Abstract
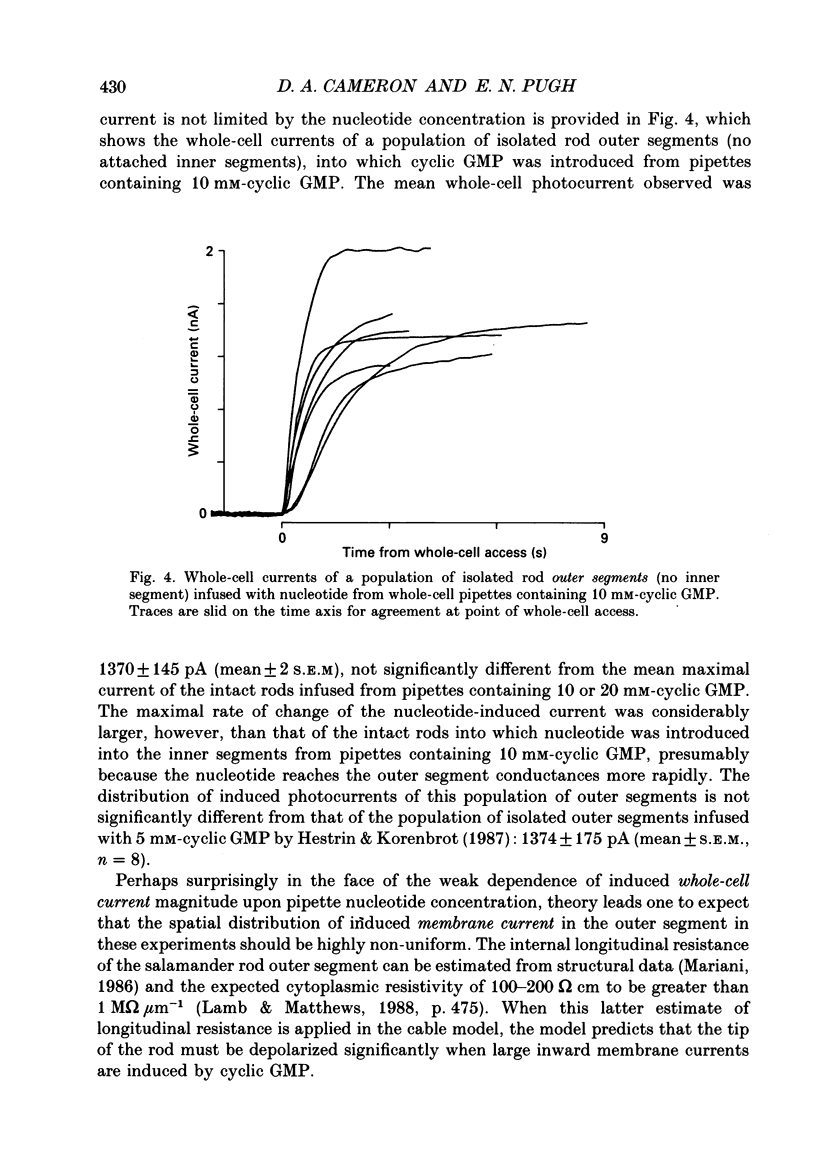
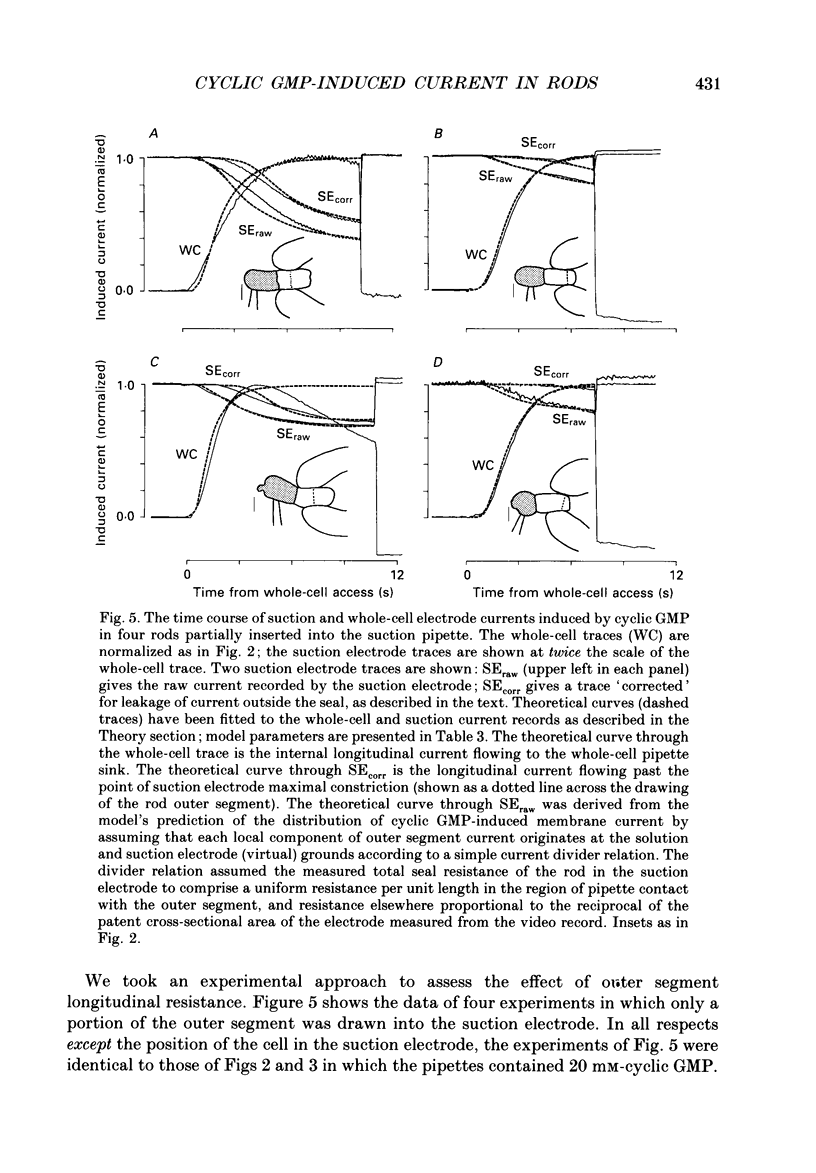
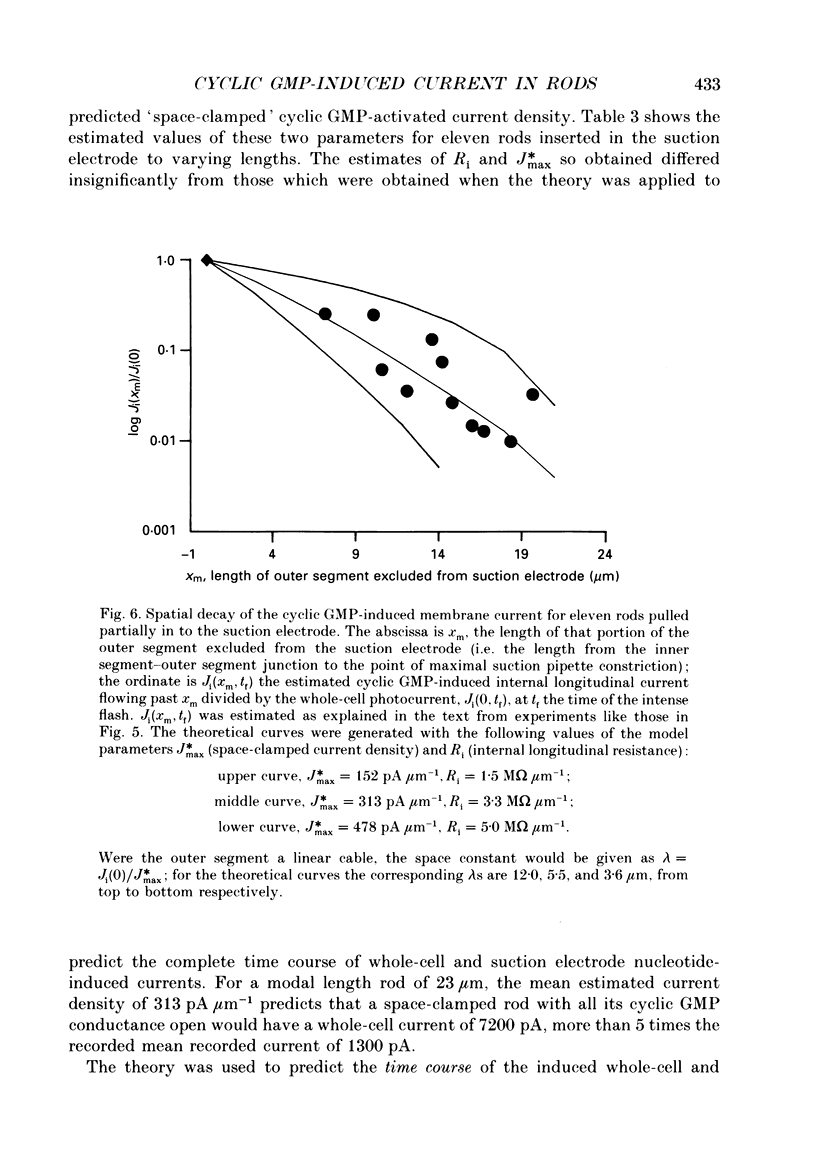
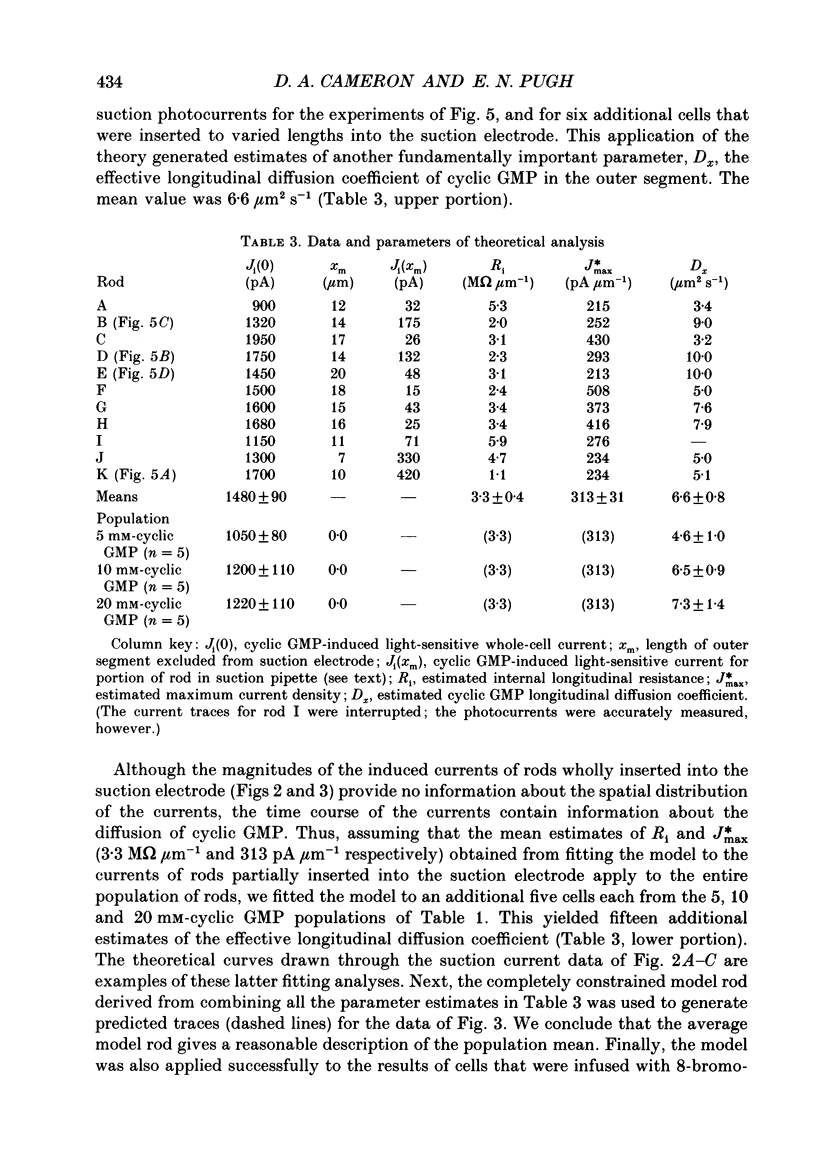
1. Cyclic GMP was introduced into isolated salamander rods through a tight-seal electrode attached to the inner segment while the outer segment was held in a suction electrode; nucleotide-induced membrane current was recorded by both electrodes. After 3-15s of nucleotide exposure the cells were stimulated with intense, brief flashes, which suppressed 90-95% of the induced membrane current. 2. The magnitude of the induced light-sensitive current depended little on the pipette cyclic GMP concentration in the range 10-20 nM: the mean whole-cell current magnitude was 1256 +/- 160 pA (mean +/- 2 S.E.M., n = 41). 3. Experiments and analyses addressed hypotheses about the nature of the magnitude limitation on the induced current. It was shown that the spatial distribution of nucleotide, the residual series resistance of the whole-cell electrode, and the diminution of the ion gradients driving the induced current did not limit the current magnitude by more than 20%. 4. In contrast, the hypothesis that outer segment internal longitudinal resistance severely limits the magnitude of the cyclic GMP-induced current was supported by experiments in which various lengths of the outer segment were drawn into the suction electrode. These showed that the ratio of nucleotide-induced light-sensitive current collected by the suction electrode to that collected by a whole-cell electrode decreased steeply as a function of outer segment length excluded from the suction electrode, having an apparent space constant of 5-7 microns. 