in this paper have been submitted to the DDBJ/GenBank/EBI Data Bank with the following accession numbers: EhVps26, AB194069; EhVps29, AB194070; EhVps35, AB194071. This work was supported by a grant for Precursory Research for Embryonic Science and Technology (PRESTO); Japan Science and Technology Agency; Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to T.N. (15019120, 15590378); a grant for Research on Emerging and Reemerging Infectious Diseases from the Ministry of Health, Labor, and Welfare; a grant for the Project to Promote Development of Anti-AIDS Pharmaceuticals from Japan Health Sciences Foundation to T.N.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-04-0283) on August 24, 2005.
Abbreviations used: E64, trans-epoxysuccinyl-l-leucylamido-(4-guanidino)butane; ORF, open reading frame; CS, cysteine synthase; CP, cysteine protease
References
- Ali, V., Hashimoto, T., Shigeta, Y., and Nozaki, T. (2004a). Molecular and biochemical characterization of D-phosphoglycerate dehydrogenase from Entamoeba histolytica. A unique enteric protozoan parasite that possesses both phosphorylated and nonphosphorylated serine metabolic pathways. Eur. J. Biochem. 271, 2670-2681. [DOI] [PubMed] [Google Scholar]
- Ali, V., Shigeta, Y., Tokumoto, U., Takahashi, Y., and Nozaki, T. (2004b). An intestinal parasitic protist, Entamoeba histolytica, possesses a non-redundant nitrogen fixation-like system for iron-sulfur cluster assembly under anaerobic conditions. J. Biol. Chem. 279, 16863-16874. [DOI] [PubMed] [Google Scholar]
- Ankri, S., Stolarsky, T., Bracha, R., Padilla-Vaca, F., and Mirelman, D. (1999). Antisense inhibition of expression of cysteine proteinases affects Entamoeba histolytica-induced formation of liver abscess in hamsters. Infect. Immun. 67, 421-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arighi, C. N., Hartnell, L. M., Aguilar, R. C., Haft, C. R., and Bonifacino, J. S. (2004). Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165, 123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchhaus, I., Jacobs, T., Leippe, M., and Tannich, E. (1996). Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol. Microbiol. 22, 255-263. [DOI] [PubMed] [Google Scholar]
- Bruchhaus, I., Loftus, B. J., Hall, N., and Tannich, E. (2003). The intestinal protozoan parasite Entamoeba histolytica contains 20 cysteine protease genes, of which only a small subset is expressed during in vitro cultivation. Eukaryot. Cell 2, 501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci, C., Thomsen, P., Nicoziani, P., McCarthy, J., and van Deurs, B. (2000). Rab 7: a key to lysosome biogenesis. Mol. Biol. Cell 11, 467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo, G., Alifano, P., Roberti, V., Bruni, C. B., and Bucci, C. (2001). Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton, J., Bujny, M., Rutherford, A., and Cullen, P. (2005). Sorting nexins-unifying trends and new perspectives. Traffic 6, 75-82. [DOI] [PubMed] [Google Scholar]
- Carlton, J., Bujny, M., Peter, B. J., Oorschot, V. M., Rutherford, A., Mellor, H., Klumperman, J., McMahon, H. T., and Cullen, P. J. (2004). Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr. Biol. 14, 1791-1800. [DOI] [PubMed] [Google Scholar]
- Carpeniseanu, S., Hirata, K., Que, X., Orozco, E., and Reed, S.L. (2000). L6: a proteinase- and phagocytosis-deficient mutant of Entamoeba histolytica. Arch. Med. Res. 31, S237-238. [DOI] [PubMed] [Google Scholar]
- Carroll, K. S., Hanna, J., Simon, I., Krise, J., Barbero, P., and Pfeffer, S. R. (2001). Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 292, 1373-1376. [DOI] [PubMed] [Google Scholar]
- Chin, L. S., Raynor, M. C., Wei, X., Chen, H. Q., and Li, L. (2001). Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem. 276, 7069-7078. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., and Zerial, M. (2000). Purification and identification of novel Rab effectors using affinity chromatography. Methods 20, 403-410. [DOI] [PubMed] [Google Scholar]
- Diamond, L. S., Harlow, D. R., and Cunnick, C. C. (1978). A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72, 431-432. [DOI] [PubMed] [Google Scholar]
- Diamond, L. S., Mattern, C. F., and Bartgis, I. L. (1972). Viruses of Entamoeba histolytica. I. Identification of transmissible virus-like agents. J. Virol. 9, 326-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J., Chen, W., Welford, A., and Wandinger-Ness, A. (2004). The proteasome alpha-subunit XAPC7 interacts specifically with Rab7 and late endosomes. J. Biol. Chem. 279, 21334-21342. [DOI] [PubMed] [Google Scholar]
- Ghosh, P., Dahms, N. M., and Kornfeld, S. (2003). Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell Bio. 4, 202-212. [DOI] [PubMed] [Google Scholar]
