Abstract
Several fusion strategies have been developed for the expression and purification of small antimicrobial peptides (AMPs) in recombinant bacterial expression systems. However, some of these efforts have been limited by product toxicity to host cells, product proteolysis, low expression levels, poor recovery yields, and sometimes an absence of posttranslational modifications required for biological activity. For the present work, we investigated the use of the baculoviral polyhedrin (Polh) protein as a novel fusion partner for the production of a model AMP (halocidin 18-amino-acid subunit; Hal18) in Escherichia coli. The useful solubility properties of Polh as a fusion partner facilitated the expression of the Polh-Hal18 fusion protein (∼33.6 kDa) by forming insoluble inclusion bodies in E. coli which could easily be purified by inclusion body isolation and affinity purification using the fused hexahistidine tag. The recombinant Hal18 AMP (∼2 kDa) could then be cleaved with hydroxylamine from the fusion protein and easily recovered by simple dialysis and centrifugation. This was facilitated by the fact that Polh was soluble during the alkaline cleavage reaction but became insoluble during dialysis at a neutral pH. Reverse-phase high-performance liquid chromatography was used to further purify the separated recombinant Hal18, giving a final yield of 30% with >90% purity. Importantly, recombinant and synthetic Hal18 peptides showed nearly identical antimicrobial activities against E. coli and Staphylococcus aureus, which were used as representative gram-negative and gram-positive bacteria, respectively. These results demonstrate that baculoviral Polh can provide an efficient and facile platform for the production or functional study of target AMPs.
Antimicrobial peptides (AMPs) are amphiphilic, positively charged molecules (8, 9, 33, 38) that have emerged as novel antimicrobial agents for use in therapeutics, animal drugs, and food preservatives. Many different kinds of antimicrobial peptides have been identified in recent years from various organisms, including amphibians, mammals, plants, invertebrates, and prokaryotes (3, 18, 22, 35). To make AMPs more economically viable, researchers have sought to mass-produce AMPs using recombinant means such as insect/baculovirus (1)-, yeast (30)-, and Escherichia coli (6, 12, 16, 19, 28, 29, 39)-based systems. However, some of these previous studies have been limited by difficulties in recovering AMPs from engineered bacteria (especially E. coli) at high levels due to their toxicity to host cells, susceptibility to proteolytic degradation, and small size (37).
Alternative expression strategies have been developed by the fusion of AMPs with partner proteins that decrease product toxicity to host cells, enhance product stability, facilitate product recovery, and/or aid in the acquisition of biological activity of the AMP product (5, 6, 10, 11, 16, 25, 26, 29, 36, 39). Previous studies have investigated AMPs fused with several partners, including bovine prochymosin (11), maltose-binding protein (10, 25), F4 of the E. coli intracellular protein PurF (16, 28), green fluorescent protein (GFP) (36), Pap3.30 from the Pseudomonas aeruginosa bacteriophage PaP3 (29), bacterial thioredoxin (2), RepA of E. coli (39), intein (21), l-ribulokinase of Salmonella enterica serovar Typhimurium (5), the C-terminal fragment of light meromyosin (6), glutathione S-transferase (26), and immunoglobulin G (IgG)-binding domains from protein A (26). However, although some of these carrier molecules have greatly improved the stability and expression level (6, 16, 17, 29) of the target AMP in the expression host, these strategies are still limited by low recovery yields and high levels of proteolytic degradation.
Baculoviral polyhedrin (Polh), derived from the Autographa californica nuclear polyhedrosis virus, is known to protect virus particles from physical and biochemical degradation, thus allowing virions to remain infectious outside the host (31, 32). Previously, we showed that Polh could be successfully used as a fusion partner for the formation of recombinant proteins as insoluble inclusion bodies in an E. coli expression system. In addition, the recombinant Polh protein showed almost the same characteristics (rapid solubilization under alkaline conditions and degradation by specific alkaline proteases in the insect gut) as native baculoviral Polh (34).
For this study, we employed Polh as a novel fusion partner for the expression of a model AMP with the hope that the unique properties of Polh could overcome some of the limitations of previous AMP fusion proteins. We employed the halocidin 18 oligomer (Hal18) (13) as our model AMP. Native halocidin from the tunicate Halocynthia aurantium is a heterodimeric AMP that consists of two subunits, Hal18 (18 amino acid residues [WLNALLHHGLNCAKGVLA]) and Hal15 (15 amino acid residues [ALLHHGLNCAKGVLA]), which are covalently linked by an intermolecular disulfide linkage. Previous work showed that Hal18 (1.93 kDa) has a stronger antibacterial activity than either full-length halocidin or Hal15 for multidrug-resistant bacteria (13). In addition, since Hal18 is a small linear peptide, it should not be affected by folding issues, because these issues are generally not critical in small peptides that lack disulfide linkages (11), making it a good choice as a model AMP for production in E. coli.
MATERIALS AND METHODS
Bacterial strains.
E. coli TOP10 [F− mcrAΔ(mrr-hsdRMS-mcrBC)Φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG] (Invitrogen) was used to construct the recombinant plasmid and to assay the antibacterial activity of purified recombinant Hal18 toward a representative gram-negative strain. E. coli BL21 [F− ompT hsdSB (rB− mB−) gal dcm] (Novagen) was used as a host strain for expressing the recombinant Polh-Hal18 fusion protein. Staphylococcus aureus (ATCC 6538p) was used to assay the antibacterial activity of purified recombinant Hal18 toward a representative gram-positive strain.
