Abstract
Synaptotagmin (syt) I, an integral membrane protein localized to secretory vesicles, is a putative Ca2+ sensor for exocytosis. Its N terminus spans the membrane once, and its cytoplasmic domain contains two conserved C2 domains, designated C2A and C2B. The isolated C2A domain penetrates membranes in response to Ca2+; isolated C2B does not. Here, we have addressed the function of each C2 domain, but in the context of the intact cytoplasmic domain (C2A-C2B), by using fluorescent reporters placed in the Ca2+-binding loops of either C2A or C2B. Surprisingly, these reporters revealed that, analogous to C2A, a Ca2+-binding loop in C2B directly penetrates into lipid bilayers. Penetration of each C2 domain was very rapid (kon ≈1010 M−1⋅s−1) and resulted in high affinity C2A-C2B–liposome complexes (Kd ≈13–14 nM). C2B-bilayer penetration strictly depended on the presence, but not the membrane binding activity, of an adjacent C2A domain, severing C2A from C2B after protein synthesis abolished the ability of C2B to dip into bilayers in response to Ca2+. The activation of C2B by C2A was also displayed by the C2 domains of syt III but not the C2 domains of syt IV. A number of proteins contain more than one C2 domain; the findings reported here suggest these domains may harbor cryptic activities that are not detected when they are studied in isolation.
Keywords: exocytosis‖liposome‖fluorescence‖cooperativity‖fusion
Communication between neurons is mediated by the Ca2+-triggered release of neurotransmitters from docked synaptic vesicles (1). The Ca2+-binding synaptic vesicle protein, synaptotagmin (syt) I, has been proposed to function as a Ca2+ sensor that triggers release (2–4). Syt I spans the vesicle membrane once and possesses a large cytoplasmic domain largely composed of two C2 domains (5), each of which function as Ca2+-sensing modules (6–8). To understand the molecular mechanism by which syt may regulate exocytosis, the immediate consequences of Ca2+ binding have been studied. The best characterized Ca2+-triggered effector interaction is between the C2A domain and membranes. In the presence of Ca2+, two Ca2+-binding loops of C2A directly penetrate into lipid bilayers (9–11). This interaction potentially drives lipid rearrangements that underlie fusion (2, 10) as supported by a genetic study (4).
Despite its homology to C2A (≈40%), the isolated C2B domain of syt fails to penetrate membranes in response to Ca2+ (12, 13). This was somewhat surprising because the function of C2 domains, in a number of enzymes, is to mediate Ca2+-triggered translocation to membranes where they can interact with substrate (reviewed in ref. 14). In a recent study, we observed that in the context of the intact cytoplasmic domain of syt (designated C2A-C2B), mutations that abolish Ca2+-triggered C2A–membrane interactions did not abolish the ability of the cytoplasmic domain to bind liposomes in response to Ca2+ (13). These data suggested that C2B rescues activity in the mutant C2A domain or that C2A activates a cryptic membrane penetration activity within C2B.
Here, we have used site-directed fluorescent probes to distinguish between these two models. Interestingly, these data demonstrate that C2A activates a cryptic membrane penetration activity in an adjacent C2B domain. These findings establish a form of cooperativity between tandem C2 domains that was not anticipated from studies of isolated C2 domains, resulting in a model for syt–membrane interactions.
Materials and Methods
Recombinant Proteins.
cDNA encoding rat syt I (G374) (8, 15), III (16), and IV (17) were kindly provided by G. Schiavo (Imperial Cancer Research Fund), S. Seino (Chiba University, Chiba, Japan), and H. Herschman (University of California, Los Angeles), respectively. The cytoplasmic (designated C2A-C2B, residues 96–421), C2A (residues 96–265), and C2B (residues 248–421) domains of syt I (G374 version) were expressed in Escherichia coli as glutathione S-transferase fusion proteins and purified by using glutathione-Sepharose beads (Amersham Pharmacia) as described (18, 19). Point mutations and chimeras were prepared by using the overlapping primer method as described (7).
In C2A-C2B, Cys-277 was replaced with Ala, then a single Cys was placed in loop 3 of the C2A domain (F234C; indicated as C2A*-C2B) or in an analogous position in the C2B domain (I367C; indicated as C2A-C2B*). In C2AM*-C2B and C2AM-C2B*, the subscript M corresponds to D230,232N substitutions that disrupt the Ca2+ and lipid binding activity of C2A (22). cDNA encoding these constructs were subcloned into pGEX-2T (Amersham Pharmacia) using BamHI and EcoRI sites.
In his6-C2A-TC-C2B*, TC indicates a thrombin cleavage site (LVPRGS) that was inserted into the linker domain between C2A and C2B (after residue 268). This construct was His-6 tagged at its N terminus by subcloning into the pTrcHis A vector (Invitrogen) by using EcoRI and XhoI. The protein was purified on Ni-NTA-agarose (Qiagen, Chatsworth, CA) as described (19).
All chimeras were expressed as glutathione S-transferase fusion proteins using pGEX vectors; their compositions were as follows: C2AM III-C2B I, residues 290–421 of III (with two Ca2+ ligand mutations, D385,387N) and residues 264–421 of I (20); C2AM I-C2B III, residues 96–272 (containing two Ca2+ ligand mutations, D230,232N) of I and residues 430–569 of III; C2A IV-C2B, residues 152–278 of IV and 264–421 of I; and C2AM I-C2B IV, residues 96–272 of I (containing two mutations at the Ca2+ binding sites, D230,232N) and residues 288–425 of IV.
