Abstract
Background
In heart failure, sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2a) activity is decreased, resulting in abnormal calcium handling and contractile dysfunction. We have previously shown that increasing SERCA2a expression by gene transfer improves ventricular function in a rat model of heart failure created by ascending aortic constriction.
Methods and Results
In this study, we tested the effects of gene transfer of SERCA2a on survival, left ventricular (LV) volumes, and metabolism. By 26 to 27 weeks after aortic banding, all animals developed heart failure (as documented by >25% decrease in fractional shortening) and were randomized to receive either an adenovirus carrying the SERCA2a gene (Ad.SERCA2a) or control virus (Ad.βgal-GFP) by use of a catheter-based technique. Sham-operated rats, uninfected or infected with either Ad.βgal-GFP or Ad.SERCA2a, served as controls. Four weeks after gene transfer, survival in rats with heart failure treated with Ad.βgal-GFP was 9%, compared with 63% in rats receiving Ad.SERCA2a. LV volumes were significantly increased in heart failure (0.64 ± 0.05 versus 0.35 ± 0.03 mL, P < 0.02). Overexpression of SERCA2a normalized LV volumes (0.46 ± 0.07 mL) in the failing hearts. 31P NMR analysis showed a reduced ratio of phosphocreatine to ATP content in failing + Ad.βgal-GFP compared with sham + Ad.βgal-GFP (0.82 ± 0.13 versus 1.38 ± 0.14, P < 0.01). Overexpression of SERCA2a in failing hearts improved the phosphocreatine/ATP ratio (1.23 ± 0.28).
Conclusions
In this study, we show that unlike inotropic agents that improve contractile function at the expense of increased mortality and worsening metabolism, gene transfer of SERCA2a improves survival and the energy potential in failing hearts.
Keywords: gene therapy, heart failure, calcium, excitation, contractility
In cardiac muscle, both contraction and relaxation are intimately dependent on the function of the sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2a) pump, which is regulated by phospholamban. In congestive heart failure, deficiency in SERCA2a results in abnormal calcium handling and diminished contraction.1,2 In addition, a decrease in phosphorylation of phospholamban has been reported in failing hearts, along with an increase in the phospholamban/SERCA2a ratio, contributing to the contractile dysfunction in heart failure.3,4 These results are consistent with the model that a decrease in SERCA2a levels alters intracellular calcium homeostasis and contributes to contractile dysfunction in failing hearts.
Recently, we showed that restoration of SERCA2a to control levels in isolated failing human cardiomyocytes improved contraction and relaxation by correcting calcium handling.5 Furthermore, in an animal model of heart failure, adenoviral gene transfer of SERCA2a improved contractile function in vivo, demonstrating the importance of SERCA2a as a therapeutic target.6 Pharmacological agents that increase contractility, however, have been shown to worsen survival in patients with heart failure and to increase the energetic demand.7 The heart requires a continuous supply of energy in the form of ATP by mostly oxidative metabolism, with the major energy reserve molecule represented by phosphocreatine (PCr).8,9 In the normal heart, although the majority of the energy consumption is due to cross-bridge cycling, relaxation requires an energy expenditure of 15% to remove Ca2+ from the cytoplasm. This high level of energy required by SERCA2a reaction is directly related to the magnitude of the Ca2+ gradient across the SR.9 Failing hearts have a reduced PCr/ATP ratio, so less energy reserve is available for the cellular processes.
In this study, we tested the hypothesis that unlike currently used pharmacological agents that increase inotropy, reconstitution of normal levels of SERCA2a by adenoviral gene transfer would improve contractile performance as well as survival in aortic-banded rats that have developed heart failure without adversely affecting energetics.
