Abstract
Diabetic foot ulcers (DFUs) represent a serious complication of diabetes with high incidence, requiring intensive treatment, prolonged hospitalization, and high costs. It poses a severe threat to the patient's life, resulting in substantial burdens on patient and healthcare system. However, the therapy of DFUs remains challenging. Therefore, exploring cell-free therapies for DFUs is both critical and urgent. Exosomes, as crucial mediators of intercellular communication, have been demonstrated potentially effective in anti-inflammation, angiogenesis, cell proliferation and migration, and collagen deposition. These functions have been proven beneficial in all stages of diabetic wound healing. This review aims to summarize the role and mechanisms of exosomes from diverse cellular sources in diabetic wound healing research. In addition, we elaborate on the challenges for clinical application, discuss the advantages of membrane vesicles as exosome mimics in wound healing, and present the therapeutic potential of exosomes and their mimetic vesicles for future clinical applications.
Keywords: Diabetic foot ulcers, Wound healing, Exosomes, Membrane vesicles, Regenerative medicine
1. Introduction
Diabetic foot ulcers (DFUs), a serious complication of diabetes mellitus, are challenging to heal due to the susceptibility to infection, and a higher rate of recurrence, disability and mortality [1]. There are about 18.6 million diabetic foot ulcers sufferers worldwide annually, approximately 20 % of these individuals eventually require minor or major limb amputation, thus presenting a significant worldwide health problem [2]. Compared with non-diabetic skin wounds, DFUs exhibit impaired endothelial cell metabolism due to persistent hyperglycemia, blockage of angiogenesis, increased tissue inflammatory factors and reactive oxygen species (ROS), and difficulty in polarizing anti-inflammatory macrophage M2 [3,4]. Enhanced matrix metalloproteinase (MMP) protease activity leads to extracellular matrix (EMC) degradation, impaired function of keratinocytes and fibroblasts, and inhibited re-epithelialization. Previous studies also indicated that DFU wounds had increased amount of neutrophil extracellular traps (NETs), leading to worsened wound injury and infection [5]. Traditional treatments of DFUs include glycemic control, anti-infection, vascular dilation, anti-platelet, lower limb revascularization, surgical debridement, and wound management. The updated International Working Group on the Diabetic Foot (IWGDF) 2023 guideline for DFUs recommends the use of sucrose-octasulfate dressings, placental derivatives, autologous leukocytes, platelet and fibrin patches, negative pressure wound therapy, and hyperbaric oxygen to improve local hypoxia as adjuncts to promote healing of diabetic foot ulcers [6]. However, the persistently high morbidity, disability, and rising costs suggest that it is urgent to explore new treatment strategies.
In recent years, our team and others have been focusing on developing emerging strategies for diabetic wound healing, particularly involving exosomes derived from cells [7]. These exosomes carry genetic material, metabolic factors, lipids, and cell surface proteins inherited from cellular source [8]. They function in intercellular communication [9] and play a crucial role in the process of diabetic wounds healing [10,11]. Almost all cell types secrete nanometer-sized vesicles called exosomes, which measure between 30 nm and 200 nm in diameter and have a double-layered lipid membrane [11]. The biomolecules carried on exosomes have widely varying based on derived cells and isolate method. There are some ubiquitous proteins can distinguish exosomes from other extracellular vesicles, including tetraspanin proteins (CD81, CD9 and CD63), the exosome biogenesis related proteins (Hrs, flotillin, Tsg101), heat shock proteins (HSP70 and HSP90) and cytoskeletal proteins [12]. The genetic characterization and composition of exosomal nucleic acids are still not fully understood, but it is well known that exosomes can deliver genetic material such as miRNAs for therapeutic purposes [9]. The plasma membrane of exosomes is more robust and resistant to degradation than derived cells, probably due to the increased content of cholesterol, sphingolipids, and desaturated lipids [13]. Exosome formation is a sophisticated process. First, the cell membrane deforms and endocytoses to form intracellular endosomes. Then, the endosomes bud into intraluminal vesicles called multivesicular bodies (MVBs). Finally, MVBs fuse with the cell membrane, releasing exosomes into the extracellular [14,15]. Although there is no uniform standard, exosomes can be isolated using various techniques such as ultracentrifugation, immunoaffinity, microfluidic, precipitation technologies [12]. The most commonly isolate method is ultracentrifugation. Exosomes are isolated from cell debris, apoptotic bodies, and other large vesicles based on their different densities which requires ultra-high centrifugation speeds of up to 100,000 g [16]. The quantification methods of exosomes including nanoparticle tracking analysis (NTA), electron microscopy and dynamic light scattering. NTA provide direct quantification without relying on a specific maker but requires expensive instruments [17]. Electron microscopy is the most popular method for exosome characterization and quality assessments following isolation [18]. Exosomes stored in phosphate-buffered saline at −80 °C have improved stability and are further protected from cryodamage by the addition of trehalose and specific preservatives [19,20].
Exosomes derived from different cell types have been shown to play an important role in various stages of diabetic wound healing. However, due to limitations such as low production, the application prospect of exosomes may be restricted, and the advantages of exosomes-simulated vesicles are gradually being revealed. This review presents an overview of the significant and potential value of exosomes and membrane vesicles from diverse cell sources in diabetic foot wound healing.
2. Cell-based therapy and exosome-based therapy on regenerative medicine
Stem cells have been extensively researched in the field of regenerative medicine over the past few decades, demonstrating potential applications due to their regenerative properties [[21], [22], [23]]. However, there are many challenges associated with translating cellular therapy into the clinic [24]. Environmental dependence is one of the limitations for the clinical application of stem cells. When extracted and separated from their niche, the viability and quantity of stem cells may be negatively impacted [25]. Additionally, uncertainty arises regarding the clinical efficacy of stem cells due to the inability of preclinical experiments to fully replicate the physiological and genetic environments identical to human clinical conditions [26]. Another obstacle is the effect of long-term storage and freeze-thaw cycles on cell numbers and viability [27]. Therefore, the establishment of standard preservation procedures is necessary. There is a controversial issue concerning the potential tumorigenicity [[28], [29], [30]]. Stem cell transplantation may increase the risk of tumor growth and metastasis, which has been widely discussed in the field of breast reconstruction. Co-cultured adipose-derived stem cells and several human breast cancer cell lines revealed the ability of stem cells to enhance malignant features of cancer cells [30]. Ethical issues in stem cell therapy have always been controversial. While autologous stem cell transplantation mitigates the ethical risk, there are still difficulties in large-scale clinical translation [31,32].
Stem cell-based therapy was once considered a very promising emerging therapy for diabetic foot [33]. However, it also faces challenges as mentioned above. The most appropriate cell type, dosages and route of administration in clinical applications remain unclear [34]. Although mesenchymal stem cells (MSCs) can induce diabetic foot wound healing, ultrasound and dermoscopy have shown that the collagen density and epidermal thickness in areas of healed ulcers had not returned to normal, which could lead to a poor long-term prognosis [35]. To solve these problems, researchers have observed that secretome engineering, especially the use of exosomes, may enable cell-free therapy [11,33,36].
Compared to cell-based therapy, cell-derived exosomes have the ability to improve and regenerate a wide range of damaged tissues due to their stability and safer delivery of signaling molecules, such as bone, cartilage, cutaneous wounds and vascular tissue [37,38]. As nanoscale vesicles, exosomes can cross biological barriers, evade immune recognition, and target specific cells [39]. Exosomes from antigen-presenting cells demonstrate low toxicity and immunogenicity [40]. Exosomes loaded with specific small molecules, such as miRNA and drugs, can improve therapeutic efficiency due to their biocompatibility and stability [38]. Unique components on the surface of exosomes allow for direct fusion of the membrane with the target cell, facilitating efficient cellular targeting [[41], [42], [43]]. MSC-derived exosomes (MSC-Exos) offer significant advantages over MSCs themselves by effectively minimizing infusion-associated toxicity [44].
Numerous systematic reviews and studies have reported the advantage of exosome-based therapy for cutaneous wound healing [[45], [46], [47]]. Moreover, various studies have further pointed to the benefit of exosomes on diabetic wounds [10,[48], [49], [50], [51], [52], [53], [54]]. In conclusion, the comparison of cellular and exosome-based therapies demonstrates the superiority of exosome-based treatment in the context of treating and managing diabetic foot (Table 1).
Table 1.
Comparison of cell-based and exosome-based therapies.
| Cell-based therapy | Exosome-based therapy |
|---|---|
| Environmental dependence [25] | Biocompatibility and stability [36,38,39] |
| Uncertainty of clinical efficacy [26] | Efficient cellular targeting [[41], [42], [43]] |
| Storage and transportation difficulties [27] | Low immunogenicity [37,39,40] |
| The potential tumorigenicity [[28], [29], [30]] | Safety [39,44] |
| Ethical issues [31,32] | Capacity to cross biological barriers [37,39] |
| Infusion-associated toxicity [44] | Low toxicity [36,40,44] |
| Loaded with specific small molecules [36,38] |
3. Stem cells-derived exosomes
3.1. Mesenchymal stem cells-derived exosomes
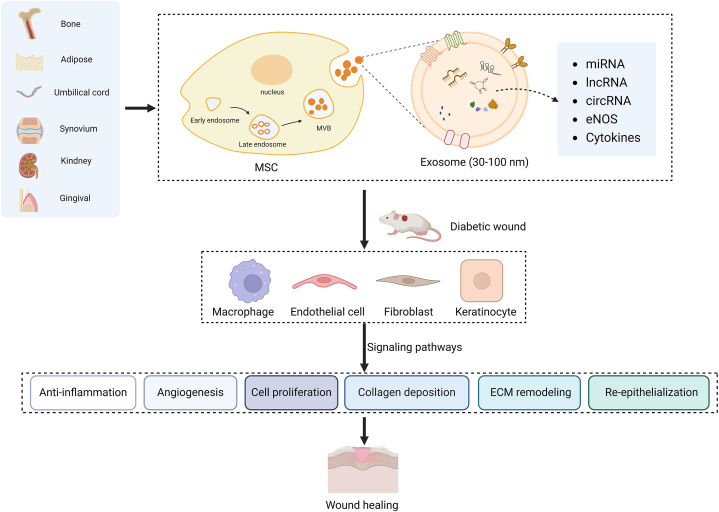
Mesenchymal stem cells possess the remarkable ability to self-renew and differentiate into various cell types [23,55]. Moreover, growth factors, cytokines, and collagen are also released, which facilitate wound healing and tissue regeneration [23]. This unique combination of properties makes mesenchymal stem cells an attractive candidate for therapeutic applications in the context of wound healing and tissue repair [24,46]. Consequently, based on the therapeutic potential, mesenchymal stem cells are considered as a promising therapy for enhancing wound healing in individuals with DFUs [56,57]. However, in the field of biomedicine, using stem cells presents ethical and potential safety issues [24]. Numerous studies indicated that mesenchymal stem cells promote wound healing through two different mechanisms [44,58]. First, mesenchymal stem cells directly differentiated into fibroblasts, myofibroblasts, and endothelial cells to promote angiogenesis and tissue repair [58]. Second, mesenchymal stem cells could release various bioactive molecules through paracrine effects to enhance cell proliferation and migration. In this context, mesenchymal stem cells-derived exosomes (MSCs-Exos) are the major delivery system [36,59]. MSCs-Exos, with a diameter ranging approximately 30–100 nm, maintain their integrity and biocompatibility through lipid bilayers, while the surface-modified proteins enhance their recognition and targeting capabilities. Moreover, abundant RNAs in MSCs-Exos can regulate receptor cells' transcription and translation [37]. Therefore, therapy based on MSCs-Exos provides an effective strategy for DFUs, mimicking the effects of stem cells while circumventing various limitations associated with stem cell therapy (Table 1). MSCs-Exos are involved in almost all stages of diabetic wound healing, including anti-inflammation, angiogenesis, collagen deposition, epithelial regeneration, and scar repair (Fig. 1).
Fig. 1.
Therapeutic potential of mesenchymal stem cell-derived exosomes in promoting wound healing in diabetic models.