Labeling of the Single Cys Mutants of C2A-C2B by 5-[[2-[(Iodoacetyl)amino]ethyl]amino]naphthalene-1-sulfonic Acid (IAEDANS).
Cys residues were labeled by incubation of proteins with a 10-fold molar excess of 1,5-IAEDANS (Molecular Probes) at 25°C for 1 h in Hepes buffer (50 mM Hepes-NaOH, pH 7.4/0.1 M NaCl). Free fluorophore was removed using Sephadex G-25 desalting columns (Amersham Pharmacia), and residual probe was removed by dialysis. The AEDANS concentration was determined using an extinction coefficient of 6.0 × 103 M−1⋅cm−1 at 337 nm (23). The protein concentration was determined by Coomassie blue staining of proteins separated by SDS/PAGE using BSA as a standard. Labeling ratios were 0.85–0.95 mole label/mole of protein.
Liposomes.
Brain-derived phosphatidylserine (PS), phosphatidylcholine (PC), 1-palmitoyl-2-stearoyl (5-doxyl)-sn-glycero-3-phosphocholine (5-doxyl-PC), 1-palmitoyl-2-stearoyl (7-doxyl)-sn-glycero-3-phosphocholine (7-doxyl-PC), and 1-palmitoyl-2-stearoyl (12-doxyl)-sn-glycero-3-phosphocholine (12-doxyl-PC) were obtained from Avanti Polar Lipids. For fluorescence studies, large (≈100 nm) unilamellar liposomes were prepared as described by Davis et al. (10).
L-3-phosphatidyl[N-methyl-3H]choline-1,2-dipalmitoyl ([3H]PC) was purchased from Amersham Pharmacia, and 3H-labeled liposome-binding assays were carried out as described by Davis et al. (10). Error bars represent the standard deviations from triplicate determinations.
Fluorescence Measurements.
Steady-state fluorescence measurements were made at 24°C using a PTI (South Brunswick, NJ) QM-1 fluorometer and felix software. Labeled protein (0.5 μM) was mixed with liposomes (11 nM liposomes = 1 mM lipid) in a cuvette using a castle-style stir bar. AEDANS was excited at 336 nm, and emission spectra were collected from 420 to 600 nm (2-nm slits). Emission spectra were corrected for blank, dilution, and instrument response. The depth of the fluorophore penetration into bilayers was calculated according to the parallax analysis described by Bai et al. (11). The distance from the bilayer center to the shallow quencher (5-doxyl-PC) was taken as 5.85 Å (24). [Ca2+]free was determined as described in Davis et al. (10). For stopped-flow rapid mixing experiments, AEDANS-labeled proteins were excited at 336 nm, and emitted light was collected by using a 470-nm cutoff filter. These experiments and calculations were carried out as described in ref. 10.
Results
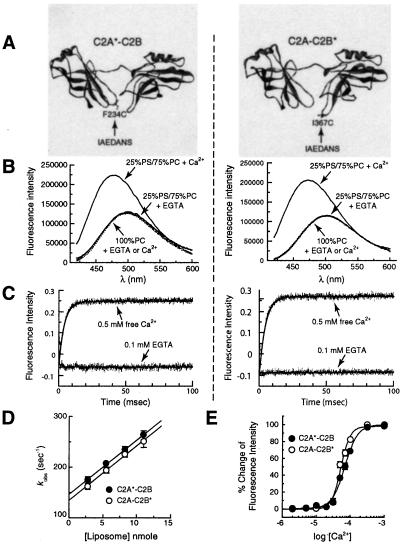
A lone Trp residue, placed at the distal tip of Ca2+-binding loop 3 of C2A, shows membrane penetration of the loop as an increase in intensity and a blue shift in the emission spectrum (9–11). To explore the possibility that C2B, in the context of C2A-C2B, also penetrates into membranes, we placed a fluorescent reporter at the analogous position in its Ca2+-binding loop 3 (position Ile-367 as determined using the crystal structures of syt I and III as templates) (20, 21). Because C2A-C2B contains a number of Trp residues that are critical for its function (data not shown), we substituted Ile-367 with a cysteine (Cys) residue and labeled it with an AEDANS. As a positive control, the equivalent position in C2A (Phe-234 in loop 3) was also changed to a Cys and labeled with AEDANS (Fig. 1A).
Figure 1.