Methods
Construction of Recombinant Adenoviruses
To construct the adenovirus containing SERCA2a cDNA, we used the method described by He et al,10 whereby the backbone vector, containing most of the adenoviral genome (pAd.EASY1), is used and the recombination is performed in Escherichia coli. SERCA2a cDNA was subcloned into the adenoviral shuttle vector (pAd.TRACK), which uses the cytomegalovirus (CMV) long terminal repeat as a promoter. The shuttle vector used also has a concomitant green fluorescent protein (GFP) under the control of a separate CMV promoter. An adenovirus containing both β-galactosidase and GFP controlled by separate CMV promoters (Ad.βgal-GFP) was used as control. The adenoviruses were propagated in 293 cells. The titers of stocks used for these studies measured by plaque assays were 3 × 1011 pfu/mL for Ad.βgal-GFP and 1.8 × 1011 pfu/mL for Ad.SERCA2a, with particle/pfu ratios of 8:1 and 18:1, respectively. These recombinant adenoviruses were tested for the absence of wild-type virus by polymerase chain reaction of the early transcriptional unit E1.
Experimental Protocol
Four-week-old Sprague-Dawley rats (Charles River, Mass; 70 to 80 g) were anesthetized with pentobarbital (65 mg/kg IP) and placed on a ventilator. A suprasternal incision was made, exposing the aortic root, and a tantalum clip with an ID of 0.58 mm (Weck, Inc) was placed on the ascending aorta. Animals in the sham group underwent a similar procedure without insertion of a clip. The supraclavicular incision was then closed, and the rats were transferred back to their cages. The supraclavicular approach was performed because during gene delivery, a thoracotomy is necessary, and if the thorax is not opened during the initial aortic banding, adhesions are avoided when gene delivery is performed.
The animals were initially divided into 2 groups: 1 group of 45 animals with aortic banding and a second group of 42 animals that were sham-operated. Three animals in the aortic banding group did not survive the initial operation, and 2 animals in the sham-operated group did not survive. In the aortic-banded animals, we waited 26 to 28 weeks for the animals to develop left ventricular (LV) dilatation before cardiac gene transfer. In this last group as well as in the sham-operated group, 14 animals did not undergo gene transfer and were followed longitudinally. The rest of the animals underwent adenoviral gene transfer with either Ad.SERCA2a or Ad.βgal-GFP.
31P NMR Measurements
Hearts were retrogradely perfused from a 100-cm hydrostatic perfusion column with modified Krebs-Henseleit buffer (mmol/L: NaCl 116, KCl 4, CaCl2 1.5, MgSO4 1.2, NaH2PO4 1.2, and NaHCO3 25, equilibrated with 95% O2/5% CO2 at 37°C) that contained 5 mmol/L glucose in a 2-L reservoir. Hearts beat spontaneously, contracting against a fluid-filled intraventricular balloon connected to a pressure transducer and inflated to an end-diastolic pressure of 5 mm Hg. A 10- to 15-mL volume of coronary effluent bathed the heart. A stable energetic state in rat hearts was confirmed from 31P NMR signals of PCr, ATP, and inorganic phosphate (Pi) as previously described.8 NMR data were collected on a Bruker 400-MHz spectrometer interfaced to a 9.4-T, vertical-bore, superconducting magnet. 31P spectra were obtained from isolated hearts perfused within a broadband, 20-mm NMR probe (Bruker Instruments). 31P NMR spectra were acquired in 128 scans with a 161-MHz, 45° excitation pulse, a 1.8-second repetition time, 35 ppm sweep width, and 8000 data points. Peak assignments were referenced to the well-established resonance signal of PCr at 0 ppm, with identification and assignment of the α-, β-, and γ-phosphate signals of ATP. Signal intensity was determined by NMR-dedicated data analysis.
Serial Echocardiographic Assessment
After 18 weeks of banding, serial echocardiograms were performed weekly in lightly anesthetized animals (pentobarbital 40 mg/kg IP). Transthoracic M-mode and 2D echocardiography was performed with a Hewlett-Packard Sonos 5500 imaging system with a 12-MHz broadband transducer. A mid–papillary level LV short-axis view was used, and measurements of posterior wall thickness, LV diastolic dimension, and fractional shortening were collected. Gene transfer was performed in all animals within 3 days of detection of a drop in fractional shortening of >25% compared with the fractional shortening at 18 weeks after banding. In the sham-operated rats, gene delivery was performed at 27 weeks.