3.1.1. Bone marrow mesenchymal stem cells-derived exosomes
Bone marrow mesenchymal stem cells (BMSCs), the adult stem cells with low immunogenicity, are widely presented in the bone marrow, and capable of self-renewal and differentiation into diverse types of tissue cells. BMSCs secrete vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and interleukin-6 (IL-6) to improve the microenvironment and regulate inflammation, immunity, fibrosis, and apoptosis, thereby promoting the angiogenic response to accelerate tissue recovery [60]. BMSCs-derived exosomes inherit these functions, primarily promoting the proliferation of endothelial cells and keratinocytes [61]. They downregulate miR-383 to increase the expression of its target factor VEGFA, promoting endothelial cell proliferation, migration, and angiogenesis, while inhibiting endothelial apoptosis under hyperglycemic conditions, thus facilitating skin wound healing in diabetic mice [52]. They could also promote the proliferation and migration of fibroblasts by downregulating miR-152-3p, mediating phosphatase and tensin homolog (PTEN) to prevent fibroblast apoptosis and inflammation, thereby accelerating DFUs healing [62]. BMSCs-derived exosomes as drug delivery systems possesses excellent biocompatibility and target recognition capabilities to improve the healing of diabetic wounds. MiR-146a-5p can be loaded into BMSCs-derived exosomes by electroporation technology, which facilitates anti-inflammatory macrophage polarization by blocking tumor necrosis factor receptor-associated factor 6 (TRAF6) expression and promote endothelial cell proliferation, migration, angiogenesis and re-epithelialization in hyperglycemia [63]. Preconditioned MSCs with chemical or biological factors may enhance the biological activity of MSCs-derived exosomes. Exosomes derived from atorvastatin pretreated BMSCs can activate the AKT/eNOS pathway both in vivo and in vitro by upregulating miR-221-3p, increasing vascular endothelial growth factor levels and promoting angiogenesis, thus exhibiting excellent wound healing promotion capabilities in diabetic wound [64]. Melatonin pretreated hBMSC-derived exosomes (MT-Exos) upregulate PTEN expression and inhibit AKT phosphorylation, increasing M2 macrophage polarization and inhibiting the inflammatory response, ultimately improving diabetic wound healing [51]. By stimulating the PI3K/AKT/eNOS signaling system, exosomes derived from pioglitazone-treated MSCs increase endothelial cell functions and angiogenesis, thus promoting diabetic wound healing [65]. Previous research has indicated that hypoxic BMSCs could exacerbate hypoxia-inducible factor (HIF)-1α-mediated transforming growth factor (TGF)-β1 secretion, downregulate SMAD2 phosphorylation levels, induce keratinocyte proliferation and migration via keratinocyte autophagy, re-epithelialization to accelerate diabetic wound healing [66]. A recent study has shown that exosomes secreted by hypoxic BMSCs have similar effects. They target MAPK-activated protein kinase 2 (MAPKAPK2) through miR-4645-5p to inhibit AKT-mTORC1 signaling pathway in keratinocytes [67].
3.1.2. Adipose-derived mesenchymal stem cell exosomes
Adipose-derived mesenchymal stem cells (ADSCs) are widely available and readily accessible multipotent adipose stem cells whose regenerative capacity has been demonstrated for cutaneous wound repair [24,[68], [69], [70]]. Exosomes from ADSCs also facilitate wound healing via a wide range of mechanisms, including anti-inflammation, anti-apoptosis, epithelial and vascular regeneration promotion, and reduction of scar formation [[71], [72], [73], [74], [75]]. Compared to ADSCs, exosomes from ADSCs are easier to store, more stable, and more biocompatible, which have been demonstrated in diabetic wound repair [45,76,77].
Studies indicated that ADSCs-Exos could inhibit cytotoxic T cells activation and proliferation, reduce IFN-γ production [78], and induce anti-inflammatory M2 macrophage polarization [79], making them a potential therapy for inflammation-related diseases. Additionally, hypoxia-induced ADSCs-Exos could upregulate miR-21-3p, miR-126-5p, miR-31-5p, and downregulate miR-99b and miR-146a to inhibit inflammation via the PI3K/AKT signaling pathway, serving as a strategy to promote diabetic wound healing [80]. They can also transfer circ-Snhg11 by the miR-144-3p/HIF-1α axis to promote M2 macrophage polarization and attenuates inflammatory factor secretion and cell apoptosis, eventually contributing to the diabetic wound healing [81]. Furthermore, ADSCs-Exos have been demonstrated to possess a remarkable protective effect on endothelial progenitor cells damaged by chronic hyperglycaemia, resulting in cell proliferation and angiogenesis [81,82], with the overexpression of nuclear factor-E2-related factor 2 (Nrf2) enhances its protective effect, as validated in diabetic rat wound [82]. ADSCs-Exos also reduce ROS production in human umbilical vein endothelial cells (HUVECs) and promote angiogenesis by modulating the mitochondrial deacetylase Sirtuin 3/superoxide dismutase (SIRT3/SOD2) pathway [82]. In diabetic mice, ADSCs-Exos stimulate monocyte/macrophage secretion of TGF-β1 and promote fibroblast function by activating the Smad3 signaling pathway, thereby accelerating diabetic wound healing [53]. Similarly, Interferon regulatory factor 1 (IRF1)-overexpressing rat ADSCs exosomes promote fibroblast proliferation, migration, and angiogenesis by regulating the miR-16-5p/SP5 axis [83]. Furthermore, exosomal vimentin from adipose progenitor cells have been shown to enhance fibroblast adaptation to external stress and inhibit stress-induced cell apoptosis [84]. Knockdown of low-density lipoprotein receptor-related protein 1 (LRP1) in fibroblasts and the use of an AKT pathway inhibitor can reverse the pro-healing effects of ADSCs-Exos on diabetic wounds, suggesting that HSP90 may be involved in the mechanism [49]. The high expression of miRNA-21 in ADSCs exosomes enhances migration and proliferation of HaCaT cells through the PI3K/AKT pathway, promoting extracellular matrix remodeling and significantly accelerating wound healing [85]. To enhance the abundance of miRNAs, miR-21-5p is loaded into hADSCs-Exos with electroporation technology to achieve more efficient therapy [86]. Exosomes can be loaded with other materials to accelerate wound healing synergistically. Hydrogel dressings have been extensively studied and utilized in wound repair due to their biocompatibility, biodegradability, and modifiability. The controlled release of exosomes from these dressings contributes to promoting diabetic wound healing [87]. Combining ADSCs-Exos with a multifunctional antimicrobial hydrogel to create FHE@exo has been shown to significantly promote endothelial cell function, increase skin adherence and reduce scarring in diabetic wound healing, proving to be more effective than single exosome therapy [88]. Incorporating exosomes from ADSCs into extracellular matrix hydrogel for topical treatment increases exosome concentration in wounds and accelerates diabetic wound healing by inhibiting inflammation, and promoting angiogenesis and cell proliferation and migration [89].
3.1.3. Umbilical cord mesenchymal stem cells-derived exosomes
Various cytokines and growth factors can be synthesized and secreted by Human umbilical cord mesenchymal stem cells (Huc MSCs), stimulating other cells to proliferate. Cytokines IL-6 and IL-8 contained in Huc MSCs exosomes can promote cell proliferation and prevent oxidative stress-induced apoptosis by activating ERK1/2 and p38 [90]. In addition, Huc MSCs exosomes transport abundant miRNAs, including miR-181, miR-21, and miR-146a, which can inhibit inflammatory responses at the wound site [91]. Moreover, endothelial cells activated by Wnt4 molecules accelerate proliferation and migration, resulting in rapid neovascularization [92]. In diabetic wound healing, Huc MSCs exosomes can also improve endothelial cell oxidative stress damage and promote angiogenesis [93]. Another study demonstrated that exosomes isolated from Huc MSCs promoted the proliferation of HUVECs and mouse embryonic fibroblasts in vitro, and in vivo, such exosomes could promote diabetic wound repair by inducing anti-inflammatory macrophages, promoting collagen deposition, and angiogenesis [94]. Meanwhile, the biological activity of derived exosomes can be enhanced by pretreating MSCs with genetic engineering techniques or biochemical factors. For example, genetically engineered Huc MSCs exosomes loaded with a large amount of eNOS under blue light irradiation significantly improved the biological function of cells grown in high levels of glucose, reduced oxidative stress-induced inflammatory factor expression and apoptosis, regulated the associated immune microenvironment and enhanced matrix remodeling, thereby promoting angiogenesis and tissue repair in chronic diabetic wounds [95]. In contrast, Huc MSCs exosomes pretreated with Nocardia rubra cell wall skeleton (Nr-CWS) stimulated the expression of circIARS1, which mediated the miR-4782-5p/VEGFA axis to promote endothelial cell proliferation and migration, thereby offering a potential strategy for diabetic wound treatment through tissue angiogenesis [96]. In a diabetic rat model, the topical application of Huc MSCs-derived exosomes wrapped in thermosensitive PF-127 hydrogel to full-thickness skin wounds resulted in increased Ki67 and CD31 expression, improved granulation tissue regeneration and upregulated VEGF and TGFβ-1 expression, thus promoted diabetic wound healing [97]. Furthermore, combining Huc MSCs-derived exosomes with polyvinyl alcohol (PVA)/alginate (Alg) nanohydrogel increased the expression of smooth muscle actin (SMA), the scavenger receptor, class B type 1 (SR-B1), and CD31, upregulated VEGF levels through ERK1/2, promoted HUVECs and angiogenesis, eventually facilitating the wound healing in diabetic rats [50]. A recent study has indicated that exosomes prepared from fresh human Huc MSCs, which combined with chitosan nanoparticles, bioactive glass (BG), and titanium dioxide (TiO2) in a composite hydrogel, can significantly enhance angiogenesis via VEGFA and VEGFR2, and accelerate full-thickness skin defect repair in diabetic mice with anti-inflammatory and antimicrobial activities [98].
3.1.4. Other mesenchymal stem cells-derived exosomes
Studies have demonstrated that synovium mesenchymal stem cells (SMSCs) could significantly enhance the proliferation of fibroblasts. SMSCs-derived exosomes overexpressing miR-126-3p can stimulate the proliferation of human fibroblasts and microvascular endothelial cells. In the diabetic rat model, these exosomes suggest accelerated epithelial regeneration of wounds by promoting cell proliferation and angiogenesis [99]. Exosomes derived from human urine-derived stem cells can promote endothelial cell function by transporting pro-angiogenic protein DMBT1, which may be one of the most promising strategies for accelerating the healing of diabetic wound [100]. Gingival MSCs-derived exosomes have been found to regulate the Wnt/β-catenin signaling pathway, promoting proliferation, migration, and angiogenesis of HUVECs in a hyperglycemic environment, while nanomaterial-encapsulated exosomes show more pronounced pro-healing effect on full-thickness wounds in diabetic mouse model, with potential for clinical translation [101]. Human amnion MSCs exosomes downregulate LATS2 expression via miR-135a and promote fibroblast migration, thereby accelerating skin wound healing in rats [102].
3.2. Other stem cells-derived exosomes
3.2.1. Endothelial progenitor cells-derived exosomes
The endothelial progenitor cells (EPCs) are multipotent stem cells that can differentiate into the endothelial cells of the body. Circulating EPCs express CD34, VEGFR2, and CD133 [103], and possess unique angiogenic and reparative functions that promote dermal wound healing [104,105]. The cellular decline and dysfunction of EPCs in diabetes lead to delayed wound healing. Targeting EPCs could accelerate the healing [106]. Exosomes derived from EPCs possess the same functions as the original cells and hold promising potential for direct application in regenerative medicine [107,108]. It has been shown that EPCs-Exos can activate the Erk1/2 pathway in vascular endothelial cells, promoting cell proliferation, migration, and vascular formation, and thus facilitating diabetic skin wound healing in rats [109]. Further study has indicated that EPC-Exos may enhance endothelial cell function via highly specific miRNA expression and downstream gene regulation [110]. A recent study has demonstrated that EPCs-Exos may inhibit ferroptosis in HUVECs and endothelial damage by upregulating miR-30e-5p, suppressing specificity protein 1 (SP1), and activating the adenosine monophosphate-activated protein kinase (AMPK) pathway [111]. In another study, EPCs-derived exosomes with miR-126-3p overexpression not only rescued the impaired proliferation and migration capabilities of HUVECs caused by hyperglycaemia but also inhibited endothelial cells pyroptosis by targeting the PI3KR2/SPRED1 signaling pathway [112]. It was found that EPC-Exos regulate the function of vascular endothelial cells and inhibit the expression of PPARG by increasing the level of miR-182-5p, thus promoting the proliferation and migration of HaCaTs and inhibiting apoptosis in a high glucose environment. These effects have been validated in diabetic mice [113].
