Abstract
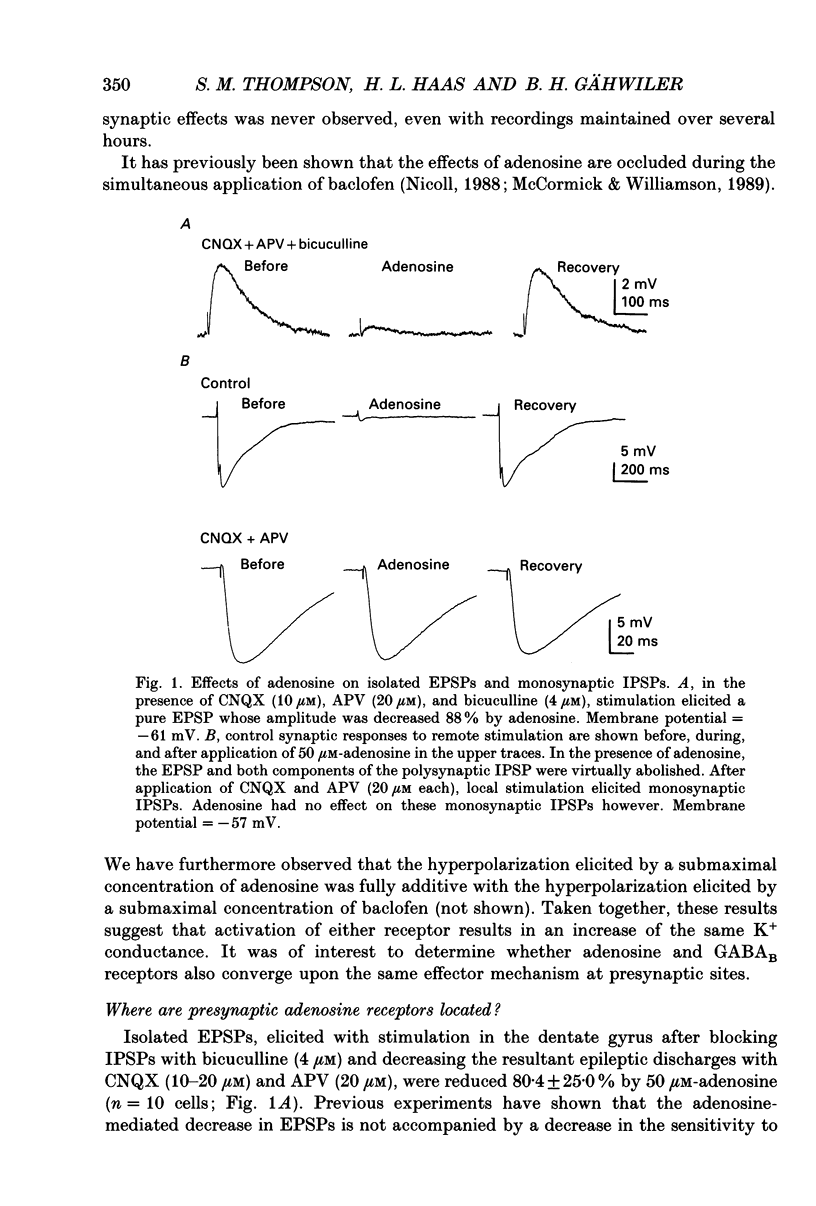
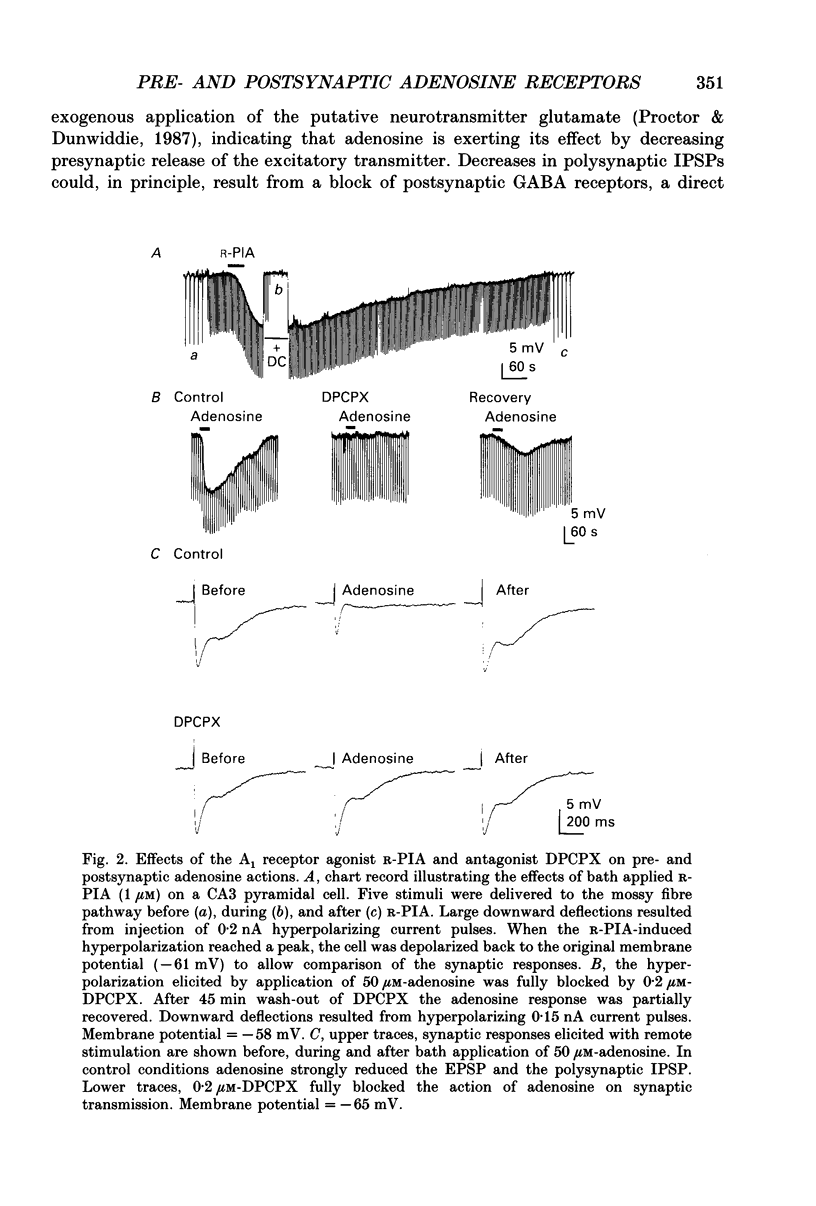
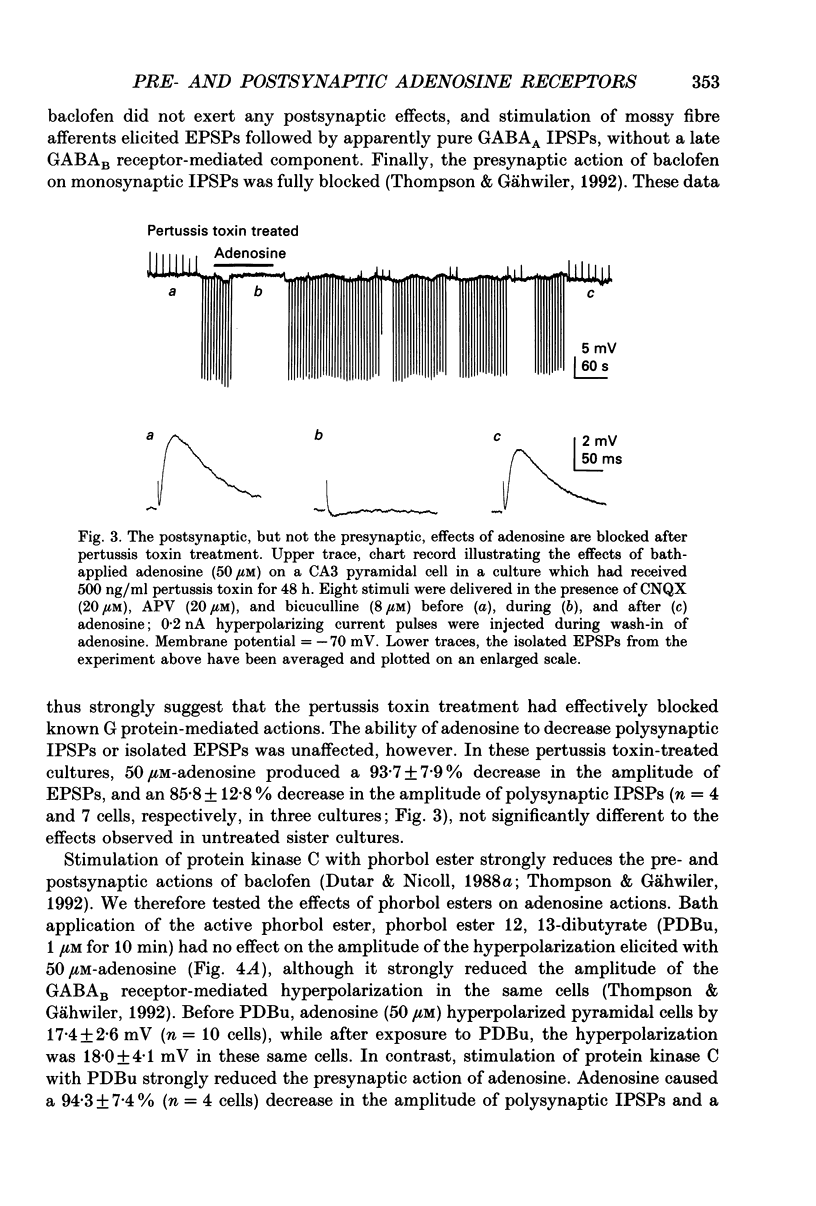
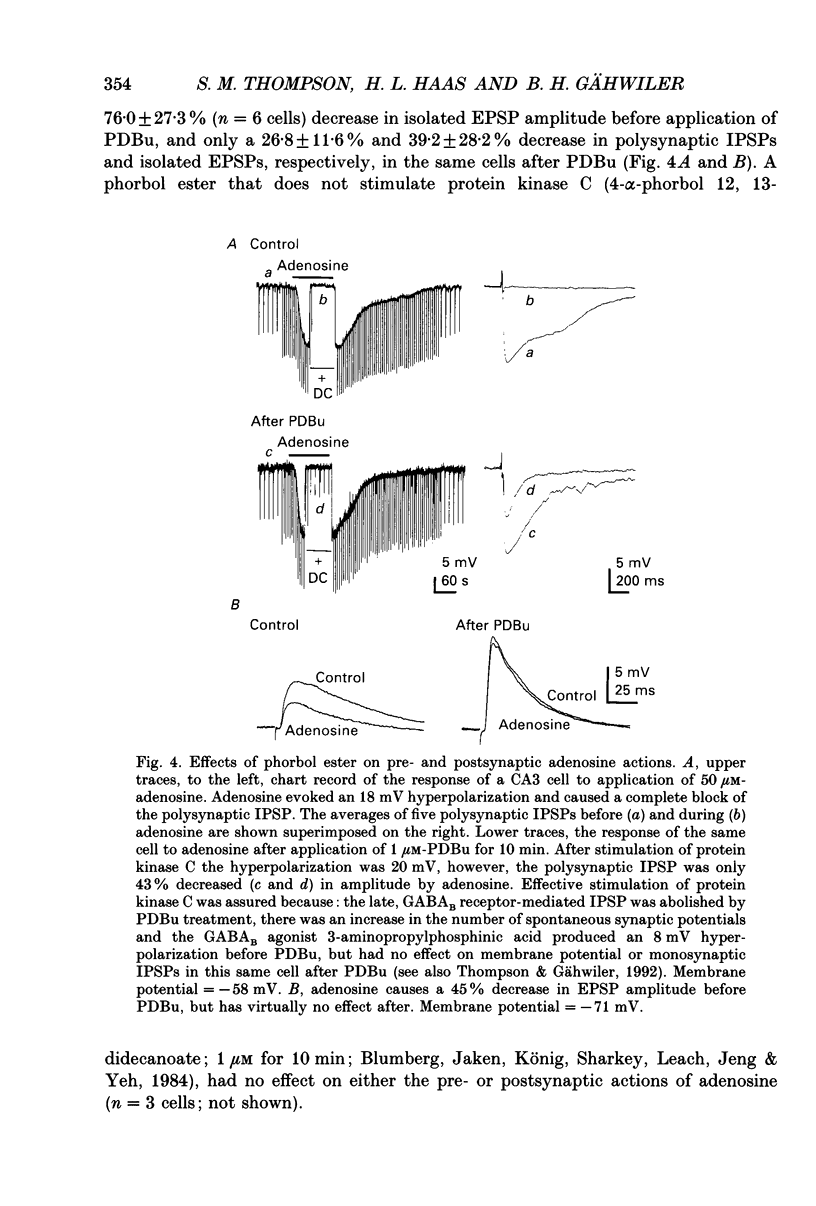
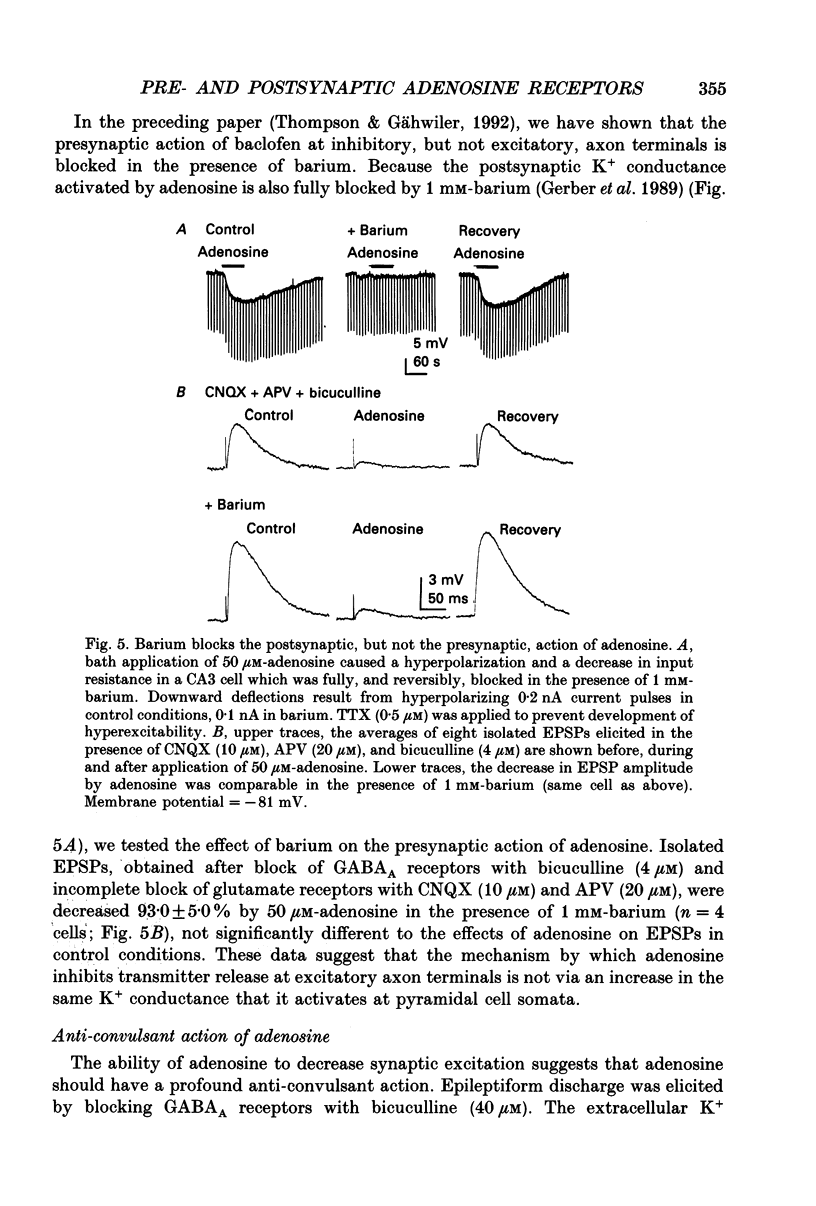
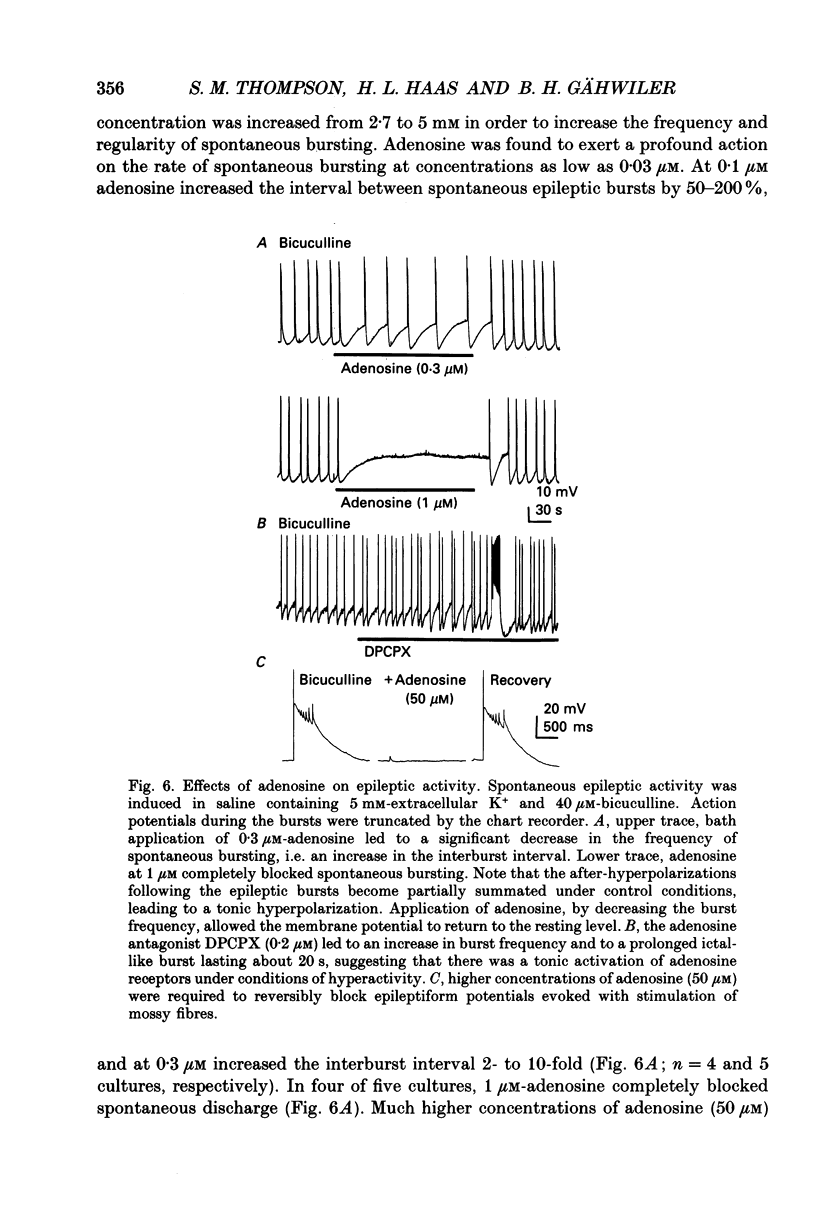
1. Intracellular microelectrode recordings were used to study the cellular location, the receptor pharmacology, and the mechanism of action of adenosine on pyramidal cells and presynaptic axonal endings in area CA3 of organotypic hippocampal slice cultures. 2. Adenosine (bath applied at 50 microM) caused a 10-15 mV hyperpolarization of CA3 cells, as well as a 75-100% decrease in the amplitude of excitatory and polysynaptic inhibitory postsynaptic potentials (EPSPs and IPSPs). Adenosine had no effect on the amplitude of monosynaptic IPSPs elicited in the presence of excitatory amino acid receptor antagonists, but did reduce the amplitude of isolated EPSPs, elicited after blocking GABAA receptors and reducing subsequent epileptic bursts with excitatory amino acid receptor antagonists. These data indicate that adenosine receptors are located on excitatory, but not inhibitory, presynaptic elements. 3. The A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, bath applied at 200 nM) blocked the pre- and postsynaptic actions of adenosine. DPCPX had no effect on the amplitude of control synaptic responses, suggesting that there is no tonic activation of adenosine receptors in hippocampal slice cultures under control conditions. The A1 receptor agonists R-N6-phenylisopropyladenosine (R-PIA) mimicked all pre- and postsynaptic actions of adenosine. 