5. A cable model of the rod was developed and used to analyse the magnitude of the nucleotide-induced currents. The data are consistent with an outer segment longitudinal resistance of 1-4 M omega microns-1, and a maximum (space-clamped) light-sensitive current density of 313 pA microns-1, equivalent to a total induced current of 7200 pA (23 microns outer segment). 6. A diffusion model was developed and combined with the non-linear cable model to provide an account for the time course of the induced membrane currents. The results are consistent with an effective longitudinal diffusion coefficient of cyclic GMP in the outer segment of 3-10 microns2 s-1, and Hill coefficient of 2-3 for the cyclic GMP gating of the light-sensitive conductance. 7. 8-Bromo-cyclic GMP also caused the light-sensitive membrane current to increase to about the same magnitude as did cyclic GMP.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Barad M. Metabolic flux of cyclic GMP and phototransduction in rabbit retina. J Physiol. 1988 Dec;406:163–179. doi: 10.1113/jphysiol.1988.sp017374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., 3rd, Walseth T. F., Heyman R. A., Barad M., Graeff R. M., Goldberg N. D. Light-induced increases in cGMP metabolic flux correspond with electrical responses of photoreceptors. J Biol Chem. 1986 Oct 5;261(28):13034–13042. [PubMed] [Google Scholar]
- Barkdoll A. E., 3rd, Pugh E. N., Jr, Sitaramayya A. Kinetics of the hydrolysis of 8-bromo-cyclic GMP by the light-activated phosphodiesterase of toad rods. J Neurochem. 1988 Mar;50(3):839–846. doi: 10.1111/j.1471-4159.1988.tb02989.x. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs W. H., Pugh E. N., Jr Kinetics and components of the flash photocurrent of isolated retinal rods of the larval salamander, Ambystoma tigrinum. J Physiol. 