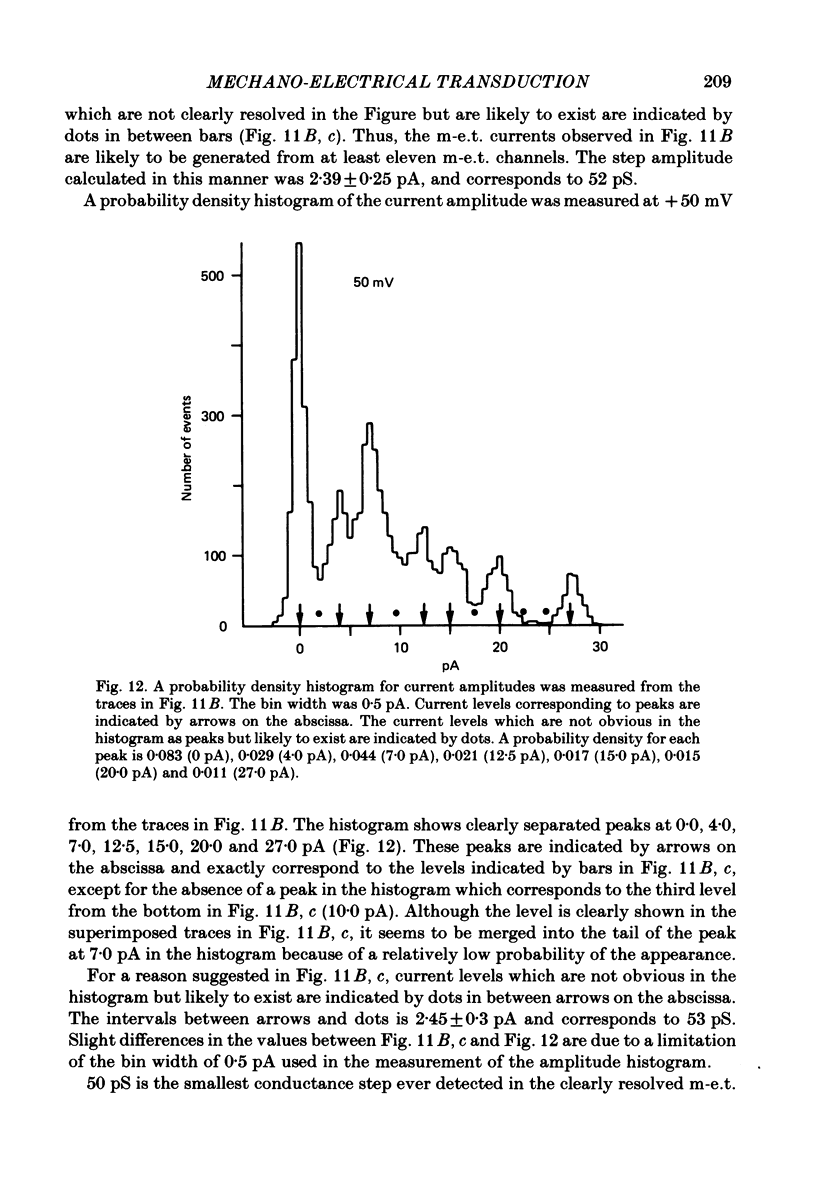
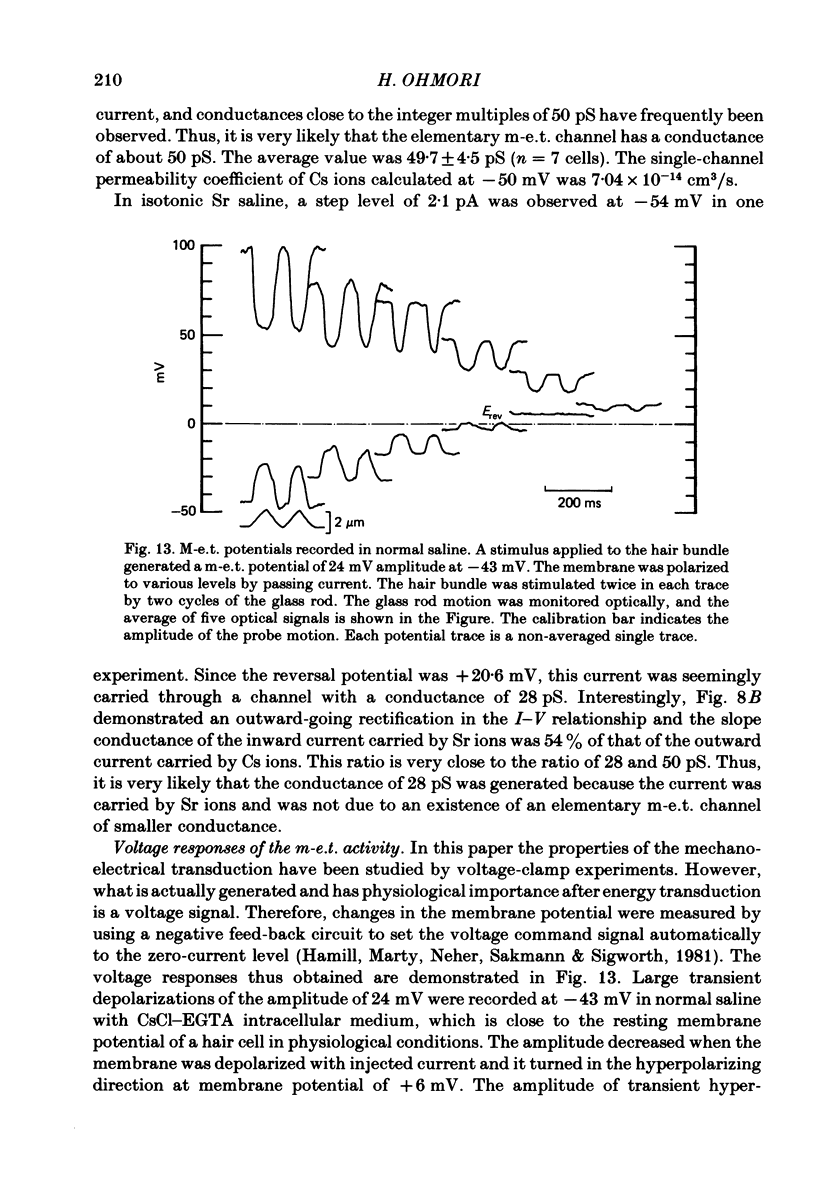
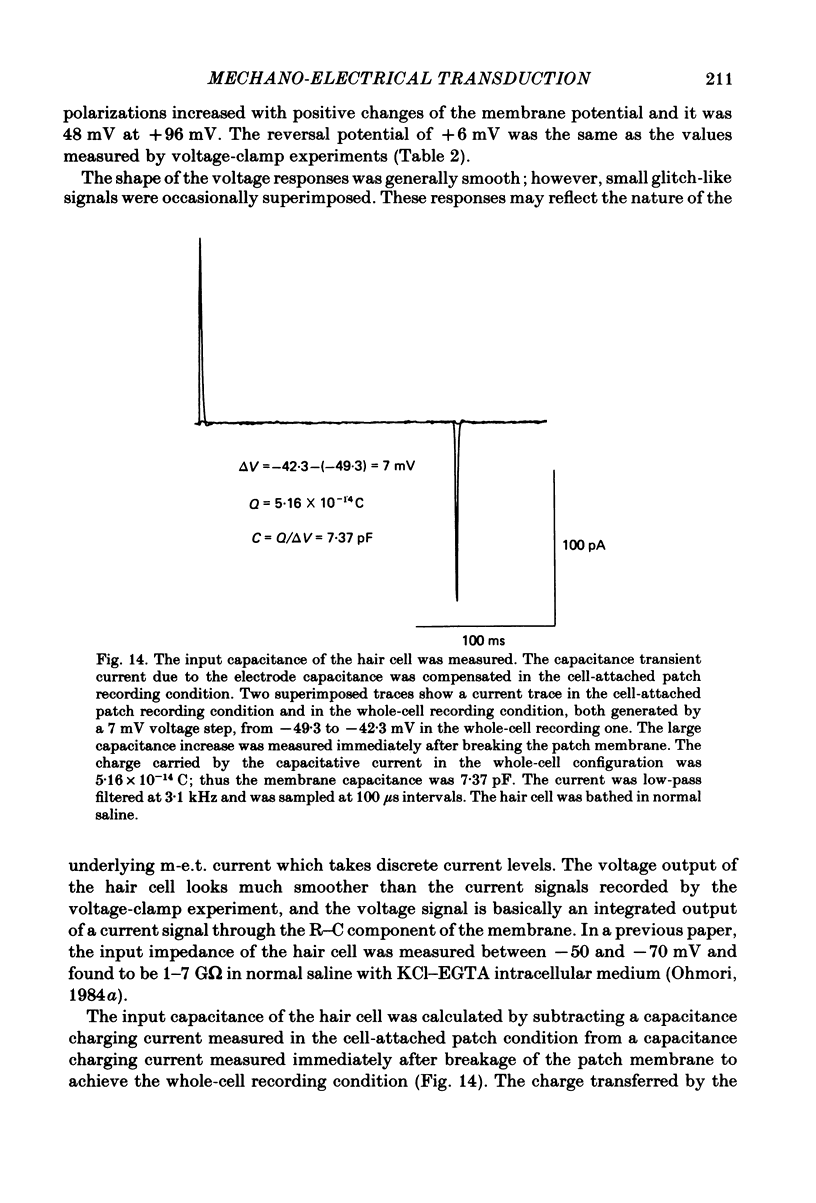
Abstract
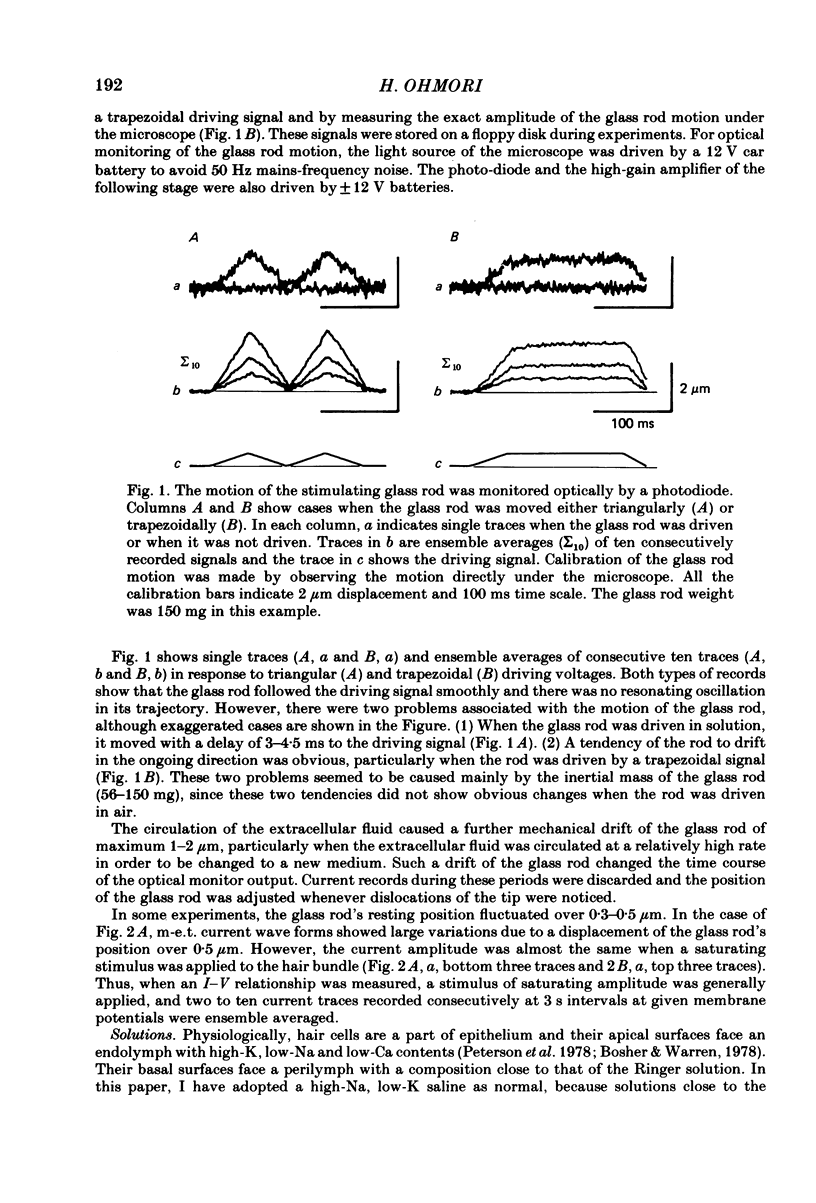
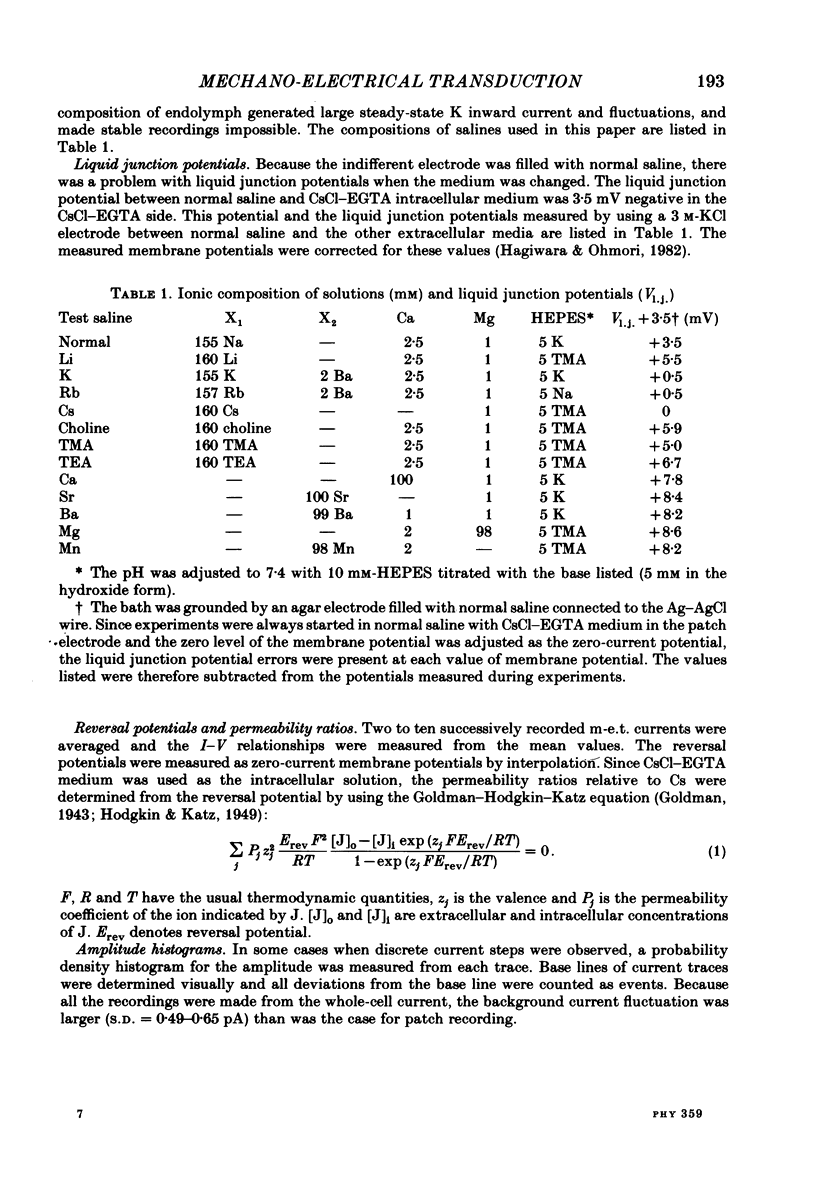
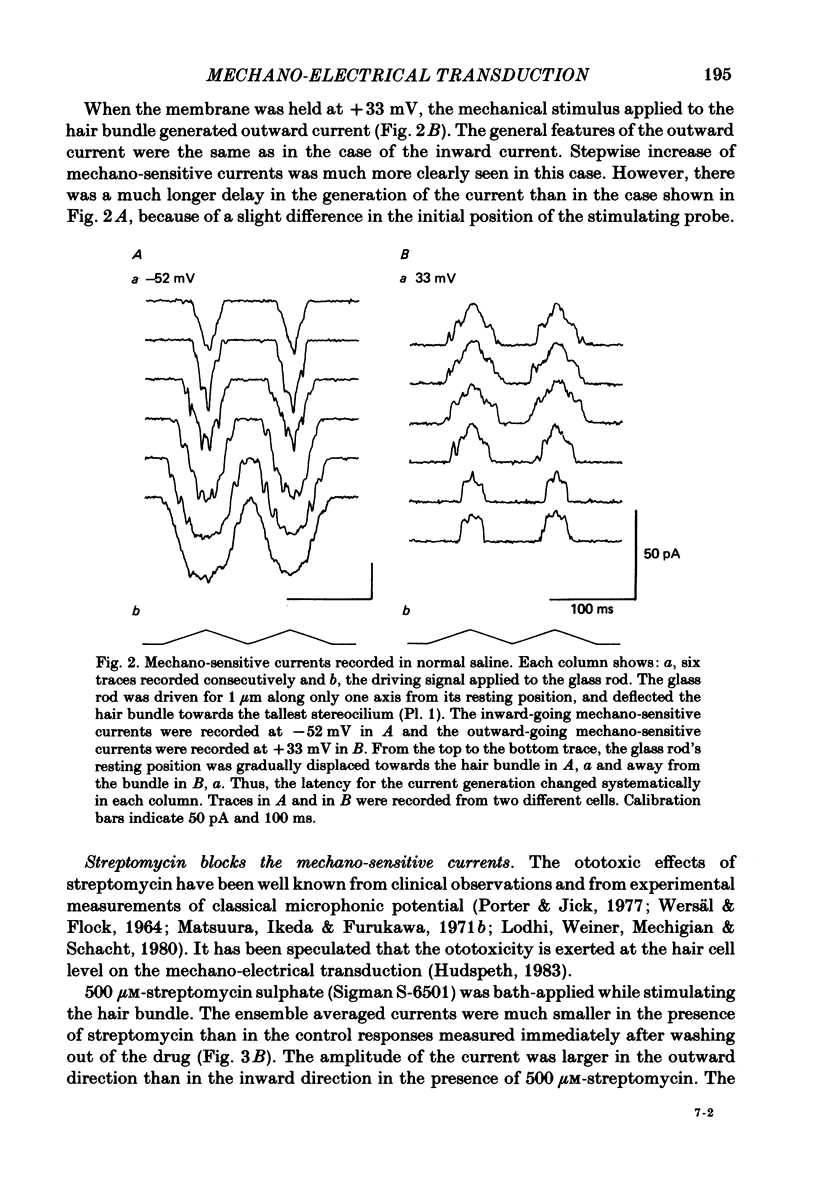
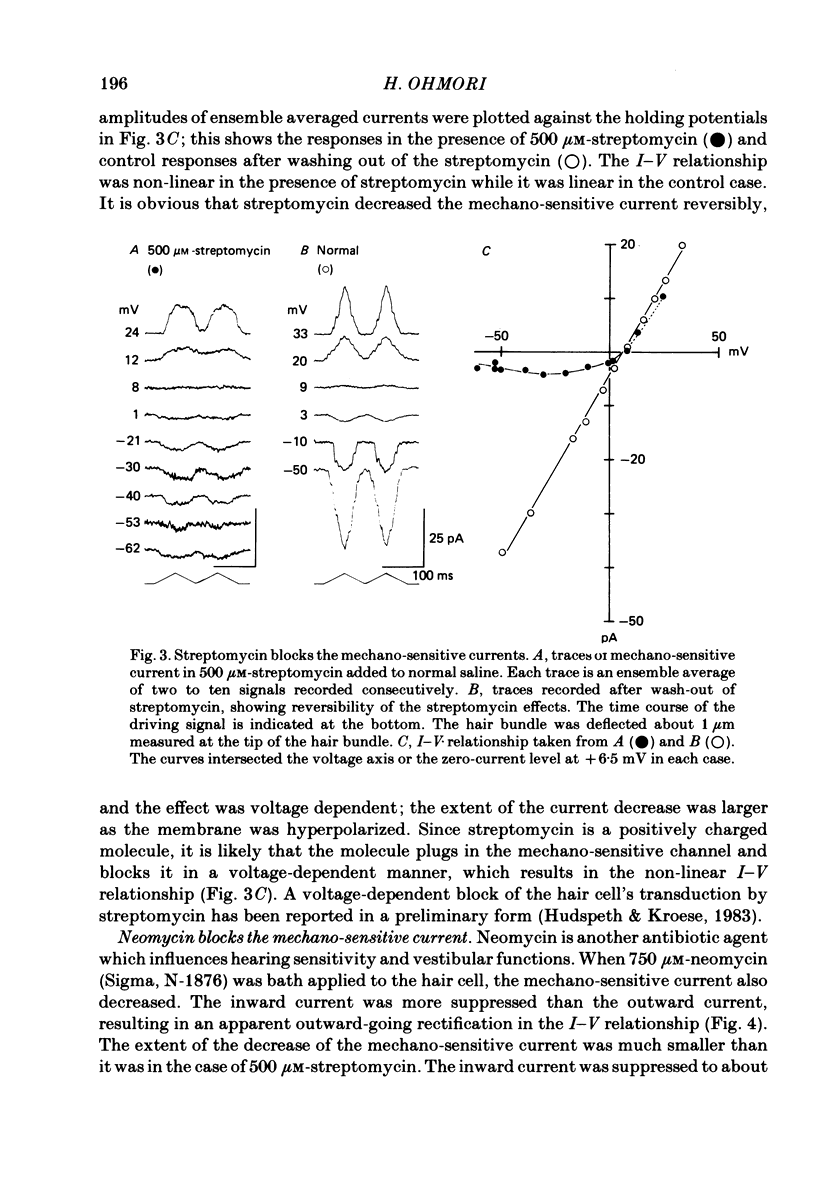
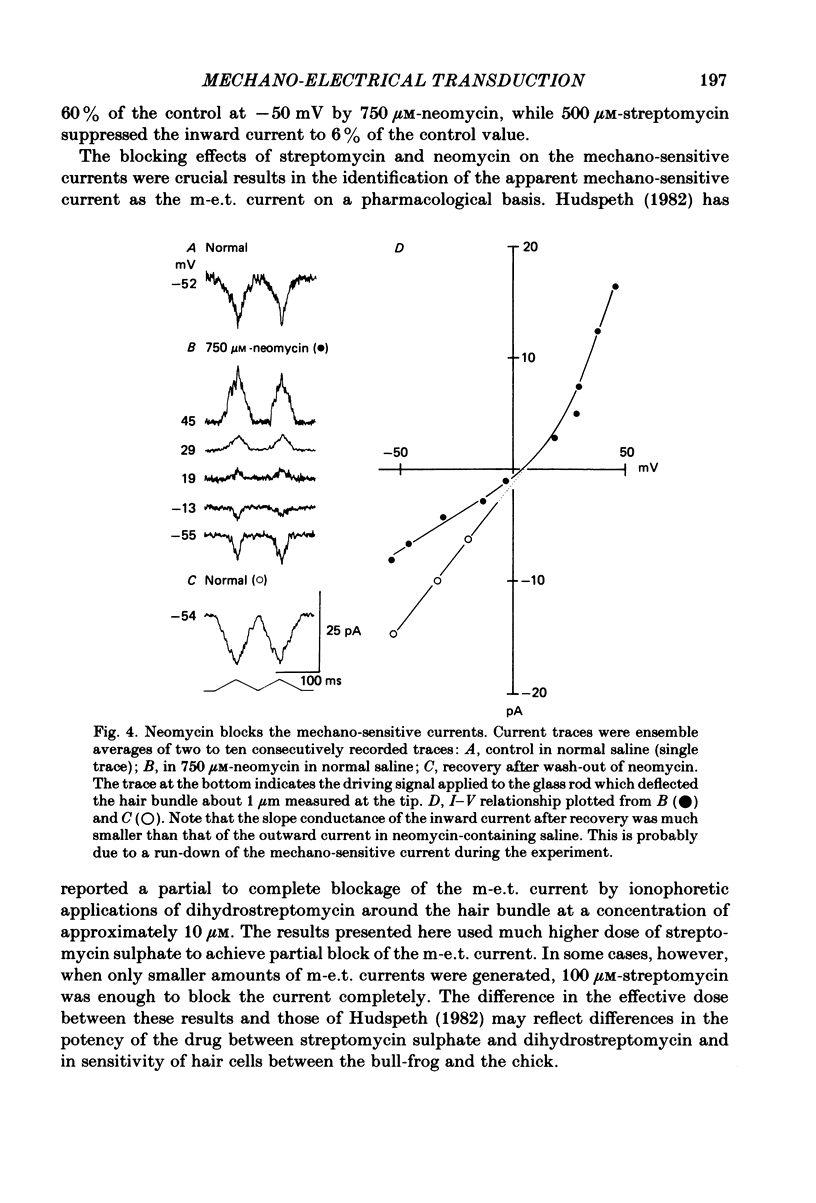
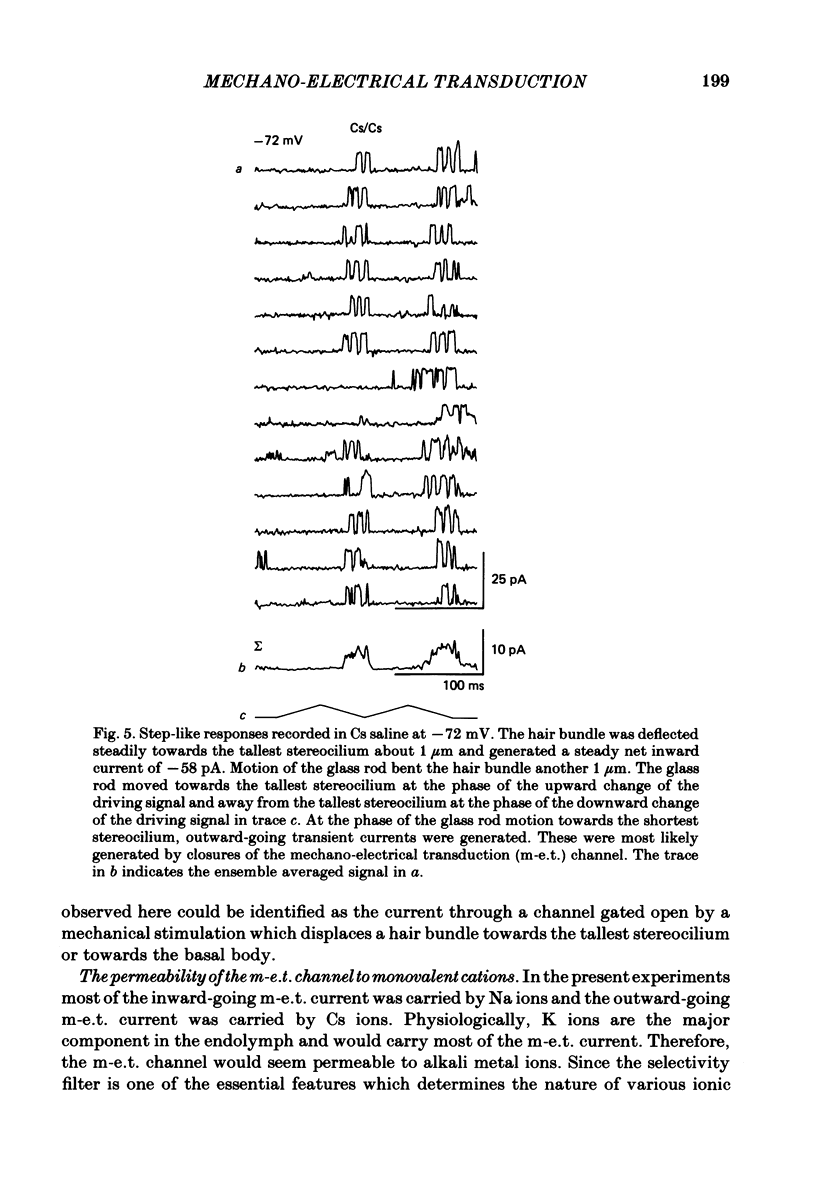
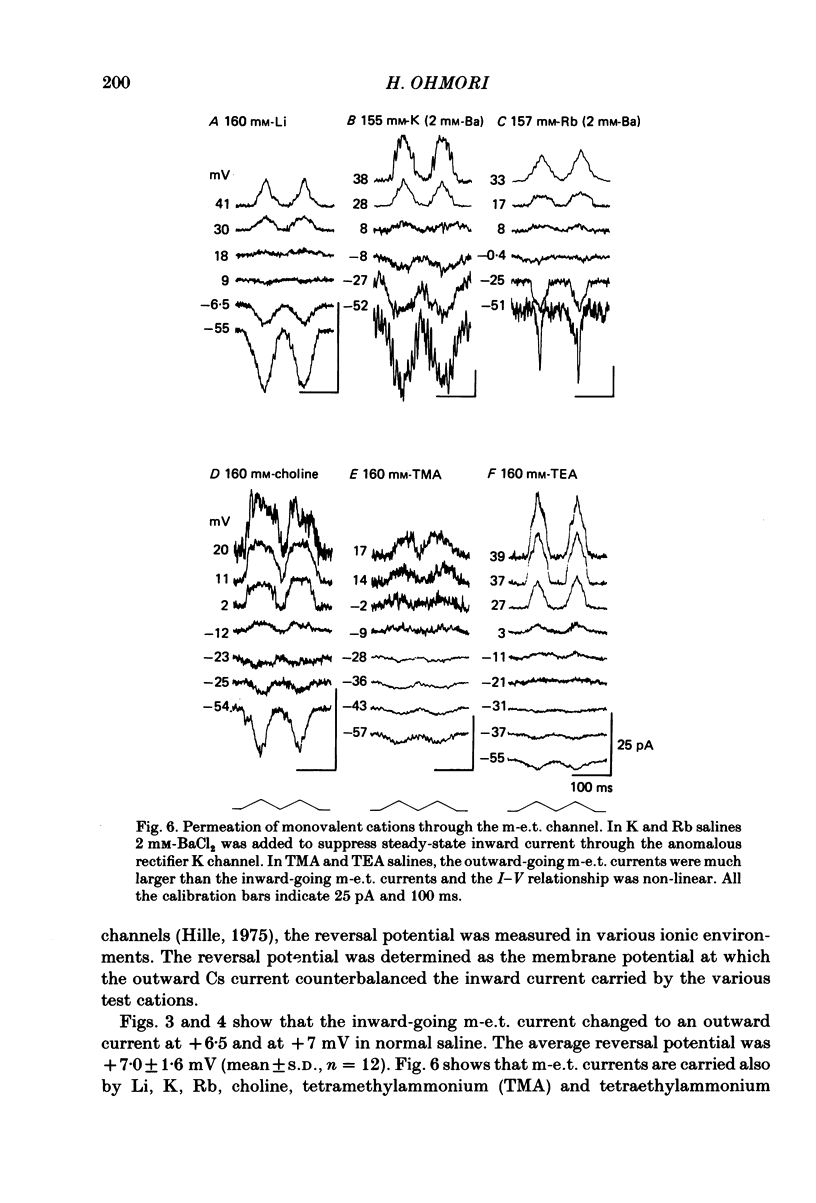
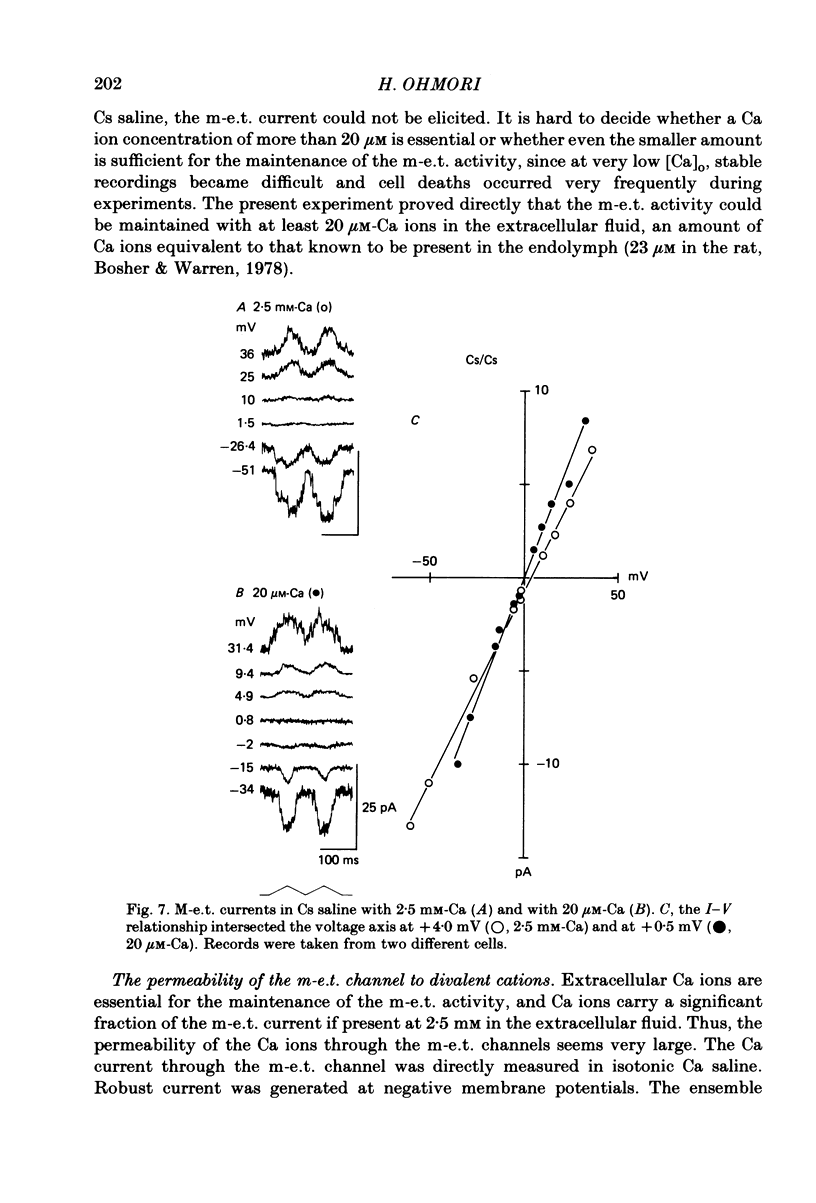
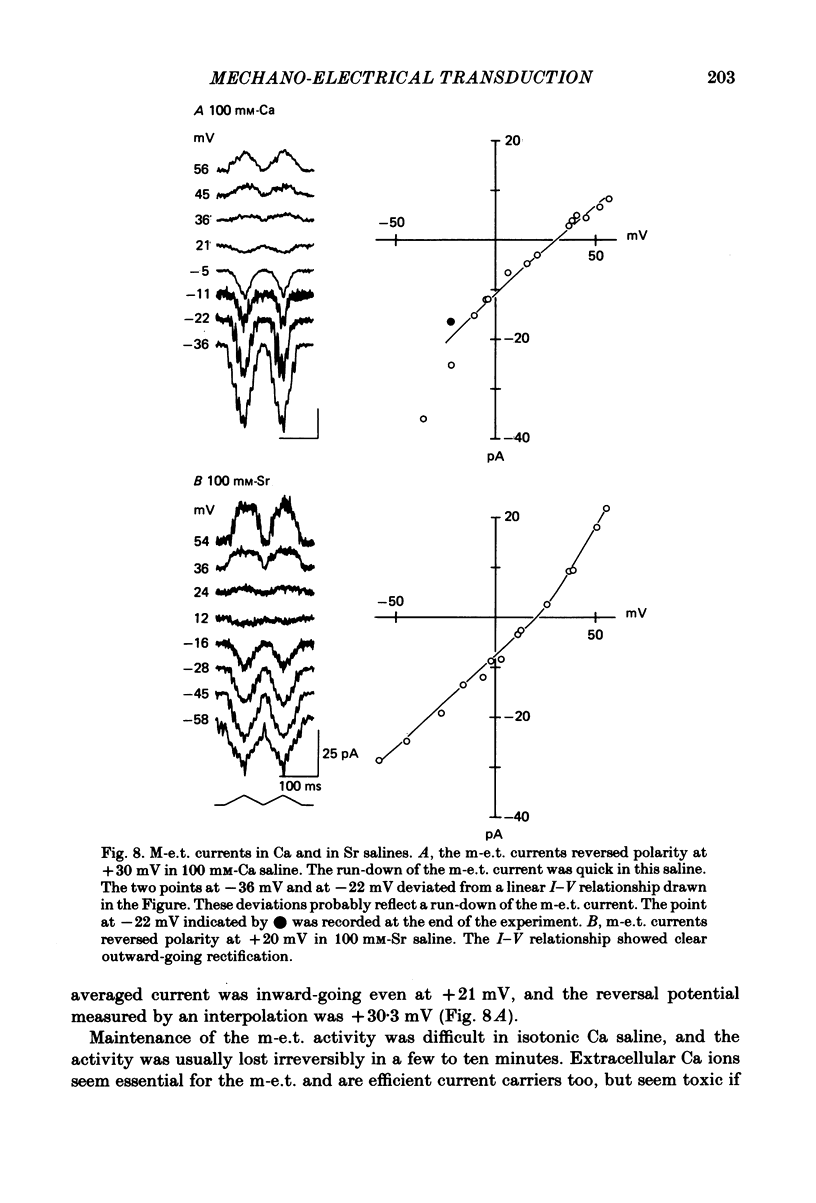
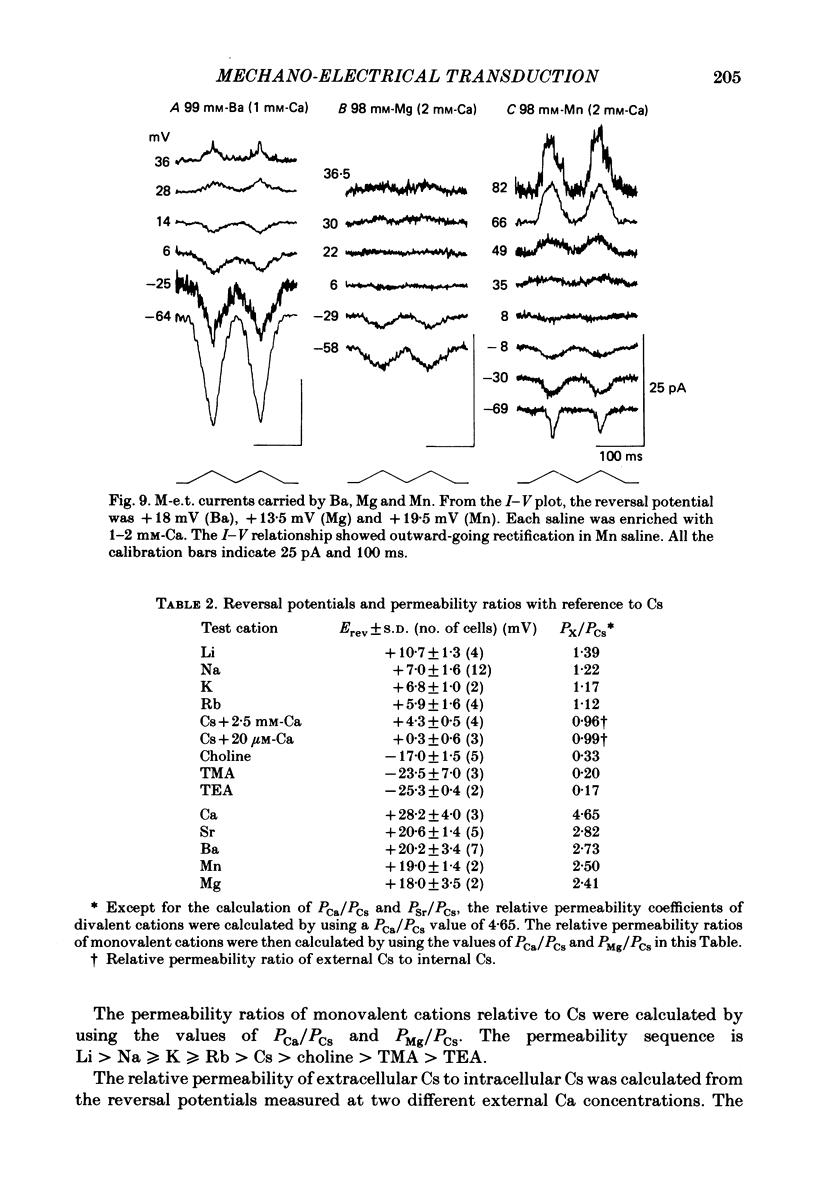
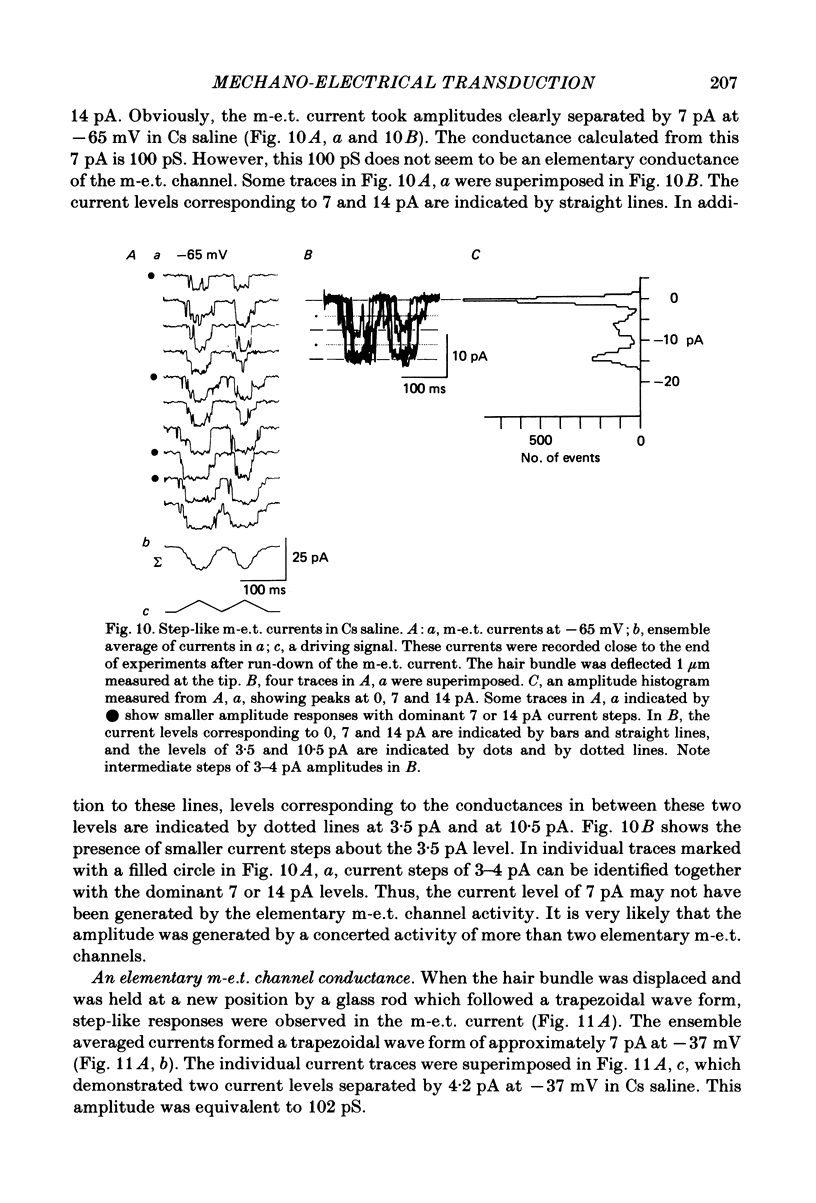
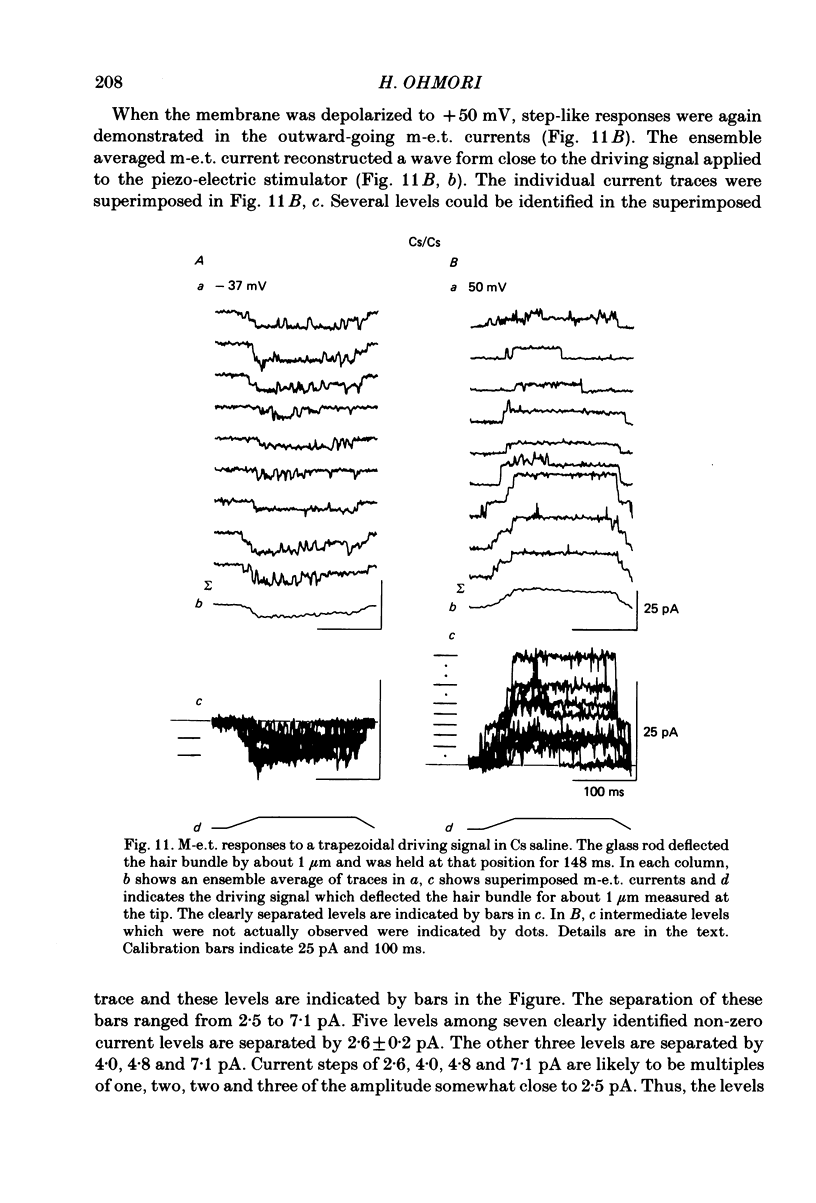
Properties of a mechano-electrical transduction channel were studied in enzymatically dissociated chick vestibular hair cells by using a whole-cell recording variation of the patch voltage-clamp technique. The apical hair bundle was stimulated by a glass rod which moved along a one-dimensional axis when stimulated by either a triangular