Plasmid construction.
Plasmid pPAP was constructed to encode the Polh-Hal18 fusion protein (Fig. 1A). First, a superlinker sequence between the BamHI and PstI sites of pSE420 (Invitrogen) was inserted into the multicloning site of pTrcHisC (Invitrogen) to construct pMPL100 (4,570 bp). The polh gene (735 bp) was obtained from the A. californica nuclear polyhedrosis virus genome (Pharmingen) using PCR amplification (primers 5′-AAGCTAGCATGCCGGATTATTCATACCGTCC-3′ and 5′-GTAGATCTATACGCCGGACCAGTGAACAG-3′) with a DNA thermal cycler (Mastercycler Personal 5332; Eppendorf Scientific) and inserted into the NheI and BglII sites of pMPL100 to construct pPolh (5,220 bp). The Hal18-encoding sequence (68 bp) was constructed by the hybridization of two synthetic oligonucleotides containing optimized E. coli major codons, BglII- and PstI-cleaved asymmetric cohesive ends, a hydroxylamine cleavage site, and a stop codon (Fig. 1B). This assembled Hal18-encoding sequence was inserted into the BglII and PstI sites of pPolh to construct a Polh-Hal18-encoding vector we designated pPAP (5,140 bp). The proteins encoded by recombinant plasmid pPAP contained hexahistidine (His6) tags at their N termini for protein purification, and their expression was under the control of isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible trc promoters.
FIG. 1.

(A) Gene map of recombinant plasmid pPAP. Abbreviations: polh, polyhedrin gene; hal18, halocidin 18-mer gene; His6, hexahistidine affinity ligand; Ptrc, trc promoter; Ampr, ampicillin resistance gene; lacIq, overexpressed lac repressor; ColE1, replication origin. (B) Design of nucleotide sequence for Hal18 peptide containing optimized E. coli codons. The hydroxylamine cleavage site (Asn-Gly) is boxed, the BglII and PstI sites are shown in bold, and the stop codon (TAA) is underlined.
Media and culture conditions.
For strain construction and protein expression, E. coli cells were grown in Luria-Bertani (LB) medium. The constructed transformant harboring the plasmid was stored at −80°C. Culture experiments were performed with 2 liters of LB medium supplemented with 50 μg/ml ampicillin (Sigma) in a 7-liter bioreactor (BioTron) at 37°C, with shaking at 250 rpm. Cell growth was monitored by measuring the optical density at 600 nm (OD600) using a UV-visible spectrophotometer (UV-1601PC; Shimadzu). When cultures reached an OD600 of 0.6, 1 mM (final concentration) IPTG was added to the culture broth for induction of the recombinant Polh-Hal18 fusion protein. At 5 h postinduction, culture samples were harvested for subsequent purification.
Purification of Polh-Hal18 fusion protein.
Harvested cells were resuspended in 3 ml of lysis buffer (buffer A; 50 mM Tris-HCl, 1 mM EDTA, 50 mM NaCl, and 1 mM phenylmethylsulfonyl fluoride, pH 8.0) per gram of cells (wet weight) and then treated with 80 μl of 10-mg/ml lysozyme per gram of cells. Samples were then frozen overnight at −20°C, after which they were thawed in water. The suspensions were passed twice through a high-pressure disrupter (Microfluidizer M-110Y; Microfluidics) at a flow rate of 450 ml/min and a pressure of 108 Pa.
The insoluble inclusion bodies were isolated by centrifugation at 14,000 × g for 30 min at 4°C and then resuspended in buffer B (100 mM NaH2PO4, 8 M urea, 10 mM Tris-HCl, pH 8.0). The resulting protein solution was clarified by centrifugation at 15,000 × g for 30 min at 4°C. The supernatant was affinity purified with Ni2+-nitrilotriacetate-agarose resin (QIAGEN) and eluted with an elution buffer (100 mM NaH2PO4, 8 M urea, 10 mM Tris-HCl, pH 5.9) containing 250 mM imidazole. The protein samples were dialyzed against 10 mM Tris-HCI (pH 12), lyophilized, and solubilized in distilled water to a final concentration of 15 mg/ml.
Cleavage and recovery of recombinant Hal18.
The purified fusion proteins (15 mg/ml) were mixed with 3 volumes of hydroxylamine cleavage reaction buffer (0.22 M Tris base, 1.7 M hydroxylamine-HCl, 4.5 M guanidine-HCl, and 1% 1-propanol, pH 9.0, with the final solution adjusted to pH 8.8) (24) and incubated at 55°C for 24 h. The cleavage reaction samples were then dialyzed against 20 mM phosphate-buffered saline (PBS; pH 7.4) and centrifuged. The supernatant containing recombinant Hal18 was saved and lyophilized. Recombinant Hal18 was finally purified by reverse-phase high-pressure liquid chromatography (RP-HPLC) with the AKTAbasic system (Amersham Biosciences) using a 4.6- by 100-mm Chromolith performance RP18e column (Merck) with a linear gradient of 20 to 40% acetonitrile containing 0.1% (vol/vol) trifluoroacetic acid at a flow rate of 3 ml/min for 1 h. The absorbance at 215 nm was monitored. Each peak was collected, lyophilized, and tested for antimicrobial activity. Total protein amounts were quantified using a commercially available kit (Bio-Rad). The molecular weight was analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) on a Voyager system 4095 mass spectrometer (Applied Biosystems).
