Abstract
Advances in the study of mRNAs have yielded major new insights into post-transcriptional control of gene expression. Focus on the spatial regulation of mRNAs in highly polarized cells has demonstrated that mRNAs translocate through cells as mRNA:protein granules (mRNPs). These complex self-assemblies containing nuclear and cytoplasmic proteins are fundamental to the coordinated translation throughout cellular development. Initial studies on translational control necessitated fixed tissue, but the last 30 years have sparked innovative live-cell studies in several cell types to deliver a far more nuanced picture of how mRNA-protein dynamics exert translational control. In this review, we weave together the events that underpin mRNA processes and showcase the pivotal studies that revealed how a multitude of protein factors engage with a transcript. We highlight a mRNA's ability to act as a ‘super scaffold’ to facilitate molecular condensate formation and further moderate translational control. We focus on the Drosophila melanogaster germline due to the extensive post-transcriptional regulation occurring during early oogenesis. The complexity of the spatio-temporal expression of maternal transcripts in egg chambers allows for the exploration of a wide range of mechanisms that are crucial to the life cycle of mRNAs.
Keywords: condensates, Drosophila oogenesis, mRNA translational control, oskar, P bodies, post transcriptional gene expression regulation
Introduction
Spatio-temporal timing of mRNA translation is critical across eukaryotic life. Precise translational silencing and localization of mRNAs are highly dynamic processes orchestrated by nuclear and cytoplasmic proteins which are recruited to the transcripts to form mRNP granules. Forming phase-separated environments within the cytoplasm, these granules lead to translational repression while still permitting transport of mRNAs to specific regions. With its highly polarized organization, the Drosophila egg chamber provides an optimal inventory for the study of these dynamic and fundamental mRNA pathways.
Drosophila melanogaster Oogenesis
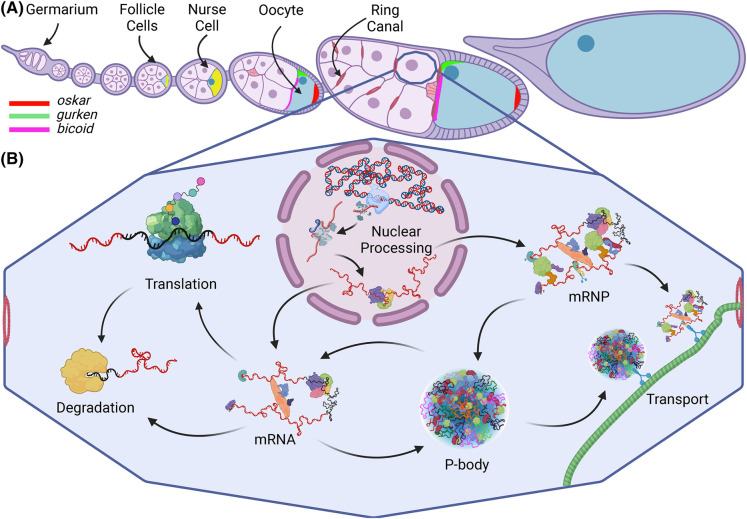
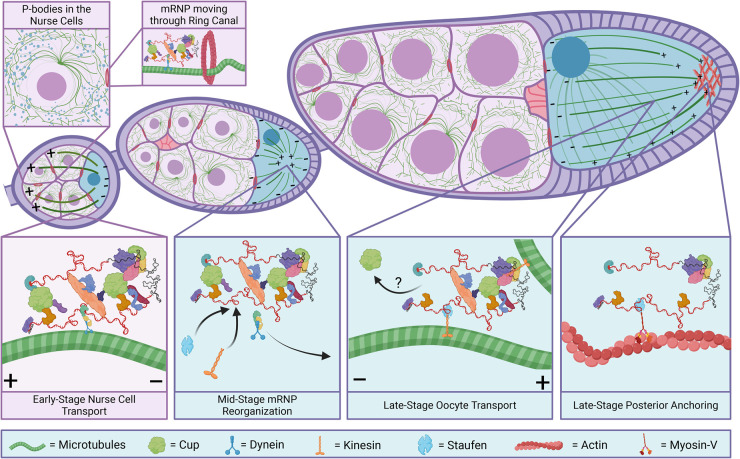
The female Drosophila germline comprises chains of multicellular egg chambers that develop through 14 stages. Egg chamber development begins at the anterior tip of the ovariole in the germarium, where germline stem cells undergo an initial asymmetric division to yield a new stem cell and a cystoblast. After four incomplete cell divisions, the cystoblast yields a germline cyst of 16 cells. One becomes the future oocyte while the other 15 develop into nurse cells. All 16 are linked by cytoplasmic bridges (ring canals) and are encapsulated by a monolayer of somatic follicle cells (Figure 1). The predominantly transcriptionally inactive oocyte receives from the nurse cells maternal mRNAs and proteins required for its future development (reviewed in [1,2]). Maternal mRNAs, therefore, relocate across significant distances via the cytoskeletal network to reach the oocyte. During their molecular voyage these mRNAs remain structurally intact and translationally repressed until cued for translation within the oocyte. Being large and transparent, egg chambers lend themselves to visualization via molecular probes and advanced microscopy, making them a uniquely popular and successful model for the study of post-transcriptional control.
Figure 1. Schematic of Drosophila melanogaster egg chamber development and mRNA life cycle.
(A) Ovariole chain of developing egg chambers depicting the pattern of localization in the oocyte of three key transcripts (oskar, gurken and bicoid) throughout oogenesis. Yellow represents the presence of all three mRNAs. (B) mRNA life cycle within a nurse cell. Created with BioRender.com.
Maternal mRNAs
A host of maternally deposited mRNAs must be localized and translated in the oocyte at precisely timed developmental stages to ensure proper embryogenesis. Coordination of this complex spatio-temporal regulation requires multiple levels of transcript-centered self-regulation throughout the mRNA's life cycle (Figure 1). Within the nucleus, primary mRNA sequences (cis-elements) mediate the direct binding of trans-acting RNA-binding proteins (RBPs) to promote nuclear egress and dictate the fate of each mRNA. Once in the cytoplasm, mRNA primary sequences and secondary structures recruit additional protein factors to generate unique mRNP macromolecular complexes. Many mRNAs are subsequently translated by ribosomes, while others are deposited at specialized cytoplasmic regions in translationally repressed states [3] (Figure 1).
Four crucial maternal transcripts that form mRNPs — oskar (osk), bicoid (bcd), gurken (grk), and nanos (nos) — have played important roles in enhancing our understanding of post-transcriptional gene regulation. For example, grk and its locally expressed protein Grk help establish the oocyte's body axes. Grk first influences the anterior-posterior axis (stages 5 and 6) followed by the dorsal-ventral axis (stage 7) [4]. Osk and nos mRNAs localize at the posterior pole while bcd mRNA is anchored at the oocyte's anterior cortex. These mRNAs remain repressed until they are translated during late stages of oogenesis (osk/nos) or early embryogenesis (bcd). Osk initiates future germline formation and, together with Nos and Bcd, establishes the embryonic anterior-posterior axis (reviewed in [5,6]). With over 250 publications on its regulation, osk mRNA is one of the most extensively studied and well-understood models of post-transcriptional regulation. Therefore, we selected it to serve as our case study for exploring various complex events occurring throughout the mRNA life cycle (Figure 1).