AEDANS reporters indicate that both C2 domains of syt I interact rapidly with membranes. (A) Molecular models depicting the labeling sites in the cytoplasmic domain of syt I (designated C2A-C2B). The fluorophore, IAEDANS, was used to label the thiol side chains of the lone Cys residues engineered into Ca2+-binding loop 3 of C2A or C2B. The label is indicated by *; C2A*-C2B harbors an AEDANS reporter labeled in the C2A domain (F234C); C2A-C2B* harbors an AEDANS reporter labeled in the C2B domain (I367C). These labels are shown by using the crystal structure of syt III (20) as a template; images were rendered by using WebLab VIEWERLITE 3.7 software. (B) Ca2+/liposome-dependent changes in the fluorescence of C2A*-C2B and C2A-C2B*. C2A*-C2B and C2A-C2B* were adjusted to 0.5 μM in Hepes and excited at 336 nm; the emission spectra were then collected from 420 to 600 nm. Spectra were first obtained in the presence of 0.1 mM EGTA and liposomes. Liposome composition (25% PS/75% PC) and concentration (11 nM liposomes, 1 mM total lipid) are the same for all experiments unless otherwise indicated. Ca2+ was then added to a final free concentration of 0.5 mM. In the presence of Ca2+ and liposomes, both C2A*-C2B and C2A-C2B* showed large increases in fluorescence intensity (71.8% and 77.8% for C2A*-C2B and C2A-C2B*, compared with the intensity in EGTA at 474 nm) and exhibited significant blue shifts (for C2A*-C2B, from 500 nm to 476 nm; for C2A-C2B*, from 502 nm to 474 nm) in their emission maxima (solid lines). Identical amounts of liposomes lacking anionic phospholipids (100% PC) did not induce fluorescence changes in the presence or absence of Ca2+ (dashed lines). (C) Stopped-flow mixing experiments demonstrate that the reporters in C2A*-C2B and C2A-C2B* rapidly penetrate membranes with similar kinetics. The fluorescence changes exhibited by C2A*-C2B or C2A-C2B* were used to monitor the kinetics of Ca2+-triggered interactions with liposomes, using an Applied Photophysics SX.18MV stopped-flow spectrometer. (Left) C2A*-C2B (1 μM) was premixed with liposomes (22 nM) and EGTA (0.1 mM), and then rapidly mixed with Ca2+ (0.5 mM final free [Ca2+]). The kinetics of this reaction are shown in the upper trace. As a control, mixing experiments were also carried out by using 0.1 mM EGTA instead of Ca2+ (lower trace), providing a minimum reference point. Data (4,000 points) were collected for 100 ms and are plotted with a best-fit single exponential function (kobs = 257 s−1). (Right) The same experiment was carried out, except using C2A-C2B* (kobs = 247 s−1). (D) Determination of kon and koff for the C2A*-C2B or C2A-C2B*⋅liposome complexes in the presence of Ca2+. Stopped-flow rapid mixing experiments were carried out as in C; kobs was determined by fitting the data with single exponential functions and plotted vs. [liposome]. The Y-intercept yields koff for the C2A*-C2B⋅liposome complex in the presence of Ca2+ (146.6 ± 6.9 s−1), and the slope yields kon (1.06 ± 0.09 × 1010 M−1⋅s−1) (10). Almost identical data were obtained for the cryptic lipid penetration activity of C2B, as revealed by analysis of C2A-C2B*; koff was 134.6 ± 9.3 s−1 and kon was 1.07 ± 0.12 × 1010 M−1⋅s−1. Error bars represent standard deviations from three independent experiments. In Ca2+, the dissociation constant for the C2A-C2B⋅liposome complex was ≈13–14 nM. (E) Steady-state Ca2+ dependency of C2A*-C2B and C2A-C2B* penetration into liposomes. The corrected emission spectra were obtained as described in B and integrated. The changes in fluorescence intensity were normalized, plotted, and fit as a function of [Ca2+]free with GRAPHPAD PRISM 2.0 software. For C2A*-C2B (●), the [Ca2+]1/2 was 69 ± 1.3 μM (Hill coefficient = 2.3); for C2A-C2B* (○), the [Ca2+]1/2 was 53 ± 1.4 μM (Hill coefficient = 3.2).
Consistent with previous studies using Trp probes, the label on the C2A domain (designated C2A*-C2B) exhibited a Ca2+-triggered increase in the fluorescence intensity and blue shift in its emission spectrum when mixed with liposomes containing acidic phospholipids (25% PS/75% PC unless otherwise indicated). These changes depended on addition of both liposomes and Ca2+ (Fig. 1B Left) (9–11) and result from the Ca2+-driven penetration of the Ca2+-bound loop, harboring the fluorescent label, into the hydrophobic phase of the lipid bilayer (refs. 9 and 11; also described in detail below). Membrane penetration requires the presence of acidic phospholipids (25% PS); the fluorescent reporters did not penetrate liposomes composed of only PC (refs. 6, 7, and 11 and data not shown). This is likely because of the need for acidic phospholipid head groups to complete the Ca2+ coordination sites; anionic lipids probably interact with basic residues in the Ca2+-binding loops (9, 21, 25, 26).
We then used this approach to address the possibility that C2B might also penetrate bilayers. As shown in Fig. 1B (Right), the fluorescence of an AEDANS label on Ca2+-binding loop 3 of C2B (designated C2A-C2B*) exhibited strikingly similar fluorescence changes upon addition of Ca2+ and liposomes; i.e., an increase in fluorescence intensity and a blue shift in the emission spectra [in the absence of liposomes, Ca2+ causes a slight (<1%) decrease in fluorescence]. These data support a model in which C2A activates a cryptic membrane penetration activity within C2B.
We next determined whether the interactions of C2A*-C2B and C2A-C2B* with membranes are rapid enough to couple Ca2+ influx to fusion using a stopped-flow rapid mixing approach. Rapid mixing of liposomes with C2A*-C2B or C2A-C2B* in EGTA resulted in an unchanging baseline fluorescence signal (Fig. 1C). Rapid mixing of liposomes with C2A*-C2B or C2A-C2B* in Ca2+ resulted in a rapid and marked increase in the fluorescence signal (Fig. 1C). These increases were well fitted with single exponential functions to determine the observed rate, kobs. We then measured kobs as a function of the [liposome]; these data are plotted in Fig. 1D; the Y-intercept yields koff and the slope yields kon. These values were 146.6 ± 6.9 s−1/1.06 ± 0.09 × 1010 M−1⋅s−1 for C2A*-C2B and 134.6 ± 9.3 s−1/1.07 ± 0.12 × 1010 M−1⋅s−1 for C2A-C2B*. If C2A and C2B function as independent domains, these studies indicate that they exhibit similar affinities for membranes; the calculated dissociation constants from these kinetics data are 13–14 nM for each of the reporter constructs. As we reported for the isolated C2A domain, the on-rates for C2A*-C2B or C2A-C2B* membrane interactions approach the collisional limit and more than satisfy the kinetic constraints of exocytosis (27, 28).