Adenoviral Delivery Protocol
The group of animals subjected to aortic banding were further subdivided into 3 additional groups of 16, 12, and 14 receiving Ad.SERCA2a, Ad.βgal-GFP, or no adenovirus, respectively. The group of sham-operated animals was also subdivided into 3 groups of 14, 12, and 14 receiving Ad.SERCA2a, Ad.βgal-GFP, or no adenovirus. The adenoviral delivery system has been described previously by our group in detail.11–13 Briefly, after the rats had been anesthetized and a thoracotomy performed, a 22-gauge catheter containing 200 mL of adenoviral solution (1010 pfu) was advanced from the apex of the LV to the aortic root. The aorta and main pulmonary artery were clamped for 20 seconds distal to the site of the catheter, and the solution was injected; then the chest was closed and the animals were allowed to recover.
Measurements of LV Volume and Elastance
Rats in the different treatment groups were anesthetized with 65 mg/kg of pentobarbital and mechanically ventilated. A 1.4F high-fidelity pressure transducer (Millar Instruments) was introduced into the LV. Four 0.7-mm piezoelectric crystals were placed over the surface of the LV along the short axis of the ventricle at the level of the mitral valve and at the apex of the LV to measure the intercrystal distances. The LV volume was derived by use of a mathematical model using Cardiosoft (Sonometrics Co). LV pressure-volume loops were generated under different loading conditions by clamping of the inferior vena cava. The end-systolic pressure-volume relationship was obtained by producing a series of pressure-volume loops and connecting the upper left corners of the individual pressure-volume loops to generate the maximal slope.
Western Blot Analysis and SERCA2a Activity
The preparation of lysates was described earlier. Briefly, lysates were prepared at 4°C in a lysis buffer containing (in mmol/L) NaCl 150, MgCl2 1, and CaCl2 1, plus detergents and proteinase inhibitors (pH 7.4). The tissue was homogenized and spun at 1400 rpm (Sorvall) for 30 minutes. The supernatant was then filtered through 4 layers of gauze and centrifuged at 15 000 rpm for 60 minutes (Beckman). SDS-PAGE was performed on the supernatant under reducing conditions on 7.5% separation gels with a 4% stacking gel in a Miniprotean II cell (Biorad). For immunoreaction, the blots were incubated with 1:2500 diluted monoclonal antibodies to SERCA2a (MA3-919; Affinity BioReagents), phospholamban (Upstate Biotechnology), or 1:1000 diluted anti-calsequestrin (MA3-913; Affinity Bioreagents) for 90 minutes at room temperature.
Crude membranes were prepared as described by Schwinger et al3 at 4°C in a buffer containing (in mmol/L) sucrose 300, PMSF 1, and PIPES 20, pH 7.4. The tissue was homogenized and spun at 8000 rpm (Beckman JA 20) for 20 minutes. The supernatant was then filtered through 4 layers of gauze and centrifuged at 35 000 rpm for 60 minutes (Sorvall). The pellet was resuspended in a 10% sucrose buffer containing (in mmol/L) KCl 400, MgCl2 0.5, CaCl2 0.5, EGTA 0.5, and PIPES 25, pH 7.0. SERCA2a activity assays were carried out on the basis of a pyruvate/NADH coupled reaction as previously described.1,13,14 Ca2+-ATPase activity was calculated as Δabsorbance/(6.22 × protein × time) in nmol ATP/(mg protein × min).
Statistics
All values are presented as mean ± SD. A 2-factor ANOVA was performed to compare the different hemodynamic parameters among the different groups. For the echocardiography data, when the variables were examined at various intervals, ANOVA with repeated measures was performed. Comparison of survival in the different groups of animals was analyzed by a log-rank test with the Kaplan-Meier method. Statistical significance was accepted at the level of P < 0.05.
