Abstract
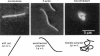
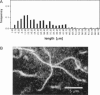
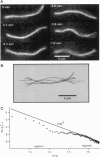
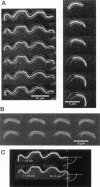
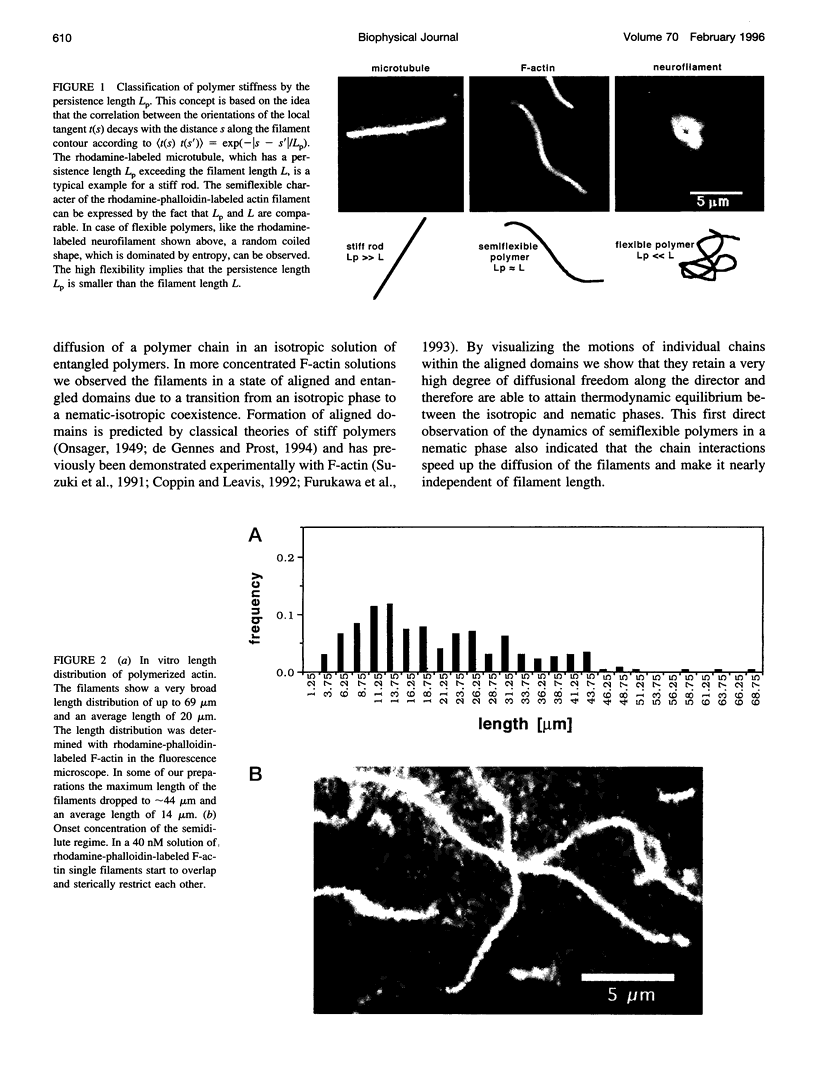
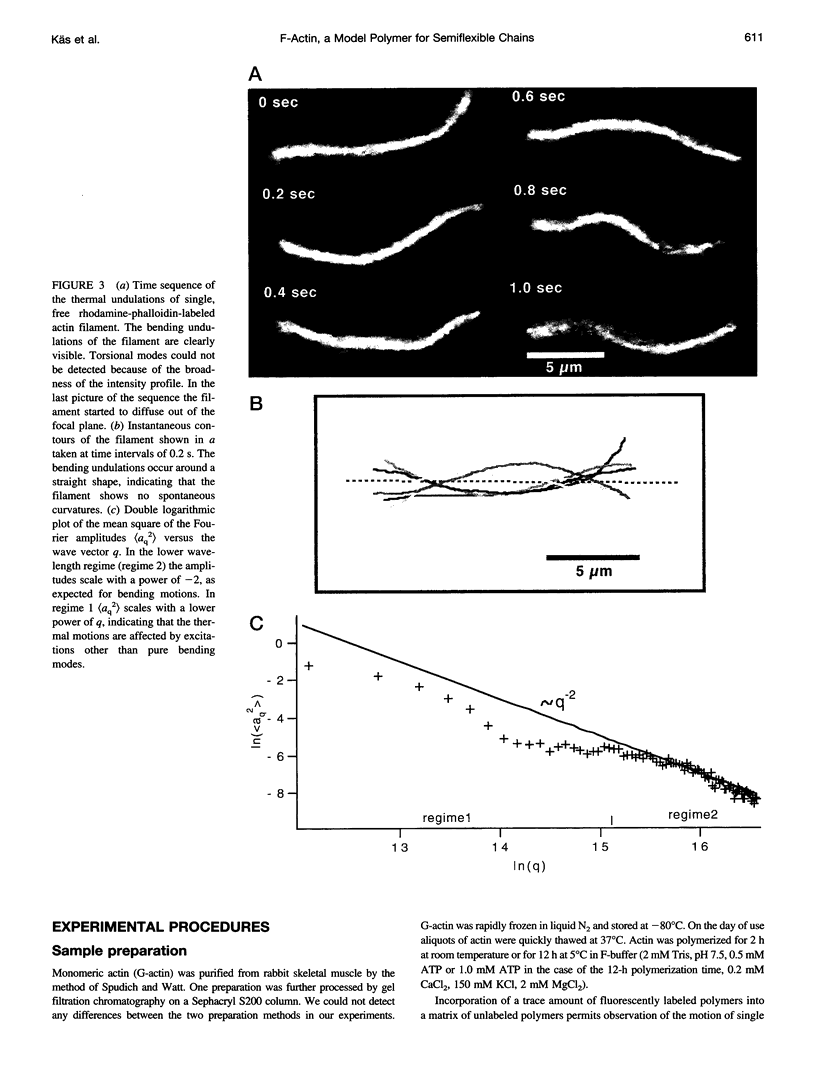
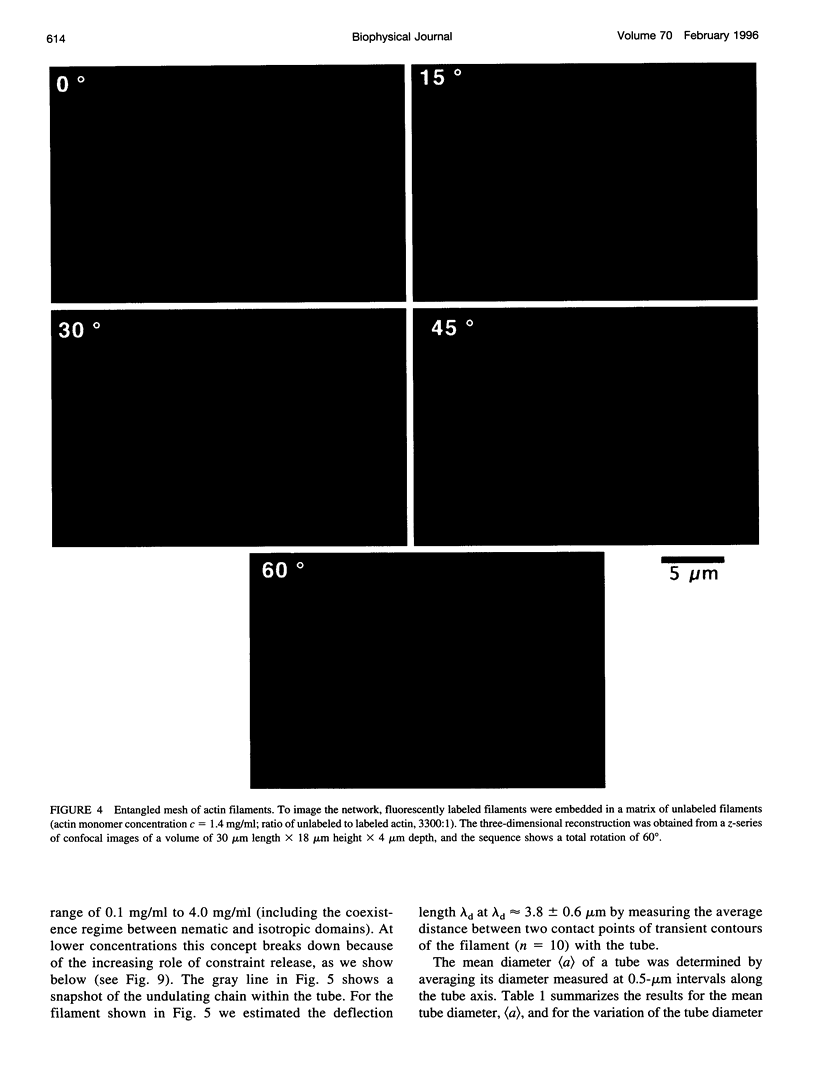
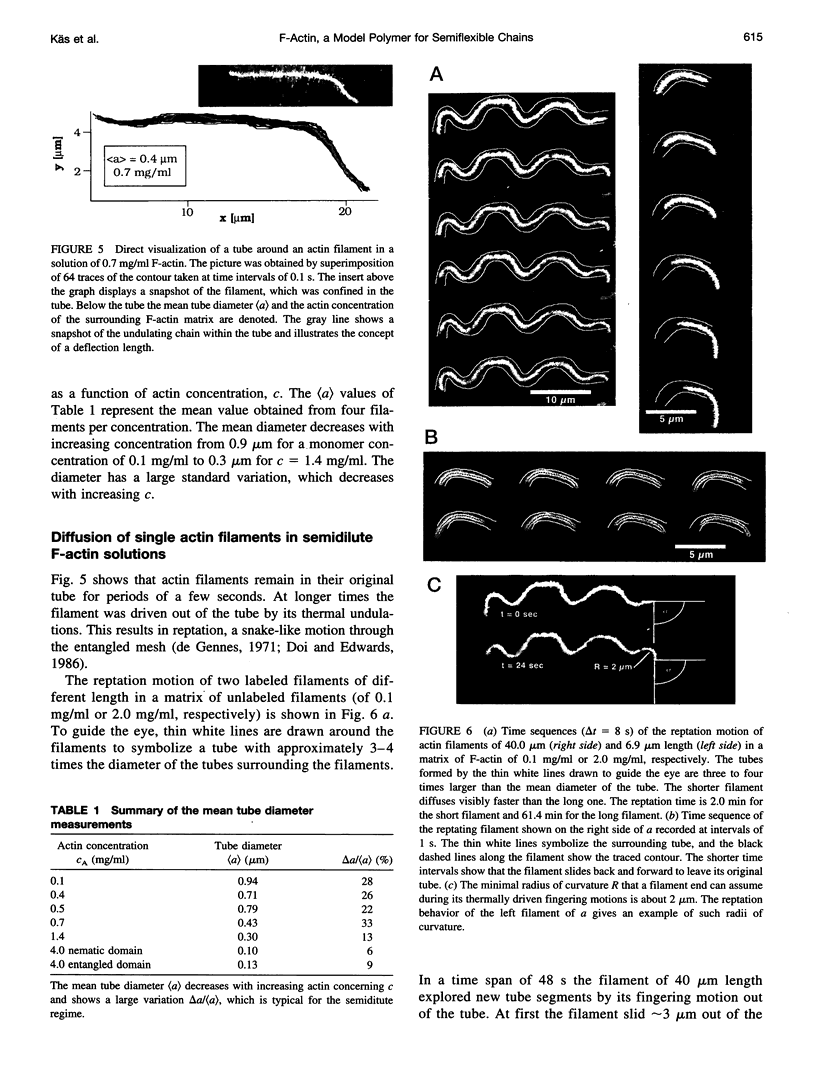
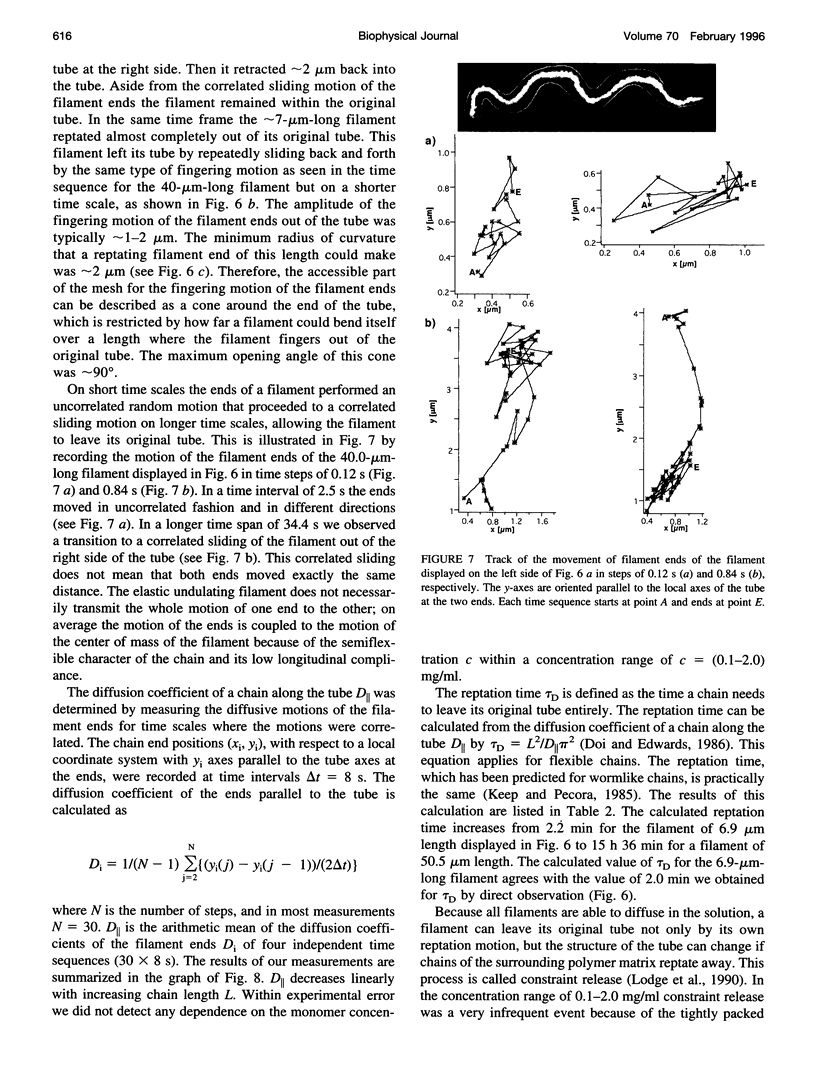
Single actin filaments were analyzed in solutions ranging from dilute (0.2 microgram/ml), where filaments interact only with solvent, to concentrations (4.0 mg/ml) at which F-actin