mRNA life cycle
Nuclear processing
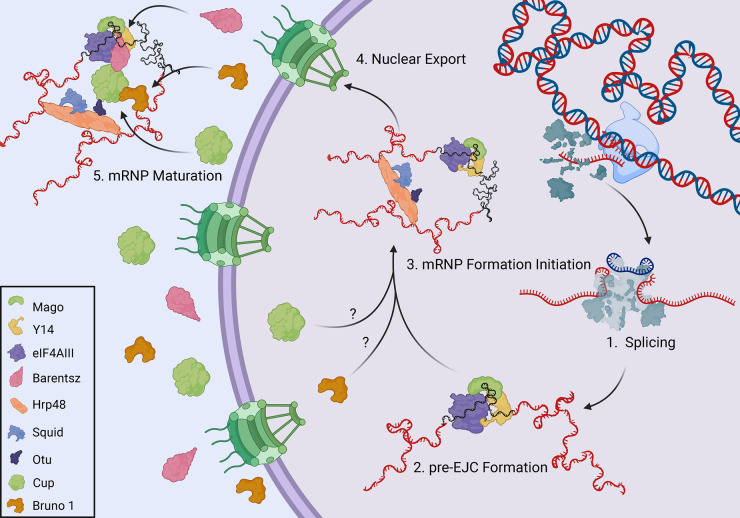
The ultimate fate of mRNA and the pathway it follows are inherent in the RNA primary sequence and are established co-transcriptionally through interactions with trans-acting nuclear proteins. This is exemplified by the formation of osk mRNP, which begins with the recruitment of the pre-Exon Junction Complex (EJC), consisting of eIF4AIII, Mago nashi (Mago), and Y14 to the transcript [7–9] (Figure 2). The presence of this complex at the junction of exons 1 and 2, upon proper splicing of intron 1, contributes to downstream events within the cytoplasm [10,11]. Hrp48 also joins osk mRNP, simultaneously binding both the 5′ and 3′ untranslated regions (UTR), thus contributing to the circularization of the transcript [12]. Hrp48 associates with nuclear proteins Otu and Squid, and together are important for the transport and localization of osk and grk mRNAs into the oocyte, reflecting a common step in these transcripts’ regulation. However, how these proteins are involved in this process is yet to be elucidated [13–15]. EJC formation is complete upon Barentsz binding at the cytoplasmic perinuclear region, which in turn recruits the motor protein Kinesin-1 to osk mRNP [16,17] (Figure 2).
Figure 2. Nuclear processing and export of osk mRNA.
Created with BioRender.com.
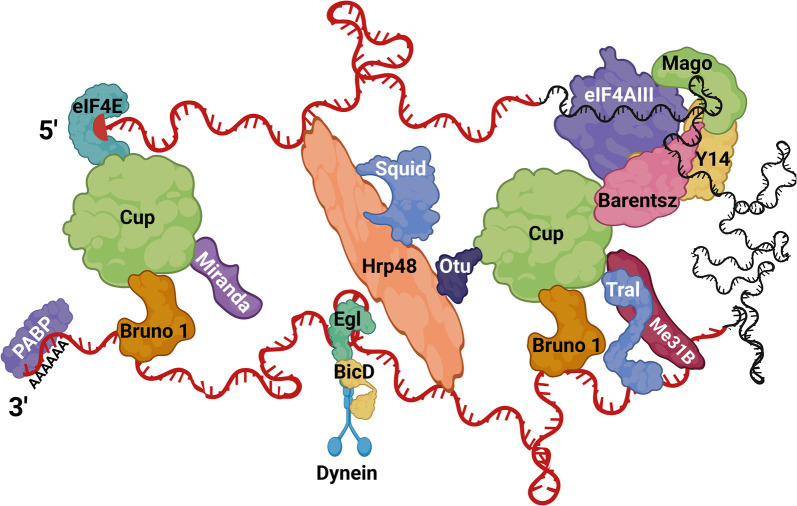
Cup is a key protein factor in the translational control of osk mRNA. Initial studies found that Cup genetically interacts with the Hrp48/Otu/Squid complex [15,18]. Furthermore, Cup localizes to the nucleus in tissue culture, and interacts with the nuclear pore complex protein Nup154, raising the possibility that it may be recruited to the complex as early as nuclear processing, perhaps initiating translational repression upon mRNA export [19,20]. Nevertheless, a recent study demonstrated that Cup is capable of direct mRNA binding, but whether Cup binds osk mRNA directly via its intrinsically disordered regions remains uncertain [21]. Moreover, proteins eIF4E and Bruno 1 may also recruit Cup to the osk mRNP [22]. As an eukaryotic translation initiation factor, eIF4E binds the m7G cap of mature mRNAs and is necessary for recruiting the translation initiation complex via binding eIF4G to facilitate cap-dependent translation [16]. Cup binds to the same site on eIF4E as eIF4G and can therefore inhibit the initiation of translation. Cup also directly binds Bruno 1 which is recruited to osk’s 3′-UTR via two Bruno Response Elements [23] (Figure 3). Both Bruno 1 and eIF4E directly bind Cup and lead to the formation of a translationally repressed mRNP. Our recent work suggests this complex may first assemble in the nucleus, an observation that needs further confirmation [24]. It has also been shown that Cup recruits Barentsz to osk mRNA, which would suggest a role for Cup in Kinesin-1 recruitment [16].
Figure 3. Hypothetical model of cytoplasmic osk mRNP.
A simplified translationally repressed osk mRNP is represented, where a subset of protein factors (not encompassing all of the literature) are recruited to different regions of the transcript, along with several factors binding in trans. 5′- and 3′- UTRs are depicted in red, and the coding region in black. Created with BioRender.com.
Cytoplasmic processing
mRNP formation
mRNAs slated for a specific region of the oocyte are transcribed in nurse cell nuclei and remain translationally silent, mediated primarily via their 3′-UTR cis-elements that recruit a cascade of numerous RBPs which take on ever more complex arrangements (reviewed in [25,26], Figure 3). Many mRNAs recruit the same proteins to maintain their stability and translational repression. For example, Bruno 1, which contains two RNA-recognition motifs (RRM), RRM 1 + 2 and an extended RRM 3, binds not only to osk mRNA but also to other maternal mRNAs including grk, cyclin A, sex lethal, and germ cell-less [27]. Bruno 1 dimerization promotes the formation of large particles that may also contribute to their translational silencing [28]. Particle formation via dimerization is not exclusive to Bruno 1, as osk 3′ UTR contains a stem–loop structure that promotes osk mRNA dimerization in vitro and its localization via ‘hitchhiking’ in vivo [29].
In a similar manner, Cup interacts with a variety of proteins and plausibly can join different mRNPs. To underscore this idea, Cup is known to interact with osk and grk mRNPs via Squid and PABP55 [18]. In addition, it is involved in cyclin A mRNA regulation possibly through Bruno 1 binding, as well as nos mRNA silencing, through Smaug, conceivably aiding in its activity [30–34]. The regulation of such diverse mRNAs by the same proteins poses an intriguing problem. A reasonable explanation may be that these proteins first associate with various mRNAs in the nurse cell cytoplasm as an evolutionarily conserved means of stabilizing the efficient and robust transport into the oocyte as silenced mRNP complexes. After entering the oocyte, individual mRNPs reorganize more narrowly to guide the specific relocation and translational timing of mRNAs.