Results
Survival
Figure 1 shows the survival curve for the 6 different groups studied. The sham-operated animals did not show any premature mortality. The sham-operated animals that were infected with either Ad.βgal-GFP or Ad.SERCA2a had early mortalities related to the surgical intervention, but then the survival curves leveled off for both sham + Ad.βgal-GFP and sham + Ad.SERCA2a. In the failing group, the noninfected animals had a survival curve that decreased steadily, and at 4 weeks the survival rate was only 18% (P < 0.0005 compared with sham). In the failing + Ad.βgal-GFP group, the survival curve also decreased, and at 4 weeks the survival rate was only 9% (P < 0.001 compared with sham + Ad.βgal-GFP). In the failing + Ad.SERCA2a group, however, the survival curve was significantly improved compared with failing + Ad.SERCA2a (P < 0.001 compared with failing + Ad.βgal-GFP).
Figure 1.
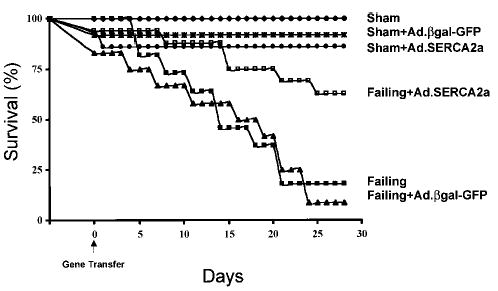
Survival-function curve of 6 different groups studied: sham, n = 14; sham + Ad.βgal-GFP, n = 12; sham + Ad.SERCA2a, n = 14; failing, n = 14; failing + Ad.βgal-GFP, n = 12; failing + SERCA2a, n = 16.
Characterization of Animals
After 18 weeks of aortic banding, the animals showed echocardiographic signs of LV hypertrophy, including an increase in wall thickness (both posterior and septal), an increase in posterior wall thickness, a decrease in LV dimensions, and an increase in fractional shortening, as shown in Table 1. After 26 to 27 weeks of banding, these animals had uniformly (1) small pericardial effusions, (2) pleural effusions, (3) an increase in lung weight, (4) ascites, and (5) dyspnea at rest, all indicative signs of severe heart failure. Echocardiographically, LV end-diastolic dimensions increased and fractional shortening decreased.
TABLE 1.
Echocardiographic Measures in Rats After Sham Surgery or Aortic Banding
| Septum, mm | PW, mm | LVEDD, mm | LVESD, mm | FS, % | |
|---|---|---|---|---|---|
| Sham | 14.9 ± 1.1 | 13.5 ± 1.0 | 66.8 ± 3.8 | 40.4 ± 6.0 | 40.0 ± 6.3 |
| Aortic banding (18 weeks) | 20.1 ± 3.9‡ | 19.8 ± 2.8‡ | 61.9 ± 6.4*‡ | 34.0 ± 6.2*‡ | 46.0 ± 8.2*‡§ |
| Aortic banding (27 weeks) | 19.7 ± 2.8† | 18.5 ± 2.3† | 69.5 ± 6.3§ | 45.1 ± 6.9‡ | 36.0 ± 10.4§ |
PW indicates posterior wall thickness during diastole; LVEDD, LV diameter at end diastole; LVESD, LV diameter at end systole; and FS, fractional shortening.
P < 0.0005 vs aortic banding (27 weeks).
P < 0.005,
P < 0.005,
P < 0.05 vs sham.
Cardiac Gene Transfer and SERCA2a Expression
Protein levels of SERCA2a were decreased in failing compared with sham LVs, as shown in Figure 2A. Adenoviral gene transfer of SERCA2a in failing hearts increased SERCA2a protein expression, restoring it to levels observed in the nonfailing hearts. Calsequestrin did not change among the different groups, nor did phospholamban. As shown in Figure 2B, tabulated ratios of SERCA2a to phospholamban and SERCA2a to calsequestrin reveal a significant decrease in failing hearts and a restoration to control levels with gene transfer of SERCA2a.
Figure 2.