Chemical synthesis of control Hal18 peptide.
Standard Hal18 was chemically synthesized with an automated solid-phase peptide synthesizer (Pioneer; Applied Biosystems) (provided by the Korea Basic Science Institute) and purified to near homogeneity by RP-HPLC. Each peak was identified by MALDI-TOF mass spectrometry.
SDS-PAGE and Western blot analysis.
The cells were harvested by centrifugation at a maximal speed (13,000 rpm) for 10 min at 4°C, and the samples were mixed with protein sample buffer (0.5 M Tris-HCl [pH 6.8], 10% glycerol, 5% sodium dodecyl sulfate [SDS], 5% β-mercaptoethanol, 0.25% bromophenol blue) and heated to 100°C for 5 min. After centrifugation for 1 min, the samples were subjected to 12% (wt/vol) SDS-polyacrylamide gel electrophoresis (SDS-PAGE) or 16% (wt/vol) Tricine SDS-PAGE. The protein bands were detected by Coomassie blue staining (Bio-Rad), silver staining (Bio-Rad), or Western blotting. For Western blot analysis, the gel was transferred onto a nitrocellulose membrane (Amersham Pharmacia) using a Mini-Trans blot cell (Bio-Rad) and Bjerrum and Schafer-Nielsen transfer buffer (48 mM Tris, 39 mM glycine, 20% methanol) for 30 min at 15 V. Proteins of interest were detected using a polyclonal rabbit anti-His6 antibody (1:2,500 [vol/vol]; Santa Cruz Biotechnology) and alkaline phosphatase-conjugated goat anti-rabbit IgG (1:50,000 [vol/vol]; Sigma). The membrane was then washed and developed colorimetrically with FAST Red TR/naphthol AS-MX (4-chloro-2-methylbenzene diazonium-3-hydroxy-2-naphthoic acid-2,4-dimethylanilide phosphate; Sigma). The membrane was scanned and its image was analyzed by Gel-Pro Analyzer software (Media Cybernetics).
Antimicrobial activity assays.
The antimicrobial activity of recombinant Hal18 was assayed using radial diffusion and MIC assays, with E. coli TOP10 serving as a representative gram-negative bacterium and S. aureus ATCC 6538p serving as a representative gram-positive bacterium. For the radial diffusion assay, the bacteria were cultured overnight in 3% tryptic soy broth (TSB; Difco), subcultured at 1:1,000 in prewarmed TSB, and grown for 2.5 h to mid-exponential growth phase at 37°C with shaking at 250 rpm. The bacterial concentration was adjusted to an OD600 of 0.3, and 1% (vol/vol) culture broth was rapidly added to previously autoclaved, warm (42°C) PBS (pH 7.4) containing 3% powdered TSB and 1% (wt/vol) low-electroendosmosis type I agarose (Sigma). The bacteria were dispersed, and the agar was plated on 100-mm petri dishes. A 3-mm-diameter gel punch was used to make evenly spaced wells. For the assay, 10 μl of solution containing recombinant Hal18 (250 mM) in acidified distilled water (0.01% acetic acid) was added to each well. The plates were then incubated for 3 h at 37°C and overlaid with 10 ml of sterile agar (6% [wt/vol] TSB and 1% agarose). After the plates were incubated for 12 h at 37°C, the diameter of the clear zone surrounding each well was measured. For the MIC assay (14), the sample peptide was dissolved to various concentrations in deionized water and added to an equal volume of a test cell culture (105 CFU/ml) with TSB medium in a 96-well tissue culture plate. The plate was incubated at 37°C overnight, and the lowest sample concentration yielding no bacterial growth was identified as the MIC.
RESULTS
Expression of Polh-Hal18 fusion protein.
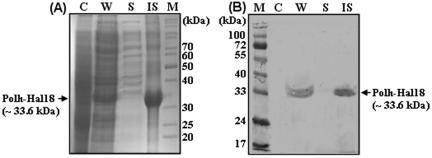
The recombinant Polh-Hal18 fusion protein was successfully expressed and showed an apparent molecular mass of about 33.6 kDa (Fig. 2), which was in good agreement with the predicted size (∼28.6 kDa for Polh, ∼2 kDa for Hal18, and ∼3 kDa for the hexahistidine affinity ligand). The expression level of the Polh-Hal18 fusion protein reached about 40% of the total cellular proteins at 5 h postinduction (Fig. 2A, lane W). Previous results from our lab (34) and others (15) have indicated that Polh forms inclusion bodies in E. coli. To directly confirm that this was the case for the recombinant Polh-Hal18 fusion protein, cells were disrupted by sonication, and SDS-PAGE and Western blot analyses were performed on whole-cell lysates, insoluble cell debris, and soluble supernatant samples (Fig. 2). The Polh-Hal18 fusion band was clearly detected with a high density in the insoluble cell debris fraction (Fig. 2A and B, lanes IS) but was not detected in the soluble supernatant fraction (Fig. 2A and B, lanes S), demonstrating that the Polh-Hal18 fusion protein was contained in the insoluble inclusion body fraction.