Biomolecular condensates
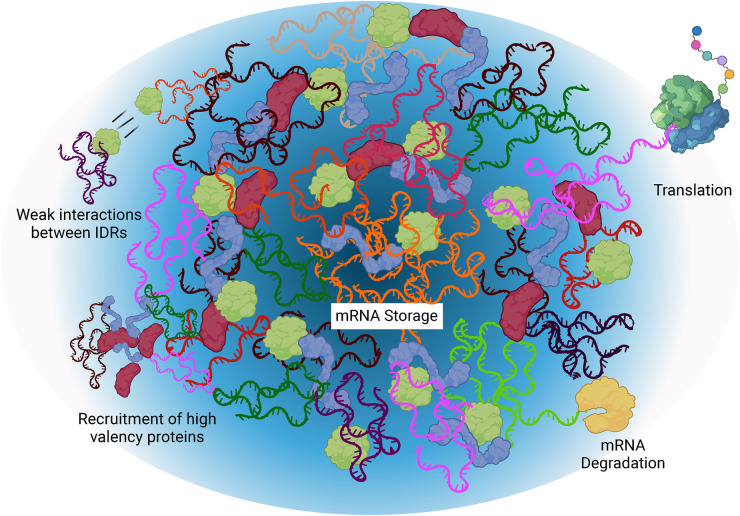
Many proteins that facilitate translational repression contain intrinsically disordered regions, which makes them highly conducive to undergoing liquid-liquid phase separation (LLPS). Separation occurs when a localized concentration of proteins and mRNAs diverges sufficiently from the surrounding cytoplasm to allow for thermodynamically favorable condensation into a separate phase of mRNPs [35–38]. Condensate formation adds another layer of regulation important for translational control since it drastically changes the availability of protein binding sites [39]. Importantly, the molecular environment of the separated phase can be attenuated rapidly by local cues to allow for dynamic changes in translational repression [40] (Figure 4).
Figure 4. Schematic of P-body condensate and the mRNA processes it supports.
Darker regions indicate the ‘core’ and lighter regions indicate the external ‘shell’. Created with BioRender.com.
mRNAs act as super-scaffolds bringing intrinsically disordered and high-valency proteins in close proximity thus nucleating condensation events [41,42]. The ‘high valency’ classification characterizes proteins that can bind many other proteins to bring about phase separation into membraneless organelles (MLO) such as P-bodies [43]. P-bodies are cytoplasmic, non-membrane bound organelles, crucial for normal cellular functions [44]. They are devoid of ribosomes and have a semi-ordered structure (reviewed in [45]) (Figure 4). In Caenorhabditis elegans and Drosophila germ granules, mRNAs exhibit self-demixing, and show a biphasic organization, where translation occurs in the outer phase, while mRNAs housed in the inner phase are translationally repressed, a mechanism that may similarly function in Drosophila P-bodies [46–52]. Maternal mRNAs exhibit differential levels of association with the P-body ‘core’ and ‘shell’ regions, which have been implied to correspond with their translational fate. For example, bcd mRNA is translationally silenced throughout oogenesis and localizes to the core, while grk mRNA must be poised for two rounds of translation and localizes to the shell region [53] (Figure 4).
Many protein components of the osk mRNP facilitate LLPS and have been shown to participate in P-body formation, including YPS, Trailer Hitch (Tral), Exuperantia, Me31B, Hsp90, and Ge1. Not surprisingly, these proteins are also implicated in osk mRNA silencing and its transport [54–59]. Interestingly, the osk transcript itself also has non-coding roles that support oogenesis, possibly in initiating P-body formation [60]. Furthermore, nucleotide mutations in osk mRNA can disrupt condensate formation in vitro, suggesting a direct role for mRNA sequences in condensate integrity [61].
P-bodies in the Drosophila germline contain proteins involved in several different aspects of RNA metabolism, including mRNA decapping activators DCP1, DCP2, and Ge1, DEAD-box RNA helicase Me31B, and translation repressors Tral and Cup amongst others (reviewed in [45]). A core component of P-bodies, Me31B was first identified as a gene essential for egg chamber development [62]. Me31B is also implicated in the translational regulation of several maternal mRNAs: osk, bcd, grk, bic-D, nos, orb, polar granule component, and germ cell-less [40,53,58,63,64]. Moreover, Me31B is important for translational silencing of osk mRNA during its transport, with this interaction likely occuring in P-bodies [24,58].
Cross-regulation seems to be a hallmark of P-bodies, as P-body proteins have been shown to regulate the expression of other P-body mRNAs that may be mediated transcriptionally, within the P-body, or through another mechanism. One such example is Me31B, where tral and cup mRNA levels decreased in me31B mutant egg chambers indicating a yet-to-be-elucidated feedback loop [65,66]. A complex cycle of regulation also extends to Cup, which associates with the protein products of the mRNAs it represses. For example, it associates with Osk protein at the posterior, where Osk is necessary for the translational activation of nos mRNA [67]. Once translated, Cup interacts with Nos protein to promote normal development of the ovarian germline [68].
Egg chambers contain a variety of MLOs, such as the perinuclear nuage in nurse cell cytoplasm and the germ granules in the oocyte (reviewed in [69]). Most other cytoplasmic MLOs not induced by stress come under the general category of ‘P-body’. Unfortunately, this simplistic classification is misleading since P-bodies exhibit substantial heterogeneity. Their complex composition with diverse proteins determines their various functions; for example, Tral and EDC3 (enhancer of Decapping 3) compete for the same FDF pocket on Me31B, making their binding mutually exclusive. When the ratio of EDC3 is higher than Tral in the P-body, mRNA degradation is favored, as EDC3 can also recruit DCP2 (Decapping protein 2). Similarly, if the ratio of Tral is higher than EDC3, Tral can recruit DCP1 (Decapping protein 1) to the P-body and leads to degradation as well. Further convolving the picture of condensate composition, Tral binds either Cup or DCP1 at the same LSm site [70]. Thus, P-bodies that contain Cup, in turn lead to translational silencing and stable mRNA storage [70]. Cup recruits the deadenylase complex CAF1-CCR4-NOT and leads to deadenylation of mRNAs while suppressing the decay activity of CAF1-CCR4-NOT itself [71]. Shortening of poly(A) tails is a widely conserved regulatory mechanism especially for maternal mRNAs, further ensuring their translational repression (reviewed in [72]). Although comprising distinct protein arrays, these MLOs are still identified simply as P-bodies. More accurate naming would reflect the fact that condensates are molecular decision-making centers where transcripts linger stably either in ‘repression-bodies’ (‘r-body’, such as Me31B-Tral-Cup) until translated or in ‘degradation-bodies’ (‘d-body’, such as Me31B-Tral-DCP1 and Me31B-EDC3-DCP2) until destined for decay.
mRNP localization
Asymmetric localization of mRNA is intrinsic to many fundamental processes, including cell motility, synaptic plasticity, and organism development. In Drosophila embryos 71% of mRNAs were found to localize asymmetrically, implying a global cellular phenomenon [73]. Drosophila oogenesis is no exception. An unbiased global analysis of mRNA localization by Jambor et al. generated a comprehensive resource, the Dresden Ovary Table, that significantly expands research support into intracellular areas of mRNA concentrations [74]. Among the 3475 mRNAs they identified to be expressed during oogenesis, 35% exhibit subcellular localization. On average, localized mRNAs, interestingly but not surprisingly, carry longer 3′-UTRs that suggest a more elaborate post-transcriptional regulation. At the same time, mRNAs localized at the posterior region also carry longer 5′-UTRs, more numerous introns and exons, and are expressed at significantly higher levels. Enrichment of non-coding regions suggests that these localized mRNAs perform a function apart from translation of their protein products alone [74].
