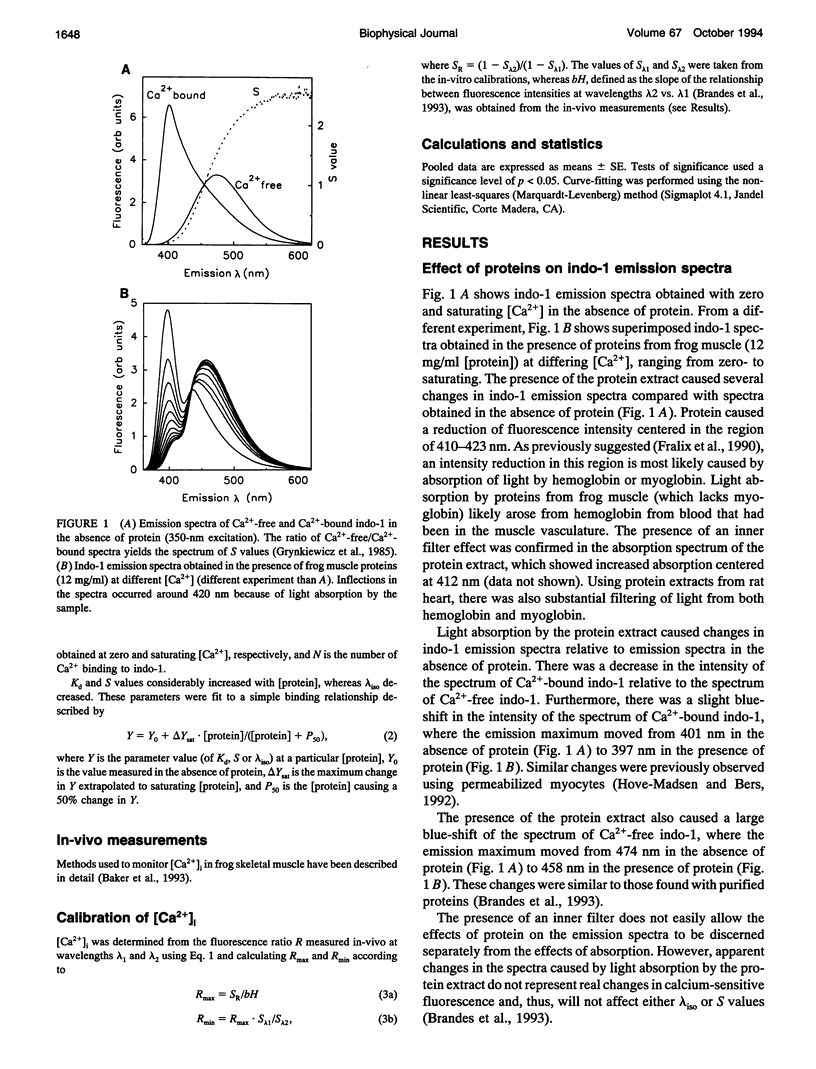
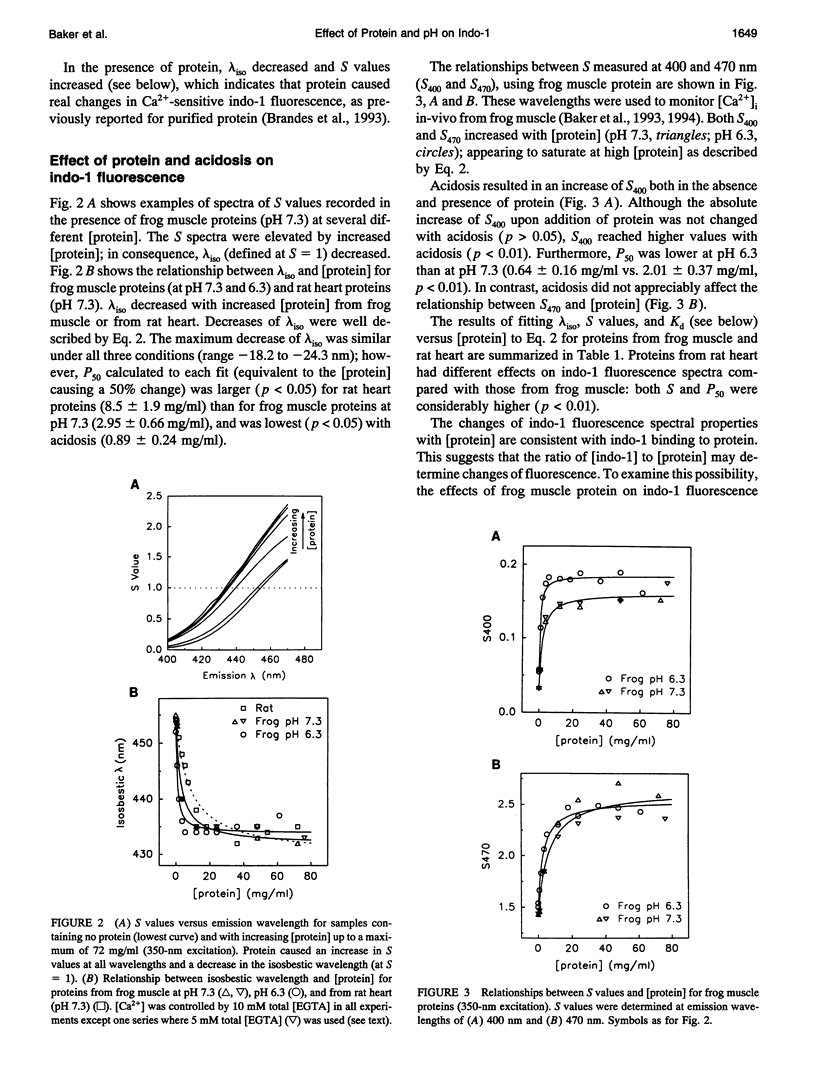
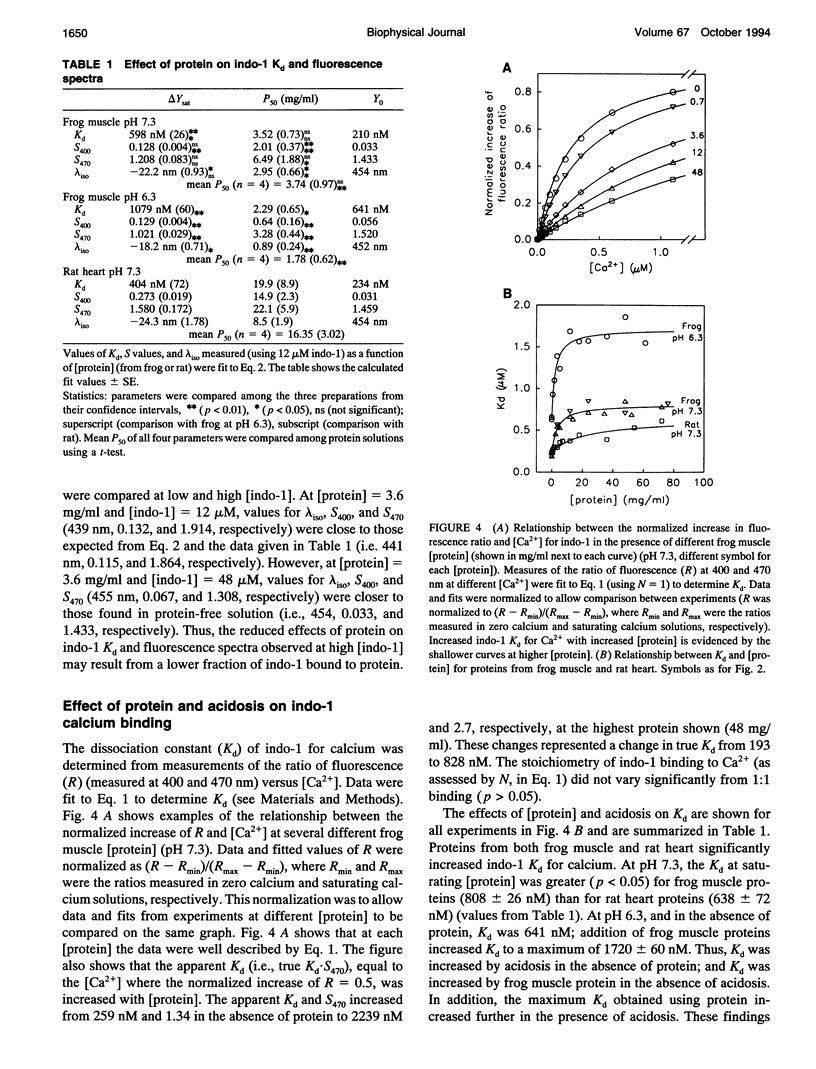
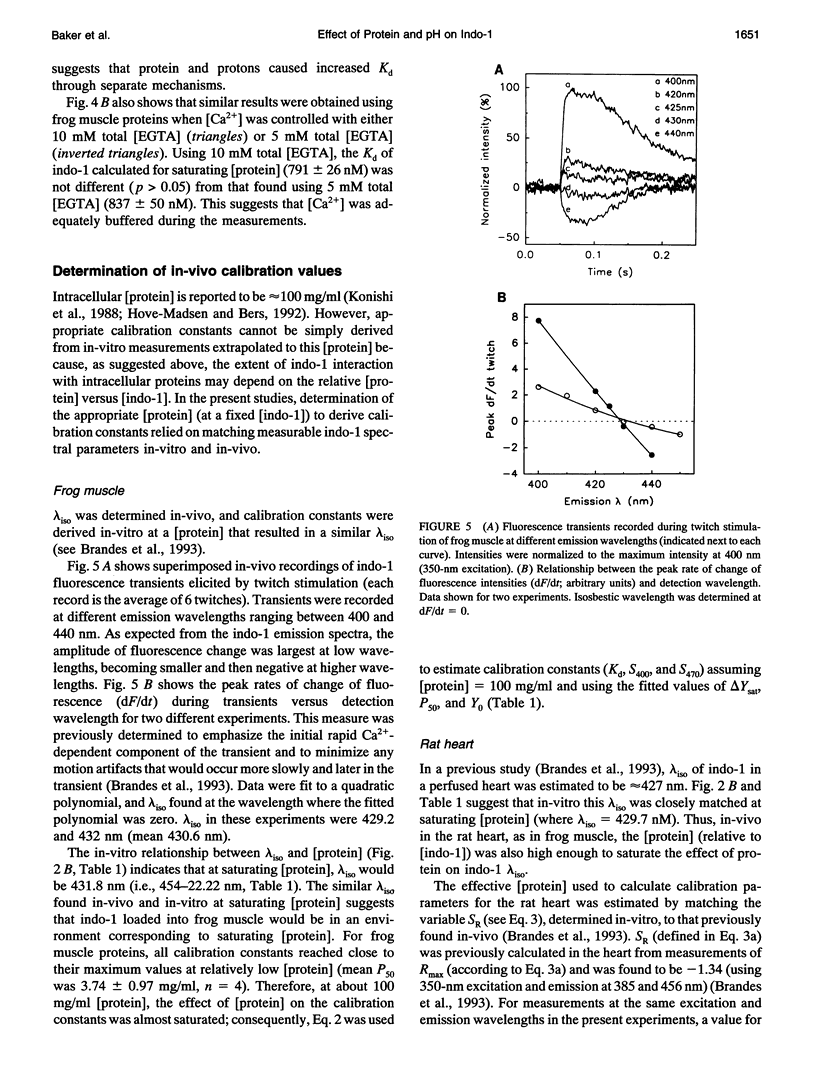
Abstract
The fluorescent indicator indo-1 is widely used to monitor intracellular calcium concentration. However, quantitation is limited by uncertain effects of the intracellular environment on indicator properties. The goal of this study was to determine the effects of protein and acidosis on the fluorescence spectra and calcium dissociation constant (Kd) of indo-1. With 350 nm excitation light, the ratio of indo-1 fluorescence in the absence versus the presence of saturating Ca2+ at wavelength lambda (S lambda) and Kd increased with [protein]. At pH 7.3, Kd, S400, and S470, which were 210 nM, 0.033, and 1.433 in the absence of protein, increased to 808 nM, 0.161, and 2.641, respectively, by adding proteins from frog muscle and to 638 nM, 0.304, and 3.039, respectively, by adding proteins from rat heart. Effects of protein on indo-1 fluorescence were reduced at higher [indo-1]. Acidosis (pH 6.3) had separate effects, which were additive