4. Pertussis toxin pretreatment (500 ng/ml for 48 h) prevented adenosine from activating postsynaptic K+ conductance, but not from inhibiting EPSPs. In contrast, stimulation of protein kinase C with phorbol ester (phorbol 12, 13-dibutyrate, 1 microM for 10 min) reduced the presynaptic, but not the postsynaptic, actions of adenosine. 5. Barium (bath applied at 1 mM) blocked the adenosine-activated K+ conductance, but not the inhibition of isolated EPSPs by adenosine. 6. Adenosine at 0.03-1 microM reduced the frequency of, or blocked, spontaneous epileptiform bursting produced by bicuculline. DPCPX (200 nM) increased the rate of spontaneous bursting, consistent with a tonic activation of adenosine receptors during hyperactivity, and led to the development of prolonged ictal-like bursts, suggesting that the endogenous release of adenosine may contribute to the termination of epileptic bursts. 7. We conclude that adenosine acts at pre- and postsynaptic receptors which are pharmacologically indistinguishable. Postsynaptically, adenosine increases a barium-sensitive K+ conductance via a pertussis toxin-sensitive GTP-binding protein. The presynaptic action of adenosine must, however, be mediated by some other mechanism.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzheimer C., Kargl L., ten Bruggencate G. Adenosinergic inhibition in hippocampus is mediated by adenosine A1 receptors very similar to those of peripheral tissues. Eur J Pharmacol. 1991 Apr 24;196(3):313–317. doi: 10.1016/0014-2999(91)90445-v. [DOI] [PubMed] [Google Scholar]