The optical reporters were also used to measure steady-state Ca2+ dependencies for the penetration of C2A and C2B into membranes, again in the context of C2A-C2B. When a reporter in C2A is monitored, the [Ca2+]1/2 for binding was 69 ± 1.4 μM (Hill coefficient = 2.3; Fig. 1E). Similar results were obtained when the reporter in C2B was monitored ([Ca2+]1/2 = 53 ± 1.3 μM; Hill coefficient = 3.2; Fig. 1E). These values are in the range of the Ca2+ requirements for secretion measured in different cell types (from ≈10 to 200 μM Ca2+; refs. 28–30).
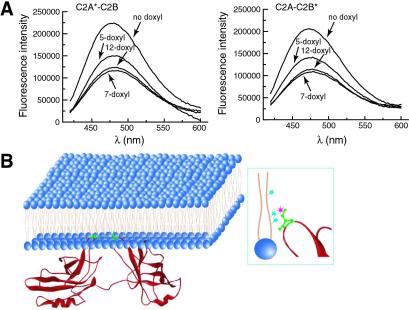
To confirm that the increase in fluorescence of C2A-C2B* results from penetration of the reporter into bilayers, we carried out quenching experiments using the membrane-embedded fluorescence quencher, doxyl-PC. Doxyl groups are efficient quenchers of AEDANS fluorescence, but because the quencher is located on the acyl chain of the lipid (Fig. 2B Right), quenching can occur only if the fluorophore penetrates into the bilayer. As shown in Fig. 2A, the reporters in C2A*-C2B and C2A-C2B* were efficiently quenched. The degree of quenching by labels at the 5, 7, and 12 positions of the acyl chain indicated that the reporters penetrated ≈1/6th into the hydrophobic core of the bilayer. These results are shown schematically in Fig. 2B, where both C2A and C2B simultaneously penetrate into the hydrophobic core of lipid bilayers, “pinning” the protein to the bilayer surface.
Figure 2.
Ca2+ binding loop 3 in the C2A and C2B domains of C2A-C2B penetrate lipid bilayers to the same extent. (A) Membrane-embedded quenchers efficiently quench the fluorescent reporters in both C2A*-C2B and C2A-C2B*. For these experiments, 0.5 μM AEDANS-labeled protein was incubated with liposomes in the presence of 0.5 mM Ca2+. Liposomes contained 25% PS, 65% PC, and 10% doxyl-PC labeled at the 5-, 7-, or 12-position of the sn-2 acyl chain. The depth of penetration was calculated as described in Materials and Methods. The distance between the fluorophore and bilayer center was 10.5 ± 0.7 Å and 10.6 ± 0.6 Å for the reporters in C2A*-C2B and C2A-C2B*, respectively. Thus, loop 3 of both C2A and C2B penetrate about one-sixth into the hydrophobic core of the lipid bilayer. (B) A model depicting the Ca2+-triggered docking of C2A-C2B onto a lipid bilayer. The crystal structure of C2A-C2B was modified from Sutton et al. (20). (Right) Three cyan stars indicate doxyl quenchers labeled at the 5-, 7-, or 12-position of the sn-2 acyl chain of PC. The pink star indicates the AEDANS fluorophore attached to a Cys residue (green) engineered into Ca2+-binding loop 3 (red) of either C2A or C2B.
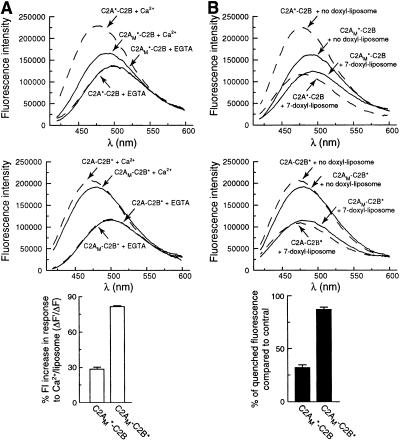
To rule out the possibility that the lipid binding activity of C2A “drags” C2B into membranes, we generated a construct harboring mutations that abolish C2A–membrane interactions (C2AM-C2B). We then placed fluorophores in loop 3 of either C2 domain (designated C2A*M-C2B and C2AM-C2B*). The mutations in C2A strongly inhibited the increase in fluorescence of the C2A probe that results from insertion into bilayers (Fig. 3A Upper), excluding the model in which C2B “repairs” mutations within C2A. In contrast, these mutations had only slight effects on the increase in fluorescence of the fluorescent reporter in C2B (Fig. 3A Middle and Lower). To confirm that mutations in C2A affect the penetration of C2A, but have little effect on the penetration of C2B, we used membrane-embedded quenchers as described above. The relative degree of quenching of reporters placed in the C2A domain was diminished by Ca2+-ligand mutations in C2A (Fig. 3B Upper). In contrast, the Ca2+ ligand mutations in C2A had little effect on the strong quenching of the fluorophore on C2B (Fig. 3B Middle and Lower). These experiments clearly demonstrate that the ability of C2B to penetrate lipid bilayers is not by means of a passive process in which C2A “drags” C2B. Rather, when C2A is adjacent to C2B, a cryptic lipid penetration activity within C2B is activated. It is interesting that this activation occurs even by using a C2A domain, which itself is not active (discussed further below).