forms a nematic phase. A persistence length of approximately 1.8 microns and an average length of approximately 22 microns (Kaufmann et al., 1992) identify actin as a model for studying the dynamics of semiflexible polymers. In dilute solutions the filaments exhibit thermal bending undulations in addition to diffusive motion. At higher semidilute concentrations (1.4 mg/ml) three-dimensional reconstructions of confocal images of fluorescently labeled filaments in a matrix of unlabeled F-actin reveal steric interactions between filaments, which account for the viscoelastic behavior of these solutions. The restricted undulations of these labeled chains reveal the virtual tube formed around a filament by the surrounding actin. The average tube diameter <a> scales with monomer concentration c as <a> varies; is directly proportional to c-(0.5 +/- 0.15). The diffusion of filaments in semidilute solutions (c = (0.1-2.0) mg/ml) is dominated by diffusion along the filament contour (reptation), and constraint release by remodeling of the surrounding filaments is rare. The self-diffusion coefficient D parallel along the tube decreases linearly with the chain length for semidilute solutions. For concentrations > 2.5 mg/ml a transition occurs from an isotropic entangled phase to a coexistence between isotropic and nematic domains. Analysis of the molecular motions of filaments suggests that the filaments in the aligned domains are in thermal equilibrium and that the diffusion coefficient parallel to the director D parallel is nearly independent of filament length. We also report the novel direct observation of u-shaped defects, called hairpins, in the nematic domains.