Biological condensates provide plausible mechanisms for asymmetric mRNA localization. Since condensates contain multiple RNPs, their coupling to the microtubule network may be more energetically efficient than individual RNP transport (Figure 5). Consistent with this view is the association of the microtubular motor protein Dynein with P-bodies, hinting at a role for P-bodies in long-distance localization [75]. A recent study by Baker et al. supports this connection by showing that Egalitarian, a Dynein adaptor protein, directly interacts with Me31B [76]. Drosophila oogenesis serves as a model for this mode of directed transport since normal cell function requires transcript movement over considerable distances at rates greater than passive diffusion can provide. However, some mRNPs, for example grk and osk, are known to move via microtubules independently of condensates, leading some to conclude that condensates are a consequence of normal mRNA movement [77]. More likely, many mRNPs may translocate from nurse cells to the oocyte by varying means that reflect the dynamic nature of the microtubule network.
Figure 5. The microtubule network and mRNP transport throughout oogenesis.
Created with BioRender.com.
During stages 2–6 of egg chamber development, the microtubular network is arranged with its minus (−) ends within the oocyte while the plus (+) ends extend into the nurse cells (Figure 5). Transcripts therefore associate with (−)-directed motor proteins to enter the oocyte. At stage 6, signals from Grk to the somatic follicle cells cause the microtubules to reorganize, orienting the microtubule (+) ends toward the center of the oocyte. Starting at stage 8, localized inhibition of PAR-1 kinase diminishes microtubule nucleation at the oocyte posterior resulting in the redirection of microtubule (+) ends toward the posterior egg chamber for the remainder of late oogenesis, and concludes with nurse cell dumping and ooplasmic streaming [78,79].
Localization of mRNAs relies on cis-elements in the mRNA (mostly in the 3′-UTR) bound by trans-acting RBPs that can further recruit other factors in fine-tuning mRNA regulation (reviewed in [80,81]). These mRNPs can self-regulate their own dynamic participation in active transport via motor proteins on microtubules, along with passive transport by cytoplasmic streaming and/or localized degradation. Localizing elements in the transcript can take a variety of forms; for example, primary sequences or complex secondary structures that act as destination zip codes within the cytoplasm (reviewed in [3,82–84]). Yet, these sequences alone do not fully explain mRNA localization; Jambor et al. have demonstrated differential localization at various developmental stages even without changes in the primary sequence [74].
How cells resolve this divergent localization continues to be a perplexing process, but the emerging picture indicates that an exchange of mRNP factors must be involved. Once again, osk mRNA serves as an example: its transport from nurse cells to the oocyte until stage 6 is facilitated mainly by the Bicaudal-D/Egalitarian/Dynein complex that distributes osk mRNA into the oocyte [77,85–87]. Microtubules rearrange during stages 7 and 8, and osk mRNA concentrates in the middle of the oocyte. By stage 9, microtubule (+) ends become slightly enriched at the posterior of the oocyte, thereby ensuring that osk mRNA, now transported by Kinesin-1, reaches its proper location [88,89] (Figure 5).
Staufen, a double-stranded RBP, mediates the switch from Dynein to Kinesin-1 and is responsible for osk mRNA localization to the posterior cortex of the oocyte [90,91]. Staufen associates with the complex only after the mRNP reaches the oocyte and the microtubules undergo reorganization [92]. After Staufen joins the complex, Egalitarian dissociates from the mRNP to allow for the switch to Kinesin-1 [91,93]. Once the complex reaches the posterior of the oocyte, an actin motor protein, Myosin-V, mediates osk mRNA anchoring by out-competing Kinesin-1 due to the low concentration of microtubules [94,95]. Similar competition between two motor proteins was observed in neurons [96]. These molecular orchestrations ensure proper cortical localization of osk mRNA along with its anchoring at the posterior cortex and persistent maintenance by the long Osk isoform to actin filaments [94].
By contrast, at the oocyte's anterior half, a higher density of microtubules favors Staufen binding followed by the transport of osk mRNA toward the posterior of the oocyte [92,94,97]. Staufen is also involved in the anterior localization of bcd mRNA [98]. How Staufen accomplishes localization of bcd and osk mRNAs to opposite ends of the oocyte remains an enigma. Different mRNP components, for example Miranda, may point to an explanation for this juxtaposition of roles. Miranda-GFP expression in the female germline leads to an ectopic localization of Staufen/osk mRNA complex at the anterior due to coupling between Staufen/osk and the bcd mRNA localization pathway [99]. Compellingly, Cup directly interacts with Miranda and also associates with Staufen at the same sites where Miranda/Staufen associates, leading to a complex, hypothetical model where Miranda cannot bind to Staufen in the presence of Cup, thereby allowing correct localization of osk mRNA to the posterior [100]. It would be interesting to determine whether egg chambers that express Miranda-GFP also exhibit reduced colocalization of Cup with osk mRNA. A second plausible explanation involves Exuperantia, which is necessary for the anterior localization of bcd mRNA, but it is not thought to be a member of the osk mRNP. In addition, bcd mRNA anchoring in the anterior depends on Dynein and is independent of the oocyte's microtubule network [101–103].
Further underscoring this conceptualization, the Bono group determined that there is a highly complex network of six evolutionarily conserved RBPs (Staufen, Vasa, eIF4AIII, Nanos, Hrp48 and Glorund) that are crucial for the regulation of the four patterning mRNAs [104]. Their research illuminates the complex interplay between these RBPs and how they collectively orchestrate the differential cytoplasmic fates of mRNAs. For instance, while Cup is a crucial component of both grk and osk mRNPs, Staufen is only part of osk mRNP [16–18,92,97]. This exemplifies the regulation of grk and osk mRNAs to involve unique sets of RBPs and suggests that the differential functions of these mRNPs resides within the distinct selection and incorporation of various factors.
Translation
Once the mRNP complex reaches its destination, translational de-repression is triggered by mechanisms that remain poorly understood during oogenesis. The silencing factors must be removed and the mRNP undergoes reorganization. This mechanism is better grasped in the early embryo, where during the maternal-to-zygotic transition, an influx of signals leads to the degradation of P-body proteins and a general release from translational repression of the transcripts contained in the condensate [105]. This level of regulation means that a wide array of transcripts can be readily released simultaneously from translational repression. A caveat to this mechanism is that alterations in the cellular environment, such as changes in salt concentration or ATP depletion, can result in the hardening or dissolution of condensates, thereby impacting the proper post-transcriptional regulation of mRNA and leading to pathological outcomes [106,107].
Perspectives
Translational control is a complex process epitomized in the Drosophila melanogaster germline where maternal mRNAs are precisely and dynamically regulated at the post-transcriptional level. Not surprisingly, many aspects of RNA processing, including RNA export, localization and translational repression, are now known to be dysregulated in, and contribute to, numerous diseases, such as neurodegeneration, cancer and infectious diseases [108,109]. MLO continue to emerge in disease etiology, thus, it is important to understand the basic mechanisms governing post-transcriptional control and how these processes are converted to drive disease. D. melanogaster egg chambers provide an exceptional system for studying such aspects of basic mRNA biology. Their genetic malleability, together with numerous genetic toolkits as well as the ease of manipulation of a developing multicellular system, allow for specialized techniques such as super-resolution visualization and in vivo biochemical interactome assays, making them ideal to carry out such research.
RNP granules play key roles in organizing spatial and temporal regulation of RNA-dependent processes. The diversity is supplied by the dynamic exchanges of trans-acting proteins that are orchestrated throughout an mRNA's life cycle. Cytoplasmic organelles without membranes are now center-stage players in cell biology, especially where multifaceted mRNA regulation (localization, translation, and stability) occurs. The D. melanogaster egg chamber serves as a model for long-range mRNA cytoplasmic transport and presents an innovative vehicle to enhance our understanding of both mRNP cellular transport and interactions with P-bodies.