FIG. 2.
Coomassie blue-stained SDS-PAGE gel (A) and Western blot analysis (B) monitoring the expression of Polh-Hal18 as inclusion bodies. A polyclonal anti-His6 antibody was used to detect the Western blot. Lanes: M, protein molecular weight marker; C, whole cells before induction; W, whole cells after induction; S, soluble supernatant after sonication and centrifugation; IS, insoluble cell debris after sonication and centrifugation.
Purification of recombinant Hal18.
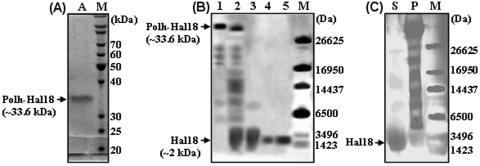
Because the Polh-Hal18 fusion protein was completely insoluble in E. coli, the isolation of inclusion bodies allowed us to recover the protein with an initial purity of about 65% (data not shown). The Polh-Hal18 fusion protein included a His6 affinity tag, and thus affinity purification under denaturing conditions was used to obtain samples with a higher purity. We found it difficult to elute the Polh-Hal18 fusion protein under low-pH conditions (pH 4.5), and thus 250 mM imidazole was added to the elution buffer (Fig. 3A, lane A). This allowed us to purify the recombinant Polh-Hal18 fusion protein with an ∼86% purity (Table 1).
FIG. 3.
(A) SDS-PAGE analysis of affinity-purified Polh-Hal18 fusion protein; (B) Tricine-SDS-PAGE analysis of recombinant Hal18 recovery from Polh-Hal18 fusion proteins; (C) Tricine-SDS-PAGE analysis of cleaved fusion protein fractions during dialysis and centrifugation. Coomassie blue was used for the staining of SDS-PAGE gels. Lanes: M, protein molecular weight marker; A, affinity-purified Polh-Hal18 fusion protein; 1, affinity-purified Polh-Hal18 fusion protein; 2, hydroxylamine cleavage reaction sample; 3, recovered recombinant Hal18 in the soluble supernatant after dialysis and centrifugation; 4, recombinant Hal18 purified by RP-HPLC; 5, synthetic Hal18 (control); S, soluble supernatant after dialysis and centrifugation; P, insoluble pellet after dialysis and centrifugation.
TABLE 1.
Summary of recovery of recombinant Hal18
| Step | Total proteina (mg) | Amt of Hal18 (mg) | Recovery (%) | Purityd (%) |
|---|---|---|---|---|
| Affinity purification | 29.91b | 1.76c | 100c | |
| Cleavage, dialysis, and centrifugation | 3.28 | 0.82 | 46.6 | 25.0 |
| RP-HPLC purification | 0.57 | 0.52 | 29.5 | 91.2 |
| Reference 6 | 11.0 | |||
| Reference 11 | 14.2 | |||
| Reference 28 | 18.9 |
Determined by a total protein assay.
Contained the Polh-Hal18 fusion protein with an 86% purity, as determined by densitometric analysis of SDS-PAGE gels.
Theoretical; calculated from the difference in the estimated molecular masses of Hal18 and Polh-Hal18.
Determined by integration of the HPLC peak.
The purified Polh-Hal18 fusion protein was cleaved by hydroxylamine in freshly prepared cleavage buffer (pH 9.0) containing 4.5 M guanidine-HCl. Optimization experiments showed that <50% of the fusion protein was cleaved in older buffer, guanidine-HCl-free buffer, or buffer that contained 6 M urea instead of guanidine-HCl (data not shown). Under the optimized conditions, most of the Polh-Hal18 fusion protein was successfully cleaved (Fig. 3B, lane 2). After dialysis of the protein against 20 mM PBS (pH 7.4), we removed the guanidine-HCl and decreased the pH to 7.4, causing the larger Polh-containing fragments to become insoluble while the smaller Hal18 peptides remained soluble. This special property of Polh allowed us to separate cleaved recombinant Hal18 peptides from precipitated Polh proteins by simple centrifugation (Fig. 3B, lanes 3 and 4). From approximately 30 mg of input purified fusion protein, we recovered about 0.82 mg of cleaved recombinant Hal18 showing a broad band upon gel analysis and having an ∼47% yield and ∼25% purity (Fig. 3B, lane 3, and Table 1). We then performed RP-HPLC to further purify the recombinant Hal18 to a higher degree than that obtained by simple dialysis and centrifugation (Fig. 3B, lane 4). The resulting peptide had a molecular mass of ∼2 kDa, which was almost identical to that of the synthetic version (1.93 kDa) (Fig. 3B, lane 5). The full isolation process generated 0.52 mg of recombinant Hal18, with a 30% recovery yield and 91% purity, from approximately 30 mg of input purified fusion protein, including 1.76 mg (theoretical value) of the recombinant Hal18 peptide (Table 1).
Antimicrobial activity of recombinant Hal18.
