Abstract
Cerebral cavernous malformations (CCMs) are congenital vascular anomalies of the central nervous system that can result in hemorrhagic stroke, seizures, recurrent headaches, and focal neurologic deficits. Mutations in the gene KRIT1 are responsible for type 1 CCM (CCM1). We report that a novel gene, MGC4607, exhibits eight different mutations in nine families with type 2 CCM (CCM2). MGC4607, similar to the KRIT1 binding partner ICAP1α, encodes a protein with a phosphotyrosine-binding domain. This protein may be part of the complex pathway of integrin signaling that, when perturbed, causes abnormal vascular morphogenesis in the brain, leading to CCM formation.
Cerebral cavernous malformations (CCMs) are congenital vascular anomalies of the CNS, with an incidence in the general population of 0.1%–0.5% (Rigamonti et al. 1988). The lesions are characterized by grossly enlarged blood vessels consisting of a single layer of endothelium and without any intervening neural tissue, ranging in diameter from a few millimeters to several centimeters (Rigamonti et al. 1988; Tomlinson et al. 1994; Gil-Nagel et al. 1995). CCMs usually present clinically during the 3rd to 5th decade of life, resulting in hemorrhagic stroke, seizures, recurrent headaches, and focal neurologic deficits (Zabramski et al. 1994, 1999). Magnetic resonance imaging (MRI) can detect these lesions and is often the only method to diagnose a clinically silent lesion. Familial forms of CCM are inherited in an autosomal dominant fashion, with three known loci on chromosomes 7q21.2 (CCM1 [MIM 116860]) (Dubovsky et al. 1995; Gunel et al. 1995; Marchuk et al. 1995), 7p15-p13 (CCM2 [MIM 603284]) (Craig et al. 1998), and 3q25.2-q27 (CCM3 [MIM 603285]) (Craig et al. 1998). The disease gene responsible for type 1 CCM (CCM1) encodes KRIT1 (KREV interaction trapped 1) (Laberge et al. 1999; Sahoo et al. 1999), a protein of unknown function containing four ankyrin domains and a C-terminal FERM domain (Serebriiskii et al. 1997). KRIT1 was recently shown to bind to ICAP1α (integrin cytoplasmic domain-associated protein) (Zhang et al. 2001; Zawistowski et al. 2002), a protein containing a phosphotyrosine-binding (PTB) domain that also binds to the β1-integrin cytoplasmic domain (Chang et al. 1997; Zhang and Hemler 1999). It is not yet understood how mutations in KRIT1 lead to the formation of CCMs, but the identification of the causative genes for CCM2 and/or CCM3 may help to elucidate the pathogenesis of CCMs.
A cohort of 37 probands with CCM was screened for KRIT1 mutations, and 10 harbored a KRIT1 mutation. The 27 non-KRIT1 (CCM1) probands were included in this study, and, where possible, additional family members were collected. The boundaries of the CCM2 locus were previously established by 1-LOD support analysis as an 11-cM interval flanked by markers D7S2846 and D7S1818 (Craig et al. 1998). Markers mapping within the interval were used to haplotype several families with CCM2, but no crossovers were identified in affected individuals. Since we were unable to genetically reduce the size of the CCM2 interval, we began to sequence positional candidate genes.
Within the genetically defined interval, 55 known or putative genes have been identified (UCSC Human Genome Assembly Web site). We initially selected eight genes for sequence analysis on the basis of biological plausibility: MGC4607, RALA (v-ral simian leukemia viral oncogene), CAMK2B (calcium/calmodulin-dependent protein kinase II β), STK17A (serine/threonine kinase 17a), CDC10 (cell division cycle 10 homolog), CDC2L5 (cell division cycle 2–like 5), HIP-55 (src homolog 3 domain–containing protein), and MYLC2A (myosin light chain 2a). The predicted gene MGC4607 was chosen because its translated protein product encodes a putative PTB domain. This same domain is found in ICAP1α, a binding partner of the CCM1 product KRIT1.