We have much to learn about the communication between the layers of mRNA regulation and the precise mechanisms underlying translational control. As technological and imaging advances continue to enlighten the world of molecular and cellular biology, new details of mRNA dynamics become unveiled. In contrast to cultured cells, D. melanogaster egg chambers contain substantially more P-bodies, which are readily visualized and can be chemically and genetically manipulated. These unique characteristics position them ideally to resolve some of the most persistent and outstanding questions in mRNA biology at the post-transcriptional level. Future work in polarized tissues can provide an important insight into MLOs' biological functions, which are much debated and still poorly understood. Deciphering how cells manage mRNA regulation in P-bodies will, therefore, be crucial to improve the management of pathological processes such as cancer progression, chemo-resistance and viral infections [45,110,111].
Acknowledgements
Due to space limitations, we apologize to those whose primary work could not be cited. We thank all members of the Bratu lab for their helpful comments. We are very grateful to Dr. Roger Persell and Dr. John McLaughlin for their editorial input and suggestions. All figures were created using the BioRender software. Funding provided by National Institute of Health (SC1GM135132) to D.P.B.
Abbreviations
- LLPS
liquid-liquid phase separation
- MLO
membrane-less organelles
- RBP
RNA-binding protein
- RRM
RNA-recognition motifs
- UTR
untranslated regions
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Hinnant, T.D., Merkle, J.A. and Ables, E.T. (2020) Coordinating proliferation, polarity, and cell fate in the Drosophila female germline. Front. Cell Dev. Biol. 8, 19 10.3389/fcell.2020.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin, J.M. and Bratu, D.P. (2015) Drosophila melanogaster oogenesis: an overview. Methods Mol. Biol. 1328, 1–20 10.1007/978-1-4939-2851-4_1 [DOI] [PubMed] [Google Scholar]
- 3.Singh, G., Pratt, G., Yeo, G.W. and Moore, M.J. (2015) The clothes make the mRNA: past and present trends in mRNP fashion. Annu. Rev. Biochem. 84, 325–354 10.1146/annurev-biochem-080111-092106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth, S. and Lynch, J.A. (2009) Symmetry breaking during Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 1, a001891 10.1101/cshperspect.a001891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato, Y. and Nakamura, A. (2012) Roles of cytoplasmic RNP granules in intracellular RNA localization and translational control in the Drosophila oocyte. Dev. Growth Differ. 54, 19–31 10.1111/j.1440-169X.2011.01314.x [DOI] [PubMed] [Google Scholar]
- 6.Lasko, P. (2012) mRNA localization and translational control in Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 4, a012294 10.1101/cshperspect.a012294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewandowski, J.P., Sheehan, K.B., Bennett, Jr, P.E. and Boswell, R.E. (2010) Mago Nashi, Tsunagi/Y14, and Ranshi form a complex that influences oocyte differentiation in Drosophila melanogaster. Dev. Biol. 339, 307–319 10.1016/j.ydbio.2009.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parma, D.H., Bennett, Jr, P.E. and Boswell, R.E. (2007) Mago Nashi and Tsunagi/Y14, respectively, regulate Drosophila germline stem cell differentiation and oocyte specification. Dev. Biol. 308, 507–519 10.1016/j.ydbio.2007.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hachet, O. and Ephrussi, A. (2001) Drosophila Y14 shuttles to the posterior of the oocyte and is required for oskar mRNA transport. Curr. Biol. 11, 1666–1674 10.1016/s0960-9822(01)00508-5 [DOI] [PubMed] [Google Scholar]
- 10.Eichler, C.E., Li, H., Grunberg, M.E. and Gavis, E.R. (2023) Localization of oskar mRNA by agglomeration in ribonucleoprotein granules. PLoS Genet. 19, e1010877 10.1371/journal.pgen.1010877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh, S., Marchand, V., Gaspar, I. and Ephrussi, A. (2012) Control of RNP motility and localization by a splicing-dependent structure in oskar mRNA. Nat. Struct. Mol. Biol. 19, 441–449 10.1038/nsmb.2257 [DOI] [PubMed] [Google Scholar]
- 12.Huynh, J.R., Munro, T.P., Smith-Litière, K., Lepesant, J.A. and St Johnston, D. (2004) The Drosophila hnRNPA/B homolog, Hrp48, is specifically required for a distinct step in osk mRNA localization. Dev. Cell 6, 625–635 10.1016/s1534-5807(04)00130-3 [DOI] [PubMed] [Google Scholar]
- 13.Goodrich, J.S., Clouse, K.N. and Schupbach, T. (2004) Hrb27c, Sqd and Otu cooperatively regulate gurken RNA localization and mediate nurse cell chromosome dispersion in Drosophila oogenesis. Development 131, 1949–1958 10.1242/dev.01078 [DOI] [PubMed] [Google Scholar]
- 14.Norvell, A., Debec, A., Finch, D., Gibson, L. and Thoma, B. (2005) Squid is required for efficient posterior localization of oskar mRNA during Drosophila oogenesis. Dev. Genes Evol. 215, 340–349 10.1007/s00427-005-0480-2 [DOI] [PubMed] [Google Scholar]
- 15.Keyes, L.N. and Spradling, A.C. (1997) The Drosophila gene fs(2)cup interacts with otu to define a cytoplasmic pathway required for the structure and function of germ-line chromosomes. Development 124, 1419–1431 10.1242/dev.124.7.1419 [DOI] [PubMed] [Google Scholar]
- 16.van Eeden, F.J., Palacios, I.M., Petronczki, M., Weston, M.J. and St Johnston, D. (2001) Barentsz is essential for the posterior localization of oskar mRNA and colocalizes with it to the posterior pole. J. Cell Biol. 154, 511–523 10.1083/jcb.200105056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilhelm, J.E., Hilton, M., Amos, Q. and Henzel, W.J. (2003) Cup is an eIF4E binding protein required for both the translational repression of oskar and the recruitment of Barentsz. J. Cell Biol. 163, 1197–1204 10.1083/jcb.200309088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clouse, K.N., Ferguson, S.B. and Schupbach, T. (2008) Squid, Cup, and PABP55B function together to regulate gurken translation in Drosophila. Dev. Biol. 313, 713–724 10.1016/j.ydbio.2007.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zappavigna, V., Piccioni, F., Villaescusa, J.C. and Verrotti, A.C. (2004) Cup is a nucleocytoplasmic shuttling protein that interacts with the eukaryotic translation initiation factor 4E to modulate Drosophila ovary development. Proc. Natl Acad. Sci. U.S.A. 101, 14800–14805 10.1073/pnas.0406451101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimaldi, M.R., Cozzolino, L., Malva, C., Graziani, F. and Gigliotti, S. (2007) Nup154 genetically interacts with Cup and plays a cell-type-specific function during Drosophila melanogaster egg-chamber development. Genetics 175, 1751–1759 10.1534/genetics.106.062844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pekovic, F., Rammelt, C., Kubíková, J., Metz, J., Jeske, M. and Wahle, E. (2023) RNA binding proteins Smaug and Cup induce CCR4-NOT-dependent deadenylation of the nanos mRNA in a reconstituted system. Nucleic Acids Res. 51, 3950–3970 10.1093/nar/gkad159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura, A., Sato, K. and Hanyu-Nakamura, K. (2004) Drosophila Cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell 6, 69–78 10.1016/s1534-5807(03)00400-3 [DOI] [PubMed] [Google Scholar]