The bactericidal activity of recombinant Hal18 cleaved from the fusion protein and recovered by dialysis and centrifugation was qualitatively determined with a radial diffusion assay using E. coli as a representative gram-negative bacterium. The soluble supernatant from the dialysis and centrifugation step (soluble Hal18 peptide) showed a bactericidal activity (Fig. 4, spot 1) comparable to that of standard synthetic Hal18 (spot 2). In contrast, no bactericidal activity was seen for the insoluble pellet from the dialysis and centrifugation step (spot 3), the affinity-purified recombinant Polh-Hal18 fusion protein (spot 6), or the other negative controls (spots 4, 5, and 7). These results confirm that biologically active cleaved recombinant Hal18 peptides were present in the soluble fraction after dialysis and centrifugation. Similar results were obtained for radial diffusion assays using the gram-positive bacterium S. aureus (data not shown).
FIG. 4.
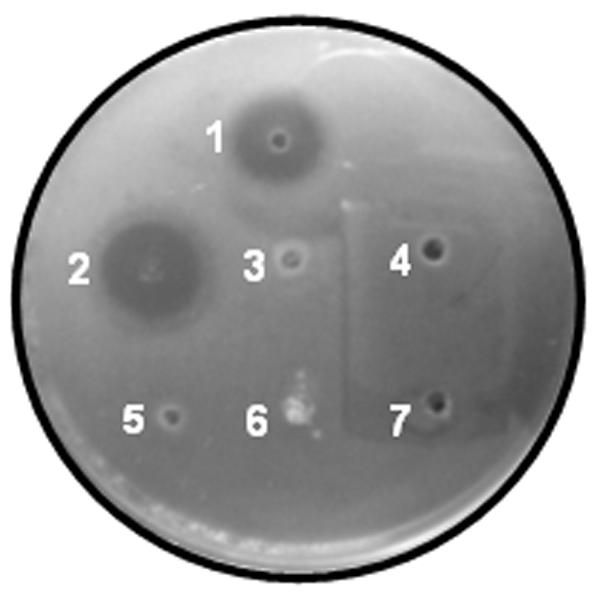
Antibacterial activity assay using purified recombinant Hal18 and E. coli. Spots: 1, recovered recombinant Hal18 (250 mM) in the soluble supernatant fraction after dialysis and centrifugation; 2, synthetic Hal18 (80 mM) as a positive control; 3, insoluble pellet fraction after dialysis and centrifugation; 4, 20 mM PBS (pH 7.4) as a negative control; 5, 10 mM Tris-HCl (pH 12) as a negative control; 6, affinity-purified Polh-Hal18 fusion protein; 7, distilled water containing 0.01% acetic acid as a negative control.
We then performed a MIC assay to quantitatively investigate the antimicrobial activity of recombinant Hal18. Recombinant Hal18 recovered by simple dialysis and centrifugation yielded MICs of 20 mM and 10 mM for E. coli and S. aureus, respectively (Table 2). The MICs of recombinant Hal18 further purified by RP-HPLC were 5 mM for E. coli and 1.25 mM for S. aureus. When we compared the MICs of RP-HPLC-purified recombinant Hal18 to those of peptides recovered by simple dialysis and centrifugation, we found that the MIC of the RP-HPLC-purified Hal18 was fourfold higher for E. coli and eightfold higher for S. aureus. Importantly, the biological activity of recombinant Hal18 was almost identical to that of the chemically synthesized Hal18 peptide used as a positive control (Table 2).
TABLE 2.
Bactericidal activities of recombinant and synthetic Hal18 peptides
| Antimicrobial peptide | MIC for organism (mM)
|
|
|---|---|---|
| E. coli | S. aureus | |
| Recombinant Hal18 purified by cleavage, dialysis, and centrifugation | 20 | 10 |
| Recombinant Hal18 purified by RP-HPLC | 5 | 1.25 |
| Synthetic Hal18 | 5 | 1.25 |
DISCUSSION
Our results clearly demonstrate that the recombinant AMP Hal18 could be efficiently produced in an E. coli expression system by fusion with the baculoviral Polh protein. We were able to avoid the use of a eukaryotic expression system (7) because refolding is not required for the biological activity of Hal18. Instead, we used an E. coli-based system, which has the benefits of simple high-density cell culture procedures, low production costs, and easy purification by inclusion body separation. For the expression of AMPs in E. coli, selection of the proper fusion partner can protect the host cell from product toxicity (2, 21, 28), avoid proteolysis (2, 6, 26), facilitate purification (6, 21, 29), and sometimes aid in the acquisition of biological activity by encouraging the cell machinery to remove additional amino acid residues at the N terminus (23), performing posttranslational modifications (5), or initiating self-splicing (21). However, while some fusion partners improved the stability and expression levels of AMPs in the expression host, the applicability of fusion proteins was often limited by a high level of proteolytic degradation and a poor recovery of the AMP target products.
Here we sought to identify a more beneficial fusion partner for AMP expression. We sought a partner that (i) is about three to four times larger than the target AMP, (ii) has an isoelectric point (pI) at least 2 pH units higher or lower than that of the target AMP, and (iii) is nontoxic to the host bacterium. We identified baculoviral Polh as a protein that satisfies these criteria. In addition, Polh can be rapidly solubilized under alkaline conditions but becomes insoluble under neutral conditions (15, 34), providing the potential for simple AMP purification. Our novel Polh-Hal18 fusion protein had a pI of 6.21 (compared to 8.24 for Hal18 alone and 5.83 for Polh alone), which benefits its expression in E. coli.