Figure 3.
C2A does not drag the C2B domain of C2A-C2B into membranes. In the context of C2A-C2B, neutralization of Ca2+ ligands (D230,232N) in the C2A domain significantly disrupts C2A–membrane interactions, but has little effect on C2B–membrane interactions. These experiments were carried out two ways. (A) Fluorescence spectra were obtained as described in Fig. 1B. The Ca2+ (0.5 mM)/liposome-triggered fluorescence increases were used as indicators of membrane penetration. The fluorescence spectra of C2A*-C2B is indicated with dashed lines. The fluorescence spectra of C2AM*-C2B or C2AM-C2B* [the subscript M refers to two Ca2+-ligand mutations (D230,232N) that disrupt Ca2+-triggered C2A–lipid interactions] (22) are indicated with solid lines. All samples contained anionic phospholipid liposomes. (Top) As a control, the fluorescence of C2A*-C2B in EGTA (0.1 mM) increases upon addition of Ca2+ (0.5 mM). This increase was attenuated in C2AM*-C2B. (Middle) This experiment was repeated by using C2A-C2B* as a positive control. As expected, Ca2+ drove increases in emission intensity of the reporter. This increase was only slightly attenuated when C2AM-C2B* was analyzed. (Bottom) The Ca2+-triggered increases in emission intensity for C2AM*-C2B and C2AM-C2B* were quantified and plotted. These values were obtained by normalizing the fluorescence intensity (FI) changes of C2AM*-C2B and C2AM-C2B* (ΔF′) to those of C2A*-C2B and C2A-C2B* (ΔF), respectively. (B) The extent of AEDANS fluorescence quenching obtained in the presence of Ca2+ (0.5 mM) and liposomes containing 10% membrane-embedded quencher (10% 7-doxyl PC/65% PC/25% PS) were used to quantify membrane penetration. (Top) C2A*-C2B served as a positive control; its emission spectra were efficiently quenched by the presence of the doxyl spin label (dashed lines). These data were compared with the fluorescence spectra of C2AM*-C2B, which showed a diminished degree of quenching (solid lines). (Middle) These experiments were repeated using the C2AM-C2B* version of syt. C2A-C2B* served as a positive control and was efficiently quenched by the doxyl spin label (dashed lines). C2AM-C2B* was quenched to a similar degree (solid lines) as C2A-C2B*, demonstrating that the ability of C2B to penetrate bilayers does not depend on the ability of the adjacent C2A domain to penetrate membranes. (Bottom) The degree of quenching of C2AM*-C2B and C2AM-C2B* are plotted as the fraction of quenched fluorescence to the C2A*-C2B and C2A-C2B* controls, respectively.
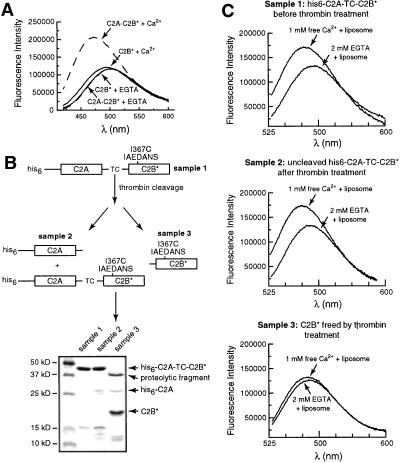
In light of these findings, we sought to better understand the mechanism by which C2A “activates” a cryptic Ca2+-triggered lipid penetration activity within C2B. When isolated C2B is expressed and purified, it fails to penetrate lipid bilayers in the AEDANS fluorescence assay (Fig. 4A). We considered two possibilities for this lack of activity: that C2A is needed for correct folding of an active C2B domain or that C2A interacts with C2B to activate it after the protein is synthesized. We addressed the question by engineering a thrombin cleavage site between C2A and C2B (Fig. 4B; designated C2A-TC-C2B). We expressed and purified a His-6-tagged version of C2A-TC-C2B and showed that the C2B domain can penetrate into lipid bilayers using an AEDANS reporter placed in loop 3 (Fig. 4C Top). We then cleaved C2B from C2A by using thrombin. Because this reaction was highly inefficient (≈3% of the protein was cleaved), we assayed whether the conditions used to carry out cleavage affected the ability of C2A-TC-C2B* to penetrate membranes and found that this activity was not diminished (Fig. 4C Middle). We purified cleaved C2B from the fusion protein and assayed its ability to interact with membranes. Analogous to C2B purified as an isolated domain (Fig. 4A); C2B* liberated from C2A also failed to penetrate membranes (Fig. 4C Bottom). These data indicate that the role of C2A is not to direct folding of C2B, but rather that C2A interacts with C2B by means of a novel mechanism that activates cryptic Ca2+-triggered membrane penetration properties within C2B, potentially by means of physical contact between the adjacent C2 domains. A recent fluorescence resonance energy transfer study indicates that C2A and C2B can come into closer proximity, albeit at millimolar divalent metal concentrations (31).