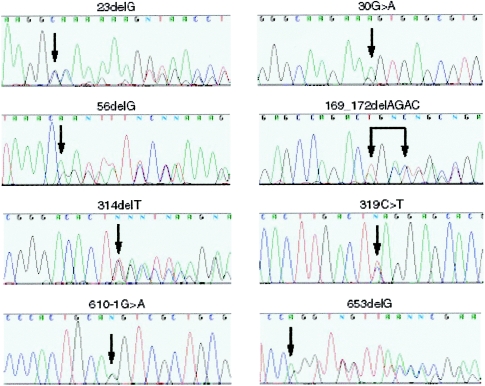
Among the panel of 27 probands without a KRIT1 mutation, we detected eight different mutations in MGC4607. One mutation (169_172delAGAC) was found in two separate families that—as a further investigation into ethnic background suggested—may be distantly related. The mutations include five frameshift, one nonsense, and two splicing mutations (table 1; fig. 1), each of which, if translated, would result in a truncated protein. The mutations map throughout the gene, including two in exon 1, suggesting that at least some may lead to loss-of-function alleles.
Table 1.
Characteristics of Nine Families with CCM2 Mutations
| Family | No. of Affected Individualsa | Exon | Nucleotide Change | Mutation Consequence | Predicted Amino Acid Change |
| 2626 | 4 | 1 | 23delG | Frameshift | 8fsX22 |
| 214 | 1 | 1 | 30G→A | Splicing alteration | K10K |
| CAV01b | 4 | 2 | 56delG | Frameshift | 19fsX22 |
| 70c | 5 | 2 | 169_172delAGAC | Frameshift | 57fsX58 |
| IFCAS-1d | 4 | 2 | 169_172delAGAC | Frameshift | 57fsX58 |
| 229 | 4 | 4 | 314delT | Frameshift | 105fsX105 |
| IFCAS-14 | 2 | 4 | 319C→T | Nonsense | Q107X |
| 2030 | 5 | 6 | 610-1G→A | Splicing alteration | NA |
| 2812 | 1 | 6 | 653delG | Frameshift | 218fsX291 |
Figure 1.
Sequence traces of the eight CCM2 mutations. Each mutation is indicated by a black arrow. The extent of a 4-bp deletion is shown with conjoined arrows.
One splice-junction mutation (610-1G→A, in family 2030) is located at the invariant G residue at the splice-acceptor site adjacent to exon 6. A second splice-site mutation was less obvious. Family 214 harbored what appeared to be a silent mutation (30G→A; K10K) in the last nucleotide of exon 1. Nonetheless, we thought that this sequence alteration might also lead to a splicing defect, because it results in a less favorable nucleotide at this position of the donor splice junction and this position is not infrequently a site of mutations affecting splicing. Sequence analysis confirmed that the 30A variant of MGC4607 was not present in 606 control chromosomes. To examine its effect on the MGC4607 mRNA, we performed expression analysis on leukocyte mRNA isolated from the proband. RT-PCR products from both the proband and control leukocyte mRNA exhibited a number of normal splice variants of the message but no additional splice variants arising from the patient mutation. However, sequence analysis of the RT-PCR products from the proband revealed only the normal allele. This suggests that the mutant 30A allele is not expressed or, more likely, that this mutation results in a grossly aberrant splice variant that is subjected to degradation.
Four of the families harboring MGC4607 mutations were previously reported as consistent with showing linkage to CCM2 (Craig et al. 1998; Squitieri et al. 2000; Dupre et al. 2003). The remaining five families with CCM2 were of insufficient power to establish linkage. Families not showing CCM2 mutations harbored either mutations in the yet undiscovered CCM3 gene or mutations undetectable by DNA sequence analysis of coding exons at the CCM1 and CCM2 loci.
The mutation in each of the nine families cosegregates with affected status for all confirmed (MRI- and/or symptom-positive) affected individuals (table 2). Except for one individual from family 2626, all symptomatic individuals had MRI data available that confirmed the presence of the CCM lesions. One mutation carrier in family 229 was apparently MRI negative at age 75 years but had suffered a stroke of unknown etiology at age 20 years and thus was classified as “presumed affected.” The families also contained a rather large number of mutation carriers that were, at the time of ascertainment, clinically silent (table 2). For nearly all of these, the lack of overt symptoms led to a presumed unaffected status, and, as a consequence, these individuals were not evaluated by MRI.
Table 2.