- Alzheimer C., Sutor B., ten Bruggencate G. Transient and selective blockade of adenosine A1-receptors by 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) causes sustained epileptiform activity in hippocampal CA3 neurons of guinea pigs. Neurosci Lett. 1989 Apr 24;99(1-2):107–112. doi: 10.1016/0304-3940(89)90273-5. [DOI] [PubMed] [Google Scholar]
- Ameri A., Jurna I. Adenosine A1 and non-A1 receptors: intracellular analysis of the actions of adenosine agonists and antagonists in rat hippocampal neurons. Brain Res. 1991 Apr 12;546(1):69–78. doi: 10.1016/0006-8993(91)91160-3. [DOI] [PubMed] [Google Scholar]
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Baraban J. M., Snyder S. H., Alger B. E. Protein kinase C regulates ionic conductance in hippocampal pyramidal neurons: electrophysiological effects of phorbol esters. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2538–2542. doi: 10.1073/pnas.82.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Chamberlin N. L., Traub R. D., Dingledine R. Role of EPSPs in initiation of spontaneous synchronized burst firing in rat hippocampal neurons bathed in high potassium. J Neurophysiol. 1990 Sep;64(3):1000–1008. doi: 10.1152/jn.1990.64.3.1000. [DOI] [PubMed] [Google Scholar]