Figure 4.
The ability of C2B to penetrate bilayers strictly depends on the presence of an adjacent C2A domain. (A) The isolated recombinant C2B domain does not penetrate into lipid bilayers. Fluorescence spectra were recorded as described in Fig. 1B; all samples contained acidic liposomes. C2A-C2B* (in 0.1 mM EGTA) served as a positive control and exhibited an increase in fluorescence upon addition of Ca2+ (0.5 mM). In contrast, isolated C2B, with the same fluorophore placed in the same position (residue 367), did not report significant changes in the presence of Ca2+ (0.5 mM)/liposomes or EGTA (0.1 mM)/liposomes. (B) Scheme showing the separation and purification of C2B* generated from C2A-C2B*. A His-6-tagged version of the cytoplasmic domain of syt, which contains a thrombin cleavage site (indicated as TC) between the C2A and C2B domains, was generated as described in Materials and Methods. This protein, his6-C2A-TC-C2B, was purified by using Ni-NTA-agarose beads. One-half of the protein immobilized on beads was eluted by using 400 mM imidazole and was designated as sample 1. Five units of thrombin was added to the other half of the bead-immobilized protein and incubated overnight at 4°C, after which the supernatant was collected and designated as sample 3. Cleavage was inefficient, and uncleaved material was eluted from the beads by first washing away the thrombin with 8 mM imidazole, followed by elution with 400 mM imidazole; this elute was designated as sample 2. Samples 1, 2, and 3 were labeled by IAEDANS as described in Materials and Methods, subjected to SDS/PAGE, and stained with Coomassie blue. The gel is shown in B (Lower). The identity of the proteins on the gel were confirmed by immunoblotting by using antibodies directed against the C2A or C2B domains of the protein (data not shown). In summary, sample 1 is his6-C2A-C2B* before thrombin treatment; sample 2 is his6-C2A-C2B* after thrombin treatment (as only 3% of the protein was cleaved, this sample is largely unchanged); and sample 3 contains C2B* that was liberated from his6-C2A-TC-C2B by thrombin treatment. (C) Removal of the C2A domain disrupts C2B–membrane interactions. The ability of the C2B* domains from samples 1, 2, and 3 (from B above) to interact with membranes was carried out as described in Fig. 1B. The fluorescence spectra obtained from samples 1, 2, and 3 are shown in Top, Middle, and Bottom, respectively (all samples contained equal molar protein, 0.5 μM). Thus, an adjacent C2A domain is essential for the Ca2+-dependent membrane penetration activity of the C2B domain.
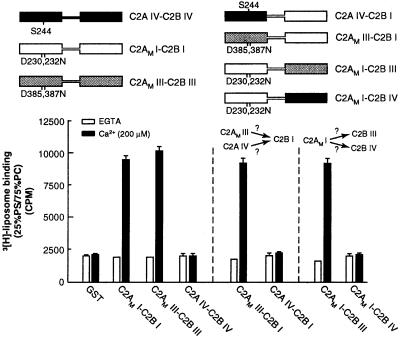
Syt IV does not bind liposomes in response to Ca2+ (32). This isoform harbors a naturally occurring serine at position 244, which corresponds to a glutamate residue at position 230 in the C2A domain of syt I. In syt I, this glutamate is a critical Ca2+ ligand; neutralization by replacement with a serine abolishes Ca2+-triggered lipid binding activity of the isolated C2A (22). “Reversal” of this mutation in the C2A domain of syt IV, by means of an S244D substitution, results in robust Ca2+-triggered lipid binding activity (33). However, all of the conserved Ca2+ ligands are present in the C2B domain of syt IV. Thus, it is surprising that the C2A domain of syt IV does not activate its C2B domain (Fig. 5). These data indicate that either C2A of IV lacks the “activation” activity for C2B or C2B from IV lacks the ability to be activated by C2A. We addressed this issue by generating chimeras between syts I and IV; as controls, syt I/III chimeras were analyzed in parallel. We first observed that the C2A domain of syt IV failed to activate the lipid binding activity of the C2B domain of syt I. In contrast, the C2AM domain of syt III (D385,387N) was able to activate C2B from isoform I. We next observed that the D230,232N mutant form of the C2A domain of syt I (C2AMI), which can activate the C2B domain of syts I and III, fails to activate the C2B domain of syt IV. These data indicate that syt IV harbors more than one loss of function; the C2A domain not only fails to penetrate membranes, it also fails to activate the C2B domain of the protein. Furthermore, the C2B of syt IV lacks the ability to be activated by a C2A domain capable of activating other C2B domains. According to these experiments, syt IV harbors additional differences in both C2 domains that disrupt its ability to interact with membranes, consistent with its putative role as an inhibitory syt (34, 35).
Figure 5.
Syt IV lacks cryptic membrane binding activity within its C2B domain. (Upper) Schematic representations of syt constructs. Regions of isoform I (open rectangles), III (shaded rectangles), or IV (black rectangles) are indicated, as are point mutations that disrupt Ca2+ and membrane-binding activity within the C2A domains of isoforms I and III (designated C2AM; corresponding to D230,232N and D385,387N in syts I and III, respectively). In syt IV, a serine residue is present at position 244; this serine abolishes Ca2+-triggered membrane-binding activity of the isolated C2A domain of the protein and is indicated. (Lower) Six micrograms of glutathione S-transferase-fused versions of the proteins shown (Upper) was immobilized on glutathione-Sepharose beads. 3H-labeled 25% PS/75% PC liposome-binding assays were carried out as described in Materials and Methods. In these experiments, C2AM I-C2B I and C2AM III-C2B III served as positive controls (13), and, as reported previously, C2A IV-C2B IV failed to bind liposomes (32). C2AM III, but not C2AM IV, activates the liposome-binding activity of C2B I. Furthermore, C2AM I activates the liposome-binding activity of C2B III but not that of C2B IV. These data reveal that the C2A domain of syt IV cannot activate the C2B domain of syt I and that the C2B domain of syt IV cannot be activated by the C2A of syt I.