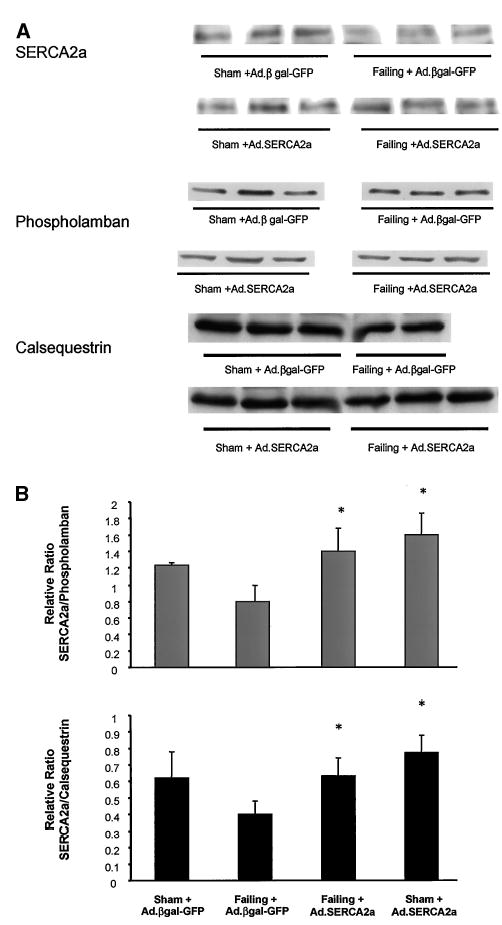
A, Immunoblots of SERCA2a, phospholamban, and calsequestrin from crude membranes of LVs from sham rats infected with Ad.βgal-GFP, failing rat hearts infected with Ad.βgal-GFP, and failing rats infected with Ad.SERCA2a. B, Relative protein levels of SERCA2a normalized to either phospholamban or calsequestrin (n = 6 in all groups). *P < 0.05 vs sham + Ad.βgal-GFP.
SERCA2a Activity
We measured SERCA2a activity at a calcium concentration of 10 mmol/L in the (1) sham + Ad.βgal-GFP, (2) failing + Ad.βgal-GFP, and (3) failing + Ad.SERCA2a groups. There was a decrease in maximal ATPase activity in the failing group (27.4 ± 4.9 versus 62.2 ± 12.8 nmol · mg−1 · min−1). Gene transfer of SERCA2a restored ATPase activity back to normal levels in the failing group 4 weeks after gene transfer (61.0 ± 8.5 nmol · mg−1 · min−1).
NMR Spectroscopy
Representative 31P NMR spectra obtained from 3 groups of rats: (1) sham + Ad.βgal-GFP, (2) failing + Ad.βgal-GFP, and (3) failing + Ad.SERCA2a, are shown in Figure 3A. These spectra show that the ratios of total amounts of PCr to ATP are lower in the failing heart than the sham heart. The integrated area for Pi was also increased in the failing heart. The overexpression of SERCA2a in failing heart restored and normalized the content of both PCr and ATP (Figure 3B). Interestingly, we found that overexpression of SERCA2a in sham-operated animals induces a reduction in PCr/ATP ratio.
Figure 3.
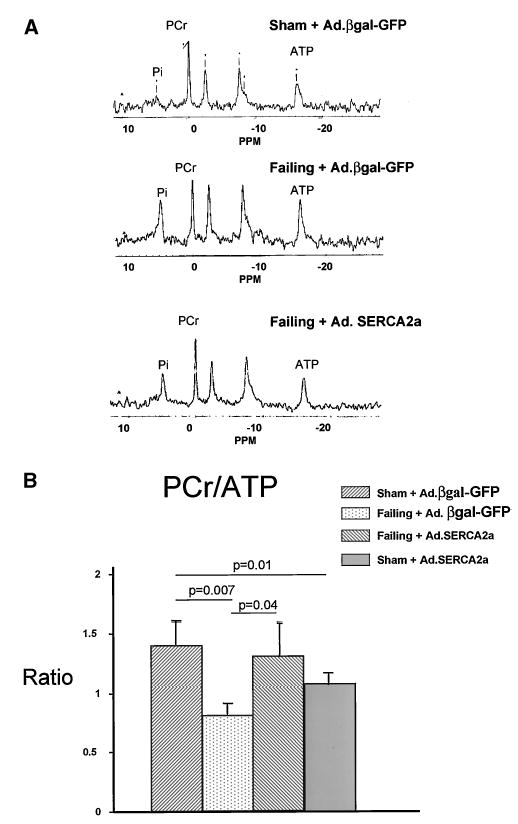
A, Representative 31P-NMR spectra of sham + Ad.βgal-GFP, failing + Ad.βgal-GFP, and failing + Ad.SERCA2a hearts. Major resonances are assigned as Pi, PCr, and α-, γ-, and β-phosphates of ATP. Integrated peak intensities are proportional to metabolite content. B, Failing spectrum illustrates that PCr-to-ATP ratio and PCr and ATP contents in failing heart are lower than in sham heart. In spectrum of failing + Ad.SERCA2a heart, PCr-to-ATP ratio is restored toward normal.