We also tested the expression of Hal18 alone in E. coli. We did not observe the expected growth inhibition in cells transformed with a Hal18-expressing construct after induction (data not shown). Furthermore, the recombinant peptide band was barely detected in these cells (data not shown). Therefore, we can surmise that solely E. coli-expressed recombinant Hal18 does not have biological activity or has minimal activity due to a very low expression level. In contrast, the expression level of the Polh-Hal18 fusion protein reached about 40% of the total cellular proteins at 5 h postinduction (Fig. 2A, lane W), indicating that the Polh-Hal18 fusion protein was efficiently expressed in E. coli cells. This high level of expression is consistent with our previous experiments involving fusions of Polh with GFP (34). In addition to the high expression levels, we also observed no protein degradation during the purification process, indicating that Polh may protect its fusion partner against proteolysis, perhaps through inclusion body formation (Fig. 2A and B, lanes IS).
Normally, recombinant fusion proteins are chemically or enzymatically cleaved to release the desired AMP product. Protease cleavage tends to be expensive, and thus researchers have sought to establish a useful chemical strategy. Previous studies have reported the release of AMPs from fusion partners by digestion with cyanogen bromide (CNBr), which can cleave peptides at methionine residues (11, 12, 16, 27). However, CNBr is highly toxic to humans, CNBr-cleaved peptides tend to have low recovery percentages (11), and the purification of these peptides can be difficult (26). In contrast, hydroxylamine is less toxic and more economic. Hydroxylamine can cleave Asn-Gly peptide linkages under relatively harsh conditions and other Asn or Gly residues can be modified to their hydroxamic acid forms. However, the biological activity of the target protein does not seem to be affected by this modification (20). Therefore, hydroxylamine may be a better choice for the cleavage of AMPs from their fusion partners. The Polh protein does not contain an Asn-Gly linkage. For this study, we used hydroxylamine to cleave Polh-Hal18 at the Asn-Gly junction between the fusion partners. The maximum cleavage rate and minimum number of side chain modifications can be achieved under optimal hydroxylamine cleavage conditions. Several conditions were suggested for the hydroxylamine cleavage reaction (2 M urea, 2 M hydroxylamine-HCl, 0.1 M Tris base, pH 9, at 45°C [4]; 0.5 M hydroxylamine-HCl, pH 8.65, at 45°C [20]; and 0.22 M Tris base, 1.7 M hydroxylamine-HCl, 4.5 M guanidine-HCl, and 1% 1-propanol, pH 9.0, at 55°C [24]). The first two conditions (4, 20) resulted in lower cleavage yields (about 30%). Therefore, we employed the last conditions (24) for hydroxylamine cleavage, and most of the Polh-Hal18 fusion proteins were successfully cleaved (Fig. 3B, lane 2). The cleavage of Polh-Hal18 fusion proteins in freshly prepared buffer was relatively efficient, whereas comparable cleavage in a guanidine-HCl-free buffer or one containing urea instead of guanidine-HCl was ineffective (<50%). This result was similar to that in a previous report (24), where cleavage yields for guanidine-HCl and urea as the denaturant were about 96% and 38%, respectively.
The Polh-linked fusion protein was rapidly solubilized in 10 mM Tris (pH 12) but became insoluble when samples were dialyzed against 20 mM PBS (pH 7.4), allowing us to easily separate the Hal18 peptide from Polh by centrifugation following the cleavage step (Fig. 3C). This primary product had the bactericidal activity of the recombinant AMP (Fig. 4), indicating that our novel Polh fusion strategy is a simple and useful platform for the functional study of AMPs. For studies requiring a more pure AMP sample, we found that RP-HPLC yielded Hal18 with a higher purity. We performed MALDI-TOF mass spectrometric analysis of the RP-HPLC-purified sample, and the molecular mass of the recombinant Hal18 was 2,140 Da, which was slightly larger than the predicted mass (1,987 Da) (data not shown). We suspect that this might be due to the formation of hydroxamic acid from Asn and Gly residues (Hal18 contains two Asn and two Gly resides) by hydroxylamine-induced modification. Using this simple and effective purification strategy, we obtained an ∼30% recovery yield, which is much higher than those of previous reports (Table 1). As suggested in previous reports (17, 21), it may be possible to increase the overall recovery yield of AMPs by the fusion of AMP multimers to a partner. Regardless, we showed herein that the fusion of a model AMP to Polh allowed effective, efficient, and simple expression and purification of the AMP from an E. coli culture.
In conclusion, we showed herein that the use of Polh as a fusion partner for a model AMP, Hal18, enabled the high-level expression and decreased proteolysis of the target Polh-Hal18 fusion protein and also facilitated the recovery of Hal18 from the fusion protein by use of a unique solubility property of Polh. Moreover, the recovered recombinant Hal18 and a synthetic control Hal18 peptide showed almost identical bactericidal activities, indicating that the expressed Hal18 AMP is biologically functional. Therefore, this baculoviral Polh fusion strategy could provide a promising new platform for production and/or functional studies of target AMPs in E. coli expression systems. Note that target AMPs should be linear peptides containing no modified amino acid residues and having no folding problems.