Comparison of MGC4607 Mutation Status with MRI Results
|
No. of Family Members with a MGC4607 Mutation, with MRI Status |
No. of Family Members without a MGC4607 Mutation, with MRI Status |
|||||
| Family | CCM Positive | CCM Negative | Unknown | CCM Positive | CCM Negative | Unknown |
| 2626 | 3 | 0 | 4a | 0 | 0 | 6 |
| 214 | 1 | 0 | 1 | 0 | 2 | 1 |
| CAV01b | 4 | 0 | 0 | 0 | 0 | 1 |
| 70c | 5 | 0 | 2 | 0 | 1 | 3 |
| IFCAS-1d | 4 | 0 | 1 | 0 | 0 | 10 |
| 229 | 4 | 1e | 1 | 0 | 1 | 0 |
| IFCAS-14 | 2 | 0 | 7 | 0 | 0 | 11 |
| 2030 | 5 | 0 | 2 | 0 | 1 | 15 |
| 2812 | 1 | 0 | 0 | 0 | 0 | 1 |
The large number of mutation-positive individuals that remain asymptomatic provides an explanation for the difficulty encountered in narrowing the candidate interval for this and other CCM loci. Asymptomatic individuals will receive a medical diagnosis of unaffected, but, without MRI examination, their status in terms of genetic linkage analysis remains uncertain. The paucity of confirmed affected individuals in most families precludes definitive linkage to a CCM locus and can even mask the autosomal dominant inheritance. Even when a family shows unequivocal linkage to the correct locus, there are generally fewer confirmed affected (MRI-positive) family members and thus fewer informative meioses that may harbor recombination events useful for refined mapping.
These data confirm the previously noted reduced penetrance within families with CCM with regard to expression of clinical symptoms. However, penetrance appears much higher in patients with MRI-diagnosed affected status. A more accurate determination of penetrance will require MRI examination of those individuals who now test positive for the mutation in their family. These data emphasize both the importance of MRI in the diagnosis of CCM and the potential for DNA-based diagnostics to identify those at risk.
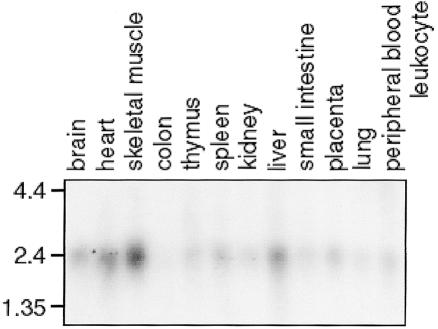
To determine the expression pattern of MGC4607, we performed northern blot analysis of human tissues, using the entire cDNA as a probe (fig. 2). MGC4607 is most highly expressed in the skeletal muscle, heart, and liver, with minimal or no expression in the colon and lung. MGC4607 is also expressed in the brain. KRIT1 and its binding partner, ICAP1α, have been also shown to be expressed in the brain (Faisst and Gruss 1998; Denier et al. 2002; Kehrer-Sawatzki et al. 2002). These expression data suggest that CCM lesion formation may not be due to an intrinsic vascular defect but, rather, that the cerebral vasculature is aberrantly responding to signals arising in the brain parenchyma. This is consistent with the histopathology of the CCM lesions, which are devoid of neuronal parenchyma within the lesions themselves. There are, however, other data that support vascular expression of the CCM1 gene product KRIT1 (Gunel et al. 2002).
Figure 2.
Northern blot analysis of MGC4607 expression. The 12 human tissues are indicated at the top, and size (in kb) is indicated on the left.
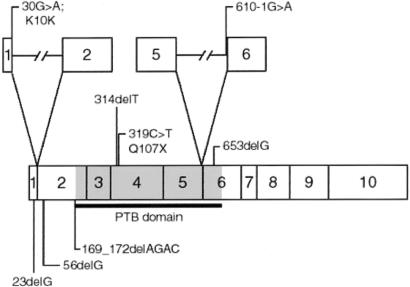
We have named this novel protein “malcavernin” for its role in the development of cerebral cavernous malformations (fig. 3). The identification of ICAP1α as a KRIT1 binding partner (Zhang et al. 2001; Zawistowski et al. 2002) suggested a model in which KRIT1 and β1-integrin compete for ICAP1α binding, possibly regulating integrin signaling (Marchuk et al. 2003). The presence of a PTB domain in malcavernin predicts a possible interaction with the CCM1 protein, KRIT1, and/or with the β1-integrin cytoplasmic tail. Integrin signaling is critical for cell-cell and cell-extracellular matrix communication, which, in turn, modulates cellular migration and morphology. A biochemical pathway is emerging for CCM pathogenesis, involving regulation of integrin signaling through a complex set of interacting proteins that may compete for binding. The proteins involved in this pathway include the CCM1 and CCM2 gene products KRIT1 and malcavernin.