3.2.2. Epidermal stem cell (ESCs) exosomes
Epidermal stem cells (ESCs) show extensive potential in wound regeneration, due to the abundance, ease of access, and minimal ethical concerns [[114], [115], [116]]. Research on diabetic wound healing indicated that ESCs expedite healing by promoting inflammation resolution, M2 macrophage polarization, vascularization, as well as cell proliferation and migration [54]. ESCs-Exos act as intercellular communication mediators, exhibiting similar functions with higher biocompatibility, lower immunogenicity, and simpler storage and transportation [54]. Mechanistically, ESCs-Exos primarily exert their effects by transferring of miRNA. For instance, ESCs-Exos enriched with miR-142-3p and miR-425-5p inhibit myofibroblast differentiation, and reduce scarring by decreasing TGF-β1 expression in dermal fibroblasts during wound healing [117]. Furthermore, the PI3K/AKT and MAPK signaling pathways play a role in ESCs-Exos' regulation of diabetic wound healing [54]. Chronic hyperglycemia causes endothelial cell dysfunction and excessive autophagy-induced apoptosis. ESCs-Exos riched with miR-200b-3p target the synapse defective rho GTPase homolog 1/RAS/ERK pathway to alleviate endothelial cell autophagy and apoptosis, with validation of their angiogenesis in db/db mouse dorsal wounds [118]. Another study confirmed that ESCs-Exos riched with miR-203a-3p could downregulate the negative regulator SOCS3 and activate the JAK2/STAT3 signaling pathway to promote M2 macrophage polarization [119]. To enhance the use of exosomes, ESCs-Exos can be merged with other materials. For example, exosomes loaded with VH298, an E3 ubiquitin ligase inhibitor, could enhance endothelial cells function and vascularization in wounds by activating the HIF-1α/VEGFA signaling pathway. Encapsulating exosomes in gelatin methacryloyl (GelMA) hydrogel for sustained release reinforces this effect, offering a new functional dressing for the treatment of diabetic wounds [120].
3.2.3. Human amniotic epithelial cells (hAEC) exosomes
Amniotic epithelial cells are low-immunogenic multipotent stem cells that produce growth factors, vascular regulatory factors, and anti-inflammatory factors that aid in skin wound healing. Isolating exosomes from amniotic cells has facilitated their use in regenerative medicine [121]. HAECs promote M2 macrophage polarization and endothelial cell function, and topical injection into full-thickness cutaneous wounds of db/db mice has confirmed the role in promoting diabetic wound healing [122]. Studies on hAEC-Exos reveals that they accelerate wound healing by stimulating fibroblast migration and proliferation through the delivery of exosomal miRNAs [123]. Another study showed that hAECs-Exos, activate the PI3K-AKT-mTOR pathway via miRNA, significantly promoting the proliferation and migration of human fibroblasts (HFBs) and the vascular regeneration activity of HUVECs, thereby inducing diabetic wound healing [124]. Additionally, hypoxia-induced hAECs-Exos are readily absorbed by keratinocytes and fibroblasts, enhancing cell proliferation and epithelial regeneration, reducing scar formation in normal wounds, and aiding in angiogenesis [125]. However, further investigation is needed to ascertain if they exert a similar impact on diabetic wounds.
4. Hemocyte-derived exosomes
4.1. Platelet-derived exosomes
Numerous studies have demonstrated that platelets can release various growth factors to regulate inflammation, stimulate angiogenesis, and promote granulation tissue growth during the wound healing process, starting from the hemostatic phase [126]. In 2023, the updated IWGDF recommended the adjunct use of platelet patches to promote the healing of diabetic foot ulcers [6]. Platelets and platelet-derived products play a significant role in diabetic wound healing, we have successfully utilized platelet-rich plasma (PRP) to accelerate diabetic ulcers healing in many cases [127]. Furthermore, we have demonstrated the antimicrobial, anti-inflammatory, and pro-cellular proliferative effects of PRP in an in vitro model of diabetic infected wound [128]. Subsequently, we isolated nanoscale cup-shaped vesicles, known as platelet-rich plasma exosomes (PRP-Exos), under different preparation conditions, which carry various proteins, mRNA, microRNA, and other bioactive substances from PRP. In vitro experiments demonstrated that PRP-Exos activated by calcium gluconate/thrombin mixture significantly promoted the proliferation and migration of HUVECs and induced tube formation through the AKT/ERK signaling pathway [129]. Therefore, PRP-Exos have the potential to promote healing of wounds. Furthermore, our study has revealed that sphingosine-1-phosphate enriched in platelet-rich plasma exosomes (PRP-Exos-S1P) transmits signals by binding to sphingosine-1-phosphate receptor 1 (S1PR1) on the membrane of skin vascular endothelial cells, thereby activating the protein kinase B/fibronectin 1 (AKT/FN1) signaling pathway to promote angiogenesis and repair in diabetic wounds [130]. Our recent study confirmed that PRP-Exos improve the healing of diabetic wound by transporting miR-26b-5p, which targets MMP-8 and subsequently inhibits NETs [131].Several recent studies have supported our findings. Guo et al. showed that PRP-Exos contain growth factors such as bFGF, VEGF, PDGF-BB and TFG-β, which promote the proliferation and migration of fibroblasts and HMEC-1 cells through YAP activation. Moreover, the pro-angiogenic and re-epithelialization effects of PRP-Exos were verified in diabetic rats [132]. Chen et al. found that lncRNA MALAT1 is downregulated in fibroblasts of diabetic foot ulcers, and the overexpression of MALAT1 by PRP-Exos enhances fibroblast viability, inhibits apoptosis and pyroptosis. This suggests the MALAT1-mediated signaling pathway probably contribute to the promoting effect of PRP-Exos on diabetic foot ulcer healing, potentially serving as a new target for diabetic foot treatment [133]. However, further exploration of its downstream regulatory mechanisms is necessary. Similarly, using PRP-Exos, Yang et al. demonstrated in vitro and vivo therapeutic potential for diabetic wounds. They hypothesized that PRP-Exos might prevent high glucose-induced ROS-dependent cell apoptosis through the PDGF-BB/JAK2/STAT3/Bcl-2 signal axis to promote fibroblast function and accelerate diabetic wound healing [134].
Additionally, novel gel dressings obtained by combining PRP-Exos, drugs, and materials such as hydrogels through bioengineering can alleviate the limitations of single drug administration and achieve complementary advantages, holding promising prospects for application in diabetic wounds. Xu et al. embedded curcuma zedoaria polysaccharide (ZWP) and PRP-Exos in chitosan/silk hydrogel sponge and verified its potential for diabetic wound healing in rats was superior to single drug treatment [135]. Zhu et al. loaded platelet-derived extracellular vesicles (PDEVs) and resveratrol (RES) nanoparticles in gelatin methacrylate/silk fibroin methacrylate (GelMA/SFMA) composite hydrogel, which suppressed iNOS expression in macrophages and promoted tube formation in HUVECs in vitro. In the diabetic mouse wound model, this gel dressing reduced the expression of iNOS and TNF-α, and upregulated anti-inflammatory factors such as Arg-1 and TGF-β1, thereby accelerating wound recovery [136]. They speculated that this regulatory mechanism may be related to the extracellular purinergic signaling pathway but did not further elucidate it. Bakadia et al. developed silk protein (SP)-based novel generation double-crosslinked hydrogel including PRP or PRP-Exos, with excellent mechanical properties, which enabled the sustained release of growth factors (GFs) and exosomes to accelerate the healing of diabetic wounds through the upregulation of GFs, the downregulation of MMP-9, and the promotion of anti-NETotic, angiogenesis, and re-epithelialization [137]. Hao et al. prepared polymer-coordinated hydrogels containing platelet-derived extracellular vesicles (pEV) and reduced graphene oxide (rGO), which exhibited excellent mechanical stability and biocompatibility in vitro and showed strong macrophage polarization and ROS scavenging. These properties were validated in diabetic rats, where GelAlg@rGO-pEV regulated the immune responses and inflammation, and promoted angiogenesis and hair follicle regeneration, thus accelerating the healing of chronic wounds [138]. Likewise, Shu et al. designed fiber-reinforced gelatin (GEL)/β-cyclodextrin (β-CD) therapeutic hydrogels encapsulating PRP-EXOs, which offer a biocompatible microenvironment and active ingredients for cell adhesion, proliferation, and skin tissue regeneration. This provides a new therapeutic platform for diabetic wound healing by modulating autophagy and inhibiting apoptosis of HUVECs and human skin fibroblasts (HSFs), inducing vascularization, collagen deposition, and re-epithelialization [139].
Like stem cells exosomes, the isolation and purification processes of platelet-derived exosomes are complex and time-consuming, and there is no efficient large-scale extraction method. Additionally, most previous studies were limited in vitro and murine animal models, so the application of platelet exosomes in clinical therapy is urgently needed to explore. A recent study provided the first evidence of the safety and therapeutic utility of clinical-grade pEVs in humans. They extracted clinical-grade pEVs on a large-scale using Ligand-based Exosome Affinity Purification chromatography. The safety of pEVs were confirmed in a randomized placebo-controlled Phase I clinical trial in healthy volunteers (ACTRN12620000944932) [140]. The trial involved a single subcutaneous injection. While the outcome indicated no adverse effects, there is no guarantee that repeated injections and systemic administration will produce the same result. The result also indicated no difference in the recovery times between the experimental and placebo groups, which may be related to the small sample size and the participants' healthy physiology. In other word, further clinical trials are needed to confirm the efficacy and safety of different route of administration and repeated doses. At the same time, the therapeutic effect of pEVs on delayed healing wounds such as DFUs should be verified. Similarly, an ongoing clinical trial (NCT06429033) aims to evaluate the safety and histological profile of purified exosome product (PEP) in healthy adults via hypodermic injection. The safety and efficacy of topical PEP in DFUs is being validated in an ongoing Phase 2a clinical trial (NCT06319287).
4.2. Leukocyte-derived exosomes
The persistent chronic inflammation in the diabetic wound microenvironment, where leukocyte dysfunction plays a critical role, contributes to delayed wound healing [[3], [4], [5]]. According to the IWGDF 2023 guidelines on diabetic foot ulcers, topical supplementation of autologous leukocytes can accelerate healing [6]. Immune cell exosomes, derived from sources such as macrophages and neutrophils, have been reported as potential factors in this process. Continuing to explore the potential of leukocyte-associated exosomes in diabetic wound healing is an important area of research.
4.2.1. Macrophage-derived exosomes
Macrophages are critical in wound healing by regulating inflammation, angiogenesis, and fibrosis [141]. However, in diabetic wounds, macrophage dysfunction, impaired polarization of M2 anti-inflammatory macrophages, increased pro-inflammatory factors and decreased anti-inflammatory factors lead to challenges during the healing process [3]. Therefore, improving macrophage function, promoting the polarization of M1-to-M2 macrophages, and activating the transdifferentiation of macrophages to fibroblasts can accelerate chronic diabetic wound healing [142]. Theoretically, exosomes derived from M2 macrophages (M2-Exos) have similar anti-inflammatory and pro-angiogenic functions as M2 macrophages. Studies have shown that M2-Exos can accelerate wound healing in diabetic wounds by promoting the switch of M1 to M2 macrophages, enhancing angiogenesis, re-epithelialization, and collagen deposition [143]. Chronic diabetic wounds with an imbalance between proinflammatory and anti-inflammatory responses could benefit from this treatment strategy. Studies on diabetic fracture healing have shown that M2-Exos induce the transformation of M1 macrophages into M2 macrophages through the simulation of the PI3K/AKT pathway [144]. It was shown that encapsulation of M2-Exos in a biodegradable polyethylene glycol (PEG) hydrogel resulted in sustained release of exosomes, maximizing their therapeutic effects in skin wound healing [145]. A specific source of macrophage-derived exosomes secreted from lean mouse adipose tissue can modulate db/db murine macrophage polarization via miR-222-3p, promoting rapid healing of diabetic wounds [146].