Effects of SERCA2a Overexpression on Pressure-Volume Relationship
Pressure-volume analysis was performed in a subset of animals. LV volumes were significantly increased in the failing rats (0.64 ± 0.05 versus 0.35 ± 0.03 mL, P < 0.02) and were decreased after SERCA2a gene transfer (0.46 ± 0.07 mL). The slope of the end-systolic pressure-volume relationship (Figure 4) was lower in failing hearts infected with Ad.βgal-GFP (n = 5) than in sham (n = 6), indicating a diminished state of intrinsic myocardial contractility: 450 ± 71 versus 718 ± 83 mm Hg/mL (P < 0.02). Gene transfer of SERCA2a restored the slope of the end-systolic pressure-volume relationship to control levels (691 ± 91 mm Hg/mL, n = 6, P < 0.03 versus failing + Ad.βgal-GFP; P < 0.1 versus sham + Ad.βgal-GFP).
Figure 4.
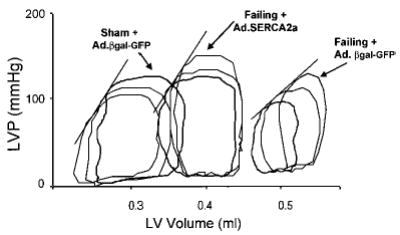
LV pressure (LVP) vs LV volume detected by piezoelectric crystals in a sham + Ad.βgal-GFP heart, a failing + Ad.βgal-GFP heart, and a failing + Ad.SERCA2a heart.
Effect on Morphological Parameters
As shown in Table 2, the failing hearts had a significant increase in heart weight when normalized to either tibial length or body mass. Gene transfer of SERCA2a in the failing heart did not have a significant effect on LV mass whether normalized to tibial length or body mass.
TABLE 2.
Morphometric Analyses
| Sham + Ad.βgal-GFP | Sham + Ad.SERCA2a | Failing + Ad.βgal-GFP | Failing + Ad.SERCA2a | |
|---|---|---|---|---|
| HW/BW × 104 | 3.7 ± 0.3 | 4.4 ± 0.6 | 4.4 ± 0.5* | 4.3 ± 0.4* |
| HW/TL × 102, g/mm | 44.8 ± 4.3 | 55.3 ± 6.2 | 50.8 ± 4.4* | 50.3 ± 6.3* |
HW indicates heart weight; BW, body weight; and TL, tibial length.
P < 0.05 vs Sham + Ad.GFP.
Discussion
In this study, we show that in an animal model of heart failure and contractile dysfunction, restoration of SERCA2a expression by cardiac gene transfer in vivo improves not only contractile function but also survival and cardiac energetics.
Abnormal SR Function and Cardiac Gene Transfer of SERCA2a
Impaired SERCA2a activity is one of the main characteristics associated with abnormal calcium handling in heart failure.1,2 A decrease in SERCA2a relative to phospholamban and a reduction of phosphorylation of phospholamban contribute to the contractile dysfunction in human heart failure.3,4 More recently, the ratio of the Na/Ca exchanger to SERCA2a has been shown to be increased in failing hearts and to be predictive of diastolic function in these hearts.15 Gene transfer of SERCA2a corrects both the SERCA2a/phospholamban ratio and the SERCA2a/Na/Ca ratio and would contribute to restoring both systolic and diastolic function.