- Gullapalli, A., Garrett, T. A., Paing, M. M., Griffin, C. T., Yang, Y., and Trejo, J. (2004). A role for sorting nexin 2 in epidermal growth factor receptor down-regulation: evidence for distinct functions of sorting nexin 1 and 2 in protein trafficking. Mol. Biol. Cell 15, 2143-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann, B. (2004). The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 5, 250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft, C. R., de la Luz Sierra, M., Barr, V. A., Haft, D. H., and Taylor, S. I. (1998). Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol. Cell. Biol. 18, 7278-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft, C. R., de la Luz Sierra, M., Bafford, R., Lesniak, M. A., Barr, V. A., and Taylor, S. I. (2000). Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol. Biol. Cell 11, 4105-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque, R., Mondal, D., Kirkpatrick, B. D., Akther, S., Farr, B. M., Sack, R. B., and Petri, W. A., Jr. (2003). Epidemiologic and clinical characteristics of acute diarrhea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 69, 398-405. [PubMed] [Google Scholar]
- Harrison, R. E., Bucci, C., Vieira, O. V., Schroer, T. A., and Grinstein, S. (2003). Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol. Cell. Biol. 23, 6494-6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg, A., Nickel, R., Lotter, H., Tannich, E., and Bruchhaus, I. (2001). Overexpression of cysteine proteinase 2 in Entamoeba histolytica or Entamoeba dispar increases amoeba-induced monolayer destruction in vitro but does not augment amoebic liver abscess formation in gerbils. Cell. Microbiol. 3, 13-20. [DOI] [PubMed] [Google Scholar]
- Huston, C. D. (2004). Parasite and host contributions to the pathogenesis of amebic colitis. Trends Parasitol. 20, 23-26. [DOI] [PubMed] [Google Scholar]
- Izumi, T., Gomi, H., Kasai, K., Mizutani, S., and Torii, S. (2003). The roles of Rab27 and its effectors in the regulated secretory pathways. Cell Struct. Funct. 28, 465-474. [DOI] [PubMed] [Google Scholar]
- Jahn, R., and Sudhof, T. C. (1999). Membrane fusion and exocytosis. Annu. Rev. Biochem. 68, 863-911. [DOI] [PubMed] [Google Scholar]
- Kotake, K., Ozaki, N., Mizuta, M., Sekiya, S., Inagaki, N., and Seino, S. (1997). Noc2, a putative zinc finger protein involved in exocytosis in endocrine cells. J. Biol. Chem. 272, 29407-29410. [DOI] [PubMed] [Google Scholar]
- Kurten, R. C., Cadena, D. L., and Gill, G. N. (1996). Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 272, 1008-1010. [DOI] [PubMed] [Google Scholar]
- Loftus, B. et al. (2005). The genome of the protist parasite Entamoeba histolytica. Nature 433, 865-868. [DOI] [PubMed] [Google Scholar]
- Mizuno, K., Kitamura, A., and Sasaki, T. (2003). Rabring7, a novel Rab7 target protein with a RING finger motif. Mol. Biol. Cell 14, 3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S. F., Ha, S. A., and Bruinsma, P. (2000). Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 151, 297-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff, A. B., Novikoff, P. M., Quintana, N., and Davis, C. (1972). Diffusion artificats in 3,3′-diaminobenzidine cytochemistry. J. Histochem. Cytochem. 20, 745-749. [DOI] [PubMed] [Google Scholar]
- Nozaki, T., Asai, T., Sanchez, L. B., Kobayashi, S., Nakazawa, M., and Takeuchi, T. (1999). Characterization of the gene encoding serine acetyltransferase, a regulated enzyme of cysteine biosynthesis from the protist parasites Entamoeba histolytica and Entamoeba dispar. Regulation and possible function of the cysteine biosynthetic pathway in Entamoeba. J. Biol. Chem. 274, 32445-32452. [DOI] [PubMed] [Google Scholar]
- Okada, M., Huston, C. D., Mann, B., Petri, Jr., W. A., Kita, K., and Nozaki, T. (2005). Proteomic analysis of phagocytosis in the enteric protozoan Entamoeba histolytica. Eukaryot. Cell 4, 827-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco, E., Suarez, M. E., and Sanchez, T. (1985). Differences in adhesion, phagocytosis and virulence of clones from Entamoeba histolytica, strain HM1: IMSS. Int. J. Parasitol. 15, 655-660. [DOI] [PubMed] [Google Scholar]
- Ozaki, N. et al. (2000). cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat. Cell Biol. 2, 805-811. [DOI] [PubMed] [Google Scholar]
- Pagano, A., Crottet, P., Prescianotto-Baschong, C., and Spiess, M. (2004). In vitro formation of recycling vesicles from endosomes requires adaptor protein-1/clathrin and is regulated by rab4 and the connector rabaptin-5. Mol. Biol. Cell 15, 4990-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Leal, J. B., and Seabra, M. C. (2001). Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313, 889-901. [DOI] [PubMed] [Google Scholar]
- Que, X., and Reed, S. L. (2000). Cysteine proteinases and the pathogenesis of amebiasis. Clin. Microbiol. Rev. 13, 196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin, J. I. (1988). Amebiasis, New York: John Wiley & Sons.