In the diabetic rat model, macrophage-derived exosomes exert anti-inflammatory effects by inhibiting the secretion of pro-inflammatory enzymes and cytokines and accelerate wound healing by inducing endothelial cell proliferation and migration, improving vascularization and re-epithelialization [147]. Comparison of M0-Exos, M1-Exos, and M2-Exos revealed that M2-Exos promote HUVEC migration and tube formation, while M1-Exos exhibit the opposite effect. High-throughput sequencing showed that miRNA-155-5p is highly expressed in M1-Exos, inhibiting the expression of growth differentiation factor 6 protein, thereby blocking angiogenesis of HUVECs [148]. Therefore, M2-Exos and miRNA-155-5p inhibitors may be effective strategies for the treatment of diabetic foot ulcers. However, the therapy with exosomes face limitations such as short half-life and instability. To overcomes these challenges, researchers have designed various multifunctional hydrogels to accelerate diabetic wound repair by promoting angiogenesis and re-epithelialization by sustained release of M2-Exos and growth factors [149,150]. An integrated hydrogel system study showed that combining anti-swelling hydrogel with anti-inflammatory M2-Exos and gold nanorods (AuNRs) photothermal effects effectively inhibits bacteria and inhibit inflammation, reduces reactive oxygen species, and angiogenesis for efficient synergistic diabetic wound treatment [151]. Another wound dressing system based on double-layer microneedles (MEs@PMN), encapsulating M2-Exos and polydopamine nanoparticles, enhances M2 macrophage polarization and can be combined with mild photothermal therapy to suppress inflammation and improve vascular regeneration, thereby accelerating diabetic wound healing [152]. Curcumin-loaded macrophage exosomes (Exos-cur) as a novel pro-healing biomaterial with better stability, anti-inflammatory, and antioxidant bioactivities were experimentally shown to inhibit inflammation, upregulate the expression of wound healing-related molecules, promote HUVEC proliferation, migration, and vascularization, optimize re-epithelialization, inhibit oxidative stress, thus accelerate diabetic rat wound healing via the Nrf2/ARE pathway [47]. A recent study reported that two-dimensional carbide (MXene)-M2-Exo nanohybrids (FM-Exo) can continuously release exosomes for up to 7 days, exhibiting a broad-spectrum antibacterial activity and immunosuppression. Mechanistically, FM-Exo can promote the function of fibroblasts and endothelial cells by activating the PI3K/AKT signaling pathway and significantly induce macrophage polarization [153].
4.2.2. Other leukocyte-derived exosomes
Neutrophils are the most abundant circulating leukocyte cells in the human body and served as the primary immune cells against pathogens with intrinsic phagocytosis [154,155]. Neutrophil-derived exosomes have been proven to be divided into anti-inflammatory and pro-inflammatory types [156]. Anti-inflammatory neutrophil-derived microvesicles (NDMVs) contain miR-126, miR-150, and miR-451a, which are taken up by monocytes to simulate M2 macrophage polarization, and may be considered as a therapeutic potential for chronic inflammatory diseases such as DFUs [157]. Dendritic cells (DCs), as the major antigen-presenting cells of the immune system, play a crucial role in initiating and regulating protective pro-inflammatory and immune responses [158]. DCs exosomes can promote angiogenesis and tissue regeneration by carrying and delivering a variety of miRNAs that modulate the function of other cells. For example, exosomes derived from mature dendritic cells (mDC-Exos) inhibit the Hippo signaling pathway by transferring miR-335 and target large tongue suppressor kinase 1 (LATS1), promoting the proliferation and osteogenic differentiation of BM-MSCs in rats with femoral defects [159]. Exosomes isolated from mouse bone marrow-derived DCs (DEX) significantly upregulate the expression of VEGF in cardiac microvascular endothelial cells (CMECs), to enhance CMEC tube formation. In vivo, DEX facilitated angiogenesis in mice by delivering miR-494-3p [160]. Therefore, DEX may represent a potential therapeutic strategy for diabetic wound healing. Additionally, exosomes derived from dendritic epidermal T cells (DETC) have been shown to accelerate re-epithelialization of skin wounds by promoting the proliferation of ESCs [161]. However, diabetic wounds often exhibit excessive inflammation and immune response. Further research and discussion are needed to effectively utilize these immune cell-derived exosomes to promote diabetic wound healing.
5. Endothelial cells-derived exosomes
Endothelial cells play a very important role in maintaining normal tissue physiology, as well as in acute and chronic inflammatory responses [162]. Targeting endothelial cells in diabetic wounds with persistent inflammation is an effective therapeutic strategy [163]. Therapy based on endothelial cell-derived exosomes may be another effective treatment modality to promote diabetic wound healing [164,165]. For example, hydrogel microneedle patches containing HUVECs-Exos achieve controlled release and transdermal delivery of exosomes around the wound, showing promise as a clinical treatment method for diabetic foot ulcers [164]. Another multifunctional acellular dermal matrix (ADM) hydrogel containing HUVEC-Exos has been proven to have the ability to promote diabetic wound healing [165]. In terms of mechanisms, hypoxic HUVECs-Exos can significantly upregulate lncHAR1B, which interacts with the transcription factor BHLHE23 to promote the expression of the KLF transcription factor 4, thereby ameliorate endothelial cell dysfunction and promote M2 macrophage polarization in diabetic wounds [48]. Overall, the specific role and molecular mechanism of HUVECs-exos in promoting diabetic wound healing are less studied and need further research.
6. Membrane vesicles
Membrane vesicles refer to artificial exosomes mimetic vesicles (EMVs) or extracellular vesicle mimetics [166,167], including cell membrane-coated synthetic nanovesicles, which serve as emerging biomaterials and drug delivery systems widely used in oncology, cardiovascular, immunology, and inflammatory disease research [[168], [169], [170], [171], [172]]. Compared with exosomes, membrane vesicles have a simpler preparation process, larger yield, and greater modifiability, which may lead to more efficient drug delivery efficiency [173]. Membrane vesicles are formed by extrusion, sonication, homogenization, and nitrogen cavitation [[174], [175], [176], [177]]. The most common method involves using an extruder to pressurize purified cell membranes or whole cells through polycarbonate membranes to prepare uniform-sized vesicles [178,179]. Similar to exosomes, various cells could be regarded as sources for drug-carrying membrane vesicles. Notably, nanoscale membrane vesicles derived from plants also have the potential as drug delivery systems due to their unique bioregulatory activity and editable nature [180]. In addition to obtaining membrane vesicles of single-cell origin, hybrid biomimetic membrane nanoparticles can be prepared using membranes from different cells [181,182].
EMVs are comparable to exosomes but have a hundred-fold higher yield and significantly higher levels of RNAs and proteins expression [183,184]. Another advantage of membrane vesicles is the varied modifiability, altering the expression of specific proteins or changing the ratio of cell membrane components by modulating the genetic information of the source cells or directly modifying the cell membrane to form specific membrane vesicles. Glycosylated platelet membrane vesicles prepared by co-incubating platelets with fucose and enzymes at room temperature have enhanced cell adhesion capabilities due to changes in membrane glycoprotein function [185]. The alteration of the cholesterol ratio can change membrane fluidity and rigidity. During vesicle preparation, adding free cholesterol directly to platelet membrane suspension and incubating it at room temperature for 30 min before extrusion results in more stable and drug-loaded platelet membrane vesicles enriched in cholesterol [176].
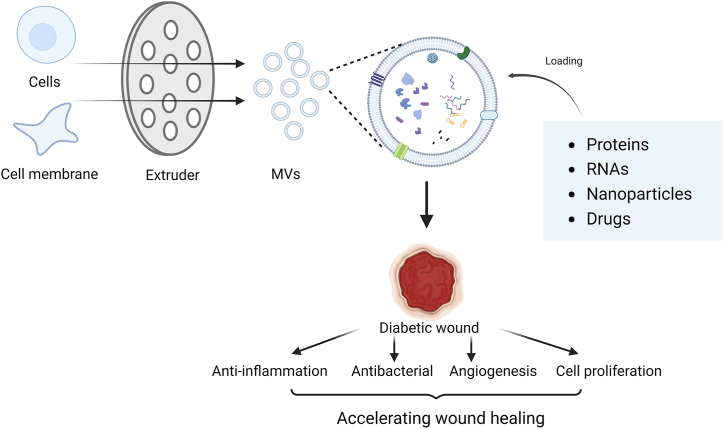
In the field of wound healing, membrane vesicles play a pro-healing role by transporting RNAs and proteins from source cells [184,186], as well as external drugs through co-extrusion [178,187], to regulate inflammation, angiogenesis, and re-epithelialization (Fig. 2). However, compared to exosomes, the research of membrane vesicles on wound healing, especially in diabetic wounds is limited, and further studies are urgently needed.
Fig. 2.
Continuous extrusion for cells or cell membranes with porous polycarbonate membranes could be utilized to produce vesicles with uniform size. These membrane vesicles load effective molecules to accelerate diabetic wound healing.
6.1. Stem cell membrane vesicles
The high expression of chemokine receptors on the membrane of stem cells endows them with the powerful anti-inflammatory and anti-injurious capabilities. Through bioengineering techniques, stem cells can be engineered to overexpress CXCR4, enhancing their homing effect on damaged tissues [188]. Stem cell membrane vesicles are considered to be a drug delivery system with significant advantages and clinical potential compared to others for promoting skin regeneration and wound healing [189]. Among them, mesenchymal stem cell membrane vesicles exhibit targeting and immune evasion properties, offering broad application potential in tissue regeneration, anti-tumor therapy, and anti-inflammatory research [190]. Local application of hUMSC EMV significantly induced regeneration of injured skin in mice, and it has been confirmed that EMV promoted the proliferation, migration, and tube formation of HUVECs in vitro experiments [184]. HUMSCs membrane vesicles conjugated with hydrogel scaffolds and were found to promote normal mouse wound healing more effectively than exosome hydrogels and proteomic analysis showed enrichment of mitochondrial-derived oxidative phosphorylation-related proteins [186]. Drug-loaded exosome mimetic vesicles were prepared by co-extrusion of the drug with induced pluripotent stem cell-derived endothelial cells (iPS-ECs) with abundant CXCR4, enabling homing to endothelial cells. These drug-loaded vesicles can promote endothelial cell function through the HIF-1α/VEGFA pathway to induce angiogenesis, and promote diabetic wound healing [178].
6.2. Platelet membrane vesicles
Platelet membrane vesicles have the ability to evade phagocytosis in the circulation due to cell membrane surface factors like CD47 [191]. Membrane vesicles engineered with glycosylated platelets and encapsulating interleukin-10 nanoparticles have the capacity to promote M2-type macrophage polarization to alleviate inflammation while promoting endothelial cell repair [185]. Platelet membrane encapsulated nanoparticles with bFGF and VEGFA gene plasmids can effectively target burn sites in rats via tail vein injection, and promote tissue healing [187]. Platelet membrane-coated therapeutic nanoparticles form multifunctional antibacterial agents (CSO@PM) with potent bactericidal and anti-inflammatory effects, as well as promote re-epithelialization and collagen deposition in wounds infected with drug-resistant bacteria, potentially aiding in the healing of diabetic foot ulcers [192]. In a recent study, Fe/Zn metal-organic frameworks were encapsulated in platelet membranes to create nanozymes with accelerated peroxidase-like activity. These nanozymes demonstrated robust antibacterial capabilities, notably suppressed levels of inflammatory cytokines, and facilitated angiogenesis. They accelerated skin wound healing when applied locally, as evidenced in a mouse model with infected full-thickness skin wounds [193]. However, further research is needed to determine their impact on diabetic wounds.
6.3. Leukocyte membrane vesicles
Neutrophil-derived membrane vesicles have a high affinity for the site of inflammation and have been used for drug delivery research in cancer and inflammatory diseases [194,195]. Membrane vesicles derived from HL-60 neutrophils form a drug delivery system that specifically targets inflamed brain endothelium in a mouse model of ischemic stroke and promotes the resolution of inflammation. This process is regulated by the expression of integrin β2 and adhesion molecule PSGL-1, both of which are expressed on neutrophil membranes [196]. Therefore, researchers believe that personalized nanoparticles formulated from neutrophil membranes have the potential to treat various inflammation-related diseases. Furthermore, human neutrophil membrane-derived nanovesicles loaded with resolvin D1 and ceftazidime specifically target inflammatory tissues and enhance the antibacterial effects, in a mouse model of bacterium-induced peritonitis [197]. In terms of systemic metabolism, studies have shown that drug-loaded membrane vesicles prepared by ultrasonication and co-extrusion of macrophage membranes with bovine serum albumin nanoparticles can effectively neutralize and remove inflammatory cytokines, as well as block immune and inflammatory cascade responses, to protect pancreatic islet cells and reduce insulin resistance, showing enormous potential in preventing and treating type 2 diabetes mellitus [198]. The use of neutrophil-derived exosome mimetics (PMNExo) for VEGF therapy delivery, combined with ECM hydrogels, has led to the development of injectable hybrid hydrogels with antimicrobial activity and wound healing properties, providing a prospective treatment platform for diabetic wound management [199]. Furthermore, the fusion of iPS-ECs membrane with M1 macrophage membrane to construct nanovesicles loaded with 4-octyl itaconate (4OI) can target M1 macrophages and endothelial cells, thereby promoting macrophage polarization and protecting endothelial cells, which in combination with injectable hydrogels, provides a novel strategy for promote diabetic wound repair [179].