or a trapezoidal command voltage. The motion of the glass rod was monitored optically using a photodiode. In response to triangular stimuli, the hair cell generated a current of triangular wave form with occasional step-like spiky or zigzag-appearing events. Control experiments confirmed that the current was generated only when the hair bundle was displaced towards the tallest stereocilium. The mechano-sensitive current was blocked by streptomycin and by neomycin. The blockage by streptomycin was clearly voltage dependent: the reduction of the current became larger with hyperpolarization of the membrane. This suggests that the positively charged antibiotic molecules plug the mechanically gated channels. From the evidence presented in 3 and 4 above, the mechano-sensitive current recorded here was identified as the mechano-electrical transduction (m-e.t.) current. The permeability of the m-e.t. channel to various monovalent cations was determined from reversal potential measurements. Since a CsCl-EGTA intracellular medium was used, all the permeabilities were calculated relative to PCs. The sequence of permeabilities was Li greater than Na greater than or equal to K greater than or equal to Rb greater than Cs greater than choline greater than TMA greater than TEA. External Ca ions were indispensable for the recording of transduction current and Sr ions could replace Ca ions without loss of the transduction activity. The minimum [Ca]o for stable generation of the m-e.t. current was 20 microM in Cs saline. The addition of 50-200 microM-Ca to the isotonic Ba saline could maintain the m-e.t. current. The m-e.t. current was observed in isotonic Ca and in Sr salines. Isotonic Ba, Mg and Mn salines were enriched with 1-2 mM-Ca in order to generate the m-e.t. current. The permeabilities of the divalent cations relative to Cs were calculated from the reversal potentials, and the sequence of permeabilities