- Reddy, J. V., and Seaman, M. N. (2001). Vps26p, a component of retromer, directs the interactions of Vps35p in endosome-to-Golgi retrieval. Mol. Biol. Cell 12, 3242-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, D., and Lohia, A. (2004). Sequence divergence of Entamoeba histolytica tubulin is responsible for its altered tertiary structure. Biochem. Biophys. Res. Commun. 319, 1010-1016. [DOI] [PubMed] [Google Scholar]
- Saito-Nakano, Y., Loftus, B. J., Hall, N., and Nozaki, T. (2005). The diversity of Rab GTPases in Entamoeba histolytica. Exp. Parasitol. 110, 244-252. [DOI] [PubMed] [Google Scholar]
- Saito-Nakano, Y., Yasuda, T., Nakada-Tsukui, K., Leippe, M., and Nozaki, T. (2004). Rab5-associated vacuoles play a unique role in phagocytosis of the enteric protozoan parasite Entamoeba histolytica. J. Biol. Chem. 279, 49497-49507. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., and Russell, D. W. (2001). Molecular Cloning, New York: Cold Spring Harbor Laboratory Press.
- Seaman, M. N. (2004). Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 165, 111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M. N. (2005). Recycle your receptors with retromer. Trends Cell Biol. 15, 68-75. [DOI] [PubMed] [Google Scholar]
- Seaman, M. N., and Williams, H. P. (2002). Identification of the functional domains of yeast sorting nexins Vps5p and Vps17p. Mol. Biol. Cell 13, 2826-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. P., Feng, Y., Cooper, K. L., Welford, A. M., and Wandinger-Ness, A. (2003). Human VPS34 and p150 are Rab7 interacting partners. Traffic 4, 754-771. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., and Olkkonen, V. M. (2001). The Rab GTPase family. Genome Biol. 2, REVIEWS3007. [DOI] [PMC free article] [PubMed]
- Sun, L., Bittner, M. A., and Holz, R. W. (2003). Rim, a component of the presynaptic active zone and modulator of exocytosis, binds 14-3-3 through its N terminus. J. Biol. Chem. 278, 38301-38309. [DOI] [PubMed] [Google Scholar]
- Takai, Y., Sasaki, T., and Matozaki, T. (2001). Small GTP-binding proteins. Physiol. Rev. 81, 153-208. [DOI] [PubMed] [Google Scholar]
- Tuvim, M. J., Adachi, R., Hoffenberg, S., and Dickey, B. F. (2001). Traffic control: Rab GTPases and the regulation of interorganellar transport. News Physiol. Sci. 16, 56-61. [DOI] [PubMed] [Google Scholar]
- Vayssie, L., Vargas, M., Weber, C., and Guillen, N. (2004). Double-stranded RNA mediates homology-dependent gene silencing of gamma-tubulin in the human parasite Entamoeba histolytica. Mol. Biochem. Parasitol. 138, 21-28. [DOI] [PubMed] [Google Scholar]
- Verges, M., Luton, F., Gruber, C., Tiemann, F., Reinders, L. G., Huang, L., Burlingame, A. L., Haft, C. R., and Mostov, K. E. (2004). The mammalian retromer regulates transcytosis of the polymeric Ig receptor. Nat. Cell Biol. 6, 763-769. [DOI] [PubMed] [Google Scholar]
- Willhoeft, U., Hamann, L., and Tannich, E. (1999). A DNA sequence corresponding to the gene encoding cysteine proteinase 5 in Entamoeba histolytica is present and positionally conserved but highly degenerated in Entamoeba dispar. Infect. Immun. 67, 5925-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2, 107-117. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Zhang, Z., Alexander, D., Bracha, R., Mirelman, D., and Stanley, S. L., Jr. (2004). Expression of amoebapores is required for full expression of Entamoeba histolytica virulence in amebic liver abscess but is not necessary for the induction of inflammation or tissue damage in amebic colitis. Infect. Immun. 72, 678-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, B., Ma, Y. C., Ostrom, R. S., Lavoie, C., Gill, G. N., Insel, P. A., Huang, X. Y., and Farquhar, M. G. (2001). RGS-PX1, a GAP for GalphaS and sorting nexin in vesicular trafficking. Science 294, 1939-1942. [DOI] [PubMed] [Google Scholar]