In our study, we showed that SERCA2a protein levels were restored to normal levels in the failing hearts and that this effect was sustained for up to 4 weeks. This seemed somewhat surprising, because first-generation adenoviruses induce transient expression peaking at 7 to 10 days and disappearing after 10 days.16 Endogenous turnover of SERCA2a, however, is ≈14 to 15 days in young rats and longer in older rats,17 which would explain the sustained levels of SERCA2a.
SERCA2a Expression and Cardiac Energetics
Decreased energy reserve via the creatine kinase reaction is a characteristic finding in both human and experimental heart failure.9,18,19 This decrease in energy reserve contributes to the development of contractile dysfunction in heart failure.19 In addition, an increase in intracellular Pi has been shown to reduce SR calcium loading and to depress calcium-induced calcium release.20 Local ATP regeneration by the creatine kinase system is one mechanism the cell can use to improve Ca2+ uptake by the SR in conditions in which an excessive increase in cytoplasmic [Ca2+] may have deleterious effects. Recently, Tian et al9 showed that pharmacological inhibition of creatine kinase resulted in altered energetics and induced abnormal Ca2+ handling and contractile dysfunction in the rat. In our experiments, restoring SERCA2a levels to normal induced an improvement in the ratio of PCr to ATP.
The findings of improved cardiac energetics in heart failure was somewhat surprising, because an increase in contractility by SERCA2a overexpression would be anticipated to increase ATP hydrolysis, thereby driving PCr down. Indeed, this increase in ATP hydrolysis is consistent with our observation of reduced PCr/ATP in the group of sham-operated hearts that were overexpressing SERCA2a. These results are also consistent with previous results showing that PCr/ATP was decreased in the phospholamban-deficient hearts relative to the wild-type hearts.21
In heart failure, however, elevated calcium levels would increase energy demand. To maintain low levels of diastolic Ca2+, a high level of free energy released from ATP hydrolysis (|ΔGp|) is necessary. To maintain the normal Ca2+ gradient (≈10 000-fold between the cytosol and the SR), the SERCA2a reaction requires a |ΔGp| of ≥52 kJ/mol, 85% to 90% of it from ATP.9 Therefore, of all the ATPase reactions in cardiac myocytes, the SERCA2a reaction is the most vulnerable to a decrease in |ΔGp|.
Survival After Gene Transfer: Therapeutic Implications
In this model of heart failure, SERCA2a overexpression improved parameters of inotropy and normalized contractile reserve. These effects translate into an inotropic intervention. Other inotropic interventions, however, have been shown clinically to increase mortality.22 There are, however, significant differences between enhancing inotropy with pharmacological agents that usually increase cAMP and gene transfer of SERCA2a. Unlike agents that increase cAMP, thereby increasing intracellular Ca2+, restoration of SERCA2a levels decreases diastolic Ca2+. Furthermore, it has been shown that sustained elevations of resting Ca2+ lead to activation of serine-threonine phosphatases, including calcineurin, inducing hypertrophy and cell death.23 Therefore, a decrease in diastolic Ca2+ may in effect reduce the proapoptotic and prohypertrophy signaling. Heart failure is associated with an increased incidence of ventricular arrhythmias, and triggered activity is a probable mechanism of arrhythmogenesis in heart failure. The increase in intracellular calcium secondary to SERCA2a downregulation increases the arrhythmogenic potential. Preventing an increase in intracellular calcium by overexpression of SERCA2a prevents the induction of triggered activity. Furthermore, improvement in energetics is another important finding in this study that may have a direct influence on survival.
Conclusions
Our results demonstrate that restoring SERCA2a expression can improve not only systolic and diastolic performance in failing hearts but also survival and cardiac energetics. Furthermore, SERCA2a normalization halts the adverse remodeling that occurs with congestive heart failure. This study validates the feasibility of cardiac gene transfer in failing hearts as a therapeutic modality.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health: HL-50361 and HL-57623 (Dr Hajjar); HL-59521 and HL-61557 (Dr Rosenzweig); HL-49574 (Dr Gwathmey); and HL-62702 (Dr Lewandowski); and a Doris Duke Charitable Foundation Clinician Scientist Award and American Federation of Aging research grant (Dr Hajjar). Dr Rosenzweig is an Established Investigator of the American Heart Association.