among divalent cations was Ca greater than Sr greater than Ba greater than Mn greater than Mg. Step-like m-e.t. currents were observed in Cs saline. The smallest step amplitude with clear resolution had a conductance of 49.7 +/- 4.5 pS (mean +/- S.D., n = 7 cells). This is likely to be an elementary m-e.t. channel conductance.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagger-Sjöbäck D., Wersäll J. The sensory hairs and tectorial membrane of the basilar papilla in the lizard Calotes versicolor. J Neurocytol. 1973 Sep;2(3):329–350. doi: 10.1007/BF01104034. [DOI] [PubMed] [Google Scholar]
- Bosher S. K., Warren R. L. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature. 1978 Jun 1;273(5661):377–378. doi: 10.1038/273377a0. [DOI] [PubMed] [Google Scholar]
- CITRON L., EXLEY D., HALLPIKE C. S. Formation, circulation and chemical properties of the labyrinthine fluids. Br Med Bull. 1956 May;12(2):101–104. doi: 10.1093/oxfordjournals.bmb.a069529. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Mechanical stimulation and micromanipulation with piezoelectric bimorph elements. J Neurosci Methods. 1980 Dec;3(2):183–202. doi: 10.1016/0165-0270(80)90025-4. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Kellner J., Mannherz H. G., Gröschel-Stewart U., Kendrick-Jones J., Scholey J. Absence of myosin-like immunoreactivity in stereocilia of cochlear hair cells. Nature. 1982 Dec 9;300(5892):531–532. doi: 10.1038/300531a0. [DOI] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Hillman D. E., Lewis E. R. Morphological basis for a mechanical linkage in otolithic receptor transduction in the frog. Science. 1971 Oct 22;174(4007):416–419. doi: 10.1126/science.174.4007.416. [DOI] [PubMed] [Google Scholar]
- Hillman D. E. New ultrastructural findings regarding a vestibular ciliary apparatus and its possible functional significance. Brain Res. 1969 Apr;13(2):407–412. doi: 10.1016/0006-8993(69)90301-1. [DOI] [PubMed] [Google Scholar]