Discussion
C2 domains are widespread conserved motifs; to date, 123 human genes encoding proteins that contain C2 domains have been identified (36). These domains are ≈140 residues in length and fold into compact eight-stranded β-sandwiches with flexible loops that protrude from one end (14, 21). In many, but not all, cases, these loops (1 and 3) mediate the binding of divalent metals, which often seems to regulate the binding of the C2 domain to other molecules including lipids and proteins (reviewed in ref. 14). In some proteins, the function of C2 domains seems to be straightforward. For example, Ca2+ triggers the translocation of C2 domain harboring phospholipases to membranes where their catalytic domains can efficiently turn-over substrate. In other cases, e.g., SYT, potential functions for C2 domains are less clear. syt was the first membrane protein identified that harbored C2 domains. Biochemical studies demonstrated that the C2A domain of syt I is an autonomously folding Ca2+ and lipid-binding module (6, 7). In the Ca2+-bound state, the Ca2+-binding loops of C2A rapidly penetrate into lipid bilayers at Ca2+ concentrations that trigger exocytosis (9–11). Positively charged amino acids within the loops interact with acidic phospholipid head groups. Neutralization of one of the positively charged residues in Ca2+-binding loop 3 has been reported to increase the Ca2+ requirements for lipid binding and to reduce evoked secretion at hippocampal synapses (4). Together, these data are consistent with a model in which Ca2+-triggered syt–membrane interactions serve as a coupling step in exocytosis. The isolated C2B domain of syt I also “senses” Ca2+ (8), but in contrast to C2A, Ca2+ does not drive the penetration of isolated C2B into membranes (12, 13).
More recently, it was discovered that the initial syt clone harbored a mutation that disrupts activities within the C2B domain (8), prompting a reevaluation of the role of C2A and C2B in mediating Ca2+-triggered interactions with membranes. New data demonstrated that mutations that abolished the Ca2+-triggered lipid binding activity of C2A did not abolish the overall Ca2+-triggered lipid binding activity of C2A-C2B; yet, isolated C2B fails to efficiently bind liposomes in response to Ca2+ (13). Here, we report that C2B penetrates into membranes in response to Ca2+, but only when tethered to an adjacent C2A domain. Interestingly, the penetration of C2B does not depend on the lipid-binding activity of C2A. However, severing C2A from C2B, after protein synthesis, disrupted the ability of the Ca2+-binding loop of C2B to insert into membranes. These data demonstrate that C2A does not activate C2B by directing folding during protein synthesis. This finding is not surprising because isolated C2B still senses Ca2+ and does not seem to be misfolded (8). Thus, C2A actively influences the properties of C2B.
This is a striking example of the evolution of multidomain proteins in which activities/properties arise or are lost by means of the use of domain repeats. It is possible that the C2B domain of syt I lost autonomous activity during evolution, giving rise to this form of cooperativity. This cooperativity may underlie the regulation of both C2 domains by molecules that interact with either C2A or C2B. For Ca2+-triggered lipid-binding activity, this is true of syts I and III (13) but not syt IV. We found that the C2 domains of syt IV exhibit a number of differences in comparison to syt I: not only is its C2A domain incapable of binding lipids in response to Ca2+, it is incapable of activating the lipid-binding activity within the C2B domain of syt I. Furthermore, the C2B domain of syt IV cannot be activated by the C2A domain of syt I (Fig. 5). These properties of syt IV are consistent with a proposed role as a seizure-induced (17) inhibitory isoform of the protein (34, 35).
A model for the structure of the syt–membrane complex is shown in Fig. 2B. In the absence of Ca2+, syt forms a weak precomplex with membranes (data not shown). In this view, Ca2+ influx would drive penetration of C2A and C2B into the vesicle or plasma membrane (11) with very rapid kinetics (Fig. 1C). If Ca2+-triggered syt–membrane interactions are crucial for exocytosis, neutralization of Ca2+ ligands within C2A may result in a mild phenotype because the mutant protein would retain the ability to penetrate bilayers in response to Ca2+. Penetration into the plasma membrane could help pull the bilayers together to facilitate soluble N-ethylmaleimide-sensitive fusion protein attachment receptor catalyzed fusion. For example, in at least some fusion events, a dimple in the plasma membrane forms as the target membrane is pulled toward the vesicle membrane (reviewed in ref. 37).
Acknowledgments
We thank X. Xia, E. Mussak, M. Jackson, C. Earles, A. Bhalla, and H. J. Kim for their help. This study was supported by National Institutes of Health Grant NIGMS GM 56827, American Heart Association Grant 9750326N, and a grant from the Milwaukee Foundation. E.R.C. is a Pew Scholar in the Biomedical Sciences. J.B. is supported by an American Health Association Predoctoral Fellowship.