1987 Dec;394:529–572. doi: 10.1113/jphysiol.1987.sp016884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Goodman M. G., Weigle W. O. Bromination of guanosine and cyclic GMP confers resistance to metabolic processing by B cells. J Immunol. 1982 Dec;129(6):2715–2717. [PubMed] [Google Scholar]
- Hestrin S., Korenbrot J. I. Effects of cyclic GMP on the kinetics of the photocurrent in rods and in detached rod outer segments. J Gen Physiol. 1987 Oct;90(4):527–551. doi: 10.1085/jgp.90.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. Control of light-sensitive current in salamander rods. J Physiol. 1988 Sep;403:439–471. doi: 10.1113/jphysiol.1988.sp017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R. External and internal actions in the response of salamander retinal rods to altered external calcium concentration. J Physiol. 1988 Sep;403:473–494. doi: 10.1113/jphysiol.1988.sp017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeish P. R., Schwartz E. A., Tachibana M. Control of the generator current in solitary rods of the Ambystoma tigrinum retina. J Physiol. 1984 Mar;348:645–664. doi: 10.1113/jphysiol.1984.sp015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani A. P. Photoreceptors of the larval tiger salamander retina. Proc R Soc Lond B Biol Sci. 1986 May 22;227(1249):483–492. doi: 10.1098/rspb.1986.0035. [DOI] [PubMed] [Google Scholar]