Acknowledgments
This work was supported by the Marine Bioprocess Research Center of the Marine Bio 21 program funded by the Ministry of Maritime Affairs & Fisheries, Korea, and by the Brain Korea 21 program issued by the Ministry of Education, Korea.
REFERENCES
- 1.Andersons, D., A. Engstrom, S. Josephson, L. Hansson, and H. Steiner. 1991. Biologically active and amidated cecropin produced in a baculovirus expression system from a fusion construct containing the antibody-binding part of protein A. Biochem. J. 280:219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrell, P. J., O. W. Liew, and A. J. Conner. 2004. Expressing an antibacterial protein in bacteria for raising antibodies. Protein Expr. Purif. 33:153-159. [DOI] [PubMed] [Google Scholar]
- 3.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 4.Bornstein, P., and G. Balian. 1977. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 47:132-145. [DOI] [PubMed] [Google Scholar]
- 5.Callaway, J. E., J. Lai, B. Haselbeck, M. Baltaian, S. P. Bonnesen, J. Weickmann, G. Wilcox, and S. P. Lei. 1993. Modification of the C terminus of cecropin is essential for broad-spectrum antimicrobial activity. Antimicrob. Agents Chemother. 37:1614-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipakova, I., E. Hostinova, J. Gasperik, and V. Velebny. 2004. High-level expression and purification of a recombinant hBD-1 fused to LMM protein in Escherichia coli. Protein Expr. Purif. 37:207-212. [DOI] [PubMed] [Google Scholar]
- 7.Datar, R. V., T. Cartwright, and C. G. Rosen. 1993. Process economics of animal cell and bacterial fermentations: a case study analysis of tissue plasminogen activator. Biotechnology 11:349-357. [DOI] [PubMed] [Google Scholar]
- 8.Devine, D. A., and R. E. Hancock. 2002. Cationic peptides: distribution and mechanisms of resistance. Curr. Pharm. Des. 8:703-714. [DOI] [PubMed] [Google Scholar]
- 9.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 10.Hara, S., and M. Yamakawa. 1996. Production in Escherichia coli of moricin, a novel type antibacterial peptide from the silkworm Bobyx mori. Biochem. Biophys. Res. Commun. 220:664-669. [DOI] [PubMed] [Google Scholar]
- 11.Haught, C., G. D. Davis, R. Subramanian, K. W. Jackson, and R. G. Harrison. 1998. Recombinant production and purification of novel antisense antimicrobial peptide in Escherichia coli. Biotechnol. Bioeng. 57:55-61. [DOI] [PubMed] [Google Scholar]
- 12.Hwang, S. W., J. H. Lee, H. B. Park, S. H. Pyo, J. E. So, H. S. Lee, S. S. Hong, and J. H. Kim. 2001. A simple method for the purification of an antimicrobial peptide in recombinant Escherichia coli. Mol. Biotechnol. 18:193-198. [DOI] [PubMed] [Google Scholar]
- 13.Jang, W. S., K. N. Kim, Y. S. Lee, M. H. Nam, and I. H. Lee. 2002. Halocidin: a new antimicrobial peptide from hemocytes of the solitary tunicate Halocynthia aurantium. FEBS Lett. 521:81-86. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. N., A. L. Barry, T. L. Gavan, and J. A. Washington. 1985. Susceptibility tests: microdilution and macrodilution broth procedures, p. 972-977. In E. H. Lennette, A. Balow, W. J. Hausler, and H. J. Shadomy (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, D.C.
- 15.Lavallee, C., M. Arella, S. Belloncik, and Y. Furuichi. 1993. Expression in Escherichia coli of the cloned polyhedrin gene of Bombyx mori cytoplasmic polyhedrosis virus. Protein Expr. Purif. 4:570-579. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J. H., J. H. Kim, S. W. Hwang, W. J. Lee, H. K. Yoon, H. S. Lee, and S. S. Hong. 2000. High-level expression of antimicrobial peptide mediated by a fusion partner reinforcing formation of inclusion bodies. Biochem. Biophys. Res. Commun. 277:575-580. [DOI] [PubMed] [Google Scholar]
- 17.Lee, J. H., M. S. Kim, J. H. Cho, and S. C. Kim. 2002. Enhanced expression of tandem multimers of the antimicrobial peptide buforin II in Escherichia coli by the DEAD-box protein and trxB mutant. Appl. Microbiol. Biotechnol. 58:790-796. [DOI] [PubMed] [Google Scholar]
- 18.Martin, E., T. Ganz, and R. I. Lehrer. 1995. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 58:128-136. [DOI] [PubMed] [Google Scholar]
