Abstract
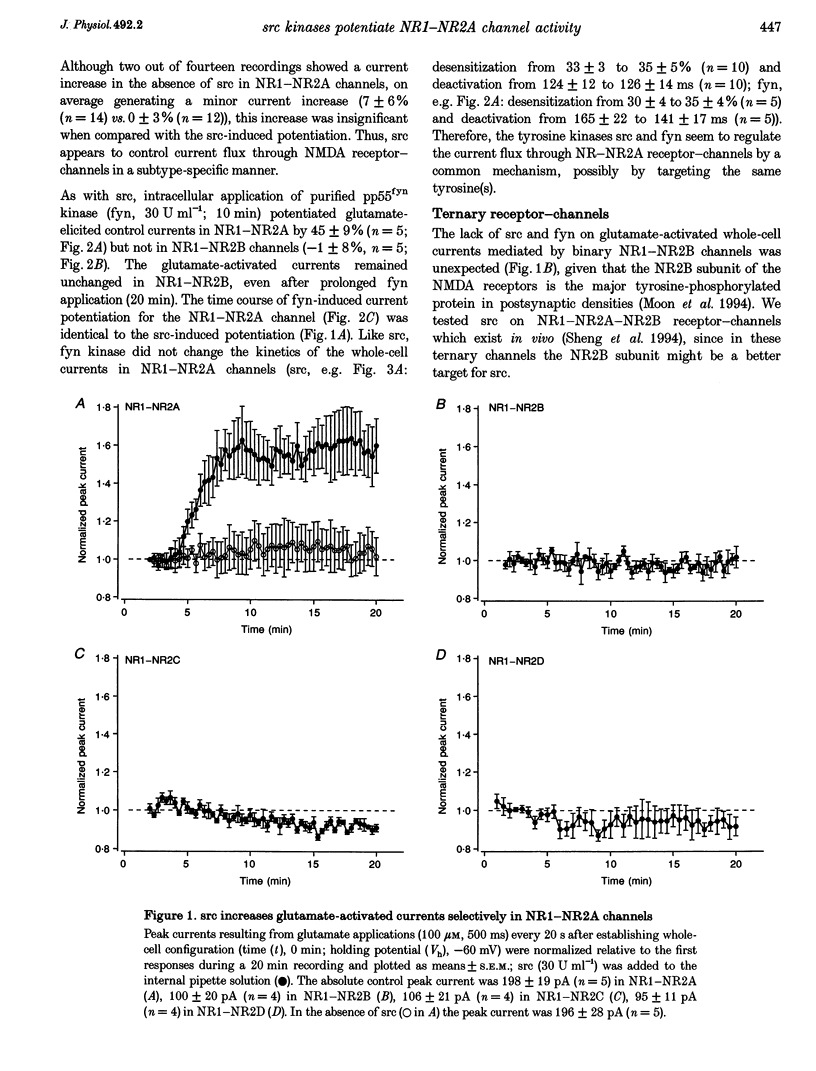
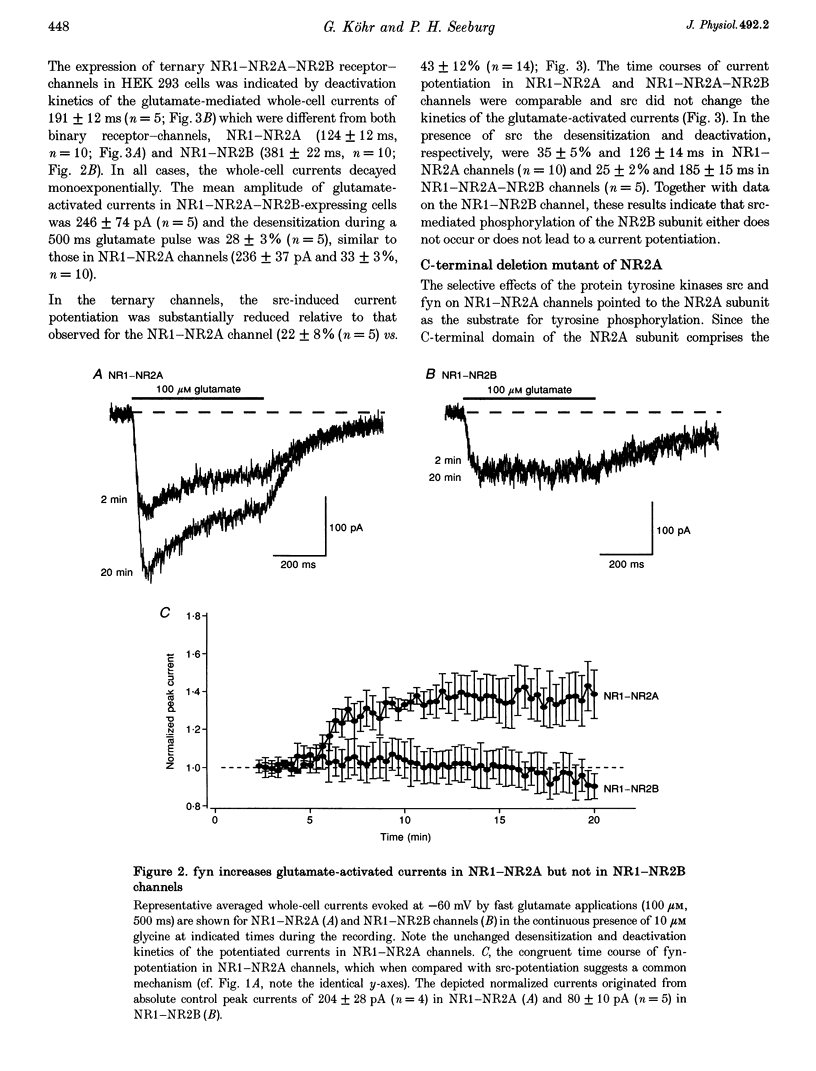
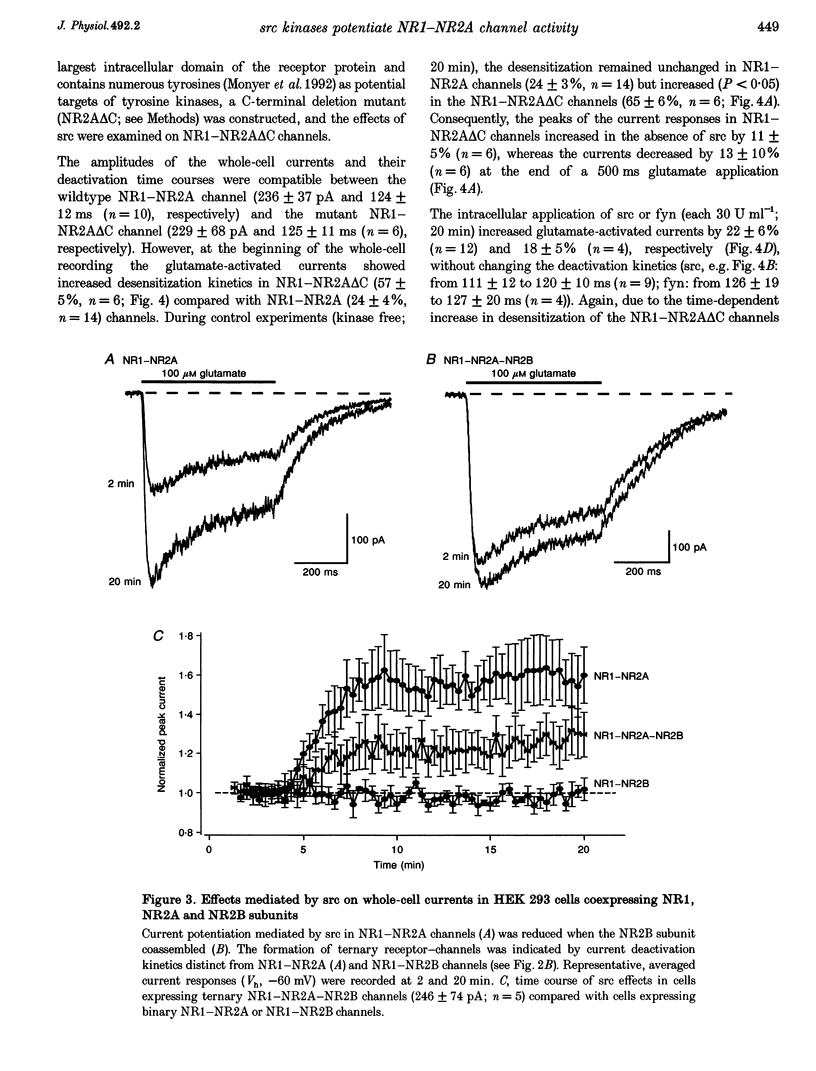
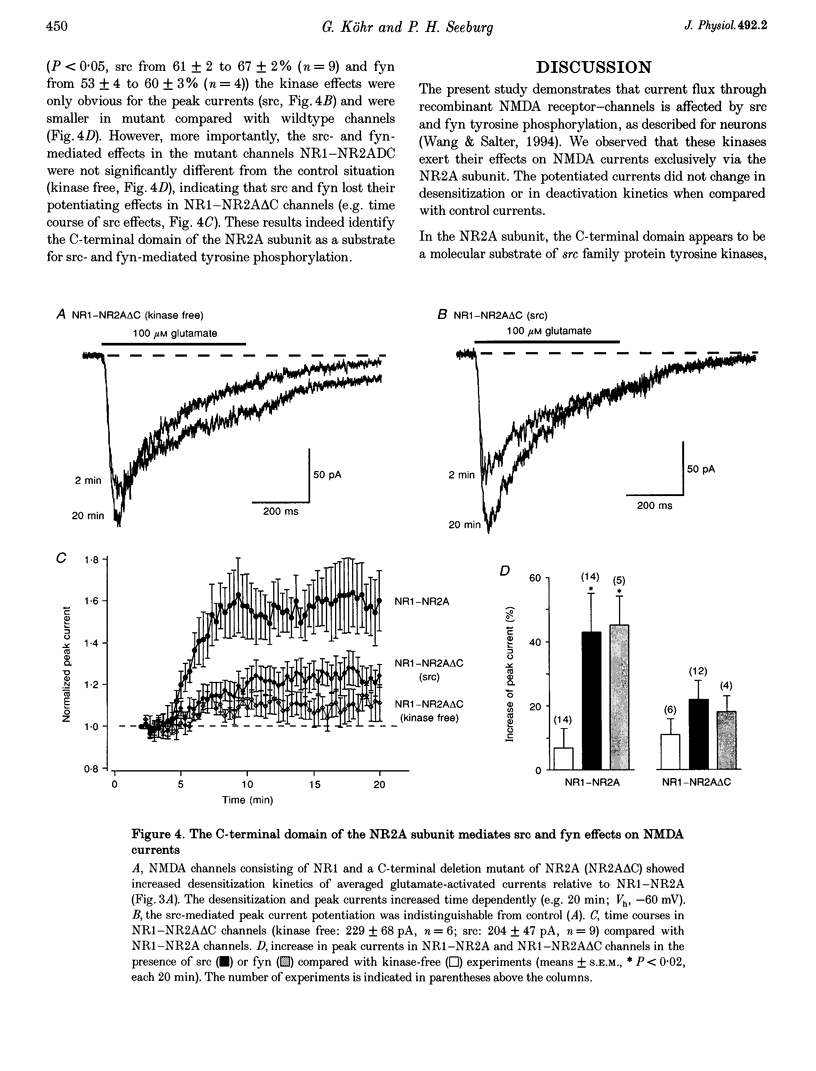
1. Tyrosine kinases regulate NMDA receptor-channel activity in cultured neurons, and NMDA receptor subunits are tyrosine phosphorylated in the brain. 2. Heteromeric NMDA receptor-channels were transiently expressed in human embryonic kidney (HEK) 293 cells and glutamate (100 microM)-activated whole-cell currents (500 ms) were studied when tyrosine kinases of the src gene family were included in the pipette solution. 3. Glutamate-activated currents (evoked every 20 s for up to 20 min) were increased by src and fyn kinases without affecting the desensitization and deactivation kinetics in NR1-NR2A but the kinases had no effects in NR1-NR2B, NR1-NR2C and NR1-NR2D receptor-channels, suggesting that a phosphorylation site in NR2A is targeted. 