Full text
PDF


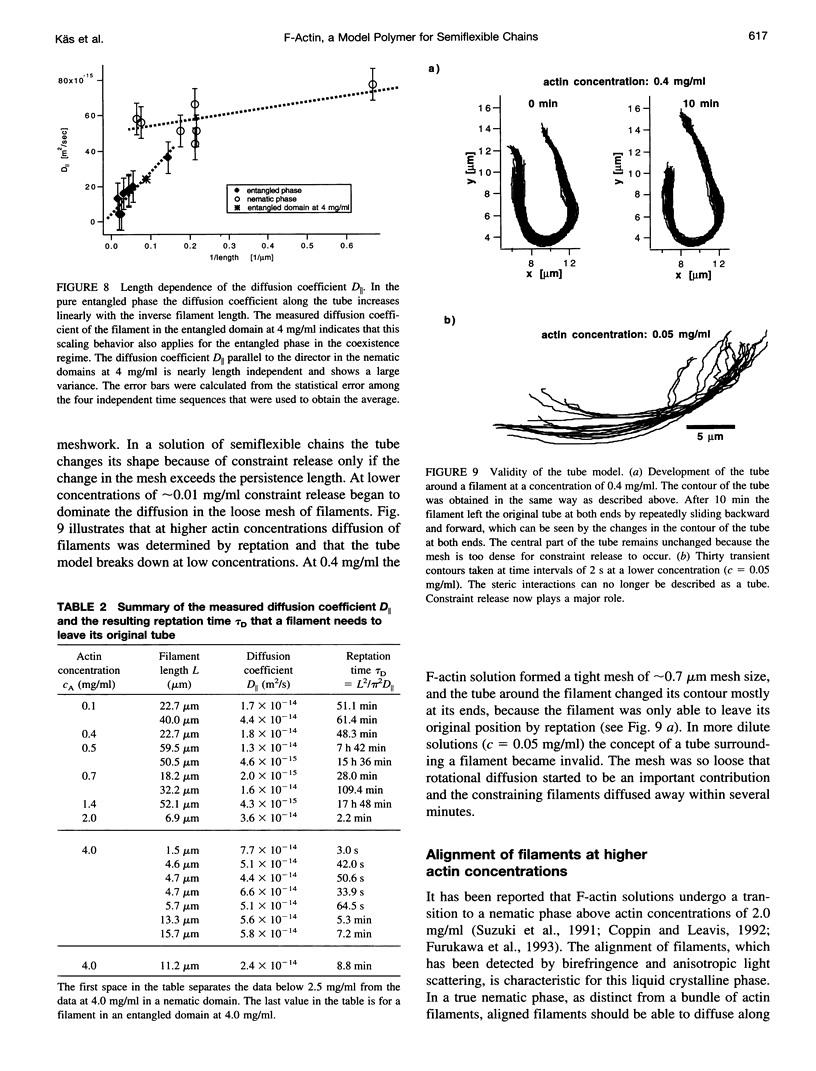
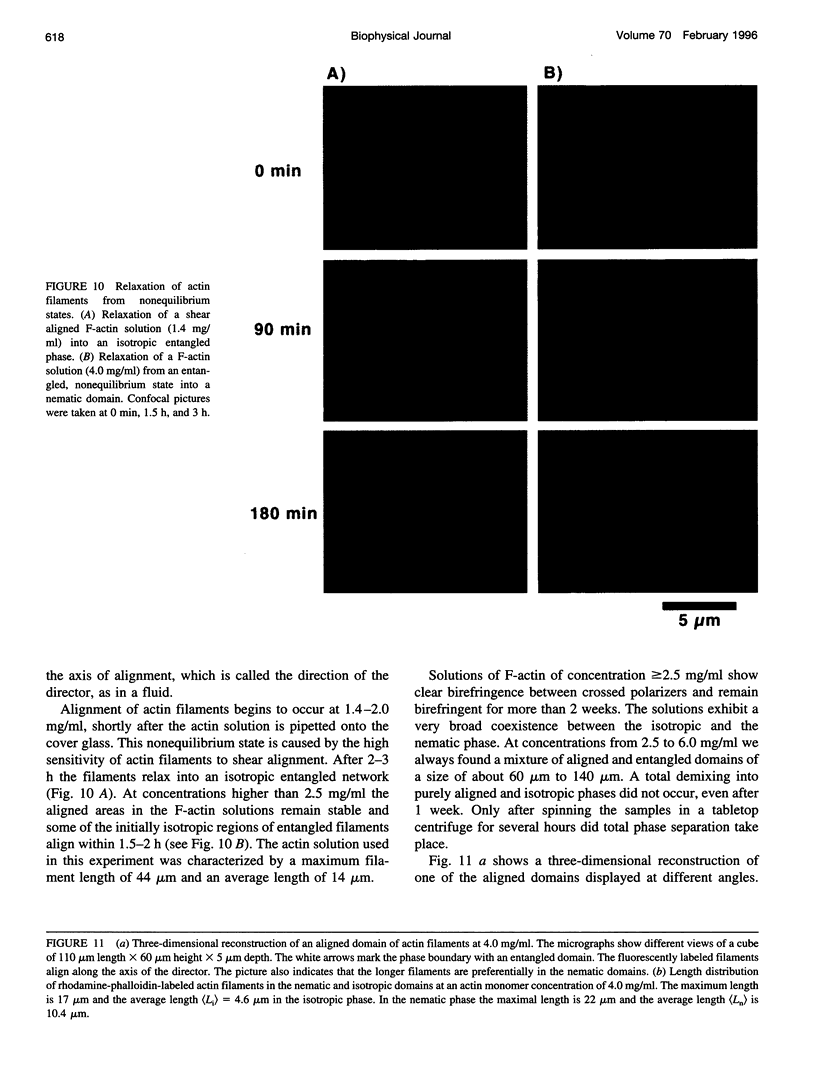
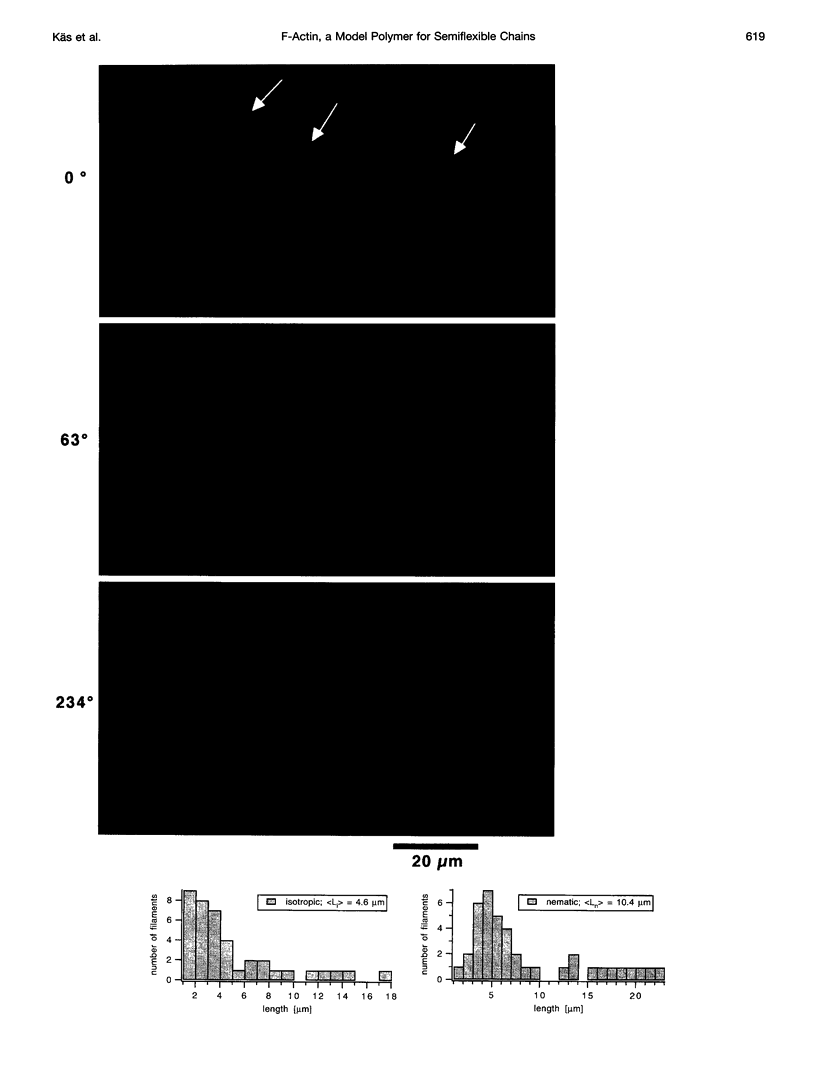
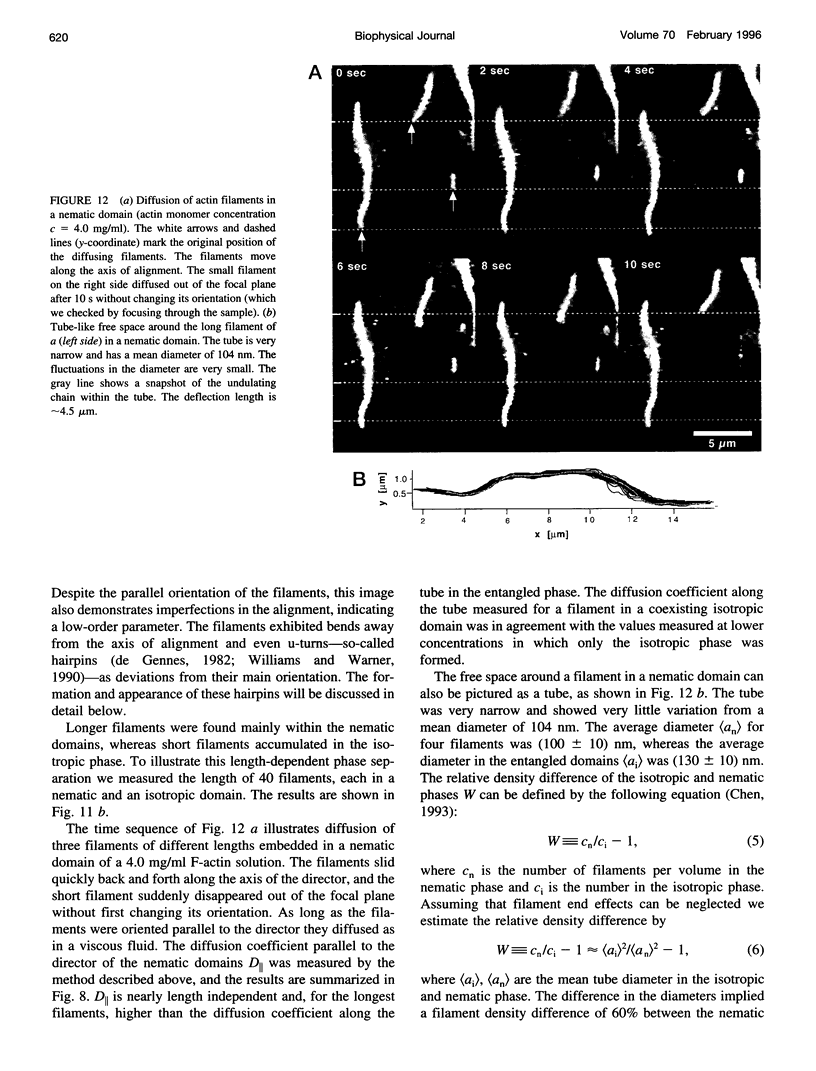


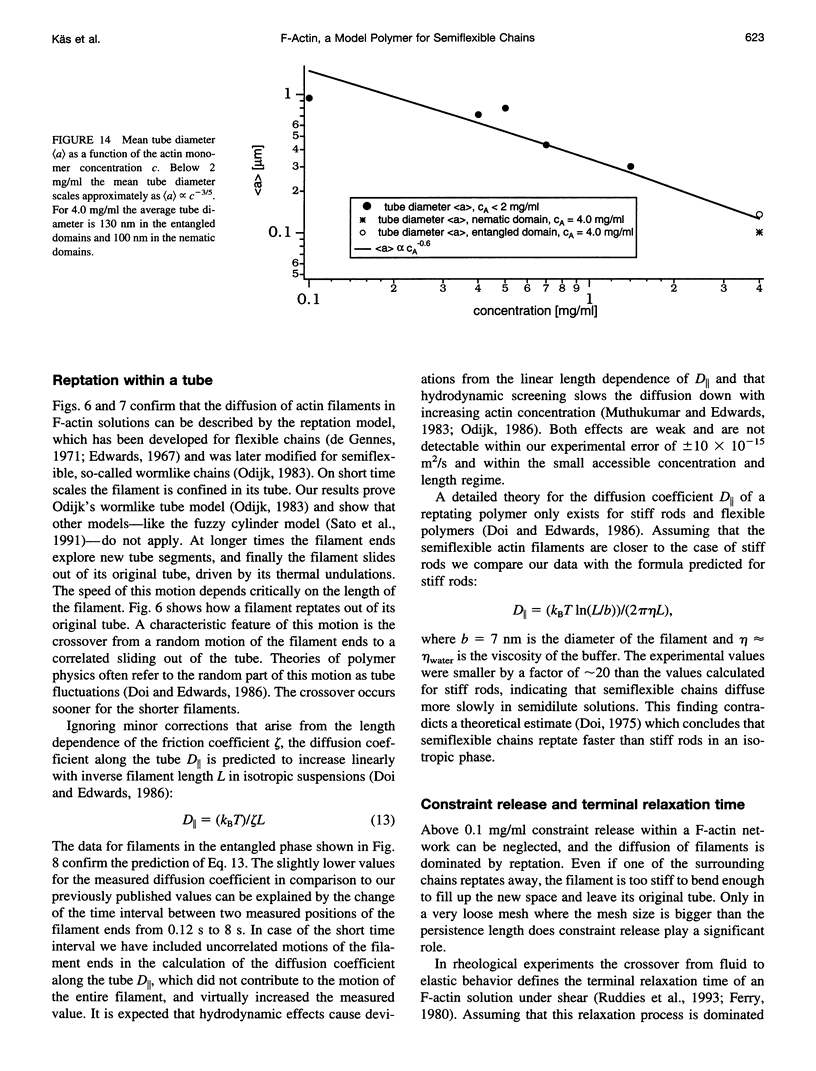


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer A., Millonig R. C., Sütterlin R., Engel A., Pollard T. D., Aebi U. The structural basis for the intrinsic disorder of the actin filament: the "lateral slipping" model. J Cell Biol. 1991 Nov;115(3):689–703. doi: 10.1083/jcb.115.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella J. F., Torres M. A. Interaction of Cap Z with actin. The NH2-terminal domains of the alpha 1 and beta subunits are not required for actin capping, and alpha 1 beta and alpha 2 beta heterodimers bind differentially to actin. J Biol Chem. 1994 Mar 4;269(9):6992–6998. [PubMed] [Google Scholar]
- Coppin C. M., Leavis P. C. Quantitation of liquid-crystalline ordering in F-actin solutions. Biophys J. 1992 Sep;63(3):794–807. doi: 10.1016/S0006-3495(92)81647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson E. L. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu Rev Biophys Biophys Chem. 1988;17:397–430. doi: 10.1146/annurev.bb.17.060188.002145. [DOI] [PubMed] [Google Scholar]
- Furukawa R., Kundra R., Fechheimer M. Formation of liquid crystals from actin filaments. Biochemistry. 1993 Nov 23;32(46):12346–12352. doi: 10.1021/bi00097a010. [DOI] [PubMed] [Google Scholar]
- Hendricks J, Kawakatsu T, Kawasaki K, Zimmermann W. Confined semiflexible polymer chains. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1995 Mar;51(3):2658–2661. doi: 10.1103/physreve.51.2658. [DOI] [PubMed] [Google Scholar]
- Ishijima A., Doi T., Sakurada K., Yanagida T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature. 1991 Jul 25;352(6333):301–306. doi: 10.1038/352301a0. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Euteneuer U., Traub P., Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991 Apr;113(1):155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Hvidt S., Käs J., Lerche D., Maggs A., Sackmann E., Schliwa M., Stossel T. P. The mechanical properties of actin gels. Elastic modulus and filament motions. J Biol Chem. 1994 Dec 23;269(51):32503–32513. [PubMed] [Google Scholar]
- Janmey P. A., Peetermans J., Zaner K. S., Stossel T. P., Tanaka T. Structure and mobility of actin filaments as measured by quasielastic light scattering, viscometry, and electron microscopy. J Biol Chem. 1986 Jun 25;261(18):8357–8362. [PubMed] [Google Scholar]
- Kaufmann S., Käs J., Goldmann W. H., Sackmann E., Isenberg G. Talin anchors and nucleates actin filaments at lipid membranes. A direct demonstration. FEBS Lett. 1992 Dec 14;314(2):203–205. doi: 10.1016/0014-5793(92)80975-m. [DOI] [PubMed] [Google Scholar]
- Käs J., Strey H., Sackmann E. Direct imaging of reptation for semiflexible actin filaments. Nature. 1994 Mar 17;368(6468):226–229. doi: 10.1038/368226a0. [DOI] [PubMed] [Google Scholar]
- Perkins T. T., Smith D. E., Chu S. Direct observation of tube-like motion of a single polymer chain. Science. 1994 May 6;264(5160):819–822. doi: 10.1126/science.8171335. [DOI] [PubMed] [Google Scholar]
- Radzihovsky L, Frey E. Kinetic theory of flux-line hydrodynamics: Liquid phase with disorder. Phys Rev B Condens Matter. 1993 Oct 1;48(14):10357–10381. doi: 10.1103/physrevb.48.10357. [DOI] [PubMed] [Google Scholar]
- Ruddies R., Goldmann W. H., Isenberg G., Sackmann E. The viscoelasticity of entangled actin networks: the influence of defects and modulation by talin and vinculin. Eur Biophys J. 1993;22(5):309–321. doi: 10.1007/BF00213554. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Finzi L., Bustamante C. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science. 1992 Nov 13;258(5085):1122–1126. doi: 10.1126/science.1439819. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Maeda T., Ito T. Formation of liquid crystalline phase of actin filament solutions and its dependence on filament length as studied by optical birefringence. Biophys J. 1991 Jan;59(1):25–30. doi: 10.1016/S0006-3495(91)82194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmuth W. D., Austin R. H. DNA electrophoresis in microlithographic arrays. Nature. 1992 Aug 13;358(6387):600–602. doi: 10.1038/358600a0. [DOI] [PubMed] [Google Scholar]