- 19.Miller, K. W., R. Schamber, Y. L. Chen, and B. Ray. 1998. Production of active chimeric pediocin AcH in Escherichia coli in the absence of processing and secretion genes from the Pediococcus pap operon. Appl. Environ. Microbiol. 64:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milner, S. J., S. M. Thomas, F. J. Ballard, and G. L. Francis. 1996. Optimization of the hydroxylamine cleavage of an expressed fusion protein to produce recombinant human insulin-like growth factor (IGF)-I. Biotechnol. Bioeng. 50:265-272. [DOI] [PubMed] [Google Scholar]
- 21.Morassutti, C., F. D. Amicis, A. Bandiera, and S. Marchetti. 2005. Expression of SMAP-29 cathelicidin-like peptide in bacterial cells by intein-mediated system. Protein Expr. Purif. 39:160-168. [DOI] [PubMed] [Google Scholar]
- 22.Noga, E. J., and U. Silphaduang. 2003. Piscidins: a novel family of peptide antibiotics from fish. Drug News Perspect. 16:87-92. [DOI] [PubMed] [Google Scholar]
- 23.Pang, S. Z., S. M. Oberhaus, J. L. Rasmussen, D. C. Knipple, J. R. Bloomquist, D. H. Dean, K. D. Bowman, and J. C. Sanford. 1992. Expression of a gene encoding a scorpion insectotoxin peptide in yeast, bacteria and plants. Gene 116:165-172. [DOI] [PubMed] [Google Scholar]
- 24.Park, H. B., S. H. Pyo, S. S. Hong, and J. H. Kim. 2001. Optimization of the hydroxylamine cleavage of an expressed fusion protein to produce a recombinant antimicrobial peptide. Biotechnol. Lett. 23:637-641. [Google Scholar]
- 25.Pierce, J. C., W. L. Maloy, L. Salvador, and C. F. Dungan. 1997. Recombinant expression of the antimicrobial peptide polyphemusin and its activity against the protozoan oyster pathogen Perkinsus marinus. Mol. Mar. Biol. Biotechnol. 6:248-259. [PubMed] [Google Scholar]
- 26.Piers, K. L., M. H. Brown, and E. W. Hancock. 1993. Recombinant DNA procedures for producing small antimicrobial cationic peptides in bacteria. Gene 134:7-13. [DOI] [PubMed] [Google Scholar]
- 27.Ponti, D., G. Mignogna, M. L. Mangoni, D. De Biase, M. Simmaco, and D. Barra. 1999. Expression and activity of cyclic and linear analogues of esculentin-1, an antimicrobial peptide from amphibian skin. Eur. J. Biochem. 263:921-927. [DOI] [PubMed] [Google Scholar]
- 28.Pyo, S. H., J. H. Lee, H. B. Park, J. S. Cho, H. R. Kim, B. H. Han, and Y. S. Park. 2004. Expression and purification of a recombinant buforin derivative from Escherichia coli. Process Biochem. 39:1731-1736. [Google Scholar]
- 29.Rao, X. C., S. Li, J. C. Hu, X. L. Jin, X. M. Hu, J. J. Huang, Z. J. Chen, J. M. Zhu, and F. Q. Hu. 2004. A novel carrier molecule for high-level expression of peptide antibiotics in Escherichia coli. Protein Expr. Purif. 36:11-18. [DOI] [PubMed] [Google Scholar]
- 30.Reichhart, J. M., I. Petit, M. Legrain, J. L. Dimarcq, E. Keppi, J. P. Lecocq, J. A. Hoffmann, and T. Achstetter. 1992. Expression and secretion in yeast of active insect defensin, an inducible antimicrobial peptide from the fleshfly Phormia terranovae. Invertebr. Reprod. Dev. 21:15-24. [Google Scholar]
- 31.Rohrmann, G. F. 1986. Polyhedrin structure. J. Gen. Virol. 67:1499-1513. [DOI] [PubMed] [Google Scholar]
- 32.Rohrmann, G. F. 1992. Baculovirus structural proteins. J. Gen. Virol. 73:749-761. [DOI] [PubMed] [Google Scholar]
- 33.Segrest, J. P., H. D. Loof, J. G. Dohlman, C. G. Brouillette, and G. M. Anantharamaiah. 1990. Amphipathic helix motif: classes and properties. Proteins Struct. Funct. Genet. 8:103-117. [DOI] [PubMed] [Google Scholar]
- 34.Seo, J. H., L. Li, J. S. Yeo, and H. J. Cha. 2003. Baculoviral polyhedrin as a novel fusion partner for formation of inclusion body in Escherichia coli. Biotechnol. Bioeng. 84:467-473. [DOI] [PubMed] [Google Scholar]
- 35.Sima, P., I. Trebichavsky, and K. Sigler. 2003. Mammalian antibiotic peptides. Folia Microbiol. (Prague) 48:123-137. [DOI] [PubMed] [Google Scholar]
- 36.Skosyrev, V. S., N. V. Rudenko, A. V. Yakhnin, V. E. Zagranichny, L. I. Popova, M. V. Zakharov, A. Y. Gorokhovatsky, and L. M. Vinokurov. 2003. EGFP as a fusion partner for the expression and organic extraction of small polypeptides. Protein Expr. Purif. 27:55-62. [DOI] [PubMed] [Google Scholar]
- 37.Valore, E. V., and T. Ganz. 1997. Laboratory production of antimicrobial peptides in native conformation. Methods Mol. Biol. 78:115-131. [DOI] [PubMed] [Google Scholar]
- 38.Zaslo, V. M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, L., T. Falla, M. Wu, S. Fidai, J. Burian, W. Kay, and R. E. Hancock. 1998. Determinants of recombinant production of antimicrobial cationic peptides and creation of peptide variants in bacteria. Biochem. Biophys. Res. Commun. 247:674-680. [DOI] [PubMed] [Google Scholar]