References
- 1.Schmidt U, Hajjar RJ, Helm PA, et al. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 2.Gwathmey JK, Copelas L, MacKinnon R, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 3.Schwinger RH, Bohm M, Schmidt U, et al. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+ -ATPase activity of cardiac sarcoplasmic reticulum from dilated cardio-myopathy patients compared with patients with nonfailing hearts. Circulation. 1995;92:3220–3228. doi: 10.1161/01.cir.92.11.3220. [DOI] [PubMed] [Google Scholar]
- 4.Schwinger RH, Munch G, Bolck B, et al. Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol. 1999;31:479–491. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 5.del Monte F, Harding SE, Schmidt U, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto MI, del Monte F, Schmidt U, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial. Am Heart J. 1999;138:78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 8.Lewandowski ED, Damico LA, White LT, et al. Cardiac responses to induced lactate oxidation: NMR analysis of metabolic equilibria. Am J Physiol. 1995;269:H160–H168. doi: 10.1152/ajpheart.1995.269.1.H160. [DOI] [PubMed] [Google Scholar]
- 9.Tian R, Halow JM, Meyer M, et al. Thermodynamic limitation for Ca2+ handling contributes to decreased contractile reserve in rat hearts. Am J Physiol. 1998;275:H2064–H2071. doi: 10.1152/ajpheart.1998.275.6.H2064. [DOI] [PubMed] [Google Scholar]
- 10.He TC, Zhou S, da Costa LT, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajjar RJ, Schmidt U, Matsui T, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajjar RJ, del Monte F, Matsui T, et al. Prospects for gene therapy for heart failure. Circ Res. 2000;86:616–621. doi: 10.1161/01.res.86.6.616. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto MI, del Monte F, Schmidt U, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt U, Hajjar RJ, Kim CS, et al. Human heart failure: cAMP stimulation of SR Ca2+-ATPase activity and phosphorylation level of phospholamban. Am J Physiol. 1999;277:H474–H480. doi: 10.1152/ajpheart.1999.277.2.H474. [DOI] [PubMed] [Google Scholar]
- 15.Hasenfuss G, Schillinger W, Lehnart SE, et al. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 16.Guzman RJ, Lemarchand P, Crystal RG, et al. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 17.Martonosi A, Halpin RA. Sarcoplasmic reticulum, 17: the turnover of proteins and phospholipids in sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1972;152:440–450. doi: 10.1016/0003-9861(72)90237-8. [DOI] [PubMed] [Google Scholar]
- 18.Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardio-myopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 19.Liao R, Nascimben L, Friedrich J, et al. Decreased energy reserve in an animal model of dilated cardiomyopathy: relationship to contractile performance. Circ Res. 1996;78:893–902. doi: 10.1161/01.res.78.5.893. [DOI] [PubMed] [Google Scholar]
- 20.Smith GL, Duncan AM, Neary P, et al. Pi inhibits the SR Ca2+ pump and stimulates pump-mediated Ca2+ leak in rabbit cardiac myocytes. Am J Physiol. 2000;279:H577–H585. doi: 10.1152/ajpheart.2000.279.2.H577. [DOI] [PubMed] [Google Scholar]
- 21.Chu G, Luo W, Slack JP, et al. Compensatory mechanisms associated with the hyperdynamic function of phospholamban-deficient mouse hearts. Circ Res. 1996;79:1064–1076. doi: 10.1161/01.res.79.6.1064. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson LW. Inotropic therapy for heart failure. N Engl J Med. 1998;339:1848–1850. doi: 10.1056/NEJM199812173392511. [DOI] [PubMed] [Google Scholar]
- 23.Lim HW, Molkentin JD. Calcineurin and human heart failure. Nat Med. 1999;5:246–247. doi: 10.1038/6430. [DOI] [PubMed] [Google Scholar]