7. Discussion and perspective
In conclusion, Exosomes have powerful pro-healing effects in diabetic wound and have safer and lower-immunity compared with cellular therapies. The toxicological characteristics of exosomes derived from human ADSCs have been evaluated through animal and cell experiments, demonstrating their safety for local treatment [200]. Furthermore, modification with genetic engineering techniques or chemical biofactors can increase the biological functionality of exosomes, thereby enhancing their therapeutical effect. Combining exosomes with hydrogels and nanomaterials can also accelerate wound healing, providing better therapeutic options for diabetic patients. In addition to regulating immune inflammation, angiogenesis, and cell proliferation, exosomes can also promote physiological processes of peripheral nerve, muscles and bone repair in the lower limbs. For example, ADSCs-Exos inhibit alkaline ceramidase 2 through miRNA-125b-5p to promote the proliferation and migration of myoblasts in diabetic mice, playing a crucial role in the repair of ischemic muscles in diabetic lower limbs [201].
Therefore, Exos have enormous potential as a therapeutic strategy to promote the healing of diabetic foot. However, despite the progress in preclinical experimental studies, there are still many challenges to be addressed before exosomes can be used in clinical settings. First, most of the current animal experiments are conducted based on acute diabetic wound models in mice and rats. The efficacy and safety of exosomes from different cellular sources need to be validated in animal models that closely resemble human anatomy to facilitate their use in future clinical trials. The safety and efficacy of exosomes in non-diabetic wound healing have been demonstrated in several completed clinical studies [140,202,203]. For example, a pilot case-control interventional study demonstrated the clinical efficacy of autologous serum-derived EVs in facilitating the healing process of chronic venous ulcers (CS2/1095/0090491) [203]. Additionally, ADSC-Exos showed both efficacy and safety in the treatment of acne scars in a double-blind randomized clinical trial [202]. However, their role in diabetic wound has not been validated in humans. Furthermore, exosomes from various cell types exhibit functional and property differences. The senescence phenotype SMCs-Exos may induce inflammaging as a side effect through their secretome [204]. The safety and efficacy of different administration routes may vary, requiring more short-term and long-term health safety assessments to confirm their safety. Additionally, issues regarding the large-scale production of exosomes and efficiency have yet to be determined. Currently, there is a lack of official worldwide standards for preparation, storage, and transportation of exosomes. The US Food and Drug Administration (FDA) has issued cautions that no exosome products are currently approved for routine clinical [205]. The regulation of exosomes is currently undergoing global improvement, and they should be regulated similarly to biological agents. Before being granted clinical approval, two key aspects need to be addressed. Firstly, there is need to understand the composition and efficacy of each type of exosomes. Secondly, legislation surrounding the regulation of exosomes needs to be refined.
Membrane vesicles have been becoming a novel delivery system for drugs and biomolecules due to their simple production process, abundant yield, high drug loading rate, and adjustability in regenerative medicine. How to utilize membrane vesicles to promote diabetic foot skin wound healing is still worth exploring and researching.
CRediT authorship contribution statement
Zhou Yang: Conceptualization, Investigation, Writing – original draft. Mengling Yang: Investigation, Conceptualization, Writing – original draft. Shunli Rui: Funding acquisition, Investigation. Wei Hao: Visualization, Writing – review & editing. Xiaohua Wu: Validation, Writing – review & editing. Lian Guo: Project administration, Visualization. David G. Armstrong: Writing – review & editing, Conceptualization, Data curation. Cheng Yang: Funding acquisition, Investigation, Project administration, Writing – review & editing. Wuquan Deng: Supervision, Funding acquisition, Project administration, Resources, Writing – review & editing.
Ethics statement
This narrative review did not involve any direct experiments on creatures, only summarized the existing data that has been published, and does not require the approval and review by the Ethics Commission. This review was completed in accordance with the ethical standard of Heliyon.
Declaration of competing interest
We hereby declare that there are no conflicts of interest to report. This encompasses financial, consultative, institutional, and other forms of conflicts that might bias the work submitted. We understand the importance of this declaration in maintaining the integrity of the scientific record and assert that no such conflicts exist.
These disclosures do not alter our adherence to Elsevier policies on sharing data and materials in the review and publication of our manuscript. We have disclosed all information regarding any potential conflicts of interest to the best of our knowledge and believe.
Thank you for your consideration of our work. We trust that this declaration will assist the editorial process and contribute to the integrity of the scholarly record.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2030 Major Science and Technology Innovation Program) (Project No. 2023ZD0509402), the National Nature Science Foundation of China (Grant No. 82370903), the Natural Science Foundation of Chongqing Municipal Science and Technology Bureau (Grant No. CSTB2022NSCQ-MSX0489), and TCM Regulating Metabolic Disease Key Laboratory of Sichuan Province (Grant No. WL2021002).
Contributor Information
Cheng Yang, Email: happyyangc@163.com.
Wuquan Deng, Email: wuquandeng@cqu.edu.cn.
References
- 1.Armstrong D.G., Boulton A.J.M., Bus S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong D.G., Tan T.W., Boulton A.J.M., et al. Diabetic foot ulcers: a review. JAMA. 2023;330(1):62–75. doi: 10.1001/jama.2023.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monaghan M.G., Borah R., Thomsen C., et al. Thou shall not heal: overcoming the non-healing behaviour of diabetic foot ulcers by engineering the inflammatory microenvironment. Adv. Drug Deliv. Rev. 2023;203 doi: 10.1016/j.addr.2023.115120. [DOI] [PubMed] [Google Scholar]
- 4.Mu R., Campos de Souza S., Liao Z., et al. Reprograming the immune niche for skin tissue regeneration - from cellular mechanisms to biomaterials applications. Adv. Drug Deliv. Rev. 2022;185 doi: 10.1016/j.addr.2022.114298. [DOI] [PubMed] [Google Scholar]
- 5.Yang S., Wang S., Chen L., et al. Neutrophil extracellular traps delay diabetic wound healing by inducing endothelial-to-mesenchymal transition via the Hippo pathway. Int. J. Biol. Sci. 2023;19(1):347–361. doi: 10.7150/ijbs.78046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P., Vilorio N.C., Dhatariya K., et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (iwgdf 2023 update) Diabetes Metab Res Rev. 2024 Mar;40(3) doi: 10.1002/dmrr.3644. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tkach M., Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Isaac R., Reis F.C.G., Ying W., et al. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744–1762. doi: 10.1016/j.cmet.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L., Qin J., Xing J., et al. The mechanisms of exosomes in diabetic foot ulcers healing: a detailed review. J. Mol. Med. (Berl.) 2023;101(10):1209–1228. doi: 10.1007/s00109-023-02357-w. [DOI] [PubMed] [Google Scholar]
- 11.Feng J., Yao Y., Wang Q., et al. Exosomes: potential key players towards novel therapeutic options in diabetic wounds. Biomed. Pharmacother. 2023;166 doi: 10.1016/j.biopha.2023.115297. [DOI] [PubMed] [Google Scholar]
- 12.Miron R.J., Zhang Y. Understanding exosomes: Part 1-characterization, quantification and isolation techniques. Periodontol. 2000;94(1):231–256. doi: 10.1111/prd.12520. [DOI] [PubMed] [Google Scholar]
- 13.Skotland T., Hessvik N.P., Sandvig K., et al. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019;60(1):9–18. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocucci E., Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015 Jun;25(6):364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Jia Y., Yu L., Ma T., et al. Small extracellular vesicles isolation and separation: current techniques, pending questions and clinical applications. Theranostics. 2022;12(15):6548–6575. doi: 10.7150/thno.74305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh Y.Q., Almughlliq F.B., Vaswani K., et al. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front Biosci (Landmark Ed) 2018;23(5):865–874. doi: 10.2741/4621. [DOI] [PubMed] [Google Scholar]
- 17.Soo C.Y., Song Y., Zheng Y., et al. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012;136(2):192–197. doi: 10.1111/j.1365-2567.2012.03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii N., Noguchi K., Ikemoto M.J., et al. Optimizing exosome preparation based on size and morphology: insights from electron microscopy. Microsc. Microanal. 2023;29(6):2068–2079. doi: 10.1093/micmic/ozad103. [DOI] [PubMed] [Google Scholar]
- 19.Bosch S., de Beaurepaire L., Allard M., et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016;6 doi: 10.1038/srep36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh J.A., Goberdhan D.C.I., O'Driscoll L., et al. Minimal information for studies of extracellular vesicles (Misev2023): from basic to advanced approaches. J. Extracell. Vesicles. 2024 Feb;13(2) doi: 10.1002/jev2.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapana J., Weinerman J., Lee D., et al. Cell-based therapy in the treatment of musculoskeletal diseases. Stem Cells Transl Med. 2024;13(10):959–978. doi: 10.1093/stcltm/szae049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yahyazadeh R., Baradaran Rahimi V., Askari V.R. Stem cell and exosome therapies for regenerating damaged myocardium in heart failure. Life Sci. 2024 Aug 15;351 doi: 10.1016/j.lfs.2024.122858. [DOI] [PubMed] [Google Scholar]
- 23.Fu X., Liu G., Halim A., et al. Mesenchymal stem cell migration and tissue repair. Cells. 2019 Jul 28;8(8) doi: 10.3390/cells8080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazini L., Rochette L., Admou B., et al. Hopes and limits of adipose-derived stem cells (adscs) and mesenchymal stem cells (mscs) in wound healing. Int. J. Mol. Sci. 2020 Feb 14;21(4) doi: 10.3390/ijms21041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roato I., Alotto D., Belisario D.C., et al. Adipose derived-mesenchymal stem cells viability and differentiating features for orthopaedic reparative applications: banking of adipose tissue. Stem Cells Int. 2016;2016 doi: 10.1155/2016/4968724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonseca A.C., Melchels F.P.W., Ferreira M.J.S., et al. Emulating human tissues and organs: a bioprinting perspective toward personalized medicine. Chem Rev. 2020 Oct 14;120(19):11128–11174. doi: 10.1021/acs.chemrev.0c00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Y., Ge G., Yang P., et al. An update on adipose-derived stem cells for regenerative medicine: where challenge meets opportunity. Adv. Sci. 2023 Jul;10(20) doi: 10.1002/advs.202207334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Liu Y., Chen Y., et al. Adipose-derived stem cells: current applications and future directions in the regeneration of multiple tissues. Stem Cells Int. 2020;2020 doi: 10.1155/2020/8810813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang J., Chen F., Liu D., et al. Adipose tissue-derived stem cells in breast reconstruction: a brief review on biology and translation. Stem Cell Res. Ther. 2021 Jan 6;12(1):8. doi: 10.1186/s13287-020-01955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koellensperger E., Bonnert L.C., Zoernig I., et al. The impact of human adipose tissue-derived stem cells on breast cancer cells: implications for cell-assisted lipotransfers in breast reconstruction. Stem Cell Res. Ther. 2017 May 25;8(1):121. doi: 10.1186/s13287-017-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo B., Parham L. Ethical issues in stem cell research. Endocr. Rev. 2009 May;30(3):204–213. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovell-Badge R., Anthony E., Barker R.A., et al. Isscr guidelines for stem cell research and clinical translation: the 2021 update. Stem Cell Rep. 2021 Jun 8;16(6):1398–1408. doi: 10.1016/j.stemcr.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho J., Yue D., Cheema U., et al. Innovations in stem cell therapy for diabetic wound healing. Adv. Wound Care. 2023 Nov;12(11):626–643. doi: 10.1089/wound.2021.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nalisa D.L., Moneruzzaman M., Changwe G.J., et al. Stem cell therapy for diabetic foot ulcers: theory and practice. J. Diabetes Res. 2022;2022 doi: 10.1155/2022/6028743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maksimova N.V., Michenko A.V., Krasilnikova O.A., et al. Mesenchymal stromal cell therapy alone does not lead to complete restoration of skin parameters in diabetic foot patients within a 3-year follow-up period. Bioimpacts. 2022;12(1):51–55. doi: 10.34172/bi.2021.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hade M.D., Suire C.N., Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021 Aug 1;10(8) doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y., Liu G., Zhang K., et al. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res. Ther. 2021 Oct 30;12(1):561. doi: 10.1186/s13287-021-02629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahabipour F., Barati N., Johnston T.P., et al. Exosomes: nanoparticulate tools for rna interference and drug delivery. J. Cell. Physiol. 2017 Jul;232(7):1660–1668. doi: 10.1002/jcp.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das C.K., Jena B.C., Banerjee I., et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol. Pharm. 2019 Jan 7;16(1):24–40. doi: 10.1021/acs.molpharmaceut.8b00901. [DOI] [PubMed] [Google Scholar]