- 23.Snee, M., Benz, D., Jen, J. and Macdonald, P.M. (2008) Two distinct domains of Bruno bind specifically to the oskar mRNA. RNA Biol. 5, 1–9 10.4161/rna.5.1.5735 [DOI] [PubMed] [Google Scholar]
- 24.Bayer, L.V., Milano, S., Formel, S.K., Kaur, H., Ravichandran, R., Cambeiro, J.A.et al. (2023) Cup is essential for oskar mRNA translational repression during early Drosophila oogenesis. RNA Biol. 20, 573–587 10.1080/15476286.2023.2242650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhukova, M., Schedl, P. and Shidlovskii, Y.V. (2024) The role of secondary structures in the functioning of 3′ untranslated regions of mRNA: a review of functions of 3′ UTRs’ secondary structures and hypothetical involvement of secondary structures in cytoplasmic polyadenylation in Drosophila. Bioessays 46, e2300099 10.1002/bies.202300099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuersten, S. and Goodwin, E.B. (2003) The power of the 3’ UTR: translational control and development. Nat. Rev. Genet. 4, 626–637 10.1038/nrg1125 [DOI] [PubMed] [Google Scholar]
- 27.Reveal, B., Garcia, C., Ellington, A. and Macdonald, P.M. (2011) Multiple RNA binding domains of Bruno confer recognition of diverse binding sites for translational repression. RNA Biol. 8, 1047–1060 10.4161/rna.8.6.17542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chekulaeva, M., Hentze, M.W. and Ephrussi, A. (2006) Bruno acts as a dual repressor of oskar translation, promoting mRNA oligomerization and formation of silencing particles. Cell 124, 521–533 10.1016/j.cell.2006.01.031 [DOI] [PubMed] [Google Scholar]
- 29.Jambor, H., Brunel, C. and Ephrussi, A. (2011) Dimerization of oskar 3′ UTRs promotes hitchhiking for RNA localization in the Drosophila oocyte. RNA 17, 2049–2057 10.1261/rna.2686411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimura, I. and Lilly, M.A. (2006) Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev. Cell 10, 127–135 10.1016/j.devcel.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 31.Dahanukar, A. and Wharton, R.P. (1996) The Nanos gradient in Drosophila embryos is generated by translational regulation. Genes Dev. 10, 2610–2620 10.1101/gad.10.20.2610 [DOI] [PubMed] [Google Scholar]
- 32.Sinsimer, K.S., Jain, R.A., Chatterjee, S. and Gavis, E.R. (2011) A late phase of germ plasm accumulation during Drosophila oogenesis requires Lost and Rumpelstiltskin. Development 138, 3431–3440 10.1242/dev.065029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macdonald, P.M. and Smibert, C.A. (1996) Translational regulation of maternal mRNAs. Curr. Opin. Genet. Dev. 6, 403–407 10.1016/s0959-437x(96)80060-8 [DOI] [PubMed] [Google Scholar]
- 34.Nelson, M.R., Leidal, A.M. and Smibert, C.A. (2004) Drosophila Cup is an eIF4E-binding protein that functions in Smaug-mediated translational repression. EMBO J. 23, 150–159 10.1038/sj.emboj.7600026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brangwynne, C.P., Eckmann, C.R., Courson, D.S., Rybarska, A., Hoege, C., Gharakhani, J.et al. (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- 36.Van Treeck, B., Protter, D.S.W., Matheny, T., Khong, A., Link, C.D. and Parker, R. (2018) RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc. Natl Acad. Sci. U.S.A. 115, 2734–2739 10.1073/pnas.1800038115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borcherds, W., Bremer, A., Borgia, M.B. and Mittag, T. (2021) How do intrinsically disordered protein regions encode a driving force for liquid-liquid phase separation? Curr. Opin. Struct. Biol. 67, 41–50 10.1016/j.sbi.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banani, S.F., Lee, H.O., Hyman, A.A. and Rosen, M.K. (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 10.1038/nrm.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tibble, R.W., Depaix, A., Kowalska, J., Jemielity, J. and Gross, J.D. (2021) Biomolecular condensates amplify mRNA decapping by biasing enzyme conformation. Nat. Chem. Biol. 17, 615–623 10.1038/s41589-021-00774-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sankaranarayanan, M., Emenecker, R.J., Wilby, E.L., Jahnel, M., Trussina, I., Wayland, M.et al. (2021) Adaptable P body physical states differentially regulate bicoid mRNA storage during early Drosophila development. Dev. Cell 56, 2886–2901.e2886 10.1016/j.devcel.2021.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranganathan, S. and Shakhnovich, E. (2021) Effect of RNA on morphology and dynamics of membraneless organelles. J. Phys. Chem. B 125, 5035–5044 10.1021/acs.jpcb.1c02286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schütz, S., Nöldeke, E.R. and Sprangers, R. (2017) A synergistic network of interactions promotes the formation of in vitro processing bodies and protects mRNA against decapping. Nucleic Acids Res. 45, 6911–6922 10.1093/nar/gkx353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittag, T. and Parker, R. (2018) Multiple modes of protein-protein interactions promote RNP granule assembly. J. Mol. Biol. 430, 4636–4649 10.1016/j.jmb.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Protter, D.S.W., Rao, B.S., Van Treeck, B., Lin, Y., Mizoue, L., Rosen, M.K.et al. (2018) Intrinsically disordered regions can contribute promiscuous interactions to RNP granule assembly. Cell Rep. 22, 1401–1412 10.1016/j.celrep.2018.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilby, E.L. and Weil, T.T. (2023) Relating the biogenesis and function of P bodies in Drosophila to human disease. Genes (Basel) 14, 1675 10.3390/genes14091675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen, X., Wang, K., Mufti, F.U.D., Xu, D., Zhu, C., Huang, X.et al. (2024) Germ granule compartments coordinate specialized small RNA production. Nat. Commun. 15, 5799 10.1038/s41467-024-50027-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiappetta, A., Liao, J., Tian, S. and Trcek, T. (2022) Structural and functional organization of germ plasm condensates. Biochem. J. 479, 2477–2495 10.1042/bcj20210815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hakes, A.C. and Gavis, E.R. (2023) Plasticity of Drosophila germ granules during germ cell development. PLoS Biol. 21, e3002069 10.1371/journal.pbio.3002069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubstenberger, A., Courel, M., Benard, M., Souquere, S., Ernoult-Lange, M., Chouaib, R.et al. (2017) P-body purification reveals the condensation of repressed mRNA regulons. Mol. Cell 68, 144–157.e145 10.1016/j.molcel.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 50.Ramat, A., Haidar, A., Garret, C. and Simonelig, M. (2023) Germ granule higher-order organization coordinates their different functions. bioRxiv 10.1101/2023.11.24.568558 [DOI] [PMC free article] [PubMed]