- Colmers W. F., Pittman Q. J. Presynaptic inhibition by neuropeptide Y and baclofen in hippocampus: insensitivity to pertussis toxin treatment. Brain Res. 1989 Sep 25;498(1):99–104. doi: 10.1016/0006-8993(89)90403-4. [DOI] [PubMed] [Google Scholar]
- Doerner D., Pitler T. A., Alger B. E. Protein kinase C activators block specific calcium and potassium current components in isolated hippocampal neurons. J Neurosci. 1988 Nov;8(11):4069–4078. doi: 10.1523/JNEUROSCI.08-11-04069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Forda S. R., Scott R. H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol. 1986 Apr;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Prestwich S. A. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature. 1985 Jul 11;316(6024):148–150. doi: 10.1038/316148a0. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Calcium channel currents and their inhibition by (-)-baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol. 1987 May;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M. Purinergic mechanisms in epilepsy. Prog Neurobiol. 1988;31(2):85–108. doi: 10.1016/0301-0082(88)90028-7. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Fredholm B. B. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther. 1989 Apr;249(1):31–37. [PubMed] [Google Scholar]
- Dunwiddie T. V., Hoffer B. J., Fredholm B. B. Alkylxanthines elevate hippocampal excitability. Evidence for a role of endogenous adenosine. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jul;316(4):326–330. doi: 10.1007/BF00501365. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Taylor M., Cass W. A., Fitzpatrick F. A., Zahniser N. R. Arachidonic acid metabolites do not mediate modulation of neurotransmitter release by adenosine in rat hippocampus or striatum. Brain Res. 1990 Sep 10;527(1):76–80. doi: 10.1016/0006-8993(90)91062-l. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988 Nov;8(11):4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Franklin P. H., Zhang G., Tripp E. D., Murray T. F. Adenosine A1 receptor activation mediates suppression of (-) bicuculline methiodide-induced seizures in rat prepiriform cortex. J Pharmacol Exp Ther. 1989 Dec;251(3):1229–1236. [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V., Bergman B., Lindström K. Levels of adenosine and adenine nucleotides in slices of rat hippocampus. Brain Res. 1984 Mar 12;295(1):127–136. doi: 10.1016/0006-8993(84)90823-0. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Proctor W., Van der Ploeg I., Dunwiddie T. V. In vivo pertussis toxin treatment attenuates some, but not all, adenosine A1 effects in slices of the rat hippocampus. Eur J Pharmacol. 1989 Aug 15;172(3):249–262. doi: 10.1016/0922-4106(89)90055-2. [DOI] [PubMed] [Google Scholar]