- 40.Clayton A., Harris C.L., Court J., et al. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of Cd55 and Cd59. Eur. J. Immunol. 2003 Feb;33(2):522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 41.Salunkhe S., Dheeraj Basak M., et al. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release. 2020 Oct 10;326:599–614. doi: 10.1016/j.jconrel.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 42.Liang Y., Duan L., Lu J., et al. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viaud S., Ploix S., Lapierre V., et al. Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-Γ. J. Immunother. 2011 Jan;34(1):65–75. doi: 10.1097/CJI.0b013e3181fe535b. [DOI] [PubMed] [Google Scholar]
- 44.Lotfy A., AboQuella N.M., Wang H. Mesenchymal stromal/stem cell (Msc)-Derived exosomes in clinical trials. Stem Cell Res. Ther. 2023 Apr 7;14(1):66. doi: 10.1186/s13287-023-03287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An Y., Lin S., Tan X., et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021 Mar;54(3) doi: 10.1111/cpr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y.Z., Gou M., Da L.C., et al. Mesenchymal stem cells for chronic wound healing: current status of preclinical and clinical studies. Tissue Eng Part B Rev. 2020 Dec;26(6):555–570. doi: 10.1089/ten.TEB.2019.0351. [DOI] [PubMed] [Google Scholar]
- 47.Li D., Zhang C., Gao Z., et al. Curcumin-loaded macrophage-derived exosomes effectively improve wound healing. Mol. Pharm. 2023 Sep 4;20(9):4453–4467. doi: 10.1021/acs.molpharmaceut.3c00062. [DOI] [PubMed] [Google Scholar]
- 48.Cheng P., Xie X., Hu L., et al. Hypoxia endothelial cells-derived exosomes facilitate diabetic wound healing through improving endothelial cell function and promoting M2 macrophages polarization. Bioact. Mater. 2024 Mar;33:157–173. doi: 10.1016/j.bioactmat.2023.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren S., Chen J., Guo J., et al. Exosomes from adipose stem cells promote diabetic wound healing through the ehsp90/lrp1/akt Axis. Cells. 2022 Oct 14;11(20) doi: 10.3390/cells11203229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Zhang P., Gao X., et al. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl. 2021 Jan;120 doi: 10.1016/j.msec.2020.111671. [DOI] [PubMed] [Google Scholar]
- 51.Liu W., Yu M., Xie D., et al. Melatonin-stimulated msc-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the pten/akt pathway. Stem Cell Res. Ther. 2020 Jun 29;11(1):259. doi: 10.1186/s13287-020-01756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han Z.F., Cao J.H., Liu Z.Y., et al. Exosomal lncrna klf3-as1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res. Clin. Pract. 2022 Jan;183 doi: 10.1016/j.diabres.2021.109126. [DOI] [PubMed] [Google Scholar]
- 53.Hsu H.H., Wang A.Y.L., Loh C.Y.Y., et al. Therapeutic potential of exosomes derived from diabetic adipose stem cells in cutaneous wound healing of Db/Db mice. Pharmaceutics. 2022 Jun 6;14(6) doi: 10.3390/pharmaceutics14061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang P., Theocharidis G., Vlachos I.S., et al. Exosomes derived from epidermal stem cells improve diabetic wound healing. J. Invest. Dermatol. 2022 Sep;142(9):2508. doi: 10.1016/j.jid.2022.01.030. 17.e13. [DOI] [PubMed] [Google Scholar]
- 55.Ding D.C., Shyu W.C., Lin S.Z. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 56.El Hage R., Knippschild U., Arnold T., et al. Stem cell-based therapy: a promising treatment for diabetic foot ulcer. Biomedicines. 2022 Jun 25;10(7) doi: 10.3390/biomedicines10071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou L., Zhang X., Du H. Advances in mesenchymal stromal cells and nanomaterials for diabetic wound healing. Diabetes Metab Res Rev. 2023 May;39(4) doi: 10.1002/dmrr.3638. [DOI] [PubMed] [Google Scholar]
- 58.Kern S., Eichler H., Stoeve J., et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cell. 2006 May;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 59.Long R., Wang S. Exosomes from preconditioned mesenchymal stem cells: tissue repair and regeneration. Regen Ther. 2024 Mar;25:355–366. doi: 10.1016/j.reth.2024.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinnaird T., Stabile E., Burnett M.S., et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004 Mar 19;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 61.Pomatto M., Gai C., Negro F., et al. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int. J. Mol. Sci. 2021 Apr 8;22(8) doi: 10.3390/ijms22083851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li B., Luan S., Chen J., et al. The msc-derived exosomal lncrna H19 promotes wound healing in diabetic foot ulcers by upregulating Pten via microrna-152-3p. Mol. Ther. Nucleic Acids. 2020 Mar 6;19:814–826. doi: 10.1016/j.omtn.2019.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou X., Ye C., Jiang L., et al. The bone mesenchymal stem cell-derived exosomal Mir-146a-5p promotes diabetic wound healing in mice via macrophage M1/M2 polarization. Mol. Cell. Endocrinol. 2024 Jan 1;579 doi: 10.1016/j.mce.2023.112089. [DOI] [PubMed] [Google Scholar]
- 64.Yu M., Liu W., Li J., et al. Exosomes derived from atorvastatin-pretreated Msc accelerate diabetic wound repair by enhancing angiogenesis via Akt/Enos pathway. Stem Cell Res. Ther. 2020 Aug 12;11(1):350. doi: 10.1186/s13287-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu Y., Tao R., Chen L., et al. Exosomes derived from pioglitazone-pretreated Mscs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021 May 21;19(1):150. doi: 10.1186/s12951-021-00894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi Y., Wang S., Zhang W., et al. Bone marrow mesenchymal stem cells facilitate diabetic wound healing through the Restoration of epidermal cell autophagy via the Hif-1α/Tgf-β1/smad pathway. Stem Cell Res. Ther. 2022 Jul 15;13(1):314. doi: 10.1186/s13287-022-02996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi Y., Wang S., Liu D., et al. Exosomal mir-4645-5p from hypoxic bone marrow mesenchymal stem cells facilitates diabetic wound healing by restoring keratinocyte autophagy. Burns Trauma. 2024;12 doi: 10.1093/burnst/tkad058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim W.S., Park B.S., Sung J.H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009 Jul;9(7):879–887. doi: 10.1517/14712590903039684. [DOI] [PubMed] [Google Scholar]
- 69.Yu J., Wang M.Y., Tai H.C., et al. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018 Sep 1;77:191–200. doi: 10.1016/j.actbio.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 70.Hassanshahi A., Hassanshahi M., Khabbazi S., et al. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019 Jun;234(6):7903–7914. doi: 10.1002/jcp.27922. [DOI] [PubMed] [Google Scholar]
- 71.Cai Y., Li J., Jia C., et al. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res. Ther. 2020 Jul 22;11(1):312. doi: 10.1186/s13287-020-01831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu N., Xie Y., Zhen Y., et al. Free-cell therapeutics and mechanism of exosomes from adipose-derived stem cells in promoting wound healing: current understanding and future applications. Chin Med J (Engl) 2022 Aug 5;135(15):1803–1805. doi: 10.1097/CM9.0000000000001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y., Zhao B., Zhang X.L., et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hadsc) and hadsc-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res. Ther. 2021 May 1;12(1):257. doi: 10.1186/s13287-021-02287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iannotta D., Yang M., Celia C., et al. Extracellular vesicle therapeutics from plasma and adipose tissue. Nano Today. 2021 Aug;39 doi: 10.1016/j.nantod.2021.101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li C., Wei S., Xu Q., et al. Application of Adscs and their exosomes in scar prevention. Stem Cell Rev Rep. 2022 Mar;18(3):952–967. doi: 10.1007/s12015-021-10252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trzyna A., Banaś-Ząbczyk A. Adipose-derived stem cells secretome and its potential application in "stem cell-free therapy". Biomolecules. 2021 Jun 13;11(6) doi: 10.3390/biom11060878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gadelkarim M., Abushouk A.I., Ghanem E., et al. Adipose-derived stem cells: effectiveness and advances in delivery in diabetic wound healing. Biomed. Pharmacother. 2018 Nov;107:625–633. doi: 10.1016/j.biopha.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 78.Blazquez R., Sanchez-Margallo F.M., de la Rosa O., et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front. Immunol. 2014;5:556. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao H., Shang Q., Pan Z., et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018 Feb;67(2):235–247. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 80.Wang J., Wu H., Peng Y., et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of pi3k/akt pathways. J Nanobiotechnology. 2021 Jul 7;19(1):202. doi: 10.1186/s12951-021-00942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi R., Jin Y., Zhao S., et al. Hypoxic adsc-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and Induction of M2-like macrophage polarization. Biomed. Pharmacother. 2022 Sep;153 doi: 10.1016/j.biopha.2022.113463. [DOI] [PubMed] [Google Scholar]
- 82.Li X., Xie X., Lian W., et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018 Apr 13;50(4):1–14. doi: 10.1038/s12276-018-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu M., Tu J., Huang J., et al. Exosomal Irf1-loaded rat adipose-derived stem cell Sheet contributes to wound healing in the diabetic foot ulcers. Mol Med. 2023 Apr 25;29(1):60. doi: 10.1186/s10020-023-00617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parvanian S., Zha H., Su D., et al. Exosomal vimentin from adipocyte progenitors protects fibroblasts against osmotic stress and inhibits apoptosis to enhance wound healing. Int. J. Mol. Sci. 2021 Apr 28;22(9) doi: 10.3390/ijms22094678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang C., Luo L., Bai X., et al. Highly-expressed micorna-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the hacat cells by increasing the Mmp-9 expression through the Pi3k/Akt pathway. Arch. Biochem. Biophys. 2020 Mar 15;681 doi: 10.1016/j.abb.2020.108259. [DOI] [PubMed] [Google Scholar]
- 86.Lv Q., Deng J., Chen Y., et al. Engineered human adipose stem-cell-derived exosomes loaded with Mir-21-5p to promote diabetic cutaneous wound healing. Mol. Pharm. 2020 May 4;17(5):1723–1733. doi: 10.1021/acs.molpharmaceut.0c00177. [DOI] [PubMed] [Google Scholar]
- 87.Yan Z., Zhang T., Wang Y., et al. Extracellular vesicle biopotentiated hydrogels for diabetic wound healing: the art of living nanomaterials combined with soft scaffolds. Mater Today Bio. 2023 Dec;23 doi: 10.1016/j.mtbio.2023.100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C., Wang M., Xu T., et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song Y., You Y., Xu X., et al. Adipose-derived mesenchymal stem cell-derived exosomes biopotentiated extracellular matrix hydrogels accelerate diabetic wound healing and skin regeneration. Adv. Sci. 2023 Oct;10(30) doi: 10.1002/advs.202304023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang B., Shen L., Shi H., et al. Exosomes from human umbilical cord mesenchymal stem cells: identification, purification, and biological characteristics. Stem Cells Int. 2016;2016 doi: 10.1155/2016/1929536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ti D., Hao H., Fu X., et al. Mesenchymal stem cells-derived exosomal micrornas contribute to wound inflammation. Sci. China Life Sci. 2016 Dec;59(12):1305–1312. doi: 10.1007/s11427-016-0240-4. [DOI] [PubMed] [Google Scholar]
- 92.Zhang B., Wu X., Zhang X., et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the wnt4/Β-catenin pathway. Stem Cells Transl Med. 2015 May;4(5):513–522. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan C., Xv Y., Lin Z., et al. Human umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing via ameliorating oxidative stress and promoting angiogenesis. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.829868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teng L., Maqsood M., Zhu M., et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate diabetic wound healing via promoting M2 macrophage polarization, angiogenesis, and collagen deposition. Int. J. Mol. Sci. 2022 Sep 9;23(18) doi: 10.3390/ijms231810421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao X., Fu L., Zou H., et al. Optogenetic engineered umbilical cord msc-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J Nanobiotechnology. 2023 Jun 2;21(1):176. doi: 10.1186/s12951-023-01886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Q., Guo L., Wang J., et al. Exosomes derived from Nr-Cws pretreated Mscs facilitate diabetic wound healing by promoting angiogenesis via the Circiars1/Mir-4782-5p/Vegfa Axis. Chin. J. Nat. Med. 2023 Mar;21(3):172–184. doi: 10.1016/S1875-5364(23)60419-4. [DOI] [PubMed] [Google Scholar]