4. In a mutant channel consisting of NR1 and a C-terminal deletion mutant of NR2A (NR2A delta C), src and fyn kinases lost their potentiating effects indicating that the phosphorylation of tyrosine(s) in the C-terminal domain of NR2A affects the current flux through native NMDA receptor-channels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Mayer M. L. Structure and function of glutamate and nicotinic acetylcholine receptors. Curr Opin Neurobiol. 1995 Jun;5(3):310–317. doi: 10.1016/0959-4388(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Huang X. Y., Morielli A. D., Peralta E. G. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993 Dec 17;75(6):1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- Huganir R. L. Regulation of the nicotinic acetylcholine receptor by serine and tyrosine protein kinases. Adv Exp Med Biol. 1991;287:279–294. doi: 10.1007/978-1-4684-5907-4_23. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Köhr G., Eckardt S., Lüddens H., Monyer H., Seeburg P. H. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 1994 May;12(5):1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Huganir R. L. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. J Biol Chem. 1995 Aug 25;270(34):20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995 Aug 31;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Medina I., Filippova N., Charton G., Rougeole S., Ben-Ari Y., Khrestchatisky M., Bregestovski P. Calcium-dependent inactivation of heteromeric NMDA receptor-channels expressed in human embryonic kidney cells. J Physiol. 1995 Feb 1;482(Pt 3):567–573. doi: 10.1113/jphysiol.1995.sp020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moon I. S., Apperson M. L., Kennedy M. B. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss S. J., Gorrie G. H., Amato A., Smart T. G. Modulation of GABAA receptors by tyrosine phosphorylation. Nature. 1995 Sep 28;377(6547):344–348. doi: 10.1038/377344a0. [DOI] [PubMed] [Google Scholar]
- Roche K. W., Tingley W. G., Huganir R. L. Glutamate receptor phosphorylation and synaptic plasticity. Curr Opin Neurobiol. 1994 Jun;4(3):383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Rundown of N-methyl-D-aspartate channels during whole-cell recording in rat hippocampal neurons: role of Ca2+ and ATP. J Physiol. 1993 Oct;470:705–729. doi: 10.1113/jphysiol.1993.sp019884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Cummings J., Roldan L. A., Jan Y. N., Jan L. Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994 Mar 10;368(6467):144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A. Channel regulation. Ion channel control by tyrosine phosphorylation. Curr Biol. 1994 Mar 1;4(3):242–245. doi: 10.1016/s0960-9822(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Valenzuela C. F., Machu T. K., McKernan R. M., Whiting P., VanRenterghem B. B., McManaman J. L., Brozowski S. J., Smith G. B., Olsen R. W., Harris R. A. Tyrosine kinase phosphorylation of GABAA receptors. Brain Res Mol Brain Res. 1995 Jul;31(1-2):165–172. doi: 10.1016/0169-328x(95)00048-w. [DOI] [PubMed] [Google Scholar]
- Wang Y. T., Salter M. W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994 May 19;369(6477):233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]