- 51.Trcek, T., Douglas, T.E., Grosch, M., Yin, Y., Eagle, W.V.I., Gavis, E.R.et al. (2020) Sequence-independent self-assembly of germ granule mRNAs into homotypic clusters. Mol. Cell 78, 941–950.e912 10.1016/j.molcel.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valentino, M., Ortega, B.M., Ulrich, B., Doyle, D.A., Farnum, E.D., Joiner, D.A.et al. (2022) Computational modeling offers new insight into Drosophila germ granule development. Biophys. J. 121, 1465–1482 10.1016/j.bpj.2022.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weil, T.T., Parton, R.M., Herpers, B., Soetaert, J., Veenendaal, T., Xanthakis, D.et al. (2012) Drosophila patterning is established by differential association of mRNAs with P bodies. Nat. Cell Biol. 14, 1305–1313 10.1038/ncb2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilhelm, J.E., Buszczak, M. and Sayles, S. (2005) Efficient protein trafficking requires Trailer hitch, a component of a ribonucleoprotein complex localized to the ER in Drosophila. Dev. Cell 9, 675–685 10.1016/j.devcel.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 55.Pisa, V., Cozzolino, M., Gargiulo, S., Ottone, C., Piccioni, F., Monti, M.et al. (2009) The molecular chaperone Hsp90 is a component of the cap-binding complex and interacts with the translational repressor Cup during Drosophila oogenesis. Gene 432, 67–74 10.1016/j.gene.2008.11.025 [DOI] [PubMed] [Google Scholar]
- 56.Nakamura, A., Amikura, R., Hanyu, K. and Kobayashi, S. (2001) Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development 128, 3233–3242 10.1242/dev.128.17.3233 [DOI] [PubMed] [Google Scholar]
- 57.Fan, S.J., Marchand, V. and Ephrussi, A. (2011) Drosophila Ge-1 promotes P body formation and oskar mRNA localization. PLoS One 6, e20612 10.1371/journal.pone.0020612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mansfield, J.H., Wilhelm, J.E. and Hazelrigg, T. (2002) Ypsilon Schachtel, a Drosophila Y-box protein, acts antagonistically to Orb in the oskar mRNA localization and translation pathway. Development 129, 197–209 10.1242/dev.129.1.197 [DOI] [PubMed] [Google Scholar]
- 59.Wilhelm, J.E., Mansfield, J., Hom-Booher, N., Wang, S., Turck, C.W., Hazelrigg, T.et al. (2000) Isolation of a ribonucleoprotein complex involved in mRNA localization in Drosophila oocytes. J. Cell Biol. 148, 427–440 10.1083/jcb.148.3.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanke, M., Jambor, H., Reich, J., Marches, B., Gstir, R., Ryu, Y.H.et al. (2015) Oskar RNA plays multiple noncoding roles to support oogenesis and maintain integrity of the germline/soma distinction. RNA 21, 1096–1109 10.1261/rna.048298.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bose, M., Rankovic, B., Mahamid, J. and Ephrussi, A. (2023) An architectural role of oskar mRNA in granule assembly. bioRxiv 10.1101/2023.08.31.555701 [DOI] [PMC free article] [PubMed]
- 62.de Valoir, T., Tucker, M.A., Belikoff, E.J., Camp, L.A., Bolduc, C. and Beckingham, K. (1991) A second maternally expressed Drosophila gene encodes a putative RNA helicase of the “DEAD box” family. Proc. Natl Acad. Sci. U.S.A. 88, 2113–2117 10.1073/pnas.88.6.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gotze, M., Dufourt, J., Ihling, C., Rammelt, C., Pierson, S., Sambrani, N.et al. (2017) Translational repression of the Drosophila nanos mRNA involves the RNA helicase Belle and RNA coating by Me31B and Trailer hitch. RNA 23, 1552–1568 10.1261/rna.062208.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flora, P., Wong-Deyrup, S.W., Martin, E.T., Palumbo, R.J., Nasrallah, M., Oligney, A.et al. (2018) Sequential regulation of maternal mRNAs through a conserved cis-acting element in their 3′ UTRs. Cell Rep. 25, 3828–3843.e3829 10.1016/j.celrep.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broyer, R.M., Monfort, E. and Wilhelm, J.E. (2017) Cup regulates oskar mRNA stability during oogenesis. Dev. Biol. 421, 77–85 10.1016/j.ydbio.2016.06.040 [DOI] [PubMed] [Google Scholar]
- 66.Kara, E., McCambridge, A., Proffer, M., Dilts, C., Pumnea, B., Eshak, J.et al. (2023) Mutational analysis of the functional motifs of the DEAD-box RNA helicase Me31B/DDX6 in Drosophila germline development. FEBS Lett. 597, 1848–1867 10.1002/1873-3468.14668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ottone, C., Gigliotti, S., Giangrande, A., Graziani, F. and Verrotti di Pianella, A. (2012) The translational repressor Cup is required for germ cell development in Drosophila. J. Cell Sci. 125, 3114–3123 10.1242/jcs.095208 [DOI] [PubMed] [Google Scholar]
- 68.Verrotti, A.C. and Wharton, R.P. (2000) Nanos interacts with cup in the female germline of Drosophila. Development 127, 5225–5232 10.1242/dev.127.23.5225 [DOI] [PubMed] [Google Scholar]
- 69.Lehmann, R. (2016) Germ plasm biogenesis–an oskar-centric perspective. Curr. Top. Dev. Biol. 116, 679–707 10.1016/bs.ctdb.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tritschler, F., Eulalio, A., Helms, S., Schmidt, S., Coles, M., Weichenrieder, O.et al. (2008) Similar modes of interaction enable Trailer Hitch and EDC3 to associate with DCP1 and Me31B in distinct protein complexes. Mol. Cell. Biol. 28, 6695–6708 10.1128/MCB.00759-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Igreja, C. and Izaurralde, E. (2011) Cup promotes deadenylation and inhibits decapping of mRNA targets. Genes Dev. 25, 1955–1967 10.1101/gad.17136311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Passmore, L.A. and Coller, J. (2022) Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat. Rev. Mol. Cell Biol. 23, 93–106 10.1038/s41580-021-00417-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lecuyer, E., Yoshida, H., Parthasarathy, N., Alm, C., Babak, T., Cerovina, T.et al. (2007) Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell 131, 174–187 10.1016/j.cell.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 74.Jambor, H., Surendranath, V., Kalinka, A.T., Mejstrik, P., Saalfeld, S. and Tomancak, P. (2015) Systematic imaging reveals features and changing localization of mRNAs in Drosophila development. Elife 4, e05003 10.7554/eLife.05003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loschi, M., Leishman, C.C., Berardone, N. and Boccaccio, G.L. (2009) Dynein and Kinesin regulate stress-granule and P-body dynamics. J. Cell Sci. 122, 3973–3982 10.1242/jcs.051383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker, F.C., Neiswender, H., Veeranan-Karmegam, R. and Gonsalvez, G.B. (2021) In vivo proximity biotin ligation identifies the interactome of Egalitarian, a Dynein cargo adaptor. Development 148, dev199935 10.1242/dev.199935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clark, A., Meignin, C. and Davis, I. (2007) A Dynein-dependent shortcut rapidly delivers axis determination transcripts into the Drosophila oocyte. Development 134, 1955–1965 10.1242/dev.02832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steinhauer, J. and Kalderon, D. (2006) Microtubule polarity and axis formation in the Drosophila oocyte. Dev. Dyn. 235, 1455–1468 10.1002/dvdy.20770 [DOI] [PubMed] [Google Scholar]
- 79.Parton, R.M., Hamilton, R.S., Ball, G., Yang, L., Cullen, C.F., Lu, W.et al. (2011) A PAR-1-dependent orientation gradient of dynamic microtubules directs posterior cargo transport in the Drosophila oocyte. J. Cell Biol. 194, 121–135 10.1083/jcb.201103160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meignin, C. and Davis, I. (2010) Transmitting the message: intracellular mRNA localization. Curr. Opin. Cell Biol. 22, 112–119 10.1016/j.ceb.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 81.Besse, F. and Ephrussi, A. (2008) Translational control of localized mRNAs: restricting protein synthesis in space and time. Nat. Rev. Mol. Cell Biol. 9, 971–980 10.1038/nrm2548 [DOI] [PubMed] [Google Scholar]