- Matthews G. Comparison of the light-sensitive and cyclic GMP-sensitive conductances of the rod photoreceptor: noise characteristics. J Neurosci. 1986 Sep;6(9):2521–2526. doi: 10.1523/JNEUROSCI.06-09-02521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Single-channel recordings demonstrate that cGMP opens the light-sensitive ion channel of the rod photoreceptor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):299–302. doi: 10.1073/pnas.84.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Boswell K. H., Muneyama K., Simon L. N., Robins R. K., Shuman D. A. Synthesis and biochemical studies of various 8-substituted derivatives of guanosine 3',5'-cyclic phosphate, inosine 3',5'-cyclic phosphate, and xanthosine 3',5'-cyclic phosphate. Biochemistry. 1973 Dec 18;12(26):5310–5319. doi: 10.1021/bi00750a014. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol G. D., Miller W. H. Cyclic GMP injected into retinal rod outer segments increases latency and amplitude of response to illumination. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5217–5220. doi: 10.1073/pnas.75.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Sather W. A., Detwiler P. B. Intracellular biochemical manipulation of phototransduction in detached rod outer segments. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9290–9294. doi: 10.1073/pnas.84.24.9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf J. L. Dependence of the single photon response on longitudinal position of absorption in toad rod outer segments. J Physiol. 1983 Oct;343:147–159. doi: 10.1113/jphysiol.1983.sp014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Walz B. Elemental distribution in Rana pipiens retinal rods: quantitative electron probe analysis. J Physiol. 1985 Jan;358:183–195. doi: 10.1113/jphysiol.1985.sp015547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Yamanaka G., Eckstein F., Baylor D. A., Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]