- 97.Yang J., Chen Z., Pan D., et al. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and Complete skin regeneration. Int J Nanomedicine. 2020;15:5911–5926. doi: 10.2147/IJN.S249129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shang S., Zhuang K., Chen J., et al. A bioactive composite hydrogel dressing that promotes healing of both acute and chronic diabetic skin wounds. Bioact. Mater. 2024 Apr;34:298–310. doi: 10.1016/j.bioactmat.2023.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tao S.C., Guo S.C., Li M., et al. Chitosan wound dressings incorporating exosomes derived from Microrna-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med. 2017 Mar;6(3):736–747. doi: 10.5966/sctm.2016-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen C.Y., Rao S.S., Ren L., et al. Exosomal Dmbt1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8(6):1607–1623. doi: 10.7150/thno.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Z., Yang S., Li X., et al. Local transplantation of Gmsc-derived exosomes to promote vascularized diabetic wound healing by regulating the Wnt/Β-catenin pathways. Nanoscale Adv. 2023 Jan 31;5(3):916–926. doi: 10.1039/d2na00762b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao S., Chen T., Hao Y., et al. Exosomal Mir-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting Lats2 expression. Stem Cell Res. Ther. 2020 Feb 13;11(1):56. doi: 10.1186/s13287-020-1570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Altabas V., Marinković Radošević J., Špoljarec L., et al. The impact of modern anti-diabetic treatment on endothelial progenitor cells. Biomedicines. 2023 Nov 14;11(11) doi: 10.3390/biomedicines11113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J.H., Ji S.T., Kim J., et al. Specific Disruption of Lnk in murine endothelial progenitor cells promotes dermal wound healing via enhanced vasculogenesis, activation of myofibroblasts, and suppression of inflammatory cell recruitment. Stem Cell Res. Ther. 2016 Oct 28;7(1):158. doi: 10.1186/s13287-016-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang C., Wang Q., Gao W., et al. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomater. 2018 Mar 15;69:156–169. doi: 10.1016/j.actbio.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 106.Wan G., Chen Y., Chen J., et al. Regulation of endothelial progenitor cell functions during hyperglycemia: new therapeutic targets in diabetic wound healing. J. Mol. Med. (Berl.) 2022 Apr;100(4):485–498. doi: 10.1007/s00109-021-02172-1. [DOI] [PubMed] [Google Scholar]
- 107.Chen K., Li Y., Xu L., et al. Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment. Stem Cell Res. Ther. 2022 Jun 7;13(1):238. doi: 10.1186/s13287-022-02921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xing Z., Zhao C., Liu H., et al. Endothelial progenitor cell-derived extracellular vesicles: a novel candidate for regenerative medicine and disease treatment. Adv Healthc Mater. 2020 Jun;9(12) doi: 10.1002/adhm.202000255. [DOI] [PubMed] [Google Scholar]
- 109.Zhang J., Chen C., Hu B., et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through erk1/2 signaling. Int. J. Biol. Sci. 2016;12(12):1472–1487. doi: 10.7150/ijbs.15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu J., Bai S., Cao Y., et al. Mirna-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab Syndr Obes. 2020;13:1259–1270. doi: 10.2147/DMSO.S243549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xia J., Song X., Meng J., et al. Endothelial progenitor cells-derived exosomes transfer Microrna-30e-5p to regulate Erastin-induced ferroptosis in human umbilical vein endothelial cells via the specificity protein 1/adenosine monophosphate-activated protein kinase Axis. Bioengineered. 2022 Feb;13(2):3566–3580. doi: 10.1080/21655979.2022.2025519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiong W., Zhang X., Zhou J., et al. Astragaloside Iv promotes exosome secretion of endothelial progenitor cells to regulate Pi3kr2/Spred1 signaling and inhibit pyroptosis of diabetic endothelial cells. Cytotherapy. 2024 Jan;26(1):36–50. doi: 10.1016/j.jcyt.2023.08.013. [DOI] [PubMed] [Google Scholar]
- 113.Li P., Hong G., Zhan W., et al. Endothelial progenitor cell derived exosomes mediated Mir-182-5p delivery accelerate diabetic wound healing via down-regulating pparg. Int. J. Med. Sci. 2023;20(4):468–481. doi: 10.7150/ijms.78790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Y., Zhang J., Yue J., et al. Epidermal stem cells in skin wound healing. Adv. Wound Care. 2017 Sep 1;6(9):297–307. doi: 10.1089/wound.2017.0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang R., Liu F., Wang J., et al. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res. Ther. 2019 Jul 29;10(1):229. doi: 10.1186/s13287-019-1312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao P., Dang Z., Liu M., et al. Molecular hydrogen promotes wound healing by inducing early epidermal stem cell proliferation and extracellular matrix deposition. Inflamm. Regen. 2023 Mar 28;43(1):22. doi: 10.1186/s41232-023-00271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duan M., Zhang Y., Zhang H., et al. Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res. Ther. 2020 Oct 23;11(1):452. doi: 10.1186/s13287-020-01971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu H., Yang H., Wang Z., et al. Epidermal stem cell derived exosomes alleviate excessive autophagy induced endothelial cell apoptosis by delivering mir200b-3p to diabetic wounds. J. Invest. Dermatol. 2024;144(5):1134–1147. doi: 10.1016/j.jid.2023.08.030. e2. [DOI] [PubMed] [Google Scholar]
- 119.Yang H., Xu H., Wang Z., et al. Analysis of Mir-203a-3p/Socs3-mediated induction of M2 macrophage polarization to promote diabetic wound healing based on epidermal stem cell-derived exosomes. Diabetes Res. Clin. Pract. 2023 Mar;197 doi: 10.1016/j.diabres.2023.110573. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y., Cao Z., Wei Q., et al. Vh298-Loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by Hif-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022 Jul 15;147:342–355. doi: 10.1016/j.actbio.2022.05.018. [DOI] [PubMed] [Google Scholar]
- 121.Farhadihosseinabadi B., Farahani M., Tayebi T., et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells, Nanomed. Biotechnol. 2018;46(sup2):431–440. doi: 10.1080/21691401.2018.1458730. [DOI] [PubMed] [Google Scholar]
- 122.Zheng Y., Zheng S., Fan X., et al. Amniotic epithelial cells accelerate diabetic wound healing by modulating inflammation and promoting neovascularization. Stem Cells Int. 2018;2018 doi: 10.1155/2018/1082076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao B., Li X., Shi X., et al. Exosomal Micrornas derived from human amniotic epithelial cells accelerate wound healing by promoting the proliferation and migration of fibroblasts. Stem Cells Int. 2018;2018 doi: 10.1155/2018/5420463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wei P., Zhong C., Yang X., et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via pi3k-Akt-Mtor-mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8 doi: 10.1093/burnst/tkaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Noh C.H., Park S., Seong H.R., et al. An exosome-rich conditioned Medium from human amniotic membrane stem cells facilitates wound healing via increased Reepithelization, collagen synthesis, and angiogenesis. Cells. 2023 Nov 24;12(23) doi: 10.3390/cells12232698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Golebiewska E.M., Poole A.W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015 May;29(3):153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deng W., Boey J., Chen B., et al. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J. Wound Care. 2016 Jul 2;25(7):393–397. doi: 10.12968/jowc.2016.25.7.393. [DOI] [PubMed] [Google Scholar]
- 128.Li T., Ma Y., Wang M., et al. Platelet-rich plasma plays an antibacterial, anti-inflammatory and cell proliferation-promoting role in an in vitro model for diabetic infected wounds. Infect. Drug Resist. 2019;12:297–309. doi: 10.2147/IDR.S186651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rui S., Yuan Y., Du C., et al. Comparison and investigation of exosomes derived from platelet-rich plasma activated by different agonists. Cell Transplant. 2021 Jan-Dec;30 doi: 10.1177/09636897211017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen T., Song P., He M., et al. Sphingosine-1-Phosphate derived from Prp-exos promotes angiogenesis in diabetic wound healing via the S1pr1/Akt/Fn1 Signalling pathway. Burns Trauma. 2023;11 doi: 10.1093/burnst/tkad003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rui S., Dai L., Zhang X., et al. Exosomal Mirna-26b-5p from Prp suppresses nets by targeting Mmp-8 to promote diabetic wound healing. J Control Release. 2024 Aug;372:221–233. doi: 10.1016/j.jconrel.2024.06.050. [DOI] [PubMed] [Google Scholar]
- 132.Guo S.C., Tao S.C., Yin W.J., et al. Exosomes derived from platelet-rich plasma promote the Re-epithelization of chronic cutaneous wounds via activation of yap in a diabetic rat model. Theranostics. 2017;7(1):81–96. doi: 10.7150/thno.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen C., Wang Q., Li D., et al. Malat1 participates in the role of platelet-rich plasma exosomes in promoting wound healing of diabetic foot ulcer. Int. J. Biol. Macromol. 2023 May 31;238 doi: 10.1016/j.ijbiomac.2023.124170. [DOI] [PubMed] [Google Scholar]
- 134.Cao W., Meng X., Cao F., et al. Exosomes derived from platelet-rich plasma promote diabetic wound healing via the Jak2/Stat3 pathway. iScience. 2023 Nov 17;26(11) doi: 10.1016/j.isci.2023.108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu N., Wang L., Guan J., et al. Wound healing effects of a curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int. J. Biol. Macromol. 2018 Oct 1;117:102–107. doi: 10.1016/j.ijbiomac.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 136.Zhu W., Dong Y., Xu P., et al. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022 Dec;154:212–230. doi: 10.1016/j.actbio.2022.10.038. [DOI] [PubMed] [Google Scholar]
- 137.Bakadia B.M., Qaed Ahmed A.A., Lamboni L., et al. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact. Mater. 2023 Oct;28:74–94. doi: 10.1016/j.bioactmat.2023.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hao P.C., Burnouf T., Chiang C.W., et al. Enhanced diabetic wound healing using platelet-derived extracellular vesicles and reduced graphene oxide in polymer-coordinated hydrogels. J Nanobiotechnology. 2023 Sep 4;21(1):318. doi: 10.1186/s12951-023-02068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shu Q.H., Zuo R.T., Chu M., et al. Fiber-reinforced gelatin/Β-cyclodextrin hydrogels loaded with platelet-rich plasma-derived exosomes for diabetic wound healing. Biomater. Adv. 2023 Nov;154 doi: 10.1016/j.bioadv.2023.213640. [DOI] [PubMed] [Google Scholar]
- 140.Johnson J., Law S.Q.K., Shojaee M., et al. First-in-Human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J. Extracell. Vesicles. 2023 Jul;12(7) doi: 10.1002/jev2.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016 Mar 15;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shen T., Dai K., Yu Y., et al. Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater. 2020 Nov;117:192–203. doi: 10.1016/j.actbio.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 143.Kim H., Wang S.Y., Kwak G., et al. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv. Sci. 2019 Oct 16;6(20) doi: 10.1002/advs.201900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Y., Lin Q., Zhang H., et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact. Mater. 2023 Oct;28:273–283. doi: 10.1016/j.bioactmat.2023.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kwak G., Cheng J., Kim H., et al. Sustained exosome-guided macrophage polarization using hydrolytically degradable peg hydrogels for cutaneous wound healing: identification of key proteins and mirnas, and sustained release formulation. Small. 2022 Apr;18(15) doi: 10.1002/smll.202200060. [DOI] [PubMed] [Google Scholar]
- 146.Xia W., Liu Y., Jiang X., et al. Lean adipose tissue macrophage derived exosome confers immunoregulation to improve wound healing in diabetes. J Nanobiotechnology. 2023 Apr 12;21(1):128. doi: 10.1186/s12951-023-01869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li M., Wang T., Tian H., et al. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif. Cells, Nanomed. Biotechnol. 2019 Dec;47(1):3793–3803. doi: 10.1080/21691401.2019.1669617. [DOI] [PubMed] [Google Scholar]
- 148.Lou R., Chen J., Zhou F., et al. Exosomal mirna-155-5p from M1-polarized macrophages suppresses angiogenesis by targeting Gdf6 to interrupt diabetic wound healing. Mol. Ther. Nucleic Acids. 2023 Dec 12;34 doi: 10.1016/j.omtn.2023.102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu P., Xiong Y., Chen L., et al. Angiogenesis-based diabetic skin reconstruction through multifunctional hydrogel with sustained releasing of M2 macrophage-derived exosome. Chem. Eng. J. 2022 Mar 1:431. [Google Scholar]