- 82.Mofatteh, M. and Bullock, S.L. (2017) SnapShot: subcellular mRNA localization. Cell 169, 178–178.e171 10.1016/j.cell.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 83.Pratt, C.A. and Mowry, K.L. (2013) Taking a cellular road-trip: mRNA transport and anchoring. Curr. Opin. Cell Biol. 25, 99–106 10.1016/j.ceb.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Das, S., Vera, M., Gandin, V., Singer, R.H. and Tutucci, E. (2021) Intracellular mRNA transport and localized translation. Nat. Rev. Mol. Cell Biol. 22, 483–504 10.1038/s41580-021-00356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navarro, C., Puthalakath, H., Adams, J.M., Strasser, A. and Lehmann, R. (2004) Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nat. Cell Biol. 6, 427–435 10.1038/ncb1122 [DOI] [PubMed] [Google Scholar]
- 86.Sladewski, T.E., Billington, N., Ali, M.Y., Bookwalter, C.S., Lu, H., Krementsova, E.B.et al. (2018) Recruitment of two Dyneins to an mRNA-dependent Bicaudal D transport complex. Elife 7, e36306 10.7554/eLife.36306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen, R.S. (2002) Oocyte patterning: Dynein and Kinesin, inc. Curr. Biol. 12, R797–R799 10.1016/s0960-9822(02)01310-6 [DOI] [PubMed] [Google Scholar]
- 88.Nashchekin, D., Fernandes, A.R. and St Johnston, D. (2016) Patronin/shot cortical foci assemble the noncentrosomal microtubule array that specifies the Drosophila anterior-posterior axis. Dev. Cell 38, 61–72 10.1016/j.devcel.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nieuwburg, R., Nashchekin, D., Jakobs, M., Carter, A.P., Khuc Trong, P., Goldstein, R.E.et al. (2017) Localised dynactin protects growing microtubules to deliver oskar mRNA to the posterior cortex of the Drosophila oocyte. Elife 6, e27237 10.7554/eLife.27237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brendza, R.P., Serbus, L.R., Duffy, J.B. and Saxton, W.M. (2000) A function for Kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science 289, 2120–2122 10.1126/science.289.5487.2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gáspár, I., Phea, L.J., McClintock, M.A., Heber, S., Bullock, S.L. and Ephrussi, A. (2023) An RNA-based feed-forward mechanism ensures motor switching in oskar mRNA transport. J. Cell Biol. 222, e202301113 10.1083/jcb.202301113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mhlanga, M.M., Bratu, D.P., Genovesio, A., Rybarska, A., Chenouard, N., Nehrbass, U.et al. (2009) In vivo colocalisation of oskar mRNA and trans-acting proteins revealed by quantitative imaging of the Drosophila oocyte. PLoS One 4, e6241 10.1371/journal.pone.0006241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McClintock, M.A., Dix, C.I., Johnson, C.M., McLaughlin, S.H., Maizels, R.J., Hoang, H.T.et al. (2018) RNA-directed activation of cytoplasmic dynein-1 in reconstituted transport RNPs. Elife 7, e36312 10.7554/eLife.36312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu, W., Lakonishok, M., Liu, R., Billington, N., Rich, A., Glotzer, M.et al. (2020) Competition between Kinesin-1 and Myosin-V defines Drosophila posterior determination. Elife 9, e54216 10.7554/eLife.54216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krauss, J., Lopez de Quinto, S., Nusslein-Volhard, C. and Ephrussi, A. (2009) Myosin-V regulates oskar mRNA localization in the Drosophila oocyte. Curr. Biol. 19, 1058–1063 10.1016/j.cub.2009.04.062 [DOI] [PubMed] [Google Scholar]
- 96.Kapitein, L.C., van Bergeijk, P., Lipka, J., Keijzer, N., Wulf, P.S., Katrukha, E.A.et al. (2013) Myosin-V opposes microtubule-based cargo transport and drives directional motility on cortical actin. Curr. Biol. 23, 828–834 10.1016/j.cub.2013.03.068 [DOI] [PubMed] [Google Scholar]
- 97.Zimyanin, V.L., Belaya, K., Pecreaux, J., Gilchrist, M.J., Clark, A., Davis, I.et al. (2008) In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell 134, 843–853 10.1016/j.cell.2008.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrandon, D., Elphick, L., Nüsslein-Volhard, C. and St Johnston, D. (1994) Staufen protein associates with the 3'UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell 79, 1221–1232 10.1016/0092-8674(94)90013-2 [DOI] [PubMed] [Google Scholar]
- 99.Chang, C.W., Nashchekin, D., Wheatley, L., Irion, U., Dahlgaard, K., Montague, T.G.et al. (2011) Anterior-posterior axis specification in Drosophila oocytes: identification of novel bicoid and oskar mRNA localization factors. Genetics 188, 883–896 10.1534/genetics.111.129312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piccioni, F., Ottone, C., Brescia, P., Pisa, V., Siciliano, G., Galasso, A.et al. (2009) The translational repressor Cup associates with the adaptor protein Miranda and the mRNA carrier Staufen at multiple time-points during Drosophila oogenesis. Gene 428, 47–52 10.1016/j.gene.2008.09.019 [DOI] [PubMed] [Google Scholar]
- 101.Cha, B.J., Koppetsch, B.S. and Theurkauf, W.E. (2001) In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell 106, 35–46 10.1016/S0092-8674(01)00419-6 [DOI] [PubMed] [Google Scholar]
- 102.Wang, S. and Hazelrigg, T. (1994) Implications for bcd mRNA localization from spatial distribution of Exu protein in Drosophila oogenesis. Nature 369, 400–403 10.1038/369400a0 [DOI] [PubMed] [Google Scholar]
- 103.Trovisco, V., Belaya, K., Nashchekin, D., Irion, U., Sirinakis, G., Butler, R.et al. (2016) Bicoid mRNA localises to the Drosophila oocyte anterior by random Dynein-mediated transport and anchoring. Elife 5, e17537 10.7554/eLife.17537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bansal, P., Madlung, J., Schaaf, K., Macek, B. and Bono, F. (2020) An interaction network of RNA-binding proteins involved in Drosophila oogenesis. Mol. Cell. Proteomics 19, 1485–1502 10.1074/mcp.RA119.001912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang, M., Ly, M., Lugowski, A., Laver, J.D., Lipshitz, H.D., Smibert, C.A.et al. (2017) Me31B globally represses maternal mRNAs by two distinct mechanisms during the Drosophila maternal-to-zygotic transition. Elife 6, e27891 10.7554/eLife.27891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin, Y. and Brangwynne, C.P. (2017) Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 10.1126/science.aaf4382 [DOI] [PubMed] [Google Scholar]
- 107.Alberti, S. and Hyman, A.A. (2021) Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213 10.1038/s41580-020-00326-6 [DOI] [PubMed] [Google Scholar]
- 108.Borden, K.L.B. (2020) The nuclear pore complex and mRNA export in cancer. Cancers (Basel) 13, 42 10.3390/cancers13010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alberti, S. and Dormann, D. (2019) Liquid-liquid phase separation in disease. Annu. Rev. Genet. 53, 171–194 10.1146/annurev-genet-112618-043527 [DOI] [PubMed] [Google Scholar]
- 110.Anderson, P., Kedersha, N. and Ivanov, P. (2015) Stress granules, P-bodies and cancer. Biochim. Biophys. Acta 1849, 861–870 10.1016/j.bbagrm.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reineke, L.C. and Lloyd, R.E. (2013) Diversion of stress granules and P-bodies during viral infection. Virology 436, 255–267 10.1016/j.virol.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]