- Gerber U., Greene R. W., Haas H. L., Stevens D. R. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J Physiol. 1989 Oct;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. W., Haas H. L. Adenosine actions on CA1 pyramidal neurones in rat hippocampal slices. J Physiol. 1985 Sep;366:119–127. doi: 10.1113/jphysiol.1985.sp015788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L., Ryan-Jastrow T. 2-Chloroadenosine reduces the N calcium current of cultured mouse sensory neurones in a pertussis toxin-sensitive manner. J Physiol. 1989 Apr;411:585–595. doi: 10.1113/jphysiol.1989.sp017592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981 Dec;4(4):329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Greene R. W. Endogenous adenosine inhibits hippocampal CA1 neurones: further evidence from extra- and intracellular recording. Naunyn Schmiedebergs Arch Pharmacol. 1988 May;337(5):561–565. doi: 10.1007/BF00182732. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Scholfield C. N. Somatically recorded Ca-currents in guinea-pig hippocampal and olfactory cortex neurones are resistant to adenosine action. Neurosci Lett. 1984 Sep 7;50(1-3):13–18. doi: 10.1016/0304-3940(84)90454-3. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D. 'Crosstalk': a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur J Biochem. 1991 Jan 1;195(1):9–27. doi: 10.1111/j.1432-1033.1991.tb15671.x. [DOI] [PubMed] [Google Scholar]
- Jarvis M. F., Williams M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989 Sep 13;168(2):243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Teyler T. J. Adenosine depresses excitatory but not fast inhibitory synaptic transmission in area CA1 of the rat hippocampus. Neurosci Lett. 1991 Jan 14;122(1):50–52. doi: 10.1016/0304-3940(91)90190-5. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. L., Skerritt J. H., Werz M. A. Adenosine agonists reduce voltage-dependent calcium conductance of mouse sensory neurones in cell culture. J Physiol. 1986 Jan;370:75–90. doi: 10.1113/jphysiol.1986.sp015923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Okada Y., Ozawa S. Inhibitory action of adenosine on synaptic transmission in the hippocampus of the guinea pig in vitro. Eur J Pharmacol. 1980 Dec 19;68(4):483–492. doi: 10.1016/0014-2999(80)90424-0. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Dunwiddie T. V. Adenosine inhibits calcium spikes in hippocampal pyramidal neurons in vitro. Neurosci Lett. 1983 Feb 21;35(2):197–201. doi: 10.1016/0304-3940(83)90550-5. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Dunwiddie T. V. Pre- and postsynaptic actions of adenosine in the in vitro rat hippocampus. Brain Res. 1987 Nov 17;426(1):187–190. doi: 10.1016/0006-8993(87)90441-0. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Miller R. J. Analysis of adenosine actions on Ca2+ currents and synaptic transmission in cultured rat hippocampal pyramidal neurones. J Physiol. 1991 Apr;435:373–393. doi: 10.1113/jphysiol.1991.sp018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Intracellular analysis of a postsynaptic action of adenosine in the rat hippocampus. Eur J Pharmacol. 1982 Apr 23;79(3-4):193–199. doi: 10.1016/0014-2999(82)90625-2. [DOI] [PubMed] [Google Scholar]