to those of protein: in the absence of protein, acidosis increased Kd to 640 nM; frog muscle proteins further increased Kd to 1700 nM. Acidosis also changed S lambda slightly. In summary, interaction with protein or protons alters indo-1 calcium-binding and fluorescence. These findings are consistent with several previous studies and suggest that indo-1 calibration constants need to be derived in the presence of appropriate types of protein, ratio of [indo-1]/[protein], and pH.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989 Aug;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx P. H., Ter Keurs H. E. Fluorescent properties of rat cardiac trabeculae microinjected with fura-2 salt. Am J Physiol. 1993 Apr;264(4 Pt 2):H1098–H1110. doi: 10.1152/ajpheart.1993.264.4.H1098. [DOI] [PubMed] [Google Scholar]
- Baker A. J., Longuemare M. C., Brandes R., Weiner M. W. Intracellular tetanic calcium signals are reduced in fatigue of whole skeletal muscle. Am J Physiol. 1993 Mar;264(3 Pt 1):C577–C582. doi: 10.1152/ajpcell.1993.264.3.C577. [DOI] [PubMed] [Google Scholar]
- Brandes R., Figueredo V. M., Camacho S. A., Baker A. J., Weiner M. W. Investigation of factors affecting fluorometric quantitation of cytosolic [Ca2+] in perfused hearts. Biophys J. 1993 Nov;65(5):1983–1993. doi: 10.1016/S0006-3495(93)81275-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fralix T. A., Heineman F. W., Balaban R. S. Effects of tissue absorbance on NAD(P)H and Indo-1 fluorescence from perfused rabbit hearts. FEBS Lett. 1990 Mar 26;262(2):287–292. doi: 10.1016/0014-5793(90)80212-2. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harkins A. B., Kurebayashi N., Baylor S. M. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J. 1993 Aug;65(2):865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove-Madsen L., Bers D. M. Indo-1 binding to protein in permeabilized ventricular myocytes alters its spectral and Ca binding properties. Biophys J. 1992 Jul;63(1):89–97. doi: 10.1016/S0006-3495(92)81597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi H., Peeters G. A., Barry W. H. Evidence that binding of Indo-1 to cardiac myocyte protein does not markedly change Kd for Ca2+. Cell Calcium. 1991 Jun;12(6):415–422. doi: 10.1016/0143-4160(91)90067-o. [DOI] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N., Harkins A. B., Baylor S. M. Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibers. Biophys J. 1993 Jun;64(6):1934–1960. doi: 10.1016/S0006-3495(93)81564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr The effects of pH and temperature on fluorescent calcium indicators as determined with Chelex-100 and EDTA buffer systems. Biochem Biophys Res Commun. 1990 Aug 31;171(1):102–108. doi: 10.1016/0006-291x(90)91362-v. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Westerblad H., Allen D. G. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol. 1991 Feb;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., duBell W. H., Stern M. D., Sollott S. J., Ziman B. D., Silverman H. S., Capogrossi M. C., Talo A., Lakatta E. G. Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol. 1992 Feb;447:83–102. doi: 10.1113/jphysiol.1992.sp018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R., Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993 Jul;466:611–628. [PMC free article] [PubMed] [Google Scholar]