Abbreviations
- syt
synaptotagmin
- PS
phosphatidylserine
- PC
phosphatidylcholine
- IAEDANS
5-[[2-[(iodoacetyl)amino]ethyl]amino]naphthalene-1-sulfonic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Augustine G J. Curr Opin Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 2.Brose N, Petrenko A G, Südhof T C, Jahn R. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 3.Littleton J T, Bai J, Vyas B, Desai R, Baltus A E, Garment M B, Carlson S D, Ganetzky B, Chapman E R. J Neurosci. 2001;21:1421–1433. doi: 10.1523/JNEUROSCI.21-05-01421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Chacon R, Konigstorfer A, Gerber S H, Garcia J, Matos M F, Stevens C F, Brose N, Rizo J, Rosenmund C, Südhof T C. Nature (London) 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 5.Perin M S, Fried V A, Mignery G A, Jahn R, Südhof T C. Nature (London) 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 6.Davletov B, Südhof T. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- 7.Chapman E R, Jahn R. J Biol Chem. 1994;269:5735–5741. [PubMed] [Google Scholar]
- 8.Desai R, Vyas B, Earles C, Littleton J T, Kowalchyck J, Martin T F J, Chapman E R. J Cell Biol. 2000;150:1125–1135. doi: 10.1083/jcb.150.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman E R, Davis A F. J Biol Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 10.Davis A F, Bai J, Fasshauer D, Wolowick M J, Lewis J L, Chapman E R. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 11.Bai J, Earles C, Lewis J, Chapman E R. J Biol Chem. 2000;275:25427–25435. doi: 10.1074/jbc.M906729199. [DOI] [PubMed] [Google Scholar]
- 12.Schiavo G, Gu Q M, Prestwich G D, Söllner T H, Rothman J E. Proc Natl Acad Sci USA. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earles C A, Bai J, Wang P, Chapman E R. J Cell Biol. 2001;154:1117–1123. doi: 10.1083/jcb.200105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nalefski E A, Falke J J. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborne S L, Herreros J, Bastiaens P I H, Schiavo G. J Biol Chem. 1999;274:59–66. doi: 10.1074/jbc.274.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Mizuta M N, Inagaki N, Nemoto Y, Matsukura S, Takahashi M, Seino S. J Biol Chem. 1994;269:11675–11678. [PubMed] [Google Scholar]
- 17.Vician L, Lim I K, Ferguson G, Tocco G, Baudry M, Herschman H R. Proc Natl Acad Sci USA. 1995;92:2164–2168. doi: 10.1073/pnas.92.6.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman E R, Hanson P I, An S, Jahn R. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- 19.Chapman E R, An S, Edwardson J M, Jahn R. J Biol Chem. 1996;271:5844–5849. doi: 10.1074/jbc.271.10.5844. [DOI] [PubMed] [Google Scholar]
- 20.Sutton R B, Ernst J A, Brunger A T. J Cell Biol. 1999;147:589–598. doi: 10.1083/jcb.147.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton R B, Davletov B A, Berghuis A M, Südhof T C, Sprang S R. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Rizo J, Südhof T C. Biochemistry. 1998;37:12395–12403. doi: 10.1021/bi9807512. [DOI] [PubMed] [Google Scholar]
- 23.Hudson E N, Weber G. Biochemistry. 1973;12:4154–4161. doi: 10.1021/bi00745a019. [DOI] [PubMed] [Google Scholar]
- 24.Chung L A, Lear J D, DeGrado W F. Biochemistry. 1992;31:6608–6616. doi: 10.1021/bi00143a035. [DOI] [PubMed] [Google Scholar]
- 25.Grobler J A, Essen L O, Williams R L, Hurley J H. Nat Struct Biol. 1996;3:788–795. doi: 10.1038/nsb0996-788. [DOI] [PubMed] [Google Scholar]
- 26.Chae Y K, Abildgaard F, Chapman E R, Markley J L. J Biol Chem. 1998;273:25659–25663. doi: 10.1074/jbc.273.40.25659. [DOI] [PubMed] [Google Scholar]
- 27.Llinas R, Steinberg I Z, Walton K. Biophys J. 1981;33:323–352. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidelberger R, Heinemann C, Matthews G. Nature (London) 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 29.Bollman J H, Sakmann B, Borst J G G. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 30.Schneggenburger R, Neher E. Nature (London) 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 31.Garcia R A, Forde C, Godwin H A. Proc Natl Acad Sci USA. 2000;97:5883–5888. doi: 10.1073/pnas.100127197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman E R, Desai R, Davis A F, Tornhel C. J Biol Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- 33.VonPoser C, Ichtchenko K, Shao X, Rizo J, Südhof T C. J Biol Chem. 1997;272:14314–14319. doi: 10.1074/jbc.272.22.14314. [DOI] [PubMed] [Google Scholar]
- 34.Littleton J T, Sereno T L, Rubin G M, Ganetzky B, Chapman E R. Nature (London) 1999;400:757–760. doi: 10.1038/23462. [DOI] [PubMed] [Google Scholar]
- 35.Wang C-T, Grishanin R, Earles C A, Chang P Y, Martin T F J, Chapman E R, Jackson M B. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- 36.International Human Genome Sequencing Consortium. Nature (London) 2001;409:860–921. [Google Scholar]
- 37.Monck J R, Fernandez J M. Neuron. 1994;12:707–716. doi: 10.1016/0896-6273(94)90325-5. [DOI] [PubMed] [Google Scholar]