- Stratton K. R., Cole A. J., Pritchett J., Eccles C. U., Worley P. F., Baraban J. M. Intrahippocampal injection of pertussis toxin blocks adenosine suppression of synaptic responses. Brain Res. 1989 Aug 14;494(2):359–364. doi: 10.1016/0006-8993(89)90604-5. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn H. R., Welsh J. E., Rubio R., Berne R. M. Changes in brain adenosine during bicuculline-induced seizures in rats. Effects of hypoxia and altered systemic blood pressure. Circ Res. 1980 Oct;47(4):568–577. doi: 10.1161/01.res.47.4.568. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., McCarren M., Snyder S. H., Alger B. E. Cholinergic phosphatidylinositol modulation of inhibitory, G protein-linked neurotransmitter actions: electrophysiological studies in rat hippocampus. Proc Natl Acad Sci U S A. 1987 May;84(10):3467–3471. doi: 10.1073/pnas.84.10.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. W., Rothman S. M. Adenosine inhibits excitatory but not inhibitory synaptic transmission in the hippocampus. J Neurosci. 1991 May;11(5):1375–1380. doi: 10.1523/JNEUROSCI.11-05-01375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström T., Vernet L., Ungerstedt U., Tossman U., Jonzon B., Fredholm B. B. Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci Lett. 1982 Apr 16;29(2):111–115. doi: 10.1016/0304-3940(82)90338-x. [DOI] [PubMed] [Google Scholar]