Figure 3.
Schematic diagram of malcavernin. Each exon is shown as a box with the corresponding exon number. The PTB domain is shown in gray. The location and description of the eight CCM2 mutations are shown. Mutations affecting splice junctions are shown above, with the introns indicated by lines.
Acknowledgments
We are grateful to the patients and their families for participation in this study. This work was supported by National Institutes of Health grant NS43543 and American Heart Association grant (Bugher Foundation Award) 0070028N (both to D.A.M.).
Electronic-Database Information
The URLs for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CCM1, CCM2, and CCM3)
- University of California, Santa Cruz (UCSC), Human Genome Assembly Web site, http://genome.ucsc.edu/ (for published assembly and genome browser)
References
- Chang DD, Wong C, Smith H, Liu J (1997) ICAP-1, a novel β1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of beta1 integrin. J Cell Biol 138:1149–1157 10.1083/jcb.138.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig HD, Gunel M, Cepeda O, Johnson EW, Ptacek L, Steinberg GK, Ogilvy CS, Berg MJ, Crawford SC, Scott RM, Steichen-Gersdorf E, Sabroe R, Kennedy CTC, Mettler G, Beis MJ, Fryer A, Awad IA, Lifton RP (1998) Multilocus linkage identifies two new loci for a Mendelian form of stroke, cerebral cavernous malformation, at 7p15-13 and 3q25.2-27. Hum Mol Genet 7:1851–1858 10.1093/hmg/7.12.1851 [DOI] [PubMed] [Google Scholar]
- Denier C, Gasc JM, Chapon F, Domenga V, Lescoat C, Joutel A, Tournier-Lasserve E (2002) Krit1/cerebral cavernous malformation 1 mRNA is preferentially expressed in neurons and epithelial cells in embryo and adult. Mech Dev 117:363–367 10.1016/S0925-4773(02)00209-5 [DOI] [PubMed] [Google Scholar]
- Dubovsky J, Zabramski JM, Kurth J, Spetzler RF, Rich SS, Orr HT, Weber JL (1995) A gene responsible for cavernous malformations of the brain maps to chromosome 7q. Hum Mol Genet 4:453–458 [DOI] [PubMed] [Google Scholar]
- Dupre N, Verlaan DJ, Hand CK, Laurent SB, Turecki G, Davenport WJ, Acciarri N, Dichgans J, Ohkuma A, Siegel AM, Rouleau GA (2003) Linkage to the CCM2 locus and genetic heterogeneity in familial cerebral cavernous malformation. Can J Neurol Sci 30:122–128 [DOI] [PubMed] [Google Scholar]
- Faisst AM, Gruss P (1998) Bodenin: a novel murine gene expressed in restricted areas of the brain. Dev Dyn 212:293–303 [DOI] [PubMed] [Google Scholar]
- Gil-Nagel A, Wilcox KJ, Stewart JM, Anderson VE, Leppik IE, Rich SS (1995) Familial cerebral cavernous angioma: clinical analysis of a family and phenotypic classification. Epilepsy Res 21:27–36 10.1016/0920-1211(95)00005-U [DOI] [PubMed] [Google Scholar]
- Gunel M, Awad IA, Anson J, Lifton RP (1995) Mapping of a gene causing cerebral cavernous malformation to 7q11.2-q21. Proc Nat Acad Sci 92:6620–6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunel M, Laurans MS, Shin D, DiLuna ML, Voorhees J, Choate K, Nelson-Williams C, Lifton RP (2002) KRIT1, a gene mutated in cerebral cavernous malformation, encodes a microtubule-associated protein. Proc Natl Acad Sci USA 99:10677–10682 10.1073/pnas.122354499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer-Sawatzki H, Wilda M, Braun VM, Richter HP, Hameister H (2002) Mutation and expression analysis of the KRIT1 gene associated with cerebral cavernous malformations (CCM1). Acta Neuropathol (Berl) 104:231–240 [DOI] [PubMed] [Google Scholar]