- 150.Xiong Y., Chen L., Liu P., et al. All-in-One: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small. 2022 Jan;18(1) doi: 10.1002/smll.202104229. [DOI] [PubMed] [Google Scholar]
- 151.Li W., Wu S., Ren L., et al. Development of an antiswelling hydrogel system incorporating M2-exosomes and photothermal effect for diabetic wound healing. ACS Nano. 2023 Nov 14;17(21):22106–22120. doi: 10.1021/acsnano.3c09220. [DOI] [PubMed] [Google Scholar]
- 152.Zeng J., Sun Z., Zeng F., et al. M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mater Today Bio. 2023 Jun;20 doi: 10.1016/j.mtbio.2023.100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang X., Ma J., Xue K., et al. Highly bioactive mxene-M2-exosome nanocomposites promote angiogenic diabetic wound repair through reconstructing high glucose-derived immune inhibition. ACS Nano. 2024 Feb 6;18(5):4269–4286. doi: 10.1021/acsnano.3c09721. [DOI] [PubMed] [Google Scholar]
- 154.Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu X., Gao Q., Wu S., et al. Optically manipulated neutrophils as native microcrafts in vivo. ACS Cent. Sci. 2022 Jul 27;8(7):1017–1027. doi: 10.1021/acscentsci.2c00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kolonics F., Kajdácsi E., Farkas V.J., et al. Neutrophils produce proinflammatory or anti-inflammatory extracellular vesicles depending on the environmental conditions. J. Leukoc. Biol. 2021 Apr;109(4):793–806. doi: 10.1002/JLB.3A0320-210R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Youn Y.J., Shrestha S., Lee Y.B., et al. Neutrophil-derived trail is a proinflammatory subtype of neutrophil-derived extracellular vesicles. Theranostics. 2021;11(6):2770–2787. doi: 10.7150/thno.51756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Worbs T., Hammerschmidt S.I., Förster R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017 Jan;17(1):30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 159.Cao Z., Wu Y., Yu L., et al. Exosomal mir-335 derived from mature dendritic cells enhanced mesenchymal stem cell-mediated bone regeneration of bone defects in athymic rats. Mol Med. 2021 Feb 26;27(1):20. doi: 10.1186/s10020-021-00268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu H., Zhang Y., Yuan J., et al. Dendritic cell-derived exosomal mir-494-3p promotes angiogenesis following myocardial infarction. Int. J. Mol. Med. 2021 Jan;47(1):315–325. doi: 10.3892/ijmm.2020.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu M., Liu Z., Chen Y., et al. Dendritic epidermal T cells secreting exosomes promote the proliferation of epidermal stem cells to enhance wound Re-epithelialization. Stem Cell Res. Ther. 2022 Mar 21;13(1):121. doi: 10.1186/s13287-022-02783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007 Oct;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 163.Huang X., Liang P., Jiang B., et al. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020 Oct 15;259 doi: 10.1016/j.lfs.2020.118246. [DOI] [PubMed] [Google Scholar]
- 164.Yuan M., Liu K., Jiang T., et al. Gelma/pegda microneedles patch loaded with huvecs-derived exosomes and tazarotene promote diabetic wound healing. J Nanobiotechnology. 2022 Mar 19;20(1):147. doi: 10.1186/s12951-022-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiang K., Chen J., Guo J., et al. Multifunctional adm hydrogel containing endothelial cell-exosomes for diabetic wound healing. Mater Today Bio. 2023 Dec;23 doi: 10.1016/j.mtbio.2023.100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nasiri Kenari A., Cheng L., Hill A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods. 2020 May 1;177:103–113. doi: 10.1016/j.ymeth.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 167.Li Y.J., Wu J.Y., Liu J., et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. 2021 Aug 12;19(1):242. doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fang R.H., Gao W., Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023 Jan;20(1):33–48. doi: 10.1038/s41571-022-00699-x. [DOI] [PubMed] [Google Scholar]
- 169.Ma J., Jiang L., Liu G. Cell membrane-coated nanoparticles for the treatment of bacterial infection. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 Sep;14(5) doi: 10.1002/wnan.1825. [DOI] [PubMed] [Google Scholar]
- 170.Wang X., Xia Z., Wang H., et al. Cell-membrane-coated nanoparticles for the fight against pathogenic bacteria, toxins, and inflammatory cytokines associated with sepsis. Theranostics. 2023;13(10):3224–3244. doi: 10.7150/thno.81520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bose R.J., Ha K., McCarthy J.R. Bio-inspired nanomaterials as novel options for the treatment of cardiovascular disease. Drug Discov. Today. 2021 May;26(5):1200–1211. doi: 10.1016/j.drudis.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang Q., Dehaini D., Zhang Y., et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018 Dec;13(12):1182–1190. doi: 10.1038/s41565-018-0254-4. [DOI] [PubMed] [Google Scholar]
- 173.Yurkin S.T., Wang Z. Cell membrane-derived nanoparticles: emerging clinical opportunities for targeted drug delivery. Nanomedicine (Lond) 2017 Aug;12(16):2007–2019. doi: 10.2217/nnm-2017-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Winkler K., Leneweit G., Schubert R. Characterization of membrane vesicles in plant extracts. Colloids Surf. B Biointerfaces. 2005 Oct 10;45(2):57–65. doi: 10.1016/j.colsurfb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 175.Gao J., Chu D., Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J Control Release. 2016 Feb 28;224:208–216. doi: 10.1016/j.jconrel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ying M., Zhuang J., Wei X., et al. Remote-loaded platelet vesicles for disease-targeted delivery of therapeutics. Adv. Funct. Mater. 2018 May 30;28(22) doi: 10.1002/adfm.201801032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Le Q.V., Lee J., Lee H., et al. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm. Sin. B. 2021 Aug;11(8):2096–2113. doi: 10.1016/j.apsb.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang W., Wang L., Guo H., et al. Dapagliflozin-loaded exosome mimetics facilitate diabetic wound healing by hif-1α-mediated enhancement of angiogenesis. Adv Healthc Mater. 2023 Mar;12(7) doi: 10.1002/adhm.202202751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang W., Yang X., Huang X., et al. Bioinspired nanovesicles released from injectable hydrogels facilitate diabetic wound healing by regulating macrophage polarization and endothelial cell dysfunction. J Nanobiotechnology. 2023 Oct 3;21(1):358. doi: 10.1186/s12951-023-02119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu C., Yu Y., Fang L., et al. Plant-derived nanoparticles and plant virus nanoparticles: bioactivity, health management, and delivery potential. Crit. Rev. Food Sci. Nutr. 2023 Apr;27:1–17. doi: 10.1080/10408398.2023.2204375. [DOI] [PubMed] [Google Scholar]
- 181.Dehaini D., Wei X., Fang R.H., et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017 Apr;29(16) doi: 10.1002/adma.201606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huang R., Zhang L., Li X., et al. Anti-Cxcr2 antibody-coated nanoparticles with an erythrocyte-platelet hybrid membrane layer for atherosclerosis therapy. J Control Release. 2023 Apr;356:610–622. doi: 10.1016/j.jconrel.2023.02.036. [DOI] [PubMed] [Google Scholar]
- 183.Jo W., Kim J., Yoon J., et al. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014 Oct 21;6(20):12056–12064. doi: 10.1039/c4nr02391a. [DOI] [PubMed] [Google Scholar]
- 184.Zhang Z., Mi T., Jin L., et al. Comprehensive proteomic analysis of exosome mimetic vesicles and exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2022 Jul 15;13(1):312. doi: 10.1186/s13287-022-03008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li F., Rong Z., Chen T., et al. Glycosylation-engineered platelet membrane-coated interleukin 10 nanoparticles for targeted inhibition of vascular restenosis. Int J Nanomedicine. 2023;18:5011–5030. doi: 10.2147/IJN.S423186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhu J., Liu Z., Wang L., et al. Exosome mimetics-loaded hydrogel accelerates wound repair by transferring functional mitochondrial proteins. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.866505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang B., Chen J., Zhang C., et al. Biomimetic nanoparticles of platelet membranes carrying bfgf and vegfa genes promote deep burn wound healing. Int Immunopharmacol. 2023 Dec;125(Pt A) doi: 10.1016/j.intimp.2023.111164. [DOI] [PubMed] [Google Scholar]
- 188.Fan L., Wei A., Gao Z., et al. Current progress of mesenchymal stem cell membrane-camouflaged nanoparticles for targeted therapy. Biomed. Pharmacother. 2023 May;161 doi: 10.1016/j.biopha.2023.114451. [DOI] [PubMed] [Google Scholar]
- 189.Zhang W., Huang X. Stem cell-based drug delivery strategy for skin regeneration and wound healing: potential clinical applications. Inflamm. Regen. 2023 Jun 30;43(1):33. doi: 10.1186/s41232-023-00287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bose R.J., Kim B.J., Arai Y., et al. Bioengineered stem cell membrane functionalized nanocarriers for therapeutic targeting of severe hindlimb ischemia. Biomaterials. 2018 Dec;185:360–370. doi: 10.1016/j.biomaterials.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 191.Wang S., Duan Y., Zhang Q., et al. Drug targeting via platelet membrane-coated nanoparticles. Small Struct. 2020 Oct;1(1) doi: 10.1002/sstr.202000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Peng Z., Zhang X., Yuan L., et al. Integrated endotoxin-adsorption and antibacterial properties of platelet-membrane-coated copper silicate hollow microspheres for wound healing. J Nanobiotechnology. 2021 Nov 22;19(1):383. doi: 10.1186/s12951-021-01130-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shi Q., Zhao Y., Liu M., et al. Engineering platelet membrane-coated bimetallic mofs as biodegradable nanozymes for efficient antibacterial therapy. Small. 2023 Dec;27 doi: 10.1002/smll.202309366. [DOI] [PubMed] [Google Scholar]
- 194.Chu D., Dong X., Shi X., et al. Neutrophil-based drug delivery systems. Adv Mater. 2018 May;30(22) doi: 10.1002/adma.201706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang H., Zang J., Zhao Z., et al. The advances of neutrophil-derived effective drug delivery systems: a key review of managing tumors and inflammation. Int J Nanomedicine. 2021;16:7663–7681. doi: 10.2147/IJN.S328705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dong X., Gao J., Zhang C.Y., et al. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019 Feb 26;13(2):1272–1283. doi: 10.1021/acsnano.8b06572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gao J., Dong X., Su Y., et al. Human neutrophil membrane-derived nanovesicles as a drug delivery platform for improved therapy of infectious diseases. Acta Biomater. 2021 Mar 15;123:354–363. doi: 10.1016/j.actbio.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu L., Yuan A., Tian X., et al. Cell-membrane-coated cationic nanoparticles disguised as macrophages for the prevention and treatment of type 2 diabetes mellitus. ACS Appl. Mater. Interfaces. 2022 Nov 16;14(45):50499–50506. doi: 10.1021/acsami.2c12218. [DOI] [PubMed] [Google Scholar]
- 199.Yu Y., Jin H., Li L., et al. An injectable, activated neutrophil-derived exosome mimetics/extracellular matrix hybrid hydrogel with antibacterial activity and wound healing promotion effect for diabetic wound therapy. J Nanobiotechnology. 2023 Aug 30;21(1):308. doi: 10.1186/s12951-023-02073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ha D.H., Kim S.D., Lee J., et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul. Toxicol. Pharmacol. 2020 Aug;115 doi: 10.1016/j.yrtph.2020.104686. [DOI] [PubMed] [Google Scholar]
- 201.Guo J., Yang X., Chen J., et al. Exosomal mir-125b-5p derived from adipose-derived mesenchymal stem cells enhance diabetic hindlimb ischemia repair via targeting alkaline ceramidase 2. J Nanobiotechnology. 2023 Jun 12;21(1):189. doi: 10.1186/s12951-023-01954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kwon H.H., Yang S.H., Lee J., et al. Combination treatment with human adipose tissue stem cell-derived exosomes and fractional Co2 laser for acne scars: a 12-week prospective, double-blind, randomized, split-face study. Acta Derm. Venereol. 2020 Nov 4;100(18) doi: 10.2340/00015555-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gibello L., D'Antico S., Salafia M., et al. First pilot case-control interventional study using autologous extracellular vesicles to treat chronic venous ulcers unresponsive to conventional treatments. Pharmacol. Res. 2023 Apr;190 doi: 10.1016/j.phrs.2023.106718. [DOI] [PubMed] [Google Scholar]
- 204.Smirnova A., Yatsenko E., Baranovskii D., et al. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: the risk of senescent drift induction in secretome-based therapeutics. Mil Med Res. 2023 Nov 30;10(1):60. doi: 10.1186/s40779-023-00498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang C.K., Tsai T.H., Lee C.H. Regulation of exosomes as biologic medicines: regulatory challenges faced in exosome development and manufacturing processes. Clin Transl Sci. 2024 Aug;17(8) doi: 10.1111/cts.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]