- Hillman D. E. Observations on morphological features and mechanical properties of the peripheral vestibular receptor system in the frog. Prog Brain Res. 1972;37:69–75. doi: 10.1016/S0079-6123(08)63894-7. [DOI] [PubMed] [Google Scholar]
- Hino N., Ochi R., Yanagisawa T. Inhibition of the slow inward current and the time-dependent outward current of mammalian ventricular muscle by gentamicin. Pflugers Arch. 1982 Sep;394(3):243–249. doi: 10.1007/BF00589099. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982 Jan;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Annu Rev Neurosci. 1983;6:187–215. doi: 10.1146/annurev.ne.06.030183.001155. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi S., Weiner N. D., Mechigian I., Schacht J. Ototoxicity of aminoglycosides correlated with their action on monomolecular films of polyphosphoinositides. Biochem Pharmacol. 1980 Feb 15;29(4):597–601. doi: 10.1016/0006-2952(80)90382-2. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Ikeda K., Furukawa T. Effects of Na + , K + , and ouabain on microphonic potentials of the goldfish inner ear. Jpn J Physiol. 1971 Oct;21(5):563–578. doi: 10.2170/jjphysiol.21.563. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Ikeda K., Furukawa T. Effects of streptomycin, kanamycin, quinine, and other drugs on the microphonic potentials of goldfish sacculus. Jpn J Physiol. 1971 Oct;21(5):579–590. doi: 10.2170/jjphysiol.21.579. [DOI] [PubMed] [Google Scholar]
- Miller M. R. Scanning electron microscope studies of the papilla basilaris of some turtles and snakes. Am J Anat. 1978 Mar;151(3):409–435. doi: 10.1002/aja.1001510306. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Mechanoelectrical transducer has discrete conductances in the chick vestibular hair cell. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1888–1891. doi: 10.1073/pnas.81.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. J Physiol. 1984 May;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J., Jick H. Drug-induced anaphylaxis, convulsions, deafness, and extrapyramidal symptoms. Lancet. 1977 Mar 12;1(8011):587–588. doi: 10.1016/s0140-6736(77)92011-6. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Kurtz G. Inhibition of membrane calcium activation by neomycin and streptomycin in crab muscle fibers. Pflugers Arch. 1974;349(4):337–349. doi: 10.1007/BF00588419. [DOI] [PubMed] [Google Scholar]
- Takasaka T., Smith C. A. The structure and innervation of the pigeon's basilar papilla. J Ultrastruct Res. 1971 Apr;35(1):20–65. doi: 10.1016/s0022-5320(71)80141-7. [DOI] [PubMed] [Google Scholar]
- WERSAELL J., FLOCK A. SUPPRESSION AND RESTORATION OF THE MICROPHONIC OUTPUT FROM THE LATERAL LINE ORGAN AFTER LOCAL APPLICATION OF STREPTOMYCIN. Life Sci. 1964 Oct;3:1151–1155. doi: 10.1016/0024-3205(64)90132-8. [DOI] [PubMed] [Google Scholar]