- Laberge S, Labauge P, Marechal E, Maciazek J, Tournier-Lasserve E (1999) Genetic heterogeneity and absence of founder effect in a series of 36 French cerebral cavernous angiomas families. Eur J Hum Genet 7:499–504 [DOI] [PubMed] [Google Scholar]
- Marchuk DA, Gallione CJ, Morrison LA, Clericuzio CL, Hart BL, Kosofsky BE, Louis DN, Gusella JF, Davis LE, Prenger VL (1995) A locus for cerebral cavernous malformations maps to chromosome 7q in two families. Genomics 28:311–314 10.1006/geno.1995.1147 [DOI] [PubMed] [Google Scholar]
- Marchuk DA, Srinivasan S, Squire TL, Zawistowski JS (2003) Vascular morphogenesis: tales of two syndromes. Hum Mol Genet 12:R97–R112 10.1093/hmg/ddg103 [DOI] [PubMed] [Google Scholar]
- Rigamonti D, Hadley MN, Drayer BP, Johnson PC, Hoenig-Rigamonti K, Knight JT, Spetzler RF (1988) Cerebral cavernous malformations. Incidence and familial occurrence. N Engl J Med 319:343–347 [DOI] [PubMed] [Google Scholar]
- Sahoo T, Johnson EW, Thomas JW, Kuehl PM, Jones TL, Dokken CG, Touchman JW, Gallione CJ, Lee-Lin SQ, Kosofsky B, Kurth JH, Louis DN, Mettler G, Morrison L, Gil-Nagel A, Rich SS, Zabramski JM, Boguski MS, Green ED, Marchuk DA (1999) Mutations in the gene encoding KRIT1, a Krev-1/rap 1a binding protein, cause cerebral cavernous malformations. Hum Mol Genet 8:2325–2333 10.1093/hmg/8.12.2325 [DOI] [PubMed] [Google Scholar]
- Serebriiskii I, Estojak J, Sonoda G, Testa JR, Golemis EA (1997) Association of Krev-1/rap 1a with Krit1, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21-22. Oncogene 15:1043–1049 10.1038/sj.onc.1201268 [DOI] [PubMed] [Google Scholar]
- Squitieri F, Maglione V, Buzzi MG, Nargi E, Novelletto A, Cannella M, Simonelli M, Colonnese C, Simonelli P, Innocenzi G, Gabliardi FM, Caruso R, Ragona G, Cantore GP (2000) Cavernous angiomas of the nervous system in Italy: clinical and genetic study. Neurol Sci 21:129–134 10.1007/s100720070087 [DOI] [PubMed] [Google Scholar]
- Steichen-Gersdorf, E, Felber S, Fuchs W, Russeger L, Twerdy K (1992) Familial cavernous angioma of the brain: observations in a four generation family. Eur J Pediatr 151:861–863 [DOI] [PubMed] [Google Scholar]
- Tomlinson FH, Houser OW, Scheithauer BW, Sundt TM Jr, Okazaki H, Parisi JE (1994) Angiographically occult vascular malformations: a correlative study of features on magnetic resonance imaging and histological examination. Neurosurgery 34:792–799; discussion 799–800 [DOI] [PubMed] [Google Scholar]
- Zabramski JM, Henn JS, Coons S (1999) Pathology of cerebral vascular malformations. Neurosurg Clin N Am 10:395–410 [PubMed] [Google Scholar]
- Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, Brown B, Rigamonti D, Brown G (1994) The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 80:422–432 [DOI] [PubMed] [Google Scholar]
- Zawistowski JS, Serebriiskii IG, Lee MF, Golemis EA, Marchuk DA (2002) KRIT1 association with the integrin-binding protein ICAP-1: a new direction in the elucidation of cerebral cavernous malformations (CCM1) pathogenesis. Hum Mol Genet 11:389–396 10.1093/hmg/11.4.389 [DOI] [PubMed] [Google Scholar]
- Zhang J, Clatterbuck RE, Rigamonti D, Chang DD, Dietz HC (2001) Interaction between krit1 and icap1α infers perturbation of integrin β1-mediated angiogenesis in the pathogenesis of cerebral cavernous malformation. Hum Mol Genet 10:2953–2960 10.1093/hmg/10.25.2953 [DOI] [PubMed] [Google Scholar]
- Zhang XA, Hemler ME (1999) Interaction of the integrin β1 cytoplasmic domain with ICAP-1 protein. J Biol Chem 274:11–19 10.1074/jbc.274.1.11 [DOI] [PubMed] [Google Scholar]